Abstract
Imaging mass spectrometry (IMS) has recently established itself in the field of “spatial metabolomics.” Merging the sensitivity and fast screening of high-throughput mass spectrometry with spatial and temporal chemical information, IMS visualizes the production, location, and distribution of metabolites in intact biological models. Since metabolite profiling and morphological features are combined in single images, IMS offers an unmatched chemical detail on complex biological and microbiological systems. Thus, IMS-type “spatial metabolomics” emerges as a powerful and complementary approach to genomics, transcriptomics, and classical metabolomics studies. In this review, we summarize the current state-of-the-art IMS methods with a strong focus on desorption electrospray ionization (DESI)-IMS. DESI-IMS utilizes the original principle of electrospray ionization, but in this case solvent droplets are rastered and desorbed directly on the sample surface. The rapid and minimally destructive DESI-IMS chemical screening is achieved at ambient conditions and enables the accurate view of molecules in tissues at the µm-scale resolution. DESI-IMS analysis does not require complex sample preparation and allows repeated measurements on samples from different biological sources, including microorganisms, plants, and animals. Thanks to its easy workflow and versatility, DESI-IMS has successfully been applied to many different research fields, such as clinical analysis, cancer research, environmental sciences, microbiology, chemical ecology, and drug discovery. Herein we discuss the present applications of DESI-IMS in natural product research.
Key words: DESI-IMS, imaging mass spectrometry, chemical imaging, metabolomics, natural products
Abbreviations
- BOT
borderline ovarian tumors
- CMC
carboxymethyl cellulose
- DESI
desorption electrospray ionization
- DMBA
7,12-Dimethylbenz[a]anthracene
- ESI
electrospray ionization
- HGSC
high-grade serous carcinoma
- IMS
imaging mass spectrometry
- LAESI
laser ablation electrospray ionization
- MALDI
matrix assisted laser desorption/ionization
- m/z
mass-to-charge ratio
- nano-DESI
nano desorption electrospray ionization
- PTFE
polytetrafluoroethylen
- SIMS
secondary ion mass spectrometry
- VLCFA
very long chain fatty acid
Introduction
During the last 50 years, natural product research has benefited from the rapid advancement of MS (mass spectrometry) instrumentation, which enabled highly sensitive analysis of biological samples 1 . New MS ionization sources such as ESI 2 and MALDI 3 have revolutionized analytical chemistry, leading their inventors to receive the Nobel Prize in Chemistry in 2002. These “soft” ionization techniques enable the direct observation of natural compounds as intact ions and forever transformed MS from a niche tool of analytical chemistry to a ubiquitous technique utilized in life sciences research. Coupled to GC (gas chromatography) and LC (liquid chromatography), hyphenated MS instruments are now established worldwide as indispensable tools for high-throughput workflows. Being the most sensitive and the most powerful method, LC-MS nowadays represents the backbone of targeted and untargeted metabolomics approaches to detect and elucidate extremely low-abundance metabolites occurring in any type of biomass used in natural product research 4 . However, MS does not provide any information concerning the spatial and temporal distribution of metabolites in a biological sample. IMS complements traditional metabolomics and chemical analyses by combining the qualitative and quantitative molecular information with spatiotemporal information, providing the capability to map specific molecules to 2D or 3D coordinates of the original sample 5 .
IMS started developing more than two decades ago as the high-resolution frontier of material science and biology 3 , 6 , 7 , and later gained large popularity in peptide and protein analyses, particularly in medicine and cancer diagnostics 8 , 9 , 10 , 11 . As of today, IMS has established itself as a highly efficient and reliable tool in capturing the image of both large and small molecules in various life science disciplines. IMS techniques combine the specificity, selectivity and sensitivity of MS with spatially resolved chemical information 3 . The ionization source or sampling probe is rastered across the sample surface to induce desorption of organic compounds into the gas phase, where they get ionized and differentiated through their m/z 12 . This process is repeated sequentially over the whole sample until the mass spectra associated with every position on the sample are acquired. The ions of interest are then displayed in different colors and superimposed on the sampleʼs picture, correlating with the natural distribution and the abundance of each m/z ion throughout the sample. The higher the abundance of the molecular ion at a certain raster position, the greater the intensity of color that is displayed 13 . IMS does not require traditional sample extraction, which not only saves time and a tedious procedure but also eliminates problems (e.g., the production of artifacts). Moreover, depending on the type of IMS application, analysis of volatile and nonvolatile compounds may be achieved at the same time 14 . An IMS dataset contains a picture for each detected analyte 15 . IMS provides an untargeted in situ analysis of molecular species from complex samples to single cells, and from biological macromolecules to small metabolites, where metabolic signatures can be correlated with the histological and morphological features 16 . It provides scientists “molecular eyes” or “molecular microscopes” for analyzing the surface, tissue, and even cellular and subcellular metabolomes 5 as well as studying the dynamics and biological functions of metabolites of interests in a given organism. By providing a simplified analytical workflow and a chemical specificity at the morphological level that was never possible before, IMS is now emerging as a revolutionary field at the interface between chemistry and (micro)biology. Not surprisingly, the IMS approach has been employed in over 100 microbiological studies since its first use in this field in the early 2000s 12 . Currently, it is being successfully applied to several areas including biomarker identification, tissue specific biosynthetic processes, microbe-microbe and host-microbe interactions, functional ecology, drug discovery, and chemical ecology.
Early IMS techniques, such as SIMS and MALDI, require the sample to be ionized at high vacuum. SIMS was first reported by Castaing and Soldzian in 1962 17 and is the oldest IMS technique. Under high vacuum, a beam of high energy primary ions is focused onto the sample, physically perturbing the surface by a process known as “sputtering” 18 , generating secondary ions, which are analyzed by the mass spectrometer 19 . One type of SIMS experiment called dynamic SIMS uses a continuous beam of primary ions to ablate the surface of the sample, allowing 3D as well as 2D imaging experiments 20 , 21 . The primary advantage of SIMS is spatial resolution, as the ion beam can be focused down to 50 nm, enabling imaging on a subcellular level 22 . SIMS-IMS has been applied to the subcellular localization of biomarkers and metallo-drugs in cancer cells 23 , 24 , as well as antibiotics in individual bacteria 25 . SIMS imaging has also been used to study nitrogen fixation in microbial communities 26 and marine invertebrates 27 , as well as carbon flux in terrestrial worms 28 . As a result of the high-energy beam of ions, SIMS typically exhibits hard ionization, producing many fragments instead of molecular ions, potentially leading to the loss of information.
The well-established technique of MALDI-MS has a characteristically soft ionization, which produces a high ratio of molecular-to-fragment ions. MALDI imaging was first reported by Caprioli et al. 8 , who mapped certain peptides and proteins in various coated tissue sections or blotted imprint of the sections 8 . In a typical MALDI-IMS experiment, the sample is coated or co-crystalized with a light-absorbent matrix and irradiated with a pulse from a UV or IR laser. The matrix absorbs the radiation, transferring energy to the sample and aiding in ionization 29 . MALDI has several disadvantages, including high chemical noise in the low mass range (< 300 m/z ) originating from the matrix components that may suppress crucial small molecule ions and the requirement for the sample to be mounted on a conductive surface. The laser desorption/ionization process also destroys the sample during MALDI-IMS. However, in comparison to several other IMS techniques discussed below, MALDI-IMS has superior spatial resolution (from > 5 µm up to > 100 µm) 12 . Other advantages of MALDI-IMS include the wide mass range (from 300 m/z up to > 5000 m/z ) with which it can operate, good tolerance of variations in sample geometry, and a wide variety of established protocols for imaging, especially of microbial colonies 30 . With selection of a proper matrix, many types and sizes of compounds, ranging from proteins to lipids, peptides or secondary metabolites, can be visualized 12 . MALDI-IMS is the most established and most frequently used IMS technique in natural product research. Mainly led by the Dorrestein group at UC San Diego (USA), MALDI-IMS has been used in many excellent studies, for example, in identification of the biosynthetic origin of natural products from a bacterium-sponge symbiont 31 , mechanisms of coral associated bacterium to protect its host from pathogenic fungi 32 , and in examination of complex molecular interplays including suppression, enhanced production, biotransformation, and other metabolic exchanges in polymicrobial co-cultures 33 . MALDI imaging has been widely applied to natural product research, examining the spatial distribution of compounds in plants 34 , 35 , 36 , 37 , 38 , 39 , bacteria 40 , 41 , cyanobacteria 31 , 42 , and various marine invertebrates 31 , 42 , 43 , 44 , 45 , 46 .
In contrast to SIMS and MALDI, which operate under vacuum, recent IMS applications are embracing techniques that can be performed at ambient conditions with as little sample preparation as possible, such as LAESI-IMS and DESI-IMS. LAESI-IMS, invented by Nemes and Vertes and first described in 2007 47 , combines a mid-IR laser with an electrospray source. The sample is ablated by the laser, and the resulting cloud of particles is ionized as it passes through the electrospray plume 48 . The primary advantages of LAESI-IMS are that non-flat samples may be analyzed and that very little sample preparation is required 49 . Since the laser ablation removes a layer of the sample with each pulse, LAESI-IMS may be used for 3D as well as 2D sample analysis 50 . One key limitation of LAESI-IMS is that the sample needs to be rich in water in order to absorb energy sufficient for ablation from the laser pulse 47 . As LAESI-IMS physically removes layers of the sample during ablation, it is not suitable for analyses in which the sample needs to be analyzed repeatedly (e.g., for a time course study). Applications of LAESI in natural product research include the investigation of bacterial biofilms 51 , 52 and in the imaging of living plant tissues 50 , 53 , 54 . Combining LAESI-IMS analysis with bioassay has been shown to be a powerful tool for drug discovery 55 .
