Root-specific expression of a cytokinin-degrading CKX gene in barley roots causes formation of a larger root system leading to higher element content in shoot organs and improved drought tolerance.
Abstract
Root size and architecture are important crop plant traits, as they determine access to water and soil nutrients. The plant hormone cytokinin is a negative regulator of root growth and branching. Here, we generated transgenic barley (Hordeum vulgare) plants with an enlarged root system by enhancing cytokinin degradation in roots to explore the potential of cytokinin modulations in improving root functions. This was achieved through root-specific expression of a CYTOKININ OXIDASE/DEHYDROGENASE gene. Enhanced biomass allocation to roots did not penalize shoot growth or seed yield, indicating that these plants were not source limited. In leaves of transgenic lines, the concentrations of several macroelements and microelements were increased, particularly those with low soil mobility (phosphorus, manganese, and zinc). Importantly, seeds contained up to 44% more zinc, which is beneficial for human nutrition. Transgenic lines also demonstrated dampened stress responses to long-term drought conditions, indicating lower drought sensitivity. Taken together, this work demonstrates that root engineering of cereals is a promising strategy to improve nutrient efficiency, biofortification, and drought tolerance.
The size and architecture of root systems are important traits of crop plants. Plant roots perform many essential functions, including taking up water and nutrients, storing reserves, anchoring the plant to the soil, and establishing biotic interactions in the rhizosphere. The root system’s size and architecture determine its ability to fulfill these diverse functions. This is relevant for crop plants, as shortages of water and nutrients limit growth and grain yield in many agroecosystems (Lynch, 1995). Drought accounts for more loss in plant productivity than any other abiotic factor, and climate change models predict an increase of drought stress in several crop production regions worldwide (Hochholdinger, 2016). Another critical yield-limiting factor is the access of roots to soil nutrients. Increasing crop yields and the expansion of agriculture to marginal lands increase demand for soil nutrients, which needs to be covered by fertilization. Fertilizers are expensive, and especially nitrate and phosphate leaching or runoff cause the eutrophication of surface waters and contamination of groundwater. To address these problems, plant breeding aims to develop varieties with improved nutrient use efficiency and improved drought tolerance. One way to improve nutrient and water use efficiency and to secure plant productivity under nonoptimal conditions may be achieved by improving the root systems of crop plants (Lynch and Brown, 2012; Comas et al., 2013; White et al., 2013; Rogers and Benfey, 2015; Koevoets et al., 2016).
In general, root length and root density are positively correlated with mineral element uptake, particularly for elements with limited solubility (Marschner, 2002). Root architecture also determines access to water, and under certain conditions, a correlation has been found between root system size and tolerance to drought stress (Price et al., 2002; Tuberosa et al., 2002; Comas et al., 2013). For example, drought-tolerant rice (Oryza sativa) varieties have been found to form a deeper and more highly branched root system than drought-sensitive varieties (Price and Tomos, 1997). Other studies have revealed a positive correlation between root traits and crop performance or grain yield under drought (de Dorlodot et al., 2007; Kell, 2011; Uga et al., 2013; Hufnagel et al., 2014; Meister et al., 2014). Consequently, root architectural traits optimizing soil exploration in time and space are among those traits that are considered to be relevant in crop breeding programs (Lynch and Brown, 2012; Comas et al., 2013; White et al., 2013; Rogers and Benfey, 2015; Koevoets et al., 2016).
Optimizing the root system by classical breeding strategies requires detailed measurements of roots, which are hidden below ground. In addition, root system architecture is a complex trait, governed by many intrinsic and extrinsic factors and involving numerous genes (Lynch and Brown, 2012). These difficulties make it challenging to breed specifically for improved root systems. However, a targeted approach to improve root systems is desirable, not only for breeding purposes but also to study the functional relevance of the size of the root system. Transgenic lines and their nontransgenic counterparts would be near-isogenic lines differing in only one or two genes, which is advantageous for functional studies.
The plant hormone cytokinin (CK) has been recognized as a major negative regulator of root development. In Arabidopsis (Arabidopsis thaliana), CK regulates the elongation of primary roots (Werner et al., 2003) and the initiation of lateral roots (Laplaze et al., 2007; Chang et al., 2013) and acts as a positional cue regulating the distance between lateral roots (Bielach et al., 2012; Chang et al., 2015). Different approaches have been used to reduce the CK status of Arabidopsis, including the overexpression of CK-degrading CYTOKININ OXIDASE/DEHYDROGENASE (CKX) genes (Werner et al., 2001, 2003), mutation of CK synthesis genes (Miyawaki et al., 2006), mutation of CK receptor genes (Riefler et al., 2006), or suppression of the CK signaling pathway (Mason et al., 2005; Heyl et al., 2008). Collectively, these studies have shown that, under standard growth conditions, the root CK status is above the optimal level for root growth. Furthermore, proof has been obtained in dicotyledonous model plants that increasing CK degradation in roots by root-specific expression of a CKX gene leads to the formation of an enhanced root system, causes increased accumulation of several micronutrients and macronutrients in the aerial plant parts, and improves survival under harsh drought conditions (Werner et al., 2010). This approach demonstrated that a single dominant gene can be used to regulate a complex trait.
CK also is involved in regulating plant responses to the deficiency of several essential nutrients, including phosphorus (Franco-Zorrilla et al., 2002, 2005), sulfur (Maruyama-Nakashita et al., 2004), iron (Séguéla et al., 2008), sodium (Mason et al., 2010), and potassium (Nam et al., 2012), or to the toxicity of arsenic (Mohan et al., 2016). Since the expression of CK-related genes is responsive to soil conditions, indicating that the environment influences cellular CK homeostasis (Ramireddy et al., 2014), an altered CK status of the root may improve the acquisition of soil nutrients (Werner et al., 2010).
Much less is known about CK and its potential usefulness to manipulate the root system in cereal plants, which would be a primary target for application because of their agricultural relevance. Cereal plants have more complex root systems than Arabidopsis, as these consist of embryonic primary and seminal roots and postembryonic crown roots (Rogers and Benfey, 2015). Monocot plants such as maize (Zea mays) and rice possess a similar set of CK genes as dicot plants (Chu et al., 2011; Tsai et al., 2012), and some of these genes have been shown to be involved in regulating crown root formation in rice (Kitomi et al., 2011; Gao et al., 2014; Zhao et al., 2015). The inhibition of lateral root initiation but stimulation of lateral root elongation by CK in rice (Rani Debi et al., 2005) suggest that CK functions are generally quite similar between monocots and dicots, but there also might be key differences that must be identified to inform efforts to improve root traits in grasses. Also, the higher content and activity of the generally less active cis-zeatin (cZ)-type CKs as compared with trans-zeatin (tZ)-type CKs in some monocot plants suggest that dicot and monocot plants differ in some aspects of CK metabolism, transport, or signaling (Lomin et al., 2011; Kudo et al., 2012).
We selected barley (Hordeum vulgare), which is an important European cereal crop species, for our root-engineering approach. The barley root consists of several seminal roots derived from the embryo and a nodal root system derived from the nodes at the base of the main stem or of the tillers. Previous attempts to manipulate the barley root system by the ectopic expression of CKX genes have proven difficult, as strong detrimental effects on shoot development and reproduction could not be avoided (Mrízová et al., 2013; Pospíšilová et al., 2016). This indicated that a successful approach may depend on restricting the manipulation of CKX gene expression to roots. Therefore, we attempted here to enhance the root system of barley plants by ectopic expression in roots of CKX genes characterized previously in Arabidopsis. As it was a priori not obvious which CKX enzyme from Arabidopsis would be most suitable to achieve root enhancement in barley, we compared the two prototypic enzymes CKX1 and CKX2, which, in Arabidopsis, affect different CK pools because of their distinct subcellular localization and substrate specificities (Werner et al., 2003; Galuszka et al., 2007). CKX1 and CKX2 belong to two different evolutionary branches of the CKX protein family that had formed before monocots and dicots diverged (Schmülling et al., 2003). In this work, we show that it is possible by genetic engineering of CK breakdown to generate barley plants that have an enhanced root system but largely unaffected shoot development. Comparison with their untransformed counterparts revealed the increased accumulation of several soil nutrients in their leaves and seeds as well as a reduced sensitivity to long-term drought conditions.
RESULTS
Identification and Validation of Root-Specific Promoters for Root Engineering in Barley
To alter root growth and development by enhanced expression of CKX genes, the use of a root-specific promoter appeared indispensable (Werner et al., 2010). For our experiments in barley, we searched for a root-specific promoter in rice, because of the close genetic relationship between rice and barley and the availability of transcriptome data and genome sequences from rice. By mining publicly available transcriptome data (Winter et al., 2007; Hruz et al., 2008; Sato et al., 2013), we identified several rice genes that were expressed exclusively or preferentially in root tissues at different developmental stages, including RETROTRANSPOSON PROTEIN (RET; LOC_Os10g31730), EXPRESSED PROTEIN (EPP; LOC_Os04g11040), and PEROXIDASE PROTEIN (PER; LOC_Os03g25330; Supplemental Fig. S1A). All three genes were expressed in roots under different growth conditions and at different developmental stages, including vegetative and reproductive growth stages of field-grown rice cultivars (Sato et al., 2013). In order to validate the root-specific expression of these genes, reverse transcription-quantitative PCR (RT-qPCR) analysis was carried out with RNA from different tissues of soil-grown rice. In roots, transcript levels of RET, EPP, or PER were more than 2,000-, 300-, or 10-fold higher, respectively, than in any of the other organs tested (Supplemental Fig. S1B). Subsequently, expression of the GUS reporter gene under the control of ∼2 kb of the EPP and PER promoters was tested in Arabidopsis. GUS staining was found almost exclusively in roots at different developmental stages of Arabidopsis (Supplemental Fig. S2). Within the primary root, expression was restricted to the transition zone and the vascular tissue, and no expression was observed in primary and lateral root meristems. These results indicated that the EPP and PER promoters mediate root-specific expression in monocotyledonous and dicotyledonous species, thus being suitable to drive CKX gene expression in our approach with transgenic barley.
Generation of Transgenic Barley Plants with Increased CKX Activity in Roots
For root engineering in barley, we used two prototypic CKX genes of Arabidopsis, CKX1 and CKX2. The two CKX gene products, CKX1 and CKX2, differ in their subcellular localization and biochemical characteristics. A tagged CKX1 protein was shown to localize predominantly to the endoplasmic reticulum (Niemann et al., 2018), while a CKX2-GFP fusion protein localized to the endoplasmic reticulum and possibly also to the apoplastic space (Werner et al., 2003; Zürcher et al., 2016). Furthermore, the CKX1 protein preferred CK ribosides and N9-glucosides as substrates, while N6-isopentenyladenine (iP) and iP riboside were preferred by CKX2 (Galuszka et al., 2007). Root-specific expression of either CKX gene strongly enhanced root growth in tobacco (Nicotiana tabacum) and Arabidopsis (Werner et al., 2010), but only enhanced CKX1 expression had a strong negative impact on shoot growth (Werner et al., 2001, 2003).
