Abstract
The CXC chemokine CXCL12 is an important factor in physiological and pathological processes, including embryogenesis, hematopoiesis, angiogenesis and inflammation, because it activates and/or induces migration of hematopoietic progenitor and stem cells, endothelial cells and most leukocytes. Therefore, CXCL12 activity is tightly regulated at multiple levels. CXCL12 has the unique property of existing in six splice variants in humans, each having a specific tissue distribution and in vivo activity. Controlled splice variant transcription and mRNA stability determine the CXCL12 expression profile. CXCL12 fulfills its functions in homeostatic and pathological conditions by interacting with its receptors CXC chemokine receptor 4 (CXCR4) and atypical chemokine receptor 3 (ACKR3) and by binding to glycosaminoglycans (GAGs) in tissues and on the endothelium to allow a proper presentation to passing leukocytes. Homodimerizaton and heterodimerization of CXCL12 and its receptors can alter their signaling activity, as exemplified by the synergy between CXCL12 and other chemokines in leukocyte migration assays. Receptor binding may also initiate CXCL12 internalization and its subsequent removal from the environment. Furthermore, CXCL12 activity is regulated by posttranslational modifications. Proteolytic removal of NH2- or COOH-terminal amino acids, citrullination of arginine residues by peptidyl arginine deiminases or nitration of tyrosine residues reduce CXCL12 activity. This review summarizes the interactions of CXCL12 with the cellular environment and discusses the different levels of CXCL12 activity regulation.
Keywords: ACKR3, chemokine, CXCL12, CXCR4, regulation
Introduction
Chemotactic cytokines or chemokines are a large group of low molecular weight proteins that promote migration and adhesion of their target cell populations. Structurally, they are divided into four groups based on the position of their conserved NH2-terminal cysteine residues. Whereas the largest subgroup of chemokines, the CC chemokines, have two adjacent conserved cysteine residues, C chemokines have only one NH2-terminal cysteine residue. CXC and CX3C chemokines have one or three other amino acids, respectively, in between their conserved NH2-terminal cysteine residues.1 Chemokines fulfill their biological functions by activating their respective seven-transmembrane domain G protein-coupled receptors (GPCRs), similarly categorized as CC chemokine receptors (CCRs), XCRs, CXCRs and CX3CRs.2
Functionally, chemokines can be divided into inflammatory and homeostatic chemokines based on their inducible or constitutive production, respectively. One such homeostatic CXC chemokine is CXCL12. CXCL12 was initially discovered as pre-B cell growth factor (PBGF) and found to be indispensable for homeostatic processes such as lymphopoiesis and embryogenesis.3 Soon thereafter, it was found that PBGF was expressed constitutively by bone marrow stromal cells and was thus named stromal cell-derived factor-1 (SDF-1).4 In the bone marrow, CXCL12 is responsible for the retention of hematopoietic progenitor and stem cells.5,6 CXCL12 stands out compared to other members of the CXC chemokine family regarding its chromosomal location. While most genes for CXC chemokines are located on chromosome 4q21, the gene encoding CXCL12 is located on chromosome 10q11.7,8 In addition, CXCL12 is the only CXC chemokine with differential mRNA splicing. Six different splice variants have been identified in humans (CXCL12α to φ) and three (CXCL12α to γ) have been identified in mice.8,9 Furthermore, CXCL12 belongs to a limited group of cytokines and growth factors that show 90% or higher homology between humans and mice on both the genome and protein level.8 Orthologs of CXCL12 and CXCR4 with a remarkably similar amino-acid sequence are also found in less evolved species like frogs (X. tropicalis) and even zebrafish (D. rerio), suggesting an ancestral origin and an important biological role for CXCL12 that provided the evolutionary pressure to retain this protein and to prevent mutations.10,11
In contrast to most receptors for inflammatory chemokines, CXCR4 only has CXCL12 as a ligand. In addition, CXCR4 is the only chemokine receptor of which knockout mice die perinatally. Mice lacking CXCR4 show lethally defective cardiac ventricular septa and embryonic hematopoiesis and neurogenesis, a phenotype similar to that of CXCL12 knockout mice.3,12,13 ACKR3 knockout mice show defective and lethal cardiac development, stressing the importance of the CXCL12/CXCR4/ACKR3 system in cardiogenesis.14,15
Because CXCR4 and ACKR3 are expressed on many cell types, regulation of CXCL12 activity is crucial for balanced homeostasis. In this manuscript, we will discuss the regulatory processes that control CXCL12 function, including controlled transcription, tissue distribution of splice variants, protein availability and cooperativity and posttranslational modifications, such as truncation, citrullination and nitration. The importance of correct CXCL12 regulation will be stressed by examples in which certain imbalances in the CXCL12/CXCR4/ACKR3 axis are associated with diseases, including cancer, multiple sclerosis and rheumatoid arthritis.
Interactions of cxcl12 with its environment
CXCL12 functions by activating CXCR4 and ACKR3
Signal transduction pathways downstream of CXCR4
CXCR4 (CD184) was initially discovered as leukocyte-derived seven-transmembrane domain receptor (LESTR).16 It is a rhodopsin-like chemokine receptor with seven transmembrane domains that is typically categorized as a G protein-coupled receptor (GPCR). Soon after, LESTR/fusin was shown to be a co-factor for human immunodeficiency virus (HIV)-1 cell entry.17 The same year, two groups separately showed that the chemokine CXCL12, at that point still called SDF-1, acted as the natural ligand for this receptor and could block infection of T cells by HIV-1 strains that use LESTR/fusin as coreceptor.18,19 Based on its connection with CXCL12, LESTR/fusin was renamed CXCR4 and categorized as a chemokine receptor.
The importance of this receptor is highlighted by the discovery of a continuously increasing number of cell types that express it on their surface membrane. These cell types comprise most leukocyte subsets, hematopoietic progenitor and stem cells found in circulation and cells of lymphoid organs like the bone marrow, thymus and lymph nodes. Endothelial cells and stromal and epithelial cells in the bone marrow, lung and small intestine also express CXCR4.20 Moreover, unlike other chemokine receptors, knocking out CXCR4 causes perinatal death.21 Indeed, Cxcr4 knockout mice show severe defects in hematopoiesis, neurogenesis, vascularization and cardiogenesis.12 In addition, it was discovered that CXCR4 exists as two splice variants, that is, CXCR4-A and CXCR4-B. CXCR4-B is more abundantly expressed and is the result of mRNA splicing. CXCR4-A is translated from unspliced mRNA that has a different start codon than CXCR4-B. As a result, the NH2 terminus of CXCR4-A is four amino acids longer than that of CXCR4-B and differs by five amino acids.22 While Duquenne et al. 23 observed no difference in activity between these two receptor splice variants, Gupta et al. 22 reported reduced activity for CXCR4-A. In any case, both receptors are functional, and CXCR4-A is thought to function as a safety back-up receptor for CXCR4-B. Indeed, given the importance of CXCR4 expression in embryonic development, in the case of absent CXCR4 mRNA splicing, an unspliced mRNA can still be translated into a functional receptor. Accordingly, a functional unspliced CXCR4 was detected in mice.24 This is not surprising, as CXCR4 is highly conserved among species and shows striking homology (89%) between humans and mice.20
CXCL12 activation is dependent on the first eight NH2-terminal amino acids of CXCL12, of which the first two amino acids, Lys and Pro, are absolutely necessary. Furthermore, an NH2-terminal CXCR4-binding motif with the amino-acid sequence RFFESH improves the binding of CXCL12 to its receptor (both CXCR4-binding sites are shown in Figure 1a). The discovery of the necessity of these two sites for CXCR4 activation gave rise to a two-step model, as was previously demonstrated for the complement protein and chemoattractant C5a and its receptor.26 The RFFESH motif is responsible for initial contact between CXCL12 and CXCR4 and induces a conformational change that allows the NH2-terminal amino acids to activate the receptor.27 However, recent studies have shown that the interaction between chemokines and their receptors is more complex and involves several other domains in both the chemokine and the receptor. The evolution toward a multi-step model is reviewed in detail by Kleist et al. 28
Figure 1.
