North American oaks are more resistant to xylem embolism than previously thought, suggesting that avoiding stem embolism is a critical component of drought tolerance in woody trees.
Abstract
Although recent findings suggest that xylem embolism represents a significant, drought-induced damaging process in land plants, substantial debate surrounds the capacity of long-vesseled, ring-porous species to resist embolism. We investigated whether recent methodological developments could help resolve this controversy within Quercus, a long-vesseled, ring-porous temperate angiosperm genus, and shed further light on the importance of xylem vulnerability to embolism as an indicator of drought tolerance. We used the optical technique to quantify leaf and stem xylem vulnerability to embolism of eight Quercus species from the Mediterranean-type climate region of California to examine absolute measures of resistance to embolism as well as any potential hydraulic segmentation between tissue types. We demonstrated that our optical assessment reflected flow impairment for a subset of our sample species by quantifying changes in leaf hydraulic conductance in dehydrating branches. Air-entry water potential varied 2-fold in leaves, ranging from −1.7 ± 0.25 MPa to −3.74 ± 0.23 MPa, and 4-fold in stems, ranging from −1.17 ± 0.04 MPa to −4.91 ± 0.3 MPa. Embolism occurred earlier in leaves than in stems in only one out of eight sample species, and plants always lost turgor before experiencing stem embolism. Our results show that long-vesseled North American Quercus species are more resistant to embolism than previously thought and support the hypothesis that avoiding stem embolism is a critical component of drought tolerance in woody trees. Accurately quantifying xylem vulnerability to embolism is essential for understanding species distributions along aridity gradients and predicting plant mortality during drought.
Drought can cause major damage to plant communities (Adams et al., 2010) and reduce plant primary productivity. Although species-specific damage thresholds are not yet fully understood (Anderegg et al., 2012), recent findings indicate that postdrought recovery of gas exchange in conifer and angiosperm plant species can be predicted by properties of the water transport system (Brodribb and Cochard, 2009; Anderegg et al., 2015; Skelton et al., 2017b). Under drought stress, the continuous column of water in the plant xylem experiences increasing tension caused by declining water potential at the sites of evaporation (usually in the leaf mesophyll). Eventually, air is drawn into the water transport system, forming embolism in the xylem conduits (Sperry and Ikeda, 1997). Although plants have developed several mechanisms to restore vessel functionality by refilling embolized vessels (Brodersen et al., 2010), those that have experienced a loss of hydraulic conductance due to embolism formation within the xylem do not always recover full hydraulic functionality following rehydration and often suffer reduced gas-exchange capacity as a result (Skelton et al., 2017b). Plants often die when water potentials drop below those associated with extensive hydraulic dysfunction (greater than 50% loss of hydraulic conductance in conifers and greater than 88% in angiosperms; Brodribb and Cochard, 2009; Choat, 2013; Urli et al., 2013; Brodribb et al., 2014).
Consequently, the capacity of plants to resist embolism formation in the xylem is hypothesized to be a major component of plant drought tolerance and survival. This is consistent with two observations: (1) in situ, species tend to maintain positive safety margins between plant water potential and water potential values that induce extensive embolism in stems (Choat et al., 2012); and (2) the capacity of xylem to withstand water deficit is correlated significantly with the aridity of the environment that species tend to inhabit for many taxa (Brodribb and Hill, 1999; Pockman and Sperry, 2000; Maherali et al., 2004; Choat et al., 2012; Blackman et al., 2014; Larter et al., 2017), but not all (Brodribb et al., 2014). Imperfect correlations between xylem resistance to embolism and aridity in these taxa may arise in part due to plants relying on additional traits or behaviors to convey drought tolerance or to avoid exposure to drought, such as low minimum leaf conductance, deep rooting, or drought-induced leaf shedding (Ackerly, 2004; Brodribb and Holbrook, 2004; West et al., 2012; Brodribb et al., 2014; Maréchaux et al., 2015; Hochberg et al., 2017).
Stomatal closure before embolism formation has been theoretically advanced as another fundamental component of plant drought tolerance (Jones and Sutherland, 1991; Sperry et al., 2002; Delzon and Cochard, 2014; Martin-StPaul et al., 2017). Early stomatal closure serves to reduce water loss and maintain a positive safety margin between plant water potential and water potential-inducing embolism, thus allowing plants to avoid water deficit-induced damage, except under severe drought conditions (Hochberg et al., 2017; Skelton et al., 2017a,b; Charrier et al., 2018). Current empirical data sets indicate that most, but not all, plants tend to exhibit conservative, positive safety margins (Brodribb and Holbrook, 2003; Choat et al., 2012; Skelton et al., 2015), supporting the hypothesis that stomata have evolved to close before water potential-induced embolism. However, relatively few studies have explicitly investigated the precise timing of stomatal closure in relation to the point of xylem embolism formation (Hochberg et al., 2017; Skelton et al., 2017a), and it is necessary to address this fundamental aspect of plant physiology.
Thus, although xylem resistance to embolism and early stomatal closure during periods of water deficit appear to be important properties of drought tolerance in land plants, fundamental questions about these properties remain unresolved. Critically, the xylem vulnerability to embolism of many plant taxa remains unmeasured or uncertain because of the methodological difficulties of studying xylem under tension or potential methodological artifacts related to specific techniques for assessing xylem vulnerability to embolism (Melcher et al., 2012; Sperry et al., 2012; Cochard et al., 2013; Wheeler et al., 2013; Rockwell et al., 2014). Of particular concern are long-vesseled angiosperm species (including many tropical trees, temperate ring-porous trees, and woody vines), as these species are thought to be prone to an open-vessel artifact, potentially resulting in spuriously low resistance to embolism. Several studies have suggested that the xylem of some plant species might be highly vulnerable to embolism, having produced so-called r-shaped xylem vulnerability to embolism curves (i.e. exponential curves, rather than the more commonly observed sigmoidal curves; Jacobsen et al., 2007b; Sperry et al., 2012). Yet, many of the species thought to be highly vulnerable to embolism occur in semiarid or water-stressed environments, frequently experience low water potentials, and, if these values are correct, would thus either be experiencing regular damage or regular xylem refilling to maintain hydraulic function (Bucci et al., 2003; Jacobsen et al., 2007a,b; Nardini et al., 2008; Johnson et al., 2009, 2011; Ogasa et al., 2013; Trifilò et al., 2015).
In addition, to better understand the capacity of a single species to withstand embolism, it is important to examine points of hydraulic failure within distinct plant tissues (Zimmermann, 1978; Tyree and Ewers, 1991), since different tissues can vary in their capacity to withstand xylem embolism (Tyree et al., 1993; Johnson et al., 2011). The so-called vulnerability segmentation between tissues possibly serves to create hydraulic fuses within the plant to further protect the more valuable tissues from drought damage. This hypothesis is consistent with observations that more distal tissues in woody trees, particularly leaves of drought-deciduous species, often are more vulnerable to water deficit than stems or large branches (Cochard et al., 1992; Tyree et al., 1993; Choat et al., 2005; Johnson et al., 2011; Hochberg et al., 2017) and with a lack of segmentation shown in evergreen herbaceous species that proportion biomass relatively evenly among tissues (Skelton et al., 2017a). However, few studies have explored variation in the degree of segmentation between tissues among closely related species; consequently, the factors contributing to interspecific variation in segmentation remain unclear.
Recent advancements in visual or optical techniques for quantifying xylem vulnerability to embolism offer potential to investigate embolism formation within different species and among distinct tissues to address current uncertainties and controversies in xylem physiology in plants (Brodribb et al., 2016b, 2017; Skelton et al., 2017a). Here, we investigated patterns in leaf and stem xylem vulnerability to embolism in Quercus (oaks), a diverse, long-vesseled (Jacobsen et al., 2007b, 2012; Hacke et al., 2009), ring-porous temperate angiosperm genus, with species distributions spanning aridity gradients in western North America. Our main objectives were to examine (1) how the capacity to withstand embolism within leaves and stems varies across Quercus species; (2) whether any segmentation between leaves and stems occurs within species; and (3) whether vulnerability to embolism in leaves and stems is a critical component of drought tolerance in this ecologically important genus. We hypothesized that embolism avoidance in stems is a critical component of drought tolerance in these long-lived woody tree species and, thus, made four main predictions: (1) stems will display low vulnerability to embolism; (2) leaves will embolize earlier than stems in response to water deficit; (3) stomatal closure will occur before embolism in stems of all species; and (4) species occurring in more arid environments will display greater resistance to embolism in stems than those restricted to more mesic environments. To test these predictions, we selected eight deciduous and evergreen Quercus species with varying climatic niches from the Mediterranean-type climate region of California and quantified leaf and stem xylem vulnerability to embolism using newly developed optical techniques. For a subset of our species, we also quantified leaf hydraulic conductance in response to water deficit, to demonstrate that our optical curves reflect changes in xylem capacity to transport water. Finally, we quantified the turgor loss point (TLP) of five sample species to gain a proxy for stomatal closure.
