Abstract
Drug efflux by intestinal P-glycoprotein (P-gp) is recognized as a significant biochemical barrier affecting oral absorption for a number of drugs apart from the cytochrome P450 3A enzyme. Various conflicting reports have been published regarding the effects of grapefruit juice (GFJ) on P-gp mediated drug efflux, in which GFJ has been shown to have no effect, as an inhibitor effect or activation of the enzyme. Therefore the present study’s objective was to provide clarification of previous findings, adopting a two-way approach, involving both single dose and multiple dosage regimens. Diltiazem (DTZ) 15 mg/kg was administered concomitantly with 5 ml/kg of GFJ to one group (n = 6) of male Wistar rats and another group (n = 6) of animals were provided distilled water with DTZ (the control). A third group of rats was administered GFJ orally for six days and on seventh day GFJ and DTZ were administered concomitantly. The Cmax and AUC of DTZ were decreased significantly in the presence of multiple dose treatment of GFJ. These data were also decreased in presence of simultaneous treatment of single dose GFJ. In vitro metabolism studies and gut sac experiments were conducted in order to understand the mechanism involved. In the liver S9 fraction prepared from the rats treated with multiple doses of GFJ, DTZ metabolism was significantly increased compared to the control. Furthermore, the amount of drug transported from the duodenum was reduced in GFJ treated rats compared to that of the control (1581.0 ± 7.8 nM vs 1084.81 ± 6.1 nM, respectively). Grapefruit juice was also reported to inhibit the organic anion transporting polypeptide (OATP), an influx transporter thus reducing the blood levels of OATP substrates which was evident from the in vitro studies. The amount of drug transported from the duodenum was reduced in the presence of pravastatin, a specific OATP inhibitor (1581.0 ± 7.8 nM to 1265.0 ± 5.5 nM). Oral single dose exposure to GFJ showed no effect on P-gp, whereas multiple dose administration of GFJ resulted in increased levels of P-gp expression and decreased levels of OATP, thus showing a varied effect on intestinal absorption, and therefore overcoming the inhibition of DTZ metabolism in rats.
1. Introduction
A grapefruit-drug interaction was first reported in 1989 (Shimomura et al. 2003). Grapefruit juice (GFJ) contains various furanocoumarins and flavanoids which are postulated to influence drug interactions. Furanocoumarins were shown to inhibit the first-pass metabolism of certain drugs that are metabolized by cytochrome P450 3A (CYP 3A). These compounds are abundant in the grapefruit flesh, sac, peel, and seed. The main mechanism of the grapefruit- drug interaction was understood to be due to inhibition of CYP 3A in the gut wall, and it is most important for drugs with low oral bioavailability (i.e., drugs with high first-pass metabolism) (Bailey et al. 1991). However, the effect of GFJ on P-gp is the subject of much controversy. In short GFJ has been shown to both inhibit and activate the P-gp drug efflux transporter in vitro (Schultze et al. 1986).
The benzothiazepine calcium-channel antagonist, diltiazem (DTZ), belongs to the most commonly prescribed drug for the treatment of angina and hypertension (Buckley et al. 1990). The oral bioavailability of DTZ is approximately 40–50% due to extensive presystemic metabolism (Kane et al. 2000). In addition almost 21% of the population consumes GFJ (Schmiedlin et al. 1999). If the patients under treatment with DTZ consume GFJ, serious adverse effects can be observed. Previously, it was reported that drugs which were substrates of CYP 3A such as calcium channel antagonists, antihistamines, benzodiazepines and others would interact with GFJ. Therefore the mechanism involved in such food-drug interactions was believed to be the enzyme inhibitory activity of components present in GFJ (Tian et al. 2002). However recent studies indicated that the absorption of drugs such as indinavir, saquinavir, digoxin, vinblastine, fexofenadine, which are not metabolized by CYP 3A, were also influenced by GFJ (Tian et al. 2002). This suggests the possible role of the transporter protein, P-gp, is also responsible for such food-drug interactions. Immunohistochemical analysis using a monoclonal antibody provided evidence for localization of P-gp in a wide range of tissues, particularly in columnar epithelial cells of the lower gastrointestinal tract (GIT), capillary endothelial cells of the brain and testis, biliary canalicular surface of hepatocytes as well as other tissues such as the apical surface of the proximal tubule in the kidney (Varma 2005). Due to selective distribution at the port of drug entry and exit, P-gp has been speculated to play a major physiological role in absorption, distribution and excretion of xenobiotics. Thus, P-gp plays a major role in influencing the pharmacokinetics of many drugs.
Many studies have been conducted to understand the influence of GFJ on CYP 3A and P-gp, although one of them dominates over the other depending on the type of substrate. The bioavailability of lovastatin was increased by 15fold when administered with GFJ (Kantola 1998). This increase in bioavailability was reported to be due to inhibition of CYP enzymes. The area under the concentration curve (AUC) and time of maximum absorption (Tmax) of amlodipine were increased by 116% and 115%, respectively with GFJ. Observations supporting the above hypothesis have also been reported with amiodarone (Libersa et al. 2000) and cyclosporine (Edwards 1999). This increase in the concentration levels was due to inhibition of P-gp as well. In contrast to these studies, which supported the inhibition of P-gp with GFJ, Soldner et al. (1999) reported an increase in activity of P-gp by using vinblastine, cyclosporine, losartan (CYP 3A and P-gp substrates), digoxin, fexofenadine (P-gp substrates) and no effect on nifedipine or felodipine (CYP 3A substrates). The same results were observed when nifedipine was administered intraduodenally. The area under the curve was reduced by 50% as well as a decrease in Tmax (50%) when GFJ was administered concomitantly (Mohri et al. 2000). Chronic administration of GFJ reduced the absorptive transport of paclitaxel causing the efflux ratio (ER) to increase much greater than 1 (ER = 20.8) (Varma 2005). GFJ was shown to decrease the organic anion transporting polypeptide (OATP) levels as well, an influx transporter. GFJ when administered along with fexofenadine, the AUC and maximum plasma concentration Cmax of fexofenadine were decreased to 30% and 40% respectively compare to control. This decrease in blood levels of fexofenadine was attributed to the inhibition of OATP by GFJ (Dresser et al. 2002).