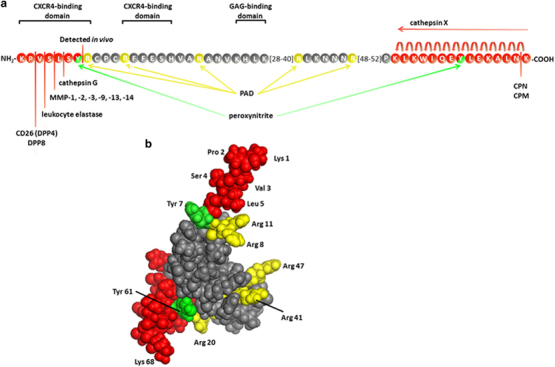
Posttranslational modifications of CXCL12α. (a) The amino-acid sequence (using the one-letter code) of CXCL12α is shown with the indicated GAG and receptor-binding domains. The enzymes responsible for NH2- and COOH-terminal truncation are indicated, and the cleaved amino acids are shown in red. Arg residues susceptible to citrullination by PAD activity are shown in yellow, and Tyr residues that may be nitrated are shown in green. (b) A 3D model of CXCL12α shows the localization of the potentially removed (red), citrullinated (yellow) and nitrated (green) amino acids. These amino acids are indicated with their three-letter code on the 3D model drawn from PDB accession code 2 kec.25
Figure 2 summarizes the principal signal transduction pathways that are activated by CXCL12. Activation of CXCR4 mainly induces G protein-coupled signal transduction, starting with the dissociation of the Gβγ and Gα subunits that are bound to the DRYLAIV amino-acid sequence in the second intracellular loop of the receptor. CXCR4 can be coupled to Gαi, Gαq or Gα12/13, resulting in activation of diverse signaling pathways.29 This results in a complex signaling cascade involving, among others, the mitogen-activated protein kinase (MAPK), phospholipase C and phosphatidylinositol-3-kinase pathways and ending with cellular migration or activation of adhesion molecules.30 Despite a lack of consensus in the matter, it was shown that CXCR4 could also signal independently of G proteins by the recruitment of Janus kinase (JAK)2 and JAK3 after receptor activation and homodimerization. The recruited kinases activate each other through transphosphorylation and subsequently phosphorylate CXCR4, allowing the signal transducer and activator of transcription (STAT) molecules STAT1, STAT2, STAT3 and STAT5 to be recruited and activated.31,32,33 Activated STAT dimers are known transcription factors and are additionally linked to Gαi-dependent intracellular calcium mobilization and chemotaxis.34 However, another group of researchers opposed these findings, and in several cell lines, JAK-deficient lymphocytes and primary human mononuclear cells, they showed that CXCL12 did not initiate signal transduction by JAK phosphorylation and did not subsequently induce STAT phosphorylation.35 β-Arrestin is also recruited to CXCR4 following its activation, showing diverse effects following its recruitment. It can directly activate signaling pathways, as has been shown for p38 MAPK,36 or it can serve as a scaffold protein that clusters proteins in the same pathway closely together.37 On the other hand, β-arrestin recruitment to CXCR4 also sterically blocks other signal transduction pathways and actively stimulates receptor internalization by facilitating clathrin and adaptin recruitment to the cell membrane.38
Figure 2.
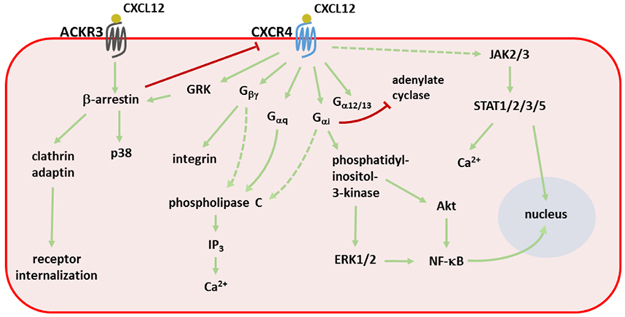
Signal transduction pathways activated by CXCL12. Stimulation of both CXCR4 and ACKR3 triggers a signaling cascade involving many second messengers. The primary pathways are depicted with green arrows, whereas inhibitory interactions are shown with a red line. Dashed lines represent signaling pathways that are without consensus in the literature. ERK1/2, extracellular signal-regulated kinase 1/2; GRK, G protein-coupled receptor kinase; IP3, inositol 3-phosphate; JAK2/3, Janus kinase 2/3; NF-κB, nuclear factor kappa-light-chain enhancer of activated B cells; PI3K, phosphatidylinositol-3-kinase; PLC, phospholipase C; STAT, signal transducer and activator of transcription.
CXCL12 is the only chemokine ligand for CXCR4, a unique feature in the promiscuous chemokine-receptor relationships. However, other non-chemokine ligands can bind and induce signal transduction via CXCR4, that is, macrophage migration inhibitory factor (MIF), extracellular ubiquitin and the HIV envelope protein gp120. Antagonizing ligands for CXCR4 are endogenous human β3-defensin and the viral chemokine mimicry protein viral macrophage inflammatory protein-II (vMIP-II). Interactions of these molecules with CXCR4 are reviewed elsewhere.20
ACKR3 has both scavenging and signaling functions
ACKR3, initially discovered as the orphan chemokine receptor RDC-1/CXCR7, binds CXCL11 and CXCL12 with high affinity.39,40,41 Binding of CXCL12 occurs with an affinity that is ten times higher than that for CXCR4 (K D of 0.4 nm for ACKR3).39 Similar to CXCR4, ACKR3 also binds gp120 from some viral strains and serves as a coreceptor for HIV entry.42 MIF also binds ACKR3 and activates platelets, stimulating their survival.43 ACKR3 is expressed on many cell types, including hematopoietic cells, neuronal progenitor cells and activated endothelial cells.20 ACKR3 activation mediates increased cell survival and adhesion, which are important characteristics that stimulate tumor growth.44 Several tumor types have been shown to express ACKR3.44,45 ACKR3−/− knockout mice show defective cardiac development with increased postnatal lethality.14,15 This phenotype is caused by loss of scavenging of probably both CXCL12 and adrenomedullin, another non-chemokine ligand of ACKR3.46,47 CXCL12- or CXCL11-induced signal transduction through ACKR3 is not initiated through the classical G proteins. Although ACKR3 has two amino-acid substitutions in the typical DRYLAIV motif for G protein binding, restoration of this motif does not enable ACKR3 to signal through G proteins.46 Instead, signal transduction is initiated by β-arrestins, and hence, it is categorized as an atypical chemokine receptor.2,48 However, this was challenged by Ödemis et al. 49 and colleagues, who reported G protein-mediated signaling through ACKR3 in rodent astrocytes and human glioma cells. Activation of ACKR3 by CXCL12 also results in MAPK-mediated signal transduction and migration of T cells and neural progenitor cells.39,50,51,52 In addition to its signaling properties, ACKR3 primarily has a scavenging function and removes CXCL12 from the environment.45,53,54 Together with the high CXCL12 binding affinity, this sequestering is achieved by the continuous internalization and recycling of ACKR3. In contrast to CXCR4, ACKR3 internalization occurs even without ligand binding and does not result in receptor degradation.46
Dimerization of both CXCL12 and its receptors alters signal transduction
Constitutive homodimerization of CXCR4 has been demonstrated even without ligand stimulation.55,56,57 In the other direction, sulfation of specific CXCR4 Tyr residues stimulates CXCL12 dimerization and results in a preference of CXCR4 for the dimerized ligand.58 This equilibrium of monomeric versus dimeric CXCL12 is important because each of these states triggers distinct signal transduction through CXCR4. However, there is no consensus on the resulting biased signaling; while one group reported equal G protein-mediated signaling and bias for monomeric CXCL12 toward β-arrestin, another group showed a bias toward G protein signal transduction for monomeric and β-arrestin signaling for dimeric CXCL12.59,60 In addition, ACKR3 preferentially removes CXCL12 monomers from the environment.60
Heterodimerization of CXCL12 with other molecules also occurs and has effects on CXCR4 signaling. For example, heterodimerization of high-mobility group box 1 (HMGB1) with CXCL12 is necessary for the attraction of monocytes through CXCR4 to sites of tissue injury.61 Furthermore, CXCL12 has been shown to heterodimerize with other platelet chemokines (CCL5, CXCL4 and CXCL7).62,63 Heterodimerization between ACKR3 and CXCR4 was demonstrated in transfected cells and resulted in increased calcium mobilization and an altered ERK1/2 phosphorylation pattern after CXCL12 stimulation.14 However, other groups reported that interaction between CXCR4 and ACKR3 inhibited G protein-mediated signaling through CXCR4.64,65 Decaillot et al. 65 showed that CXCR4 and ACKR3 heterodimers constitutively recruited β-arrestin to the cell membrane instead, stimulating downstream second messengers, such as p38 and ERK1/2. Several groups have reported that the in vitro chemotactic response of several cell types is increased due to ACKR3 and CXCR4 heterodimerization.64,65,66 The complexity of the interplay between CXCR4 and ACKR3 is further increased by the observation that the CXCR4 levels on a cell membrane are inversely correlated with those of ACKR3.67 CXCR4 also forms heterodimers with many other membrane molecules, including other chemokine receptors. These interactions are reviewed in detail elsewhere.28
Glycosaminoglycans are crucial elements in chemokine presentation
Important interaction partners for chemokines, other than their receptors, are glycosaminoglycans (GAGs) such as heparin and heparan sulfate. GAGs are long, unbranched polymers of negatively charged sulfated disaccharide units with enormous heterogeneity. They make up the glycan part of proteoglycans and form an extracellular, negatively charged matrix that allows interaction with positively charged protein structures. GAGs are critical components for the chemotactic activity of chemokines because the chemokine–GAG interaction is indispensable in the formation of chemokine gradients.68 For CXCL12, the importance of GAG binding for in vivo functionality has been shown. Mutant mice expressing CXCL12α, β and γ isoforms, which are functional on CXCR4 but unable to bind GAGs, had an increased number of circulating CD34+ cells and showed impaired revascularization due to the lack of infiltrating cells in ischemic areas.69 Moreover, the interaction with GAGs is crucial for proper in vivo CXCL12 function because the interaction with GAGs protects CXCL12 from NH2-terminal truncation and inactivation by CD26.70
Structural investigation of CXCL12 showed that a cluster of positively charged amino acids is present in the first β-strand of the amino-acid sequence, that is, the BBXB domain, where B stands for a basic amino acid and X for any other residue. In the case of CXCL12, this domain consists of Lys24, His25, Leu26 and Lys27, as shown in Figure 1a.71 Changing Lys and His residues in this domain to Ser resulted in reduced GAG and endothelial cell binding of CXCL12 and reduced transendothelial migration of peripheral blood mononuclear cells (PBMCs) in vitro.71,72 Moreover, due to this mutation, CXCL12 lost its potency to attract lymphocytes and monocytes to air pouches in vivo and even antagonized the functionality of wild-type CXCL12.72 Although this domain is undoubtedly necessary for GAG binding, other positively charged amino acids are also involved in this interaction. It was shown that NH2-terminal truncation of CXCL12 by one or two amino acids reduced the heparin-binding potential, as a positively charged Lys is removed.73,74,75 Furthermore, the splice variant CXCL12γ has a COOH-terminal extension of 20 amino acids compared with CXCL12α. This unstructured tail consists of 60% positively charged amino acids, mostly arranged in BBXB GAG-binding domains. As a result, CXCL12γ has a 10 times higher affinity for heparin compared to CXCL12α.76,77 Interaction of this positively charged tail with GAGs is necessary for CXCL12γ to achieve an appropriate orientation of its NH2 terminus to activate CXCR4. In the absence of GAGs, the COOH terminus of CXCL12γ interacts with sulfated amino acids of the CXCR4 NH2–terminus and blocks further activation.78
The tertiary and quaternary structure of CXCL12 is also important for GAG binding. A linear peptide containing the first 34 amino acids, including the BBXB domain, bound to heparin with far less affinity compared with folded, intact CXCL12.71 Sadir and colleagues showed that Arg41 and Lys43 are also involved in GAG binding and are located in close proximity to the BBXB domain in the folded protein. Furthermore, GAG-binding promotes CXCL12 homodimerization, which has effects on signaling through its receptors, as explained in the previous section.79 Upon CXCL12 dimerization, the GAG-binding tertiary structures of each CXCL12 monomer are oriented toward each other, creating a highly positive cluster that further potentiates GAG interactions.73
Regulatory processes for cxcl12 activity
As CXCL12 is a crucial chemokine in many homeostatic processes, such as neurogenesis, embryogenesis, angiogenesis and lymphopoiesis, and plays a role in inflammatory processes, its activity is tightly controlled. CXCL12 activity is regulated at many levels, that is, transcription, differential mRNA splicing, posttranslational modifications and protein availability and cooperativity. These regulatory processes are summarized in Figure 3.
