Abstract
Background
The aim of this study was to investigate the effects of TNF-α and IL-10 on the expression of ICAM-1 and CD31 in human coronary artery endothelial cells (HCAEC).
Material/Methods
HCAEC was treated with 0, 2.5 μg/l, 5 μg/l, and 10 μg/l of TNF-α for 2 h, 6 h, and 10 h, and with 0 μg/l, 10 μg/l, 100 μg/l, and 200 μg/l of IL-10 for 5 h, 10 h and 15 h, respectively. RNA inference of TNF-αR was performed with siRNA. Real-time PCR, Western blot analysis, and ELSA were used to detect the mRNA level and protein level of ICAM-1 and CD31.
Result
TNF-α significantly increased the mRNA and protein expression of ICAM-1 (P<0.05), and 2.5 μg/l TNF-α had the most obvious effect. RNAi of TNF-αR reduced the induction of TNF-α on the mRNA and protein expression of ICAM-1 (P<0.05). TNF-α significantly decreased the CD31 in the supernatant (P<0.05), and 2.5 μg/l TNF-α had the most obvious effect. IL-10 significantly decreased the ICAM-1 protein level. IL-10 decreased the mRNA expression and the protein expression of CD31. The effect on mRNA was not significant (P>0.05), while the effect on the protein expression was significant (P<0.05).
Conclusions
TNF-α and IL-10 treatment can affect the expression of ICAM-1 and CD31 in HCAEC.
MeSH Keywords: Antigens, CD31; Atherosclerosis; Receptors, Interleukin-10
Background
Atherosclerosis (AS) is common in vascular diseases and seriously affects human health. The pathogenesis of AS has been widely investigated [1–3]. The role of inflammation in AS has received much attention [3]. There are inflammatory responses in AS, such as degeneration, exudation, and hyperplasia [4,5].
Inflammatory factors, such as tumor necrosis factor α (TNF-α), interleukin (IL)-10, intercellular cell adhesion molecule-1 (ICAM-1), and CD31, are closely related to AS [6]. Normal endothelial cells can regulate vascular tension, maintain vascular structure, secrete anticoagulant substances, and have anti-inflammatory effects [7]. Vascular endothelial dysfunction is characterized by vascular tension adjustment disorder, abnormal adhesion molecules, and an attenuated or even depleted endothelium-dependent relaxation response caused by increased endothelial-derived relaxation factor, which is synthetized and released by endothelial cells [6–8].
TNF-α is mainly produced by mononuclear macrophages, neutrophils, activated NK cells, and mast cells, and is involved in the inflammatory response [9]. TNF-α acts on vascular endothelial cells and enhances the expression of adhesion molecules by endothelial cells and leukocytes, which results in inflammatory cell adhesion, infiltration, and neutrophil degranulation. Then, the coagulation, thrombosis and obstruction of blood vessels are induced, which further promotes AS plaque formation [10]. The receptors of TNF-α include TNF-αR1 (low affinity) and TNF-αR2 (high affinity) [11].
IL-10 mainly regulates the production of T cells, B cells, and NK cells, and inhibits the transcription, expression, and secretion of cytokines in a variety of immunocompetent cells [8]. Thereby, IL-10 can prevent thrombosis, myocardial ischemia, and ischemia-reperfusion injury, protect the myocardium, and regulate immune cells and immune molecules [12]. IL-10 mainly participates in immune-mediated injury at the early stage, and mainly inhibits the release of inflammatory factors at the later stage [13]. Coronary artery injury occurs when IL-10 level is too low in the circulating system, which is insufficient to antagonize the effects of IL-18, TNF-α, and other inflammatory factors [9]. Increased IL-10 level may reflect the extent of systemic inflammatory response in patients with myocardial infarction [10–14].
ICAM-1 is an adhesion molecule closely related to vascular diseases [15], and is widely expressed in endothelial cells after ischemia/reperfusion. ICAM-1 is involved in white blood cell adhesion and aggregation [16], participates in lipid metabolism, and affects the levels of cholesterol, low-density lipoprotein, and high-density lipoprotein [17,18].
Immunoglobulin CD31 is highly expressed in vascular endothelial cells [19]; it participates in the maintenance of monolayer structural integrity and promotes cell adhesion, accumulation, activation, and release of platelet-leukocyte-endothelial. Low expression of CD31 increases the permeability of vascular endothelial cells, which results in vascular endothelial cell dysfunction and promotes cerebrovascular AS. In AS, platelet endothelial cell adhesion molecule-1 (PECAM-1)-dependent immunosuppression is disturbed, which in turn promotes leukocyte recruitment and AS inflammation [20].
