Abstract
Cell-based therapies are a promising alternative to grafts and organ transplantation for treating tissue loss or damage due to trauma, malfunction, or disease. Over the past two decades, mesenchymal stem cells (MSCs) have attracted much attention as a potential cell population for use in regenerative medicine. While the proliferative capacity and multilineage potential of MSCs provide an opportunity to generate clinically relevant numbers of transplantable cells, their use in tissue regenerative applications has met with relatively limited success to date apart from secreting paracrine-acting factors to modulate the defect microenvironment. Presently, there is significant effort to engineer the biophysical properties of biomaterials to direct MSC differentiation and further expand on the potential of MSCs in tissue engineering, regeneration, and repair. Biomaterials can dictate MSC differentiation by modulating features of the substrate including composition, mechanical properties, porosity, and topography. The purpose of this review is to highlight recent approaches for guiding MSC fate using biomaterials and provide a description of the underlying characteristics that promote differentiation toward a desired phenotype.
Keywords: biomaterial, mesenchymal stem cell, differentiation, tissue engineering, scaffold
Introduction
Mesenchymal stem cells (MSCs) have tremendous potential in cell-based therapies for tissue repair and regeneration due to their proliferation, multilineage potential, proangiogenic capabilities, immune regulatory and anti-inflammatory potential, and relative lack of ethical concerns compared to embryonic stem cells.1 The multilineage potential of MSCs, which may be derived from several tissue compartments, is the cornerstone for their use in tissue regeneration. Growth factors and other inductive cues effectively induce MSC differentiation, but this approach suffers from its own limitations including the costly supraphysiological dosages needed to achieve the desired phenotype, potential off-target effects of large dosages, and challenges in achieving the “optimal” release kinetics to stimulate neighboring cells. Moreover, the long-term maintenance of MSC differentiation is rapidly lost in vitro upon the removal of these cues2, as often occurs in vivo upon transplantation of induced cells. Thus, there is a significant need for effective strategies to instruct and maintain MSC differentiation, even upon cell delivery to the defect site. MSCs also respond to insoluble exogenous stimuli within their surrounding extracellular matrix (ECM) (Figure 1). The highly tailorable properties of biomaterials, commonly employed as temporary ECMs for associated cells, are catalyzing the development of new alternatives to tissue grafts.
Figure 1. The biophysical properties of the extracellular matrix instruct cell fate.
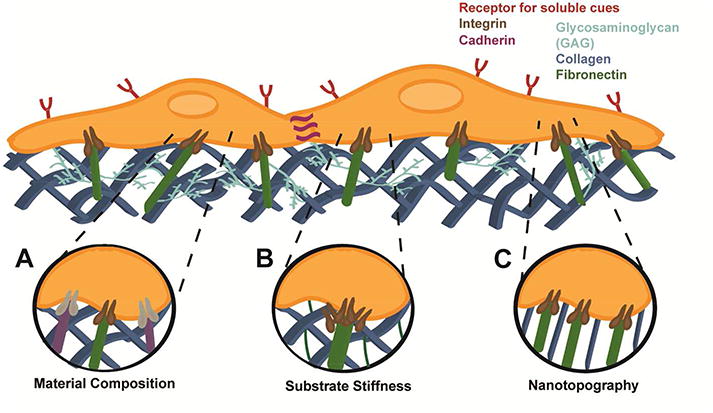
(A) Integrins (shown in brown and grey) from MSCs engage specific proteins and peptide sequences (illustrated in green and purple) in the ECM to promote adhesion, proliferation, or signal differentiation. (B) MSCs sense the underlying stiffness of the matrix by clustering transmembrane integrins, dictating cell spreading and adhesion strength, and linking the intracellular cytoskeleton to the ECM. These signals are transduced to the nucleus, modulating gene expression and resultant changes in cell phenotype. (C) Substrate topography can dictate cell alignment and increase MSC focal adhesion and actin polymerization.
MSC Populations for use in Tissue Engineering
MSCs are a heterogeneous cell population referred to by many names including mesenchymal stem cells, multipotent or mesenchymal stromal cells, progenitor cells, and support cells. Unlike embryonic stem cells, MSCs can be harvested from numerous tissue compartments of the postnatal organism for autologous use including bone marrow, adipose tissue, placenta, skin, synovium, and others, or generated from dermal skin fibroblasts (induced pluripotent stem cell-derived MSCs). While the community still lacks one specific identification marker for MSCs, a minimal criteria has been established: adherence to plastic, specific surface antigen expression (including, but not limited to CD105+, CD73+, CD90+, and CD45-), and in vitro differentiation toward osteoblasts, adipocytes, and chondrocytes.3 Although cellular therapies require an exorbitant number of MSCs that ranges several million cells per kilogram body weight, only a limited number of MSCs can be extracted from adult tissue. The potential to isolate or generate larger numbers of MSCs, such as through lipoaspiration or iPSC-derived technologies, respectively, is an important consideration when selecting the appropriate MSC source. Despite similar markers, all MSCs do not exhibit identical therapeutic or regenerative potential.4-5 The goal of this review is to highlight recent efforts using biomaterials to instruct MSC differentiation and function. We describe the use of biomaterials to direct MSC differentiation toward the osteogenic, chondrogenic, myogenic, and adipogenic lineages, while highlighting the potential of these biomaterials in regenerative therapies and tissue engineering.
Biomaterials to Guide MSC Osteogenic Differentiation for Bone Formation
The loss of bone volume due to trauma, disease, or congenital defects represents a significant challenge to patients across the lifespan. Despite bones' innate ability to repair upon injury, 5-20% of fractures do not heal due to loss of vascularity, instability, infection, soft tissue damage, or systemic disease.6 Autogenous iliac crest bone graft (ICBG) remains the gold standard treatment option to repair large bone deficits. However, challenges such as a limited supply that fails to support the requirements of large defects, chronic pain at the graft site in nearly one-third of patients, and poor survival of transplanted cells motivate the pursuit for alternative approaches to bone healing. Cell therapy is one of the most promising substitutes for ICBG, and MSCs are a prime candidate as a cell source. MSCs undergo osteogenic differentiation toward the osteoblastic lineage and secrete potent concentrations of endogenous trophic factors that promote vascularization and recruitment of reparative host cells. The success of this approach depends upon transplanting cells using carriers that instruct cell phenotype and function. Numerous osteoinductive materials are commercially available following approval by the US FDA and EU (reviewed in 7). However, intense interest remains for the development of materials-based approaches to instruct osteogenic differentiation achieved by engineering the biophysical properties of biomaterials. Several implant properties are under investigation to guide MSC differentiation to the osteogenic, chondrogenic, myogenic, and adipogenic lineages (Table 1) as highlighted below.
Table 1.
Common material biophysical properties to instruct MSC differentiation.
| Property | Material | Example | Effect | Tissue |
|---|---|---|---|---|
| COMPOSITION | bioinorganics | CaP, HAP | Increased adsorption of plasma proteins, cell adhesion, and nucleation of cell-secreted calcium | bone8-10 |
| fat11 | ||||
|
| ||||
| bioactive glasses | Dissolution products stimulate osteogenesis and trophic factor secretion | bone12-14 | ||
|
| ||||
| proteins & peptides | collagen, fibronectin, laminin | Endogenous sites for MSC adhesion and spreading | bone15-16 | |
| cartilage17 | ||||
| muscle18-20 | ||||
| fat21-22 | ||||
|
| ||||
| RGD, DGEA | Facilitate cell adhesion, may be presented from “blank slate” biomaterials | bone23-25 | ||
| cartilage26-27 | ||||
| muscle28 | ||||
|
| ||||
| decellularized tissues | demineralized bone matrix, decellularized cartilage and fat | Native ECM promotes cell adhesion and guides differentiation | bone15, 29-30 | |
| cartilage31-32 | ||||
| muscle33 | ||||
| fat34-35 | ||||
|
| ||||
| cell-secreted ECMs | Native ECM promotes cell adhesion and differentiation | bone2, 36-37 | ||
| cartilage38 | ||||
| fat37 | ||||
|
| ||||
| SUBSTRATE STIFFNESS | composite materials | PLG/HAP, collagen/DBM, silk/HA | Improved handling and degradation; promotes MSC adhesion and differentiation | bone15, 39-43 |
| muscle44 | ||||
|
| ||||
| hydrogels | alginate, hyaluronic acid, collagen, PEGDA, fibrin, polyacrylamide | Increase bulk stiffness by crosslinker concentration, resulting in increased differentiation | bone45-48 | |
| cartilage23, 26, 49-50 | ||||
| muscle51-54 | ||||
| fat22, 55-56 | ||||
|
| ||||
| SURFACE TOPOGRAPHY | fiber alignment/channels/grooves | PLG, PCL, PLLA | Control anisotropy to enhance focal adhesion | bone16, 57-58 |
| cartilage59-60 | ||||
| muscle61-64 | ||||
|
| ||||
| surface roughness | PCL, titanium | Increases MSC adhesion, focal adhesion kinase (FAK) signaling, and differentiation | bone65-68 | |
|
| ||||
| PLG | Promotes elastin and collagen deposition | muscle69 | ||
Composition
Bioinorganics play a key role in bone regeneration as defect fillers or cell carriers due to their osteoconductive potential. Calcium phosphate (CaP)-based ceramics (i.e., hydroxyapatite (HAP), β-tricalcium phosphate (β-TCP), bioactive glasses, etc.) are broadly used in the clinical setting to fill lost bone volume. In addition to their osteoconductive nature, bioactive glasses promote angiogenesis and bone formation when cells are stimulated by ions from their dissolution products.12-14 Implants formed of β-TCP8 or porous HAP9 were used as carriers for freshly isolated autologous stromal vascular fraction (SVF) to increase maxillary bone height for dental implant placement or repair the proximal humerus in two first-in-human studies. Compared to the ceramic scaffold alone, defects treated with SVF-seeded scaffolds exhibited greater bone volume. Studies in the maxilla failed to explicitly describe the contribution of transplanted cells, allowing for the possibility that SVF may contribute indirectly to bone repair by secreting trophic factors to recruit endogenous cells. However, biopsies from repair tissue in the humerus revealed human-derived bone tissue and vascular structures, confirming the ability of bioceramics to promote osteogenic differentiation in situ. The effect of bioinorganic substrates on MSC osteogenic differentiation may likely be dependent on the ceramic composition, concentration of transplanted cells, and defect site.