Due to many contradictory reports, a two-way approach, involving both single and multiple dose studies were conducted. The objective of this work was to investigate the influence of GFJ on the pharmacokinetics of DTZ and to further understand the possible mechanism of interaction that is influencing the drugs pharmacokinetics. In vitro metabolism studies were also conducted to explain the influence of CYP 3A enzymes using a primary CYP 3A substrate, felodipine. In vitro absorption studies were conducted to elucidate the actual mechanism involved in the transport of DTZ.
2. Investigations and results
2.1. Metabolism studies
To demonstrate the presence of CYP 3A enzyme in the S9 fraction used for metabolism studies and the inhibition of this enzyme by GFJ, testosterone which is specifically metabolized by CYP 3A to 6-β-hydroxy testosterone, was taken as a probe drug. There was a significant decrease in the formation of 6-β-hydroxy testosterone (31%) when incubated in GFJ pretreated rat liver S9 fraction compared to the control (66%).
These metabolism studies were conducted to confirm that there were sufficient levels of CYP 3A enzymes in the animals used and found that these levels were indeed sufficient for the metabolism of DTZ. As testosterone is specifically a CYP 3A substrate, we were able to demonstrate that there was CYP 3A enzyme in the S9 fraction and these CYP 3A levels were sufficient to metabolize testosterone. Another experiment was conducted to study the influence of testosterone on DTZ in the control rat liver S9 fraction. The % metabolism of DTZ was decreased from 88.9% in the control to 41.3% in the presence of testosterone, indicating that DTZ is a CYP 3A substrate. Therefore we were able to show that DTZ is specifically metabolized by the CYP 3A enzyme as reported by Christensen et al. (2002) and it is inhibited by GFJ. These data are provided in Table 1.
Table 1.
Metabolic stability of diltiazem in male wistar rat liver S9 fraction in presence of testosterone. Statistically significant, t-test (P < 0.05)
| Time (min) |
Diltiazem | Diltiazem with testosterone |
|---|---|---|
| 0 | 0 | 0 |
| 5 | 36.61 | 9.19 |
| 10 | 49.01 | 16.32 |
| 20 | 60.15 | 29.91 |
| 30 | 69.56 | 35.67 |
| 40 | 77.47 | 38.16 |
| 60 | 88.91 | 41.25 |
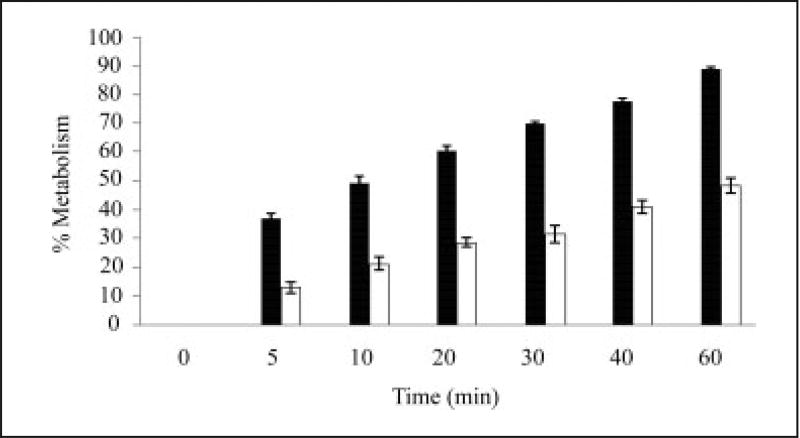
The metabolism of DTZ in the control rat liver S9 fraction was 88.9% and in the treated rat liver S9 fraction the DTZ level was 48.3%. There was a significant decrease in the metabolism of DTZ in the GFJ treated rat liver S9 fraction compared to that of the control. GFJ might have inhibited the CYP enzymes, thereby leading to the reduction of the metabolism of DTZ. These results are shown in Table 2 and Fig. 1.
Table 2.
Metabolic stability of diltiazem in male wistar rat liver S9 fraction in presence of GFJ. Statistically significant, t-test (P < 0.05)
| Time (min) |
Diltiazem | Diltiazem with GFJ |
|---|---|---|
| 0 | 0 | 0 |
| 5 | 36.61 | 12.79 |
| 10 | 49.01 | 21.11 |
| 20 | 60.15 | 28.49 |
| 30 | 69.56 | 31.38 |
| 40 | 77.47 | 40.76 |
| 60 | 88.91 | 48.26 |
Fig. 1.
%Metabolism of diltiazem in rat liver S9 fraction (n = 3). P < 0.05
■ – control, □ – in presence of grapefruit juice
* Statistically significant
2.2. Absorption studies
In the normal sac study, the mean transport of diltiazem from the mucosal to the serosal surface across normal rat intestine in the absence and the presence of GFJ (a known P-gp inducer and OATP inhibitor), verapamil (a potent P-gp inhibitor) and also under induced conditions of P-gp with rifampicin (a known CYP 3A and P-gp inducer), felodipine (a known CYP 3A inhibitor), and pravastatin (a known OATP inhibitor) was determined in the duodenum region of the rat small intestine. When the concentrations in the normal sac and the everted intestinal sac method were measured the transport of diltiazem in the normal sac was 1580.03 ± 2.06 nM whereas in the everted sac was 3975.66 ± 1.96 nM (+151.6%). The ratio of % transport of DTZ between serosal to mucosal and mucosal to serosal was more than doubled, which indicates that the net movement of DTZ across the rat duodenum is preferably more towards the secretary direction rather than absorptive direction (Koster et al. 1983). Considering these data, one could deduce that DTZ is a P-gp substrate. These results are represented in Table 3.
Table 3.