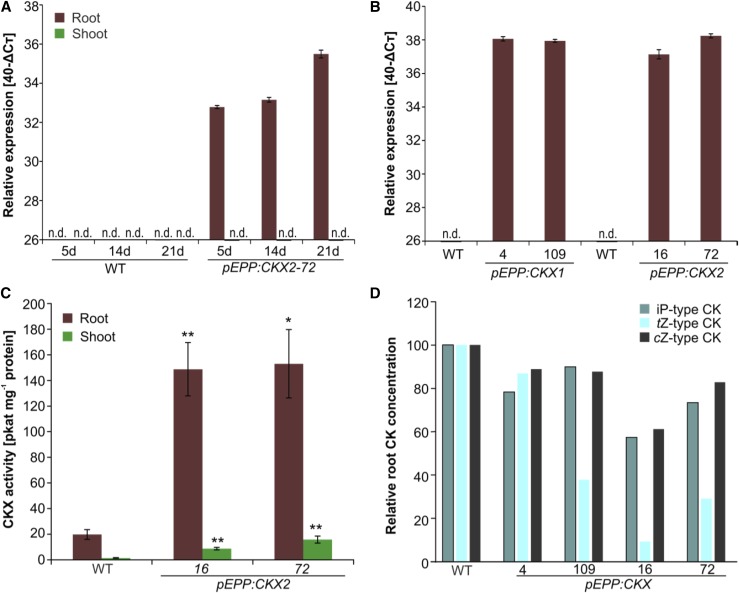
Transgenic barley plants expressing CKX1 or CKX2 under the control of the three root-specific promoters pEPP, pPER, and pRET were generated using Agrobacterium tumefaciens-mediated transformation of the spring barley variety Golden Promise (Bartlett et al., 2008). Expression of the transgenes was tested by RT-qPCR in several independent homozygous lines of the T3 generation. Since pEPP:CKX lines were mostly used for further analyses, we document the characterization of these lines in more detail. The root specificity of CKX2 transgene expression in the line pEPP:CKX2-72 was confirmed 5, 14, and 21 d after germination, whereas in the shoot tissue no expression was detected (Fig. 1A). For further studies, two independent transgenic barley lines with high expression levels were selected for each of the two CKX homologs (i.e. pEPP:CKX1-4, pEPP:CKX1-109, pEPP:CKX2-16, and pEPP:CKX2-72). In all four lines, transcript levels of the transgene were high in the roots (Fig. 1B). Subsequently, CKX activity was tested in 3-week-old soil-grown transgenic plants expressing the CKX2 gene and compared with the wild type. The analysis was limited to CKX2, as CKX1 activity is very low in barley and difficult to quantify (Galuszka et al., 2007). Both pEPP:CKX2 lines showed greater than 7.5-fold higher CKX activities in roots when compared with roots of untransformed plants (Fig. 1C). An increase in CKX activity also was observed in the shoot tissue of transgenic lines, but there the enzyme activity was only about 6% to 11% of the activity found in roots (Fig. 1C).
Figure 1.
Characterization of transgenic barley lines with root-specific expression of CKX genes. A, Expression of CKX2 under the control of the EPP promoter in roots at different developmental stages. B, Expression of CKX1 or CKX2 in roots of different 3-week-old transgenic barley lines (T3 generation). For A and B, RT-qPCR was performed using three biological replicates for each line and the barley reference gene ubiquitin-conjugating enzyme (HvUBC10) as the reference gene. Relative expression of the transgene is shown as 40-ΔCT value, with 26 being the threshold value for expressed genes. Data represent means ± sd. C, CKX enzyme activity in wild-type (WT) and transgenic lines expressing CKX2. The CKX enzyme activity assay was performed with root extracts of 3-week-old soil-grown plants using iP as a substrate. Four biological replicates pooled from two to three plants were analyzed for each genotype. Data represent means ± se. D, CK concentrations in roots of transgenic barley plants compared with wild-type roots. CK metabolites were quantified in 3-week-old plants grown in a hydroponic system. The total content of each major group of CK metabolites in the wild type was set to 100%. Four biological replicates of two to three plants were analyzed for each genotype. Complete data and statistics are shown in Supplemental Table S1. Asterisks indicate statistically significant differences from the wild type determined using a two-tailed Student’s t test (*, P < 0.05 and **, P < 0.01). n.d., not detected.
Quantification of CK metabolites in the roots of 3-week-old plants of the transgenic barley lines revealed a trend of lower levels of CK metabolites compared with the wild type (Fig. 1D; Supplemental Table S1). In particular, the root concentration of the free base iP, which was the only bioactive CK detected, was only between 21% and 47% of the concentration in wild-type roots. The total content of iP-type and cZ-type CKs in transgenic roots was in the range of 57% to 89% of that of the wild type. The reduction was stronger for tZ-type CKs: three transgenic lines contained 9% to 38% of the concentration of the wild type, while the line pEPP:CKX1-4 contained 87% of tZ-type CKs in its roots (Fig. 1D; Supplemental Table S1). A large part of the reduction was due to a lower concentration of CK sugar conjugates, which are biologically inactive CK storage forms (Sakakibara, 2006) and represented the bulk of CK metabolites (Supplemental Table S1). The strong reduction of their concentration indicated reduced availability of the physiologically active form tZ, which, by itself, was below the detection level. The total concentration of cZ-type CKs was slightly lower in roots of the transgenic lines. In shoots, the total concentration of iP-type CKs was unchanged, while the concentration of tZ-type CKs in lines 16 and 72 was about 2-fold higher than in the wild type but unchanged in two other lines; in this case, this difference also was due mainly to an altered concentration of the corresponding conjugates (Supplemental Table S1).
Transgenic Barley Plants Exhibit Enlarged Root Systems
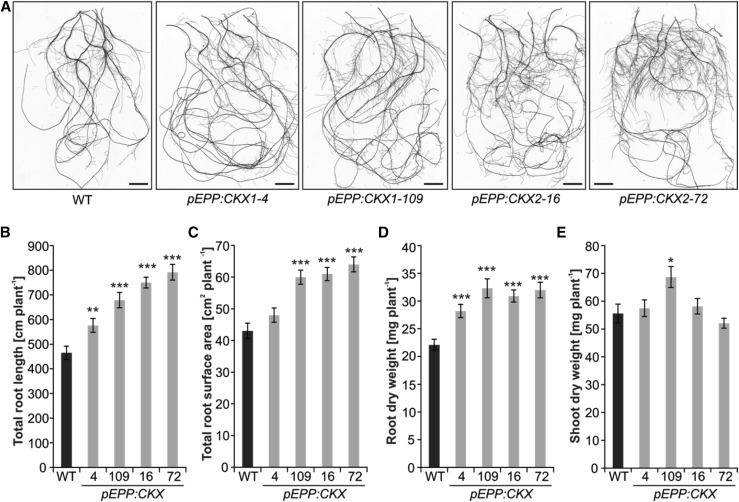
We then investigated whether enhanced CKX expression in roots of transgenic barley would alter plant growth. Visual inspection of roots from 2-week-old hydroponically grown plants indicated that the size of the root system was apparently increased in transgenic plants compared with the wild type (Fig. 2A). Quantitative analysis revealed an increase of the total root length by 24% to 70% and of the total root surface area by 12% to 50% in transgenic plants compared with the wild type (Fig. 2, B and C). Root biomass of transgenic plants was increased by up to 47% in comparison with wild-type roots (Fig. 2D). In contrast, the shoot biomass of the transgenic lines was comparable to that of the wild type, except for line pEPP:CKX1-109, which showed a 15% increase in shoot biomass (Fig. 2E). The differential root growth increased the root-to-shoot biomass ratio by 16% to 50% in the transgenic lines. The analysis of 3-week-old plants grown in hydroponics confirmed that transgenic plants developed a larger root system size with increased biomass but essentially without reduction in shoot biomass (Supplemental Fig. S3, A–C). Growth of two transgenic lines also was tested in soil-filled rhizoboxes (Supplemental Fig. S3D). Also under these conditions, root size and root-to-shoot biomass ratio of these plants were increased by about 30% and 35% to 75%, respectively, as compared with the wild type (Supplemental Fig. S3, E–G). Notably, pRET:CKX and pPER:CKX transgenic lines also developed a larger root system (Supplemental Fig. S4).
Figure 2.
Root-specific expression of CK oxidases enhances root system size. A, Root phenotypes of 2-week-old transgenic lines grown in hydroponic culture. Representative images of individual plants of the wild type (WT) and each transgenic line are shown. Bars = 2 cm. B to E, Total root length (B), total root surface area (C), root biomass (dry weight; D), and shoot biomass (dry weight; E) of 2-week-old plants. Total root length and surface area were calculated using the WinRHIZO software. Data represent means ± se (n = 20). Asterisks indicate significant differences from the wild type as determined by a two-tailed Student’s t test (*, P < 0.05; **, P < 0.01; and ***, P < 0.001).
Root-Specific Expression of CKX Does Not Cause a Yield Penalty
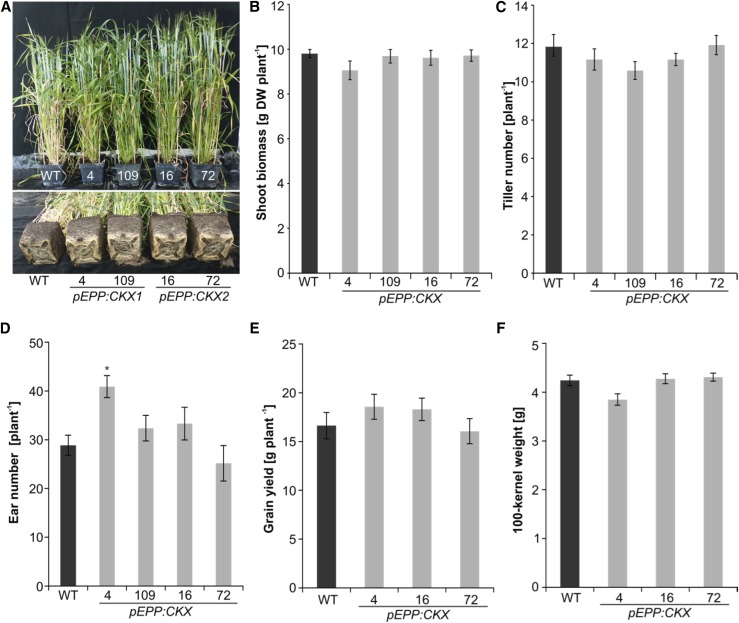
Next, we analyzed whether the increased root size of transgenic plants would have an impact on seed yield. Visual inspection of 3-month-old soil-grown transgenic plants confirmed their enhanced root system at this late developmental stage (Fig. 3A). However, growth and development of the shoots of these plants appeared to be similar to those of wild-type plants. For example, plant height and the number of days to reach the heading stage were similar in all lines (data not shown). Likewise, shoot biomass (on a dry weight basis) of 70-d-old transgenic plants was comparable to that of the wild type (Fig. 3B). Furthermore, yield-related traits like the number of tillers per plant (Fig. 3C), the number of ears per plant (Fig. 3D), total grain yield per plant (Fig. 3E), and the hundred-kernel weight (Fig. 3F) were not significantly different from those of the wild type at P < 0.05. Only line pEPP:CKX1-4 showed a significantly (P < 0.01) larger number of ears per plant; however, this increase did not affect total grain yield (Fig. 3E). Taken together, the root-specific expression of CKX genes caused root enhancement but did not significantly affect shoot growth or seed yield in the transgenic lines.
Figure 3.
Root-specific expression of CK oxidases does not cause a yield penalty. A, Root and shoot phenotypes of 12-week-old plants grown in soil-filled pots. B and C, Shoot biomass (B) and number of tillers (C) of 10-week-old transgenic plants. D to F, Number of filled spikes (D), total yield per plant (E), and hundred-kernel weight (F) of wild-type (WT) and transgenic plants. Data represent means ± se (n = 15–20). The asterisk indicates a significant difference from the wild type as determined by a two-tailed Student’s t test (*, P < 0.01).
Root-Specific Expression of CKX Enhances Mineral Element Accumulation in Leaves and Seeds
One important function of roots is to acquire mineral elements from the soil. The ability of roots to efficiently explore and exploit the available soil volume is particularly relevant for nutrients with low soil mobility. Therefore, an enhanced growth of roots might contribute to an improved extraction of such nutrients from the soil. To study whether the increased root size had an impact on element content of aboveground plant parts, we determined the concentrations of 16 different elements in leaves and seeds of the transgenic lines.