Figure 3.
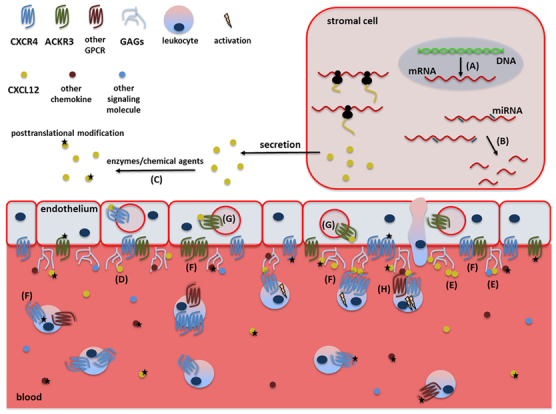
Levels of CXCL12 activity regulation. CXCL12 activity is controlled at multiple levels, starting with (a) controlled transcription and mRNA splicing of CXCL12 variants and (b) subsequent regulation of mRNA stability by microRNA (miRNA). After translation, CXCL12 and its receptor may be posttranslationally modified by several enzymes or chemical agents (c). On the endothelium, interaction of CXCL12 with GAGs is necessary for its immobilization on blood vessels and its presentation to passing leukocytes (d). This GAG interaction favors CXCL12 homo- and heterodimerization (e) and protects CXCL12 from posttranslational modifications. The seven-transmembrane domain receptors CXCR4 and ACKR3 are activated by CXCL12 and are able to form homo- or heterodimers among each other or with other chemokine receptors (f). ACKR3 internalizes CXCL12 and alters its gradient (g), whereas synergy between CXCL12 and other chemokines increases the response of target cells (h).
Controlled transcription of CXCL12 splice variants
CXCL12 is an important chemokine in homeostasis and is therefore continuously produced by different cell types. In addition, conditions such as hypoxia and growth arrest are able to induce increased CXCL12 expression.80 For example, augmented CXCL12 production is necessary to increase angiogenesis and assists in tissue healing and wound repair. In these situations, hypoxia-inducible factor-1 (HIF-1) upregulates the production of CXCL12 by endothelial cells, resulting in increased attraction of progenitor cells.81,82 Upregulation of CXCL12 by hypoxia also occurs during cancer development to promote angiogenesis, as has been demonstrated for ovarian cancer.83 Hypoxia also induces expression of CXCL12 receptors, for example, CXCR4 on endothelial and melanoma cells and ACKR3 on glioma cells.84,85,86 For example, preconditioning mesenchymal stem cells with hypoxia prior to engraftment increases CXCR4 and ACKR3 expression through HIF-1 and results in increased migration, adhesion and survival of these cells.87 On the other hand, CXCL12 expression can also be downregulated in tumor-associated mesenchymal stem cells by interaction of these cells with transforming growth factor-β (TGF-β), an event that promotes breast cancer metastasis.88
As mentioned before, CXCL12 exists in six different splice variants in humans (CXCL12α to φ) and three in mice (CXCL12α to γ).8,9 These splice variants are encoded by the same Cxcl12 gene and share the first three exons. They differ by the fourth exon, which determines the length of the splice variant. Indeed, all the CXCL12 isoforms share the first 67 amino acids and differ in length, with 68, 72, 98, 119, 69 and 79 amino acids for CXCL12α to φ, respectively.9 The importance of these splice variants is demonstrated by the different activities they show in various processes. For example, CXCL12α, CXCL12β and CXCL12ɛ are able to increase the survival rate of hematopoietic progenitor cells in vitro, while the other isoforms do not. All CXCL12 variants show anti-HIV-1 activity but with varying potencies, with CXCL12γ being the most potent.89 This isoform is, on the other hand, rather weak in inducing in vitro chemotaxis compared to the most studied form CXCL12α.76,77,78,89 CXCL12γ also exhibits lower activation of CXCR4-mediated signal transduction via CXCR4.78 In contrast, CXCL12γ is the most active variant regarding chemotaxis in vivo, probably due to the increased resistance of this isoform toward enzymatic inactivation.77 Indeed, as already mentioned, CXCL12γ has the highest affinity for GAGs, and this interaction offers protection toward proteolytic inactivation.70,76,77
In general, CXCL12α and to a lesser extent CXCL12β are the most abundant in adult tissues and are the only variants detected in bone marrow. CXCL12γ is mostly present in the heart, while CXCL12δ, CXCL12ɛ and CXCL12φ are most abundantly expressed in the pancreas. Remarkably, a shift in the expression pattern of the CXCL12 isoforms occurs from fetal to adult tissues. CXCL12γ, for example, is not expressed in fetal tissues. In addition to CXCL12α and CXCL12β, CXCL12δ is mostly expressed in fetal liver and lungs, while this splice variant is primarily present in the pancreas in adults.9 The different tissue distribution of the CXCL12 splice variants suggests a regulated mRNA splicing or a regulated stability of the spliced mRNA. Recently, it was discovered that miRNA miR-141 controlled CXCL12β mRNA stability.90 In inflamed colon sections from Crohn’s disease patients, miR-141 downregulation coincided with increased CXCL12β mRNA and protein levels, whereas healthy tissues showed high miR-141 levels and a low incidence of CXCL12β. The mRNA and protein levels of the other CXCL12 splice variants were unaltered in healthy or inflamed colon sections. Administration of pre-miR-141 to mice with experimental colitis, an animal model for Crohn’s disease, resulted in a reduction in CXCL12β protein levels and an amelioration of the disease severity, thus stressing the importance of correct regulation of CXCL12 variants at the mRNA level.90
CXCL12 protein availability and cooperativity
Further control of CXCL12 is exerted at the protein level. CXCL12 can be removed from the environment by the activity of its receptors CXCR4 and ACKR3. CXCR4 is only internalized after stimulation with CXCL12 and is subsequently degraded, leading to downmodulation of CXCR4 expression on the cell membrane. CXCR4 is therefore less suited for continuous CXCL12 sequestration. However, ACKR3 internalization following CXCL12 activation does not lead to receptor degradation. Moreover, ACKR3 continuously cycles between the cell membrane and the cytoplasm and has a higher binding affinity for CXCL12 than CXCR4.39,46 In this way, ACKR3 actively participates in maintaining CXCL12 gradients, which is, as mentioned before, of critical importance for the migration of target cells. This was demonstrated by the loss of polarity and directional migration of primordial germ cells in zebrafish mutants uniformly overexpressing CXCL12.53 High CXCL12 concentrations reduce the presence of CXCR4 on the target cell membrane and therefore hamper continuous directional migration. The same phenotype was observed in ACKR3 knockdown mutant zebrafish. Thus, high CXCL12 concentrations do not induce a migratory response, and ACKR3 creates functional CXCL12 gradients.53 Therefore, Luker and colleagues hypothesized that ACKR3 promotes tumor growth and metastasis by scavenging CXCL12 from the tumor microenvironment. By doing so, CXCR4+ cancer cells remain responsive to CXCL12 and can escape from the primary tumor and metastasize to other organs that express CXCL12.45 The scavenging activity of ACKR3 is also important during the onset of multiple sclerosis. It was shown that removal of CXCL12 from the basolateral side of the blood brain barrier by ACKR3 results in a shift in membrane polarity and a subsequent infiltration of mononuclear cells into the central nervous system.91
In addition to regulation of its availability, the chemotactic potency of CXCL12 is fine-tuned through cooperation with other chemokines. Chemokine synergy is an interesting phenomenon with regard to protein cooperativity and occurs when the stimulatory effect of two chemokines on the same cell type is higher than the stimulation effect of each ligand separately. CXCL12 can therefore empower the potency of other chemokines. For example, CXCL12, which is a weak neutrophil chemoattractant, synergizes with the potent neutrophil recruiting protein CXCL8 to increase neutrophil migration. CXCL12 also synergizes with the CXCR3 ligands CXCL9, CXCL10 and CXCL11 and multiple members of the CC chemokine family to attract many other cell types, such as B and T cells, dendritic cells, monocytes and CD34+ progenitor cells.92,93 In addition, as previously mentioned, CXCL12 forms heterodimers with HMGB1 to attract monocytes to sites of injury in a CXCR4-dependent manner.61
Posttranslational modifications control CXCL12 activity
After controlled transcription and translation, CXCL12 activity is further regulated posttranslationally through enzymatic or chemical modifications. These changes alter several aspects of CXCL12, including GAG-binding properties and receptor binding and activation. The modifications that have been detected to occur are NH2-terminal and COOH-terminal truncation, citrullination of Arg residues and nitration of Tyr residues. The modified amino acids and the responsible agents are listed in Tables 1 and 2 and are indicated on the amino acid sequence of CXCL12 shown in Figure 1a. To appreciate the localization of these amino acids in the tertiary structure of CXCL12, the modified amino acids are also highlighted in a 3D model of the chemokine in Figure 1b.
