Abstract
Empathy and vicarious learning of fear are increasingly understood as separate phenomena, but the interaction between the two remains poorly understood. We investigated how social (vicarious) fear learning is affected by empathic appraisals by asking participants to either enhance or decrease their empathic responses to another individual (the demonstrator), who received electric shocks paired with a predictive conditioned stimulus. A third group of participants received no appraisal instructions and responded naturally to the demonstrator. During a later test, participants who had enhanced their empathy evinced the strongest vicarious fear learning as measured by skin conductance responses to the conditioned stimulus in the absence of the demonstrator. Moreover, this effect was augmented in observers high in trait empathy. Our results suggest that a demonstrator’s expression can serve as a “social” unconditioned stimulus (US), similar to a personally experienced US in Pavlovian fear conditioning, and that learning from a social US depends on both empathic appraisals and the observers’ stable traits.
Keywords: observational aversive learning, social conditioning, skin conductance, emotions, expressions, open data
In social species, including Homo sapiens, expressions of fear and distress are extremely salient cues that have rapid and strong impact on observers (Adolphs, 2013). This profound impact stems from the important survival value of learning about potential threats in the environment and quickly understanding the intentions, thoughts, and feelings of other individuals. Remarkably, no studies of humans have examined how learning about threats by observing others is modulated by empathy, understanding and sharing their emotional experiences. This is especially surprising in light of the fact that the processes underlying social learning and empathy are likely to be intimately interconnected in real life, given that from early development (Klinnert, Campos, & Source, 1983) throughout adulthood (Blair, 2003; Olsson & Ochsner, 2008), people habitually interpret the meaning of others’ emotional expressions to better understand and learn about their environment. Indeed, research on social fear learning in humans and other species has shown that the mere sight of a conspecific’s (demonstrator’s) distress can serve as a potent proxy for the individual’s own, direct, experience, thereby offering a more safe and efficient route to learning as compared with individual trial and error (Askew & Field, 2008; Bandura, 1965; Goubert, Vlaeyen, Crombez, & Craig, 2011; Olsson & Phelps, 2007; Rachman, 1979).
How, then, does empathy affect social fear learning? Previous research provides support for two alternative predictions. According to the first, learning to fear a stimulus by observing a demonstrator’s fear reactions should be insensitive to cognitive manipulations, just as direct (Pavlovian) fear conditioning is. Backing for this prediction comes from findings, across species, of strong and instant observational fear learning supported by a neural system, including the amygdala, that is known to be involved in Pavlovian conditioning (Debiec & Sullivan, 2014; Hooker, Germine, Knight, & D’Esposito, 2006; Knapska et al., 2006; LeDoux, 2015; Meffert, Brislin, White, & Blair, 2015; Olsson, Nearing, & Phelps, 2007). Additional support comes from research showing that vicariously transmitted fear is similar to Pavlovian conditioning in that it is expressed in the absence of awareness of the conditioned stimulus (Olsson & Phelps, 2004).
The second prediction suggests that attributions of mental states to the demonstrator should affect the strength of learning by means of modifying the value of the demonstrator’s emotional expressions. This prediction receives tentative support from earlier studies showing that knowledge about the demonstrator’s aversive experiences interacts with perceptual information to instigate an aversive response in the observer (Berger, 1962; Hygge & Öhman, 1978). Yet attributions of mental states to the demonstrator have never been experimentally manipulated to directly examine the causal impact of empathy on learning. Research outside the learning field has provided additional support for this second prediction by establishing close links between emotions experienced by the self and others. For example, empathic responses are supported, in part, by the same neural mechanisms as self-experienced distress and pain (Lamm, Decety, & Singer, 2011; Singer, Kiebel, Winston, Dolan, & Frith, 2004) and attributions of mental states to the other (Shamay-Tsoory, Aharon-Peretz, & Perry, 2009; Wagner, Kelley, & Heatherton, 2011; Zaki & Ochsner, 2012).
Note that empathic responses depend predictably on attributions of mental states and traits to the target persons. For example, observers who believe that social targets are competitive or untrustworthy exhibit inhibited spontaneous empathic responses (Cikara, Bruneau, & Saxe, 2011; Lanzetta & Englis, 1989; Singer et al., 2006), whereas those who perceive targets as cooperators (Lanzetta & Englis, 1989) or attend to targets’ painful experiences (Lamm, Batson, & Decety, 2007) exhibit enhanced empathic responses. In fact, many perspective-taking manipulations strongly alter individuals’ empathic experience and social behavior across a variety of contexts (Batson et al., 2003; Galinsky & Moskowitz, 2000). These studies, taken together with an imaging study (Olsson et al., 2007) showing that activity in empathy-related brain regions during observation of a demonstrator’s pain predicted the strength of learning demonstrated on a subsequent test, support the conjecture that empathic appraisals directly affect vicarious fear learning. Empathy might have an impact on learning because the demonstrator’s expression of fear and pain serves as a “social” unconditioned stimulus, or US, similar to a personally experienced US in Pavlovian conditioning. Accordingly, the quality of the social US might determine the outcome of vicarious learning much as the quality of a directly experienced, tactile US, such as a mild electric shock, determines the outcome of Pavlovian conditioning. On the basis of this associative model of social fear learning, we predicted that deliberate attempts to enhance and decrease empathic appraisals of a demonstrator’s emotional responses should facilitate and inhibit, respectively, vicarious fear learning.