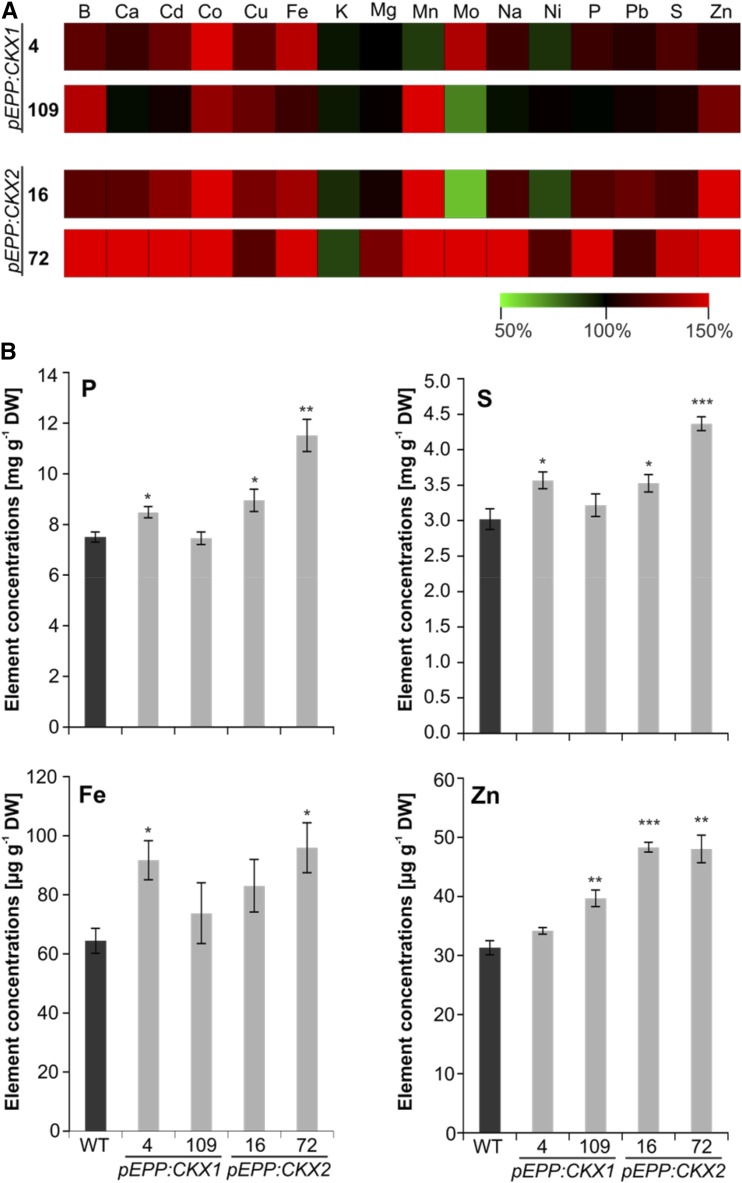
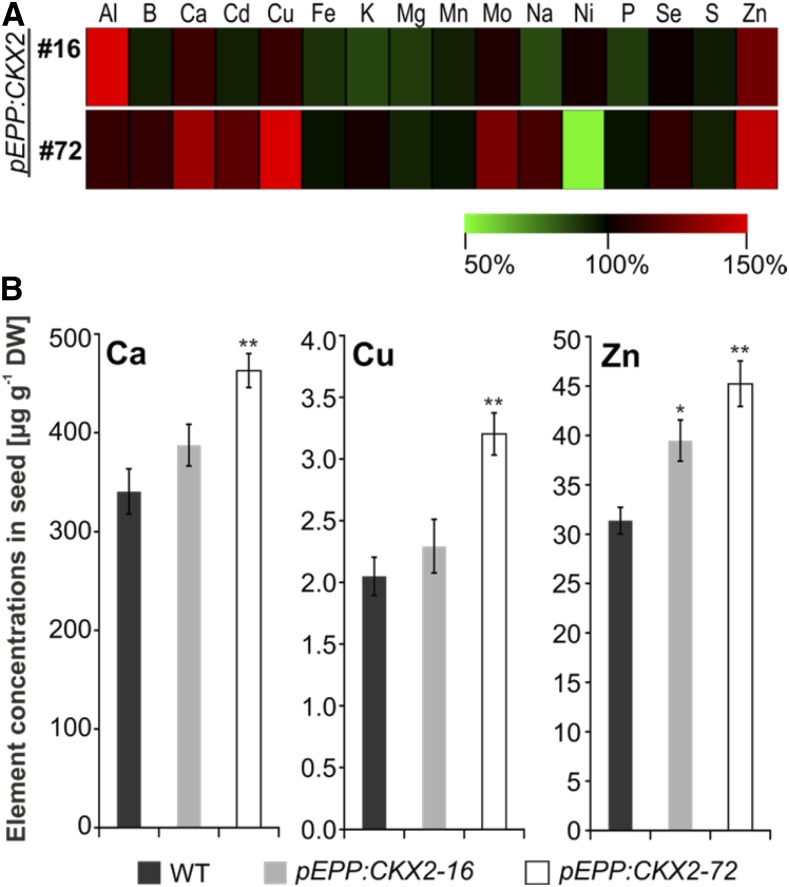
In leaves from 8-week-old soil-grown transgenic plants, concentrations of numerous mineral elements were higher than in wild-type plants, and there was a stronger increase in lines expressing CKX2 than in those expressing CKX1 (Fig. 4A; Supplemental Table S2). Of the five macroelements (phosphorus [P], potassium [K], sulfur [S], magnesium [Mg], and calcium [Ca]), the concentrations of P and S were increased by 13% to 53% and 17% to 45% in three of the four lines, respectively (Fig. 4). In contrast, leaf concentrations of K tended to be reduced slightly (i.e. by 5%–13% in all four transgenic lines; Fig. 4; Supplemental Table S2). Similarly, the concentrations of several microelements were increased in at least three of the four transgenic lines: copper (Cu; 20%–28%), manganese (Mn; 51%–70%), and zinc (Zn; 7%–54%). The concentrations of other elements (Ca, iron [Fe], and boron [B]) were increased compared with the wild type as well, but the difference was usually only statistically significant either in two lines, pEPP:CKX1-4 and pEPP:CKX2-72, or in pEPP:CKX2-72 alone (Fig. 4; Supplemental Table S2).
Figure 4.
Mineral element concentrations in shoots. A, Relative changes in mineral element concentrations in transgenic lines compared with the wild type (WT). The concentration of each mineral element in wild-type shoots was set to 100%, and relative differences in transgenic lines are shown in a heat map generated using Multiexperiment Viewer version 4.9 (Saeed et al., 2003). The complete data set is shown in Supplemental Table S2. B, Concentrations of P, S, Fe, and Zn in 8-week-old soil-grown shoots. Four biological replicates for each genotype were analyzed, each containing shoots from two to three plants. Data represent means ± se. Asterisks indicate significant differences from the WT as determined by a two-tailed Student’s t test (*, P < 0.05; **, P < 0.01; and ***, P < 0.001). DW, dry weight.
The enhanced ability of transgenic lines to accumulate nutrients also was evaluated in plants grown under drought stress. Under drought conditions, nutrient uptake is generally lower due to a decrease in transpiration rate and reduced nutrient uptake into root cells (Levitt, 1980). In our experiment, drought stress did not strongly affect the concentrations of mineral elements in any of the genotypes (Supplemental Table S3). Transgenic plants grown under well-watered or drought stress conditions showed enhanced accumulation of several elements to a similar extent as in the experiment described above, although they were grown in a different soil. In particular, the enhanced accumulation of Mn (+33%–212%) and P (+18%–35%) in both transgenic lines under all conditions indicated that this is a stable trait.
We then investigated whether the increase in nutrient accumulation found in leaves is reflected by a similar increase in seeds. To this end, we analyzed element concentrations in dried seeds of the two CKX2-expressing lines, as these showed the highest leaf element accumulation, and the wild type. In contrast to leaves, the concentrations of most of the elements were similar in all lines (Supplemental Table S4). However, the concentrations of Ca, Cu, and Zn were increased consistently in seeds of transgenic plants (Fig. 5A; Supplemental Table S4). The increase was significant for Zn in both lines (26% and 44%, respectively), whereas the increases for Ca and Cu were significant only in line pEPP:CKX2-72 (Fig. 5B; Supplemental Table S4). Taken together, in seeds of transgenic barley plants, an increased concentration was found for a subgroup of elements that were increased in leaves.
Figure 5.
Mineral element concentrations in seeds. A, Relative changes in mineral element concentrations in transgenic lines compared with the wild type (WT). The concentration of each mineral element in wild-type seeds was set to 100%, and relative differences in transgenic lines are shown in a heat map generated using Multiexperiment Viewer version 4.9 (Saeed et al., 2003). The complete data set is shown in Supplemental Table S4. B, Concentrations of Ca, Cu, and Zn in seeds of transgenic lines in comparison with wild-type seeds. Four biological replicates for each genotype were analyzed, each containing seeds from two to three plants. Data represent means ± se. Asterisks indicate significant differences from the wild type as determined by a two-tailed Student’s t test (*, P < 0.05 and **, P < 0.01). DW, Dry weight.
Transgenic Plants Withstand Long-Term Drought Better Than the Wild Type
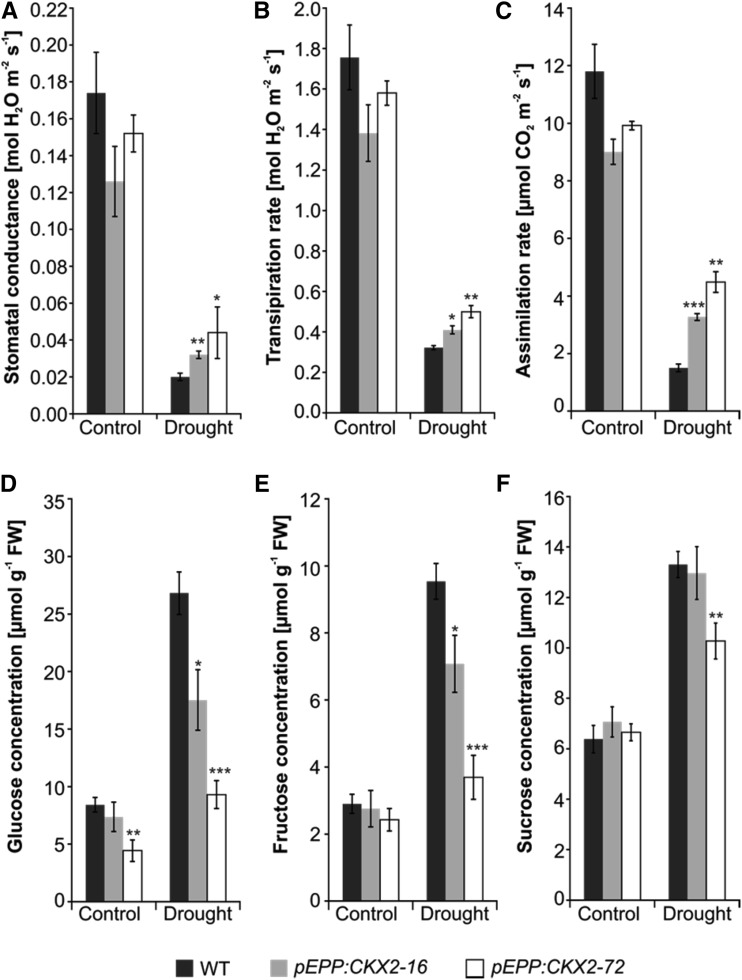
As crop plants frequently experience water stress, we explored the response of transgenic barley plants to long-term drought conditions. The two pEPP:CKX2 transgenic lines that showed the strongest root growth were compared with the wild type. Drought stress was imposed for two weeks on plants having eight to nine tillers by maintaining the moisture of the growth substrate at 10% using a soil moisture meter, corresponding to 20% to 25% of field capacity. Several parameters indicating stress responses were monitored, including stomatal conductance, transpiration rate, and carbon assimilation, all impacting photosynthesis.