Table 1.
CXCL12α truncations and their effects
| Modifying enzyme | Missing amino acids | Effect on activity | Effect on GAG binding | Ref. | |
|---|---|---|---|---|---|
| NH2-terminal truncation | CD26 (DPP4)aDPP8a | NH2-KP | Inactivated on CXCR4Reduced on ACKR3 | Reduced | 27, 74, 75, 94, 95, 96, 97, 98, 99, 100, 101, 102 |
| Leukocyte elastase | NH2-KPV | Inactivated | Reduced | 27, 103 | |
| MMP-1, 2, 3, 9, 13, 14 | NH2-KPVS | Inactivated | ND | 27, 104 | |
| Cathepsin G | NH2-KPVSL | Inactivated | Reduced | 27, 105 | |
| ND | NH2-KPVSLSY | Inactivated | Reduced | 74 | |
| COOH-terminal truncation | CPN | K-COOH | Reduced | Reduced | 97, 106, 107 |
| CPM | |||||
| Cathepsin Xa | KLKWIGEYLEKALNK-COOH | Reduced | ND | 108 |
Abbreviation: ND, not determined.
aActivity of this enzyme was also detected on CXCL12β.
Table 2.
Side chain modifications and their effects on CXCL12α
| Modifying agent | Altered amino acids | Effect on activity | Effect on GAG binding | Ref. | |
|---|---|---|---|---|---|
| Arg citrullination | PAD2 | Arg8Cit | Reduced on CXCR4Unaltered on ACKR3 | ND | 109 |
| Arg8Cit, Arg12Cit, Arg20Cit | Inactivated on CXCR4Reduced on ACKR3 | ND | 109 | ||
| Arg8Cit, Arg12Cit, Arg20Cit, Arg41Cit, Arg46Cit | Inactivated on CXCR4Inactivated on ACKR3 | ND | 109 | ||
| Tyr nitration | Peroxynitrite | Tyr7N-Tyr | Reduced | Unaltered | 110, 111 |
Abbreviations: ND, not determined; N-Tyr, nitrotyrosine.
Truncation by various enzymes inactivates CXCL12
An intensively studied protease in the regulation of chemokine activity is the serine protease dipeptidyl peptidase IV (DPP4; CD26). This exopeptidase exists as a soluble enzyme or as a membrane-bound protease on endothelial cells, activated lymphocytes, fibroblasts and epithelial cells. CD26 specifically cleaves proteins that contain an Ala or Pro residue at the penultimate position in their amino acid sequence.112 CD26 can readily cleave CXCL12α and CXCL12β in vitro.94,95 In fact, CXCL12α proved to be the most suited chemokine substrate for CD26, showing the shortest half-life values in in vitro truncation experiments.96 Later experiments showed that CXCL12α is truncated after in vitro incubation with human serum and after intravenous injection in mice.97,113 In vivo circulating, CD26-cleaved CXCL12α was detected in murine, rhesus monkey and human plasma.74,114,115 Moreover, the use of CD26 knockout mice or treatment of mice and rhesus monkeys with the CD26 inhibitor MK-0626 resulted in a decreased level of truncated CXCL12α.114,115
Binding of CXCL12α to heparin protected it from CD26-mediated cleavage, suggesting that CXCL12α in tissues is less prone to enzymatic inactivation than CXCL12α in the blood stream.70 Because all CXCL12 splice variants have the same NH2 terminus, one could expect a similar disposition toward truncation by CD26. Both CXCL12α and CXCL12β are described as suitable substrates for CD26.94,95,96,97,113 However, depending on the number of positively charged amino acids in the additional fourth exon, the different CXCL12 splice variants may be better protected from NH2-terminal truncation by a stronger interaction with GAGs, as is the case for CXCL12γ.76
Once truncated, the resulting CXCL12α form, CXCL12(3–68), had reduced heparin and CXCR4 binding capacity and was unable to activate CXCR4, as was indicated by the loss of calcium-dependent signaling and chemotaxis in peripheral blood-derived lymphocytes.27,94,95,97 Although CXCL12(3–68) no longer activated CXCR4, it still had enough receptor-binding affinity to desensitize CXCR4 for further calcium signaling.94 In addition, phosphorylation of the second messengers Akt and ERK was abolished in T cells and cells transfected with CXCR4.75,98 Interestingly, while CXCL12(3–68) was unable to recruit β-arrestin 2 to the cell membrane following CXCR4 activation, this recruitment still occurred after ACKR3 activation, albeit to a lesser extent.75 Furthermore, CD26 inhibited the angiogenic properties of CXCL12 in vitro. Loss of Akt and ERK1/2 phosphorylation and impaired in vitro tube formation and cell migration was observed after stimulation of endothelial cells with CXCL12(3–68).75,99 Moreover, in vitro migration of CD34+ hematopoietic progenitor cells improved when a CD26 inhibitor (diprotin A) or CD26−/− cells were used, indicating that the chemotactic activity of CXCL12α is reduced due to CD26 activity on progenitor cells.100
In vivo, CXCL12-induced lymphocyte extravasation was successfully maintained by administering the CD26 inhibitor sitagliptin to mice,75 and homing of progenitor cells after engraftment was also greatly increased after administration of diprotin A to mice or by using CD26−/− progenitor cells.101 Interestingly, CD26 is upregulated on CD34+ progenitor cells by granulocyte-colony stimulating factor (G-CSF).116 Thus, G-CSF contributes to the mobilization of bone marrow progenitor cells into circulation through inactivation of CXCL12 by increasing CD26 expression and stimulating release of the granulocyte-derived serine proteases elastase and cathepsin G in the bone marrow.117 Administration of the CXCR4 small molecule antagonist AMD3100 in combination with G-CSF is therefore now an approved treatment for stem cell mobilization in patients suffering from non-Hodgkin’s lymphoma or multiple myeloma.118
Leukocyte elastase inactivates CXCL12 by removing the first three amino acids because it specifically cleaves after an NH2-terminal Xaa-Pro-Val sequence.103 Removal of the first five amino acids by cathepsin G also inactivates CXCL12.105 Another soluble protease that cleaves CXCL12, DPP8, is related to DPP4 and shows the same substrate specificity. However, the half-life of CXCL12α and CXCL12β is far greater when incubated with DPP8 compared with DPP4. Moreover, DPP8 is an intracellular protease and can therefore only cleave CXCL12 after its internalization or when DPP8 is released into the environment by cell lysis.102 Finally, NH2-terminal truncation and inactivation by several members of the matrix metalloproteinase family have also been described to occur; MMP-1, -2, -3, -9, -13 and -14 remove the first four amino acids of CXCL12.104 The presence of circulating truncated CXCL12 variants in the blood of patients treated with G-CSF was assessed using mass spectrometry, and intact CXCL12 was detected, as well as truncated variants missing 2, 3, 5 and 7 amino acids.74
In addition to the NH2 terminus, the COOH terminus of CXCL12 can be enzymatically truncated. The COOH-terminal Lys of CXCL12α can be removed by both the soluble carboxypeptidase N (CPN) and the anchored CPM.106,107 Removal of this Lys results in a moderate decrease in the ability of CXCL12α to induce chemotaxis and cell proliferation and to bind GAGs, making CXCL12α more available for further NH2-terminal truncation.97,106,107 However, the reducing effects are far less pronounced compared to the NH2-terminal truncation of CXCL12α. Moreover, CXCL12α is the only CXCL12 splice variant that contains a COOH-terminal Lys, since the other splice variants have an additional COOH-terminal exon. Only CXCL12φ can be an additional substrate for CPN because this protease also cleaves COOH-terminal Arg residues. However, to date, this modification has not been detected in this CXCL12 splice variant. In contrast, all CXCL12α detected in human plasma had its COOH-terminal Lys removed.74 Another enzyme reported to cleave the CXCL12 COOH-terminus is cathepsin X. This enzyme is secreted by hematopoietic cells and non-hematopoietic bone marrow cells and has exopeptidase activity. In vitro digestion experiments on CXCL12α and CXCL12β showed that they both are COOH-terminally truncated by cathepsin X activity, an event that reduced their activity. The removal of amino acids proceeds sequentially until a Pro residue is present in the amino acid sequence, as cathepsin X is unable to remove this amino acid.108