Compared to other IMS techniques, DESI-IMS presents several advantages that render it well suited for natural products chemistry. Because SIMS-IMS and MALDI-IMS are both performed under vacuum, the samples must usually be freeze-dried before the analysis, making these techniques incompatible with living tissue. DESI differs from SIMS, MALDI, and LAESI in that it is considered a minimally destructive ionization method, allowing samples to be repeatedly analyzed in a time course experiment. The relatively limited sample preparation required for DESI-IMS analysis combined with minimally destructive sampling of living tissue enable DESI-IMS to fill a niche inaccessible by other IMS methodologies. These properties make DESI-IMS a potent tool for the investigation of microbe-microbe and host-microbe interactions. In this review, we focus on DESI-IMS technique, discussing the principles of the ionization mechanism, instrument optimization, sample preparation, and current applications in natural products chemistry, including terrestrial or marine microorganisms, plants, and animals, and finally its wide use in medical biochemistry and clinical research.
DESI-IMS
In 2004, DESI was introduced as a novel ESI technique by Cooksʼ group at Purdue University (USA) 56 . DESI relies on a soft ionization technique similar to ESI in LC-MS, which delivers mass spectra with very low fragmentation in either positive or negative ionization mode. The simple workflow and the ease with which a DESI source can be connected to existing mass spectrometers contribute to rising popularity of DESI in different fields of analytical chemistry. One of the main advantages in comparison to other IMS techniques is that DESI does not require extensive sample preparation such as matrix fixation in MALDI-IMS, resulting in a simplified analytical procedure for a rapid spatial and temporal identification of chemicals in intact biological samples, all under ambient conditions 56 , 57 . DESI-IMS can be applied to investigate highly complex sample surfaces, from polymeric materials to biological tissues and even fluids 58 , allowing for the detection and semi-quantification of a variety of polar or non-polar small molecules 56 , 59 , 60 , 61 .
Chemical and physical aspects of the ionization mechanism
In DESI, high-velocity ionized solvent droplets desorb the analytes of interest directly from the sample surface, where compounds are solvated in a thin film. To achieve the chemical desorption, solvents are electro-sprayed under high voltage through an emitter capillary producing charged “primary” droplets, which are nebulized and then directed toward the sample. Metabolites located on the sample surface are in this way desorbed into gaseous “secondary” droplets delivering molecular ions entering the MS inlet where m/z values are measured 62 , 63 ( Fig. 1 ). Depending on the polarity of the metabolite of interest, different spray solvents from the DESI source can be used for imaging. For the analysis of both polar and non-polar compounds, standard solvents such as MeOH or ACN are frequently used, usually with the addition of 2 – 5% H 2 O, but different solvent compositions have been employed according to the specific needs and application type 64 , 65 . Overall, DESI-IMS has been shown to have a high degree of tolerance toward salt adducts formation and ionization suppression 66 . Interestingly, while the electrodynamics of droplets formation from the liquid ESI cone-jet have been thoroughly studied 67 , the physical mechanism responsible for progeny droplet formation and impact at the sample surface during the DESI event is still elusive and not completely understood. Due to the high-pressure environment and the high kinetic energy of the molecules produced in DESI 58 , the desorption process does not seem to depend on the physical particle sputtering mechanisms that govern gas ion collisions techniques such as SIMS 18 , 58 , 68 , 69 . Instead, several studies on ion-surface scattering processes in vacuum suggest that, during DESI, the molecules from surfaces may be ionized and released following a mechanisms known as “chemical sputtering” 70 , 71 . Fluid dynamics studies that simulated the electrochemical desorption mechanisms of DESI hypothesized that stochastic momentum-transfer events are the major forces leading to the formation of analyte-containing droplets 63 , 72 . These results also highlight the strong dependence on the contact angle and surface wettability of the materials as some of the major variables that govern the DESI process and that eventually affect the way metabolites are picked up from the surfaces and analyzed 72 . A modified version of DESI is nano-DESI, in which the microscale solvent extraction and desorption are achieved using two separate capillaries surrounding the sample in a “liquid bridge” 73 , 74 . Notably, nano-DESI is most suitable for direct sampling and IMS chemical profiling of small-scale wet surfaces that would suffer ablation from the strong nebulizing gas of DESI such as living microbial colonies in agar media and associated biofilms 74 , 75 , 76 .
Fig. 1.
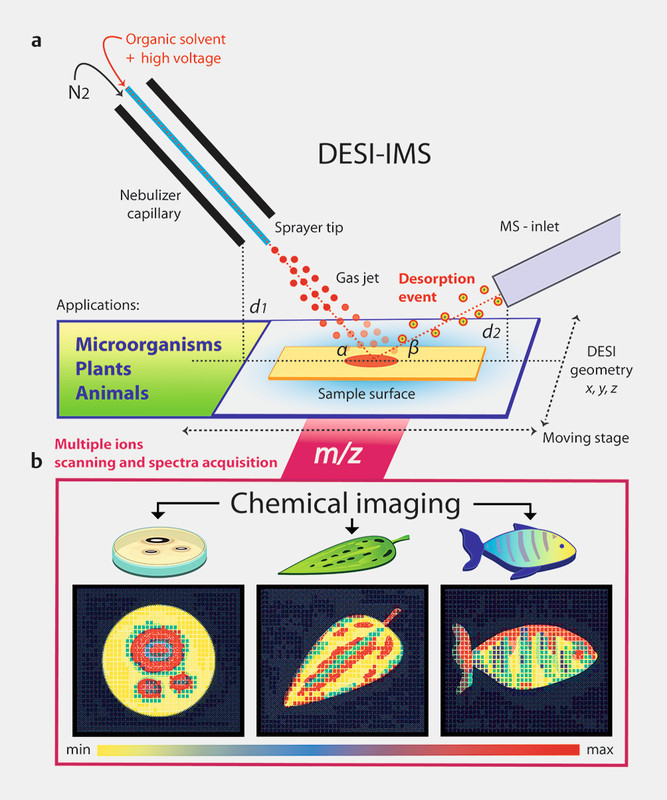
Graphic representation of the DESI-IMS workflow. a The ionization mechanism and desorption event are achieved directly at the sample surface to deliver molecular images (adapted from Takáts et al., 2004) 56 . b Relative intensities observed for multiple ions ( m/z ) represented with different colors in various biological systems (i.e., polymicrobial culture, plant and animal, respectively). Sensitive parameters, which strongly affect the resolution include the geometry between the DESI components ( x, y, z axis), such as the distance of the sample surface to the sprayer tip ( d 1) and to the MS inlet ( d 2).
DESI instrumental parameters and geometry
Due to the sensitive intrinsic properties, optimization of different parameters prior to DESI analysis is routinely required in order to obtain the best desorption and ionization of metabolites in the sample and the highest reproducibility between experiments. The correct adjustment of these parameters will strongly influence the outcome of the analysis and the final imaging resolution. Among the most critical parameters are the electrospray solvent flow rate (e.g., 0.1 – 5 µL/min) and voltage (1 – 8 kV), and the overall geometry of the DESI system, particularly the distance of sample surface to the DESI spray nozzle tip ( d 1) and to the MS-inlet ( d 2), as well as their respective incident and collection degree angles ( α and β ) 58 , 77 , 78 ( Fig. 1 ). Common application guidelines of the instrument recommend utilizing a geometry configuration with larger tip-to-surface distances ( d 1 = 5 – 8 mm), smaller incident angles ( α = 20 – 50°), and higher voltages (3 – 8 kV) for small molecules such as lipids or aromatic hydrocarbons, and opposite range of settings ( d 1 = 1 – 2 mm; α = 60 – 90°, 1 – 4 kV) for larger molecules, such as proteins or nucleic acids 58 . Solvent flow rate and sprayer distance from the sample surface influence the quality of the analysis as they affect droplet sizes and their speed. At 2 µL/min solvent flow rate and 1130 kPa nebulizing gas pressure, droplets have been measured to have an average diameter of 2 – 4 µm and an impact velocity of 120 m/s 63 . High nebulizing flow rates are generally preferred as they produce smaller and faster droplets, which increase the desolvation efficiency. However, the upper limit is reached when droplets generated are too small and may evaporate before reaching the surface resulting in a lack of signal. Thus, given each geometry setup, the relationship between solvent and gas flow rates will strongly determine the final imaging resolution 77 . A typical spatial resolution can range between 50 and 200 µm, with an average resolution of 40 – 60 µm 79 and the highest resolution reported at 35 µm during phospholipid analysis in mouse brain tissue in 2012 79 , 80 , 81 . Increased resolution can be achieved by nano-DESI thanks to nano-spray ability to deliver droplets at lower velocity (4 m/s), allowing a gentle deposition of charged reagents with minimal splashing and a spatial imaging to < 12 µm 73 , 82 .
Sample preparation
DESI-IMS has been successfully applied in several areas ranging from clinical medical research to pharmacology, and from natural product chemistry to chemical ecology. According to the application and the nature of the biological tissue, sample preparation may differ. DESI analysis is usually restricted to flat and preferably hard surfaces. Samples with smooth and regular surfaces can be usually analyzed directly with no sample preparation. In case of non-flat surfaces (for instance animal organs and tissues), cryosections can be prepared following commonly established IMS protocols 83 . In case of plants, preparation of DESI samples can be achieved in different ways. Flowers and leaves are often problematic as they present soft, irregular, and very absorbent surfaces, which may result in low or instable signal during imaging. In addition, most land plants contain wax cuticles on their leaf surface that are highly hydrophobic and can be difficult to penetrate by spraying solvents. As an alternative to direct analysis on plant surfaces, indirect sample analysis can be performed via “imprinting” of metabolites for instance on glass slides. However, ablation of metabolites deposited on the glass surface may occur rapidly, resulting in a loss of signal intensity over repeated measurements. Sorbent materials such as porous PTFE or TLC (thin-layer chromatography) silica plates appear more suitable for indirect tissue analysis by sample imprinting. Both PTFE and TLC plates have good absorbing properties that retain metabolites from the plants until desorption with DESI and have been applied for the imprinting of various plant organs and tissues 70 , 71 , 72 , 83 . TLC is a cheaper solution with similar performance to PTFE, but overall PTFE is currently reported as the best solution for DESI-IMS analysis of plant materials in terms of both reproducibility and quantitative ability 81 , 85 , 86 , 87 .