The slow resorption of many bioinorganics, coupled with their brittle handling characteristics, has spurred the development of composite materials formed of bioceramics and synthetic or natural materials to improve handling and versatility. These materials exhibit greater compressive properties as a function of ceramic loading, improved nucleation of cell-secreted calcium, and overall enhanced osteogenic potential that promotes osteogenic differentiation of MSCs in vitro39-41, 70-71 and bone formation in vivo43, 72-73. For example, silk/HAP composites fabricated by direct-write assembly stimulated MSC osteogenic differentiation regardless of filament spacing74, providing expanded opportunities for applying other stimuli (i.e., mechanical forces, osteoinductive cues, co-cultures) to generate osteogenic grafts. However, changes in construct composition due to increased ceramic loading, which directly increases mechanical properties of the scaffold, confound the determination of the true material contribution toward osteogenic differentiation.
Substrate stiffness
Mechanical properties of the underlying matrix, particularly materials with increased stiffness, can induce osteogenic differentiation of MSCs toward the osteoblastic lineage75-76 and activate osteoinductive growth factor pathways77. The role of implant stiffness on MSC differentiation within 3D scaffolds was examined using decellularized cancellous bone coated with increasing concentrations of collagen containing equal masses of HAP.15 Compared to cancellous bone alone, all materials coated with collagen/HAP exhibited increased local and bulk stiffness and enhanced osteogenic differentiation in vitro. Constructs with the highest bulk modulus yielded the greatest expression of osteogenic markers in vivo when implanted in an ectopic site. Although the scaffolds had similar microstructures derived from the underlying decellularized bone, the concentration of collagen was greatest in stiffer constructs, providing another means for enhanced osteogenesis through increased cell adhesion.
Surface roughness
The importance of surface roughness on MSC osteogenic differentiation was demonstrated using polycaprolactone (PCL) substrates possessing average roughnesses varying from the sub-micron to nearly 5 μm range with decreasing peak distances.65 Compared to flat surfaces, materials with large roughness and correspondingly narrower distances between roughness elements increased MSC osteogenic differentiation. Similar trends were observed when MSCs were cultured on titanium surfaces with sub-nano to sub-micron surface features.66 Increasing surface topography, which enhances focal adhesions and actin polymerization, can increase MSC osteogenic differentiation67 and VEGF secretion68. While potentially useful as an implant when cells colonize the surface, there are remaining challenges for its use in a 3D environment to achieve cell entrapment in 3D biomaterials.
Fiber alignment
The alignment of underlying ECM fibers has been studied using electrospun biomaterials, resulting in substrates with a microscopic structure similar to native tissue extracellular matrix. Fiber alignment can be further regulated by the properties of the collector. For example, slower rotation speeds yield fibers with random orientation, while faster speeds result in more highly aligned fibers. The incorporation of collagen into nanofibers of PLG and PCL promoted early adhesion, proliferation, and osteogenic gene expression in ASCs compared to cells on fibers lacking collagen, and this effect was further increased on aligned fibers.16 Compared to randomly oriented fibers, MSC migration was accelerated when seeded on aligned poly(L-lactic acid) (PLLA) fibers in vitro.57 Acellular aligned implants significantly increased new bone formation in murine calvarial defects compared to implants formed of randomly oriented fibers, resulting in new bone with similar underlying morphological structure of the implants. In contrast, others reported that randomly aligned PLLA fibers enhanced osteogenic differentiation in vitro, inducing cartilage formation and subsequent bone formation in a murine tendon model.58 These data suggest that fiber composition and alignment are key contributors to MSC response for forming mineralized tissues, and the site of implantation may direct endogenous host cell response toward bone formation.
Scaffold porosity
Scaffold porosity is a critical material design parameter to enable invasion of host cells and blood vessels for providing necessary nutrients. The minimum pore size required to induce the formation of mineralized tissue is generally considered to be 50-100 μm78-79, although there is no consensus on the most effective range of pore sizes for scaffolds used in bone regeneration. Smaller pores fail to promote the formation of mineralized tissue, often resulting in the formation of weak fibrous tissue, yet polylactide-co-glycolide (PLG)/CaP composite scaffolds with pore diameters as high as 1000 μm support bone formation in vivo80. Human MSCs implanted ectopically on commercially available β-TCP scaffolds exhibited greater bone formation in vivo in the most porous scaffolds.81 These scaffolds exhibited significant differences in micro- and macro-porosity, as pore diameters of the 65% and 75% porous scaffolds differed substantially (41 vs. 136 μm, respectively). Thus, scaffold variability limited the conclusions that could be drawn regarding the interactions between scaffold void volume and pore diameter. PLG/bioactive glass composites supported the formation of mineralized tissues by MSCs in vitro as pore diameter increased from 125-300 μm to 500-850 μm.82 Increases in mineralized tissue formation were attributed to more calcium and silicon available on the pore surface, providing increased interactions between associated MSCs and two key elements within bioactive glass that induce cellular responses. To mimic the heterogeneity of pore diameter from trabecular to cortical bone, gradients in pore diameter were formed via rapid prototyping of PCL.83 There was a strong correlation between osteogenic differentiation, ECM mineralization, and pore dimensions under long-term culture.
Hydrogels
As an alternative to implantable biomaterials, hydrogels are promising platforms to exploit stem cells in tissue engineering due to their high water content, ease of entrapping cells or inductive cues, and their morphology resembling the native ECM. Hydrogels have been formed of natural and synthetic polymers, and their use in tissue engineering has been reviewed elsewhere.84-85 By manipulating the composition86, mechanical properties45-47, and presentation of polypeptide ligands and proteins that promote adhesion and activation of specific cellular signaling pathways23-25, hydrogels can induce MSC osteogenic differentiation, as well as differentiation toward other lineages. For example, alginate is a biocompatible, naturally derived polysaccharide used extensively in tissue engineering applications due to the numerous options to tailor the gel's physical properties and direct differentiation of entrapped MSCs (Figure 2).
Figure 2. Alginate is a highly tailorable biomaterial that can be manipulated to guide MSC differentiation.
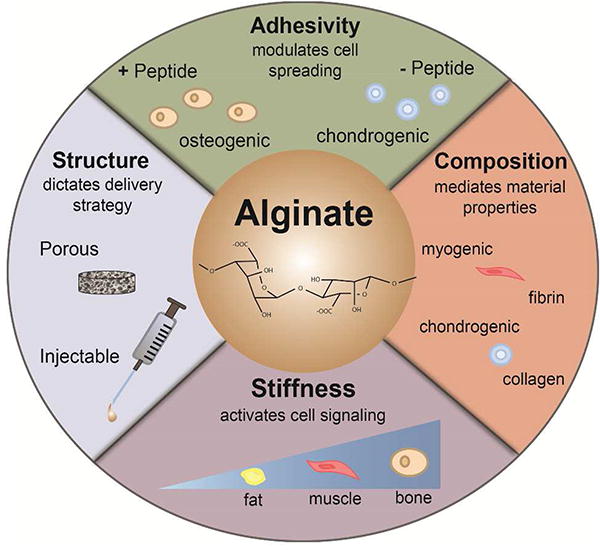
The biophysical properties of alginate can be tuned by controlling cell adhesion through chemical modification with peptides or proteins, composition by forming composites with other materials, and bulk substrate stiffness via crosslinker concentration or polymer density. MSCs engaging or entrapped in alginate can be differentiated to bone-forming osteoblasts, cartilage-forming chondrocytes, muscle-forming smooth and skeletal muscle cells, and soft tissue-filling adipocytes.
Unlike most implantable systems, hydrogels can be easily formed with reproducible changes in initial substrate stiffness achieved by controlling the concentration of crosslinkers used with constant composition. More recently, hydrogels have been designed to exhibit dynamic changes in stiffness.48 In alginate gels of different molecular weights crosslinked with varied calcium concentrations, the speed of stress relaxation could be increased in gels with similar initial elastic moduli, revealing the ability of stress relaxation in viscoelastic materials to increase cell spreading87 and osteogenic differentiation48. Nanocomposite hydrogels were formed by blending photocrosslinkable methacrylated gelatin hydrogels with nanosilicates, ultrathin nanomaterials containing SiO2, MgO, Na2O and Li2O.42 The addition of nanosilicates increased hydrogel stiffness, and murine preosteoblasts cultured on the surface of these composite gels exhibited increased osteogenic differentiation in vitro as a function of nanosilicate loading. Similar results were observed when human MSCs were entrapped in the hydrogel under 3D culture.88 The high surface area-to-volume ratio of these particulates may provide increased adhesion sites or additional cues via mechanotransduction to drive MSC osteogenic differentiation, but no studies were performed to elucidate the mechanism of this contribution.