Accumulated transport of diltiazem (nM) in duodenal, normal and everted sac (n = 3), P < 0.05
| Time (min) |
Normal sac | Everted sac |
|---|---|---|
| 1 | 0 | 0 |
| 5 | 834.93 | 1204.69 |
| 10 | 1293.33 | 2092.98 |
| 15 | 1390.05 | 2200.07 |
| 20 | 1420.76 | 2725.59 |
| 25 | 1501.07 | 3328.09 |
| 30 | 1581.96 | 3975.66 |
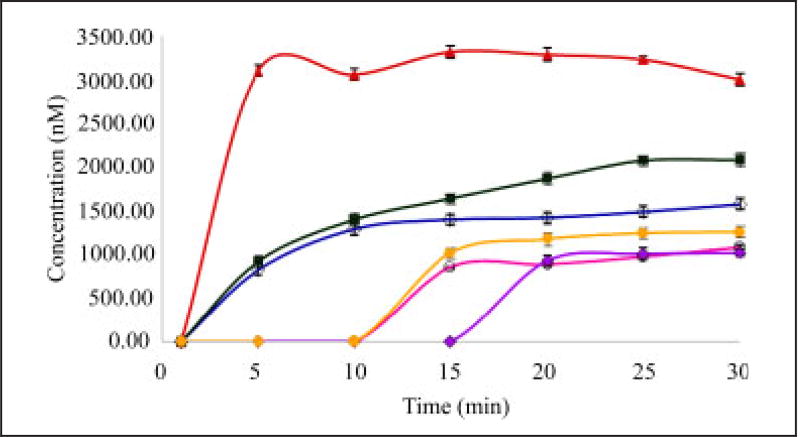
The mean cumulative concentration of DTZ in the presence of verapamil was increased from 1581.96 ± 7.88 to 3004.67± 5.18 nM in the duodenum. Verapamil is a P-gp inhibitor (efflux transporter), therefore increases the DTZ levels by inhibiting P-gp. The presence of felodipine significantly increased (P < 0.05) the mean cumulative concentration of diltiazem in the duodenum from 1581.96 ± 7.88 to 2081.00 ± 8.88 nM. Felodipine is a specific CYP450 enzyme inhibitor thus increasing the levels of DTZ by reducing its metabolism in the intestine. Rifampicin pretreatment decreased the mean cumulative concentrations of DTZ significantly from 1581.96 ± 7.88 to 1015.7 ± 9.69 nM. Rifa mpicin when given for 7 days induced P-gp, while this P-gp in turn reduced the DTZ levels. The mean cumulative concentration of diltiazem in the presence of pravastatin was decreased significantly from 1581.96 ± 7.88 to 1265.97 ± 5.51 nM. Pravastatin is an OATP inhibitor; an influx transporter thereby reducing the DTZ levels by inhibiting influx transportation. In summary, verapamil increased the mean cumulative transport by 89.93%, felodipine increased the mean cumulative transport by 31.54% and rifampicin pretreatment increased the mean cumulative efflux of diltiazem by 35.79%. However, the presence of pravastatin decreased the mean cumulative transport by 19.97% (P < 0.05).
In addition, GFJ pretreatment decreased the mean cumulative concentrations from 1581.96 ± 7.88 to 1084.04 ± 6.17 nM and reduced the mean cumulative transport by 31.47% (P < 0.05). The time course of diltiazem transport across the normal sac of the duodenum is shown in Table 4 and Fig. 2.
Table 4.
Mean ± S.D. (n = 3) Cumulative concentrations (nM) in intestinal normal sac in wistar rats. Statistically significant, t-test (P < 0.05)
| Region | Diltiazem | In presence of | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Grapefruit juice | Verapamil | Rifampicin | Felodipine | Pravastatin | ||
| Duodenum | 1581.96 ± 7.88 | 1084.04 ± 6.1 (−31.47%) | 3004.67 ± 5.18 (89.93%) | 1015.70 ± 9.69 (−35.79%) | 2084.33 ± 13.98 (31.76%) | 1265.97 ± 5.51 (−19.97%) |
Fig. 2.
Influence of grapefruit juice (GFJ), verapamil, rifampicin, felodipine on the permeation of DTZ in rat duodenum (n = 3) P < 0.05 Diltiazem levels in the absence (CTR) and presence of verapamil (VPM), rifampicin (RMP), felodipine (FDP) and pravastatin (PSTN)
◇ – control, ○ – in presence of grapefruit juice,
 – in presence of rifampicin, ▲ – in presence of verapamil, ■ – in presence of felodipine, ● – in presence of pravastatin
– in presence of rifampicin, ▲ – in presence of verapamil, ■ – in presence of felodipine, ● – in presence of pravastatin
2.3. In vivo studies
Animal experiments were conducted as per protocol 231/2000/CPCSEA. All of the rats tolerated treatments well and there were no reports of severe adverse effects during the study period. All of the concentrations were observed within the range of the calibration curve (12.62 ng/ml to 2034.2 ng/ml). In the rats, the mean and individual plasma concentration versus time profiles and pharmacokinetic parameters of DTZ before, after a single dose and pretreatment with GFJ are shown in Table 5 and Fig. 3.
Table 5.