Data analysis
The datasets produced by DESI spatial mass scanning typically contain a large set of information and thus requires intensive post-acquisition analysis for data extraction, visualization, and interpretation. In this respect, developments in bioinformatics tools such as machine learning algorithms allows for advanced high-throughput data analysis 88 . For instance, deep learning and unsupervised neural network based methods have been successfully applied for the analysis of complex dataset from 3D-DESI-MS of tumor tissue, allowing for high dimensionality reduction and data clustering normally not achievable by classical linear methods 89 . Recently, a collaborative initiative sponsored by EMBL (European Molecular Biology Laboratory) in the framework of the European Research Program Horizon 2020 tackled the need for high-throughput analysis of high dimensionality data from MALDI and DESI-IMS and developed an open-source method that is available to test in its beta uploading using the MetaSpace cloud-server ( http://metaspace2020.eu/ ). A similar project has been undertaken by the community of OpenMSI ( https://openmsi.nersc.gov ), which provides a high-performance web-based platform for the storage and management of IMS data, including tools for visualization and statistical analysis 90 .
Applications of DESI-IMS in Natural Product Research
Application of DESI-IMS on microorganisms
Since 2008, DESI-IMS has been successfully applied for chemical profiling of metabolites located on surfaces and/or internal tissues, studying single or mixed microbial cultures and chemical interactions between them ( Table 1 ). However, the use of DESI-IMS on soft and irregular surfaces (such as agar media) is still a challenge today because the strong nebulizing gas of DESI can provoke an aperture in some cultures. Indeed, direct IMS of living colonies has mostly been accomplished by nano-DESI-IMS, more suitable method to imaging wet surface, on living communities such as Shewanella oneidensis, Bacillus subtilis, Streptomyces coelicolor, Mycobacterium smegmatis, and Pseudomonas aeruginosa for investigation of various chemical families (e.g., lipopeptides, rhamnolipids, quinolones, phenazines, glycopeptidolipids) 74 . However, analysis of B. subtilis by DESI-IMS or by nano-DESI-IMS has revealed, in both cases, the presence of surfactin-type lipopeptides.
Table 1 Application of DESI-IMS in microbial natural products.
| Organism | Max no. of microorganisms in mixtures | Applications | Sample preparation | ionization mode | Known chemical family/compounds visualized | References | |
|---|---|---|---|---|---|---|---|
| Fungi | T. harzianum; M. roreri | 1 – 2 | Antagonistic interaction of fungi | Imprint | Positive | Dehydrohistydiltryptophanyldiketopiperazine; histidyltryptophanyldidetopiperazine; roquefortine C; roquefortine D; meleagrin-CH3O; glandicoline A; glandicoline B; meleagrine; epineoxaline; oxaline; peptaibols (harzianins and trichotoxins); T39 butenolide; harzianolide; sorbicillinol | 96 |
| P. restrictum | 1 | Spatial and temporal distribution of quorum sensing inhibitors metabolites from the guttate-forming fungus | Cross section (thickness: 15 µm) and imprint | Negative | 2-Hydroxyemodic acid; 1′-hydroxyisorhodoptilometrin; 1′-hydroxy-2′-ketoisorhodoptilometrin; (+)-2′-isorhodoptilometrin; desmethyldermoquinone; 2-chloroemodic acid; emodin | 94 | |
| C. aquaticus; Fusarium sp. | 2 | Interaction of an antifungal compound producing fungus and a mycotoxin producing fungus | Direct analysis using cardboard inserts and cross section | Positive | Phomopsinone A; T-2 toxin | 97 | |
| Fungi/ bacteria | B. seminalis; M. perniciosa; P. capsisi; P. palmivora; P. citrophthora | 1 – 2 | Antagonistic interaction between bacteria and fungi and oomycetes | Direct analysis on dehydrated agar plate | Positive and Negative | Glycerophosphoethanolanine and glycerophosphocholine classes; phospholipids (glycerophosphatidic acid and glycerophosphoglycerols); rhamnolipids (Rha-Rha-C14-C14 and Rha-Rha-C15-C14); pyrrolnitrin; glycrophosphoserine; glycerophosphoinositol; pyochelin | 98 |
| Bacteria | B. subtilis; S. coelicolor | 2 | Metabolic exchange between microbial species | Millipore HA filter imprint | Negative | Surfactin; plipastatin; actinorhodin | 99 |
| 16 bacterial strains | 1 | Characterization of different bacterial species | Bacterial suspensions | Positive and Negative | Lipids | 92 | |
| B. subtilis | 1 | Characterization of bacterial species | Intact biofilm on membrane (filter paper) | Positive and Negative | Lipopeptide (surfactin) | 91 | |
| E. coli | 1 | Temporal detection of central carbon analysis | Bacterial extracts on nonporous Teflon substrate | Negative | Central carbon metabolites (13 metabolites) | 95 | |
| S. wadayamensis | 1 | Protocol allowing direct IMS of agar cultures: application on metal scavengers siderophores | Direct analysis on dehydrated agar plate and imprint | Positive | Siderophore (protonated desferrioxamine; ferrioxamine B; Al-desferrioxamine B complex); antimycin isoforms | 93 | |
| Pathogens/plants | P. ultimum; S. tuberosum | – | Plant metabolic response to pathogen invasion | Imprint | Positive | Glycoalkaloids ( α -solanine and α -chaconine; solanidine; solasodenone; solanaviol; solasodiene; solaspiralidine; γ -solanine/ γ -chaconine; β -solanine and β -chaconine) | 100 |
One of the first applications of DESI-IMS directly on bacterial cultures aimed at identification of bacterial species based on their chemistry and particularly on the basis of their lipid constituents 91 , 92 . For example, the cyclic lipopeptide surfactin C15, a well-known metabolite produced by Bacillus sp., was observed on agar plates, permitting the taxonomical identification of B. subtilis directly on Petri dish 91 . DESI-IMS was also employed to analyze lipid composition on 16 bacterial samples to distinguish bacterial species and even subspecies using bacterial samples suspended in 70% EtOH. Chemotaxonomical identification of the bacterial species was based on the distribution of several major classes of lipids, including phosphotidylethanolamines, phosphotidylglycerols, and lysophospholipids, in positive and negative ionization modes. This lipidomic-type approach allowed the taxonomical characterization of several bacterial species including Staphylococcus aureus, Escherichia coli, B. subtilis, and Salmonella sp. 92 . The lipidomic analysis of bacterial strains by DESI-IMS enabled the differentiation of Gram-positive and Gram-negative bacteria 92 , providing new opportunities for microbiological research.
DESI-IMS has also been used for analyzing natural products (primary and secondary metabolites) directly from bacterial and/or fungal cultures. For instance, metal scavenging siderophores were imaged directly from agar culture of Streptomyces wadayamensis 93 , whereas several new polyhydroxyanthraquinones were identified as quorum sensing inhibitors in the guttate-forming Penicillium restrictum 94 , an endophytic fungus isolated from the stems of a milk thistle, Silybum marianum (L.) Gaertn (Asteraceae). The DESI-IMS study showed that the polyhydroxyanthraquinones were produced by fungal mycelia and were expressed differentially over time. Also, the potent quorum sensing inhibitory polyhydroxyanthraquinones were observed to be concentrated at the fungal surface, whereas less potent compounds were diffused through the culture medium 94 , illustrating the power of DESI-IMS to determine the spatiotemporal distribution as well as the specific production of natural products. Further investigation of bacterial central carbon metabolism performed by Jackson et al. 95 on E. coli allowed the detection of 13 out of 17 selected central carbon metabolites (i.e., metabolites involved in the integration of pathways of transport and oxidation of main carbon source inside the cell). Similarly, DESI-IMS was shown to be an effective method to understand chemical interactions/exchanges (e.g., antagonistic fungal interactions 96 , 97 , fungal/bacterial interactions 98 , or metabolic exchange between bacterial species 99 directly). The interaction of micro- and macroorganisms with other organisms often relies on the production of secondary metabolites. Specific metabolite exchanges such as two lactones derivative (T39 butenolide and harzianolide), one hexaketide metabolite (sorbicillinol), and an unknown metabolite ( m/z [M + H] + 319.1) were revealed during the co-culturing of the commercially relevant phytopathogenic fungus Moniliophthora roreri (causing fungal cacao pest) and the fungal biocontrol agent Trichoderma harzianum 96 . Likewise, the production of phomopsinone A ( α -pyrone derivatives) and T-2 toxin (mycotoxin) was shown during the co-culture of fungus Fusarium sp. and fungus Clohesyomyces aquaticus 97 . A proteobacterium, Burkholderia seminalis isolated from Saccharum officinarum L. (Poaceae) roots, was shown to inhibit the growth of multiple cacao pathogens by the production of rhamnolipids, and other unidentified metabolites 98 . DESI-IMS imaging of B. subtilis and S. coelicolor co-culture has highlighted the ability of B. subtilis to silence antibiotic production (in particular the production of benzo isochromane quinone polyketide actinorhodin) in S. coelicolor , giving B. subtilis a fitness advantage over S. coelicolor 99 . Such studies employing DESI-IMS do not only provide a better understanding for microbial interactions, but also offer new ecological perspectives–for example, the ability T. harzianum or B. seminalis to act as biocontrol agent against M. roreri and other cacao pathogens, respectively. Chemical imaging of microbial co-cultures has potential to be used as a tool for the discovery and the localization of new bioactive metabolites generated by the co-microorganisms. Those co-microorganisms modulate the activation or the suppression of specific metabolite production.