Under well-watered (control) conditions, stomatal conductance and transpiration rate were not significantly different between transgenic and wild-type plants (Fig. 6, A and B). Relative to control conditions, water deficit reduced stomatal conductance and transpiration rate in wild-type plants down to 11% and 18%, respectively. In transgenic plants, stomatal conductance was reduced to 25% to 29% and transpiration rate was reduced to 30% to 32% of control conditions (Fig. 6, A and B). Similarly, the CO2 assimilation rate was reduced to 13% of the control conditions in the wild type but only to 36% to 45% in the transgenic lines (Fig. 6C). Wild-type plants and transgenic plants contained similar concentrations of sugars under control conditions, and all genotypes showed increases under drought, which was significantly stronger for the wild type (Fig. 6, D–F). The accumulation of sugars is important for osmotic adjustment under drought stress (Seki et al., 2007; Todaka et al., 2017). Together, these results indicated that transgenic plants withstood prolonged water deficit better than wild-type plants, which is particularly evident from the higher CO2 assimilation rate in the transgenic plants.
Figure 6.
Root-specific expression of CKX genes increases the tolerance of long-term drought stress. Stomatal conductance (A), transpiration rate (B), assimilation rate (C), and Glc (D), Fru (E), and Suc (F) concentrations in wild-type (WT) plants and two transgenic lines are shown. Measurements were taken from five biological replicates of each genotype grown under either well-watered (control) or drought stress conditions, with four technical replications per measurement. Data represent means ± se. Asterisks indicate significant differences from the wild type as determined by a two-tailed Student’s t test (*, P < 0.05; **, P < 0.01; and ***, P < 0.001). Differences between control and drought conditions within all genotypes were statistically significant at P < 0.05. FW, Fresh weight.
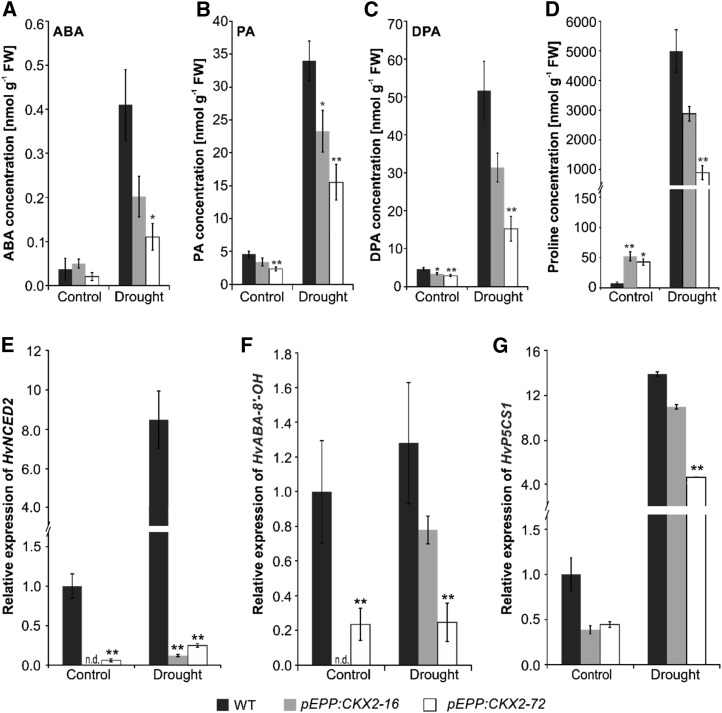
Next, we measured the concentrations of abscisic acid (ABA), which is a master regulator of the drought stress response (Shinozaki and Yamaguchi-Shinozaki, 2007), and its catabolic products phasic acid (PA) and dihydrophasic acid (DPA). PA and DPA tend to accumulate and, therefore, can be seen as indicators for the length of a stress period. Under control conditions, the steady-state levels of ABA and its catabolites were low and similar or slightly lower in transgenic as compared with wild-type plants (Fig. 7A). Drought caused an 11-fold increase in the ABA level of the wild type and a 4- to 5-fold increase in transgenic plants (Fig. 7A). The accumulation of PA and DPA in response to drought was lower in the transgenic lines than in the wild type (Fig. 7, B and C). Gene expression analysis showed that transcript levels of the key gene in ABA synthesis, 9-cis-EPOXYCAROTENOID DIOXYGENASE2 (HvNCED2), were much lower in transgenic plants than in the wild type (Fig. 7E). Under drought conditions, the HvNCED2 transcript levels in the two transgenic lines remained about 20-fold lower than in wild-type plants (Fig. 7E). The transcript levels of the gene ABA-8′-HYDROXYLASE (HvABA-8′-OH), coding for a protein that mediates the key step in ABA degradation, also were lower in the transgenic lines, but the differences were not as large as for HvNCED2 (Fig. 7F). These results showed that CKX expression in roots led to a reduced accumulation of ABA, PA, and DPA under drought, which was reflected by dampened ABA metabolism, as indicated by the transcript levels of key genes.
Figure 7.
ABA homeostasis and Pro concentrations in pEPP:CKX transgenic lines. A to D, Leaf concentrations of ABA (A), PA (B), DPA (C), and Pro (D) in wild-type (WT) and transgenic plants grown under control and drought stress conditions (n = 4–5). E to G, Relative transcript levels of a gene involved in ABA synthesis (HvNECD2; E), a gene involved in ABA degradation (HvABA-8’-OH; F), and the Pro synthesis gene (HvP5CS1; G) at the eight to nine tiller stage as determined by RT-qPCR. Total RNA was extracted from leaves of wild-type and transgenic plants grown under control and drought stress conditions. The transcript level in wild-type leaves under control conditions was set to 1 (n = 4). Data represent means ± se. Asterisks indicate significant differences from the wild type as determined by a two-tailed Student’s t test (*, P < 0.05 and **, P < 0.01). Differences between control and drought conditions within all genotypes were statistically significant at P < 0.05 except in (F). FW, Fresh weight.
It has been shown that several plant species accumulate certain amino acids upon exposure to abiotic stress, which might thus serve as metabolic stress markers (for review, see Krasensky and Jonak, 2012). A comparison of free amino acid concentrations in the shoots of plants grown under control or drought conditions revealed that, except for Met, the concentrations of all measured amino acids were severalfold higher in transgenic compared with wild-type plants (Table I). Under control conditions, the total concentration of proteinogenic amino acids was about 3-fold higher in transgenic plants in comparison with the wild type. In contrast, the concentration of Met was reduced by about 8- to 30-fold in transgenic plants. Notably, the steady-state level of the nonproteinogenic amino acid γ-aminobutyric acid (GABA) also was 4- to 5-fold higher in transgenic plants. Drought treatment caused a 6.6-fold increase in total amino acid content in the wild type, whereas it was only ∼1.7-fold higher in the transgenic plants (Table I). The three amino acids Asn, Ser, and Thr were of particular interest, as their concentrations have been shown to correlate positively with the performance of rice under drought (Degenkolbe et al., 2013). Under control conditions, their concentrations were significantly higher in transgenic plants than in wild-type plants. Following drought stress, their concentrations increased by 30-fold (Asn), 14-fold (Ser), and 6.8-fold (Thr) in the wild type but only by ∼2.5-, 1.5-, and ∼2-fold, respectively, in the transgenic lines (Table I).
Table I. Transgenic plants contain higher levels of free amino acids under control conditions.
The quantification of free amino acid contents was performed using shoot material of wild-type and transgenic plants grown under well-watered and drought stress conditions. Values are means ± se for each compound and were calculated from a minimum of four to five individual plants per genotype in each treatment. Significant differences were calculated using a two-tailed Student’s t test. Amino acid values are given in nmol g−1 fresh weight. Boldface values indicate significant differences (P < 0.05) for the same genotype under drought stress as compared to control conditions. Asterisks indicate significant differences compared with the wild type for the same treatment (*, P < 0.05; **, P < 0.01; and ***, P < 0.001).
| Amino Acid | Control | Drought Stress | ||||
|---|---|---|---|---|---|---|
| Wild Type | pEXP:CKX2 | Wild Type | pEXP:CKX2 | |||
| #16 | #72 | #16 | #72 | |||
| His | 25 ± 16 | 121 ± 11** | 82 ± 5* | 245 ± 18 | 219 ± 5 | 149 ± 10** |
| Asn | 127 ± 32 | 1,688 ± 133*** | 1,546 ± 182* | 3,846 ± 402 | 4,907 ± 185 | 3,564 ± 280 |
| Ser | 127 ± 49 | 1,268 ± 85*** | 1,009 ± 46*** | 1,815 ± 120 | 2,021 ± 33 | 1,585 ± 95 |
| Gln | 603 ± 363 | 1,303 ± 87 | 1,398 ± 168 | 2,041 ± 87 | 2,147 ± 150 | 1,643 ± 117 |
| Gly | 41 ± 27 | 174 ± 17** | 134 ± 15* | 246 ± 14 | 261 ± 3 | 245 ± 41 |
| Asp | 853 ± 616 | 2,140 ± 200 | 1,542 ± 107 | 2,169 ± 57 | 2,390 ± 82 | 2,125 ± 42 |
| Glu | 808 ± 607 | 2,970 ± 310* | 2,359 ± 144 | 3,795 ± 182 | 3,628 ± 78 | 3,406 ± 193 |
| Thr | 298 ± 207 | 1,015 ± 89* | 860 ± 43 | 2,042 ± 174 | 2,141 ± 40 | 1,620 ± 81 |
| Ala | 240 ± 178 | 1,071 ± 72** | 1,008 ± 29* | 1,331 ± 79 | 1,579 ± 45* | 1,232 ± 50 |
| Pro | 8 ± 2 | 52 ± 7** | 43 ± 5* | 4,995 ± 715 | 2,885 ± 248 | 896 ± 234** |
| Cys | 13 ± 2 | 35 ± 2*** | 43 ± 8 | 34 ± 4 | 26 ± 1 | 27 ± 1 |
| Lys | 3 ± 0.3 | 77 ± 22* | 57 ± 11* | 251 ± 21 | 175 ± 12* | 124 ± 21** |
| Tyr | 36 ± 9 | 53 ± 9 | 60 ± 18 | 88 ± 2 | 78 ± 6 | 61 ± 10 |
| Met | 302 ± 25 | 35 ± 26*** | 10 ± 5** | 10 ± 4 | 12 ± 4 | 5 ± 1 |
| Val | 70 ± 50 | 278 ± 34* | 211 ± 19* | 688 ± 87 | 647 ± 30 | 41 ± 512* |
| Ile | 32 ± 22 | 98 ± 13* | 73 ± 9 | 315 ± 50 | 233 ± 23 | 145 ± 11* |
| Leu | 32 ± 24 | 94 ± 18 | 67 ± 11 | 285 ± 40 | 195 ± 23 | 127 ± 7* |
| Phe | 31 ± 16 | 91 ± 10* | 69 ± 2 | 144 ± 12 | 104 ± 3* | 93 ± 5* |
| GABA | 29 ± 23 | 104 ± 13* | 136 ± 24* | 187 ± 23 | 169 ± 12 | 97 ± 5* |
| Total | 3,678 ± 2,268 | 12,667 ± 1,158 | 10,704 ± 851 | 24,527 ± 2,091 | 23,817 ± 983 | 17,185 ± 1,716 |
Pro is known to accumulate in considerable amounts in response to abiotic stress like drought, salt, or osmotic stress (Sharma et al., 2011; Kavi Kishor and Sreenivasulu, 2014). Under control conditions, the steady-state level of Pro was 5- to 6-fold higher in transgenic than in wild-type plants (Fig. 7D). However, Pro accumulated massively (∼620-fold increase) in the wild type in response to drought stress. In transgenic plants, Pro accumulation increased only by 20- to 50-fold compared with the control conditions and thereby remained 40% to 80% lower than in the wild type (Fig. 7D). Pro homeostasis depends on HvP5CS1 (Δ1-PYRROLINE-5-CARBOXYLATE SYNTHETASE1), which is involved in the biosynthesis of Pro from Glu (Verslues and Sharma, 2010). Examining its transcript levels showed that, following drought stress, HvP5CS1 mRNA levels increased strongly in all lines, but it remained at lower levels in the transgenic lines under both control and drought stress conditions (Fig. 7G).