Citrullination of CXCL12 is a subtle modification that reduces its activity
Citrullination, or deimination, is a posttranslational modification in which the imine group of an Arg is hydrolyzed to a ketone group, resulting in citrulline (Cit). This modification is catalyzed by peptidylarginine deiminase (PAD) and can only occur to Arg residues that are part of a peptide.119 In mammals, PAD has five paralogs that originated by gene duplication. These five PAD isozymes are expressed in distinct cell types; hematopoietic cells, such as monocytes and granulocytes, express PAD2 and PAD4.119 Although this modification is subtle, with a loss of only 1 Da, the conversion of a positively charged Arg to a neutral Cit residue can alter the protein structure and possibly influence interactions with other proteins. Citrullination is a homeostatic process, and histone citrullination is described as one of the posttranslational modifications that controls DNA condensation and therefore gene transcription.120 Moreover, citrullination is also involved in the release of neutrophil extracellular traps (NETs) where highly decondensed DNA is extruded together with antimicrobial proteins to trap pathogens.121 However, citrullination is also an important factor in autoimmune diseases, such as multiple sclerosis (MS) and rheumatoid arthritis (RA), because citrullinated proteins may trigger autoreactive T cells and can result in the production of autoantibodies. In MS, citrullination of myelin basic protein (MBP) is an important factor in disease development.122 In RA, anti-citrulline autoantibodies are used as an early diagnostic marker.123
In vitro incubation of CXCL12 with PAD2 showed that CXCL12 Arg residues were readily converted into Cit, weakening or abolishing CXCL12 activity, including receptor binding, signal transduction and chemotaxis. CXCL12 contains several NH2-terminal Arg residues, and the higher the number of citrullinated Arg, the lower the remaining CXCL12 activity on CXCR4. Interestingly, the effects of CXCL12 citrullination were less severe on ACKR3.109 In addition to CXCL12, the chemokines CXCL5, CXCL8, CXCL10 and CXCL11 are also citrullinated, generally resulting in reduced activity.124,125,126,127
Tyrosine nitration reduces CXCL12 activity
Nitration of aromatic Tyr or Trp residues occurs chemically in the presence of the highly reactive oxidant peroxynitrite that is formed by the reaction of superoxide anion and the radical nitric oxide (NO). NO fulfills many functions and is produced by nitric oxide synthases (NOS). These enzymes are constitutively expressed as neuronal (nNOS), endothelial (eNOS) and mitochondrial (mtNOS) NOS. A fourth NOS has an inducible expression (iNOS) that is controlled by inflammatory triggers and occurs mainly in macrophages, neutrophils and eosinophils that use NO to fight against pathogens and tumors.128 Nitrotyrosine formation occurs in many different proteins and is considered a marker for inflammation. Nitration of proteins can increase or decrease their activity or have no effect at all.129
In vitro nitration of chemokines has only been described for CCL2, CCL3, CCL5, CCL11, CXCL8 and CXCL12. Biochemical identification of the nitrated residues by mass spectrometry or Edman degradation sequencing has been confirmed for CCL2 and CXCL12.110,111 Eosinophil chemotaxis toward CCL5 or CCL11 that were incubated with peroxynitrite was reduced,130,131 and the neutrophil and monocyte chemotactic activity of CCL3 and neutrophil attractant potency of CXCL8 was reduced by incubation with peroxynitrite.132,133 Nitrated CCL2 was detected in tissue sections of human prostate and colon carcinoma, and colocalization of CCL2 and nitrotyrosine was found in kidneys after ischemia reperfusion injury.110,134 As a result of in vitro nitration of CCL2 by peroxynitrite, Molon et al. 110 observed abolished CD8+ T-cell migration and reduced CD14+ monocyte chemotaxis. Other groups also reported reduced monocyte chemotaxis and transendothelial migration following stimulation with nitrated CCL2.134,135 Remarkably, i.v. administration of nitrated CCL2 inhibited CCL2-mediated recruitment of leukocytes to air pouches in mice, suggesting that nitrated chemokines in circulation aid in the resolution of tissue inflammation.134 In vitro, CXCL12 is nitrated chemically by peroxynitrite incubation, but nitration also occurs naturally, as nitrated CXCL12 was isolated from cocultures of CXCL12-producing bone marrow stromal cells with leukocytes under inflammatory conditions.110,111 NH2-terminal nitration of CXCL12, designated [3-NT7]CXCL12, reduced intracellular calcium mobilization, IP3 accumulation and ERK1/2 phosphorylation, resulting in reduced in vitro lymphocyte and monocyte chemotaxis. Moreover, [3-NT7]CXCL12 was unable to induce lymphocyte extravasation after intraarticular injection in mice.111
Concluding remarks
CXCL12 has many interaction partners, specifically glycosaminoglycans and seven-transmembrane domain receptors. Furthermore, stimulation of either CXCR4 or ACKR3 may result in activation of several different signal transduction pathways. Therefore, it is not sufficient to assess only one aspect of these interactions when addressing possible changes in CXCL12 functionality. Certainly, concerning receptor bias, there have been reports of CXCL12 homodimerization or truncation resulting in skewed receptor usage.59,60,75 Moreover, when investigating the presence of different CXCL12 splice variants in different tissues, combining splice variant-specific antibodies and splice variant-specific primers to examine CXCL12 at both the protein and mRNA level ensures a cautious strategy not to miss subtle alterations in the expression of CXCL12 variants. Lastly, the array of modifying agents removing or altering amino acids from CXCL12 and thereby altering its activity cannot be overlooked. Here, mRNA cannot distinguish between native and specifically modified forms. Whereas antibodies have recently been developed that are able to specifically detect citrullinated or nitrated residues in chemokines, limited proteolytic truncation is hard to detect using immunoassays. Additional techniques, such as mass spectrometry, might prove useful to determine the fraction of CXCL12 that is posttranslationally modified. For example, Richter et al. 74 detected a COOH-terminal truncated, and thus less active, form of CXCL12α in human plasma using mass spectrometry. Therefore, it is important to approach the determination of the levels of CXCL12, or by extension any chemokine, with appropriate caution, taking into consideration the extensive regulation at multiple levels. A combination of complementary techniques is therefore necessary for a thorough understanding of the effects of chemokines in homeostatic or pathological processes.