Plant-pathogen interactions have also been analyzed by DESI-IMS, linking plant responses to microbial pathogens as reported by Tata et al. 100 . Plants represent an important source of nutrients but very few studies on plant pathosystems (i.e., ecosystems defined by parasitism) are available. DESI-IMS was used to study the fluctuations of glycoalkaloids in sprouted potatolices infected by the fungal phytopathogen Pythium ultimum . After eight days of infection, it was observed that metabolic pathways were affected by the phytopathogen to increase or decrease the levels of in total 12 glycoalkaloids 100 . Therein, the DESI-IMS study permitted to determine the plant metabolic changes on the production of glycoalkaloid after infection by the fungal phytopathogen and demonstrated its useful application in plant pathosystems.
In summary, DESI-IMS has so far been used on microorganisms for (i) taxonomical identification of species, (ii) production and spatiotemporal distribution of (surface) metabolites, (iii) identifying metabolic exchanges between bacterial species, (iv) antagonistic fungal/fungal and fungal/bacterial interactions, and (v) elucidation of metabolic responses of plants to pathogenic infections ( Table 1 ). These studies open up new perspectives in (micro)biology, and in the understanding of biological systems from a chemical angle.
Application of DESI-IMS on macroorganisms
Several examples of DESI-IMS analyses have been reported on macroorganisms, including analyses on plants, algae, and fish ( Table 2 ).
Table 2 Application of DESI-IMS on macroorganism-derived natural products.
| Organism | Applications | Sample preparation | Ionization mode | Known chemical family/compounds visualized | References | |
|---|---|---|---|---|---|---|
| Plants | C. roseus | Identifying varietal differences, toxic metabolites production, changes in metabolites during growth, pest/ pathogen attack, and natural stresses | TLC imprints | Positive | Serpentine; vindoline; catharanthine; 19- S -vindoline; like-catharanthine; perivine; alstonine; tabersonine isomers; dihydrotabersonine; ajmalicine; hydroxytarbersonine; decaetoxyvindoline; S-adenosylmethionine; akuamimicine; methoxytabersonine; lochnerinine; echitovenine; deacetoxyvindoline; vindolinine; strictosidine; anhydrovinblastine; vinblastine | 84 |
| H. vulgare | Chemical profiling | Direct and imprints on porous Teflon substrate | Positive and Negative | Hydroxynitrile glucosides (hydroxynitrile glucosides; osmaronin) | 101 | |
| H. perforatum; D. stramonium; P. somniferum | Spatial distribution on leaves, petals and/or seeds of three plants species | Imprint on porous Teflonsubstrate | Positive and Negative | Hyperforin; hypericin; adhyperforin; pseudohypericin; protopseudohypericin; protohypericin; atropine; scopolamine; hexose sugars; sucrose; papaverine; morphine | 81 | |
| H. perforatum | Methods development for the direct imaging of soft plant tissues | Direct analysis | Negative | Tetracosanic acid; hexacosanoic acid; octacosanoic acid; melissic acid; quercetin; quercitrin; isobaric mixture of isoquercitrin; hyperoside; hypericin; protohypericin; pseudohypericin; protopseudohypericin; C26, C28 and C30 fatty acids; hyperforin; rutin; hyperfirin; adhyperfin | 102 | |
| D. binectariferum | Spatial and temporal distribution of rohitukine and related compounds during various stages of seed development | TLC imprints | Positive | Rohitukine; acetylated and glycosylated rohitukine | 104 | |
| C. japonicum; L. styraciflua; O. virginiana | Rapid identification and spatially resolved relative quantification of chlorophyll degradation products in complex senescent plant tissue matrixes | Imprint using porous PTFE substrate | Positive and Negative | Cj -NCC-1 and 2; Hv -NCC-1; So -NCC-2; Ov -NCC-1 (chlorophyll catabolite, polyfunctionalized linear tetrapyrrole); phenylboronic acid with diol substituent of the chlorophyll catabolite; | 85 | |
| Algae | C. serratus | Natural products chemistry and chemical ecology to provide ecological roles of metabolites | Algal sample preserved with 10% formalin in seawater and then affixed to PTFE substrates | Negative | Bromophycolide A and B; callophycoic acid | 105 |
| P. neurymenioides | Natural products chemistry and chemical ecology to provide ecological roles of metabolites | Direct analysis by glue fixation on glass slides | Positive | Neurymenolide A | 106 | |
| Animals | D. rerio | Characterization, distribution and metabolism of a toxic ionic liquid | Cryosection using CMC (carboxymethyl cellulose), 50-µm tissue sections | Positive | Phospholipids; AMMOENG 130 | 57 |
With regard to plants, DESI-IMS has principally been applied on the imprints of leaves 81 , 84 , 85 , 101 , 102 , flowers and petals 84 , 101 , 102 , fruits 103 , and seeds 104 ( Table 2 ). The cuticle of plants is dominated by metabolites such as fatty acids with aliphatic tails longer than 22 carbons. These VLCFAs represent a physical morphological barrier in leaves and petals, presenting an obstacle for DESI-IMS analysis. To overcome this difficulty, a ternary solvent system, CHCl 3 -ACN-H 2 O (1 : 1 : 0.04), was developed and used to analyze the cuticle itself as well as the subcuticular metabolites directly by DESI-IMS 102 . The leaves and petals of Hypericum perforatum L. (Clusiaceae) were analyzed in several studies 64 , 70 , 81 , 82 , 85 , in which the detection and localization of several metabolites were reported. The phloroglucinol derivative hyperforin was shown to be localized in the translucent glands, while hypericin (an anthraquinone derivative) was present in the dark glands. A number of other phloroglucinol, flavonoid, and anthraquinone derivatives known from Hypericum sp. (adhyperforin, pseudohypericin, protopseudohypericin, protohypericin, rutin, quercetin, phloroglucinol), as well as VLCFAs, were detected by indirect analysis (imprints) 81 or direct analysis using a ternary solvent system and/or after surface extraction by CHCl 3 102 . Similarly, the distribution of leaf metabolites of Datura stramonium L. (Solanaceae) was analyzed, revealing the presence of the major tropane alkaloids atropine and scopolamine. Both compounds were located principally in the leaf ribs and veins, suggesting their transport within the plant 81 .
Similar approaches were also performed on other plants, such as Papaver somniferum L. (Papaveraceae) 81 , Catharanthus roseus (L.) G.Don (Apocynaceae) 84 , Hordeum vulgare L. (Poaceae) 101 , Cercidiphyllum japonicum Siebold & Zucc (Cercidiphyllaceae), Liquidambar styraciflua L. (Hamamelidaceae) , Ostrya virginiana K.Koch (Corylaceae) 85 , Lotus japonicus (Regel) K.Larsen (Leguminosae), Manihot esculenta Crantz (Euphorbiaceae) 87 , Ginkgo biloba L. (Ginkgoaceae) 104 , and strawberry ( Fragaria x ananassa Duch. [Rosaceae]) 103 , indicating the capability of DESI-IMS to map various classes of metabolites (e.g., terpenoid indole alkaloids, hydroxynitrile glucosides) on various plant organs ( Table 2 ). The spatial and temporal distribution of rohitukine, a chromone alkaloid with anti-inflammatory, anticancer, and immunomodulatory activities, was imaged during the seed development of Dysoxylum binectariferum Hook.f. (Meliaceae). Rohikutine accumulation was shown to be more important during seed development and its increase was specifically located in the embryo, cotyledons, and to a lesser extent in the seed coat 104 . DESI-IMS studies on leaves, flowers, or seeds were performed to determine metabolite distribution at a specific time or during various plant developmental stages.
DESI-IMS has also been used to study molecular distribution of bioactive natural products in marine organisms, such as seaweeds ( Table 2 ). The DESI-IMS analysis of the tropical red seaweed Callophycus serratus revealed the presence of the macrocyclic halogenated-benzoates, bromophycolide A and B on the surface of the alga 105 . Both compounds inhibit the growth of Lindra thalassiae , a marine fungus that infects diverse hosts ranging from brown algae to seagrasses. The natural concentrations of these two metabolites were shown to be more than sufficient to display an antifungal activity in the algal surface patches, suggesting the importance of secondary metabolites for chemically mediated biological processes 105 . The compounds were observed also within internal algal tissue by DESI-IMS. The fact that bromophycolides are not homogenously distributed on the algal surface but associated with distinct patches could be due to the presence of tissue damage, indicating the chemical defense of C. serratus to fungal infection. Seaweeds and corals often occur in close proximity in the same environment. As coral reefs are declining globally, seaweeds are commonly replacing corals. However, the mechanism of the increasing coral-algal interactions is poorly understood. Andras et al. 106 employed DESI-IMS to visualize and quantify neurymenolide A, an unusual cyclophane derivative isolated from the red alga Phacelocarpus neurymenioides. Neurymenolide A is an allelopathic agent occurring on the surface of the live alga that damages the coral Porites rus by direct contact with the alga. This study elegantly shows the potential of DESI-IMS in chemical ecology studies. In our laboratory, we have been applying DESI-IMS to Baltic Sea plants and seaweeds to determine their surface metabolome, in combination with classical metabolomics approaches ( Fig. 2 ).
Fig. 2.
