Abstract
The purpose of this study is to determine the optimal iodine contrast-to-noise ratio (CNR) achievable for different patient sizes using virtual-monoenergetic-images (VMIs) and a universal acquisition protocol on photon-counting-detector CT (PCD-CT), and to compare results to those from single-energy (SE) and dual-source-dual-energy (DSDE) CT. Vials containing 3 concentrations of iodine were placed in torso-shaped water phantoms of 5 sizes and scanned on a 2nd generation DSDE scanner with both SE and DE modes. Tube current was automatically adjusted based on phantom size with CTDIvol ranging from 5.1 to 22.3 mGy. PCD-CT scans were performed at 140 kV, 25 and 75 keV thresholds, with CTDIvol matched to the SE scans. DE VMIs were created and CNR was calculated for SE images and DE VMIs. The optimal kV (SE) or keV (DE VMI) was chosen at the point of highest CNR with no noticeable artifacts. For 10 mgI/cc vials in the 35 cm phantom, the optimal CNR of VMIs on PCD (22.6@50keV) was comparable to that of the best DSDE protocol (23.9@50keV) and was higher than that of the best SE protocol (19.7@80kV). In general, the difference of optimal CNR between PCD and SE increased with phantom size, with PCD 50 keV VMIs having an equivalent CNR (0.6% difference) with that of SE at the 25 cm phantom and 57% higher CNR at the 45 cm phantom. PCD-CT demonstrated comparable iodine CNR of VMIs to that of DSDE across patient sizes. Whereas SE and DSDE CT exams require use of patient-size-specific acquisitions settings, our findings point to the ability of PCD-CT to simplify protocol selection, using a single VMI keV setting (50 keV), acquisition kV (140 kV), and energy thresholds (25 and 75 keV) for all patient sizes, while achieving optimal or near optimal iodine CNR values.
Keywords: Computed tomography (CT), photon counting detector (PCD), dual energy, virtual monoenergetic image (VMI), kV selection
1. BACKGROUND AND PURPOSE
In conventional single energy (SE) CT with energy integrating detectors, the tube potential (kV) has a significant impact on image quality and radiation dose [1, 2]. Therefore, as part of routine clinical workflow, operators must select the appropriate tube potential based on patient size and imaging task. Inappropriate tube potential selection could result in suboptimal image quality or excessive radiation dose. In addition, dual energy (DE) CT is becoming more widely available for routine clinical use and has been shown to provide extra information to improve diagnosis in multiple clinical areas [3]. The question arises then as to whether a patient should be scanned with a SE at optimal kV protocol or a DE protocol. In addition, multiple DE acquisition modes (i.e., tube potential pairs) are available on certain scanners and the selection of an appropriate DE mode is patient size and imaging task dependent [4]. The complexity of these decisions can decrease workflow efficiency, yet inappropriate selection of SE kV or DE kV pairs can result in suboptimal exams. A simple efficient workflow that provides optimal image quality across all patient sizes, while highly desirable, seems unlikely to be achieved with either SE or DE technology.
Recently, photon-counting-detector-based CT (PCD-CT) has been studied with phantom, animal and patients using clinical dose levels and dose rates [5–9]. The energy discrimination capability of the PCD enables a single-source, single-detector, simultaneous multi-energy CT acquisition. Multiple image types can be generated using PCD-CT, including virtual monoenergetic images (VMI), which simulate the appearance of images acquired with a monoenergetic beam. The iodine contrast in VMI increases with decreased VMI photon energy (keV), yet the image noise increases at lower keV settings. It has been shown that an optimal keV exists that has the highest contrast to noise ratio (CNR) [10]. Reports have also demonstrated that the selection of keV could influence lesion conspicuity [11]. We hypothesize that VMIs acquired on a PCD-CT scanner with a fixed tube potential and created at a fixed keV can provide comparable or improved iodine CNR to SE CT with optimized kV and DSDE with optimized tube potential pairs across different patient sizes. Such a universal protocol, based on a single tube potential, a single set of energy thresholds, and single keV value for VMIs, and which provides optimized iodine CNR across patient sizes, would significantly simplify clinical protocol decisions and workflow.