RESULTS
Variation in Leaf and Stem Xylem Vulnerability to Embolism among Quercus Species
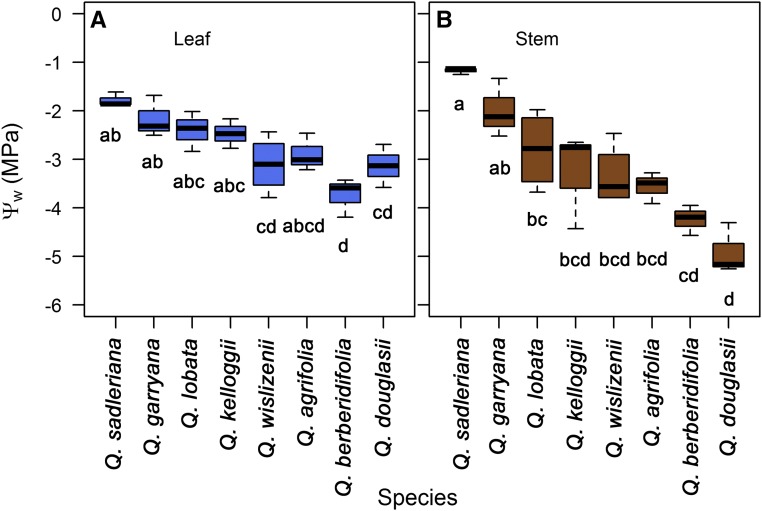
We observed considerable interspecific variation in the leaf and stem water potentials associated with embolism in our sample of eight Quercus species (Figs. 1 and 2; Table I). We used the leaf or stem vulnerability curves to quantify a standardized metric to compare species (i.e. Pe, water potential at initialization of embolism) and found that the eight sample Quercus species varied 2-fold in leaf vulnerability (Fig. 3A; Table I; F = 11.39; degrees of freedom [d.f.] = 7; P = 1.76 × 10−5) and 4-fold in stem xylem vulnerability to embolism (Fig. 3B; Table I; F = 6.89; d.f. = 7; P = 0.00564). The species most vulnerable to xylem embolism was Q. sadleriana (leaf Pe = −1.78 ± 0.08 MPa; stem Pe = −1.17 ± 0.04 MPa), while Q. berberidifolia (leaf Pe = −3.74 ± 0.23 MPa; stem Pe = −4.26 ± 0.31 MPa) and Q. douglasii (leaf Pe = −3.13 ± 0.26 MPa; stem Pe = −4.91 ± 0.3 MPa) were the least vulnerable to embolism (Fig. 3; Table I).
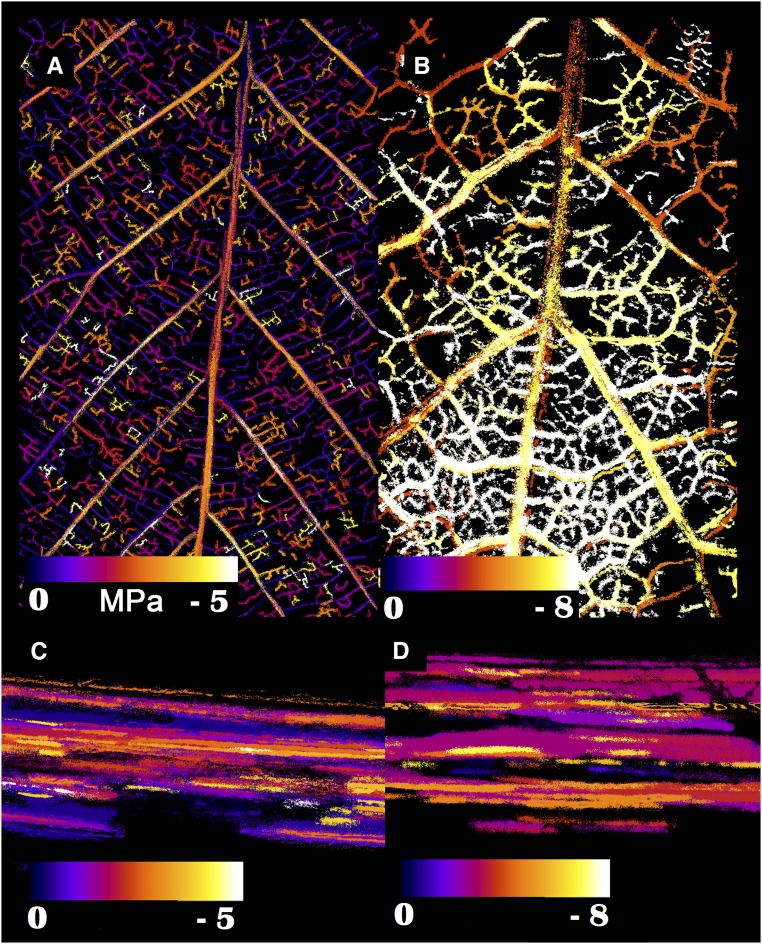
Figure 1.
Embolism events in the xylem of a Q. sadleriana leaf (A) and stem (C) and a Q. berberidifolia leaf (B) and stem (D), as observed using the optical vulnerability technique. Scale bars indicate the water potential recorded at each event and are different for each image. Q. sadleriana was the least resistant to embolism, and Q. berberidifolia was the most resistant to embolism.
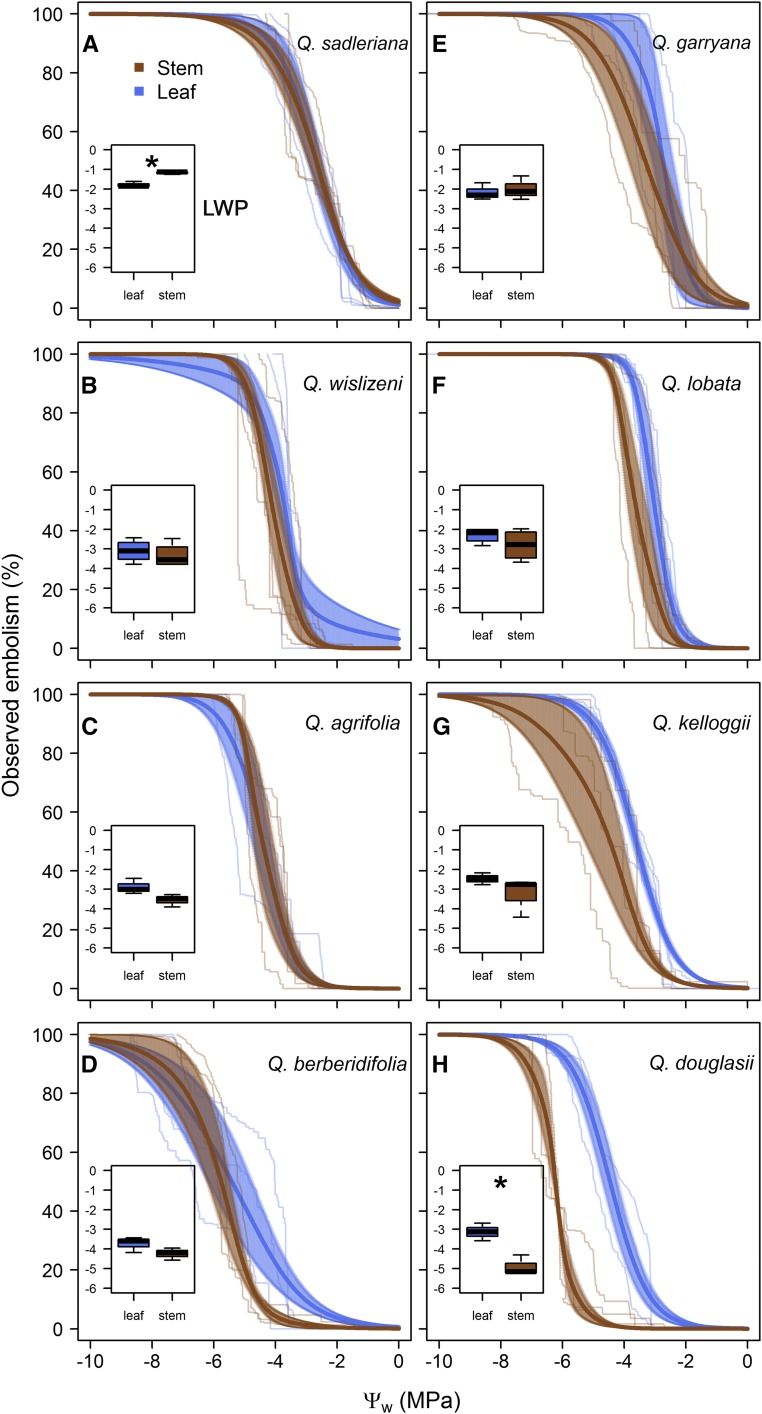
Figure 2.