Acknowledgements
This research was supported by the Interuniversity Attraction Poles Programme initiated by the Belgian Science Policy Office (I.A.P. Project 7/40), the Fund for Scientific Research of Flanders (FWO-Vlaanderen Projects G.0D25.17N, G.0764.14, and G.0D66.13), the Concerted Research Actions of the Regional Government of Flanders (GOA/12/017) and C1 funding (C16/17/010) of KU Leuven.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 2.Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 4.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim CH, Broxmeyer HE. In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor, and the bone marrow environment. Blood. 1998;91:100–110. [PubMed] [Google Scholar]
- 6.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 7.Yoshie O, Imai T, Nomiyama H. Chemokines in immunity. Adv Immunol. 2001;78:57–110. doi: 10.1016/s0065-2776(01)78002-9. [DOI] [PubMed] [Google Scholar]
- 8.Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 9.Yu L, Cecil J, Peng SB, Schrementi J, Kovacevic S, Paul D, et al. Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene. 2006;374:174–179. doi: 10.1016/j.gene.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7:243. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeVries ME, Kelvin AA, Xu L, Ran L, Robinson J, Kelvin DJ. Defining the origins and evolution of the chemokine/chemokine receptor system. J Immunol. 2006;176:401–415. doi: 10.4049/jimmunol.176.1.401. [DOI] [PubMed] [Google Scholar]
- 12.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 14.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci USA. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerrits H, van Ingen Schenau DS, Bakker NE, van Disseldorp AJ, Strik A, Hermens LS, et al. Early postnatal lethality and cardiovascular defects in CXCR7-deficient mice. Genesis. 2008;46:235–245. doi: 10.1002/dvg.20387. [DOI] [PubMed] [Google Scholar]
- 16.Loetscher M, Geiser T, O'Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269:232–237. [PubMed] [Google Scholar]
- 17.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 18.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 19.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 20.Pawig L, Klasen C, Weber C, Bernhagen J, Noels H. Diversity and inter-connections in the CXCR4 chemokine receptor/ligand family: molecular perspectives. Front Immunol. 2015;6:429. doi: 10.3389/fimmu.2015.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Power CA. Knock out models to dissect chemokine receptor function in vivo. J Immunol Methods. 2003;273:73–82. doi: 10.1016/s0022-1759(02)00419-2. [DOI] [PubMed] [Google Scholar]
- 22.Gupta SK, Pillarisetti K. Cutting edge: CXCR4-Lo: molecular cloning and functional expression of a novel human CXCR4 splice variant. J Immunol. 1999;163:2368–2372. [PubMed] [Google Scholar]
- 23.Duquenne C, Psomas C, Gimenez S, Guigues A, Carles MJ, Barbuat C, et al. The two human CXCR4 isoforms display different HIV receptor activities: consequences for the emergence of X4 strains. J Immunol. 2014;193:4188–4194. doi: 10.4049/jimmunol.1303298. [DOI] [PubMed] [Google Scholar]
- 24.Heesen M, Berman MA, Hopken UE, Gerard NP, Dorf ME. Alternate splicing of mouse fusin/CXC chemokine receptor-4: stromal cell-derived factor-1alpha is a ligand for both CXC chemokine receptor-4 isoforms. J Immunol. 1997;158:3561–3564. [PubMed] [Google Scholar]
- 25.Veldkamp CT, Ziarek JJ, Su J, Basnet H, Lennertz R, Weiner JJ, et al. Monomeric structure of the cardioprotective chemokine SDF-1/CXCL12. Protein Sci. 2009;18:1359–1369. doi: 10.1002/pro.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siciliano SJ, Rollins TE, DeMartino J, Konteatis Z, Malkowitz L, Van RG, et al. Two-site binding of C5a by its receptor: an alternative binding paradigm for G protein-coupled receptors. Proc Natl Acad Sci USA. 1994;91:1214–1218. doi: 10.1073/pnas.91.4.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, et al. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleist AB, Getschman AE, Ziarek JJ, Nevins AM, Gauthier PA, Chevigne A, et al. New paradigms in chemokine receptor signal transduction: moving beyond the two-site model. Biochem Pharmacol. 2016;114:53–68. doi: 10.1016/j.bcp.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin JB. Chemokine signaling in cancer: one hump or two? Semin Cancer Biol. 2009;19:116–122. doi: 10.1016/j.semcancer.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768:952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vila-Coro AJ, Rodriguez-Frade JM, Martin De AA, Moreno-Ortiz MC, Martinez A, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 1999;13:1699–1710. [PubMed] [Google Scholar]
- 32.Zhang XF, Wang JF, Matczak E, Proper JA, Groopman JE. Janus kinase 2 is involved in stromal cell-derived factor-1alpha-induced tyrosine phosphorylation of focal adhesion proteins and migration of hematopoietic progenitor cells. Blood. 2001;97:3342–3348. doi: 10.1182/blood.v97.11.3342. [DOI] [PubMed] [Google Scholar]
- 33.Soldevila G, Licona I, Salgado A, Ramirez M, Chavez R, Garcia-Zepeda E. Impaired chemokine-induced migration during T-cell development in the absence of Jak 3. Immunology. 2004;112:191–200. doi: 10.1111/j.1365-2567.2004.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soriano SF, Serrano A, Hernanz-Falcon P, Martin DeAA, Monterrubio M, Martinez C, et al. Chemokines integrate JAK/STAT and G-protein pathways during chemotaxis and calcium flux responses. Eur J Immunol. 2003;33:1328–1333. doi: 10.1002/eji.200323897. [DOI] [PubMed] [Google Scholar]
- 35.Moriguchi M, Hissong BD, Gadina M, Yamaoka K, Tiffany HL, Murphy PM, et al. CXCL12 signaling is independent of Jak2 and Jak3. J Biol Chem. 2005;280:17408–17414. doi: 10.1074/jbc.M414219200. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 37.Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2:129–134. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- 39.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 40.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Libert F, Parmentier M, Lefort A, Dinsart C, Van Sande J, Maenhaut C, et al. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989;244:569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu N, Soda Y, Kanbe K, Liu HY, Mukai R, Kitamura T, et al. A putative G protein-coupled receptor, RDC1, is a novel coreceptor for human and simian immunodeficiency viruses. J Virol. 2000;74:619–626. doi: 10.1128/jvi.74.2.619-626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee M, Borst O, Walker B, Fotinos A, Vogel S, Seizer P, et al. Macrophage migration inhibitory factor limits activation-induced apoptosis of platelets via CXCR7-dependent Akt signaling. Circ Res. 2014;115:939–949. doi: 10.1161/CIRCRESAHA.115.305171. [DOI] [PubMed] [Google Scholar]
- 44.Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci USA. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luker KE, Lewin SA, Mihalko LA, Schmidt BT, Winkler JS, Coggins NL, et al. Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells. Oncogene. 2012;31:4750–4758. doi: 10.1038/onc.2011.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naumann U, Cameroni E, Pruenster M, Mahabaleshwar H, Raz E, Zerwes HG, et al. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS One. 2010;5:e9175. doi: 10.1371/journal.pone.0009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein KR, Karpinich NO, Espenschied ST, Willcockson HH, Dunworth WP, Hoopes SL, et al. Decoy receptor CXCR7 modulates adrenomedullin-mediated cardiac and lymphatic vascular development. Dev Cell. 2014;30:528–540. doi: 10.1016/j.devcel.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, et al. Beta-arrestin- but not G protein-mediated signaling by the ‘decoy’ receptor CXCR7. Proc Natl Acad Sci USA. 2010;107:628–632. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ödemis V, Lipfert J, Kraft R, Hajek P, Abraham G, Hattermann K, et al. The presumed atypical chemokine receptor CXCR7 signals through G(i/o) proteins in primary rodent astrocytes and human glioma cells. Glia. 2012;60:372–381. doi: 10.1002/glia.22271. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Li G, Stanco A, Long JE, Crawford D, Potter GB, et al. CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron. 2011;69:61–76. doi: 10.1016/j.neuron.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar R, Tripathi V, Ahmad M, Nath N, Mir RA, Chauhan SS, et al. CXCR7 mediated Gialpha independent activation of ERK and Akt promotes cell survival and chemotaxis in T cells. Cell Immunol. 2012;272:230–241. doi: 10.1016/j.cellimm.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Chen Q, Zhang M, Li Y, Xu D, Wang Y, Song A, et al. CXCR7 mediates neural progenitor cells migration to CXCL12 independent of CXCR4. Stem Cells. 2015;33:2574–2585. doi: 10.1002/stem.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Beaty N, Chen S, Qi CF, Masiuk M, Shin DM, et al. The CXCR7 chemokine receptor promotes B-cell retention in the splenic marginal zone and serves as a sink for CXCL12. Blood. 2012;119:465–468. doi: 10.1182/blood-2011-03-343608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Babcock GJ, Farzan M, Sodroski J. Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J Biol Chem. 2003;278:3378–3385. doi: 10.1074/jbc.M210140200. [DOI] [PubMed] [Google Scholar]
- 56.Toth PT, Ren D, Miller RJ. Regulation of CXCR4 receptor dimerization by the chemokine SDF-1alpha and the HIV-1 coat protein gp120: a fluorescence resonance energy transfer (FRET) study. J Pharmacol Exp Ther. 2004;310:8–17. doi: 10.1124/jpet.103.064956. [DOI] [PubMed] [Google Scholar]