Plasma profile of Mean ± SD (n = 6) diltiazem concentration following administration of 15 mg/kg b wt p.o with water (control), single and multiple dose GFJ
| Parameters | units | Diltiazem | Single dose GFJ | Multiple dose GFJ | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Cmax | ng/ml | 120.78 | 16.36 | 92.82 (23.1%) | 15.12 | 40.95 (66.09%) | 23.96 |
| AUC0–24 | ng · hr/ml | 43.63 | 8.16 | 43.08 (1.26%) | 9.44 | 24.77 (43.22%) | 18.18 |
| AUC0–∞ | ng · hr/ml | 62.77 | 17.82 | 51.48 (17.98) | 10.16 | 33.71 (35.14%) | 9.31 |
| Tmax | hr | 0.08 | 0 | 0.08 (0%) | 0.07 | 0.43 (437.5%) | 0.33 |
| T1/2 | hr | 0.49 | 0.28 | 0.52 (6.12%) | 0.08 | 1.16 (136.7%) | 0.63 |
(n = 6) P < 0.05. The values mentioned in bracket are expressed as % inhibition compared to that of control
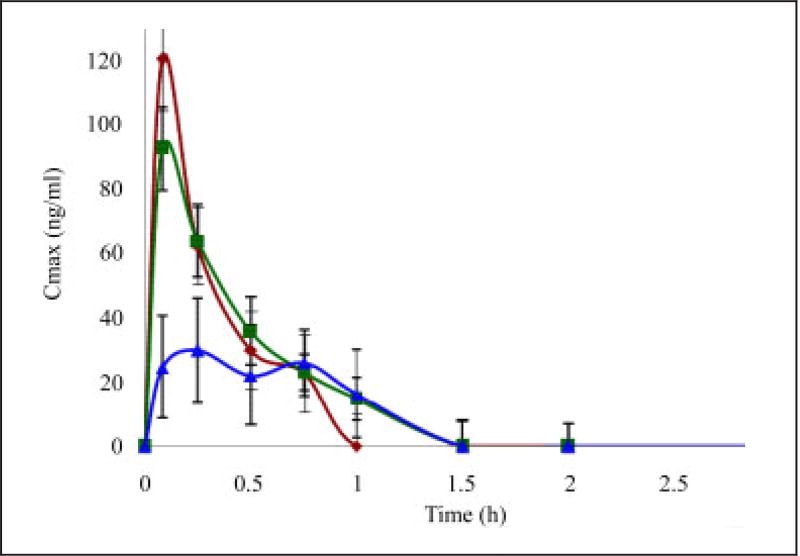
Fig. 3.
Mean plasma concentration ± SD versus time profiles of diltiazem following administration of 15mg/kg b wt p.o with water (control), single and multiple dose GFJ. (n = 6) P < 0.05
◆ – control, ■ – grapefruit juice (single day study), ▲ – grapefruit juice (7 day study)
The maximum plasma concentration reached (Cmax) for diltiazem in the presence of a single dose and multiple dose GFJ are in the ratio of 0.76 : 0.33 whereas the systemic exposures (AUC0–t) are in the ratio of 0.98 : 0.56 compared to the control. Therefore there was a significant reduction in the Cmax (120.78 ± 16.36 ng/ml to 40.95 ± 23.1 ng/ml) and AUC0–t (43.63 ± 8.16 µg · h/ml to 24.77 ± 18.18 µg · h/ml) in multiple doses of GFJ however there was no significant change in the Cmax (120.78 ± 82.57 ng/ml to 92.82 ± 15.12 ng/ml) and AUC0–t (43.63 ± 8.16 µg · h/ml to 43.08 ± 9.44 µg · h/ml) of a single dose of GFJ. The maximum absorption of DTZ was decreased slightly in the presence of single administration of GFJ and decreased significantly (p < 0.05) in the presence of multiple dose administration of GFJ. The time to reach maximum absorption (Tmax) was increased from 0.08 h to 0.43 h in the presence of multiple doses of GFJ but did not show the similar effect in the presence of a single dose of GFJ. AUC0–α decreased from 62.77 ± 17.82 µg · h/ml to 33.71 ± 9.31 µg · h/ml (P < 0.05) in the case of a multiple dose of GFJ, whereas there was no significant change in the single dose of GFJ (62.77 ± 17.82 to 51.48 ± 10.16). T1/2 increased significantly from 0.49 ± 0.28 h to 1.16 ± 0.63 h in a multiple dose of GFJ, however in the single dose of GFJ there was no significant change (0.49 ± 0.28 h to 0.39 ± 0.08 h).
Grapefruit juice treatment decreased the Cmax, AUC0–t and AUC0–α of DTZ by 66.0% (P < 0.01), 43.2% (P < 0.005), 35.1% (P < 0.05) respectively with multiple dosing. The same parameters were decreased by 23.14%, 1.26%, 17.98% (P < 0.05) with a single GFJ dose respectively. GFJ increased the Tmax by 437.5% (P < 0.05) in the multiple dose but no change in Tmax was observed in this single dose study. T1/2 was increased by 136.7% (P > 0.05) in the multiple dosing and similarly there was no statistically significant change in T1/2 during single dosing of GFJ.
3. Discussion
The present study describes the effect of GFJ (15 mg/kg/day dosed once for 7 days), a known P-gp modulator and, CYP 3A and OATP inhibitor, on the oral pharmacokinetics of diltiazem. DTZ is mainly used in the treatment of hypertension and angina. It is mainly metabolized to desacetyl diltiazem, demethyl diltiazem and deacetyldemethyl diltiazem in which CYP 3A mediates this oxidation.
Our in vitro metabolism study data demonstrate that GFJ is an inhibitor of CYP 3A. The finding of the present study is in contrast to Mohri et al. (2000) who reported that GFJ induces CYP 3A upon long-term administration (nifedipine/intraduodenally). These researchers found that nifedipine levels increased by approximately 1.62 times in rats. However, these present in vitro metabolism studies are in good agreement with those of Christensen et al. (2002) who reported that diltiazem metabolism is inhibited upon its simultaneous administration with GFJ. DTZ metabolism was decreased when incubated in control rat liver S9 fraction along with testosterone compared to the incubation with DTZ alone. As previously stated, testosterone is a specific CYP 3A inhibitor. Therefore, since DTZ metabolism was decreased in GFJ’s presence, we are able to attribute the DTZ metabolism to an inhibition of CYP 3A enzymes by GFJ. In addition in vitro transport studies performed on DTZ in the presence of felodipine, a known CYP 3A inhibitor also suggest that DTZ is a specific CYP 3A substrate and its metabolism might be inhibited by GFJ.