Optical vulnerability curves for leaves (blue) and stems (brown) of all eight sample Quercus species: Q. sadleriana (A; the least resistant to embolism); Q. wislizenii (B); Q. agrifolia (C); Q. berberidifolia (D); Q. garryana (E); Q. lobata (F); Q. kelloggii (G); and Q. douglasii (H). Solid dark lines and shading indicate the mean observed embolism ± se for each tissue type (n = 3). Light lines indicate raw curves for each individual. The insets are box plots showing the mean Pe for leaves and stems for each species. Asterisks indicate species in which the values for leaves and stems were significantly different.
Table I. Comparison of mean Pe and water potential associated with 50% loss of hydraulic conductance (P50) values for leaves (n = 3) and stems (n = 3) of our eight sample Quercus species.
Values shown are means ± se (n = 3).
| Species | Tissue | Trait | |
|---|---|---|---|
| Pe | P50 | ||
| MPa | |||
| Q. sadleriana | Leaf | −1.78 ± 0.08 | −2.72 ± 0.25 |
| Stem | −1.17 ± 0.04 | −2.74 ± 0.20 | |
| Q. garryana | Leaf | −1.70 ± 0.25 | −2.81 ± 0.27 |
| Stem | −1.99 ± 0.35 | −3.32 ± 0.56 | |
| Q. kelloggii | Leaf | −2.47 ± 0.18 | −3.62 ± 0.13 |
| Stem | −3.28 ± 0.58 | −4.73 ± 0.64 | |
| Q. lobata | Leaf | −2.4 ± 0.24 | −3.02 ± 0.18 |
| Stem | −2.8 ± 0.40 | −3.58 ± 0.25 | |
| Q. wislizenii | Leaf | −3.10 ± 0.29 | −3.77 ± 0.13 |
| Stem | −3.35 ± 0.31 | −4.10 ± 0.24 | |
| Q. agrifolia | Leaf | −2.89 ± 0.22 | −4.47 ± 0.31 |
| Stem | −3.56 ± 0.19 | −4.32 ± 0.26 | |
| Q. douglasii | Leaf | −3.13 ± 0.26 | −4.45 ± 0.24 |
| Stem | −4.91 ± 0.3 | −6.27 ± 0.06 | |
| Q. berberidifolia | Leaf | −3.74 ± 0.23 | −5.52 ± 0.62 |
| Stem | −4.24 ± 0.18 | −5.95 ± 0.38 | |
Figure 3.
Interspecific comparisons of the Pe (means ± se; n = 3) for leaves (A) and stems (B). Letters below each box plot indicate significant differences between species.
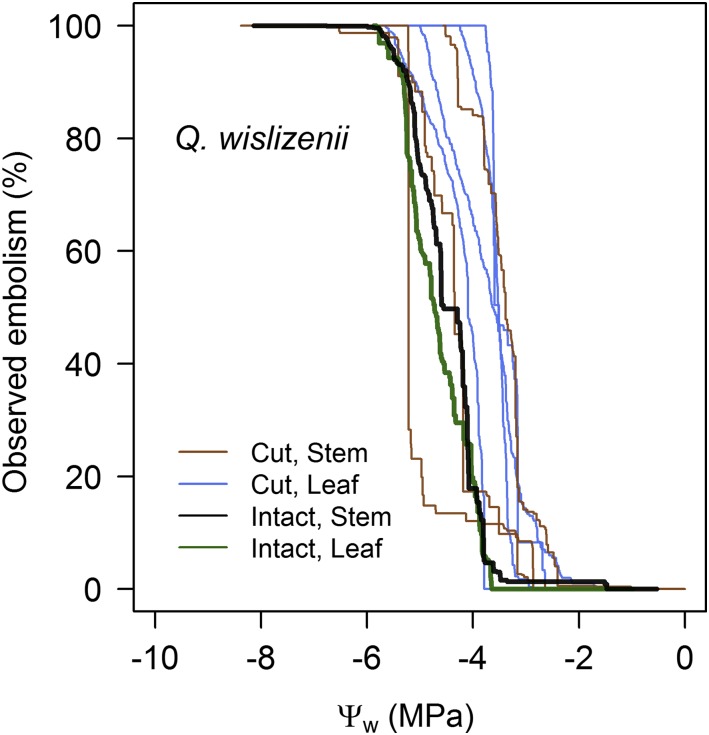
We observed low vulnerability to embolism in both cut and intact individuals of Q. wislizenii (Fig. 4). Leaves of the potted, intact individuals were slightly less vulnerable to embolism (Pe = −3.71 MPa) than leaves of the cut branches (Pe = −2.89 ± 0.22 MPa; Fig. 4). Stems of the potted, intact individuals (Pe = −3.71 MPa) were highly consistent with stems from the cut branches (−3.56 ± 0.19 MPa; Fig. 4).
Figure 4.
Comparison of vulnerability curves generated on cut leaves and branches and on a leaf and branch of a fully intact, healthy, well-watered Q. wislizenii individual that had experienced no prior water stress.
Although we observed substantial interspecific variation in the magnitude of the water potentials associated with embolism in leaves and stems, the shape of the vulnerability curves was similar for all species. Specifically, in each of our eight sample species, the progression of total cumulative embolism in the xylem of leaves and stems when plotted against leaf or stem water potential approximately followed a sigmoidal pattern (Fig. 2). The shape of the vulnerability curves observed in intact, potted individuals of Q. wislizenii was also sigmoid and highly consistent with our observations of cut branches from field-grown plants of the same species (Fig. 4).
Vulnerability Segmentation in Quercus Species
Our data show that leaf xylem was as vulnerable to embolism as stem xylem in all species, except Q. sadleriana and Q. douglasii (Fig. 2, insets). Leaves were more vulnerable than stems in Q. douglasii (Ψleaf – Ψstem = 1.6 MPa; t = −4.46; d.f. = 3.89; P = 0.01), while the reverse was observed in Q. sadleriana (Ψleaf – Ψstem = −0.61 MPa; t = 6.52; d.f. = 2.87; P = 0.008).
Relationship between Turgor Loss and Xylem Vulnerability to Embolism
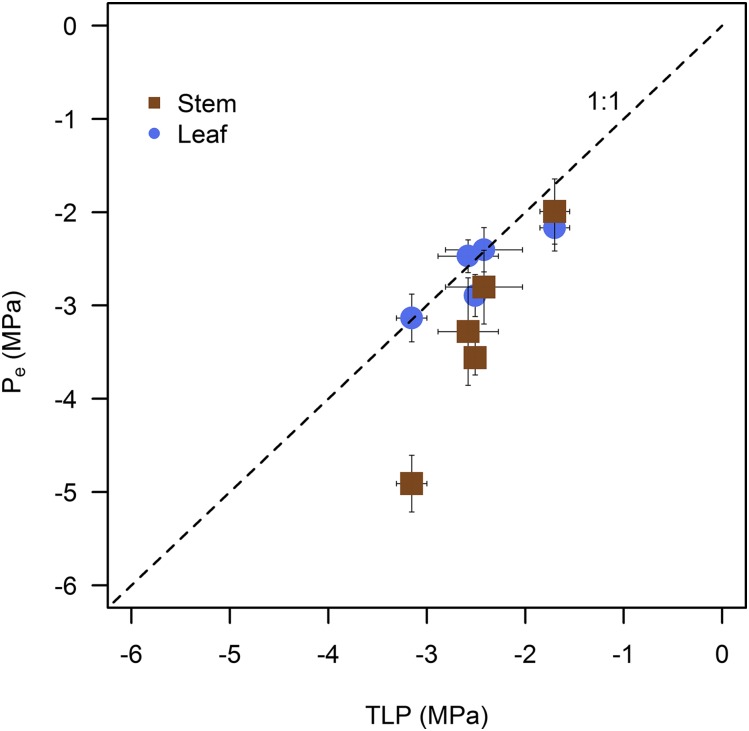
The safety margin between the water potential associated with turgor loss and that associated with the onset of embolism within leaves (i.e. TLP – Ψe, in MPa) tended to be very small in all species (Fig. 5). Safety margins ranged from +0.38 MPa in Q. agrifolia (i.e. turgor loss before embolism) to −0.11 MPa in Q. kelloggii (Fig. 5). Safety margins between turgor loss and the onset of xylem embolism within stems were much greater and tended to increase with greater resistance to embolism formation (Fig. 5). In stems, safety margins ranged from +1.76 MPa in Q. douglasii to +0.29 MPa in Q. garryana (Fig. 5).