In addition, individuals vary strongly in their abilities to decode and resonate with others’ emotions. Indeed, past research has documented considerable interindividual difference in empathic ability as measured by self-reports (Davis, 1983; Mehrabian, 1996), physiological concurrence over time (Levenson & Ruef, 1992), accurate understanding of others’ emotions (Mayer, Salovey, & Caruso, 2008; Zaki, Bolger, & Ochsner, 2008), and activity in brain regions implicated in empathic processes, such as the medial prefrontal, insular, and temporal cortices (e.g., Singer et al., 2004; Zaki & Ochsner, 2012). Finally, individual differences and contextual factors often interact to produce empathy (Keysers & Gazzola, 2014; Zaki, 2014). For instance, individuals high and low in empathy may differ from each other with respect to prosociality or the accuracy of their social inferences, but only when targets’ group membership or expressivity provides an opportunity for individual differences to manifest themselves (Stürmer, Snyder, Kropp, & Siem, 2006; Zaki et al., 2008). We therefore expected that individual differences in traitlike empathic ability might affect learning, possibly by interacting with state-level manipulations to enhance the effect of empathic appraisals.
Another potentially important aspect of vicarious fear learning is the relevance of the demonstrator’s distress to the observer’s own current or future situation. For example, learning to fear a dog by observing another individual’s reactions to it might be more efficient if you expect to encounter the dog yourself in the near future. An alternative prediction relies on the assumption that the disadvantage of not encoding life-dependent information, such as what causes pain to others, outweighs the extra effort of learning “false positives.” If such a better-safe-than-sorry learning strategy is at play, there would be no, or little, difference in learning as a function of whether or not the learner expects to be in the same situation as the demonstrator.
The Present Study
The primary goal of the current study was to examine the role of empathy in social fear learning. To this end, we combined nomothetic and idiographic approaches (Cronbach, 1957; Kosslyn et al., 2002), measuring both the general effect of manipulating empathy appraisals and the impact of individual variability in trait empathy. The effect of empathy appraisals was examined by manipulating the instructions given to participants before they underwent a vicarious fear-learning procedure; some received standard instructions to either enhance or decrease empathy (high- and low-empathy groups, respectively), and others received no empathy-related instructions (no-instruction group). Individual variability was assessed using the Balanced Emotional Empathy Scale (BEES; Mehrabian, 1996). The secondary goal of this study was to examine the role of self-relevance of the demonstrator’s distress. To this end, each participant completed two observational-learning procedures that manipulated whether or not the participant expected to undergo the same learning procedure as the demonstrator at a later time.
Method
Participants
Participants were recruited in the Columbia University community through flyers and recruitment notices and were compensated with $15 or 2 course credits. Members of this community were eligible if they were over the age of 18 and had no previous experience with experiments involving shocks. One hundred twenty-nine participants completed the study. A target of 40 participants per group was established before data collection began, to be in keeping with previous studies of vicarious fear learning. Nineteen participants (12 men) who reported after the experiment that they did not believe they would receive shocks, despite the instructions they were given, were excluded from the analyses. In addition, 9 participants (4 men) who showed no measurable skin conductance responses (SCRs) and 1 male outlier in the low-empathy group (i.e., SCR more than 2.5 SD from the mean of that group) were excluded from the analyses. Thus, the final sample included 47 men and 53 women, ages 18 through 35 years (high-empathy group: n = 35; no-instruction group: n = 31; low-empathy group: n = 34).
Stimuli
The basic experiment involved an E-Prime program (Version 2.0; Psychology Software Tools, Inc., Pittsburgh, PA) that displayed colored squares one at a time against a black background on the computer screen. Two sets of colors were used: red/green and yellow/blue. The two sets were assigned to the two observational-learning conditions (high and low self-relevance) in a counterbalanced fashion. In the red/green set, the red square served as the conditioned stimulus (CS+) and was associated with a shock to the demonstrator; the green square served as the control stimulus (CS–) and was never associated with a shock. In the blue/yellow set, the yellow and blue squares served as the CS+ and CS–, respectively.