The purpose of this study is to determine the optimal iodine contrast-to-noise ratio (CNR) achievable for different object sizes using virtual-monoenergetic-images (VMIs) and a universal acquisition protocol on photon-counting-detector CT (PCD-CT), and to compare results to single-energy (SE) and dual-source-dual-energy (DSDE) CT.
2. METHODS AND MATERIALS
Phantoms and Imaging Protocols
Vials containing 3 concentrations of iodine (5, 10, and 20 mgI/cc) were placed in torso-shaped water phantoms that mimic the attenuation of an abdomen for different patients sizes (lateral widths of 25, 30, 35, 40, and 45 cm). Phantoms were scanned on a 2nd generation dual-source CT scanner (Definition Flash, Siemens Healthcare, Forchheim, Germany) with both SE and DE modes. For SE, tube potentials of 80, 100, 120, 140 kV were used with collimation of 128 × 0.6 mm and helical pitch of 0.6. Tube current was automatically adjusted based on phantom size (CareDose4D, 120 kV, 200 QRM), with CTDIvol ranging from 5.1 to 22.3 mGy for phantom sizes from 25 to 45 cm. The same phantoms were also scanned using 2 DE modes (i.e. tube potential pairs): 80/Sn140, and 100/Sn140 kV, with Sn indicating a tin filter added to the 140 kV beam. Collimation for DE modes was set as 32 × 0.6 mm and pitch as 0.6. Tube current was adjusted so that the CTDIvol was matched to that of the SE scans for each size of phantom.
The same phantoms were also scanned on a research PCD-CT scanner (Figure 1), which was built based on a 2nd generation dual-source CT scanner (i.e., the same scanner model used for DSDE measurements). The PCD-CT scanner has an energy integrating detector subsystem covering 50 cm field of view (FOV) and a PCD subsystem covering a 27.5 cm FOV. Either 2 or 4 energy thresholds can be selected, which resulted in 2 or 4 energy bins. In addition, two ultra-high-spatial-resolution imaging modes, with detector size of 0.25 mm × 0.25 mm at the iso-center, are available. This enables simultaneous multi-energy CT data acquisition with image thickness of 0.25 mm [12, 13]. Detailed descriptions of the system can be found elsewhere [5]. In this study, PCD scans were all performed at 140 kV, with energy thresholds of 25 and 75 keV, a collimation of 32 × 0.5 mm and a helical pitch of 0.6. The tube current was adjusted so that the CTDIvol was matched for each size of phantom to that of the SE and DE scans from the dual source scanner.
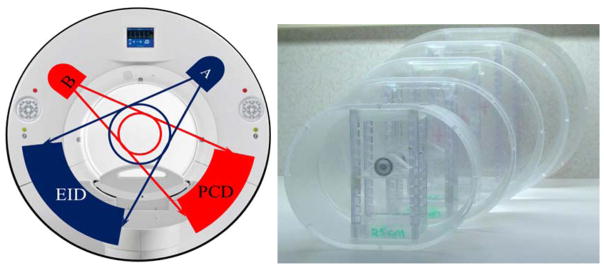
Figure 1.
The PCD-CT scanner (left) and water phantoms (right) used in this study.
Data Analysis
All images were reconstructed with a quantitative, medium smooth kernel (D30), and 60 mm field of view centered on the iodine-containing vials. For both DSDE and PCD-CT scans, VMIs with noise reduction techniques (Mono+, Siemens Healthcare, Forchheim, Germany) were generated using an offline tool (eXamine, Siemens Healthcare, Forchheim, Germany) [14]. For each image set (SE, DE VMI, and PCD VMI), signal and noise were measured for each vial and the background water area, from which CNR was calculated. The optimal kV for SE and optimal keV for multi-energy scans were chosen at the point of highest CNR with the following criteria: 1) no noticeable artifacts; 2) −10 HU ≤ mean CT number of water background ≤ +10 HU; 3) image noise lower than twice the noise level of SE at 120 kV [4]. CNR results were then compared among SE, DSDE, and PCD-CT techniques.