Tissue engineering has long embraced the idea of borrowing cues from nature to build replacement tissues and effective biomaterials. To that end, decellularized allogenic and xenogenic tissues are under examination to stimulate osteogenic differentiation and bone formation. Demineralized bone matrix is widely used in the clinical setting to function as a bone graft extender.89 Decellularized cartilage promoted formation of mineralized tissues by MSCs, with in vivo mineralization substantially greater than acellular implants.29 Similar results have been reported using decellularized cartilage to treat critical-sized bone defects30, 90, transplanting chondrogenically-induced MSCs on degradable scaffolds91, or seeding MSCs into hyaline cartilage grafts formed of chondrocytes and their ECM38 – the efficacy of each likely achieved through endochondral bone formation. As an alternative to bony and cartilaginous tissues, cells have been directed to secrete instructive ECMs for use as coatings on scaffolds to capitalize on the complexity of the ECM combined with the tailorability of engineered materials. Cell-secreted ECMs, which are rich in collagens, glycosaminoglycans, and other matricellular components, promote cell adhesion and proliferation92, MSC differentiation36-37, in situ bone formation when used to transplant MSCs2, 73, and biomimetic presentation of growth factors93. MSC survival is increased when transplanted on ECM-coated substrates, providing an opportunity to extend the therapeutic potential of MSCs for tissue regeneration.2, 73 However, the role of ECM biophysical cues on cell survival of implanted MSCs has not been sufficiently studied. The complexity of the ECM provides synergistic cues not available with a single peptide at a cost of stability and long-term shelf life.
Nanoparticles
While the bulk of this review has focused on materials designed to instruct cells using external cues, nanoparticle-based systems provide intracellular cues to promote osteogenic differentiation of MSCs in culture, with potentially sustained benefits upon transplantation. Nanoparticles formed of iron oxide94, silver95 or gold are under examination for their ability to stimulate osteogenic differentiation, either alone or when used as a delivery vehicle for osteoinductive factors. Prior reports demonstrated that gold nanoparticles (AuNPs) alone could enhance MSC osteogenic differentiation in culture96-97, but the performance of treated cells in a more clinically relevant environment has not been evaluated. Mechanotransduction is a potent mediator of osteogenic differentiation in MSCs, and internalization of AuNPs may induce changes in the cytoskeleton of treated cells. Yes-associated protein (YAP) has been implicated as a critical mediator in this process98, and YAP activity correlated with the osteogenic response of MSCs treated with AuNPs.97 However, these studies are consistently performed in media with osteogenic supplements, suggesting that AuNPs alone may not be sufficient to stimulate osteoinduction, and treated cells may require other external stimuli to enhance bone formation.
Biomaterials for Inducing MSC Chondrogenic Differentiation for Engineered Cartilages
Cartilage has limited potential for native healing due to poor cellularity and vascular supply that might bring repair cells into the tissue site.99 Moreover, damaged hyaline articular cartilage is commonly repaired with fibrocartilage, which lacks the robust, long-term mechanical properties necessary for a joint under such appreciable and continuous loading. Current approaches to treat degenerative cartilage include autografts, allografts, and marrow stimulation via microfracture surgery. However, challenges such as tissue site morbidity, potential for disease transmission, poor integration, and inferior quality of repair tissue motivate the pursuit of alternate methods for engineering replacement cartilage. Cell-based approaches have emerged as a strategy to engineer replacement cartilage, with notable successes clinically available using autologous articular chondrocytes. Delays and loss of chondrogenic potential during necessary culture expansion drive the exploration of alternate cell sources to generate chondrocytes. Considering their chondrogenic potential in vitro, ease of acquisition, and successful application in bone engineering, there is a need for biomaterials that augment or direct MSC chondrogenesis for repairing cartilage defects. Unlike other phenotypes, there are no available markers to distinguish differentiation between hyaline versus fibrocartilage, relying on chondrocyte-specific gene expression and synthesis of cartilage-associated ECM proteins as indicators for chondrogenic differentiation. Due to its unique mechanical properties and long-term challenges in repair, this review will be restricted to biomaterials used in guiding MSCs for engineering articular cartilage.
Hydrogels are a popular carrier to entrap MSCs for chondrogenic differentiation, as they enable efficient cell encapsulation, simultaneous entrapment of chondroinductive growth factors and cytokines (e.g., transforming growth factors TGF-β1, -β2, and -β3), and their properties can be readily controlled. Hydrogels were formed of decellularized porcine cartilage, following methacrylation and crosslinking with UV light. The resultant material properties exhibited compressive moduli near native porcine cartilage, while also supporting matrix synthesis and chondrogenic gene expression by rat MSCs.32 Human MSCs were entrapped in alginate hydrogels, whose high hydrophilicity restricts protein adsorption and cell adhesion, allowing cells to maintain a rounded shape mimicking chondrocyte morphology.23, 49 The properties of alginate require chemical modification of the polymer to present peptides to guide MSC differentiation and matrix deposition.100 Hyaluronic acid (HA) is a native component of cartilage, and MSCs may interact with HA via cell surface receptors to enhance chondrogenesis. MSCs entrapped in HA hydrogels and cultured in chondroinductive conditions exhibited chondrogenic differentiation, a response that was enhanced by the presentation of RGD adhesion ligands with increasing density.26 Hydrogels of electrospun HA were formed with distinct mechanics and RGD densities. While adhesion and proliferation of MSCs correlated with ligand density, expression of chondrogenic markers increased as a function of both RGD density and hydrogel mechanics.27 In direct comparison to PEG hydrogels, HA hydrogels enabled more robust MSC chondrogenesis and cartilaginous matrix formation both in vitro and in vivo101, yet PEG provides tremendous opportunities to engineer the microenvironment for chondrogenesis from the bottom up due to its non-fouling nature. For example, the synergy of RGD, HA, and/or collagen type 1 with substrate stiffness on chondrogenesis was recently examined using PEG hydrogels.102 Chondrogenesis was greatest in softer hydrogels containing all three ligands, evidenced by increased sulfated GAG production and chondrogenic gene expression. PEG hydrogels were successfully applied following microfracture surgery to support cartilage repair by MSCs seeping into the gel. In 18 human patients, defects treated with the implant had greater defect fill percentages, lower T2 relaxation time by MRI (reflective of water content), and reduced pain after 6 months.103 Additionally, PEG degradation can be regulated when matrix metalloproteinase (MMP)-degradable sequences are incorporated within the hydrogel backbone. Local degradation induced by co-cultures of MSCs and chondrocytes increased ECM deposition and distribution within biorthogonal-crosslinked PEG hydrogels, which was superior to either cell population alone.50 These findings suggest that scaffolds permitting cell-mediated remodeling may enable superior outcomes by promoting cellular matrix production and reveal new targets for designing materials to induce chondrogenic differentiation for cartilage engineering.
Biomaterials formed of aliphatic polyesters are commonly used to instruct MSC chondrogenic differentiation due to their FDA-approved status, breakdown into relatively harmless byproducts, and familiarity to the field. Woven fibers of polyglycolic acid (PGA) were used to form 3D constructs that supported chondrocyte function and matrix deposition in vitro, yet the scaffolds did not retain their initial biomechanical properties over 28 days due to material degradation and insufficient cartilage matrix accumulation.104 To address this shortcoming, PCL woven meshes were seeded with human ASCs and maintained without chondrogenic factors. The initial compressive properties were retained over the 28-day period and possessed a coefficient of friction similar to native articular cartilage.105 However, the neocartilage exhibited a fibrocartilage phenotype, suggesting other cues (soluble, mechanical, or both) are required to generate functional hyaline cartilage in this system. Randomly oriented, nanofibrous PCL scaffolds supported chondrogenic differentiation of MSCs in vitro59, which were seeded with human MSCs and implanted into a full thickness swine cartilage defect.106 Six months after treatment, defects treated with allogeneic chondrocytes or acellular scaffolds yielded mostly fibrocartilage, yet MSC-treated defects contained hyaline cartilage with the highest mechanical properties. Importantly, the investigators attested that nanofibrous materials are superior to hydrogels since they can be physically attached to surrounding cartilage and promoting stable integration into the defect without a periosteal membrane cover. Alternatively, acellular scaffolds formed of PLLA/PCL outperformed MSC-seeded scaffolds in stimulating formation of hyaline cartilage in rabbit osteochondral defects, perhaps due to increased homing of endogenous cells and induced chondrogenic differentiation due to the scaffold open-pore structure.60 These results demonstrate that the properties of the biomaterial dictate the function of endogenous or transplanted cells for cartilage formation, yet additional studies are necessary to evaluate the long-term success of these approaches.
Biomaterials to Induce MSC Myogenic Differentiation
The loss of functional muscle tissue suppresses patient mobility and quality of life. MSCs are a promising progenitor population for smooth muscle cells, which may be found in blood vessels, bladder, and multiple other tissues. Tissue engineering a replacement bladder would benefit from a readily available cell source, particularly as patients with bladder cancer are not candidates to use autologous urinary cells, and harvest of functional cells is invasive. Smooth muscle cells are the predominant cell type involved in maintaining bladder contractile function and structural integrity. To generate smooth muscle cells, MSCs were induced for 14 days with myogenic growth factors (TGF-β1 and platelet derived growth factor (PDGF-BB)) and seeded on nanofibrous PLLA. One month after transplantation in vivo, myogenically-induced MSCs expressed desmin and myosin at similar levels as smooth muscle cells.61 Similarly, myogenically-induced ASCs seeded on PLG scaffolds and implanted in a rat cystectomy/bladder augmentation model remained viable after transplantation, resulting in increased smooth muscle mass and transient expression of myogenic markers, giving way to endogenous cells that contributed to scaffold remodeling and maintenance.107 Scaffolds seeded with undifferentiated ASCs induced little contractility, perhaps due to slow polymer degradation that interfered with cell-cell connections. However, the need for long-term ASC differentiation (at least 6 weeks) to achieve a smooth muscle cell phenotype represents a substantial limitation that must be resolved by considering allogeneic sources or additional biomaterial modification. For example, surface nanostructure of synthetic scaffolds can be modulated by incubation in sodium hydroxide, reducing the scale of topography from a flat surface down to nanosized topography and resulting in correspondingly increased surface area.69 Bladder smooth muscle cells exhibited increased cell adhesion, proliferation, and collagen and elastin production when cultured on PLG scaffolds possessing nanotopographical features compared to those with submicron or smooth surfaces. The synergy of soluble cues and materials with engineered properties may accelerate the generation of myoblastic cells from MSCs.