AS often involves coronary endothelium, and the expression of ICAM-1 and CD31 in Human Coronary Artery Endothelial Cells (HCAEC) induced by TNF-α and IL-10 is still unclear. In the present study, HCAEC cells were used and treated with TNF-α or IL-10. The levels of CD31 and ICAM-1 were measured and analyzed.
Material and Methods
Cell culture and treatment
The HCAEC cells were obtained from Shanghai Langkang Biotech, Ltd. (Shanghai, China). They were cultured in DMEM medium supplemented with 15% fetal bovine serum and endothelial cell growth factor and kept in an incubator at 37°C and 5% CO2. The study was approved by the Ethics Committee of Inner Mongolia Medical University (number YKD2016063).
For TNF-α stimulation, cells were treated with 2.5 μg/l, 5 μg/l, and 10 μg/l of TNF-α for 2 h, 6 h, and 10 h, respectively. For IL-10 stimulation, cells were incubated with 10 μg/l, 100 μg/l, and 200 μg/l of IL-10 for 5 h, 10 h, and 15 h, respectively. The cells without TNF-α or IL-10 treatment were used as negative controls.
RNA interference
According to different treatments, cells were divided into a control group (without treatment), a TNF-α group, a TNF-α+siRNA group, and a siRNA group. Cells in the TNF-α group were treated with 2.5 μg/l TNF-α for 6 h. Cells in the siRNA group were transfected with siRNA of TNF-α receptor. In the TNF-α+siRNA group, cells were first treated with siRNA of TNF-α receptor and then with 2.5 μg/l TNF-α for 6 h. After treatment, the cells were incubated at 37°C for 24 h.
ELISA
The supernatants were collected as the samples. The levels of CD31 and ICAM-1 were determined with a quantitative sandwich enzyme immunoassay technique according to the manufacturer’s instructions (Flarebio, Wuhan, China). HRP-labeled goat anti-rabbit IgG (1: 2000) were used as the secondary antibody. The absorbance value at 450 nm wavelength was read with a microplate reader (Multiskan MK-3, Finland).
Western blotting
Cells were harvested and total protein was isolated. Protein concentration was measured by a BCA protein assay kit (Beyotime, Nanjing, China). Then, proteins were electrophoresed on 12% SDS-PAGE and transferred to a PVDF membrane. Membranes were blocked with blocking buffer (Thermo Scientific, Waltham, USA) for 1 h at room temperature and then incubated with primary antibodies against ICAM-1 and β-actin (Protein Tech, Chicago, USA) overnight at 4°C. After rinsing with PBS, membranes were incubated with the corresponding secondary antibodies (Protein Tech, Chicago, USA) for 1 h at room temperature. Finally, the antibody-bound proteins were detected by use of an ECL Western blot detection kit (Santa Cruz, CA, USA). The densities of protein bands were measured using Image J software. The relative expression of ICAM-1 was expressed by the ratio of ICAM-1 to β-actin protein.
Real-time PCR
Total RNA was isolated by Trizol method. RNA was reverse-transcribed into cDNA using the TIANscript RT Kit (TIANGEN, Beijing, China). Real-time PCR assays were performed to detect the expression levels of ICAM-1 mRNA and CD31 mRNA. GADPH was used as an internal control. Primers used in this study were designed using ABI real-time fluorescence quantitative PCR software and are shown in Table 1. The 2(−ΔΔCT) method was used to calculate the relative expression levels of ICAM-1 and CD31 mRNA. Briefly, the DCt value for each sample was calculated according to the equation: DCt [Ct (sample)–Ct (input)]. Next, the DDCt was calculated by DDCt=DCt (sample)–DCt (control). Finally, fold difference between sample and control was calculated as 2(−ΔΔCt).
Table 1.
Primers used in this study.
| Name | Sequence (5′ to 3′) |
|---|---|
| GAPDH-F | GAGTCAACGGATTTGGTCGT |
| GAPDH-R | TTGATTTTGGAGGGATCTCG |
| ICAM-1-F | TGGGCAAGAACCTTACCCTACG |
| ICAM-1-R | TTCAGTGCGGCACGAGAAATT |
| CD31-F | CATGGTGGAGCACAGTGGCA |
| CD31-R | TGGGATGGAGCAGGACAGGTT |
Statistical analysis
All results are expressed as mean ±SD, and statistical analysis was performed using SPSS statistical software (version 13.0). To compare the same measurement indexes between groups, one-way ANOVA was used if the measured data were normally distributed and the variance between groups was homogeneous. The rank sum test was used if the distribution was not normal or the variance was not homogeneous. A P value less than 0.05 was considered as statistically significant.