3. RESULTS
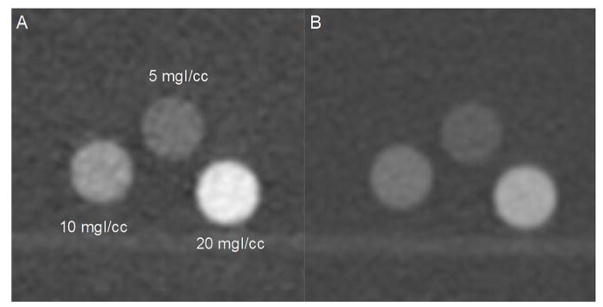
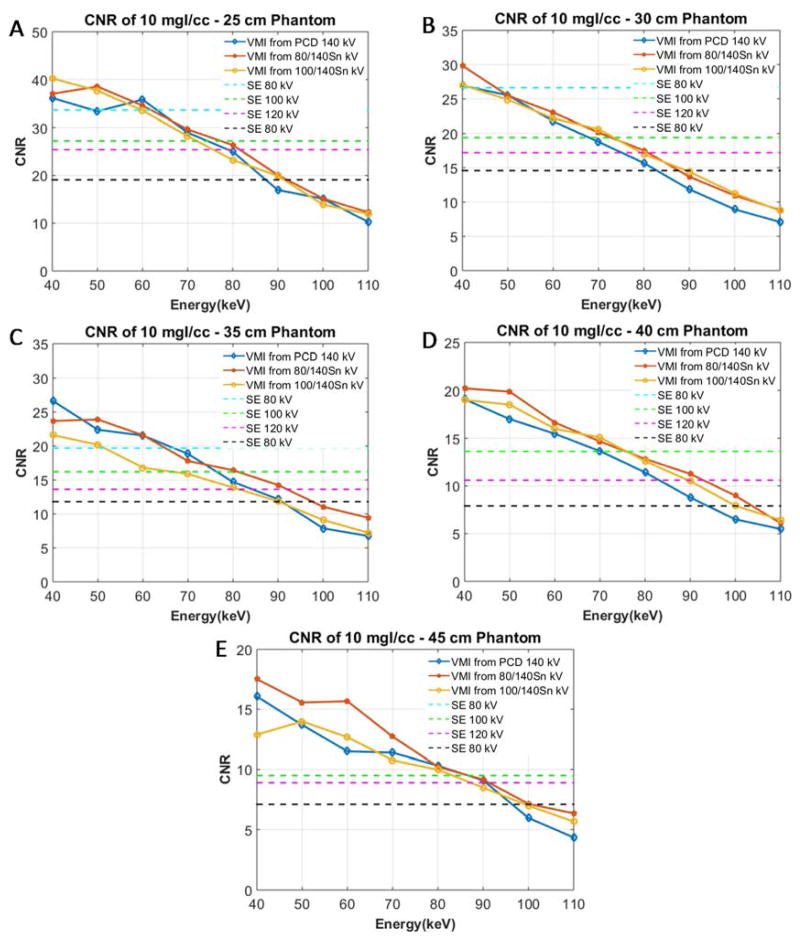
The PCD VMIs had higher iodine contrast than did the 80 kV SE images. However, image noise was also higher on the VMIs. Figure 2 shows sample images of the iodine-containing vials in the 35 cm water phantom, with a 50 keV VMI obtained from the PCD-CT scan using 140 kV, 25 and 75 keV thresholds (Figure 2A) and a SE image acquired with 80 kV (Figure 2B). Figure 3 shows the CNR of the 10 mgI/cc vials in the water phantom with different sizes (25, 30, 35, 40 and 45 cm) for PCD VMI, DSDE VMI, as well as the SE images. Table 1 summarizes the optimal CNR for each phantom size and scanning mode and the SE kV or DE VMI energy at which it was achieved. Due to the image artifacts and excessive noise, DSDE 80/Sn140 kV was excluded for water phantoms larger than 35 cm due to image artifacts. PCD VMIs at 50 keV had comparable or higher CNR than that of SE scanned at the optimal kV. This difference of CNR increased with phantom size, indicating more benefit of PCD in larger size phantoms. For 10 mgI/cc vials, the optimal CNR value for PCD 50 keV VMIs was comparable (~0.6% difference) to that of SE at the 25 cm phantom size, while at the 45 cm phantom size, the optimal CNR of PCD 50 keV VMIs was 57% higher than that of SE. The iodine CNR was very similar between PCD-CT and 100/Sn140 DSDE for all phantom sizes. For smaller size phantoms, 80/Sn140 kV DSDE had slightly higher CNR than that of PCD-CT. Figure 3 shows these data in greater detail.