Figure 5.
Comparison of TLP (means ± se; n = 3) and Pe of leaves (means ± se; n = 3; blue) and stems (means ± se; n = 3; brown) of five sample Quercus species, indicating that TLP was reached at higher water potential values than Pe of leaves and stems of most species.
Relationship between Vulnerability to Embolism and Aridity
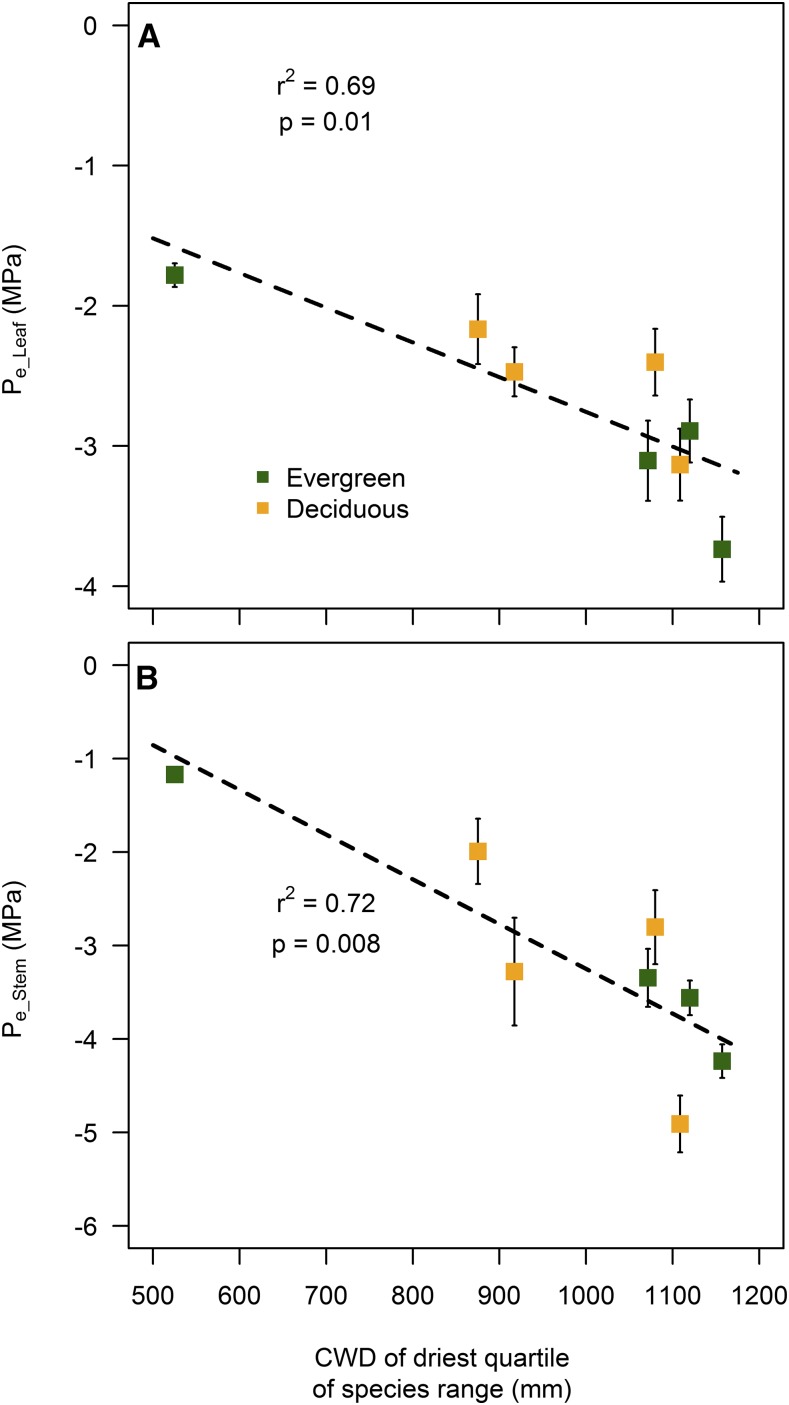
The Quercus species from the western United States occupy regions with different levels of climatic water deficit (CWD; Fig. 6), indicating that they occupy distinct climatic niches related to water availability. Species climatic niches range from that of Q. sadleriana (A), restricted to mesic sites (CWD < 600 mm), to that of Q. berberidifolia (G), inhabiting xeric sites in California. We found a significant positive correlation between the aridity of the sites that individuals tend to occupy and the resistance to embolism of both leaves (Fig. 6A) and stems (Fig. 6B). Q. sadleriana and Q. garryana, the two species with the least resistant xylem, are restricted to sites that are much more mesic than Q. berberidifolia, the species with the most resistant xylem (Fig. 6).
Figure 6.
The CWD (a proxy for the aridity of a site; mm) of the driest 25th percentile of the distribution of eight sample Quercus species is significantly associated with the Pe (means ± se; n = 3) of both leaves (A) and stems (B). Species with lower resistance to embolism occur in much more mesic sites than those with greater resistance to embolism.
DISCUSSION
Our study applies the optical method of quantifying xylem embolism resistance to investigate fundamental physiological and ecological questions associated with xylem embolism in plants. Specifically, our observations of variation in resistance to embolism within leaves and stems in Quercus species, in combination with observations of points of turgor loss, leaf habit, and climatic niches, allow us to draw several important conclusions about the aspects of drought tolerance within this ecologically important genus. First, our observations of the capacity of western North American Quercus species to resist embolism within leaves and stems indicate that Quercus species may be considerably more resistant to water deficit than previously thought (Jacobsen et al., 2007a,b; Sperry et al., 2012). Second, our finding that leaves were at least as resistant to embolism as stems in seven out of eight species indicates that leaves do not serve as hydraulic fuses in this genus, contrary to the segmentation hypothesis. Third, the fact that turgor loss occurs either at or before the point of incipient embolism in leaves and stems supports the prediction that stomata will close earlier than the onset of xylem embolism during periods of water deficit (Martin-StPaul et al., 2017). Finally, the clear positive association between the aridity of the sites that a species occupies and the capacity to withstand embolism supports the notion that xylem resistance to embolism is an important component of drought tolerance.
High Resistance of Quercus Species to Embolism
Substantial debate currently surrounds the capacity of long-vesseled, ring-porous temperate plants to resist embolism. The finding that all eight of our sample Quercus species have P50 values below −2 MPa suggests that trees and shrubs in this ecologically important genus might be more resistant to embolism than previously thought. This conclusion is at odds with several previous results showing stems in some Quercus species to be highly vulnerable (Jacobsen et al., 2007b; Sperry et al., 2012). However, our findings are consistent with a recent assessment of xylem vulnerability to embolism in European oaks showing that species from this genus are highly resistant to embolism (Lobo et al., 2018). Our findings also agree with several recent studies that have similarly suggested that other long-vesseled, woody species are more resistant to embolism than previously reported, including grapevine (Vitis vinifera; Choat et al., 2010; Hochberg et al., 2017; Charrier et al., 2018) and olive (Olea europaea; Rodriguez-Dominguez et al., 2018). It has been suggested previously that vulnerability curves generated on short branch segments (less than 30 cm) of long-vesseled plants using the centrifuge technique might suffer from an open-vessel artifact (Sperry et al., 2012; Cochard et al., 2013) and might underestimate vulnerability to embolism (Choat et al., 2010). Our findings provide important support for this claim in an ecologically diverse and often ecologically dominant North American genus.
In addition, the finding that all sample Quercus species displayed sigmoid-shaped curves contrasts with previous reports of exponential or r-shaped curves in this genus (Jacobsen et al., 2007b; Sperry et al., 2012). In particular, our observation that Q. wislizenii stems display sigmoidal vulnerability curves is at odds with previously published reports of r-shaped curves in this species (Jacobsen et al., 2007b). Notably, the previously published r-shaped curves were generated from relatively short branches using the centrifuge technique. Here, we were able to validate our observations of sigmoid vulnerability curves obtained from cut branches of Q. wislizenii by observing embolism formation in intact trees of this species (Fig. 4), confirming our result. This important finding suggests that r-shaped curves in other Quercus species are likely to be a product of an open-vessel artifact associated with the centrifuge technique and should be revisited.