Results
Effect of human TNF-α on ICAM-1 in HCAEC
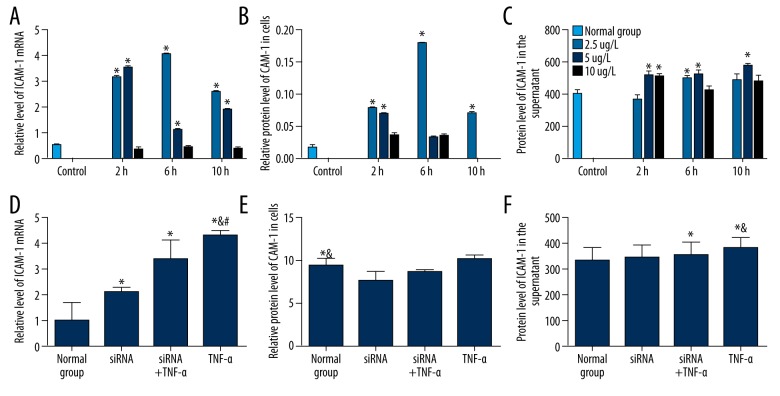
To investigate the proinflammatory effect of TNF-α on coronary artery endothelial cells, HCAEC was treated with different concentrations of TNF-α for different times. The ICAM-1 mRNA was detected by qRT-PCR. ICAM protein level was detected by Western blot and ELISA. Compared with control, TNF-α significantly increased the mRNA (Figure 1A) and protein expression of ICAM-1 (Figure 1B, 1C) (P<0.05); the optimal concentration of TNF-α was 2.5 μg/l and the optimal treatment time was 6 h. However, after blocking TNF-αR expression by siRNA, the effect of TNF-α on the mRNA (Figure 1D) and protein expression of ICAM-1 (Figure 1E, 1F) was abolished. These results indicate that TNF-α can induce the increase of ICAM-1 in HCAEC cells.
Figure 1.
Effect of human TNF-α on ICAM-1 in HCAEC. (A–C) The level of ICAM was detected after treatment with different concentrations of TNF-α for indicated times. The ICAM-1 mRNA level (A), protein expression in membrane (B), and in the supernatant (C) were detected by qRT-PCR, Western Blot, and ELISA, respectively. The concentrations of TNF-α are labeled on the right. (D–F) The level of ICAM was detected after treatment with TNF-α and TNF-αR siRNA. The ICAM-1 mRNA level (D), protein expression in membrane (E), and in the supernatant (F) were detected by qRT-PCR, Western blot, and ELISA, respectively. Normal Group, cells without treatment; TNF-α, cells were treated with 2.5 μg/l TNF-α for 6 h; siRNA, cells were transfected with siRNA of TNF-α receptor; TNF-α+siRNA, cells were first transfected with siRNA of TNF-α receptor and then with 2.5 μg/l TNF-α for 6 h; * P<0.05 compared with the normal group; & P<0.05 compared with the siRNA group; # P<0.05 compared with siRNA+TNF-α group.