It has been suggested that the interaction between GFJ and drugs may be caused by a combination of inhibition of CYP 3A activity and P-gp transport during the intestinal absorption process. However, the effect of GFJ on P-gp has been controversial since GFJ has been shown to both inhibit and activate the P-gp drug efflux transporter in vitro (Christensen et al. 2002).
In this present study, we investigated transport of DTZ at the intestinal level to determine the influence of GFJ, rifampicin (P-gp inducers), verapamil (P-gp inhibitor) and pravastain (OATP inhibitor) in the rat-normal sac of the duodenum. Results of our in vitro studies revealed that diltiazem transport across the small intestine is very much affected by these known P-gp modulators and the OATP inhibitor. In this study, the mean ± S.D. cumulative exsorption concentrations of DTZ after pretreatment with GFJ and rifampicin were decreased, whereas the concentrations were increased in the presence of verapamil. Even though DTZ is a highly permeable drug as per BCS classification, its transport from serosal to mucosal is more than 2-fold compared to its mucosal to serosal transport. Therefore the net movement of DTZ across the rat duodenum is in the secretary direction, which proves that DTZ may be a P-gp substrate and thus any supplement (in this study, GFJ) that influences P-gp also influences the DTZ levels. Our in vitro intestinal transport studies performed on GFJ treated duodenum resulted in a decrease in DTZ transport from the mucosal to serosal surface. These data suggests that GFJ has induced intestinal P-gp and thus has resulted in the reduced DTZ levels compared to the control. This observation indicated the role of P-gp, an efflux pump on diltiazem absorption. The DTZ transport from the mucosal to the serosal side was also reduced in the presence of pravastatin, a known OATP inhibitor suggesting that DTZ might be an OATP substrate. As GFJ is also an OATP inhibitor upon chronic administration, the reduced DTZ transport in GFJ-treated duodenum can also be attributed to inhibition of OATP.
The pretreatment with GFJ significantly decreased the Cmax, AUC0–α and AUC0–t of DTZ by 66.1%, 35% and 43% respectively. There were no significant changes in pharmacokinetic parameters of DTZ with the single dose, but there was a significant change with the multiple dose of GFJ.
The observed pharmacokinetic data of diltiazem after treatment indicated that the influence of the P-gp modulator, GFJ, on the oral pharmacokinetics of DTZ, especially by acting through intestinal P-gp apart from other regions such as the liver and the biliary canaliculi. Intestinal P-gp is known to localize in the brush border membrane to pump drugs from the serosal side into the mucosal side (Sigusch et al. 1994). Induction of intestinal P-gp increased drug exsorption and thus decreased net drug absorption. The increase in the Tmax of the multiple dose regimen by 437% compared to that of the control provides an indication that the reduced drug levels could be due to decreased absorption of DTZ at the intestinal level. This decrease in the drug levels is due to the exsorption of drug, which in turn is due to induction of P-gp by GFJ. There was no change in the Tmax in the single dose regimen suggesting that GFJ is not producing an effect on P-gp upon single dose treatment. The significant pharmacokinetic changes observed in Cmax and Tmax clearly indicate that the absorption of DTZ might have been influenced through P-gp and OATP by GFJ. The reason for the significant increase in Tmax may be due to the induction of P-gp and inhibition of OATP. As GFJ induced P-gp, the drug is effluxed from the blood into the intestine reducing the absorption rate and thus resulting in an increased Tmax. The inhibition of OATP by GFJ, which is evident from the in vitro results, has further reduced the blood levels of DTZ resulting in a reduced Cmax compared to the control. However, the possible role of CYP 3A on the pharmacokinetics of DTZ cannot be ruled out directly, as it is believed that metabolism of DTZ is through CYP 3A, and GFJ is also an inhibitor of CYP 3A. Our in vitro metabolism studies also demonstrated that DTZ metabolism is inhibited in GFJ-treated S9 fractions. Therefore, whatever changes that occurred are likely due to the influence of GFJ on P-gp, OATP and CYP 3A in the intestine.
If GFJ influences both P-gp and CYP enzymes to the same extent, there should not be any change in the DTZ levels in treated animals compared to the control. However, this present study’s in vivo data provides evidence that the plasma levels of DTZ levels were much less in the treated group compared to the control, indicating that GFJ is influencing P-gp more profoundly, compared to CYP enzymes. Therefore, these results indicate that before the drug is available for metabolism in the liver and intestine, it is being effluxed at the intestinal absorption level, leading to the reduced blood levels of DTZ. The reduction in blood levels due to induction of CYP enzymes by GFJ has been ruled out, since the in vitro metabolism results suggest that the GFJ is inhibiting these enzymes. Moreover, there was no influence of GFJ when it was administered concomitantly with DTZ (Sigusch et al. 1994). Our single dose data is supported by Sigusch et al in which these researchers reported that grapefruit juice does not influence the bioavailability of DTZ (Sigusch et al. 1994). Upon single dose administration of GFJ, there may be inhibition of CYP 3A and induction of P-gp but most likely not significantly. However, there was a significant influence on the pharmacokinetic parameters of DTZ upon chronic administration of GFJ. This data is supported by Varma et al. (2005) who reported that chronic administration of grapefruit juice induces P-gp. Soldner et al. (1999) performed in vitro experiments in MDCK-MDR1 cells and reported that GFJ activates P-gp and is therefore in good agreement with our results. It has also been reported that grapefruit juice is an inhibitor of OATP (Breschi et al. 1981). Dresser et al. (2002) demonstrated from their in vitro studies that GFJ inhibited rat OATP, utilizing fexofenadine as a model drug. In this work these researchers showed that the fexofenadine Cmax and AUC0–t levels were decreased from 288 µg/ml to 110 µg/ml and 1330 µg · h/ml to 439 µg · h/ml respectively due to inhibition of OATP. These researchers also reported from the in vitro experimental data that the furanocoumarins and bioflavanoids present in GFJ reduced the OATP activity significantly to reduce the model drug levels. Our in vitro experiments with the specific OATP inhibitor (pravastatin) and the Dresser et al. (2002) studies on rat OATP confirm that GFJ was not only inhibiting CYP 3A and inducing P-gp but also inhibiting OATP. Therefore, GFJ is inducing P-gp and inhibiting OATP, there is an additive effect in the reduction of transport of DTZ from the intestine into the systemic circulation, thus reducing the blood levels of DTZ to a more significant extent.