DISCUSSION
CKX Transgene Expression Is a Valuable Approach for Genetic Engineering of the Root System
This work showed that it is possible to generate barley plants with an enhanced root system by ectopic expression of a single CKX gene without affecting shoot development. Apparently, the enlarged root system was formed without metabolic costs for the shoot, as plant height, heading time, shoot biomass, number of tillers and ears per plant, hundred-kernel weight, and total grain yield were similar in the transgenic lines and control lines. This indicates that these plants were not source limited and the carbon fixed by the shoot was sufficient to support stronger root growth. This is an important result strengthening the prospects of using root enhancement as a strategy to improve crop plant performance (de Dorlodot et al., 2007; Meister et al., 2014; Rogers and Benfey, 2015; Hochholdinger, 2016; Koevoets et al., 2016). It is in contrast to the long-held view that carbon fixation becomes a limiting factor when plants invest more carbon into root biomass. In such a case, enhanced root growth would establish a competing sink that supports root growth at the expense of shoot development. These results support the idea that the capacity for tissue formation and growth (sink strength) may be the limiting factor for growth, rather than the provision of carbohydrates produced by photosynthesis. Therefore, these data are consistent with the suggested shift of paradigm from a source- to a sink-oriented view when looking at endogenous determinants of plant growth (Körner, 2015). Following this view, the CK concentrations in roots of wild-type plants may be considered as higher than optimal for strong root growth. Lowering the concentrations of CKs by genetic engineering suppresses the negative impact of CKs on root development and, thus, enhances sink strength of the root. In Arabidopsis the combination of root-specific CKX gene expression with the expression of transgenes enhancing shoot growth led to an increase of overall plant growth (Vercruyssen et al., 2011). This suggests that transgenic approaches may be used in a combinatorial manner for the growth promotion of belowground and aboveground organs.
To genetically engineer plants with a root-specific enhancement of CK breakdown, the careful selection of a suitable promoter to regulate CKX gene expression is of great importance. We compared three root-specific promoters of rice to drive CKX gene expression and found the EPP promoter to be the most suitable one. However, the pPER:CKX and pRET:CKX fusion constructs also yielded transgenic plants with an enhanced root system and without provoking detrimental effects on shoot growth. Previous attempts to direct CKX gene expression to roots of monocot plants led to plants that formed smaller shoots with severely compromised fertility (Pospíšilová et al., 2016) or plants that were infertile (Mrízová et al., 2013), presumably because the chosen promoters lacked specificity. The root-specific expression of OsCKX4 in rice also caused the formation of a stronger root system, but shoot traits were not documented (Gao et al., 2014).
The ectopic expression of both prototypic CKX enzymes of Arabidopsis yielded qualitatively similar effects, but CKX2 had a stronger impact on root system size and element accumulation than CKX1. For example, the total root length of the plants expressing CKX2 was increased by 60% to 70%, whereas it was only 20% to 40% in the plants expressing CKX1. Such a difference between the two genes also has been noted in Arabidopsis and tobacco (Werner et al., 2010), suggesting that the affected subcellular CK pools have a distinct regulatory and apparently evolutionarily conserved impact on these traits. This indicates that, despite different subcellular distribution and CK metabolite profiles in Arabidopsis and barley (Jiskrová et al., 2016), there must be a common impact of CKX enzymes on the composition or pool sizes of bioactive CKs.
Root-Specific Expression of CKX Causes Enhanced Element Accumulation in Leaves and Seeds
The enhanced root growth in transgenic lines contributed to an improved uptake of several elements from the soil and their increased accumulation in shoot organs. In leaves of transgenic barley plants, the concentrations of several essential elements, in particular P, Mn, and Zn, were increased significantly by up to more than 100% in at least three of the four analyzed transgenic lines. The enhanced accumulation of these elements was confirmed in two different substrates and under different growth conditions (well watered versus drought stress), indicating that this was a stable trait. Among the two different CKX genes, CKX2 expression had a stronger impact on the accumulation of additional elements, including S. The increased uptake of these elements is beneficial, as they are limiting in numerous agricultural soils and their availability may limit low-input agriculture. Therefore, the development of crop genotypes with root traits increasing element acquisition should increase growth and yield on infertile soils (White et al., 2013). For example, it has been shown for transgenic tobacco plants with an enhanced root system that their accumulation of higher levels of Mg correlates with increased chlorophyll content and better growth on medium with low Mg content (Werner et al., 2010).
The increased element accumulation in transgenic barley is consistent with the view that, among the root traits, total root length is key in the acquisition of sparingly soluble elements (White and Broadley, 2009; White and Greenwood, 2013). Indeed, it has been shown in rice that lateral roots, which contribute the largest portion to total root length, contribute significantly to the acquisition of P, Mn, and Zn (Liu et al., 2013). However, not all elements respond in the same way to an enhanced root system. In fact, for most of them the accumulation in leaves was not altered, suggesting that, besides a larger soil volume exploited by the enhanced root system of CKX-overexpressing plants, additional factors must play a role. The involvement of CK in regulating the response to the availability of P, S, Fe, Na, and K is well known (Franco-Zorrilla et al., 2002, 2005; Maruyama-Nakashita et al., 2004), suggesting that, besides better exploitation of the soil space by the larger root system, an altered response to P and S availability may have contributed to their increased uptake. The increased expression of genes encoding transporters for phosphate, sulfate, Mn, and Zn, as found for CK-deficient roots of Arabidopsis (Werner et al., 2010; Brenner and Schmülling, 2012), could make a relevant contribution. The recent finding that CK regulates the formation of passage cells in the Casparian strip, which has an impact on the transfer of soil-derived elements to the vasculature (Andersen et al., 2018), suggests an additional possibility in which an altered root CK status may influence nutrient uptake. Altered root exudation of element-mobilizing compounds or altered interaction of CK-deficient roots with root microbiota (Cosme et al., 2016) also may play a role. However, to what extent these different factors might contribute to enhanced element acquisition is unclear at present. Notably, root-specific CKX gene expression in the dicots Arabidopsis and tobacco caused the accumulation of the same type of elements in leaves as in barley, which suggests that their acquisition depends on common mechanisms that appear to be evolutionarily stable. The reasons why such mechanisms linked to the hormone CK are under selection remain unknown.
An Increase in Seed Zn Content Contributes to Biofortification
A specific and important aspect of Zn accumulation is that this element not only strongly and consistently increased in leaves but also in seeds, where it can make a valuable contribution to human nutrition. This is relevant, as insufficient uptake of Zn by humans causes malnutrition and related health disorders in more than 2 billion people worldwide (WHO, 2016). Zn deficiency is particularly widespread in developing countries, where nutrition depends mainly on plant-based diets and where the lack of Zn contributes significantly to infant mortality (White and Broadley, 2011). Therefore, several breeding programs have been initiated to increase the Zn content in cereal grains (Palmgren et al., 2008; White and Broadley, 2011; Borrill et al., 2014).
Our results showed that root enhancement induced by CK deficiency increased the accumulation of Zn in seeds by 26% to 44%. This increase raised the concentration of Zn from approximately 31 mg kg−1 in the wild type to 40 to 45 mg kg−1 in seeds of both tested transgenic lines. This is even more than the target Zn concentration of 38 mg kg−1, as set by the HarvestPlus program for wheat (Triticum aestivum) grains (White and Broadley, 2011). For comparison, the enrichment of Zn in maize, rice, or wheat grains that was achieved by the application of Zn-enriched fertilizers to the soil was in the range of 23%, 7%, or 19%, and that achieved by foliar application was in the range of 30%, 25%, or 63%, respectively (Joy et al., 2015). Lonergan et al. (2009) identified in barley several quantitative trait loci that increased the grain Zn content by 53% to 75%. Considering the costs required for Zn fertilization, achieving Zn enrichment through root enhancement, as described here, presents a cost-effective and sustainable strategy contributing to genetic biofortification, which could be used in a complementary manner with other efforts (Borrill et al., 2014). In another transgenic approach, endosperm-specific expression of the Zn transporter gene METAL TOLERANCE PROTEIN1 doubled Zn accumulation in the endosperm of barley plants, which was most likely conferred by enhanced vacuolar Zn loading (Menguer et al., 2018). Future studies should reveal the molecular mechanism(s) underlying Zn biofortification in CKX transgenic plants and also study the tissue localization and chemical speciation of Zn, which affect Zn bioavailability for human consumption.
Transgenic Plants with Enhanced Root System Size Are Less Sensitive to Drought Stress
Several parameters indicate that the transgenic barley lines generated here were less sensitive to drought stress. The first response to drought is a reduction in stomatal conductance to decrease water loss by transpiration (Medrano et al., 2002). However, this response impedes plant growth, as it restricts CO2 entry and, consequently, lowers the photosynthetic rate. For optimal growth under drought, a compromise between carbon assimilation and water transpiration has to be reached. In this study, transgenic barley plants displayed a 1.5- to 2-fold higher stomatal conductance and, accordingly, an almost 2-fold higher carbon assimilation rate compared with wild-type plants under long-term drought conditions. Apparently, such an effective drought-avoidance mechanism may be at least partially attributed to the enhanced root system. Several root traits, including the biomass, length, density, and depths of roots, have been correlated previously with drought tolerance in different crop species and are proposed to contribute to plant performance under drought conditions (Meister et al., 2014; Kashiwagi et al., 2015; Gagné-Bourque et al., 2016). Model species with larger root systems are more tolerant to drought as well (Werner et al., 2010; Macková et al., 2013), which may be caused partially by the role of CK in regulating the response to drought (Nishiyama et al., 2011; Nguyen et al., 2016). Interestingly, a recent report shows that root growth is required to sense and inform about the availability of water and induce root branching in the search for water (Robbins and Dinneny, 2018). It could be that the continued growth of roots of CKX-modulated lines facilitates this process and improves access to water. Noteworthy, CK also can act directly in leaves to affect stomatal behavior. Thus, increasing CK production in leaves during periods of severe drought stress caused drought tolerance (Rivero et al., 2007).
Drought induces the accumulation of ABA, as this hormone has an important function in orchestrating the response to drought stress (Verslues, 2016). An increase in ABA can be regarded as an indicator of drought stress. In both the CKX-overexpressing lines and the wild type, the concentrations of ABA and its catabolites PA and DPA increased under experimental drought conditions. However, the concentrations reached in the transgenic lines were only about half of those in the wild type, indicating that the former experienced a weaker stress level. Such a lower ABA accumulation might be due at least partially to a lower basal expression level as well as to reduced responses to drought of the ABA biosynthesis gene HvNCED2 and the ABA catabolism gene HvABA-8′-OH (Fig. 7). It has been shown in different perennial grass species that a lower level of ABA accumulation correlates with drought tolerance (Wang et al., 2003; DaCosta and Huang, 2007). This could be due to the avoidance of detrimental effects of increased ABA levels on photosynthesis and growth (Sreenivasulu et al., 2012). In addition, altered CK levels in CKX-overexpressing lines also may interfere directly with ABA homeostasis. There is evidence from Arabidopsis for cross talk between CK and ABA signaling pathways on the level of hormone biosynthesis and transcriptional regulation (Nishiyama et al., 2011; O’Brien and Benková, 2013). However, the precise regulatory circuits relevant for CK-ABA cross talk in CKX-transgenic barley remain to be clarified.