Effect of human TNF-α on CD31 in HCAEC
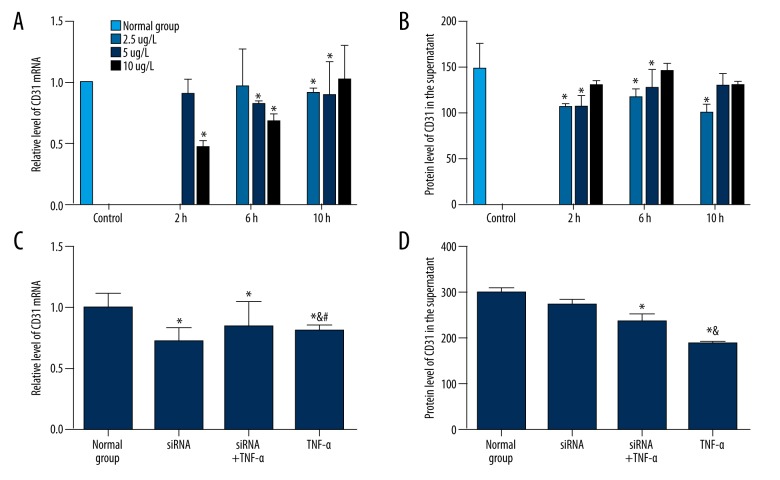
To determine the effect of TNF-α on CD31, qRT-PCR and ELISA were performed to detect the mRNA and protein level of CD31, respectively. As shown in Figure 2A, the effect of TNF-α on CD31 mRNA was not significant (P>0.05). TNF-α significantly decreased CD31 protein in the culture supernatant (P<0.05), and treatment with 5 μg/l TNF-α for 6 h had the most obvious effect (Figure 2B). RNAi of TNF-αR had no statistically significant effect on the blocking effect of TNF-α on the mRNA expression and protein expression of CD31 (Figure 2C, 2D). These results indicate that TNF-α can inhibit the CD31 protein level in the supernatant, but has no obvious influence on mRNA level of CD31 in HCAEC.
Figure 2.
Effect of human TNF-α on CD31 in HCAEC. (A, B) The level of CD31 was detected after treatment with different concentrations of TNF-α for indicated times. The CD31 mRNA level (A) and protein expression in the supernatant (B) were detected by qRT-PCR and ELISA, respectively. The concentrations of TNF-α is labeled on the right. (C, D) The level of ICAM was detected after treatment with TNF-α and TNF-αR siRNA. The CD31 mRNA level (C) and protein expression in the supernatant (D) were detected by qRT-PCR and ELISA, respectively. Normal group, cells without treatment; TNF-α, cells were treated with 2.5 μg/l TNF-α for 6 h; siRNA, cells were transfected with siRNA of TNF-α receptor; TNF-α+siRNA, cells were first transfected with siRNA of TNF-α receptor and then with 2.5 μg/l TNF-α for 6 h; * P<0.05 compared with normal group; & P<0.05 compared with siRNA group; # P<0.05 compared with siRNA+TNF-α group.
Effect of human IL-10 on ICAM-1 in HCAEC
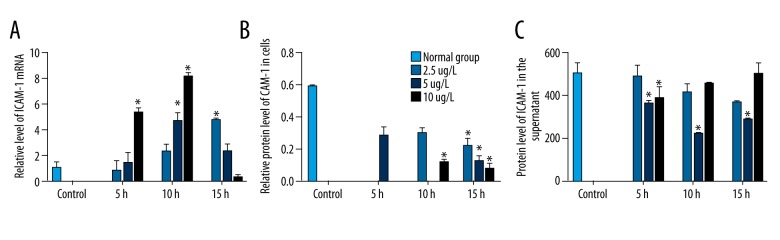
To investigate the inhibitory effect of IL-10 on coronary artery endothelial cells, HCAECs were was treated with different concentrations of IL-10 for different times. The ICAM-1 mRNA was detected by PCR and ICAM-1 protein was measured by Western blot and ELISA. Compared with control, IL-10 increased the mRNA expression of ICAM-1 (Figure 3A). IL-10 significantly decreased the ICAM-1 protein level in cells and in the supernatant (P<0.05, Figure 3B, 3C). The optimal concentration of IL-10 was 100 μg/L and the optimal treatment time was 10 h.
Figure 3.
Effect of human IL-10 on ICAM-1 in HCAEC. The level of ICAM was detected after treatment with different concentrations of IL-10 for indicated times. The ICAM-1 mRNA level (A), the ICAM-1 protein expression in membrane (B), and in the supernatant (C) were detected by qRT-PCR, Western blot, and ELISA, respectively. The concentrations of IL-10 are labeled on the right. * P<0.05 compared with normal group.
Effect of human IL-10 on CD31 in HCAECs
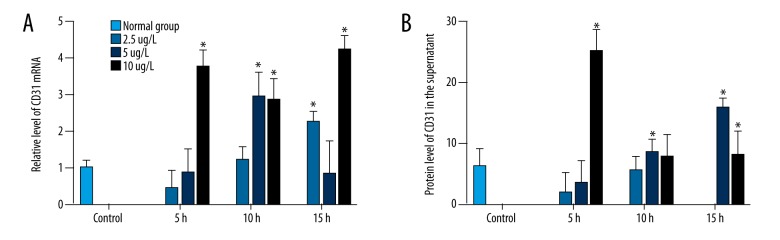
The effect of IL-10 on CD31 expression was measured by qRT-PCR and ELISA, respectively. Compared with control, 100 and 200 μg/l of IL-10 increased the mRNA expression and the protein expression of CD31 (Figure 4A, 4B), and the effect on mRNA was significant (P>0.05). These results demonstrate that IL-10 can inhibit inflammation by increasing the expression of CD31.