In summary, normal sac studies in the rat have clearly addressed that the alterations in the oral pharmacokinetics of DTZ could be due to changes at the absorption site, i.e. in the intestine. The extent of inhibition of CYP enzymes, OATP and induction of P-gp depends upon the levels of furanocoumarins and flavanoids in the GFJ, thus influencing systemic drug levels. Since, the efflux of drug from the blood to the intestine is increased by grapefruit juice, there is slow absorption of the drug and hence an increase in Tmax and ultimately leading to a decrease in the Cmax. Thus GFJ does not demonstrate an influence upon simultaneous administration with DTZ, whereas it induces P-gp and may inhibit OATP upon its chronic administration. Future investigations will study the effect of OATP on DTZ, as well as attempting to provide more clarification of these complex interactions.
4. Experimental
4.1. Materials
Diltiazem Hydrochoride was kindly donated by Parke Davis (Hyderabad, India). Grapefruit juice (GFJ) was purchased from Ocean Spray Cranberries Inc, U.S.A. Rifampicin, pravastatin, verapamil, ketoconazole were kindly donated by Glenmark Pharmaceuticals Limited (India). Wistar rats inbred at Glenmark Pharmaceuticals Limited (India) were procured and used. Felodipine was kindly donated by Zydus cadila HC Limited, (Ahmedabad, India). NADPH was purchased from SRL Biosciences (Mumbai, India). Krebs’s buffer was purchased from Hi Media Ltd (Mumbai, India). Glucose was purchased from E. Merck Limited (Mumbai, India). Methanol, acetonitrile and triethanolamine were of HPLC grade (Thomas Baker, Mumbai, India).
4.2. In vitro studies
4.2.1. Absorption studies of diltiazem using the normal sac method
The effect of GFJ on the transport of DTZ was studied by observing the direct effect on intestinal transport in untreated as well as treated rats with GFJ for 7 days in excised duodenum by using the normal sac method. Male Wister rats were fasted overnight with free access to water before the experiments. The whole small intestine was flushed with 50 ml of ice-cold saline (Claris Life Sciences, Ahmedabad, India) with the animal under anesthesia with urethane (1.5 g/kg.b.wt/ip). The rat was exsanguinated, and the duodenum was isolated. A 5 cm section of the duodenum without eversion was prepared. DTZ (100 µM) was dissolved in pH 7.4 isotonic Krebs Buffer (KBS) containing 25 mM glucose. The drug solution (200 µl) was then introduced into the mucosal side, and both ends of the sac were ligated tightly. The sac containing probe drug solution was immersed in an organ bath (Hugo Sachs, Germany) containing 25 mM glucose as that on the mucosal side. The medium was pre-warmed at 37 °C and pre-oxygenated with 5% CO2/95% O2 for 15 min. Under bubbling with a CO2/O2 mixture gas, the transport of the DTZ from the mucosal to serosal surface was measured by sampling the serosal medium periodically for 30 min. Integrity of the preparation throughout the experiment was checked by adding 200 µM phenolsulfonphthalein to the mucosal or serosal side. At 15 min, 0.1 ml samples of the mucosal side were mixed with 1 ml of NaOH and the absorbance was measured at 550 nm (Breschi et al. 1981; Koster et al. 1983).
Everted sac studies were conducted to confirm that the DTZ is a P-gp substrate by using DTZ (100 µM) in the same way as that of normal sac study except that the duodenum is everted (Saitoh et al. 1995).
To understand the DTZ transport mechanism, the transport of DTZ in the presence of various P-gp inhibitors and inducers were studied, using 100 µM verapamil (P-gp inhibitor), 60 mg/kg b.wt/p.o for 7 days of rifampicin (P-gp inducer), 100 µM felodipine (CYP 3A inhibitor) and 100 µM pravastatin (OATP inhibitor).
4.2.2. Preparation of rat liver S9 fraction
Two groups of male wistar rats (n = 2) were administered with saline (control) and treated with GFJ orally for 7 days. The control and treated animals were fasted overnight for 12 h and anesthetized with urethane (1.5 gm/kg.b.wt/ip). The rat liver S9 fractions were prepared from the above groups according to the method cited in the literature (Yoshihara et al. 2004) and the abdominal cavity of the animal was opened with a vertical abdominal incision. The portal vein was isolated and cannulated with a polyethylene intramedic tubing PE100. The rat liver was perfused with ice cold homogenization medium (0.05 M Tris 0.154 M KCl, and 0.01 M MgCl2) at the rate of 15 ml/min using a peristaltic roller pump (Ecoline, Harvard apparatus, GmbH Germany) with a slit of the inferior vena cava below the kidneys until the liver was completely cleared of blood remnants. The perfused liver was excised and kept in a beaker containing an ice cold homogenizing buffer. The liver was rinsed three times with the ice cold homogenizing buffer and kept in an ice bath until further processing. Small pieces of liver were made with surgical scissors in a petridish kept on an ice bath. The 20 (% w/v) liver homogenate was made in homogenizing buffer (0.05 M Tris 0.154 M KCl, and 0.01 M MgCl2) using a motor driven porter elvehjm tissue homogenizer (Glas-col, Canada). This homogenate was centrifuged at 9000 g for 20 min at 4 °C in a high speed centrifuge (Heraeus, GmbH Germany). The supernatant, S9 fractions, was estimated for protein content by using Barfoed’s reagent and was diluted to obtain the final protein concentration of 10 mg/ml with homogenization buffer. These fractions were stored at −80 °C in a freezer (Heraeus, GmbH Germany) until further studies were conducted.