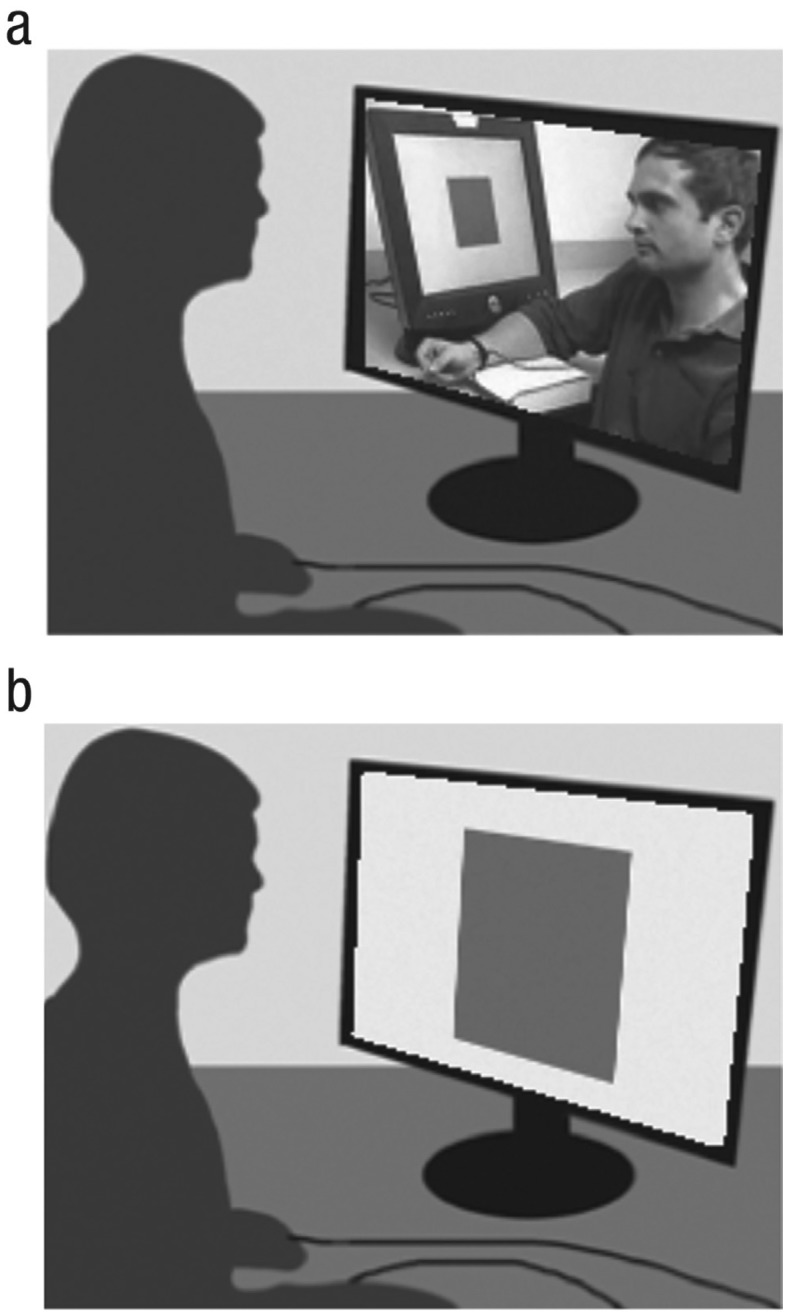
For the observation stage (see Design and Procedure), we recorded movies of demonstrators participating in a differential Pavlovian fear-conditioning experiment using the stimuli just described (see Fig. 1a). Previous research with monkeys (Cook & Mineka, 1990) and humans (Olsson & Phelps, 2004) has shown that watching a movie of a demonstrator reacting to a CS+ can be as effective in transmitting fear as watching the event live. Four movies were made so that each of the two color sets was matched with a male and a female demonstrator. The movies showed a demonstrator seated in front of a PC monitor with shock electrodes attached to his or her wrist. The CS+ and CS– were each presented to the demonstrator five times for 10 s in a pseudorandomized order and interleaved with an intertrial interval that ranged from 10 to 14 s (M = 12 s). In each movie, three of the CS+ presentations coterminated with a shock administered to the demonstrator’s wrist, and none of the CS– presentations were paired with a shock. The demonstrator briefly expressed discomfort by frowning and jerking the arm when receiving a shock.
Fig. 1.
The vicarious fear-learning paradigm. Each self-relevance condition consisted of two consecutive stages: In the observation stage (a), the participants watched a movie of another person (the demonstrator) occasionally receiving shocks paired with one of two colored squares (the conditioned stimulus, or CS+), but never with the other colored square (the control stimulus, or CS–). During the test stage (b), participants were presented with the same colored squares displayed on the screen. Although they expected to receive shocks during this stage, they never did; this ensured that learning of the contingency between the CS+ and shock remained indirect (social) in nature.
For the test stage of the study (see Design and Procedure), participants were presented with the same CS+ and CS– that they had just watched being presented to the demonstrator in the movie (see Fig. 1b). To ensure that the only source of contingency learning was indirect, or social, we did not deliver any electric shocks to the participants during this stage. The conditioned fear response was assessed with the SCR, measured through disposable Ag-AgCl electrodes attached to the distal phalanges of the second and third digits of the left hand. The SCR signal was amplified and recorded with a BIOPAC 150 System SRC module connected to a PC and continuously recorded at a rate of 200 samples per second. Off-line analysis of the analogue SCR waveforms was conducted with AcqKnowledge software (BIOPAC Systems, Inc., Goleta, CA).
Design and procedure
Each participant was assigned to one of the three empathic-appraisal groups: high-empathy, low-empathy, or no-instruction. All participants were submitted to the two self-relevance conditions that manipulated whether the participants believed that they themselves would (high self-relevance) or would not (low self-relevance) be participating in the same experiment as the demonstrator at a later time.