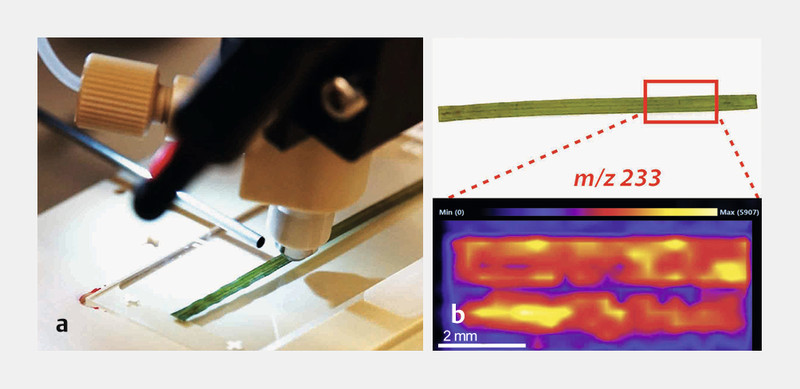
Application of DESI-IMS in our laboratory at GEOMAR for surface chemical imaging of marine plants from the Baltic Sea. a Direct surface analysis of a seagrass and ( b ) metabolite localization and relative abundance of m/z ion 233 [M + H] + , achieved using a rapid scanning on glass imprints at 200 µm resolution (covered area approximately 12 mm 2 ).
There is only one study that has reported the use of DESI-IMS on the whole body zebrafish ( Danio rerio ) by cryosectioning 43 . This study aimed at localizing the accumulation of a mildly toxic ionic liquid AMMOENG 130 in zebrafish tissues, after exposure of the zebrafish to varying concentrations of this liquid. The ionic liquids are found in large quantities in detergents and softeners, and are potentially toxic. In particular, AMMOENG 130 was found to be very toxic against D. rerio (i.e., more toxic than 13 out of 15 common ionic liquids). DESI-IMS experiment on D. rerio revealed the accumulation of this toxin in the respiratory and nervous system of the fish, suggesting that it is potentially a neurotoxin. Consequently, DESI-IMS analysis of macroorganisms opens new avenues in other research fields such as ecotoxicology for biomonitoring of toxic compounds.
Application of DESI-IMS in medicine and clinical research
Similar to MALDI-IMS, the dominant application area of DESI-IMS is medical research, where it enables a rapid and accurate method to visualize biomarkers in biological tissues. In particular, DESI-IMS has been demonstrated to be very efficient in lipid composition analysis. Lipids play key roles in various cytological processes and in cell signaling; hence, the analysis of lipid composition in biological tissues is crucial to support morphological investigations, diagnosis, and characterization of pathophysiological processes such as neurodegenerative and cardiovascular diseases, as well as neoplastic processes. Tissue profiling of lymphoma, gliomas, gastric, liver, prostate, and breast cancer have all showed how drastic change in lipid composition can be used as biomarkers. DESI-IMS has been applied in medical research as reported by at least five publications in 2017 and more than 14 publications since 2007 ( Table 3 ). Selected examples are described below to illustrate medical aspects of DESI-IMS applications.
Table 3 Application DESI-IMS in medicine and clinical research.
| Tissues/samples | Applications | Sample preparation | Ionization mode | Known chemical family/compounds visualized | References | |
|---|---|---|---|---|---|---|
| n. a.: information not available | ||||||
| Medicine | Tissues smears | Cancer profiling | Tumor sectioned (cryosection) on glass slides with PTFE spots | Negative | Lipids (fatty acids and phospholipids) | 111 |
| Human brain tumors | Molecular diagnosis of human brain tumors | n. a. | Negative | Lipids | 107 | |
| Squamous cell carcinomas | Rapid determination of tumor stroma ratio | Tissue slices using frozen tumor samples (cryosection) | Negative | Lipids | 113 | |
| Tumor and normal breast tissues | Lipidomic comparison of normal rat breast tissues and neoplastic tissues from animals belonging to the DMBA chemical carcinogenesis rat model | Tissues (cryosection) | Negative | Lipids | 114 | |
| Human tissue specimens including normal ovarian tissues, BOT and HGSC samples | Identification of potential predictive markers of disease aggressiveness: application on serious ovarian tumors | Tissues (cryosection) | Positive and Negative | Saturated and unsaturated fatty acids; sphingolipids; ceramides; cardiolipins; glycerophosphoethanolamines; glycerophosphoglycerols; glycerophosphoserines; glycerophosphoinositols; glycerophosphocholines; triacylglycerols; sphingomyelins | 115 | |
| Cerebrum tumor tissue | Correlation between lipidomic data and glioma diagnostic information | Tissues (cryosection) | Positive and Negative | Lipids | 112 | |
| Human cancer tissues and human fluids (blood, urine) | Cancer diagnostic and paper spray ionization for therapeutic drug monitoring | Tissues (cryosection) and direct fluids on paper spray | Positive | Glycerophosphocholines | 116 | |
| Urine samples | Metabolites and drugs analysis | Extracted urine samples on Teflon slides | Positive | Temazepam; oxazepam; desmethyldiazepam; para-hydroxytemazepam; alprazolam; codeine; morphine; oxymorphone; amphetamine; 11-Nor-9-carboxy-delta9-tetrahydroxydrocannabinol; delta9-tetrahydrocannabinol | 117 | |
| Histological sections of brain, lung, kidney and testis | Spatial distribution of drugs directly from histological sections | Histological sections | Positive | Clozapine; N-desmethylclozapine; clozapine-N-oxide | 60 | |
| Human tissue samples | Detection of metastatic breast and thyroid cancer in lymph nodes | Tissue samples (cryosection) | Negative | Lipids | 118 | |
| Prostate tissue | Diagnosis of prostate cancer | Tissue sections (cryosection) | Negative | Krebs cycle metabolites; carbohydrates; lipids | 108 | |
| Tumor tissues | 3D-DESI imaging to study metabolic heterogeneity of cancer | Tissues sections | Negative | n. a. | 89 | |
| Mouse brain tissue | Lipidomic profiling in individual mouse preimplantation embryos and oocytes | Fresh dry samples | Negative | Lipids; phospholipids | 119 | |
| Oocytes and preimplantation embryos of bovine species | Lipidomic profiling | Brain tissue sections | Positive | Lipid metabolism (free fatty acids; phospholipids, cholesterol-related molecules; triacylglycerols) | 120 | |
Eberlin et al. 107 used lipidomic data as biomarkers to rapidly classify 36 human gliomas including oligodendroglioma, astrocytoma, and oligostrocytoma at different histologic grades and varied tumor cell concentrations. The combination of DESI-IMS, multivariate analysis, and machine learning was applied to recognize glioma subtypes and grade on the basis of the World Health Organization tumor classification system. The analysis resulted in a recognition capability of greater than 99% and a cross-validation of more than 97% based on 128 peaks for the subtype, 123 peaks for the grade, and 136 peaks for the concentration. Furthermore, small molecule analysis (Krebs cycle intermediates metabolites and carbohydrates) combined with lipid composition was used to distinguish prostate cancer from normal tissues and to improve the accuracy of prostate cancer identification 108 . These models permitted the differentiation of prostate cancer from benign specimens with nearly 90% accuracy per patient. Glucose/citrate ratio, in parallel to lipids composition, could also be used to accurately monitor the occurrence of prostate cancer 108 . In the majority of cancer diagnoses by DESI-IMS, lipid composition was of key importance to distinguish tumor tissues from normal tissues ( Table 3 ) 108 . DESI-IMS was used to record the spatial intensity distribution of a drug, clozapine, directly from histological sections of brain, lung, kidney, and testis 60 , in addition to cancer diagnosis applications. In another study, Miyamoto et al. 109 combined microscopy, MALDI-IMS and nano-DESI to reveal changes in glucose metabolism and the accumulation of sphingomyelin metabolites in the glomeruli of the diabetic mice fed with high-fat diet. This shows the great potential of the technique in various areas of medical research, including metabolic disorders.
On the whole, DESI-IMS has been successfully applied in various research fields including microbiology, ecotoxicology, ecology, forensics, embryology, chemistry, and medicine, presenting a fast, easy, and reliable direct/indirect analysis of biological samples. DESI-IMS has found applications in other research fields–for instance, in astrophysics to detect and identify the spatial distribution of organic compounds on meteoritesʼ surface 110 indicating its potential in so many other disciplines.
Conclusions and Future Perspectives
IMS techniques have already opened new avenues in several areas of life sciences, including medicine, microbiology, and natural product chemistry. The IMS surpasses all existing molecular analysis methods (i.e., genomics, transcriptomics and metabolomics) by giving crucial and simultaneous spatial information on molecules. Therefore, various IMS techniques with different ionization modes and workflows are being adopted by scientists to address questions presented by spatial metabolomics on various biological systems. However, all IMS techniques have drawbacks and limitations in instrumentation, sample preparation, metabolites annotation, resolution, and data analysis. Considering their ever-increasing popularity, it is foreseeable that the IMS techniques will rapidly improve to move forward and find even more applications in life and medical sciences, and potentially in industry. Specifically, IMS techniques will bring metabolomics research to a paradigm shift and completely change the way how we interrogate biological systems and provide new approaches in drug discovery and medicine. With future developments, IMS has the power to transform our ability to interrogate natural processes for example, better understanding host-microbe and microbe-microbe interaction, biosynthesis of natural products, chemical ecology, virulence factors, and disease states. Continuous improvement will be crucial for the advancement of IMS and its contribution in our understanding of life and metabolic processes.
Acknowledgements
SP acknowledges the Foundation Blanceflor Boncompagni Ludovisi scholarship. DF is supported by the MarPipe project (improving the flow in the pipeline of the next generation of marine biodiscovery scientists) funded through the European Commission H2020-MSCA-ITN-ETN scheme, GA721421.