4.2.3. Incubation with rat liver S9 fraction
Incubation mixtures (total volume 0.5 ml) consisted of 420 µl of 100 mM Tris buffer (pH 7.4), 25 µl of 2 mM NADPH, 50 µl of control rat liver S9 fraction (to obtain a final concentration of 1 mg protein/ml) and 5 µl of diltiazem (final concentration of 12.5 µM). This experiment was performed in triplicate in treated and control rat liver S9 fractions. Before addition of NADPH, preincubation was performed in a shaker water bath (SW-22, Julabo, Switzerland) at 37 °C for 5 min so that the mixture equilibrated with the experimental conditions. The enzymatic reaction was initiated by adding the NADPH and allowed to proceed at 37 °C in the shaker water bath. At 0, 5, 10, 20, 30, 40, 60 min, 100 µl of acetonitrile was added and mixed well to terminate the reaction in each vial. These samples were centrifuged at 10,000 rpm for 10 min and the supernatant was injected into an HPLC for the analysis of diltiazem. All incubation mixtures were performed in triplicate.
4.3. In vivo studies
Three groups of male wistar rats weighing 180–250 g were used throughout the study. The first group of rats (n = 6) received a single dose of 15 mg/kg.b.wt/p.o DTZ in water through oral gavage after overnight fasting (12 h before dosing). These rats were simultaneously administered water at 5 ml/kg/p.o in view of the design of simultaneous food-drug interaction studies and to keep the volume of the fluids the same in all of the groups. The second group of rats (n = 6) were administered a single dose of 15 mg/kg/p.o DTZ in water and 5 ml/kg of GFJ was given concomitantly through oral gavage after overnight fasting (12 h before dosing) condition. A multiple dose study was conducted in the third group of rats (n = 6). These animals were pretreated with GFJ (5 ml/kg/p.o) and water (5 ml/kg/p.o) for 6 days while on the seventh day GFJ (5 ml/kg/p.o) was given concomitantly with DTZ (5 ml/kg/p.o). The blood samples were collected from all of the groups at pre-dose, 0.083, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 24 h post-dose and were kept in an ice bath until further experimentation. These samples were further processed for plasma by centrifuging at 4 °C for 10 min at 1000 × g by a Megafuge 3SR (Heraeus, Germany) and then stored at −80 °C until further analysis. These samples were analyzed by HPLC-UV for estimating the levels of DTZ.
4.4. Chemical analysis
4.4.1. Chromatographic conditions
Diltiazem levels were analyzed by using the reverse-phase HPLC method described by Al-Saidan et al. (2005) with a minor modification of the mobile phase composition. The HPLC system (Waters) equipped with Empower software was used to quantify the DTZ in samples at 238 nm. Plasma samples and in vitro absorption samples were analyzed for DTZ using a reversed phase analytical Hypersil BDS C-18 stainless steel column (250 mm length and 4.6 mm internal diameter packed with porous silica spheres of 5 µ diameter, 100 A0 pore diameter). The mobile phase used was a mixture of acetonitrile and water (35 : 65) containing 0.25% v/v of triethylamine (pH adjusted to 3.0 with 5% orthophosphoric acid). The flow rate of 0.8 mL/min was used for the in vivo study and the in vitro absorption study samples. A gradient method consisting of 100 mM ammonium acetate buffer, acetonitrile and methanol was used for in vitro metabolism study samples. The method produced a linear calibration curve over the range of 12.5 to 2000 ng/ml of DTZ in plasma (R2 = 0.999). The method was specific to diltiazem and none of the other drugs used in this study had interference with diltiazem quantification.
4.4.2. Pretreatment of biological samples for HPLC
To 100 µl of plasma, 5 µl of omeprazole (20 µg/ml) were added as an internal standard and vortexed for 30 s. To this mixture, 1.5 ml of methyl tertiary butyl ether (TBME) was added and vortexed on a cyclo-mixer (37600 mixer, Thermolyne, USA) for 10 min and centrifuged at 10,000 rpm (Megafuge 3SR, Heraeus, GmbH Germany) for 10 min. The supernatant was then separated into a glass tube and evaporated under dried nitrogen (Nitrogen Evaporator, Zymark, USA) at 40 °C. The final residue was reconstituted with 100 µl of mobile phase and injected into the HPLC-UV for the quantification of diltiazem.
4.5. Pharmacokinetic analysis
The pharmacokinetic parameters, peak plasma concentrations [Cmax] and time to reach peak concentration [tmax] were directly obtained from concentration-time data. In the present study, AUC0–t refers to the AUC from 0 to 24 h, which was determined by the linear trapezoidal rule, and AUC0–∞ refers to the AUC from 0 to infinity. The AUC0–∞ was calculated using the formula AUC0–t + [Clast/K] where Clast is the concentration in µg/ml at the last time point and K is the elimination rate constant.
Various pharmacokinetic parameters such as area under the curve [AUC], elimination half life [T1/2] volume of distribution [V/f] and the total clearance [CL/f] for each subject were obtained using a non-compartmental pharmacokinetic analysis from Win Nonlin 5.1 (Pharsight Corporation, North Carolina, USA).
4.6. Statistical analysis
The mean pharmacokinetic parameters of DTZ obtained before and after pretreatment with GFJ were compared by ANOVA using Win Nonlin 5.1 (Pharsight Corporation, North Carolina, USA) and the in vitro metabolism and the in vitro absorption results were compared by a student t-test using Graph Pad Prism version 3.02 (Graph Pad Inc., USA). A value of P < 0.05 was considered to be statistically significant.