Lack of Vulnerability Segmentation in Quercus Species
Our study reports evidence of a lack of vulnerability segmentation in six out of eight sample North American Quercus species. Differences between the mean water potential associated with embolism formation in leaves and those in stems were statistically significant in only two cases (Q. douglasii and Q. sadleriana, and the latter had more vulnerable stems than leaves). These results are consistent with previous findings for European Quercus species (Cochard et al., 1992) and suggest that, when segmentation between leaves and stems exists, it might often be slight. Thus, our finding that the mean water potential associated with embolism formation in leaves was similar to that in stems contradicts the hydraulic fuse hypothesis (that leaves will embolize before stems to protect the more valuable tissues).
Although there was no clear general relationship between leaf habit and segmentation, leaf shedding during severe drought events may play a substantial role in avoiding stem embolism in certain Quercus species. We note that Q. douglasii, the species with the greatest degree of segmentation between leaves and stems and a species most resistant to xylem embolism, also is considered to be one of the only drought-deciduous oak species in California (i.e. it drops its leaves during periods of severe water deficit; Griffin, 1973). Recently, leaf shedding also was found to be associated with leaf embolism and avoidance of extensive stem embolism in other drought-deciduous woody plant species (Hochberg et al., 2017), indicating that this process might be an important additional component of drought tolerance. Future studies should investigate the complexity of leaf habit, further exploring differences between drought-deciduous and winter-deciduous leaf habits in drought-prone habitats.
Xylem Vulnerability and Stomatal Closure in Quercus Species
In a subset of the species studied, we observed that the point of bulk leaf turgor loss occurs very close to or before incipient embolism in the leaf and consistently before incipient embolism within stems, further enhancing support for the hypothesis that the avoidance of embolism within stems is a critical component of drought tolerance in Quercus. This finding is consistent with those of other studies on different plant genera showing that stomatal closure preempts xylem embolism (Brodribb et al., 2003; Trifilò et al., 2014; Martin-StPaul et al., 2017; Li et al., 2018) and suggests that early stomatal closure might be a widespread strategy to prevent embolism formation that has evolved within land plants.
Our finding that safety margins between TLP and Pe in leaves are small is somewhat surprising, since a recent study found that the water potentials associated with stomatal closure and incipient embolism were similar for only a small number of species, with most species closing their stomata long before embolism (Martin-StPaul et al., 2017). However, our findings are consistent with a study that found a strong 1:1 correspondence between the water potential at stomatal closure and incipient embolism formation across 12 ecologically diverse species from temperate Australian woodlands (Li et al., 2018). If avoiding water potentials associated with embolism is important to long-lived oaks, then close coordination between stomatal closure and Pe suggests that minimum stomatal conductance is low in these species. Although this hypothesis has been demonstrated to be true in conifer species (Brodribb et al., 2014), it has yet to be tested in angiosperms. Our results also indicate that safety margins between TLP and Pe were larger in stems than in leaves and increased with greater resistance to embolism. This finding provides further evidence that avoiding embolism in stems is critical to avoiding drought-induced damage in oaks.
Although it has been suggested that some plants rely on osmotic adjustment during a season to maintain water uptake and cell turgor (Chaves et al., 2009; Blum, 2017), the close association between Pe in leaves and TLP observed at the beginning of the season in Quercus leaves indicates that osmotic adjustment during a season is likely to be relatively small in these species. These findings are consistent with a previous suggestion that a lack of osmotic adjustment might be inherent in certain North American Quercus species (Abrams, 1990).
Xylem Vulnerability to Embolism and Drought Tolerance
Low xylem vulnerability to embolism in both stems and leaves of California Quercus species is associated with increasing aridity of the sites that species are able to occupy, providing additional support for our hypothesis that xylem vulnerability to embolism is a key component of drought tolerance in Quercus. This hypothesis is further supported by historical observations of minimum seasonal water potentials in two California Quercus species (Q. douglasii and Q. agrifolia) during severely dry years, which closely match our Pe values for these species. Midday leaf water potential values have fallen as low as −4.8 MPa for individuals of Q. douglasii (stem Pe = −4.91 ± 0.3 MPa) and −2.9 MPa for individuals of Q. agrifolia (stem Pe = −3.56 ± 0.19 MPa; Griffin, 1973; Osuna et al., 2015). Both studies noted little or no drought damage in individuals of these species during the severely dry periods, findings that are consistent with the hypothesis that water potentials associated with embolism in stems represent critical thresholds of drought-induced damage.
Plant functional traits that vary systematically across environmental gradients are considered adaptive because they enhance species performance and survival under particular environmental conditions (Ackerly, 2003). Our study provides evidence that the vulnerability to embolism of western North American Quercus species is an adaptive trait strongly linked to site aridity and the capacity to withstand drought. This conclusion is highly consistent with previous studies on other plant genera that show that interspecific variation in leaf resistance to embolism influences species distributions across water availability gradients at local scales (Nardini et al., 2012), broad climatic scales (Blackman et al., 2014), and at the dry end of species geographical ranges (Blackman et al., 2012).
CONCLUSION
Our findings that xylem embolism in Quercus occurs at low water potentials, primarily after turgor loss, and that species-specific vulnerability to embolism decreases with increasing aridity of sites that species occupy indicate that the capacity to resist xylem embolism is a key component of drought tolerance within western North American Quercus species. Ultimately, our data provide additional support for the framework of drought tolerance among land plants that suggests that (1) stomatal closure occurs before embolism and (2) embolism avoidance, particularly in stems, is a critical component of drought tolerance. They also suggest that previous observations of r-shaped curves in Quercus species and other long-vesseled angiosperms should be revisited (Maherali et al., 2004; Cavender-Bares et al., 2005; Jacobsen et al., 2007a,b; Choat et al., 2012; Sperry et al., 2012; Trifilò et al., 2015). Resolving these concerns will be important for answering fundamental questions about the adaptive capacity of xylem embolism resistance across land plant groups, the importance of dynamic processes of xylem conduit refilling, the ubiquity of early stomatal closure (Skelton et al., 2015), and the influence of xylem vulnerability on species distributions and community composition.
Finally, in instances where plant functional traits have a known mechanistic relationship with specific environmental stresses, such as drought or CWD, these traits offer insight into how specific changes in the environment might affect future species distributions and vegetation structure and function. Previous studies within western North America have indicated that changes in CWD associated with global climate change might play an important role in determining shifts in plant communities (McIntyre et al., 2015). Although these studies have suggested that Quercus species might be less negatively affected by changes in CWD than other tree species in the region, our data suggest instead that Quercus species might be closely adapted to specific CWD conditions and, therefore, potentially highly sensitive to increases in CWD. Combining observations of in situ minimum plant water potential with site-specific estimates of aridity (such as CWD) to estimate a safety margin from incipient embolism will be useful for predicting the drought conditions that may cause damage and for predicting the sensitivity of plant communities to potential future changes in climate.
MATERIALS AND METHODS
Sampling Strategy and Study Species
Eight species of Quercus (Fagaceae), an ecologically dominant genus in western North America known to have long vessels, as shown by analyses of several species (Zimmermann and Jeje, 1981; Jacobsen et al., 2007b, 2012; Hacke et al., 2009), were sampled. Species were chosen to capture variation in leaf habit (deciduous and evergreen), taxonomic groups, and distributional ranges along the west coast of North America (to capture potential variation in climatic niches). Our study species were all long-lived woody tree or shrub species that grow to between 2 and ∼35 m in height. Specifically, we selected four evergreen species and four deciduous species: Quercus agrifolia (section: Erythrobalanus, or red oaks) is a widespread, evergreen tree species; Quercus berberidifolia (section: Lepidobalanus, or white oaks) is a large, evergreen woody shrub or small tree found in chaparral or coastal sage scrub communities; Quercus wislizenii (section: Erythrobalanus) is a large evergreen tree; Quercus sadleriana (section: Quercus) is a medium-sized evergreen understory shrub found in coniferous forests in northern California and southwestern Oregon; Quercus garryana (section: Lepidobalanus) is a deciduous woody tree species occurring in California and Oregon; Quercus douglasii (section: Lepidobalanus) is a deciduous woody tree species endemic to California; Quercus kelloggii (section: Erythrobalanus) is a deciduous woody tree species; and Quercus lobata (section: Lepidobalanus) is a large deciduous, overstory, woody tree species. Our primary study site, Pepperwood Preserve in Sonoma County, has seven co-occurring Quercus species and is located on the west coast of California (38° 34′ 59.64′′ N, 122° 44′ 21.81′′ W, 441 m elevation). Vegetation at the site is currently predominantly mixed evergreen and deciduous woodland, which occupies vast tracts of California. Q. sadleriana has a more limited distribution in high-rainfall regions of northern California and was sampled at the Six Rivers National Forest in Humboldt County. Q. wislizenii was sampled from the Hopland Research and Extension Center and the Sierra Foothills Research and Extension Center.