MSCs undergo myogenic differentiation in the presence of soluble cues, but the underlying ECM provides instructive cues. Biomaterials may be engineered to present protein/peptide coatings or patterned surfaces that guide differentiation (Figure 3). MSCs exhibited increased smooth muscle actin expression when seeded on an endothelial cell-derived matrix compared to individual matrix proteins.33 Additionally, the capacity of human MSCs to undergo vascular cell differentiation was examined when seeding MSCs on PGA meshes coated with ECM proteins. Compared to other matricellular proteins or uncoated substrates, materials coated with fibronectin induced the greatest expression of myogenic markers in MSCs18-19, yet others reported that laminin-coated TCP outperformed fibronectin.20 These data demonstrate the importance of ECM protein-integrin interactions for promoting the myogenic phenotype of MSCs and provide an opportunity to augment the bioactivity of underlying substrates to instruct MSC fate.
Figure 3. Biomaterial topography instructs MSC adhesion and alignment to guide cell phenotype.
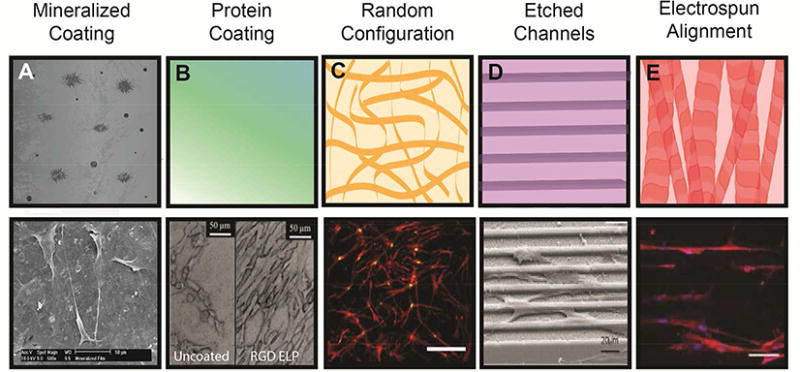
(A) Top: Polymer coating with biomineral increases protein adsorption, cell adhesion, and spreading. Bottom: MSC spreading on biomineralized PLG films.10 (B) Top: Coating with functional proteins regulates integrin engagement. Bottom: MSC adhesion to RGD-linked elastin-like peptide-coated titanium.137 (C) Top: Randomly configured nanofibers in ECM for increased surface area of cell adhesion. Bottom: MSCs on decellularized MSC-secreted ECM undergo osteogenic differentiation.2 (D) Top: Defined surface structures guide cell spreading and orientation. Bottom: MSCs on micropatterned polyimide undergo osteogenic differentiation.138 (E) Top: Aligned electrospun nanofibers guide cell orientation and enhance FAK signaling. Bottom: MSCs on aligned PLLA/PCL exhibit alignment at Day 1 and subsequent increases in myogenic differentiation.64 Reproduced with permission from Refs. 2, 10, 64, 137-138 Copyrights 2010 John Wiley and Sons, 2016 Elsevier, 2016 American Chemical Society, 2015 Elsevier, 2013 John Wiley and Sons.
Myogenic differentiation of MSCs has been enhanced by anisotropic topography and increased stiffness.108-110 Rat MSCs expressed more desmin and myosin heavy chain when cultured on grooved substrates that provided an anisotropic culture environment versus flat surfaces.62 However, it is unknown whether this phenotype is retained upon removal from the grooved surface, a key issue for maximizing the translational potential of this approach. The mechanical properties of the underlying substrate are another important contributor to differentiation, with stiffer substrates promoting a myogenic phenotype better than softer, compliant materials. MSCs cultured on stiff collagen-coated culture dishes exhibited greater calponin expression, a marker for smooth muscle cells, compared to cells on weaker collagen gels. This effect was even more pronounced in the presence of TGF-β on both materials.111 Conversely, MSCs on collagen gels exhibited higher expression of adipogenic markers, perhaps mediated by differences in cell adhesion strength to each material. Similarly, myosin heavy chain expression, another indicator of the smooth muscle cell phenotype, was maximized in MSCs cultured on stiffer silk fibers in the presence of TGF-β.44 The effect of substrate stiffness on myogenic differentiation has been demonstrated using PDMS, as MSCs seeded on stiffer substrates (>30 kPa) exhibited increased myogenic differentiation compared to cells on more compliant gels (<20 kPa), while constraining cell shape can synergistically promote myogenic differentiation.51 Similar results were observed using silk nanofiber scaffolds, with greater MyoD1 and desmin expression observed in MSCs on stiffer substrates.63 However, it is unclear whether the smooth muscle cell phenotype is retained over time as cells remodel the materials and the compressive moduli weaken with polymer degradation. To explore this issue, the contribution of gel viscoelasticity on MSC myogenic differentiation was examined using PDMS hydrogels. Compared to stiff gels, MSCs seeded on gels with increased creep (i.e., substrate loss modulus) exhibited increased myogenic differentiation due to reductions in isometric cytoskeletal tension.52
There is a tremendous clinical need for available skeletal muscle cells to address muscle loss due to trauma or disease and restore mobility. Previous attempts to transplant cells into fibrotic muscle tissue have been unsuccessful in achieving a functional myogenic phenotype. To investigate strategies to induce progenitor cells toward the myogenic lineage, ASCs were cultured on polyacrylamide gels of increasing stiffness to establish the physical properties which induce maximum myogenic gene expression in the absence of myogenic growth factors. Compared to bone marrow-derived MSCs, ASCs maintained on stiff polyacrylamide gels underwent nuclear fusion more rapidly and efficiently, yet MSCs did not exhibit stiffness-dependent fusion.53 Unlike MSCs, differentiated multinucleated ASCs regained this phenotype when chemically disrupted and allowed to refuse, while also retaining the phenotype upon transfer to non-permissive stiff substrates.
Substrate stiffness alone may be insufficient to guide MSCs toward the myogenic phenotype and may require soluble cues to activate key signaling pathways, such as Notch, to efficiently induce these cells. Notch signaling, a key mechanism during mammalian development and stem cell regulation, is activated by engagement of a Notch receptor with Jagged or Delta-like ligand, modulates transcription in several cell types, and mediates myogenic differentiation.112 As an alternative to stimulating cells using soluble Notch ligands in culture media, the presentation of Jagged1-encoding peptides from polymeric substrates induced myogenic differentiation in MSCs in a dose-dependent manner.113 These strategies demonstrate the role of biomaterials in directing myogenic differentiation of MSCs and provide opportunities to generate large numbers of muscle cells for various applications.
Biomaterials for Cell-Based Adipose Tissue Engineering
Patient needs for soft tissue repair span the replacement of lost tissue volume following tumor resection to elective procedures performed to increase definition and tone. Like many other tissue repair strategies, autologous tissue grafts are the preferred tissue source for this application.114 However, soft tissue or fat grafts suffer from erratic graft resorption in reconstructive surgery, occurring in up to 80% of patients, and loss of filler architecture over time in elective plastic surgery114-115. Injectable soft tissue fillers including collagen, HA, HAP, and PLLA are effective to restore tissue volume but exhibit only temporary effects.11, 116-117 The high degree of versatility with each of these materials allows for their sculpting to individual defects or injuries, yet these materials suffer from unnatural texturing, leakage, and extrusion, motivating a more natural approach to soft tissue repair.115
Early biomaterial-based adipogenic differentiation utilized naturally-derived materials to direct adult stem cells down an adipogenic lineage. Preadipocytes in collagen alone penetrated the scaffold in vivo, yet mature adipocytes were only found at the scaffold's outer edge, suggesting that cell growth and differentiation were potentially restricted by pore size.21 MSCs on silk fibroin underwent adipogenic differentiation in vivo, while scaffolds formed of collagen or PLA suffered from unacceptably rapid degradation that prevented their identification and recovery.55 Composite biomaterials containing collagen have been developed to advance early successes while adding tailorability of the material.118 Collagen was blended into Pluronic F-127, a synthetic hydrogel FDA-approved for use in humans, to promote MSC adhesion over that observed with Pluronic F-127 alone.56 The addition of collagen unexpectedly resulted in a single cluster of cells, an effect that was more pronounced with higher collagen concentrations, thus preventing the homogeneous distribution of cells necessary for clinical use. Additionally, the presentation of collagen to MSCs in PEG hydrogels induced greater adipogenic differentiation than laminin or fibronectin, confirming the importance of integrin-ligand interactions and spreading for MSC adipogenic differentiation.22
Decellularized ECM is a promising material for MSC adipogenic differentiation due to its inherent structural and compositional complexity retained following decellularization. Adipogel is a decellularized ECM product derived from adipose tissue that directs preadipocyte and ASC adipogenic differentiation in the absence of adipogenic soluble cues.34 The product has now been amassed from several species including mouse, rat, pig, and human.35 The potency of adipogenic ECM has led to its combination with other materials including hydrogels formed from chondroitin sulphate119 and hyaluronan120.
The retention of predefined shape and dimensions of implants is critical to maintain desired function and aesthetics when replacing lost soft tissue. Compared to natural materials, synthetic biomaterials provide increased reproducibility in material properties and degradation. Adipogenically-induced MSCs suspended in PEG cylinders exhibited full retention of original shape and dimensions after implantation for four weeks, along with increased opacity reflective of tissue formation.121 Conversely, cylinders formed from a porous collagen sponge loaded with MSCs lost 35-65% of their original dimensions during the same period. Adipogenically-induced MSCs transplanted on injectable PLG microspheres achieved increased tissue volume and adipogenic markers in vivo compared to undifferentiated MSCs.122 Despite the application of biomaterials that promote adipogenic differentiation of stem and progenitor cells, these approaches have not yet produced fully functional and vascularized adipose tissue that is ready for clinical implantation. Biomaterials which induce the deposition of an adipogenic ECM while resorbing over time, allowing the invasion and proliferation of cells with adipogenic potential, may provide a clinically successful platform for patients in need of soft tissue reconstruction.