Footnotes
Conflict of Interest The authors declare that they have no conflicts of interest.
References
- 1.Jarmusch A K, Cooks R G. Emerging capabilities of mass spectrometry for natural products. Nat Prod Rep. 2014;31:730–738. doi: 10.1039/c3np70121b. [DOI] [PubMed] [Google Scholar]
- 2.Fenn J, Mann M, Meng C, Wong S, Whitehouse C. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka K, Waki H, Ido Y, Akita S, Yoshida Y, Yoshida T, Matsuo T. Protein and polymer analyses up to m/z 100 000 by laser ionization time-of-flight mass spectrometry . Rapid Commun Mass Spectrom. 1988;2:151–153. [Google Scholar]
- 4.Aksenov A A, da Silva R, Knight R, Lopes N P, Dorrestein P C. Global chemical analysis of biology by mass spectrometry. Nat Rev Chem. 2017;1:1–20. [Google Scholar]
- 5.Petras D, Jarmusch A K, Dorrestein P C. From single cells to our planet-recent advances in using mass spectrometry for spatially resolved metabolomics. Curr Opin Chem Biol. 2017;36:24–31. doi: 10.1016/j.cbpa.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Chabala J M, Soni K K, Li J, Gavrilov K L, Levi-Setti R. High-resolution chemical imaging with scanning ion probe SIMS. Int J Mass Spectrom Ion Process. 1995;143:191–212. [Google Scholar]
- 7.Chaurand P, Schwartz S A, Billheimer D, Xu B J, Crecelius A, Caprioli R M. Integrating histology and imaging mass spectrometry. Anal Chem. 2004;76:1145–1155. doi: 10.1021/ac0351264. [DOI] [PubMed] [Google Scholar]
- 8.Caprioli R M, Farmer T B, Gile J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal Chem. 1997;69:4751–4760. doi: 10.1021/ac970888i. [DOI] [PubMed] [Google Scholar]
- 9.Stoeckli M, Chaurand P, Hallahan D E, Caprioli R M. Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues. Nat Med. 2001;7:493–496. doi: 10.1038/86573. [DOI] [PubMed] [Google Scholar]
- 10.Masumori N, Thomas T Z, Chaurand P, Case T, Paul M, Kasper S, Caprioli R M, Tsukamoto T, Shappell S B, Matusik R J. A probasin-large T antigen transgenic mouse line develops prostate adenocarcinoma and neuroendocrine carcinoma with metastatic potential. Cancer Res. 2001;61:2239–2249. [PubMed] [Google Scholar]
- 11.Caldwell R L, Caprioli R M. Tissue profiling by mass spectrometry. Mol Cell Proteomics. 2005;4:394–401. doi: 10.1074/mcp.R500006-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Dunham S JB, Ellis J F, Li B, Sweedler J V. Mass spectrometry imaging of complex microbial communities. Acc Chem Res. 2016;50:96–104. doi: 10.1021/acs.accounts.6b00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esquenazi E, Dorrestein P C, Gerwick W H. Probing marine natural product defenses with DESI-imaging mass spectrometry. Proc Natl Acad Sci. 2009;106:7269–7270. doi: 10.1073/pnas.0902840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L P, Feng B S, Yang J W, Chang C L, Bai Y, Liu H W. Applications of ambient mass spectrometry in high-throughput screening. Analyst. 2013;138:3097–3103. doi: 10.1039/c3an00119a. [DOI] [PubMed] [Google Scholar]
- 15.McDonnell A L, Heeren R M. Imaging mass spectrometry. Mass Spectrom Rev. 2007;26:606–643. doi: 10.1002/mas.20124. [DOI] [PubMed] [Google Scholar]
- 16.Ho Y N, Shu L J, Yang Y L. Imaging mass spectrometry for metabolites: technical progress, multimodal imaging, and biological interactions. Wiley Interdiscip Rev Syst Biol Med. 2017;9:1–32. doi: 10.1002/wsbm.1387. [DOI] [PubMed] [Google Scholar]
- 17.Castaing R, Slodzian G. Microanalyse par émission secondaire. J Microsc (Paris) 1962;1:395–410. [Google Scholar]
- 18.Smentkowski V S. Trends in sputtering. Prog Surf Sci. 2000;64:1–58. [Google Scholar]
- 19.Liebl H. Ion microprobe mass analyzer. J Appl Phys. 1967;38:5277–5283. [Google Scholar]
- 20.Boxer S G, Kraft M L, Weber P K. Advances in imaging secondary ion mass spectrometry for biological samples. Annu Rev Biophys. 2009;38:53–74. doi: 10.1146/annurev.biophys.050708.133634. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher J S, Vickerman J C. Secondary ion mass spectrometry: characterizing complex samples in two and three dimensions. Anal Chem. 2013;85:610–639. doi: 10.1021/ac303088m. [DOI] [PubMed] [Google Scholar]
- 22.Chandra S, Smith D R, Morrison G H. Peer reviewed: a subcellular imaging by dynamic SIMS ion microscopy. Anal Chem. 2000;72:104A–114A. doi: 10.1021/ac002716i. [DOI] [PubMed] [Google Scholar]
- 23.Wu K, Jia F, Zheng W, Luo Q, Zhao Y, Wang F. Visualization of metallodrugs in single cells by secondary ion mass spectrometry imaging. JBIC J Biol Inorg Chem. 2017;22:653–661. doi: 10.1007/s00775-017-1462-3. [DOI] [PubMed] [Google Scholar]
- 24.Chandra S, Tjarks W, Lorey D R, Barth R F. Quantitative subcellular imaging of boron compounds in individual mitotic and interphase human glioblastoma cells with imaging secondary ion mass spectrometry (SIMS) J Microsc. 2008;229:92–103. doi: 10.1111/j.1365-2818.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- 25.Tian H, Six D A, Krucker T, Leeds J A, Winograd N. Subcellular chemical imaging of antibiotics in single bacteria using C60-secondary ion mass spectrometry. Anal Chem. 2017;89:5050–5057. doi: 10.1021/acs.analchem.7b00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dekas A E, Poretsky R S, Orphan V J. Deep-sea Archaea fix and share nitrogen in methane-consuming microbial consortia. Science. 2009;326:422–426. doi: 10.1126/science.1178223. [DOI] [PubMed] [Google Scholar]
- 27.Lechene C P, Luyten Y, McMahon G, Distel D L. Quantitative imaging of nitrogen fixation by individual bacteria within animal cells. Science. 2007;317:1563–1566. doi: 10.1126/science.1145557. [DOI] [PubMed] [Google Scholar]
- 28.Vidal A, Remusat L, Watteau F, Derenne S, Quenea K. Incorporation of 13C labelled shoot residues in Lumbricus terrestris casts: a combination of transmission electron microscopy and nanoscale secondary ion mass spectrometry . Soil Biol Biochem. 2016;93:8–16. [Google Scholar]
- 29.Karas M, Bachmann D, Bahr U, Hillenkamp F. Matrix-assisted ultraviolet laser desorption of non-volatile compounds. Int J Mass Spectrom Ion Process. 1987;78:53–68. [Google Scholar]
- 30.Watrous J D, Dorrestein P C. Imaging mass spectrometry in microbiology. Nat Rev Microbiol. 2011;9:683–694. doi: 10.1038/nrmicro2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simmons T L, Coates R C, Clark B R, Engene N, Gonzalez D, Esquenazi E, Dorrestein P C, Gerwick W H. Biosynthetic origin of natural products isolated from marine microorganism-invertebrate assemblages. Proc Natl Acad Sci U S A. 2008;105:4587–4594. doi: 10.1073/pnas.0709851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moree W J, Yang J Y, Zhao X, Liu W T, Aparicio M, Atencio L, Ballesteros J, Sanchez J, Gavilan R G, Gutiarrez M, Dorrestein P C. Imaging mass spectrometry of a coral microbe interaction with fungi. J Chem Ecol. 2013;39:1045–1054. doi: 10.1007/s10886-013-0320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moree W J, Phelan V V, Wu C H, Bandeira N, Cornett D S, Duggan B M, Dorrestein P C. Interkingdom metabolic transformations captured by microbial imaging mass spectrometry. Proc Natl Acad Sci. 2012;109:13811–13816. doi: 10.1073/pnas.1206855109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li B, Bhandari D R, Römpp A, Spengler B. High-resolution MALDI mass spectrometry imaging of gallotannins and monoterpene glucosides in the root of Paeonia lactiflora. Sci Rep. 2016;6:36074. doi: 10.1038/srep36074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiono K, Hashizaki R, Nakanishi T, Sakai T, Yamamoto T, Ogata K, Harada K, Ohtani H, Katano H, Taira S. Multi-imaging of cytokinin and abscisic acid on the roots of rice ( Oryza sativa ) using matrix-assisted laser desorption/ionization mass spectrometry . J Agric Food Chem. 2017;65:7624–7628. doi: 10.1021/acs.jafc.7b02255. [DOI] [PubMed] [Google Scholar]
- 36.Wu W, Liang Z, Zhao Z, Cai Z. Direct analysis of alkaloid profiling in plant tissue by using matrix-assisted laser desorption/ionization mass spectrometry. J Mass Spectrom. 2007;42:58–69. doi: 10.1002/jms.1138. [DOI] [PubMed] [Google Scholar]
- 37.Shroff R, Vergara F, Muck A, Svatos A, Gershenzon J. Nonuniform distribution of glucosinolates in Arabidopsis thaliana leaves has important consequences for plant defense . Proc Natl Acad Sci. 2008;105:6196–6201. doi: 10.1073/pnas.0711730105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson D MG, Carolan V A, Crosland S, Sharples K R, Clench M R. Examination of the distribution of nicosulfuron in sunflower plants by matrix-assisted laser desorption/ionisation mass spectrometry imaging. Rapid Commun Mass Spectrom. 2009;23:1321–1327. doi: 10.1002/rcm.3973. [DOI] [PubMed] [Google Scholar]