Vulnerability to Embolism
Large branches of at least six different individuals of each species were collected at predawn from healthy-looking individuals in the field. To avoid any potential artifact associated with open vessels, we ensured that the cut branches were longer than the species’ maximum recorded vessel length (Supplemental Table S1). Where we were unable to determine maximum vessel length for a species (Q. sadleriana), we ensured that the branches were cut from the base of the root collar. Upon excision, branches were immediately placed in at least two plastic bags with damp paper towels to prevent further water loss and transported back to the laboratory at the University of California, Berkeley, for processing. There, we used an optical method to capture embolism in both leaves and branches using flatbed scanners in a dark, temperature-controlled room. For each species, at least three branches from different individuals were used to capture embolism events within the leaves according to the methods described by Brodribb et al. (2016b), and at least three branches from different individuals were used to capture embolism events within small branches (less than 0.5 cm in diameter) according to the methods described by Brodribb et al. (2017). Full details, including an overview of the technique, image processing, as well as scripts to guide image capture and analysis, are available at http://www.opensourceov.org. Extensive validation of the techniques can be found in several recent publications (Brodribb et al., 2016a,b, 2017; Skelton et al., 2017a,b). Briefly, for leaves, we secured a healthy, intact leaf between two microscope slides on a flatbed scanner (Epson Perfection V800 or V850 Scanner; Epson America) using duct tape. We scanned each leaf in transmission mode (as opposed to reflective mode, to allow light to pass through the leaf xylem) at least once every 4 min for a period of a few days (usually less than 4 d). For stems, we carefully removed a small section of bark to expose the xylem, placed it face down on the scanner, and secured it in place using duct tape. Stems were scanned in reflective mode, which allowed us to observe embolism within the outer few layers of xylem in each stem. Using branches of small size for observation reduced, but did not entirely exclude, the possibility that our method might have missed significant radial variation in embolism within branches (i.e. between rings).
As branches were being scanned for leaf or stem embolism, we simultaneously monitored stem and leaf xylem water potential of each individual to measure the level of hydration of each branch. For stem xylem water potential, we placed a stem psychrometer (ICT International) on a large branch neighboring each scanned branch at more than 60 cm from the cut end of the main branch. Stem psychrometers were connected to the xylem, sealed with high-vacuum grease (Dow Corning), and secured with Parafilm (Bemis) to prevent moisture loss. Stem xylem water potential was recorded every 10 min for the duration of the scanning process. We verified the accuracy of the stem psychrometer readings for each branch by periodically measuring leaf xylem water potential using a Scholander-type pressure chamber (PMS Instruments; Supplemental Fig. S1). For leaf xylem water potential, we excised at least two leaves neighboring the scanned leaves, immediately wrapped them in a moist paper towel and aluminum foil, and placed them in plastic bags to prevent further water loss. We measured the xylem water potential of each leaf using the Scholander-type pressure chamber. Since branches were largely equilibrated because of being kept in the dark, variation among neighboring leaves was slight (always less than 0.1 MPa).
Upon completion, image sequences were analyzed to identify embolism events, seen as changes in the reflection of the stem xylem or changes in the transmission of light through the leaf xylem. Image subtraction of subsequent images conducted in ImageJ (National Institutes of Health) was used to reveal rapid changes in light transmission or contrast produced by each embolism event. Slow movements of the stems or leaves caused by drying could easily be distinguished from embolism events and were filtered from the analysis. Embolism events were thresholded, allowing automated counting of each event using the analyze-stack function in ImageJ.
From the thresholded stack of embolism events, we could extract a time-resolved count of embolism events (using the time stamp of each image). We then converted the raw embolism counts to a percentage of total pixels embolized, producing a data set of time-resolved percentage embolism. The time-resolved percentage embolism data were combined with the water potential time line to estimate the leaf or stem xylem water potential associated with each embolism event. Vulnerability to embolism was recorded as the relationship between percentage embolism and water potential. We extracted the Pe (MPa), defined as the leaf or stem xylem water potential associated with greater than 5% embolism for each branch. From these data, we calculated a mean Pe ± se for leaves and stems of each species. Several other metrics of xylem vulnerability to embolism have been used extensively in the literature, such as the P50 (MPa). Pe was used to identify a critical threshold of embolism. Previous studies have suggested that the point of air-entry (i.e. Pe) represents a point of incipient damage to plant functionality (Skelton et al., 2017b). For consistency with other studies, we also report P50 values (Table I).
Reliability of the Optical Vulnerability Curves
We conducted two experiments to demonstrate that our optical vulnerability curves reliably capture the capacity of plants to withstand embolism. In the first experiment, we aimed to determine, for a subset of our eight sample species, whether the observed embolism accurately quantifies changes in hydraulic conductance. To achieve this, we quantified leaf hydraulic conductance in response to water deficit by using the rehydration kinetics method (Skelton et al., 2017b) on cut branches of two sample species, Q. kelloggii (deciduous) and Q. agrifolia (evergreen). Briefly, for each species, we collected three additional branches from different individuals and dried them on the benchtop. Periodically, we quantified kleaf and water potential (using the methods described by Skelton et al. [2017b]) to obtain a complete vulnerability curve for each individual. We then compared the response of kleaf to the percentage embolism obtained using the optical vulnerability technique. In both species, percentage embolism measured using the optical technique was associated with the loss of leaf hydraulic conductance (Supplemental Fig. S2). In both species, kleaf also remained relatively constant over an initial range of leaf water potentials but declined upon further dehydration (Supplemental Fig. S2). Uncompromised water transport followed by a rapid decline in hydraulic functional capacity corroborates our observations of an initial lack of embolism followed by a rapid accumulation of embolism events (Supplemental Fig. S2).
The aim of our second experiment was to demonstrate that our observations of embolism in cut branches accurately reflect total embolism in intact samples, since the vulnerability curves constructed with the optical method might be influenced by the minimum pressure to which the samples were exposed previously or by an artifact associated with cutting branches. In particular, we wanted to exclude the possibility that our cut branches had already experienced embolism because of prior water stress or cutting. To do so, we quantified embolism in leaves and stems of healthy, well-watered, intact Q. wislizenii individuals that we obtained from a plant nursery. We followed the same protocol outlined earlier to capture embolism formation within newly and fully expanded leaves and stems of intact, hydrated individuals. We were careful to observe the minimum water potential that our potted individuals experienced before being set up on the scanner. Since the minimum water potential that our potted Q. wislizenii individuals had experienced (−0.68 MPa) was well above the point of incipient embolism in leaves (−3.71 MPa) and stems (−3.78 MPa), we were able to exclude the possibility of prior stress causing embolism in these tissues. We report the results from this second experiment in “Results.”
Each time that branches were collected for optical curves, the in situ midday water potential of leaves from the same individuals for each of our study species also was sampled. These data indicate that water potentials experienced by the trees in the field before sampling remained well above those associated with embolism for all species (Supplemental Table S1).
Pressure-Volume Curves and TLP
To determine a proxy for the point of stomatal closure, we measured the water potential corresponding to bulk leaf turgor loss (TLP; MPa). Previous studies have shown that stomatal aperture is reduced significantly at leaf TLP (Brodribb et al., 2003; Buckley et al., 2003). The point of bulk leaf cell turgor was determined for individuals of five sample species by the relationship between Ψleaf and water content in the leaf. Branches from well-hydrated individuals of each sample species were cut under water and allowed to hydrate to greater than −0.1 MPa. From these branches, at least three leaves per species were removed and used to quantify leaf pressure-volume curves using the bench-drying technique (Tyree and Hammel, 1972). The Ψleaf values and leaf weight were measured periodically until Ψleaf stopped declining or desiccation-induced cell damage was observed in the leaves. At this stage, leaves were placed in a drying oven for at least 2 d for complete desiccation to determine dry weight. For each leaf, relative water content was determined and plotted against Ψleaf, and the Ψleaf at turgor loss was determined as the point of inflection between the linear and nonlinear portions of the plot. A mean TLP ± se (n = 3) was calculated for each species.