CKX Expression in Roots Prepares Barley Plant Metabolism for Adverse Growth Conditions
Besides an altered ABA content, we observed several other metabolic changes in leaves of transgenic lines that could support an improved tolerance to drought. Drought stress led to an increase in overall amino acid content (Table I). Several of the amino acids (Pro, Asn, Ser, Thr, and GABA) are not only considered as metabolic markers for drought and similar stress conditions but have proven beneficial during stress acclimation or avoidance (Krasensky and Jonak, 2012). Under control conditions, transgenic barley plants displayed severalfold higher concentrations of these amino acids. Under drought conditions, their concentrations increased less strongly in CKX-transgenic barley, indicating, similar to the behavior of ABA, reduced drought sensitivity. Several studies indicated that increased basal levels of certain amino acids might contribute to reduced sensitivity to drought conditions. The analysis of drought-induced changes in the metabolome of two different barley cultivars revealed that the level of Pro was already high in the drought-tolerant cultivar in the absence of drought stress (Chmielewska et al., 2016). The analysis of a diverse population of rice cultivars identified a positive correlation of the levels of Asn, Ser, and Thr under control conditions with the performance of the respective cultivar under drought stress (Degenkolbe et al., 2013). In Arabidopsis, higher susceptibility to drought stress correlated with a low content of GABA, and increasing the endogenous GABA level rescued this defect (Mekonnen et al., 2016). It has been argued that increased basal levels of these amino acids represent a biochemical predisposition acting as an efficient drought tolerance mechanism (Chmielewska et al., 2016). As almost nothing is known about the links between CK and amino acid metabolism, future studies should aim at understanding the mechanisms underlying the observed metabolic changes in CKX-transgenic barley plants and reveal their functional relevance.
CONCLUSION
This study has demonstrated the feasibility of generating cereal plants with a larger root system by increasing CK breakdown in the root, which increases the accumulation of certain elements in leaves and seeds and decreases the sensitivity to drought stress. The ability to promote the expansion of the root system in a targeted fashion is an important technological advance. For example, it could be combined with other approaches, such as the expression of DEEPER ROOTING1, which impacts the angle of lateral roots and causes roots to grow deeper, improving access to water in deep soil layers (Uga et al., 2013). We anticipate that our approach described here can be used for other monocot species as well, including wheat and maize, although these have distinct types of roots and root architecture.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The spring barley (Hordeum vulgare) variety Golden Promise (Forster, 2001) was used for transformation. Transgenic plants were generated using Agrobacterium tumefaciens-mediated transformation of immature embryos (Jacobsen et al., 2006; Horvath et al., 2017). A. tumefaciens strain AGL1 containing the binary vector pIPKb001(Himmelbach et al., 2007) was used for all transformations. The determination of the number of T-DNA insertions and the identification of homozygous individuals in the T1 progeny (obtained by self-fertilization of regenerated T0 plants) were carried out by qPCR performed on genomic DNA by iDNA Genetics. Unless stated otherwise, plants were grown under controlled greenhouse conditions (20°C/16°C, 16-h/8-h light/dark cycle, 500 μmol m−2 s−1 by metal halide lamps [HQI] supplemented with tungsten bulbs).
RNA Isolation and RT-qPCR Analysis
Total RNA was extracted from tissues using TRIzol reagent (Invitrogen) following the manufacturer’s protocol. RNA was purified using the RNayes MinElute clean-up kit (Qiagen). The removal of genomic DNA was achieved using RQ1 RNase-Free DNase (Promega). Two micrograms of total RNA was used for cDNA synthesis using the RevertAid First Strand cDNA Synthesis Kit of Fermentas and oligo(dT) primers. To test cDNA yield, qPCR was performed using primers of HvUBC10 (UniGene Hv.27073). Supplemental Table S5 lists the primer sequences used in this study. The cDNA samples were used to determine gene expression levels by RT-qPCR according to Cortleven et al. (2014).
CKX Enzyme Activity Assay
The CKX enzyme activity assay was performed by using leaf and root material from 3-week-old soil-grown transgenic plants according to Galuszka et al. (2007). Briefly, samples were powdered in liquid nitrogen and protein was extracted by incubating them in 1.5-fold excess (v/w) of the extraction buffer (0.2 m Tris-HCl, pH 8, 0.3% Triton X-100 (v/v), and 1 mm phenylmethylsulfonyl fluoride). For the enzyme activity assay, 2,6-dichlorophenol indophenol was used as the electron acceptor and iP as substrate. Further quantification of protein content in samples was estimated by using the Bradford method (Bradford, 1976) with bovine serum albumin as the standard.
Phytohormone Measurements
The quantification of ABA and its degradation products was performed as described by Hosseini et al. (2016). Briefly, frozen leaf material was ground in liquid nitrogen before extraction in ice-cold MeOH:formic acid:water (v/v/v, 15:1:4). ABA, PA, and DPA were separated in an ultra-performance liquid chromatography system using a high-capacity column (Eclipse Plus C18) using a gradient consisting of 0.1% (v/v) formic acid and liquid chromatography-mass spectrometry-grade MeOH. Mass spectrometry was performed using a 6490 triple Quad tandem mass spectrometer (Agilent) using [2H6]ABA as an internal standard. For CK analysis 100 mg of freeze-dried root material was extracted using MeOH:water (1:1, v/v) and purified by solid-phase extraction. CKs were eluted with 1 mL of 0.35 m ammonia dissolved in 60% MeOH. Dried eluents were resolved in 50 to 100 µL of 25% MeOH and quantified by liquid chromatography-electrospray ionization-tandem mass spectrometry (UPLC-Xevo-TQ-MS; Waters). Mass spectrometry data were processed using TargetLynx version 4.1 SCN 904 with internal standard correction. Details of CK extraction, separation, and quantification, including external and internal standards, have been described (Eggert and von Wirén, 2017).
Quantification of Root System Size and Biomass
Barley seeds were germinated on moistened filter paper in the dark for 3 d and then transferred to light for another 2 d. Thereafter, the seedlings were transferred to a hydroponic system and cultivated for another 15 d. For the hydroponic system 0.1-strength Hoagland solution [1 mm KH2PO4, 0.5 mm KNO3, 0.4 mm Ca(NO3), 0.2 mm MgSO4, 0.1 mm FeNaEDTA, 0.01 mm H3BO3, 2 μm MnSO4, 0.2 μm ZnSO4, 0.2 μm CuSO4, 0.1 μm Na2MoO4, and 0.02 mm NaCl] was used (Krämer et al., 1996). The 12 L of nutrient solution per box was properly aerated and changed every 4 to 5 d. Then, roots and shoots were separated and their fresh weights were determined. Later, samples were dried in an oven at 80°C for 68 h, and the dry weight was recorded.
For the quantification of root system architecture of soil-grown transgenic plants, soil-filled rhizoboxes were used. Rhizoboxes (height 31 cm, width 24 cm, length 32 cm; Schütt Labortechnik) were assembled as described by Golldack et al. (2004) and Reibe et al. (2015). The rooting compartment was separated by two pieces of nylon gauze (1-μm mesh size; Heidland) between two plexiglass frames. In each rhizobox, three of these plexiglass frame-nylon gauze constructions were inserted (Supplemental Fig. S3D), and pure soil was filled into the rhizoboxes. Eight biological replicates for each genotype were used, resulting in eight rhizoboxes. In each rhizobox, three pregerminated (5-d-old) wild-type and transgenic barley seedlings were inserted between the nylon gauze and grown for 23 d (Supplemental Fig. S3D). After harvest, plants were separated into shoots and roots grown between the nylon gauze. Roots were carefully lifted from within the frames and the gauze and cautiously washed to remove bound soil particles. Before scanning, roots were spread out in a root-positioning tray (20 × 30 cm) to minimize overlap and scanned with a flatbed scanner (Epson EU-88). Grayscale images obtained in tiff format were analyzed with WinRHIZO (Pro version 2005a; Regent Instruments). Afterward, separated shoots and roots were dried at 80°C for 68 h, and the dry weight was recorded.
Quantification of Shoot and Seed Element Contents
Seeds of four independent transgenic lines and the wild type were germinated on filter paper in vitro. Three days after germination, seedlings were transferred to the greenhouse into an unfertilized (type 0) soil supplied by Einheitserde. The composition of unfertilized soil was tested and certified by Institut Koldingen as described by Drechsler et al. (2015). Plants were grown for 4 weeks by supplementing equal amounts of fertilizer solution every 2 or 3 d depending on soil moisture. The fertilizer solution was based on the composition of 0.5× Murashige and Skoog medium containing 10 mm KNO3, 10 mm NaH2PO4, 1 mm MgSO4, 1 mm CaCl2, 50 μm NaFeEDTA, 50 μm H3BO3, 50 μm MnSO4, 18.5 μm ZnSO4, 50 nm CuSO4, 50 nm CoCl2, 0.5 μm NaMoO4, and 2 mm MES. The solution was adjusted to pH 5.7 with 1 m KOH. Leaf samples from 2-month-old plants were dried for 72 h at 65°C, and equal amounts were weighed into polytetrafluoroethylene tubes and digested with an HNO3 + H2O2 mixture in a pressurized microwave digestion system (MARS from CEM). The concentrations of macroelements and microelements were analyzed by inductively coupled plasma optical emission spectrometry (iCAP 6500 dual OES spectrometer; Thermo Fisher Scientific) with certified standard reference samples as a control. The element contents in shoots of drought-stressed plants and from seed samples were determined as outlined above.
Growth Conditions for Drought Stress Treatment
Seeds of Golden Promise and the transgenic barley lines pEPP:CKX2-16 and pEPP:CKX2-72 were germinated separately in seed germination trays in a climate-controlled growth chamber for 2 weeks. Then, germinated seeds were transferred to the cold room for vernalization at 8°C for an additional 2 weeks. Thereafter, 4-week-old plants were sown in 5-L pots filled with 2 kg of peat-based growth substrate in five independent replications. The temperature in the greenhouse was approximately 16°C at night and 20°C during the day with a 16-h/8-h light/dark cycle.
The soil moisture content was monitored using the moisture meter HH2 coupled with the soil moisture sensor SM200 (Delta T Devices). Under the control condition, the moisture meter device showed 40% soil moisture level, corresponding to 100% field capacity. The 10% soil moisture corresponded to 20% to 25% field capacity (Lancashire et al., 1991), which causes severe drought stress in barley (Seiler et al., 2011; Hosseini et al., 2016). When the plants had formed between eight and nine tillers, watering was stopped for about 3 d to lower soil moisture to 10%. These drought stress conditions were maintained for 2 weeks with daily monitoring of soil moisture. Control plants were held continuously at 100% field capacity. The whole shoots were harvested after 2 weeks of drought stress for further physiological, biochemical, and molecular analyses.
Quantification of Photosynthesis-Related Parameters
Infrared gas analysis was carried out on individual fully emerged flag leaves of all three barley lines at 12 d after flowering using an LCpro+ device (ADC Bioscientific). A constant supply of 400 µL L−1 CO2 (flow rate, 200 μmol s−1) was provided by a CO2 cartridge at a photon flux density of 900 μmol m−2 s−1 by a mixed red/blue LED light source mounted above the leaf chamber head. The net assimilation rate, internal CO2 concentration, stomatal conductance, and transpiration rate were all recorded from five individual plants growing under either well-watered or drought stress conditions, with four technical replications per measurement. All the parameters were recorded in the morning hours starting from 10 am to 1 pm. The instrument was stabilized for 30 min in the greenhouse where measurements were taken. The measurements were only taken once after the internal CO2 concentration had stabilized (2–3 min after insertion of the leaf into the measuring chamber).