Figure 4.
Effect of human IL-10 on CD31 in HCAEC. The level of CD31 was detected after treatment with different concentrations of IL-10 for indicated times. The CD31 mRNA level (A) and protein expression in the supernatant (B) were detected by qRT-PCR and ELISA, respectively. The concentrations of IL-10 are labeled on the right. Cells were treated with different concentrations of IL-10 for indicated times. * P<0.05 compared with normal group.
Discussion
AS is the most common and important vascular disease; it can involve the coronary artery, carotid artery, and other arteries [21–23]. It is reported that TNF-α can positively regulate the expression of ICAM-1 in a dose- and time-dependent manner [24,25]. In the present study, we found that TNF-α showed an inducible effect on mRNA and protein levels of ICAM-1 in a time-dependent manner. After TNF-α stimulation, ICAM-1 on endothelial cells and leukocytes was increased, which thereby increases the adhesion between leukocytes and the vascular endothelium, mediates the inflammatory response, and thus promotes the process of AS [26]. RNAi of TNF-αR blocked the effect of TNF-α on ICAM-1, indicating that TNF-αR mediates the effect of TNF-α on ICAM-1 expression.
In this study, we found that TNF-α decreased the expression of CD31 protein. CD31 is highly expressed in all vascular endothelial cells of mature individuals, mainly located in the junction of endothelial cells, and is involved in the maintenance of structural integrity of vascular endothelial cell monolayers. Under physiological conditions, CD31 supports endothelial barrier function [27]. In atherosclerotic vasculitis, the function of CD31 is stimulated by proinflammatory cytokines and adhesion factors [28]. In inflammatory atherosclerosis, the expression of CD31 is decreased and its function is impaired, which impairs the barrier function of endothelial cells [29]. Therefore, we hypothesized that the decrease in CD31 reduces the barrier function of endothelial cells and results in increased adhesion of leukocytes and reduced vascular integrity, thus promoting inflammation and the formation of arterial plaques. Furthermore, RNAi of TNF-αR does not significantly affect the inhibitory effect of TNF-α on CD31, suggesting that TNF-αR does not mediate the effect of TNF-α on CD31.
Our study showed that IL-10 increased the mRNA level of ICAM-1 in HCAEC but decreased the protein expression of ICAM-1, and the effect was correlated with treatment time and IL-10 concentration. This suggests that IL-10 has a positive effect on ICAM-1 transcription, but has an inhibitory effect on the protein expression of ICAM-1, suggesting that the inhibitory effect of IL-10 on inflammation is mediated by the protein level of ICAM-1. A study has also shown [30] that IL-10 inhibits inflammation at low concentrations and short duration, and promotes inflammation at high concentrations and long durations, which is consistent with the effect of IL-10 on the level of ICAM-1 protein. The expression and regulation of ICAM-1 may have a certain time delay [31], which is consistent with our results.
Our results also showed that IL-10 increased the mRNA level of CD31, but the effect was not significant. IL-10 significantly increased the protein expression of CD31, suggesting that IL-10 enhances the barrier function of endothelial cells and inhibits AS by up-regulating CD31, resulting in increased neutrophils and decreased adhesion of other leukocytes to endothelial cells. Therefore, it enhances vascular integrity, reduces movement of white blood cells into the intima medium, delays the development of inflammation, and prevents atherosclerosis [32].
Our study has several limitations. First, only mechanism research was conducted in this study. Second, the combined effects of TNF-α and IL-10 were not investigated. Third, the other downstream targets of TNF-α and IL-10 were not analyzed. Future studies, such as functional studies of TNF-α and IL-10, are warranted.
Conclusions
We found that TNF-α promotes the mRNA and protein expression of ICAM-1 through TNF-αR. TNF-α inhibits the protein level of CD31. This inhibitory effect on CD31 is not through TNF-αR. The effects of TNF-α on ICAM-1 and CD-31 may further play a positive role in promoting AS. IL-10 inhibits the expression of ICAM-1, promotes CD31 expression, and inhibits inflammation.