Acknowledgments
This project was partially supported by Grant Number P20RR021929 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
References
- Al-Saidan SM, Krishnaiah YS, Patro SS, Satyanaryana V. In vitro and in vivo evaluation of guar gum matrix tablets for oral controlled release of water-soluble diltiazem hydrochloride. AAPS Pharm Sci Tech. 2005;6:E14–21. doi: 10.1208/pt060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DS, Munoz JC, Arnold J. Interaction of citrus juices with felodipine and nefedipine. Lancet. 1991;337:268–269. doi: 10.1016/0140-6736(91)90872-m. [DOI] [PubMed] [Google Scholar]
- Breschi C, Carelli V, Di Colo G, Nannipieri E. Effect of tissue degeneration on drug transfer across in vitro rat intestine. Farmaco – Ed. Sc. 1981;36:166–180. [PubMed] [Google Scholar]
- Buckley MMT, Grant SM, Goa KL, McTabish D, Sorkin EM. Diltiazem A reappraisal of its pharmacological properties and therapeutic use. Drugs. 1990;39:757–806. doi: 10.2165/00003495-199039050-00009. [DOI] [PubMed] [Google Scholar]
- Christensen H, Asberg A, Holmboe H, Berg KJ. Coadministration of grapefruit juice increases systemic exposure of diltiazem in healthy volunteers. Eur J Clin Pharmacol. 2002;58:515–520. doi: 10.1007/s00228-002-0516-8. [DOI] [PubMed] [Google Scholar]
- Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- Edwards DJ, Fitzimmons ME, Schuetz EG, Yasuda KMP, Warbasse LH, Woster PM, Schuetz JD, Watkins P. 6′,7′-Dihydroxybergamottin in grapefruit juie and Seville orange juice: Effects on cyclosporine disposition, enterocyte CYP 3A, and P-glycoprotein. Clin Pharmacol Ther. 1999;65:237–244. doi: 10.1016/S0009-9236(99)70102-5. [DOI] [PubMed] [Google Scholar]
- Kane GC, Lipsky JJ. Drug-grapefruit juice interactions. Mayo Clin Proc. 2000;75:933–942. doi: 10.4065/75.9.933. [DOI] [PubMed] [Google Scholar]
- Kantola T, Kivisto KT, Neuvonen PJ. Grapefruit juice greatly increases serum concentrations of lovastatin and lovastatin acid. Clin. Pharmacol. Ther. 1998;63:397–402. doi: 10.1016/S0009-9236(98)90034-0. [DOI] [PubMed] [Google Scholar]
- Koster AS, Noordhoek J. Glucuronidation in isolated perfused rat intestinal segments after mucosal and serosal administration of 1-naphthol. J Pharm Exp Ther. 1983;226:533–538. [PubMed] [Google Scholar]
- Libersa CC, Brique SA, Motte KB, Caron JF, Guedon-Moreau LM, Humbert L. Dramatic inhibition of amiodarone metabolism induced by grapefruit juice. Br J Clin Pharmacol. 2000;49:373–378. doi: 10.1046/j.1365-2125.2000.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri K, Uesawa Y, Sagawa K. Effects of long-term grapefruit juice ingestion on nifedipine pharmacokinetics: induction of rat hepatic P-450 by grapefruit juice. Drug Met Disp. 2000;28:482–486. [PubMed] [Google Scholar]
- Saitoh H, Aungst BJ. Possible involvement of multiple P-glycoprotein-mediated Efflux systems in the transport of verapamil and other organic cations across rat intestine. Pharm Res. 1995;12:1304–1310. doi: 10.1023/a:1016217505990. [DOI] [PubMed] [Google Scholar]
- Schmiedlin-Ren P, Edwards DJ, Fitzsimmons ME, He K, Lown KS, Woster PM, Tahman A, Thummel KE, Fisher JM, Hollenberg PF, Watkins PB. Mechanism of enhanced oral availability of CYP 3A substrates by grapefruit constituents: decreased enterocyte CYP 3A concentration and mechanism-based inactivation by furanocoumarins. Drug Met Disp. 1999;25:1228–1234. [PubMed] [Google Scholar]
- Schulte KL, Meyer-Sabellek WA, Haertenberger A, Thiede HM, Roecker L, Distler A, Gotzen R. Antihypertensive and metabolic effects of diltiazem and nifedipine. Hypertension. 1986;8:859–65. doi: 10.1161/01.hyp.8.10.859. [DOI] [PubMed] [Google Scholar]
- Shimomura S, Wanwimolruk S, Chen JJ. Drug interactions with grapefruit juice: an evidence-based overview. Pharmacy Times. 2003 ( https://secure.pharmacytimes.com/lessons/200303–02.asp)
- Sigusch H, Henschel L, Kraul H, Merkel U, Hoffmann A. Lack of effect of grapefruit juice on diltiazem bioavailability in human subjects. Pharmazie. 1994;49:675–679. [PubMed] [Google Scholar]
- Soldner A, Christians U, Susanto M, Wacher VJ, Silverman JA, Benet LS. Grapefruit juice activates P-glycoprotein-mediated drug transport. Pharm. Res. 1999;16:478–485. doi: 10.1023/a:1011902625609. [DOI] [PubMed] [Google Scholar]
- Tian R, Koyabu N, Takanaga H, Matsuo H, Ohtani H, Sawada Y. Effects of grapefruit juice and orange juice on the intestinal efflux of P-glycoprotein substances. Pharm Res. 2002;19:802–809. doi: 10.1023/a:1016100715125. [DOI] [PubMed] [Google Scholar]
- Varma MVS, Panchangula R, Bansal T, Kaul CL. Co-treatment with grapefruit juice inhibits while chronic administration activates intestinal P-glycoprotein-mediated drug efflux. Pharmazie. 2005;60:922–927. [PubMed] [Google Scholar]
- Yoshihara SI, Mizutare T, Makishima M, Suzuki N, Fujimoto N, Igarashi K, Ohta S. Potent estrogenic metabolites of bisphenol A and bisphenol B formed by rat liver S9 fraction: their structures and estrogenic potency. Toxicol Sci. 2004;78:50–59. doi: 10.1093/toxsci/kfh047. [DOI] [PubMed] [Google Scholar]