Quantification of Free Amino Acids
Free amino acids were extracted as described by Hosseini et al. (2016). Shoots of barley plants were ground in liquid nitrogen, and 50 mg of finely powdered fresh material were extracted using 1 mL of ice-cold MeOH and chloroform (v/v, 1:1). The fluorescing reagent 6-aminoquinolyl-N-hydroxysuccinimidylcarbamate (ACQ) was used for the derivatization and detection of amino acids. ACQ was dissolved in 3 mg mL−1 acetonitrile and incubated at 55°C for 10 min. Then, 20 mL of plant extract was derivatized in a cocktail containing 20 µL of ACQ and 160 µL of a 0.2 m boric acid buffer (pH 8.8) in a final volume of 200 µL. The solution was incubated at 55°C for 10 min. The separation of derivatized samples was carried out with a reverse-phase HPLC system (Waters) consisting of a gradient pump (Alliance 2795 HT; Waters), a degassing module, an autosampler, and a 2475 fluorescence detector (Waters). A reverse-phase column (XBridge; 150 mm, 5 µm; Waters) was used for the separation and detection of amino acids at an excitation wavelength of 300 nm and an emission wavelength of 400 nm. The gradient was accomplished with buffer A containing 140 mm sodium acetate, pH 5.8 (Suprapur; Merck), and 7 mm triethanolamine (Sigma). Acetonitrile (Roti C Solv HPLC; Roth) and purest HPLC water (Geyer) were used as eluents B and C. Chromatograms were recorded using the software program Empower Pro (Waters).
Accession Numbers
Accession numbers are as follows: AtCKX1, AT2G41520; AtCKX2, AT2G19500; EPP, LOC_Os04g11040; RET, LOC_Os10g31730; PER, LOC_Os03g25330; HvNCED2, AB239298.1; HvABA-8′-OH, DQ145932; and HvP5CS1, AK251855.1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Identification and validation of root-specific promoters of rice.
Supplemental Figure S2. Validation of root-specific promoters of rice in transgenic Arabidopsis plants.
Supplemental Figure S3. Root size of barley plants expressing pEPP:CKX.
Supplemental Figure S4. Shoot and root size of barley plants expressing pRET:CKX or pPER:CKX.
Supplemental Table S1. Concentrations of CK metabolites in roots and shoots of 3-week-old barley plants.
Supplemental Table S2. Element concentrations in shoots of transgenic barley plants.
Supplemental Table S3. Element concentrations in shoots of transgenic barley plants grown under well-watered and drought stress conditions.
Supplemental Table S4. Element concentrations in seeds of transgenic barley plants.
Supplemental Table S5. Sequences of primers used in the study.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Magdalena Schubert and Hans-Henning Steinbiß for excellent technical support in the production of transgenic plants and Jochen Kumlehn for vector pIPKb001.
Footnotes
This work was supported by the German Federal Ministry for Education and Research (BMBF) to T.S. and H.G. in the frame of the PLANT-KBBE program and the project "ROOT - Root enhancement for crop improvement".
Articles can be viewed without a subscription.
References
- Andersen TG, Naseer S, Ursache R, Wybouw B, Smet W, De Rybel B, Vermeer JEM, Geldner N (2018) Diffusible repression of cytokinin signalling produces endodermal symmetry and passage cells. Nature 555: 529–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JG, Alves SC, Smedley M, Snape JW, Harwood WA (2008) High-throughput Agrobacterium-mediated barley transformation. Plant Methods 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielach A, Podlesáková K, Marhavý P, Duclercq J, Cuesta C, Müller B, Grunewald W, Tarkowski P, Benková E (2012) Spatiotemporal regulation of lateral root organogenesis in Arabidopsis by cytokinin. Plant Cell 24: 3967–3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrill P, Connorton JM, Balk J, Miller AJ, Sanders D, Uauy C (2014) Biofortification of wheat grain with iron and zinc: integrating novel genomic resources and knowledge from model crops. Front Plant Sci 5: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brenner WG, Schmülling T (2012) Transcript profiling of cytokinin action in Arabidopsis roots and shoots discovers largely similar but also organ-specific responses. BMC Plant Biol 12: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ramireddy E, Schmülling T (2013) Lateral root formation and growth of Arabidopsis is redundantly regulated by cytokinin metabolism and signalling genes. J Exp Bot 64: 5021–5032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ramireddy E, Schmülling T (2015) Cytokinin as a positional cue regulating lateral root spacing in Arabidopsis. J Exp Bot 66: 4759–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewska K, Rodziewicz P, Swarcewicz B, Sawikowska A, Krajewski P, Marczak Ł, Ciesiołka D, Kuczyńska A, Mikołajczak K, Ogrodowicz P, et al. (2016) Analysis of drought-induced proteomic and metabolomic changes in barley (Hordeum vulgare L.) leaves and roots unravels some aspects of biochemical mechanisms involved in drought tolerance. Front Plant Sci 7: 1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ZX, Ma Q, Lin YX, Tang XL, Zhou YQ, Zhu SW, Fan J, Cheng BJ (2011) Genome-wide identification, classification, and analysis of two-component signal system genes in maize. Genet Mol Res 10: 3316–3330 [DOI] [PubMed] [Google Scholar]
- Comas LH, Becker SR, Cruz VM, Byrne PF, Dierig DA (2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortleven A, Nitschke S, Klaumünzer M, Abdelgawad H, Asard H, Grimm B, Riefler M, Schmülling T (2014) A novel protective function for cytokinin in the light stress response is mediated by the Arabidopsis histidine kinase2 and Arabidopsis histidine kinase3 receptors. Plant Physiol 164: 1470–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosme M, Ramireddy E, Franken P, Schmülling T, Wurst S (2016) Shoot- and root-borne cytokinin influences arbuscular mycorrhizal symbiosis. Mycorrhiza 26: 709–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaCosta M, Huang B (2007) Drought survival and recuperative ability of bentgrass species associated with changes in abscisic acid and cytokinin production. J Am Soc Hortic Sci 132: 60–66 [Google Scholar]
- de Dorlodot S, Forster B, Pagès L, Price A, Tuberosa R, Draye X (2007) Root system architecture: opportunities and constraints for genetic improvement of crops. Trends Plant Sci 12: 474–481 [DOI] [PubMed] [Google Scholar]
- Degenkolbe T, Do PT, Kopka J, Zuther E, Hincha DK, Köhl KI (2013) Identification of drought tolerance markers in a diverse population of rice cultivars by expression and metabolite profiling. PLoS ONE 8: e63637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler N, Zheng Y, Bohner A, Nobmann B, von Wirén N, Kunze R, Rausch C (2015) Nitrate-dependent control of shoot K homeostasis by the nitrate transporter1/peptide transporter family member NPF7.3/NRT1.5 and the stelar K+ outward rectifier SKOR in Arabidopsis. Plant Physiol 169: 2832–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert K, von Wirén N (2017) Response of the plant hormone network to boron deficiency. New Phytol 216: 868–881 [DOI] [PubMed] [Google Scholar]
- Forster BP. (2001) Mutation genetics of salt tolerance in barley: an assessment of Golden Promise and other semi-dwarf mutants. Euphytica 120: 317–328 [Google Scholar]
- Franco-Zorrilla JM, Martin AC, Solano R, Rubio V, Leyva A, Paz-Ares J (2002) Mutations at CRE1 impair cytokinin-induced repression of phosphate starvation responses in Arabidopsis. Plant J 32: 353–360 [DOI] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Martín AC, Leyva A, Paz-Ares J (2005) Interaction between phosphate-starvation, sugar, and cytokinin signaling in Arabidopsis and the roles of cytokinin receptors CRE1/AHK4 and AHK3. Plant Physiol 138: 847–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagné-Bourque F, Bertrand A, Claessens A, Aliferis KA, Jabaji S (2016) Alleviation of drought stress and metabolic changes in timothy (Phleum pratense L.) colonized with Bacillus subtilis B26. Front Plant Sci 7: 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galuszka P, Popelková H, Werner T, Frébortová J, Pospíšilová H, Mik V, Köllmer I, Schmülling T, Frébort I (2007) Biochemical characterization of cytokinin oxidases/dehydrogenases from Arabidopsis thaliana expressed in Nicotiana tabacum L. J Plant Growth Regul 26: 255–267 [Google Scholar]
- Gao S, Fang J, Xu F, Wang W, Sun X, Chu J, Cai B, Feng Y, Chu C (2014) CYTOKININ OXIDASE/DEHYDROGENASE4 integrates cytokinin and auxin signaling to control rice crown root formation. Plant Physiol 165: 1035–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golldack J, Augustin C, Lentzsch P, Werner A (2004) Pathozones of genetic subtypes of Gaeumannomyces graminis in cereals. Soil Biol Biochem 36: 145–154 [Google Scholar]
- Heyl A, Ramireddy E, Brenner WG, Riefler M, Allemeersch J, Schmülling T (2008) The transcriptional repressor ARR1-SRDX suppresses pleiotropic cytokinin activities in Arabidopsis. Plant Physiol 147: 1380–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelbach A, Zierold U, Hensel G, Riechen J, Douchkov D, Schweizer P, Kumlehn J (2007) A set of modular binary vectors for transformation of cereals. Plant Physiol 145: 1192–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F. (2016) Untapping root system architecture for crop improvement. J Exp Bot 67: 4431–4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath M, Steinbiss HH, Reiss B (2017) Gene targeting without DSB induction is inefficient in barley. Front Plant Sci 7: 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SA, Hajirezaei MR, Seiler C, Sreenivasulu N, von Wirén N (2016) A potential role of flag leaf potassium in conferring tolerance to drought-induced leaf senescence in barley. Front Plant Sci 7: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P (2008) Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufnagel B, de Sousa SM, Assis L, Guimaraes CT, Leiser W, Azevedo GC, Negri B, Larson BG, Shaff JE, Pastina MM, et al. (2014) Duplicate and conquer: multiple homologs of PHOSPHORUS-STARVATION TOLERANCE1 enhance phosphorus acquisition and sorghum performance on low-phosphorus soils. Plant Physiol 166: 659–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J, Venables I, Wang MB, Matthews P, Ayliffe M, Gubler F (2006) Barley (Hordeum vulgare L.). Methods Mol Biol 343: 171–183 [DOI] [PubMed] [Google Scholar]
- Jiskrová E, Novák O, Pospíšilová H, Holubová K, Karády M, Galuszka P, Robert S, Frébort I (2016) Extra- and intracellular distribution of cytokinins in the leaves of monocots and dicots. N Biotechnol 33: 735–742 [DOI] [PubMed] [Google Scholar]
- Joy EJM, Stein AJ, Young SD, Ander EL, Watts MJ, Broadley MR (2015) Zinc-enriched fertilisers as a potential public health intervention in Africa. Plant Soil 389: 1–24 [Google Scholar]
- Kashiwagi J, Krishnamurthy L, Purushothaman R, Upadhyaya HD, Gaur PM, Gowda CLL, Ito O, Varshney RK (2015) Scope for improvement of yield under drought through the root traits in chickpea (Cicer arietinum L.). Field Crops Res 170: 47–54 [Google Scholar]
- Kavi Kishor PB, Sreenivasulu N (2014) Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ 37: 300–311 [DOI] [PubMed] [Google Scholar]
- Kell DB. (2011) Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration. Ann Bot 108: 407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitomi Y, Ito H, Hobo T, Aya K, Kitano H, Inukai Y (2011) The auxin responsive AP2/ERF transcription factor CROWN ROOTLESS5 is involved in crown root initiation in rice through the induction of OsRR1, a type-A response regulator of cytokinin signaling. Plant J 67: 472–484 [DOI] [PubMed] [Google Scholar]
- Koevoets IT, Venema JH, Elzenga JTM, Testerink C (2016) Roots withstanding their environment: exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front Plant Sci 7: 1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. (2015) Paradigm shift in plant growth control. Curr Opin Plant Biol 25: 107–114 [DOI] [PubMed] [Google Scholar]
- Krämer U, Cotter-Howells JD, Charnock JM, Baker AJM, Smith JAC (1996) Free histidine as a metal chelator in plants that accumulate nickel. Nature 379: 635–638 [Google Scholar]
- Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63: 1593–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Makita N, Kojima M, Tokunaga H, Sakakibara H (2012) Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-zeatin-O-glucosyltransferase in rice. Plant Physiol 160: 319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancashire P, Bleiholder H, Boom TPL, Strauss R, Weber E, Witzenberger A (1991) A uniform decimal code for growth stages of crops and weeds. Ann Appl Biol 119: 561–601 [Google Scholar]