After participants signed the informed-consent document, the SCR and shock electrodes were attached, and participants were informed that they would receive shocks that were uncomfortable, but not painful. All participants were told that the experiment would begin with an “observation stage,” in which they would watch a movie of another person receiving shocks associated with one of two colors, followed by a “test stage.” Participants were told that they would go through the observation and test stages twice over the course of the study.
Before each movie, participants were given information related to both empathic appraisals and the particular self-relevance of the video that they were about to watch (for the verbatim instructions, see the Supplemental Material available online). Participants in the high-empathy group were informed that the person in the movie rated the shocks as painful, and they were asked to pay attention to his or her discomfort. Participants in the low-empathy group were told that the person in the movie acted as if the—hardly noticeable—shocks were painful. This group was asked to pay attention to the actor’s expressions, but only as cues to understand the relationship between the colors on the screen and the shocks. Participants in the no-instruction group were given no information about the demonstrator’s experiences and no instructions regarding empathic appraisals.
The self-relevance instructions were given to each participant directly after the empathic-appraisal instructions. In the high-self-relevance condition, participants were told that the experiment they were about to watch was the same as the one they were to participate in themselves after the movie. In the low-self-relevance condition, participants were instead told that they would later be asked to compare the content of the movie with another movie. Each participant completed the procedure (observation stage followed by test stage) for the high-self-relevance condition and the low-self-relevance condition; the order of the conditions was counterbalanced across participants.
Regardless of the self-relevance condition, after the observation stage, and just before the test stage began, all participants were told that they were about to participate in the same experiment as the person whom they had just watched in the movie. In the following test stage, the CS+ and the CS– were each presented five times using the same parameters as in the movie. As already noted, participants did not receive shocks during either test stage. After the first test stage, participants were told they had not received shocks because they had been allotted to a control experiment with no shocks for the first part of the study. These instructions were designed to maintain the anticipation of receiving the shocks during the second test stage. After the two self-relevance conditions were completed, the electrodes were removed, and participants completed self-report questionnaires.
SCR parameters and data analysis
Following standard procedure (Lykken & Venables, 1971; Olsson et al., 2007), we measured SCR for each trial (i.e., stimulus presentation) as the largest base-to-peak amplitude difference in skin conductance (in microsiemens). For the response to the CS+ and CS– during test, we used the interval from 0.5 to 4.5 s after the onset of the stimulus, and for the response to the social US during the observation stage, we used the interval from 0.5 to 4.5 s after onset of the shock to the demonstrator and the corresponding interval during CS– presentations to the demonstrator (i.e., when no shock was presented). The minimal response criterion was 0.02 µS. Responses that did not pass this criterion were scored as 0. The SCR data were low-pass-filtered and smoothed and square-root-transformed to normalize the distributions. To minimize variance of no psychological interest, such as that due to individual differences in sweat-gland properties, we divided each SCR response by the individual participant’s maximal response, separately for the test and observation stages (Lykken & Venables, 1971).
Data were analyzed separately for the observation and the test stages and for the empathic-appraisal and self-relevance conditions. The main measures of interest were the conditioned fear response (SCR to the CS+ minus SCR to the CS– during test; see Table S1 in the Supplemental Material for mean SCRs to the CS+ and CS- during the test stage) and the unconditioned response (SCR watching shock minus SCR watching no shock to the demonstrator). The first CS+ and CS– trials were excluded from the calculation of the mean conditioned response because the SCR on the first trials following a contextual shift (from the observation to the test stage) was expected to be larger than the SCR on subsequent trials, and of little psychological interest in the current learning paradigm.
Self-report measures
Participants completed the BEES (Mehrabian, 1996), which is designed to measure emotional empathy, by rating the extent to which they agreed with 15 positively worded items and 15 negatively worded items (i.e., statements that a consistent responder would agree with and disagree with, respectively; e.g., “Unhappy movie endings haunt me for hours afterward” and “I cannot feel much sorrow for those who are responsible for their own misery”), using a 9-point scale from −4 (very strong disagreement) to 4 (very strong agreement). Responses had high internal consistency (α = .87). In addition, participants completed a demographics questionnaire and were asked if they had believed that they would receive shocks throughout the experiment.