Footnotes
Conflicts of interest
None.
Source of support: This study was supported by a grant from the Inner Mongolia Natural Science Foundation (No. 2013MS1135)
References
- 1.Liu JT. [Advances in inflammatory mechanisms of AS pathogenesis]. Journal of Xian Jiaotong University (Medical Sciences) 2015;36:141–52. [in Chinese] [Google Scholar]
- 2.Sun ZH. Atherosclerosis and atheroma plaque rupture: Normal anatomy of vasa vasorum and their role associated with atherosclerosis. ScientificWorldJournal. 2014;25:1–6. doi: 10.1155/2014/285058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäck M, Weber C, Lutgens E. Regulation of atherosclerotic plaque inflammation. J Intern Med. 2015;278(5):462–82. doi: 10.1111/joim.12367. [DOI] [PubMed] [Google Scholar]
- 4.Chiaretti A, Capozzi D, Mariotti P, et al. Increased levels of neurotrophins in the cerebrospinal fluid of children with Epstein-Barr virus meningoencephalitis. Int J Infect Dis. 2014;20:52–57. doi: 10.1016/j.ijid.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Eder P, Lykowska-Szuber L, Iwanik K, et al. The influence of anti-TNF therapy on CD31 and VEGF expression in colonic mucosa of Crohn’s disease patients in relation to mucosal healing. Folia Histochem Cytobiol. 2016;6:1–20. doi: 10.5603/FHC.a2016.0008. [DOI] [PubMed] [Google Scholar]
- 6.Huang D, Zhao Q, Liu H, et al. PPAR-α agonist WY-14643 inhibits LPS-induced inflammation in dynovial fibroblasts via NF-κB pathway. J Mol Neurosci. 2016;59:1–10. doi: 10.1007/s12031-016-0775-y. [DOI] [PubMed] [Google Scholar]
- 7.Gerdes N, Seijkens T, Lievens D, et al. Platelet CD40 exacerbates atherosclerosis by transcellular activation of endothelial cells and leukocytes. Arterioscler Thromb Vasc Biol. 2016;36:482–90. doi: 10.1161/ATVBAHA.115.307074. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Qi G, Wang L, et al. Deficiency of programmed cell death 4 results in increased IL-10 expression by macrophages and thereby attenuates atherosclerosis in hyperlipidemic mice. Cell Mol Immunol. 2016;13:524–34. doi: 10.1038/cmi.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren ZQ, Liu N, Zhao K. Micro RNA-19a suppresses IL-10 in peripheral B cells from patients with atherosclerosis. Cytokine. 2016;86:86–91. doi: 10.1016/j.cyto.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Zhang W, Huang T, et al. Relationship between inflammatory factors TNF-α, IL-6, IL-17 and rheumatoid arthritis complicated with atherosclerosis. Immunol J. 2017 [in Press] [Google Scholar]
- 11.Tay C, Liu YH, Hosseini H, et al. B cell-specific depletion of TNFα inhibits atherosclerosis development and plaque vulnerability to rupture by reducing cell death and inflammation. Cardiovasc Res. 2016;111:385–97. doi: 10.1093/cvr/cvw186. [DOI] [PubMed] [Google Scholar]
- 12.Stöger JL, Boshuizen MC, Brufau G, et al. Deleting myeloid IL-10 receptor signalling attenuates atherosclerosis in LDLR−/− mice by altering intestinal cholesterol fluxes. Thromb Haemost. 2016;116:565–77. doi: 10.1160/TH16-01-0043. [DOI] [PubMed] [Google Scholar]
- 13.Meng K, Bangwei WU, Huang Y, et al. Neutralization of interleukin 23 attenuates angiotensin II-induced atherosclerosis in ApoE−/− mice. J Clin Cardiol. 2016;5:82–85. [Google Scholar]
- 14.Dražen P, Nikolajević SJ, Šantl LM, et al. Polymorphism rs5498 of the ICAM-1 gene affects the progression of carotid atherosclerosis in patients with type 2 diabetes mellitus. Lipids Health Dis. 2016;15:1–7. doi: 10.1186/s12944-016-0247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzolla V, Armani A, Mammi C, et al. Essential role of ICAM-1 in aldosterone-induced atherosclerosis. Int J Cardiol. 2017;232:233–42. doi: 10.1016/j.ijcard.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang JX, Pan YY, Ge JH, et al. Tanshinone II A attenuates TNF-α-induced expression of VCAM-1 and ICAM-1 in endothelial progenitor cells by blocking activation of NF-κB. Cell Physiol Biochem. 2016;40:195–206. doi: 10.1159/000452537. [DOI] [PubMed] [Google Scholar]