Climatic Niche
To determine the aridity associated with the climatic niche of each species, we quantified the CWD (mm; Flint et al., 2013) associated with all recorded observations of Quercus individuals within the Calflora database (Baldwin et al., 2017). For each observation (i.e. coordinate), we calculated mean monthly CWD between 1981 and 2010 using a state-wide basin characterization model (Flint et al., 2013). We subsequently determined the driest 25th percentile of the 30-year average CWD values for each species and used this measure as a proxy for the aridity that each species experienced in its home range.
Statistical Analysis and Fit
The relationship between cumulative percentage embolism and leaf water potential was fit according to a sigmoid function:
where a corresponds to the sensitivity to decreasing Ψ and b is the Ψ associated with 50% embolism (i.e. P50).
To test for differences in Pe and P50 between species, ANOVA, in addition to a posthoc Tukey’s honestly significant difference test, was used. To evaluate differences between the Pe of leaves and Pe of stems for each species, we used Student’s t test on each sample species.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Relationship between leaf water potential and stem water potential.
Supplemental Figure S2. Relationship between leaf hydraulic conductance and observed xylem embolism.
Supplemental Table S1. Maximum vessel length and observed minimum midday water potential of sample species.
Acknowledgments
We thank the staff at Pepperwood Preserve for their ongoing support and for allowing us to use the preserve for plant material. We also thank Prahlad Popper for field assistance with identifying oaks and several undergraduate research assistants at the University of California, Berkeley, for their efforts in data collection and image processing, including Janette Bustos, Eric Garcia, and Joseph Munneke. We thank Tim Brodribb and Chris Lucani from the University of Tasmania for generous technical support and feedback with the optical method. The article also benefitted from excellent feedback from three independent reviewers.
Footnotes
This work was supported by National Science Foundation grant 1457400 to D.D.A., T.E.D., and S.E.T.
Articles can be viewed without a subscription.
References
- Abrams MD. (1990) Adaptations and responses to drought in Quercus species of North America. Tree Physiol 7: 227–238 [DOI] [PubMed] [Google Scholar]
- Ackerly DD. (2003) Community assembly, niche conservatism, and adaptive evolution in changing environments. Int J Plant Sci 164: S165–S184 [Google Scholar]
- Ackerly DD. (2004) Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol Monogr 74: 25–44 [Google Scholar]
- Adams HD, Macalady AK, Breshears DD, Allen CD, Stephenson NL, Saleska SR, Huxman TE, McDowell NG (2010) Climate-induced tree mortality: earth system consequences. Eos (Wash DC) 91: 153–154 [Google Scholar]
- Anderegg WRL, Berry JA, Field CB (2012) Linking definitions, mechanisms, and modeling of drought-induced tree death. Trends Plant Sci 17: 693–700 [DOI] [PubMed] [Google Scholar]
- Anderegg WRL, Flint A, Huang C, Flint L, Berry JA, Davis FW, Sperry JS, Field CB (2015) Tree mortality predicted from drought-induced vascular damage. Nat Geosci 8: 367–371 [Google Scholar]
- Baldwin BG, Thornhill AH, Freyman WA, Ackerly DD, Kling MM, Morueta-Holme N, Mishler BD (2017) Species richness and endemism in the native flora of California. Am J Bot 104: 487–501 [DOI] [PubMed] [Google Scholar]
- Blackman CJ, Brodribb TJ, Jordan GJ (2012) Leaf hydraulic vulnerability influences species’ bioclimatic limits in a diverse group of woody angiosperms. Oecologia 168: 1–10 [DOI] [PubMed] [Google Scholar]
- Blackman CJ, Gleason SM, Chang Y, Cook AM, Laws C, Westoby M (2014) Leaf hydraulic vulnerability to drought is linked to site water availability across a broad range of species and climates. Ann Bot 114: 435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A. (2017) Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ 40: 4–10 [DOI] [PubMed] [Google Scholar]
- Brodersen CR, McElrone AJ, Choat B, Matthews MA, Shackel KA (2010) The dynamics of embolism repair in xylem: in vivo visualizations using high-resolution computed tomography. Plant Physiol 154: 1088–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Cochard H (2009) Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol 149: 575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb T, Hill RS (1999) The importance of xylem constraints in the distribution of conifer species. New Phytol 143: 365–372 [Google Scholar]
- Brodribb TJ, Holbrook NM (2003) Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol 132: 2166–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM (2004) Stomatal protection against hydraulic failure: a comparison of coexisting ferns and angiosperms. New Phytol 162: 663–670 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM, Edwards EJ, Gutiérrez MV (2003) Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ 26: 443–450 [Google Scholar]
- Brodribb TJ, McAdam SAM, Jordan GJ, Martins SCV (2014) Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proc Natl Acad Sci USA 111: 14489–14493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Bienaimé D, Marmottant P (2016a) Revealing catastrophic failure of leaf networks under stress. Proc Natl Acad Sci USA 113: 4865–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Skelton RP, McAdam SAM, Bienaimé D, Lucani CJ, Marmottant P (2016b) Visual quantification of embolism reveals leaf vulnerability to hydraulic failure. New Phytol 209: 1403–1409 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Carriqui M, Delzon S, Lucani C (2017) Optical measurement of stem xylem vulnerability. Plant Physiol 174: 2054–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Sternberg LDSL (2003) Dynamic changes in hydraulic conductivity in petioles of two savanna tree species: factors and mechanisms contributing to the refilling of embolized vessels. Plant Cell Environ 26: 1633–1645 [Google Scholar]
- Buckley TN, Mott KA, Farquhar GD (2003) A hydromechanical and biochemical model of stomatal conductance. Plant Cell Environ 26: 1767–1785 [Google Scholar]
- Cavender-Bares J, Cortes P, Rambal S, Joffre R, Miles B, Rocheteau A (2005) Summer and winter sensitivity of leaves and xylem to minimum freezing temperatures: a comparison of co-occurring Mediterranean oaks that differ in leaf lifespan. New Phytol 168: 597–612 [DOI] [PubMed] [Google Scholar]
- Charrier G, Delzon S, Domec JC, Zhang L, Delmas CEL, Merlin I, Corso D, King A, Ojeda H, Ollat N, et al. (2018) Drought will not leave your glass empty: low risk of hydraulic failure revealed by long-term drought observations in world’s top wine regions. Sci Adv 4: o6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103: 551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B. (2013) Predicting thresholds of drought-induced mortality in woody plant species. Tree Physiol 33: 669–671 [DOI] [PubMed] [Google Scholar]
- Choat B, Ball MC, Luly JG, Holtum JAM (2005) Hydraulic architecture of deciduous and evergreen dry rainforest tree species from north-eastern Australia. Trees (Berl) 19: 305–311 [Google Scholar]
- Choat B, Drayton WM, Brodersen C, Matthews MA, Shackel KA, Wada H, McElrone AJ (2010) Measurement of vulnerability to water stress-induced cavitation in grapevine: a comparison of four techniques applied to a long-vesseled species. Plant Cell Environ 33: 1502–1512 [DOI] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG, et al. (2012) Global convergence in the vulnerability of forests to drought. Nature 491: 752–755 [DOI] [PubMed] [Google Scholar]
- Cochard H, Breda N, Granier A, Aussenac G (1992) Vulnerability to air embolism of three European species (Quercus petraea (Matt) Liebl, Q. pubescens Willd, Q. robur L). Ann Sci 49: 225–233 [Google Scholar]
- Cochard H, Badel E, Herbette S, Delzon S, Choat B, Jansen S (2013) Methods for measuring plant vulnerability to cavitation: a critical review. J Exp Bot 64: 4779–4791 [DOI] [PubMed] [Google Scholar]
- Delzon S, Cochard H (2014) Recent advances in tree hydraulics highlight the ecological significance of the hydraulic safety margin. New Phytol 203: 355–358 [DOI] [PubMed] [Google Scholar]