Figure 2.
Images of 3 iodine vials within a 35 cm water tank: 50 keV VMI from PCD data acquired at 140 kV (A), and from conventional SE CT data acquired at 80 kV (B). Window/Level = 1800/400 HU
Figure 3.
CNR of VMIs at each keV and SE images at each kV for the 10 mgI/cc solution for 25 cm (A), 30 cm (B), 35 cm (C), 40 cm (D) and 45 cm (E) water phantoms.
Table 1.
CNR values for various scan modes for the 10 mgI/cc solution and different sizes of water phantom, and the SE tube potential (kV) or DE VMI energy (keV) at which it was achieved. The use of a single DE VMI energy (50 keV) would allow for the use of a universal PCD scan protocol and VMI setting, with iodine CNR at all phantom sizes either essentially equivalent to, or greater than, achieved with a SE protocol at a kV chosen according to patient size.
| Scanning Mode | 25 cm | 30 cm | 35 cm | 40 cm | 45 cm |
|---|---|---|---|---|---|
| SE | 33.6@80kV | 26.6@80kV | 19.7@80kV | 13.6@100kV | 8.7@120kV |
| DSDE 80/Sn140 kV | 38.5@50keV | 25.5@50keV | 23.9@50keV | N/A | N/A |
| DSDE 100/Sn140 kV | 37.7@50keV | 24.9@50keV | 20.2@50keV | 18.5@50keV | 13.9@50keV |
| PCD 140 kV | 33.4@50keV | 25.5@50keV | 22.6@50keV | 17.8@50keV* | 13.7@50keV* |
N/A = acceptable image quality could not be achieved with this scan mode and phantom size
CT number of water was outside of our ±10 HU range due to known calibration issues with large phantom sizes. The 60 keV setting met the CT number criteria but had lower iodine CNR.
4. DISCUSSIONS AND CONCLUSIONS
In this study, we investigated the feasibility of using a single universal protocol of 140 kV, 25 keV and 75 keV thresholds, for all patient sizes on a PCD-CT to simplify clinical workflow while maintaining iodine CNR equal to or greater than achieved using SE CT and the optimal kV for each patient size. The results demonstrated that for small to medium water phantoms (25–35 cm), PCD VMIs exhibited comparable CNR to SE protocols, while for phantoms larger than 35 cm, the iodine CNR of PCD VMIs was superior to that of SE protocols. The CNR performance of VMI generated from PCD-CT protocol was comparable to that from DSDE. For the PCD data, 50 keV VMI images at 40 and 45 cm phantom sizes had water CT numbers lower than −10 HU. This is a likely resolvable issue by improving calibrations and beam hardening corrections for large attenuation objects. These findings suggest that all patients, regardless of size, can be scanned on the PCD-CT with a universal protocol at 140 kV with comparable or better CNR than that of SE scans with optimized kV selection, an approach that will significantly simplify the clinical workflow and reduce inadvertent selection of an inferior kV setting. In addition, the results in this study suggest that the keV selection for PCD-CT VMIs can be size independent. Future studies should focus on lesion detectability using a reader study approach to further evaluate the clinical performance of the proposed universal PCD protocols, acquired using a single source, a single acquisition protocol (having a single kV and set energy thresholds), and the use of a single VMI energy setting (keV).