- 57.Percherancier Y, Berchiche YA, Slight I, Volkmer-Engert R, Tamamura H, Fujii N, et al. Bioluminescence resonance energy transfer reveals ligand-induced conformational changes in CXCR4 homo- and heterodimers. J Biol Chem. 2005;280:9895–9903. doi: 10.1074/jbc.M411151200. [DOI] [PubMed] [Google Scholar]
- 58.Veldkamp CT, Seibert C, Peterson FC, De la Cruz NB, Haugner JC, III, Basnet H, et al. Structural basis of CXCR4 sulfotyrosine recognition by the chemokine SDF-1/CXCL12. Sci Signal. 2008;1:ra4. doi: 10.1126/scisignal.1160755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drury LJ, Ziarek JJ, Gravel S, Veldkamp CT, Takekoshi T, Hwang ST, et al. Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proc Natl Acad Sci USA. 2011;108:17655–17660. doi: 10.1073/pnas.1101133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ray P, Lewin SA, Mihalko LA, Lesher-Perez SC, Takayama S, Luker KE, et al. Secreted CXCL12 (SDF-1) forms dimers under physiological conditions. Biochem J. 2012;442:433–442. doi: 10.1042/BJ20111341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–563. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carlson J, Baxter SA, Dreau D, Nesmelova IV. The heterodimerization of platelet-derived chemokines. Biochim Biophys Acta. 2013;1834:158–168. doi: 10.1016/j.bbapap.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 63.von Hundelshausen P, Agten SM, Eckardt V, Blanchet X, Schmitt MM, Ippel H, et al. Chemokine interactome mapping enables tailored intervention in acute and chronic inflammation. Sci Transl Med. 2017;9:eaah6650. doi: 10.1126/scitranslmed.aah6650. [DOI] [PubMed] [Google Scholar]
- 64.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113:6085–6093. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- 65.Decaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J Biol Chem. 2011;286:32188–32197. doi: 10.1074/jbc.M111.277038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hernandez L, Magalhaes MA, Coniglio SJ, Condeelis JS, Segall JE. Opposing roles of CXCR4 and CXCR7 in breast cancer metastasis. Breast Cancer Res. 2011;13:R128. doi: 10.1186/bcr3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 68.Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE. Regulation of protein function by glycosaminoglycans—as exemplified by chemokines. Annu Rev Biochem. 2005;74:385–410. doi: 10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- 69.Rueda P, Richart A, Recalde A, Gasse P, Vilar J, Guerin C, et al. Homeostatic and tissue reparation defaults in mice carrying selective genetic invalidation of CXCL12/proteoglycan interactions. Circulation. 2012;126:1882–1895. doi: 10.1161/CIRCULATIONAHA.112.113290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sadir R, Imberty A, Baleux F, Lortat-Jacob H. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. J Biol Chem. 2004;279:43854–43860. doi: 10.1074/jbc.M405392200. [DOI] [PubMed] [Google Scholar]
- 71.Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, et al. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J Biol Chem. 1999;274:23916–23925. doi: 10.1074/jbc.274.34.23916. [DOI] [PubMed] [Google Scholar]
- 72.O'Boyle G, Mellor P, Kirby JA, Ali S. Anti-inflammatory therapy by intravenous delivery of non-heparan sulfate-binding CXCL12. FASEB J. 2009;23:3906–3916. doi: 10.1096/fj.09-134643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sadir R, Baleux F, Grosdidier A, Imberty A, Lortat-Jacob H. Characterization of the stromal cell-derived factor-1alpha-heparin complex. J Biol Chem. 2001;276:8288–8296. doi: 10.1074/jbc.M008110200. [DOI] [PubMed] [Google Scholar]
- 74.Richter R, Jochheim-Richter A, Ciuculescu F, Kollar K, Seifried E, Forssmann U, et al. Identification and characterization of circulating variants of CXCL12 from human plasma: effects on chemotaxis and mobilization of hematopoietic stem and progenitor cells. Stem Cells Dev. 2014;23:1959–1974. doi: 10.1089/scd.2013.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Janssens R, Mortier A, Boff D, Ruytinx P, Gouwy M, Vantilt B, et al. Truncation of CXCL12 by CD26 reduces its CXC chemokine receptor 4- and atypical chemokine receptor 3-dependent activity on endothelial cells and lymphocytes. Biochem Pharmacol. 2017;132:92–101. doi: 10.1016/j.bcp.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 76.Laguri C, Sadir R, Rueda P, Baleux F, Gans P, Arenzana-Seisdedos F, et al. The novel CXCL12gamma isoform encodes an unstructured cationic domain which regulates bioactivity and interaction with both glycosaminoglycans and CXCR4. PLoS One. 2007;2:e1110. doi: 10.1371/journal.pone.0001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rueda P, Balabanian K, Lagane B, Staropoli I, Chow K, Levoye A, et al. The CXCL12gamma chemokine displays unprecedented structural and functional properties that make it a paradigm of chemoattractant proteins. PLoS One. 2008;3:e2543. doi: 10.1371/journal.pone.0002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Connell BJ, Sadir R, Baleux F, Laguri C, Kleman JP, Luo L, et al. Heparan sulfate differentially controls CXCL12alpha- and CXCL12gamma-mediated cell migration through differential presentation to their receptor CXCR4. Sci Signal. 2016;9:ra107. doi: 10.1126/scisignal.aaf1839. [DOI] [PubMed] [Google Scholar]
- 79.Fermas S, Gonnet F, Sutton A, Charnaux N, Mulloy B, Du Y, et al. Sulfated oligosaccharides (heparin and fucoidan) binding and dimerization of stromal cell-derived factor-1 (SDF-1/CXCL 12) are coupled as evidenced by affinity CE-MS analysis. Glycobiology. 2008;18:1054–1064. doi: 10.1093/glycob/cwn088. [DOI] [PubMed] [Google Scholar]
- 80.Santiago B, Calonge E, Del Rey MJ, Gutierrez-Canas I, Izquierdo E, Usategui A, et al. CXCL12 gene expression is upregulated by hypoxia and growth arrest but not by inflammatory cytokines in rheumatoid synovial fibroblasts. Cytokine. 2011;53:184–190. doi: 10.1016/j.cyto.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 81.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 82.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 83.Kryczek I, Lange A, Mottram P, Alvarez X, Cheng P, Hogan M, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–472. [PubMed] [Google Scholar]
- 84.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schutyser E, Su Y, Yu Y, Gouwy M, Zaja-Milatovic S, Van Damme J, et al. Hypoxia enhances CXCR4 expression in human microvascular endothelial cells and human melanoma cells. Eur Cytokine Netw. 2007;18:59–70. doi: 10.1684/ecn.2007.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Esencay M, Sarfraz Y, Zagzag D. CXCR7 is induced by hypoxia and mediates glioma cell migration towards SDF-1alpha. BMC Cancer. 2013;13:347. doi: 10.1186/1471-2407-13-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu H, Xue W, Ge G, Luo X, Li Y, Xiang H, et al. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1alpha in MSCs. Biochem Biophys Res Commun. 2010;401:509–515. doi: 10.1016/j.bbrc.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 88.Yu PF, Huang Y, Xu CL, Lin LY, Han YY, Sun WH, et al. Downregulation of CXCL12 in mesenchymal stromal cells by TGFbeta promotes breast cancer metastasis. Oncogene. 2017;36:840–849. doi: 10.1038/onc.2016.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Altenburg JD, Broxmeyer HE, Jin Q, Cooper S, Basu S, Alkhatib G. A naturally occurring splice variant of CXCL12/stromal cell-derived factor 1 is a potent human immunodeficiency virus type 1 inhibitor with weak chemotaxis and cell survival activities. J Virol. 2007;81:8140–8148. doi: 10.1128/JVI.00268-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang Z, Shi T, Zhou Q, Shi S, Zhao R, Shi H, et al. miR-141 Regulates colonic leukocytic trafficking by targeting CXCL12beta during murine colitis and human Crohn's disease. Gut. 2014;63:1247–1257. doi: 10.1136/gutjnl-2012-304213. [DOI] [PubMed] [Google Scholar]
- 91.McCandless EE, Piccio L, Woerner BM, Schmidt RE, Rubin JB, Cross AH, et al. Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. Am J Pathol. 2008;172:799–808. doi: 10.2353/ajpath.2008.070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gouwy M, Schiraldi M, Struyf S, Van Damme J, Uguccioni M. Possible mechanisms involved in chemokine synergy fine tuning the inflammatory response. Immunol Lett. 2012;145:10–14. doi: 10.1016/j.imlet.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 93.Gouwy M, Struyf S, Leutenez L, Portner N, Sozzani S, Van Damme J. Chemokines and other GPCR ligands synergize in receptor-mediated migration of monocyte-derived immature and mature dendritic cells. Immunobiology. 2014;219:218–229. doi: 10.1016/j.imbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 94.Proost P, Struyf S, Schols D, Durinx C, Wuyts A, Lenaerts JP, et al. Processing by CD26/dipeptidyl-peptidase IV reduces the chemotactic and anti-HIV-1 activity of stromal-cell-derived factor-1alpha. FEBS Lett. 1998;432:73–76. doi: 10.1016/s0014-5793(98)00830-8. [DOI] [PubMed] [Google Scholar]
- 95.Shioda T, Kato H, Ohnishi Y, Tashiro K, Ikegawa M, Nakayama EE, et al. Anti-HIV-1 and chemotactic activities of human stromal cell-derived factor-1alpha (SDF-1alpha) and SDF-1beta are abolished by CD26/dipeptidyl peptidase IV-mediated cleavage. Proc Natl Acad Sci USA. 1998;95:6331–6336. doi: 10.1073/pnas.95.11.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lambeir AM, Proost P, Durinx C, Bal G, Senten K, Augustyns K, et al. Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem. 2001;276:29839–29845. doi: 10.1074/jbc.M103106200. [DOI] [PubMed] [Google Scholar]