- 17.Lai ZZ, Ji CL, Xu XJ, et al. [Effect of tongfu yiqi decoction on TNF-α, IL-lβ and ICAM-1 in rats with sepsis]. Zhejiang Journal of Integrated Traditional Chinese and swestern Medicine. 2016;6:9–12. [in Chinese] [Google Scholar]
- 18.Wu T, Shi JX, Geng S, et al. The MK2/HuR signaling pathway regulates TNF-α-induced ICAM-1 expression by promoting the stabilization of ICAM-1 mRNA. BMC Pulm Med. 2016;16:1–11. doi: 10.1186/s12890-016-0247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang FY, Gao H, Zhao ZH. [Expression of TNF- and sICAM-1 in intracranial aneurysms]. Heilongjiang Medicine and Pharmacy. 2016;39:53–54. [in Chinese] [Google Scholar]
- 20.Chiaretti A, Capozzi D, Mariotti P, et al. Increased levels of neurotrophins in the cerebrospinal fluid of children with Epstein-Barr virus meningoencephalitis. Int J Infect Dis. 2014;5:52–57. doi: 10.1016/j.ijid.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Franz J, Brinkmann BF, König M, et al. Nanoscale imaging reveals a Tetraspanin-CD9 coordinated elevation of endothelial ICAM-1 clusters. PLoS One. 2016;11:e0146598. doi: 10.1371/journal.pone.0146598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Podgórski M, Kupczyk M, Grzelak P, et al. Inhaled corticosteroids in asthma: promoting or protecting against atherosclerosis? Med Sci Monit. 2017;23:5337–44. doi: 10.12659/MSM.904469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia Q, Liu H, Li Y, et al. Combination of magnetic resonance angiography and computational fluid dynamics may predict the risk of stroke in patients with asymptomatic carotid plaques. Med Sci Monit. 2017;23:479–88. doi: 10.12659/MSM.902995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiaretti A, Antonelli A, Piastra M, et al. Expression of neurotrophic factors in cerebrospinal fluid and plasma of children with viral and bacterial meningoencephalitis. Acta Paediatr. 2004;93:1178–84. doi: 10.1080/08035250410031314. [DOI] [PubMed] [Google Scholar]
- 25.Rieber-Mohn AB, Sugulle M, Wallukat G, et al. Auto-antibodies of the renin-angiotensin-system in preeclampsia and uteroplacental acute atherosis. Pregnancy Hypertension An International Journal of Womens Cardiovascular Health. 2017;7:61–62. [Google Scholar]
- 26.Lim JC, Ko KI, Mattos M, et al. TNF-α contributes to diabetes impaired angiogenesis in fracture healing. Bone. 2017;99:26–38. doi: 10.1016/j.bone.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rakocevie J, Orlic O, Mitrovic-Ajtic O, et al. Endothelial cells markers from clinician’s perspective. Exp Mol Pathol. 2017;102:303–13. doi: 10.1016/j.yexmp.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Fike AJ, Nguyen LT, Kumova OK, et al. Characterization of CD31 expression on murine and human neonatal T lymphoytes during development and activation. Pediatr Res. 2017;82(1):133–40. doi: 10.1038/pr.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beigi F, Patel M, Morales-Garza MA. Optimized method for isolating highly purified and fanctional porcine aortic endothelial and smooth muscle cells. J Cell Physiol. 2017;232:3139–45. doi: 10.1002/jcp.25764. [DOI] [PubMed] [Google Scholar]
- 30.Dagdeviren S, Jung DY, Friedline RH, et al. IL-10 prevents aging-associated in skeletal muscle. FASEB J. 2017;31:701–10. doi: 10.1096/fj.201600832R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pflugfelder SC, Stern M, Zhang S, et al. LFA-1/ICAM-1 interaction as a therapeutic target in dry eye disease. J Ocul Pharmacol Ther. 2017;33:5–12. doi: 10.1089/jop.2016.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cochain C, Zernecke A. Macrophages in vascular inflammation and atherosclerosis. Eur J Physiol. 2017;469:485–99. doi: 10.1007/s00424-017-1941-y. [DOI] [PubMed] [Google Scholar]