- Flint LE, Flint AL, Thorne JH, Boynton R (2013) Fine-scale hydrologic modeling for regional landscape applications: the California Basin Characterization Model development and performance. Ecol Process 2: 1–25 [Google Scholar]
- Griffin JR. (1973) Xylem sap tension in three woodland oaks of central California. Ecology 54: 152–159 [Google Scholar]
- Hacke UG, Jacobsen AL, Pratt RB (2009) Xylem function of arid-land shrubs from California, USA: an ecological and evolutionary analysis. Plant Cell Environ 32: 1324–1333 [DOI] [PubMed] [Google Scholar]
- Hochberg U, Windt CW, Ponomarenko A, Zhang YJ, Gersony J, Rockwell FE, Holbrook NM (2017) Stomatal closure, basal leaf embolism and shedding protect the hydraulic integrity of grape stems. Plant Physiol 174: 764–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen AL, Pratt RB, Davis SD, Ewers FW (2007a) Cavitation resistance and seasonal hydraulics differ among three arid Californian plant communities. Plant Cell Environ 30: 1599–1609 [DOI] [PubMed] [Google Scholar]
- Jacobsen AL, Pratt RB, Ewers FW, Davis SD (2007b) Cavitation resistance among 26 chaparral species of southern California. Ecol Monogr 77: 99–115 [Google Scholar]
- Jacobsen AL, Pratt RB, Tobin MF, Hacke UG, Ewers FW (2012) A global analysis of xylem vessel length in woody plants. Am J Bot 99: 1583–1591 [DOI] [PubMed] [Google Scholar]
- Johnson DM, Woodruff DR, McCulloh KA, Meinzer FC (2009) Leaf hydraulic conductance, measured in situ, declines and recovers daily: leaf hydraulics, water potential and stomatal conductance in four temperate and three tropical tree species. Tree Physiol 29: 879–887 [DOI] [PubMed] [Google Scholar]
- Johnson DM, McCulloh KA, Meinzer FC, Woodruff DR, Eissenstat DM (2011) Hydraulic patterns and safety margins, from stem to stomata, in three eastern U.S. tree species. Tree Physiol 31: 659–668 [DOI] [PubMed] [Google Scholar]
- Jones HG, Sutherland RA (1991) Stomatal control of xylem embolism. Plant Cell Environ 14: 607–612 [Google Scholar]
- Larter M, Pfautsch S, Domec JC, Trueba S, Nagalingum N, Delzon S (2017) Aridity drove the evolution of extreme embolism resistance and the radiation of conifer genus Callitris. New Phytol 215: 97–112 [DOI] [PubMed] [Google Scholar]
- Li X, Blackman CJ, Choat B, Rymer PD, Medlyn BE, Tissue DT (2018) Tree hydraulic traits are coordinated and strongly linked to climate-of-origin across a rainfall gradient. Plant Cell Environ 41: 646–660 [DOI] [PubMed] [Google Scholar]
- Lobo A, Torres-Ruiz JM, Burlett R, Lemaire C, Parise C, Francioni C, Tru L, Tomá I, Kehlet J, Dahl E, et al. (2018) Assessing inter- and intraspecific variability of xylem vulnerability to embolism in oaks. For Ecol Manage 424: 53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali H, Pockman WT, Jackson RB (2004) Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 85: 2184–2199 [Google Scholar]
- Maréchaux I, Bartlett MK, Sack L, Baraloto C, Engel J, Joetzjer E, Chave J (2015) Drought tolerance as predicted by leaf water potential at turgor loss point varies strongly across species within an Amazonian forest. Funct Ecol 29: 1268–1277 [Google Scholar]
- Martin-StPaul N, Delzon S, Cochard H (2017) Plant resistance to drought depends on timely stomatal closure. Ecol Lett 20: 1437–1447 [DOI] [PubMed] [Google Scholar]
- McIntyre PJ, Thorne JH, Dolanc CR, Flint AL, Flint LE, Kelly M, Ackerly DD (2015) Twentieth-century shifts in forest structure in California: denser forests, smaller trees, and increased dominance of oaks. Proc Natl Acad Sci USA 112: 1458–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher PJ, Holbrook NM, Burns MJ, Zwieniecki MA, Cobb AR, Brodribb TJ, Choat B, Sack L (2012) Measurements of stem xylem hydraulic conductivity in the laboratory and field. Methods Ecol Evol 3: 685–694 [Google Scholar]
- Nardini A, Ramani M, Gortan E, Salleo S (2008) Vein recovery from embolism occurs under negative pressure in leaves of sunflower (Helianthus annuus). Physiol Plant 133: 755–764 [DOI] [PubMed] [Google Scholar]
- Nardini A, Pedà G, La Rocca N (2012) Trade-offs between leaf hydraulic capacity and drought vulnerability: morpho-anatomical bases, carbon costs and ecological consequences. New Phytol 196: 788–798 [DOI] [PubMed] [Google Scholar]
- Ogasa M, Miki NH, Murakami Y, Yoshikawa K (2013) Recovery performance in xylem hydraulic conductivity is correlated with cavitation resistance for temperate deciduous tree species. Tree Physiol 33: 335–344 [DOI] [PubMed] [Google Scholar]
- Osuna JL, Baldocchi DD, Kobayashi H, Dawson TE (2015) Seasonal trends in photosynthesis and electron transport during the Mediterranean summer drought in leaves of deciduous oaks. Tree Physiol 35: 485–500 [DOI] [PubMed] [Google Scholar]
- Pockman WT, Sperry JS (2000) Vulnerability to xylem cavitation and the distribution of Sonoran Desert vegetation. Am J Bot 87: 1287–1299 [PubMed] [Google Scholar]
- Rockwell FE, Wheeler JK, Holbrook NM (2014) Cavitation and its discontents: opportunities for resolving current controversies. Plant Physiol 164: 1649–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Dominguez CM, Murphy MRC, Lucani C, Brodribb TJ (2018) Mapping xylem failure in disparate organs of whole plants reveals extreme resistance in olive roots. New Phytol 218: 1025–1035 [DOI] [PubMed] [Google Scholar]
- Skelton RP, West AG, Dawson TE (2015) Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proc Natl Acad Sci USA 112: 5744–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton RP, Brodribb TJ, Choat B (2017a) Casting light on xylem vulnerability in an herbaceous species reveals a lack of segmentation. New Phytol 214: 561–569 [DOI] [PubMed] [Google Scholar]
- Skelton RP, Brodribb TJ, McAdam SAM, Mitchell PJ (2017b) Gas exchange recovery following natural drought is rapid unless limited by loss of leaf hydraulic conductance: evidence from an evergreen woodland. New Phytol 215: 1399–1412 [DOI] [PubMed] [Google Scholar]
- Sperry JS, Ikeda T (1997) Xylem cavitation in roots and stems of Douglas-fir and white fir. Tree Physiol 17: 275–280 [DOI] [PubMed] [Google Scholar]
- Sperry JS, Hacke UG, Oren R, Comstock JP (2002) Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ 25: 251–263 [DOI] [PubMed] [Google Scholar]
- Sperry JS, Christman MA, Torres-Ruiz JM, Taneda H, Smith DD (2012) Vulnerability curves by centrifugation: is there an open vessel artefact, and are ‘r’ shaped curves necessarily invalid? Plant Cell Environ 35: 601–610 [DOI] [PubMed] [Google Scholar]
- Trifilò P, Barbera PM, Raimondo F, Nardini A, Gullo MA Lo (2014) Coping with drought-induced xylem cavitation: coordination of embolism repair and ionic effects in three Mediterranean evergreens. Tree Physiol 34: 109–122 [DOI] [PubMed] [Google Scholar]
- Trifilò P, Nardini A, Lo Gullo MA, Barbera PM, Savi T, Raimondo F (2015) Diurnal changes in embolism rate in nine dry forest trees: relationships with species-specific xylem vulnerability, hydraulic strategy and wood traits. Tree Physiol 35: 694–705 [DOI] [PubMed] [Google Scholar]
- Tyree MT, Ewers FW (1991) Tansley Review No. 34. The hydraulic architecture of trees and other woody plants. New Phytol 119: 345–360 [Google Scholar]
- Tyree MT, Hammel HT (1972) The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. J Exp Bot 23: 267–282 [Google Scholar]
- Tyree MT, Cochard H, Cruiziat P, Sinclair B, Ameglio T (1993) Drought-induced leaf shedding in walnut: evidence for vulnerability segmentation. Plant Cell Environ 16: 879–882 [Google Scholar]
- Urli M, Porté AJ, Cochard H, Guengant Y, Burlett R, Delzon S (2013) Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol 33: 672–683 [DOI] [PubMed] [Google Scholar]
- West AG, Dawson TE, February EC, Midgley GF, Bond WJ, Aston TL (2012) Diverse functional responses to drought in a Mediterranean-type shrubland in South Africa. New Phytol 195: 396–407 [DOI] [PubMed] [Google Scholar]
- Wheeler JK, Huggett BA, Tofte AN, Rockwell FE, Holbrook NM (2013) Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant Cell Environ 36: 1938–1949 [DOI] [PubMed] [Google Scholar]
- Zimmermann MH. (1978) Hydraulic architecture of some diffuse-porous trees. Can J Bot 56: 2286–2295 [Google Scholar]
- Zimmermann MH, Jeje AA (1981) Vessel-length distribution in stems of some American woody plants. Can J Bot 59: 1882–1892 [Google Scholar]