- 97.De La Luz SM, Yang F, Narazaki M, Salvucci O, Davis D, Yarchoan R, et al. Differential processing of stromal-derived factor-1alpha and stromal-derived factor-1beta explains functional diversity. Blood. 2004;103:2452–2459. doi: 10.1182/blood-2003-08-2857. [DOI] [PubMed] [Google Scholar]
- 98.Tilton B, Ho L, Oberlin E, Loetscher P, Baleux F, Clark-Lewis I, et al. Signal transduction by CXC chemokine receptor 4. Stromal cell-derived factor 1 stimulates prolonged protein kinase B and extracellular signal-regulated kinase 2 activation in T lymphocytes. J Exp Med. 2000;192:313–324. doi: 10.1084/jem.192.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wesley UV, Hatcher JF, Ayvaci ER, Klemp A, Dempsey RJ. Regulation of dipeptidyl peptidase IV in the post-stroke rat brain and in vitro ischemia: implications for chemokine-mediated neural progenitor cell migration and angiogenesis. Mol Neurobiol. 2016;54:4973–4985. doi: 10.1007/s12035-016-0039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Christopherson KW, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169:7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 101.Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 102.Ajami K, Pitman MR, Wilson CH, Park J, Menz RI, Starr AE, et al. Stromal cell-derived factors 1alpha and 1beta, inflammatory protein-10 and interferon-inducible T cell chemo-attractant are novel substrates of dipeptidyl peptidase 8. FEBS Lett. 2008;582:819–825. doi: 10.1016/j.febslet.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 103.Valenzuela-Fernandez A, Planchenault T, Baleux F, Staropoli I, Le-Barillec K, Leduc D, et al. Leukocyte elastase negatively regulates stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J Biol Chem. 2002;277:15677–15689. doi: 10.1074/jbc.M111388200. [DOI] [PubMed] [Google Scholar]
- 104.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 105.Delgado MB, Clark-Lewis I, Loetscher P, Langen H, Thelen M, Baggiolini M, et al. Rapid inactivation of stromal cell-derived factor-1 by cathepsin G associated with lymphocytes. Eur J Immunol. 2001;31:699–707. doi: 10.1002/1521-4141(200103)31:3<699::aid-immu699>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 106.Davis DA, Singer KE, De La Luz SM, Narazaki M, Yang F, Fales HM, et al. Identification of carboxypeptidase N as an enzyme responsible for C-terminal cleavage of stromal cell-derived factor-1alpha in the circulation. Blood. 2005;105:4561–4568. doi: 10.1182/blood-2004-12-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marquez-Curtis L, Jalili A, Deiteren K, Shirvaikar N, Lambeir AM, Janowska-Wieczorek A. Carboxypeptidase M expressed by human bone marrow cells cleaves the C-terminal lysine of stromal cell-derived factor-1alpha: another player in hematopoietic stem/progenitor cell mobilization? Stem Cells. 2008;26:1211–1220. doi: 10.1634/stemcells.2007-0725. [DOI] [PubMed] [Google Scholar]
- 108.Staudt ND, Aicher WK, Kalbacher H, Stevanovic S, Carmona AK, Bogyo M, et al. Cathepsin X is secreted by human osteoblasts, digests CXCL12 and impairs adhesion of hematopoietic stem and progenitor cells to osteoblasts. Haematologica. 2010;95:1452–1460. doi: 10.3324/haematol.2009.018671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Struyf S, Noppen S, Loos T, Mortier A, Gouwy M, Verbeke H, et al. Citrullination of CXCL12 differentially reduces CXCR4 and CXCR7 binding with loss of inflammatory and anti-HIV-1 activity via CXCR4. J Immunol. 2009;182:666–674. doi: 10.4049/jimmunol.182.1.666. [DOI] [PubMed] [Google Scholar]
- 110.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Janssens R, Mortier A, Boff D, Vanheule V, Gouwy M, Franck C, et al. Natural nitration of CXCL12 reduces its signaling capacity and chemotactic activity in vitro and abrogates intra-articular lymphocyte recruitment in vivo. Oncotarget. 2016;7:62439–62459. doi: 10.18632/oncotarget.11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mortier A, Gouwy M, Van DJ, Proost P, Struyf S. CD26/dipeptidylpeptidase IV-chemokine interactions: double-edged regulation of inflammation and tumor biology. J Leukoc Biol. 2016;99:955–969. doi: 10.1189/jlb.3MR0915-401R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Antonsson B, De Lys P, Dechavanne V, Chevalet L, Boschert U. In vivo processing of CXCL12alpha/SDF-1alpha after intravenous and subcutaneous administration to mice. Proteomics. 2010;10:4342–4351. doi: 10.1002/pmic.201000331. [DOI] [PubMed] [Google Scholar]
- 114.Busso N, Wagtmann N, Herling C, Chobaz-Peclat V, Bischof-Delaloye A, So A, et al. Circulating CD26 is negatively associated with inflammation in human and experimental arthritis. Am J Pathol. 2005;166:433–442. doi: 10.1016/S0002-9440(10)62266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang W, Choi BK, Li W, Lao Z, Lee AY, Souza SC, et al. Quantification of intact and truncated stromal cell-derived factor-1alpha in circulation by immunoaffinity enrichment and tandem mass spectrometry. J Am Soc Mass Spectrom. 2014;25:614–625. doi: 10.1007/s13361-013-0822-7. [DOI] [PubMed] [Google Scholar]
- 116.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 117.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Keating GM. Plerixafor: a review of its use in stem-cell mobilization in patients with lymphoma or multiple myeloma. Drugs. 2011;71:1623–1647. doi: 10.2165/11206040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 119.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 120.György B, Toth E, Tarcsa E, Falus A, Buzas EI. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol. 2006;38:1662–1677. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 121.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 122.Moscarello MA, Mastronardi FG, Wood DD. The role of citrullinated proteins suggests a novel mechanism in the pathogenesis of multiple sclerosis. Neurochem Res. 2007;32:251–256. doi: 10.1007/s11064-006-9144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Steiner G, Smolen J. Autoantibodies in rheumatoid arthritis and their clinical significance. Arthritis Res. 2002;4((Suppl 2)):S1–S5. doi: 10.1186/ar551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mortier A, Loos T, Gouwy M, Ronsse I, Van Damme J, Proost P. Posttranslational modification of the NH2-terminal region of CXCL5 by proteases or peptidylarginine Deiminases (PAD) differently affects its biological activity. J Biol Chem. 2010;285:29750–29759. doi: 10.1074/jbc.M110.119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yoshida K, Korchynskyi O, Tak PP, Isozaki T, Ruth JH, Campbell PL, et al. Citrullination of epithelial neutrophil-activating peptide 78/CXCL5 results in conversion from a non-monocyte-recruiting chemokine to a monocyte-recruiting chemokine. Arthritis Rheumatol. 2014;66:2716–2727. doi: 10.1002/art.38750. [DOI] [PubMed] [Google Scholar]
- 126.Proost P, Loos T, Mortier A, Schutyser E, Gouwy M, Noppen S, et al. Citrullination of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. J Exp Med. 2008;205:2085–2097. doi: 10.1084/jem.20080305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loos T, Mortier A, Gouwy M, Ronsse I, Put W, Lenaerts JP, et al. Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: a naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation. Blood. 2008;112:2648–2656. doi: 10.1182/blood-2008-04-149039. [DOI] [PubMed] [Google Scholar]
- 128.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 129.Aulak KS, Miyagi M, Yan L, West KA, Massillon D, Crabb JW, et al. Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proc Natl Acad Sci USA. 2001;98:12056–12061. doi: 10.1073/pnas.221269198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sato E, Simpson KL, Grisham MB, Koyama S, Robbins RA. Effects of reactive oxygen and nitrogen metabolites on RANTES- and IL-5-induced eosinophil chemotactic activity in vitro. Am J Pathol. 1999;155:591–598. doi: 10.1016/S0002-9440(10)65154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sato E, Simpson KL, Grisham MB, Koyama S, Robbins RA. Effects of reactive oxygen and nitrogen metabolites on eotaxin-induced eosinophil chemotactic activity in vitro. Am J Respir Cell Mol Biol. 2000;22:61–67. doi: 10.1165/ajrcmb.22.1.3644. [DOI] [PubMed] [Google Scholar]
- 132.Sato E, Simpson KL, Grisham MB, Koyama S, Robbins RA. Inhibition of MIP-1alpha-induced human neutrophil and monocyte chemotactic activity by reactive oxygen and nitrogen metabolites. J Lab Clin Med. 2000;135:161–169. doi: 10.1067/mlc.2000.104307. [DOI] [PubMed] [Google Scholar]
- 133.Sato E, Simpson KL, Grisham MB, Koyama S, Robbins RA. Reactive nitrogen and oxygen species attenuate interleukin 8-induced neutrophil chemotactic activity in vitro. J Biol Chem. 2000;275:10826–10830. doi: 10.1074/jbc.275.15.10826. [DOI] [PubMed] [Google Scholar]
- 134.Barker CE, Thompson S, O'Boyle G, Lortat-Jacob H, Sheerin NS, Ali S, et al. CCL2 nitration is a negative regulator of chemokine-mediated inflammation. Sci Rep. 2017;7:44384. doi: 10.1038/srep44384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sato E, Simpson KL, Grisham MB, Koyama S, Robbins RA. Effects of reactive oxygen and nitrogen metabolites on MCP-1-induced monocyte chemotactic activity in vitro. Am J Physiol. 1999;277((3 Pt 1)):L543–L549. doi: 10.1152/ajplung.1999.277.3.L543. [DOI] [PubMed] [Google Scholar]