Materials to Stimulate MSC Secretion of Endogenous Factors
Despite the multilineage potential of MSCs, the contribution of MSCs to tissue repair are more widely attributed to their secretion of endogenous trophic factors that recruit host cells to the defect site, promote local vascularization, and regulate the local inflammatory microenvironment.123 This is supported by the rapid depletion of implanted MSCs and host cell infiltration into biomaterial scaffolds upon implantation. MSCs seeded on PLG/HAP composite scaffolds exhibited increased production of proangiogenic VEGF and PDGF in vitro, which supported increases in vessel density in vivo.43 Increases in osteogenic differentiation observed in vitro were not detected with human cells in vivo, suggesting that MSCs secreted paracrine-acting factors to recruit host cells for tissue formation. MSCs transplanted in macroporous alginate cryogels stimulated significantly higher muscle contraction forces in damaged soleus muscle tissue compared to animals receiving empty scaffolds, which was attributed to the secretion of bioactive factors that stimulated endogenous muscle progenitor cells.28 However, the capacity of MSCs to enhance angiogenesis and tissue formation is dependent upon differentiation state.124-125 Furthermore, the immunomodulatory potential of MSCs may provide exciting opportunities for using allogeneic cell sources in tissue repair.126-127 MSCs derived from bone marrow, fat, or cord blood did not lose their immune regulatory potential during short-term culture on HAP/TCP scaffolds clinically approved in Europe.128 As MSCs underwent osteogenic differentiation during long-term culture, their potential to suppress stimulated T and NK cells was impaired. Similar results were observed when MSCs were entrapped in PEG hydrogels and implanted in an ectopic site, with undifferentiated MSCs reducing the fibrous capsule thickness around the hydrogels better than gels containing MSCs that had undergone osteogenic differentiation.129 These data demonstrate that trophic factor secretion is dependent upon differentiation state, representing a critical aspect to consider when implanting MSCs on a biomaterial for tissue repair. Paracrine factors have equal importance as biophysical cues to instruct cell function, and strategies that synergistically modulate cell fate using both soluble and insoluble cues are necessary for enabling tissue regeneration in vivo.
Future Outlook
Biomaterials represent an exciting arena in regenerative medicine to instruct cell fate, restore lost tissue volume and function, and improve patient quality of life. Considering the potency of soluble inductive cues to induce MSC differentiation, new strategies are necessary to leverage these bioactive cues when using biomaterials to instruct MSC fate. Capitalizing on their affinity for heparin, growth factors have been covalently incorporated or tethered to various biomaterials for local presentation to neighboring or entrapped cells.130-132 Spatial and temporal gradients of growth factor presentation can be achieved to activate the differentiation programs of cells as a function of position within the material. The incorporation of oxygen-generating biomaterials133 within substrates engineered to instruct cell fate may better promote MSC differentiation for tissue formation, as MSC differentiation is at least partially mediated by available oxygen.134 Lastly, accumulating evidence demonstrates the benefits of co-culturing stem and progenitor cells with primary cells to promote tissue formation using more accessible cell populations.135-136 Mixed populations can be activated to undergo differentiation or secrete paracrine-acting factors as a function of biomaterial composition and biophysical properties. The inclusion of progenitor cells that secrete bioactive trophic factors can counter senescence of other more differentiated cells and recruit endogenous cells into the defect site, while potentially reducing the number of cells required.
Despite promising advancements described herein, much work remains to successfully apply a biomaterials-mediated approach to guide MSCs for clinical use. The capacity of biophysical cues to promote survival of implanted MSCs has not been decoupled from other effects such as differentiation and trophic factor secretion. Osteogenically-induced MSCs exhibit greater survival and engraftment versus undifferentiated MSCs in vivo2, yet it is unclear whether viability is enhanced by the biophysical properties themselves or if this is mediated through directing cell fate. Furthermore, it is crucial to evaluate how the scaffold integrates with the host tissue when MSCs are transplanted on engineered platforms to instruct cell function. Failure to promote implant integration with surrounding tissue can result in inferior mechanical properties, resulting in revision procedures that are common in bone and cartilage repair. Biomaterials used for adipose tissue reconstruction with MSCs must hold their shape and resist resorption to avoid visible defects in tissue volume. Strategies to repair muscle tissue must enable the transmission of mechanical force so that innervated tissues may effectively respond to voluntary and involuntary stimuli. Conversely, if cells will be separated from the scaffold after generating enough cells with the desired phenotype, it is imperative to establish the duration that the phenotype is retained under environmental conditions similar to the delivery site. Alternative approaches may be required to sustain the established phenotype in vivo.
This review highlights recent efforts to develop and apply biomaterials that guide MSC differentiation and contributions to tissue repair. To extend the use of these materials beyond preclinical studies, their advancement into clinical use will require additional testing to demonstrate safety and efficacy. The value of a biomaterials-based approach over pharmacological methods will be established by demonstrating the reproducibility of their application and cell response in blinded studies and when used by individuals with varying skill. Additional markers are necessary to more accurately describe the MSC populations a priori (i.e., differentiation state, therapeutic potential) to directly compare the effect of these materials on cells from various donors and engineer biomaterials that target functions restricted to specific stages of cell growth. By combining such strategies with existing knowledge of biomaterial properties to instruct cell phenotype, MSCs may become an even more powerful tool in regenerating and repairing damaged tissues.
Acknowledgments
This work was supported by National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number R01 DE025475 (JKL). JW was supported by a National Science Foundation Graduate Research Fellowship (1650042). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the decision to publish, or preparation of the manuscript.
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Hoch AI, Mittal V, Mitra D, Vollmer N, Zikry CA, Leach JK. Cell-secreted matrices perpetuate the bone-forming phenotype of differentiated mesenchymal stem cells. Biomaterials. 2016;74:178–87. doi: 10.1016/j.biomaterials.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 4.Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21(14):2724–52. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 5.Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2(6):455–463. doi: 10.5966/sctm.2012-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tseng SS, Lee MA, Reddi AH. Nonunions and the potential of stem cells in fracture-healing. J Bone Joint Surg Am. 2008(90 Suppl 1):92–8. doi: 10.2106/JBJS.G.01192. [DOI] [PubMed] [Google Scholar]
- 7.Miron RJ, Zhang YF. Osteoinduction: a review of old concepts with new standards. J Dent Res. 2012;91(8):736–44. doi: 10.1177/0022034511435260. [DOI] [PubMed] [Google Scholar]
- 8.Prins HJ, Schulten EAJM, Ten Bruggenkate CM, Klein-Nulend J, Helder MN. Bone regeneration using the freshly isolated autologous stromal vascular fraction of adipose tissue in combination with calcium phosphate ceramics. Stem Cells Transl Med. 2016;5(10):1362–1374. doi: 10.5966/sctm.2015-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxer F, Scherberich A, Todorov A, Studer P, Miot S, Schreiner S, Guven S, Tchang LA, Haug M, Heberer M, Schaefer DJ, Rikli D, Martin I, Jakob M. Implantation of stromal vascular fraction progenitors at bone fracture sites: from a rat model to a first-in-man study. Stem Cells. 2016;34(12):2956–66. doi: 10.1002/stem.2478. [DOI] [PubMed] [Google Scholar]
- 10.Rao RR, He J, Leach JK. Biomineralized composite substrates increase gene expression with nonviral delivery. J Biomed Mater Res A. 2010;94(2):344–54. doi: 10.1002/jbm.a.32690. [DOI] [PubMed] [Google Scholar]
- 11.Beer K. Dermal fillers and combinations of fillers for facial rejuvenation. Dermatol Clin. 2009;27(4):427–32. v. doi: 10.1016/j.det.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Hoppe A, Guldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–74. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27(17):3249–3255. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Leu A, Leach JK. Proangiogenic potential of a collagen/bioactive glass substrate. Pharm Res. 2008;25(5):1222–9. doi: 10.1007/s11095-007-9508-9. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Dong C, Yang L, Lv Y. 3D scaffolds with different stiffness but the same microstructure for bone tissue engineering. ACS Appl Mater Interfaces. 2015;7(29):15790–802. doi: 10.1021/acsami.5b02662. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Qian Y, Xia Y, Chen G, Dai Y, Li N, Zhang F, Gu N. Enhanced osteogenesis of ADSCs by the synergistic effect of aligned fibers containing collagen I. ACS Appl Mater Interfaces. 2016;8(43):29289–29297. doi: 10.1021/acsami.6b08791. [DOI] [PubMed] [Google Scholar]