Results
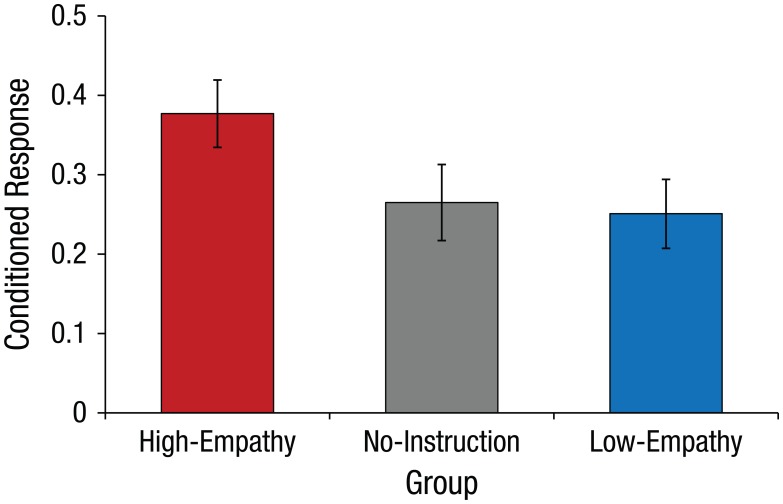
Because there was no effect of self-relevance condition, F(1, 94) = 0.3 (see Fig. S1 in the Supplemental Material), we collapsed the data across the self-relevance conditions and focused subsequent analyses on the empathy manipulation. Next, we conducted a univariate analysis of variance with the conditioned fear response during test as the dependent variable and empathic-appraisal group and gender as between-subjects factors. The analysis revealed a main effect of the empathic-appraisal manipulation, F(2, 94) = 3.2, p = .042, ηp2 = .063, which was explained by larger conditioned responses in the high-empathy group compared with the two other groups, t(97) = 2.44, p = .02, d = 0.50; this result indicated stronger learning following empathic appraisals (see Fig. 2). Moreover, a linear relationship was found among the empathic-appraisal groups; the conditioned response was largest in the high-empathy group and lowest in the low-empathy group, with the no-instruction group in between, t(97) = 2.40, p = .02, d = 0.50.
Fig. 2.
Conditioned response (expression of learned fear) during the test stage as a function of empathic-appraisal group, collapsed across the self-relevance conditions. Error bars represent ±1 SEM.
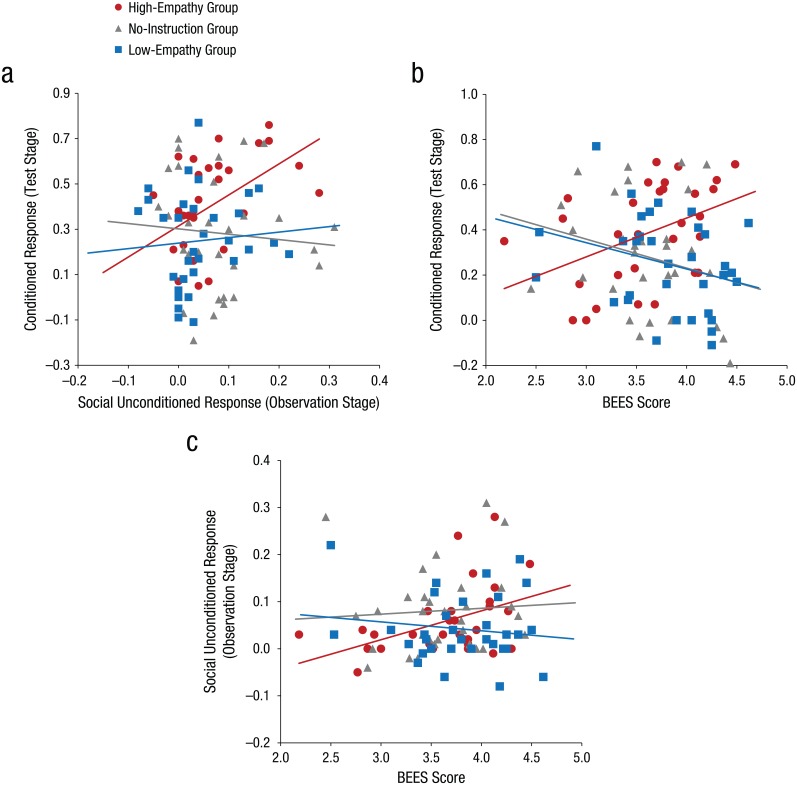
Next, we explored whether (a) the unconditioned response to the social US was predictive of learning (i.e., the conditioned response during later test) in the high-empathy group and (b) individual differences in empathy were related to individual differences in responses during the observation and test stages. Table 1 provides a summary of the relationships among social unconditioned response, conditioned response, and BEES score, separately for the three empathic-appraisal groups (see Fig. 3 for scatterplots). Although there were no reliable correlations between social unconditioned and conditioned responses in the no-instruction and low-empathy groups, social unconditioned and conditioned responses correlated positively in the high-empathy group (rs = .51, p < .05). This was consistent with our expectation that induced empathic appraisals would facilitate vicarious fear learning. Furthermore, trait empathy, as measured with the BEES, modulated the unconditioned response (rs = .41, p < .05) and the conditioned response (rs = .45, p < .05) to a similar extent in the high-empathy group. Again, in the other two groups, the correlations were not significant (ps > .1). After we partialed out BEES scores, the relationship between social unconditioned response and conditioned response was no longer reliable in the high-empathy group (rs = .18, p = .33). Taken together, our results indicate that individuals with high trait empathy flexibly modulate their learning on the basis of the level of empathy they experience in a situation, suggesting that empathic appraisals and trait empathy interact to facilitate vicarious fear learning.
Table 1.
Spearman Correlation Coefficients for the Relationships Among Balanced Emotional Empathy Scale (BEES) Score, Social Unconditioned Response, and Conditioned Response in the Three Empathic-Appraisal Groups
| Variable | Unconditioned response | Conditioned response |
|---|---|---|
| High-empathy group | ||
| BEES score | .41* | .45* |
| Unconditioned response | — | .51* |
|
| ||
| No-instruction group | ||
| BEES score | .15 | −.24 |
| Unconditioned response | — | −.17 |
|
| ||
| Low-empathy group | ||
| BEES score | −.02 | −.26 |
| Unconditioned response | — | .19 |
p < .05.