Acknowledgments
This research was supported in part by NIH Grants R01 EB016966 and C06 RR018898. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The research photon-counting CT system described herein is not commercially available.
References
- 1.Yu L, Li H, Fletcher JG, et al. Automatic selection of tube potential for radiation dose reduction in CT: a general strategy. Med Phys. 2010;37(1):234–43. doi: 10.1118/1.3264614. [DOI] [PubMed] [Google Scholar]
- 2.Hough DM, Fletcher JG, Grant KL, et al. Lowering kilovoltage to reduce radiation dose in contrast-enhanced abdominal CT: initial assessment of a prototype automated kilovoltage selection tool. AJR Am J Roentgenol. 2012;199(5):1070–7. doi: 10.2214/AJR.12.8637. [DOI] [PubMed] [Google Scholar]
- 3.McCollough CH, Leng S, Yu L, et al. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology. 2015;276(3):637–53. doi: 10.1148/radiol.2015142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michalak G, Grimes J, Fletcher J, et al. Selection of optimal tube potential settings for dual-energy CT virtual mono-energetic imaging of iodine in the abdomen. Abdom Radiol (NY) 2017;42(9):2289–2296. doi: 10.1007/s00261-017-1122-7. [DOI] [PubMed] [Google Scholar]
- 5.Kappler S, Glasser F, Janssen S, et al. A research prototype system for quantum-counting clinical CT. 7622:6. [Google Scholar]
- 6.Yu Z, Leng S, Jorgensen SM, et al. Evaluation of conventional imaging performance in a research whole-body CT system with a photon-counting detector array. Phys Med Biol. 2016;61(4):1572–95. doi: 10.1088/0031-9155/61/4/1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutjahr R, Halaweish AF, Yu Z, et al. Human Imaging With Photon Counting-Based Computed Tomography at Clinical Dose Levels: Contrast-to-Noise Ratio and Cadaver Studies. Invest Radiol. 2016;51(7):421–9. doi: 10.1097/RLI.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pourmorteza A, Symons R, Sandfort V, et al. Abdominal Imaging with Contrast-enhanced Photon-counting CT: First Human Experience. Radiology. 2016;279(1):239–45. doi: 10.1148/radiol.2016152601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Symons R, Krauss B, Sahbaee P, et al. Photon-counting CT for simultaneous imaging of multiple contrast agents in the abdomen: An in vivo study. Med Phys. 2017;44(10):5120–5127. doi: 10.1002/mp.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L, Leng S, McCollough CH. Dual-energy CT-based monochromatic imaging. AJR Am J Roentgenol. 2012;199(5 Suppl):S9–S15. doi: 10.2214/AJR.12.9121. [DOI] [PubMed] [Google Scholar]
- 11.Shuman WP, Green DE, Busey JM, et al. Dual-energy liver CT: effect of monochromatic imaging on lesion detection, conspicuity, and contrast-to-noise ratio of hypervascular lesions on late arterial phase. AJR Am J Roentgenol. 2014;203(3):601–6. doi: 10.2214/AJR.13.11337. [DOI] [PubMed] [Google Scholar]
- 12.Leng S, Yu Z, Halaweish A, et al. Dose-efficient ultrahigh-resolution scan mode using a photon counting detector computed tomography system. J Med Imaging (Bellingham) 2016;3(4):043504. doi: 10.1117/1.JMI.3.4.043504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leng S, Gutjahr R, Ferrero A, et al. Ultra-high spatial resolution multi-energy CT using photon counting detector technology. 10132:7. doi: 10.1117/12.2255589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant KL, Flohr TG, Krauss B, et al. Assessment of an advanced image-based technique to calculate virtual monoenergetic computed tomographic images from a dual-energy examination to improve contrast-to-noise ratio in examinations using iodinated contrast media. Invest Radiol. 2014;49(9):586–92. doi: 10.1097/RLI.0000000000000060. [DOI] [PubMed] [Google Scholar]