- 39.Li B, Bhandari D R, Janfelt C, Römpp A, Spengler B. Natural products in Glycyrrhiza glabra (licorice) rhizome imaged at the cellular level by atmospheric pressure matrixassisted laser desorption/ionization tandem mass spectrometry imaging . Plant J. 2014;80:161–171. doi: 10.1111/tpj.12608. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez D J, Haste N M, Hollands A, Fleming T C, Hamby M, Pogliano K, Nizet V, Dorrestein P C. Microbial competition between Bacillus subtilis and Staphylococcus aureus monitored by imaging mass spectrometry . Microbiology. 2011;157:2485–2492. doi: 10.1099/mic.0.048736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y L, Xu Y, Straight P, Dorrestein P C. Translating metabolic exchange with imaging mass spectrometry. Nat Chem Biol. 2009;5:885–887. doi: 10.1038/nchembio.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esquenazi E, Coates C, Simmons L, Gonzalez D, Gerwick W H, Dorrestein P C. Visualizing the spatial distribution of secondary metabolites produced by marine cyanobacteria and sponges via MALDI-TOF imaging. Mol Biosyst. 2008;4:562–570. doi: 10.1039/b720018h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genji T, Fukuzawa S, Tachibana K. Distribution and possible function of the marine alkaloid, norzoanthamine, in the zoanthid Zoanthus sp. using MALDI imaging mass spectrometry . Mar Biotechnol. 2010;12:81–87. doi: 10.1007/s10126-009-9202-5. [DOI] [PubMed] [Google Scholar]
- 44.Elnaggar M S, Ebada S S, Ashour M L, Ebrahim W, Singab A, Lin W, Liu Z, Proksch P. Two new triterpenoids and a new naphthoquinone derivative isolated from a hard coral-derived fungus Scopulariopsis sp . Fitoterapia. 2017;116:126–130. doi: 10.1016/j.fitote.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Waters A L, Peraud O, Kasanah N, Sims J W, Kothalawala N, Anderson M A, Abbas S H, Rao K V, Jupally V R, Kelly M, Dass A, Hill R T, Hamann M T. An analysis of the sponge Acanthostrongylophora igens microbiome yields an actinomycete that produces the natural product manzamine A . Front Mar Sci. 2014;1:1789–1802. doi: 10.3389/fmars.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yarnold J E, Hamilton B R, Welsh D T, Pool G F, Venter D J, Carroll A R. High resolution spatial mapping of brominated pyrrole-2-aminoimidazole alkaloids distributions in the marine sponge Stylissa flabellata via MALDI-mass spectrometry imaging . Mol Biosyst. 2012;8:2249–2259. doi: 10.1039/c2mb25152c. [DOI] [PubMed] [Google Scholar]
- 47.Nemes P, Vertes A. Laser ablation electrospray ionization for atmospheric pressure, in vivo , and imaging mass spectrometry . Anal Chem. 2007;79:8098–8106. doi: 10.1021/ac071181r. [DOI] [PubMed] [Google Scholar]
- 48.Nemes P, Vertes A. London: Royal Society of Chemistry; 2014. Laser Ablation Electrospray Ionization Mass Spectrometry: Mechanisms, Configurations and imaging Applications; pp. 348–371. [Google Scholar]
- 49.Bartels B, Kulkarni P, Danz N, Bocker S, Saluz H P, Svatos A. Mapping metabolites from rough terrain: laser ablation electrospray ionization on non-flat samples. RSC Adv. 2017;7:9045–9050. [Google Scholar]
- 50.Nemes P, Barton A A, Li Y, Vertes A. Ambient molecular imaging and depth profiling of live tissue by infrared laser ablation electrospray ionization mass spectrometry. Anal Chem. 2008;80:4575–4582. doi: 10.1021/ac8004082. [DOI] [PubMed] [Google Scholar]
- 51.Dean S N, Walsh C, Goodman H, Van Hoek M L. Analysis of mixed biofilm ( Staphylococcus aureus and Pseudomonas aeruginosa ) by laser ablation electrospray ionization mass spectrometry . Biofouling. 2015;31:151–161. doi: 10.1080/08927014.2015.1011067. [DOI] [PubMed] [Google Scholar]
- 52.Nemes P, Vertes A.Atmospheric-pressure molecular imaging of biological tissues and biofilms by LAESI mass spectrometry J Vis Exp 201043pii:2097doi:10.3791/2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartels B, Svatoš A. Spatially resolved in vivo plant metabolomics by laser ablation-based mass spectrometry imaging (MSI) techniques: LDI-MSI and LAESI . Front Plant Sci. 2015;6:1–7. doi: 10.3389/fpls.2015.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Etalo D, de Vos R C, Joosten M H, Hall R. Spatially-resolved plant metabolomics: some potentials and limitations of laser-ablation electrospray ionization (LAESI) mass spectrometry metabolite imaging. Plant Physiol. 2015;169:1424–1435. doi: 10.1104/pp.15.01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Motley J L, Stamps B W, Mitchell C A, Thompson A T, Cross J, You J, Powell D R, Stevenson B S, Cichewicz R H. Opportunistic sampling of roadkill as an entry point to accessing natural products assembled by bacteria associated with non-anthropoidal mammalian microbiomes. J Nat Prod. 2017;80:598–608. doi: 10.1021/acs.jnatprod.6b00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takáts Z. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306:471–472. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 57.Perez C J, Tata A, de Campos M L, Peng C, Ifa D R. Monitoring toxic ionic liquids in zebrafish ( Danio rerio ) with desorption electrospray ionization mass spectrometry imaging (DESI-MSI) . J Am Soc Mass Spectr. 2017;28:1136–1148. doi: 10.1007/s13361-016-1515-9. [DOI] [PubMed] [Google Scholar]
- 58.Takáts Z, Wiseman J M, Cooks R G. Ambient mass spectrometry using desorption electrospray ionization (DESI): instrumentation, mechanisms and applications in forensics, chemistry, and biology. J Mass Spectrom. 2005;40:1261–1275. doi: 10.1002/jms.922. [DOI] [PubMed] [Google Scholar]
- 59.Eberlin L S, Ferreira C R, Dill A L, Ifa D R, Cooks G R. Desorption electrospray ionization mass spectrometry for lipid characterization and biological tissue imaging. Biochim Biophys Acta. 2011;1811:946–960. doi: 10.1016/j.bbalip.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiseman J M, Ifa D R, Zhu Y, Kissinger C B, Manicke N E, Kissinger P T, Cooks R G, Wiseman J M, Ifa D R, Zhu Y, Kissinger C B, Manicke N E, Kissinger P T, Cooks R G. Desorption electrospray ionization mass spectrometry: imaging drugs and metabolites in tissues. Proc Natl Acad Sci U S A. 2008;105:18120–18125. doi: 10.1073/pnas.0801066105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manicke N E, Wiseman J M, Ifa D R, Cooks R G. Desorption electrospray ionization (DESI) mass spectrometry and tandem mass spectrometry (MS/MS) of phospholipids and sphingolipids: ionization, adduct formation, and fragmentation. J Am Soc Mass Spectrom. 2008;19:531–543. doi: 10.1016/j.jasms.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Harris G A, Nyadong L, Fernandez F M. Recent developments in ambient ionization techniques for analytical mass spectrometry. Analyst. 2008;133:1297–1301. doi: 10.1039/b806810k. [DOI] [PubMed] [Google Scholar]
- 63.Venter A, Sojka P E, Cooks R G. Droplet dynamics and ionization mechanisms in desorption electrospray ionization mass spectrometry. Anal Chem. 2006;78:8549–8555. doi: 10.1021/ac0615807. [DOI] [PubMed] [Google Scholar]
- 64.Green F M, Salter T L, Gilmore I S, Stokes P, OʼConnor G. The effect of electrospray solvent composition on desorption electrospray ionisation (DESI) efficiency and spatial resolution. Analyst. 2010;135:731. doi: 10.1039/b924208b. [DOI] [PubMed] [Google Scholar]
- 65.Badu-Tawiah A, Bland C, Campbell D I, Cooks R G. Non-aqueous spray solvents and solubility effects in desorption electrospray ionization. J Am Soc Mass Spectrom. 2010;21:572–579. doi: 10.1016/j.jasms.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 66.Jackson A U, Talaty N, Cooks R G, Van Berkel G J. Salt tolerance of desorption electrospray ionization (DESI) J Am Soc Mass Spectrom. 2007;18:2218–2225. doi: 10.1016/j.jasms.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 67.Collins R T, Jones J J, Harris M T, Basaran O A. Electrohydrodynamic tip streaming and emission of charged drops from liquid cones. Nat Phys. 2008;4:149–154. [Google Scholar]
- 68.Sigmund P, Bitensky I S, Jensen J. Molecule and cluster bombardment: energy loss, trajectories, and collision cascades. Nucl Instruments Methods Phys Res Sect B. 1996;112:1–11. [Google Scholar]
- 69.Kasi S R, Kang H, Sass C S, Rabalais J W. Inelastic processes in low-energy ion-surface collisions. Surf Sci Rep. 1989;10:1–104. [Google Scholar]