- 17.Goldshmid R, Cohen S, Shachaf Y, Kupershmit I, Sarig-Nadir O, Seliktar D, Wechsler R. Steric interference of adhesion supports in-vitro chondrogenesis of mesenchymal stem cells on hydrogels for cartilage repair. Sci Rep. 2015;5:12607. doi: 10.1038/srep12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs) FASEB J. 2008;22(6):1635–48. doi: 10.1096/fj.07-087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shudo Y, Cohen JE, Goldstone AB, Macarthur JW, Patel J, Edwards BB, Hopkins MS, Steele AN, Joubert LM, Miyagawa S, Sawa Y, Woo YJ. Isolation and trans-differentiation of mesenchymal stromal cells into smooth muscle cells: Utility and applicability for cell-sheet engineering. Cytotherapy. 2016;18(4):510–517. doi: 10.1016/j.jcyt.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki S, Narita Y, Yamawaki A, Murase Y, Satake M, Mutsuga M, Okamoto H, Kagami H, Ueda M, Ueda Y. Effects of extracellular matrix on differentiation of human bone marrow-derived mesenchymal stem cells into smooth muscle cell lineage: utility for cardiovascular tissue engineering. Cells Tissues Organs. 2010;191(4):269–280. doi: 10.1159/000260061. [DOI] [PubMed] [Google Scholar]
- 21.Hemmrich K, von Heimburg D. Biomaterials for adipose tissue engineering. Expert Rev Med Devic. 2006;3(5):635–645. doi: 10.1586/17434440.3.5.635. [DOI] [PubMed] [Google Scholar]
- 22.Jung JP, Bache-Wiig MK, Provenzano PP, Ogle BM. Heterogeneous differentiation of human mesenchymal stem cells in 3D extracellular matrix composites. Biores Open Access. 2016;5(1):37–48. doi: 10.1089/biores.2015.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon O, Alt DS, Linderman SW, Alsberg E. Biochemical and physical signal gradients in hydrogels to control stem cell behavior. Adv Mater. 2013;25(44):6366–72. doi: 10.1002/adma.201302364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho SS, Vollmer N, Refaat MI, Jeon O, Alsberg E, Lee MA, Leach JK. Bone Morphogenetic Protein-2 promotes human mesenchymal stem cell survival and resultant bone formation when entrapped in photocrosslinked alginate hydrogels. Adv Healthc Mater. 2016;5(19):2501–2509. doi: 10.1002/adhm.201600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive alginate hydrogels for bone tissue engineering. J Dent Res. 2001;80(11):2025–9. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 26.Schuh E, Hofmann S, Stok K, Notbohm H, Muller R, Rotter N. Chondrocyte redifferentiation in 3D: the effect of adhesion site density and substrate elasticity. J Biomed Mater Res A. 2012;100(1):38–47. doi: 10.1002/jbm.a.33226. [DOI] [PubMed] [Google Scholar]
- 27.Kim IL, Khetan S, Baker BM, Chen CS, Burdick JA. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials. 2013;34(22):5571–5580. doi: 10.1016/j.biomaterials.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pumberger M, Qazi TH, Ehrentraut MC, Textor M, Kueper J, Stoltenburg-Didinger G, Winkler T, von Roth P, Reinke S, Borselli C, Perka C, Mooney DJ, Duda GN, Geissler S. Synthetic niche to modulate regenerative potential of MSCs and enhance skeletal muscle regeneration. Biomaterials. 2016;99:95–108. doi: 10.1016/j.biomaterials.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Gawlitta D, Benders KEM, Visser J, van der Sar AS, Kempen DHR, Theyse LFH, Malda J, Dhert WJA. Decellularized cartilage-derived matrix as substrate for endochondral bone regeneration. Tissue Eng Part A. 2015;21(3-4):694–703. doi: 10.1089/ten.TEA.2014.0117. [DOI] [PubMed] [Google Scholar]
- 30.Cunniffe GM, Vinardell T, Murphy JM, Thompson EM, Matsiko A, O'Brien FJ, Kelly DJ. Porous decellularized tissue engineered hypertrophic cartilage as a scaffold for large bone defect healing. Acta Biomater. 2015;23:82–90. doi: 10.1016/j.actbio.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 31.Kiyotake EA, Beck EC, Detamore MS. Cartilage extracellular matrix as a biomaterial for cartilage regeneration. Ann Ny Acad Sci. 2016;1383:139–159. doi: 10.1111/nyas.13278. [DOI] [PubMed] [Google Scholar]
- 32.Beck EC, Barragan M, Tadros MH, Gehrke SH, Detamore MS. Approaching the compressive modulus of articular cartilage with a decellularized cartilage-based hydrogel. Acta Biomater. 2016;38:94–105. doi: 10.1016/j.actbio.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lozito TP, Kuo CK, Taboas JM, Tuan RS. Human mesenchymal stem cells express vascular cell phenotypes upon interaction with endothelial cell matrix. J Cell Biochem. 2009;107(4):714–722. doi: 10.1002/jcb.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma NS, Nagrath D, Yarmush ML. Adipocyte-derived basement membrane extract with biological activity: applications in hepatocyte functional augmentation in vitro. Faseb J. 2010;24(7):2364–2374. doi: 10.1096/fj.09-135095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poon CJ, Pereira ECMV, Sinha S, Palmer JA, Woods AA, Morrison WA, Abberton KM. Preparation of an adipogenic hydrogel from subcutaneous adipose tissue. Acta Biomater. 2013;9(3):5609–20. doi: 10.1016/j.actbio.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Decaris ML, Leach JK. Design of experiments approach to engineer cell-secreted matrices for directing osteogenic differentiation. Ann Biomed Eng. 2011;39(4):1174–1185. doi: 10.1007/s10439-010-0217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai R, Nakamoto T, Hoshiba T, Kawazoe N, Chen G. Matrices secreted during simultaneous osteogenesis and adipogenesis of mesenchymal stem cells affect stem cells differentiation. Acta Biomater. 2016;35:185–93. doi: 10.1016/j.actbio.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Lau TT, Lee LQ, Vo BN, Su K, Wang DA. Inducing ossification in an engineered 3D scaffold-free living cartilage template. Biomaterials. 2012;33(33):8406–17. doi: 10.1016/j.biomaterials.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 39.Antebi B, Cheng X, Harris JN, Gower LB, Chen XD, Ling J. Biomimetic collagen-hydroxyapatite composite fabricated via a novel perfusion-flow mineralization technique. Tissue Eng Part C Methods. 2013;19(7):487–96. doi: 10.1089/ten.tec.2012.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy WL, Kohn DH, Mooney DJ. Growth of continuous bonelike mineral within porous poly(lactide-co-glycolide) scaffolds in vitro. J Biomed Mater Res. 2000;50(1):50–58. doi: 10.1002/(sici)1097-4636(200004)50:1<50::aid-jbm8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 41.Chung EJ, Sugimoto MJ, Ameer GA. The role of hydroxyapatite in citric acid-based nanocomposites: surface characteristics, degradation, and osteogenicity in vitro. Acta Biomater. 2011;7(11):4057–63. doi: 10.1016/j.actbio.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Xavier JR, Thakur T, Desai P, Jaiswal MK, Sears N, Cosgriff-Hernandez E, Kaunas R, Gaharwar AK. Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS Nano. 2015;9(3):3109–18. doi: 10.1021/nn507488s. [DOI] [PubMed] [Google Scholar]
- 43.He J, Genetos DC, Leach JK. Osteogenesis and trophic factor secretion are influenced by the composition of hydroxyapatite/poly(lactide-co-glycolide) composite scaffolds. Tissue Eng Part A. 2010;16(1):127–37. doi: 10.1089/ten.tea.2009.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Floren M, Bonani W, Dharmarajan A, Motta A, Migliaresi C, Tan W. Human mesenchymal stem cells cultured on silk hydrogels with variable stiffness and growth factor differentiate into mature smooth muscle cell phenotype. Acta Biomaterialia. 2016;31:156–166. doi: 10.1016/j.actbio.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parekh SH, Chatterjee K, Lin-Gibson S, Moore NM, Cicerone MT, Young MF, Simon CG. Modulus-driven differentiation of marrow stromal cells in 3D scaffolds that is independent of myosin-based cytoskeletal tension. Biomaterials. 2011;32(9):2256–2264. doi: 10.1016/j.biomaterials.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen WL, Likhitpanichkul M, Ho A, Simmons CA. Integration of statistical modeling and high-content microscopy to systematically investigate cell-substrate interactions. Biomaterials. 2010;31(9):2489–97. doi: 10.1016/j.biomaterials.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Davis HE, Miller SL, Case EM, Leach JK. Supplementation of fibrin gels with sodium chloride enhances physical properties and ensuing osteogenic response. Acta Biomater. 2011;7(2):691–9. doi: 10.1016/j.actbio.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15(3):326–34. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu J, Wang W, Ludeman M, Cheng K, Hayami T, Lotz JC, Kapila S. Chondrogenic differentiation of human mesenchymal stem cells in three-dimensional alginate gels. Tissue Eng Part A. 2008;14(5):667–80. doi: 10.1089/tea.2007.0272. [DOI] [PubMed] [Google Scholar]
- 50.Sridhar BV, Brock JL, Silver JS, Leight JL, Randolph MA, Anseth KS. Development of a cellularly degradable PEG hydrogel to promote articular cartilage extracellular matrix deposition. Adv Healthc Mater. 2015;4(5):702–13. doi: 10.1002/adhm.201400695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J, Abdeen AA, Tang X, Saif TA, Kilian KA. Geometric guidance of integrin mediated traction stress during stem cell differentiation. Biomaterials. 2015;69:174–83. doi: 10.1016/j.biomaterials.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cameron AR, Frith JE, Gomez GA, Yap AS, Cooper-White JJ. The effect of time-dependent deformation of viscoelastic hydrogels on myogenic induction and Rac1 activity in mesenchymal stem cells. Biomaterials. 2014;35(6):1857–68. doi: 10.1016/j.biomaterials.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 53.Choi YS, Vincent LG, Lee AR, Dobke MK, Engler AJ. Mechanical derivation of functional myotubes from adipose-derived stem cells. Biomaterials. 2012;33(8):2482–91. doi: 10.1016/j.biomaterials.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, Zhou H, Weir MD, Xu HH, Chen Q, Trotman CA. Fast-degradable microbeads encapsulating human umbilical cord stem cells in alginate for muscle tissue engineering. Tissue Eng Part A. 2012;18(21-22):2303–14. doi: 10.1089/ten.tea.2011.0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mauney JR, Nguyen T, Gillen K, Kirker-Head C, Gimble JM, Kaplan DL. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28(35):5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vashi AV, Keramidaris E, Abberton KM, Morrison WA, Wilson JL, O'Connor AJ, Cooper-White JJ, Thompson EW. Adipose differentiation of bone marrow-derived mesenchymal stem cells using Pluronic F-127 hydrogel in vitro. Biomaterials. 2008;29(5):573–579. doi: 10.1016/j.biomaterials.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 57.Lee JH, Lee YJ, Cho HJ, Shin H. Guidance of in vitro migration of human mesenchymal stem cells and in vivo guided bone regeneration using aligned electrospun fibers. Tissue Eng Part A. 2014;20(15-16):2031–2042. doi: 10.1089/ten.tea.2013.0282. [DOI] [PubMed] [Google Scholar]