Fig. 3.
Scatterplots illustrating the correlations between (a) the social unconditioned response to the demonstrator’s expressions of distress and the conditioned response during the later test stage, (b) the conditioned response during test and Balanced Emotional Empathy Scale (BEES) score, and (c) the social unconditioned response and BEES score. The lines represent the best-fitting regression lines. Results are shown separately for the three empathic-appraisal groups. The corresponding Spearman rank correlation coefficients are shown in Table 1.
Discussion
Other peoples’ expressions of fear and distress provide information about their internal states, as well as potential dangers in the environment. However, it has been unclear how individuals’ interpretations of others’ emotional states interact with the process of learning from their emotional expressions, and if that interaction varies among people with differing empathic abilities. Here, we have shown that both empathic appraisals and trait empathy significantly affect vicarious fear learning. When participants observed another individual’s aversive experiences, those who had been instructed to actively appraise the thoughts and feelings of that distressed individual more effectively learned to fear a neutral stimulus than did those who had not been instructed to do so. This effect was mainly due to participants with high trait empathy; the explicit manipulation of empathic appraisals had a greater benefit for fear learning among participants with higher trait empathy.
In keeping with previous research, our results show that social observation, like standard Pavlovian conditioning, can potently facilitate fear responses to previously neutral stimuli (Goubert et al., 2011; Hooker et al., 2006; Kavaliers & Choleris, 2011; Knapska et al., 2006; LeDoux, 2015; Meffert et al., 2015; Olsson et al., 2007). In contrast to Pavlovian conditioning, however, social learning also involves processing a wide range of social information that can affect the quality and strength of learning. Previous studies have demonstrated that contextual appraisals of the meaning of other people’s expressions of distress and pain can profoundly affect the ensuing behavioral and neural responses in the observer. For example, believing that a suffering person is a competitor (Lanzetta & Englis, 1989) or cheater (Singer et al., 2006) downgrades empathic responses in the observer. In fact, such negative appraisals often invert the expression of empathy into schadenfreude (Cikara et al., 2011; Lanzetta & Englis, 1989). In contrast, when the suffering person belongs to the observer’s social group (Hein, Silani, Preuschoff, Batson, & Singer, 2010) or is perceived as a future collaborator (Lanzetta & Englis, 1989), or when the observer focuses attention onto the target’s painful experiences (Batson et al., 2003; Lamm et al., 2007), empathic responses and subsequent helping behavior are enhanced (Zaki, 2014). To the best of our knowledge, our study is the first to demonstrate that empathic appraisals of another individual’s feelings enhance vicarious fear learning, even when the demonstrator is no longer present.
These results support our working model of the mechanisms underlying associative learning, suggesting that the emotional expression of the demonstrator acts as a social US that affects the quality and strength of the ensuing fear memories, much as a directly experienced US does. This conclusion dovetails with a previous study showing that greater activity in empathy-related brain regions (e.g., the anterior insula and cingulate cortices) in response to the demonstrator’s expression of distress is associated with stronger learning expressed later during a test in the absence of the demonstrator (Olsson et al., 2007).
It is worth noting that our high-empathy condition included a greater attentional focus on the demonstrator’s internal states, compared with the other two empathic-appraisal conditions. Although this difference is inherent to what we intended to investigate, it limits our conclusions about the effect being exclusively due to empathy. This concern, however, is mitigated by our finding that empathic appraisals had the greatest influence in people with high trait empathy, which strengthened the claim that the appraisal effect is indeed related to emotional sharing.
Our manipulation of self-relevance did not affect vicarious learning. Although we are reluctant to speculate about the reasons for a null effect, the lack of a difference between the self-relevance conditions suggests that the underlying associative-learning mechanisms operate, at least partly, independently of expectations about the relevance of the observed situation for the future self. This would be consistent with the hypothesis that the fear-learning system automatically registers contingencies predictive of potential threat.
In sum, we have shown that empathic appraisals can enhance the strength of vicarious fear learning as expressed through autonomic responses (SCR) to the CS+ at a later time in the absence of the demonstrator. We have also shown that during empathic appraisals, the relationship between the SCR elicited by watching the demonstrator receiving shocks and later expressed learning is strengthened in participants high in trait empathy. Our results support an associative model of vicarious fear learning in which a demonstrator’s emotional expression serves as a social US analogous to a personally experienced US in Pavlovian conditioning, and that the quality of vicarious fear learning depends on appraisals of the social US.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Funding: This research was funded by National Institute of Health Grant MH076137 to K. N. Ochsner and by an Independent Starting Grant (ELSI; 284366) from the European Research Council to A. Olsson.