- Laplaze L, Benkova E, Casimiro I, Maes L, Vanneste S, Swarup R, Weijers D, Calvo V, Parizot B, Herrera-Rodriguez MB, et al. (2007) Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 19: 3889–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt J. (1980) Responses of Plants to Environmental Stresses, Ed 2 Academic Press, New York [Google Scholar]
- Liu Y, Donner E, Lombi E, Li RY, Wu ZC, Zhao FJ, Wu P (2013) Assessing the contributions of lateral roots to element uptake in rice using an auxin-related lateral root mutant. Plant Soil 372: 125–136 [Google Scholar]
- Lomin SN, Yonekura-Sakakibara K, Romanov GA, Sakakibara H (2011) Ligand-binding properties and subcellular localization of maize cytokinin receptors. J Exp Bot 62: 5149–5159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan PF, Pallotta MA, Lorimer M, Paull JG, Barker SJ, Graham RD (2009) Multiple genetic loci for zinc uptake and distribution in barley (Hordeum vulgare). New Phytol 184: 168–179 [DOI] [PubMed] [Google Scholar]
- Lynch J. (1995) Root architecture and plant productivity. Plant Physiol 109: 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Brown KM (2012) New roots for agriculture: exploiting the root phenome. Philos Trans R Soc Lond B Biol Sci 367: 1598–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macková H, Hronková M, Dobrá J, Turečková V, Novák O, Lubovská Z, Motyka V, Haisel D, Hájek T, Prášil IT, et al. (2013) Enhanced drought and heat stress tolerance of tobacco plants with ectopically enhanced cytokinin oxidase/dehydrogenase gene expression. J Exp Bot 64: 2805–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. (2002) Mineral Nutrition of Higher Plants. Academic Press, London [Google Scholar]
- Maruyama-Nakashita A, Nakamura Y, Yamaya T, Takahashi H (2004) A novel regulatory pathway of sulfate uptake in Arabidopsis roots: implication of CRE1/WOL/AHK4-mediated cytokinin-dependent regulation. Plant J 38: 779–789 [DOI] [PubMed] [Google Scholar]
- Mason MG, Mathews DE, Argyros DA, Maxwell BB, Kieber JJ, Alonso JM, Ecker JR, Schaller GE (2005) Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell 17: 3007–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Jha D, Salt DE, Tester M, Hill K, Kieber JJ, Schaller GE (2010) Type-B response regulators ARR1 and ARR12 regulate expression of AtHKT1;1 and accumulation of sodium in Arabidopsis shoots. Plant J 64: 753–763 [DOI] [PubMed] [Google Scholar]
- Medrano H, Escalona JM, Bota J, Gulías J, Flexas J (2002) Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Ann Bot 89: 895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister R, Rajani MS, Ruzicka D, Schachtman DP (2014) Challenges of modifying root traits in crops for agriculture. Trends Plant Sci 19: 779–788 [DOI] [PubMed] [Google Scholar]
- Mekonnen DW, Flügge UI, Ludewig F (2016) Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci 245: 25–34 [DOI] [PubMed] [Google Scholar]
- Menguer PK, Vincent T, Miller AJ, Brown JKM, Vincze E, Borg S, Holm PB, Sanders D, Podar D (2018) Improving zinc accumulation in cereal endosperm using HvMTP1, a transition metal transporter. Plant Biotechnol J 16: 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T (2006) Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc Natl Acad Sci USA 103: 16598–16603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan TC, Castrillo G, Navarro C, Zarco-Fernández S, Ramireddy E, Mateo C, Zamarreño AM, Paz-Ares J, Muñoz R, García-Mina JM, et al. (2016) Cytokinin determines thiol-mediated arsenic tolerance and accumulation. Plant Physiol 171: 1418–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrízová K, Jiskrová E, Vyroubalová Š, Novák O, Ohnoutková L, Pospíšilová H, Frébort I, Harwood WA, Galuszka P (2013) Overexpression of cytokinin dehydrogenase genes in barley (Hordeum vulgare cv. Golden Promise) fundamentally affects morphology and fertility. PLoS ONE 8: e79029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam YJ, Tran LS, Kojima M, Sakakibara H, Nishiyama R, Shin R (2012) Regulatory roles of cytokinins and cytokinin signaling in response to potassium deficiency in Arabidopsis. PLoS ONE 7: e47797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KH, Ha CV, Nishiyama R, Watanabe Y, Leyva-González MA, Fujita Y, Tran UT, Li W, Tanaka M, Seki M, et al. (2016) Arabidopsis type B cytokinin response regulators ARR1, ARR10, and ARR12 negatively regulate plant responses to drought. Proc Natl Acad Sci USA 113: 3090–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann MCE, Weber H, Hluska T, Leonte G, Anderson SM, Novák O, Senes A, Werner T (2018) The cytokinin oxidase/dehydrogenase CKX1 is a membrane-bound protein requiring homooligomerization in the endoplasmic reticulum for its cellular activity. Plant Physiol 176: 2024–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama R, Watanabe Y, Fujita Y, Kojima M, Werner T, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T, Sakakibara H, Schmülling T, et al. (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23: 2169–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JA, Benková E (2013) Cytokinin cross-talking during biotic and abiotic stress responses. Front Plant Sci 4: 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG, Clemens S, Williams LE, Krämer U, Borg S, Schjørring JK, Sanders D (2008) Zinc biofortification of cereals: problems and solutions. Trends Plant Sci 13: 464–473 [DOI] [PubMed] [Google Scholar]
- Pospíšilová H, Jiskrová E, Vojta P, Mrízová K, Kokáš F, Čudejková MM, Bergougnoux V, Plíhal O, Klimešová J, Novák O, et al. (2016) Transgenic barley overexpressing a cytokinin dehydrogenase gene shows greater tolerance to drought stress. N Biotechnol 33: 692–705 [DOI] [PubMed] [Google Scholar]
- Price AH, Tomos AD (1997) Genetic dissection of root growth in rice (Oryza sativa L.). II. Mapping quantitative trait loci using molecular markers. Theor Appl Genet 95: 143–152 [Google Scholar]
- Price AH, Cairns JE, Horton P, Jones HG, Griffiths H (2002) Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. J Exp Bot 53: 989–1004 [DOI] [PubMed] [Google Scholar]
- Ramireddy E, Chang L, Schmülling T (2014) Cytokinin as a mediator for regulating root system architecture in response to environmental cues. Plant Signal Behav 9: e27771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani Debi B, Taketa S, Ichii M (2005) Cytokinin inhibits lateral root initiation but stimulates lateral root elongation in rice (Oryza sativa). J Plant Physiol 162: 507–515 [DOI] [PubMed] [Google Scholar]
- Reibe K, Götz KP, Döring TF, Roß CL, Ellmer F (2015) Impact of hydro-/biochars on root morphology of spring wheat. Arch Agron Soil Sci 61: 8 [Google Scholar]
- Riefler M, Novak O, Strnad M, Schmülling T (2006) Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18: 40–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E (2007) Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci USA 104: 19631–19636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins NE II, Dinneny JR (2018) Growth is required for perception of water availability to pattern root branches in plants. Proc Natl Acad Sci USA 115: E822–E831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ED, Benfey PN (2015) Regulation of plant root system architecture: implications for crop advancement. Curr Opin Biotechnol 32: 93–98 [DOI] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al. (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378 [DOI] [PubMed] [Google Scholar]
- Sakakibara H. (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57: 431–449 [DOI] [PubMed] [Google Scholar]
- Sato Y, Takehisa H, Kamatsuki K, Minami H, Namiki N, Ikawa H, Ohyanagi H, Sugimoto K, Antonio BA, Nagamura Y (2013) RiceXPro version 3.0: expanding the informatics resource for rice transcriptome. Nucleic Acids Res 41: D1206–D1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmülling T, Werner T, Riefler M, Krupková E, Bartrina y Manns I (2003) Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J Plant Res 116: 241–252 [DOI] [PubMed] [Google Scholar]
- Séguéla M, Briat JF, Vert G, Curie C (2008) Cytokinins negatively regulate the root iron uptake machinery in Arabidopsis through a growth-dependent pathway. Plant J 55: 289–300 [DOI] [PubMed] [Google Scholar]
- Seiler C, Harshavardhan VT, Rajesh K, Reddy PS, Strickert M, Rolletschek H, Scholz U, Wobus U, Sreenivasulu N (2011) ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions. J Exp Bot 62: 2615–2632 [DOI] [PubMed] [Google Scholar]
- Seki M, Umezawa T, Urano K, Shinozaki K (2007) Regulatory metabolic networks in drought stress responses. Curr Opin Plant Biol 10: 296–302 [DOI] [PubMed] [Google Scholar]
- Sharma S, Villamor JG, Verslues PE (2011) Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol 157: 292–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58: 221–227 [DOI] [PubMed] [Google Scholar]
- Sreenivasulu N, Harshavardhan VT, Govind G, Seiler C, Kohli A (2012) Contrapuntal role of ABA: does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 506: 265–273 [DOI] [PubMed] [Google Scholar]
- Todaka D, Zhao Y, Yoshida T, Kudo M, Kidokoro S, Mizoi J, Kodaira KS, Takebayashi Y, Kojima M, Sakakibara H, et al. (2017) Temporal and spatial changes in gene expression, metabolite accumulation and phytohormone content in rice seedlings grown under drought stress conditions. Plant J 90: 61–78 [DOI] [PubMed] [Google Scholar]
- Tsai YC, Weir NR, Hill K, Zhang W, Kim HJ, Shiu SH, Schaller GE, Kieber JJ (2012) Characterization of genes involved in cytokinin signaling and metabolism from rice. Plant Physiol 158: 1666–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuberosa R, Sanguineti MC, Landi P, Giuliani MM, Salvi S, Conti S (2002) Identification of QTLs for root characteristics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes. Plant Mol Biol 48: 697–712 [DOI] [PubMed] [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, et al. (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45: 1097–1102 [DOI] [PubMed] [Google Scholar]
- Vercruyssen L, Gonzalez N, Werner T, Schmülling T, Inzé D (2011) Combining enhanced root and shoot growth reveals cross talk between pathways that control plant organ size in Arabidopsis. Plant Physiol 155: 1339–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE. (2016) ABA and cytokinins: challenge and opportunity for plant stress research. Plant Mol Biol 91: 629–640 [DOI] [PubMed] [Google Scholar]
- Verslues PE, Sharma S (2010) Proline metabolism and its implications for plant-environment interaction. The Arabidopsis Book 8: e0140, doi/10.1199/tab.0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Huang B, Xu Q (2003) Effects of abscisic acid on drought response of Kentucky bluegrass. J Am Soc Hortic Sci 128: 36–41 [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T (2001) Regulation of plant growth by cytokinin. Proc Natl Acad Sci USA 98: 10487–10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15: 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Nehnevajova E, Köllmer I, Novák O, Strnad M, Krämer U, Schmülling T (2010) Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 22: 3905–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets: iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol 182: 49–84 [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR (2011) Physiological limits to zinc biofortification of edible crops. Front Plant Sci 2: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Greenwood DJ (2013) Properties and management of cationic elements for crop growth. In Gregory PJ, Nortcliff S, eds, Soil Conditions and Plant Growth. Blackwell Publishing, Oxford, pp 160–194 [Google Scholar]
- White PJ, George TS, Gregory PJ, Bengough AG, Hallett PD, McKenzie BM (2013) Matching roots to their environment. Ann Bot 112: 207–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2016) Vitamin and Mineral Nutrition Information System. World Health Organization. www.who.int
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Cheng S, Song Y, Huang Y, Zhou S, Liu X, Zhou DX (2015) The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling. Plant Cell 27: 2469–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürcher E, Liu J, di Donato M, Geisler M, Müller B (2016) Plant development regulated by cytokinin sinks. Science 353: 1027–1030 [DOI] [PubMed] [Google Scholar]