- 58.Yin Z, Chen X, Song HX, Hu JJ, Tang QM, Zhu T, Shen WL, Chen JL, Liu HH, Heng BC, Ouyang HW. Electrospun scaffolds for multiple tissues regeneration in vivo through topography dependent induction of lineage specific differentiation. Biomaterials. 2015;44:173–185. doi: 10.1016/j.biomaterials.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 59.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26(6):599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Barron V, Neary M, Mohamed KMS, Ansboro S, Shaw G, O'Malley G, Rooney N, Barry F, Murphy M. Evaluation of the early in vivo response of a functionally graded macroporous scaffold in an osteochondral defect in a rabbit model. Ann Biomed Eng. 2016;44(5):1832–1844. doi: 10.1007/s10439-015-1473-6. [DOI] [PubMed] [Google Scholar]
- 61.Tian H, Bharadwaj S, Liu Y, Ma H, Ma PX, Atala A, Zhang Y. Myogenic differentiation of human bone marrow mesenchymal stem cells on a 3D nano fibrous scaffold for bladder tissue engineering. Biomaterials. 2010;31(5):870–7. doi: 10.1016/j.biomaterials.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang PY, Li WT, Yu J, Tsai WB. Modulation of osteogenic, adipogenic and myogenic differentiation of mesenchymal stem cells by submicron grooved topography. J Mater Sci Mater Med. 2012;23(12):3015–28. doi: 10.1007/s10856-012-4748-6. [DOI] [PubMed] [Google Scholar]
- 63.Bai S, Han H, Huang X, Xu W, Kaplan DL, Zhu H, Lu Q. Silk scaffolds with tunable mechanical capability for cell differentiation. Acta Biomater. 2015;20:22–31. doi: 10.1016/j.actbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, Wong YS, Wen F, Ng KW, Ng GK, Venkatraman SS, Boey FY, Tan LP. Human mesenchymal stem-cell behaviour on direct laser micropatterned electrospun scaffolds with hierarchical structures. Macromol Biosci. 2013;13(3):299–310. doi: 10.1002/mabi.201200318. [DOI] [PubMed] [Google Scholar]
- 65.Faia-Torres AB, Guimond-Lischer S, Rottmar M, Charnley M, Goren T, Maniura-Weber K, Spencer ND, Reis RL, Textor M, Neves NM. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials. 2014;35(33):9023–32. doi: 10.1016/j.biomaterials.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 66.Khang D, Choi J, Im YM, Kim YJ, Jang JH, Kang SS, Nam TH, Song J, Park JW. Role of subnano-, nano- and submicron-surface features on osteoblast differentiation of bone marrow mesenchymal stem cells. Biomaterials. 2012;33(26):5997–6007. doi: 10.1016/j.biomaterials.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Sun L, Danoux CB, Wang Q, Pereira D, Barata D, Zhang J, LaPointe V, Truckenmuller R, Bao C, Xu X, Habibovic P. Independent effects of the chemical and microstructural surface properties of polymer/ceramic composites on proliferation and osteogenic differentiation of human MSCs. Acta Biomater. 2016;42:364–77. doi: 10.1016/j.actbio.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 68.Saldana L, Crespo L, Bensiamar F, Arruebo M, Vilaboa N. Mechanical forces regulate stem cell response to surface topography. J Biomed Mater Res A. 2014;102(1):128–40. doi: 10.1002/jbm.a.34674. [DOI] [PubMed] [Google Scholar]
- 69.Pattison MA, Wurster S, Webster TJ, Haberstroh KM. Three-dimensional, nano-structured PLGA scaffolds for bladder tissue replacement applications. Biomaterials. 2005;26(15):2491–500. doi: 10.1016/j.biomaterials.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Morales-Hernandez DG, Genetos DC, Working DM, Murphy KC, Leach JK. Ceramic identity contributes to mechanical properties and osteoblast behavior on macroporous composite scaffolds. J Funct Biomater. 2012;3:382–397. doi: 10.3390/jfb3020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vissers CAB, Harvestine JN, Leach JK. Pore size regulates mesenchymal stem cell response to Bioglass-loaded composite scaffolds. Journal of Materials Chemistry B. 2015;3(44):8650–8658. doi: 10.1039/c5tb00947b. [DOI] [PubMed] [Google Scholar]
- 72.Kim SS, Sun Park M, Jeon O, Yong Choi C, Kim BS. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(8):1399–1409. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 73.Harvestine JN, Vollmer NL, Ho SS, Zikry CA, Lee MA, Leach JK. Extracellular matrix-coated composite scaffolds promote mesenchymal stem cell persistence and osteogenesis. Biomacromolecules. 2016;17(11):3524–3531. doi: 10.1021/acs.biomac.6b01005. [DOI] [PubMed] [Google Scholar]
- 74.Sun L, Parker ST, Syoji D, Wang X, Lewis JA, Kaplan DL. Direct-write assembly of 3D silk/hydroxyapatite scaffolds for bone co-cultures. Adv Healthc Mater. 2012;1(6):729–35. doi: 10.1002/adhm.201200057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 76.Mao AS, Shin JW, Mooney DJ. Effects of substrate stiffness and cell-cell contact on mesenchymal stem cell differentiation. Biomaterials. 2016;98:184–91. doi: 10.1016/j.biomaterials.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zouani OF, Kalisky J, Ibarboure E, Durrieu MC. Effect of BMP-2 from matrices of different stiffnesses for the modulation of stem cell fate. Biomaterials. 2013;34(9):2157–66. doi: 10.1016/j.biomaterials.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 78.Itälä AI, Ylänen HO, Ekholm C, Karlsson KH, Aro HT. Pore diameter of more than 100 microm is not requisite for bone ingrowth in rabbits. J Biomed Mater Res. 2001;58(6):679–83. doi: 10.1002/jbm.1069. [DOI] [PubMed] [Google Scholar]
- 79.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–91. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Sicchieri LG, Crippa GE, de Oliveira PT, Beloti MM, Rosa AL. Pore size regulates cell and tissue interactions with PLGA-CaP scaffolds used for bone engineering. J Tissue Eng Regen Med. 2012;6(2):155–62. doi: 10.1002/term.422. [DOI] [PubMed] [Google Scholar]
- 81.Kasten P, Beyen I, Niemeyer P, Luginbuhl R, Bohner M, Richter W. Porosity and pore size of beta-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: An in vitro and in vivo study. Acta Biomater. 2008;4(6):1904–1915. doi: 10.1016/j.actbio.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 82.Vissers CAB, Harvestine JN, Leach JK. Pore size regulates mesenchymal stem cell response to Bioglass-loaded composite scaffolds. J Mater Chem B. 2015;3(44):8650–8658. doi: 10.1039/c5tb00947b. [DOI] [PubMed] [Google Scholar]
- 83.Di Luca A, Ostrowska B, Lorenzo-Moldero I, Lepedda A, Swieszkowski W, Van Blitterswijk C, Moroni L. Gradients in pore size enhance the osteogenic differentiation of human mesenchymal stromal cells in three-dimensional scaffolds. Sci Rep. 2016;6:22898. doi: 10.1038/srep22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chemical reviews. 2001;101(7):1869–79. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 85.Short AR, Koralla D, Deshmukh A, Wissel B, Stocker B, Calhoun M, Dean D, Winter JO. Hydrogels that allow and facilitate bone repair, remodeling, and regeneration. J Mater Chem B. 2015;3(40):7818–7830. doi: 10.1039/C5TB01043H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Calabrese R, Kaplan DL. Silk ionomers for encapsulation and differentiation of human MSCs. Biomaterials. 2012;33(30):7375–85. doi: 10.1016/j.biomaterials.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, Huebsch N, Mooney DJ. Substrate stress relaxation regulates cell spreading. Nat Commun. 2015;6:6364. doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paul A, Manoharan V, Krafft D, Assmann A, Uquillas JA, Shin SR, Hasan A, Hussain MA, Memic A, Gaharwar AK, Khademhosseini A. Nanoengineered biomimetic hydrogels for guiding human stem cell osteogenesis in three dimensional microenvironments. J Mater Chem B. 2016;4(20):3544–3554. doi: 10.1039/C5TB02745D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zwingenberger S, Nich C, Valladares RD, Yao ZY, Stiehler M, Goodman SB. Recommendations and considerations for the use of biologics in orthopedic surgery. Biodrugs. 2012;26(4):245–256. doi: 10.2165/11631680-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bahney CS, Hu DP, Taylor AJ, Ferro F, Britz HM, Hallgrimsson B, Johnstone B, Miclau T, Marcucio RS. Stem cell-derived endochondral cartilage stimulates bone healing by tissue transformation. J Bone Miner Res. 2014;29(5):1269–82. doi: 10.1002/jbmr.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harada N, Watanabe Y, Sato K, Abe S, Yamanaka K, Sakai Y, Kaneko T, Matsushita T. Bone regeneration in a massive rat femur defect through endochondral ossification achieved with chondrogenically differentiated MSCs in a degradable scaffold. Biomaterials. 2014;35(27):7800–7810. doi: 10.1016/j.biomaterials.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 92.Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Min Res. 2007;22(12):1943–1956. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]
- 93.Kim IG, Hwang MP, Du P, Ko J, Ha CW, Do SH, Park K. Bioactive cell-derived matrices combined with polymer mesh scaffold for osteogenesis and bone healing. Biomaterials. 2015;50:75–86. doi: 10.1016/j.biomaterials.2015.01.054. [DOI] [PubMed] [Google Scholar]