Supplemental Material: Additional supporting information can be found at http://pss.sagepub.com/content/by/supplemental-data
Open Practices:

All data have been made publicly available via Open Science Framework and can be accessed at https://osf.io/bqzpt/. The complete Open Practices Disclosure for this article can be found at http://pss.sagepub.com/content/by/supplemental-data. This article has received the badge for Open Data. More information about the Open Practices badges can be found at https://osf.io/tvyxz/wiki/1.%20View%20the%20Badges/ and http://pss.sagepub.com/content/25/1/3.full.
References
- Adolphs R. (2013). The biology of fear. Current Biology, 23, R79–R93. doi: 10.1016/j.cub.2012.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew C., Field A. P. (2008). The vicarious learning pathway to fear 40 years on. Clinical Psychology Review, 28, 1249–1265. doi: 10.1016/j.cpr.2008.05.003 [DOI] [PubMed] [Google Scholar]
- Bandura A. (1965). Vicarious processes: A case of no-trial learning. In Berkowitz L. (Ed.), Advances in experimental social psychology (Vol. 2, pp. 1–55). New York, NY: Academic Press. [Google Scholar]
- Batson C. D., Lishner D. A., Carpenter A., Dulin L., Harjusola-Webb S., Stocks E. L., . . . Sampat B. (2003). “. . . As you would have them do unto you”: Does imagining yourself in the other’s place stimulate moral action? Personality and Social Psychology Bulletin, 29, 1190–1201. doi: 10.1177/0146167203254600 [DOI] [PubMed] [Google Scholar]
- Berger S. M. (1962). Conditioning through vicarious instigation. Psychological Review, 69, 450–466. doi: 10.1037/h0046466 [DOI] [PubMed] [Google Scholar]
- Blair R. J. R. (2003). Facial expressions, their communicatory functions and neuro–cognitive substrates. Philosophical Transactions of the Royal Society B: Biological Sciences, 358, 561–572. doi: 10.1098/rstb.2002.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikara M., Bruneau E., Saxe R. (2011). Us and them: Intergroup failures of empathy. Current Directions in Psychological Science, 20, 149–153. doi: 10.1177/0963721411408713 [DOI] [Google Scholar]
- Cook M., Mineka S. (1990). Selective associations in the observational conditioning of fear in rhesus monkeys. Journal of Experimental Psychology: Animal Behavior Process, 16, 372–389. doi: 10.1037/0097-7403.16.4.372 [DOI] [PubMed] [Google Scholar]
- Cronbach L. J. (1957). The two disciplines of scientific psychology. American Psychologist, 12, 671–684. doi: 10.1037/h0043943 [DOI] [Google Scholar]
- Davis M. H. (1983). Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology, 44, 113–126. doi: 10.1037/0022-3514.44.1.113 [DOI] [Google Scholar]
- Debiec J., Sullivan R. M. (2014). Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proceedings of the National Academy of Sciences, USA, 111, 12222–12227. doi: 10.1073/pnas.1316740111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinsky A. D., Moskowitz G. B. (2000). Perspective-taking: Decreasing stereotype expression, stereotype accessibility, and in-group favoritism. Journal of Personality and Social Psychology, 78, 708–724. doi: 10.1037/0022-3514.78.4.708 [DOI] [PubMed] [Google Scholar]
- Goubert L., Vlaeyen J. W., Crombez G., Craig K. D. (2011). Learning about pain from others: An observational learning account. The Journal of Pain, 12, 167–174. doi: 10.1016/j.jpain.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Hein G., Silani G., Preuschoff K., Batson C. D., Singer T. (2010). Neural responses to ingroup and outgroup members’ suffering predict individual differences in costly helping. Neuron, 68, 149–160. doi: 10.1016/j.neuron.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Hooker C. I., Germine L. T., Knight R. T., D’Esposito M. (2006). Amygdala response to facial expressions reflects emotional learning. The Journal of Neuroscience, 26, 8915–8922. doi: 10.1523/JNEUROSCI.3048-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hygge S., Öhman A. (1978). Modeling processes in the acquisition of fears: Vicarious electrodermal conditioning to fear-relevant stimuli. Journal of Personality and Social Psychology, 36, 271–279. doi: 10.1037/0022-3514.36.3.271 [DOI] [PubMed] [Google Scholar]
- Kavaliers M., Choleris E. (2011). Sociality, pathogen avoidance, and the neuropeptides oxytocin and arginine vasopressin. Psychological Science, 22, 1367–1374. doi: 10.1177/0956797611420576 [DOI] [PubMed] [Google Scholar]
- Keysers C., Gazzola V. (2014). Dissociating the ability and propensity for empathy. Trends in Cognitive Sciences, 18, 163–166. doi: 10.1016/j.tics.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinnert M. D., Campos J. J., Source J. (1983). Emotions as behavior regulators: Social referencing in infancy. In Plutchik R., Kellerman H. (Eds.), Emotions in early development (pp. 57–86). New York, NY: Academic Press. [Google Scholar]