- 94.Wang Q, Chen B, Cao M, Sun J, Wu H, Zhao P, Xing J, Yang Y, Zhang X, Ji M, Gu N. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials. 2016;86:11–20. doi: 10.1016/j.biomaterials.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 95.Zhang R, Lee P, Lui VC, Chen Y, Liu X, Lok CN, To M, Yeung KW, Wong KK. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomedicine. 2015;11(8):1949–59. doi: 10.1016/j.nano.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 96.Ko WK, Heo DN, Moon HJ, Lee SJ, Bae MS, Lee JB, Sun IC, Jeon HB, Park HK, Kwon IK. The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. J Colloid Interface Sci. 2015;438:68–76. doi: 10.1016/j.jcis.2014.08.058. [DOI] [PubMed] [Google Scholar]
- 97.Li J, Li JJ, Zhang J, Wang X, Kawazoe N, Chen G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale. 2016;8(15):7992–8007. doi: 10.1039/c5nr08808a. [DOI] [PubMed] [Google Scholar]
- 98.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 99.Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–21. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bidarra SJ, Barrias CC, Granja PL. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014;10(4):1646–1662. doi: 10.1016/j.actbio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 101.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15(2):243–54. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carrion B, Souzanchi MF, Wang VT, Tiruchinapally G, Shikanov A, Putnam AJ, Coleman RM. The synergistic effects of matrix stiffness and composition on the response of chondroprogenitor cells in a 3D precondensation microenvironment. Adv Healthc Mater. 2016;5(10):1192–202. doi: 10.1002/adhm.201501017. [DOI] [PubMed] [Google Scholar]
- 103.Sharma B, Fermanian S, Gibson M, Unterman S, Herzka DA, Cascio B, Coburn J, Hui AY, Marcus N, Gold GE, Elisseeff JH. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med. 2013;5(167):167ra6. doi: 10.1126/scitranslmed.3004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moutos FT, Freed LE, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater. 2007;6(2):162–7. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- 105.Moutos FT, Guilak F. Functional properties of cell-seeded three-dimensionally woven poly(epsilon-caprolactone) scaffolds for cartilage tissue engineering. Tissue Eng Part A. 2010;16(4):1291–301. doi: 10.1089/ten.tea.2009.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li WJ, Chiang H, Kuo TF, Lee HS, Jiang CC, Tuan RS. Evaluation of articular cartilage repair using biodegradable nanofibrous scaffolds in a swine model: a pilot study. J Tissue Eng Regen M. 2009;3(1):1–10. doi: 10.1002/term.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jack GS, Zhang R, Lee M, Xu Y, Wu BM, Rodriguez LV. Urinary bladder smooth muscle engineered from adipose stem cells and a three dimensional synthetic composite. Biomaterials. 2009;30(19):3259–70. doi: 10.1016/j.biomaterials.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yim EK, Darling EM, Kulangara K, Guilak F, Leong KW. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials. 2010;31(6):1299–306. doi: 10.1016/j.biomaterials.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gasiorowski JZ, Murphy CJ, Nealey PF. Biophysical cues and cell behavior: the big impact of little things. Annu Rev Biomed Eng. 2013;15:155–76. doi: 10.1146/annurev-bioeng-071811-150021. [DOI] [PubMed] [Google Scholar]
- 110.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 111.Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, Li S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials. 2011;32(16):3921–30. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morrow D, Guha S, Sweeney C, Birney Y, Walshe T, O'Brien C, Walls D, Redmond EM, Cahill PA. Notch and vascular smooth muscle cell phenotype. Circ Res. 2008;103(12):1370–82. doi: 10.1161/CIRCRESAHA.108.187534. [DOI] [PubMed] [Google Scholar]
- 113.Wen F, Wong HK, Tay CY, Yu H, Li H, Yu T, Tijore A, Boey FY, Venkatraman SS, Tan LP. Induction of myogenic differentiation of human mesenchymal stem cells cultured on Notch agonist (Jagged-1) modified biodegradable scaffold surface. ACS Appl Mater Interfaces. 2014;6(3):1652–61. doi: 10.1021/am4045635. [DOI] [PubMed] [Google Scholar]
- 114.Trojahn Kolle SF, Oliveri RS, Glovinski PV, Elberg JJ, Fischer-Nielsen A, Drzewiecki KT. Importance of mesenchymal stem cells in autologous fat grafting: a systematic review of existing studies. J Plast Surg Hand Surg. 2012;46(2):59–68. doi: 10.3109/2000656X.2012.668326. [DOI] [PubMed] [Google Scholar]
- 115.Stosich MS, Mao JJ. Stem Cell–Based Soft Tissue Grafts for Plastic and Reconstructive Surgeries. Semin Plast Surg. 2005;19(3):251–260. [Google Scholar]
- 116.Schierle CF, Casas LA. Nonsurgical rejuvenation of the aging face with injectable poly-L-lactic acid for restoration of soft tissue volume. Aesthet Surg J. 2011;31(1):95–109. doi: 10.1177/1090820X10391213. [DOI] [PubMed] [Google Scholar]
- 117.Wu DC, Karnik J, Margarella T, Nguyen VL, Calame A, Goldman MP. Evaluation of the in vivo effects of various laser, light, or ultrasound modalities on human skin treated with a collagen and polymethylmethacrylate microsphere dermal filler product. Lasers Surg Med. 2016;48(9):811–819. doi: 10.1002/lsm.22580. [DOI] [PubMed] [Google Scholar]
- 118.Natesan S, Baer DG, Walters TJ, Babu M, Christy RJ. Adipose-Derived Stem Cell Delivery into Collagen Gels Using Chitosan Microspheres. Tissue Eng Pt A. 2010;16(4):1369–1384. doi: 10.1089/ten.tea.2009.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheung HK, Han TT, Marecak DM, Watkins JF, Amsden BG, Flynn LE. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials. 2014;35(6):1914–23. doi: 10.1016/j.biomaterials.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 120.Flynn L, Prestwich GD, Semple JL, Woodhouse KA. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials. 2007;28(26):3834–42. doi: 10.1016/j.biomaterials.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 121.Stosich MS, Mao JJ. Adipose tissue engineering from human adult stem cells: Clinical implications in plastic and reconstructive surgery. Plast Reconstr Surg. 2007;119(1):71–83. doi: 10.1097/01.prs.0000244840.80661.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Choi YS, Park SN, Suh H. Adipose tissue engineering using mesenchymal stem cells attached to injectable PLGA spheres. Biomaterials. 2005;26(29):5855–5863. doi: 10.1016/j.biomaterials.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 123.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 124.Hoch AI, Binder BY, Genetos DC, Leach JK. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One. 2012;7(4):e35579. doi: 10.1371/journal.pone.0035579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rothenberg AR, Ouyang L, Elisseeff JH. Mesenchymal stem cell stimulation of tissue growth depends on differentiation state. Stem Cells Dev. 2011;20(3):405–14. doi: 10.1089/scd.2010.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 127.Griffin MD, Elliman SJ, Cahill E, English K, Ceredig R, Ritter T. Concise review: adult mesenchymal stromal cell therapy for inflammatory diseases: how well are we joining the dots? Stem Cells. 2013;31(10):2033–41. doi: 10.1002/stem.1452. [DOI] [PubMed] [Google Scholar]
- 128.Bassi G, Guilloton F, Menard C, Di Trapani M, Deschaseaux F, Sensebe L, Schrezenmeier H, Giordano R, Bourin P, Dominici M, Tarte K, Krampera M. Effects of a ceramic biomaterial on immune modulatory properties and differentiation potential of human mesenchymal stromal cells of different origin. Tissue Eng Part A. 2015;21(3-4):767–81. doi: 10.1089/ten.tea.2014.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Swartzlander MD, Blakney AK, Amer LD, Hankenson KD, Kyriakides TR, Bryant SJ. Immunomodulation by mesenchymal stem cells combats the foreign body response to cell-laden synthetic hydrogels. Biomaterials. 2015;41:79–88. doi: 10.1016/j.biomaterials.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pike DB, Cai SS, Pomraning KR, Firpo MA, Fisher RJ, Shu XZ, Prestwich GD, Peattie RA. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials. 2006;27(30):5242–5251. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 131.Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. P Natl Acad Sci USA. 2013;110(12):4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jeong SI, Jeon O, Krebs MD, Hill MC, Alsberg E. Biodegradable photo-crosslinked alginate nanofibre scaffolds with tuneable physical properties, cell adhesivity and growth factor release. Eur Cell Mater. 2012;24:331–43. doi: 10.22203/ecm.v024a24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials. 2009;30(5):757–62. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 134.He J, Genetos DC, Yellowley CE, Leach JK. Oxygen tension differentially influences osteogenic differentiation of human adipose stem cells in 2D and 3D cultures. J Cell Biochem. 2010;110(1):87–96. doi: 10.1002/jcb.22514. [DOI] [PubMed] [Google Scholar]
- 135.Lai JH, Kajiyama G, Smith RL, Maloney W, Yang F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep. 2013;3:3553. doi: 10.1038/srep03553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meretoja VV, Dahlin RL, Kasper FK, Mikos AG. Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials. 2012;33(27):6362–9. doi: 10.1016/j.biomaterials.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Raphel J, Karlsson J, Galli S, Wennerberg A, Lindsay C, Haugh MG, Pajarinen J, Goodman SB, Jimbo R, Andersson M, Heilshorn SC. Engineered protein coatings to improve the osseointegration of dental and orthopaedic implants. Biomaterials. 2016;83:269–82. doi: 10.1016/j.biomaterials.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Abagnale G, Steger M, Nguyen VH, Hersch N, Sechi A, Joussen S, Denecke B, Merkel R, Hoffmann B, Dreser A, Schnakenberg U, Gillner A, Wagner W. Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials. 2015;61:316–26. doi: 10.1016/j.biomaterials.2015.05.030. [DOI] [PubMed] [Google Scholar]