- Knapska E., Nikolaev E., Boguszewski P., Walasek G., Blaszczyk J., Kaczmarek L., Werka T. (2006). Between-subject transfer of emotional information evokes specific pattern of amygdala activation. Proceedings of the National Academy of Sciences, USA, 103, 3858–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn S. M., Cacioppo J. T., Davidson R. J., Hugdahl K., Lovallo W. R., Spiegel D., Rose R. (2002). Bridging psychology and biology: The analysis of individuals in groups. American Psychologist, 57, 341–351. doi: 10.1037/0003-066X.57.5.341 [DOI] [PubMed] [Google Scholar]
- Lamm C., Batson C. D., Decety J. (2007). The neural substrate of human empathy: Effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience, 19, 42–58. doi: 10.1162/jocn.2007.19.1.42 [DOI] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54, 2492–2502. doi: 10.1016/j.neuroimage.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Lanzetta J. T., Englis B. G. (1989). Expectations of cooperation and competition and their effects on observers’ vicarious emotional responses. Journal of Personality and Social Psychology, 56, 543–554. doi: 10.1037/0022-3514.56.4.543 [DOI] [Google Scholar]
- LeDoux J. (2015). Anxious: Using the brain to understand and treat fear and anxiety. New York, NY: Penguin Random House. [Google Scholar]
- Levenson R. W., Ruef A. M. (1992). Empathy: A physiological substrate. Journal of Personality and Social Psychology, 63, 234–246. doi: 10.1037/0022-3514.63.2.234 [DOI] [PubMed] [Google Scholar]
- Lykken D. T., Venables P. H. (1971). Direct measurement of skin conductance: A proposal for standardization. Psychophysiology, 8, 656–672. doi: 10.1111/j.1469-8986.1971.tb00501.x [DOI] [PubMed] [Google Scholar]
- Mayer J. D., Salovey P., Caruso D. R. (2008). Emotional intelligence: New ability or eclectic traits? American Psychologist, 63, 503–517. doi: 10.1037/0003-066X.63.6.503 [DOI] [PubMed] [Google Scholar]
- Meffert H., Brislin S. J., White S. F., Blair J. R. (2015). Prediction errors to emotional expressions: The roles of the amygdala in social referencing. Social Cognitive & Affective Neuroscience, 10, 537–544. doi: 10.1093/scan/nsu085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabian A. (1996). Manual for the Balanced Emotional Empathy Scale (BEES). (Available from Albert Mehrabian, 1130 Alta Mesa Rd., Monterey, CA 93940)
- Olsson A., Nearing K. I., Phelps E. A. (2007). Learning fears by observing others: The neural systems of social fear transmission. Social Cognitive and Affective Neuroscience, 2, 3–11. doi: 10.1093/scan/nsm005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A., Ochsner K. N. (2008). The role of social cognition in emotion. Trends in Cognitive Sciences, 12, 65–71. doi: 10.1016/j.tics.2007.11.010 [DOI] [PubMed] [Google Scholar]
- Olsson A., Phelps E. A. (2004). Learned fear of “unseen” faces after Pavlovian, observational, and instructed fear. Psychological Science, 15, 822–828. doi: 10.1111/j.0956-7976.2004.00762.x [DOI] [PubMed] [Google Scholar]
- Olsson A., Phelps E. A. (2007). Social learning of fear. Nature Neuroscience, 10, 1095–1102. doi: 10.1038/nn1968 [DOI] [PubMed] [Google Scholar]
- Rachman S. (1979). The return of fear. Behaviour Research and Therapy, 17, 164–166. doi: 10.1016/0005-7967(79)90028-7 [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S. G., Aharon-Peretz J., Perry D. (2009). Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132, 617–627. doi: 10.1093/brain/awn279 [DOI] [PubMed] [Google Scholar]
- Singer T., Kiebel S. J., Winston J. S., Dolan R. J., Frith C. D. (2004). Brain responses to the acquired moral status of faces. Neuron, 41, 653–662. doi: 10.1016/S0896-6273(04)00014-5 [DOI] [PubMed] [Google Scholar]
- Singer T., Seymour B., O’Doherty J. P., Stephan K. E., Dolan R. J., Frith C. D. (2006). Empathic neural responses are modulated by the perceived fairness of others. Nature, 439, 466–469. doi: 10.1038/nature04271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürmer S., Snyder M., Kropp A., Siem B. (2006). Empathy-motivated helping: The moderating role of group membership. Personality and Social Psychology Bulletin, 32, 943–956. doi: 10.1177/0146167206287363 [DOI] [PubMed] [Google Scholar]
- Wagner D. D., Kelley W. M., Heatherton T. F. (2011). Individual differences in the spontaneous recruitment of brain regions supporting mental state understanding when viewing natural social scenes. Cerebral Cortex, 21, 2788–2796. doi: 10.1093/cercor/bhr074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J. (2014). Empathy: A motivated account. Psychological Bulletin, 140, 1608–1647. doi: 10.1037/a0037679 [DOI] [PubMed] [Google Scholar]
- Zaki J., Bolger N., Ochsner K. (2008). It takes two: The interpersonal nature of empathic accuracy. Psychological Science, 19, 399–404. doi: 10.1111/j.1467-9280.2008.02099.x [DOI] [PubMed] [Google Scholar]
- Zaki J., Ochsner K. N. (2012). The neuroscience of empathy: Progress, pitfalls and promise. Nature Neuroscience, 15, 675–680. doi: 10.1038/nn.3085 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.