- 70.Cooks R G, Jo S C, Green J. Collisions of organic ions at surfaces. Applied Surface Science. 2004;231:13–21. [Google Scholar]
- 71.Gologan B, Green J R, Alvarez J, Laskin J, Cooks R G. Ion/surface reactions and ion soft-landing. Phys Chem Chem Phys. 2005;7:1490–1500. doi: 10.1039/b418056a. [DOI] [PubMed] [Google Scholar]
- 72.Costa A B, Graham Cooks R. Simulated splashes: elucidating the mechanism of desorption electrospray ionization mass spectrometry. Chem Phys Lett. 2008;464:1–8. [Google Scholar]
- 73.Badu-Tawiah A K, Eberlin L S, Ouyang Z, Cooks R G. Chemical aspects of the extractive methods of ambient ionization mass spectrometry. Annu Rev Phys Chem. 2013;64:481–505. doi: 10.1146/annurev-physchem-040412-110026. [DOI] [PubMed] [Google Scholar]
- 74.Watrous J, Roach P, Alexandrov T, Heath B S, Yang J Y, Kersten R D, van der Voort M, Pogliano K, Gross H, Raaijmakers J M, Moore B S, Laskin J, Bandeira N, Dorrestein P C. Mass spectral molecular networking of living microbial colonies. Proc Natl Acad Sci U S A. 2012;109:E1743–E1752. doi: 10.1073/pnas.1203689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lanekoff I, Geydebrekht O, Pinchuk G E, Konopka A E, Laskin J. Spatially resolved analysis of glycolipids and metabolites in living Synechococcus sp. PCC 7002 using nanospray desorption electrospray ionization . Analyst. 2013;138:1971–1978. doi: 10.1039/c3an36716a. [DOI] [PubMed] [Google Scholar]
- 76.Watrous J, Roach P, Heath B, Alexandrov T, Laskin J, Dorrestein P C. Metabolic profiling directly from the Petri dish using nanospray desorption electrospray ionization imaging mass spectrometry. Anal Chem. 2013;85:10385–10391. doi: 10.1021/ac4023154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiseman J M, Ifa D R, Venter A, Cooks R G. Ambient molecular imaging by desorption electrospray ionization mass spectrometry. Nat Protoc. 2008;3:517–524. doi: 10.1038/nprot.2008.11. [DOI] [PubMed] [Google Scholar]
- 78.Bodzon-Kulakowska A, Drabik A, Ner J, Kotlinska J H, Suder P. Desorption electrospray ionisation (DESI) for beginners – how to adjust settings for tissue imaging. Rapid Commun Mass Spectrom. 2014;28:1–9. doi: 10.1002/rcm.6755. [DOI] [PubMed] [Google Scholar]
- 79.Tillner J, Wu V, Jones E A, Pringle S D, Karancsi T, Dannhorn A, Veselkov K, McKenzie J S, Takáts Z. Faster, more reproducible DESI-MS for biological tissue imaging. J Am Soc Mass Spectrom. 2017;28:2090–2098. doi: 10.1007/s13361-017-1714-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campbell D I, Ferreira C R, Eberlin L S, Cooks R G. Improved spatial resolution in the imaging of biological tissue using desorption electrospray ionization. Anal Bioanal Chem. 2012;404:389–398. doi: 10.1007/s00216-012-6173-6. [DOI] [PubMed] [Google Scholar]
- 81.Thunig J, Hansen S H, Janfelt C. Analysis of secondary plant metabolites by indirect desorption electrospray ionization imaging mass spectrometry. Anal Chem. 2011;83:3256–3259. doi: 10.1021/ac2004967. [DOI] [PubMed] [Google Scholar]
- 82.Laskin J, Heath B S, Roach P J, Cazares L, Semmes O J. Tissue imaging using nanospray desorption electrospray ionization mass spectrometry. Anal Chem. 2012;84:141–148. doi: 10.1021/ac2021322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwartz S A, Reyzer M L, Caprioli R M. Direct tissue analysis using matrix-assisted laser desorption/ionization mass spectrometry: practical aspects of sample preparation. J Mass Spectrom. 2003;38:699–708. doi: 10.1002/jms.505. [DOI] [PubMed] [Google Scholar]
- 84.Hemalatha R G, Pradeep T. Understanding the molecular signatures in leaves and flowers by desorption electrospray ionization mass spectrometry (DESI MS) imaging. J Agric Food Chem. 2013;61:7477–7487. doi: 10.1021/jf4011998. [DOI] [PubMed] [Google Scholar]
- 85.Müller T, Oradu S, Ifa D R, Cooks G R, Kräutler B, Müller T, Oradu S, Ifa D R, Cooks R G, Kräutler B. Direct plant tissue analysis and imprint imaging by desorption electrospray ionization mass spectrometry. Anal Chem. 2011;83:5754–5761. doi: 10.1021/ac201123t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ifa D R, Manicke N E, Rusine A L, Cooks R G. Quantitative analysis of small molecules by desorption electrospray ionization mass spectrometry from polytetrafluoroethylene surfaces. Rapid Commun Mass Spectrom. 2008;22:503–510. doi: 10.1002/rcm.3377. [DOI] [PubMed] [Google Scholar]
- 87.Li B, Knudsen C, Hansen N K, Jorgensen K, Kannangara R, Bak S, Takos A, Rook F, Hansen S H, Moller B L, Janfelt C, Bjarnholt N. Visualizing metabolite distribution and enzymatic conversion in plant tissues by desorption electrospray ionization mass spectrometry imaging. Plant J. 2013;74:1059–1071. doi: 10.1111/tpj.12183. [DOI] [PubMed] [Google Scholar]
- 88.Oetjen J, Veselkov K, Watrous J, McKenzie J S, Becker M, Hauberg-Lotte L, Kobarg J H, Strittmatter N, Mróz A K, Hoffmann F, Trede D, Palmer A, Schiffler S, Steinhorst K, Aichler M, Goldin R, Guntinas-Lichius O, von Eggeling F, Thiele H, Maedler K, Walch A, Maass P, Dorrestein P C, Takáts Z, Alexandrov T. Benchmark datasets for 3D MALDI- and DESI-imaging mass spectrometry. Gigascience. 2015;4:20. doi: 10.1186/s13742-015-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Inglese P, Mckenzie J S, Mroz A, Kinross J, Veselkov K, Holmes E, Takáts Z, Nicholson J K, Glen R C. Deep learning and 3D-DESI imaging reveal the hidden metabolic heterogeneity of cancer. Chem Sci. 2017;8:3500–3511. doi: 10.1039/c6sc03738k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rübel O, Greiner A, Cholia S, Louie K, Bethel E W, Northen T R, Bowen B P. OpenMSI: a high-performance web-based platform for mass spectrometry imaging. Anal Chem. 2013;85:10354–10361. doi: 10.1021/ac402540a. [DOI] [PubMed] [Google Scholar]
- 91.Song Y, Talaty N, Datsenko K, Wanner B L, Cooks R G. In vivo recognition of Bacillus subtilis by desorption electrospray ionization mass spectrometry (DESI-MS) . R Soc Chem. 2009;134:838–841. doi: 10.1039/b900069k. [DOI] [PubMed] [Google Scholar]
- 92.Zhang J I, Talaty N, Costa A B, Xia Y, Tao W A, Bell R, Callahan J H, Cooks R G. Rapid direct lipid profiling of bacteria using desorption electrospray ionization mass spectrometry. Int J Mass Spectrom. 2011;301:37–44. [Google Scholar]
- 93.Angolini C FF, Vendramini P H, Araújo F DS, Araújo W L, Augusti R, Eberlin M N, De Oliveira L G. Direct protocol for ambient mass spectrometry imaging on agar culture. Anal Chem. 2015;87:6925–6930. doi: 10.1021/acs.analchem.5b01538. [DOI] [PubMed] [Google Scholar]
- 94.Figueroa M, Jarmusch A K, Raja H A, El-Elimat T, Kavanaugh J S, Horswill A R, Cooks R G, Cech N B, Oberlies N H. Polyhydroxyanthraquinones as quorum sensing inhibitors from the guttates of Penicillium restrictum and their analysis by desorption electrospray ionization mass spectrometry . J Nat Prod. 2014;77:1351–1358. doi: 10.1021/np5000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jackson A U, Werner S R, Talaty N, Song Y, Campbell K, Cooks R G, Morgan J A. Targeted metabolomic analysis of Escherichia coli by desorption electrospray ionization and extractive electrospray ionization mass spectrometry . Anal Biochem. 2008;375:272–281. doi: 10.1016/j.ab.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 96.Tata A, Perez C, Campos M L, Bayfield M A, Eberlin M N, Ifa D R. Imprint desorption electrospray ionization mass spectrometry imaging for monitoring secondary metabolites production during antagonistic interaction of fungi. Anal Chem. 2015;87:12298–12305. doi: 10.1021/acs.analchem.5b03614. [DOI] [PubMed] [Google Scholar]
- 97.Sica V P, Raja H A, El-Elimat T, Oberlies N H. Mass spectrometry imaging of secondary metabolites directly on fungal cultures. RSC Adv. 2014;4:63221–63227. [Google Scholar]
- 98.Araújo F DS, Vieira R L, Molano E PL, Maximo H J, Dalio R JD, Vendramini P H, Araújo W L, Eberlin M N. Desorption electrospray ionization mass spectrometry imaging reveals chemical defense of Burkholderia seminalis against cacao pathogens . RSC Adv. 2017;7:29953–29958. [Google Scholar]
- 99.Watrous J, Hendricks N, Meehan M, Dorrestein P C. Capturing bacterial metabolic exchange using thin film desorption electrospray ionization-imaging mass spectrometry. Anal Chem. 2010;82:1598–1600. doi: 10.1021/ac9027388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tata A, Perez C J, Hamid T S, Bayfield M A, Ifa D R. Analysis of metabolic changes in plant pathosystems by imprint imaging DESI-MS. J Am Soc Mass Spectrom. 2015;26:641–648. doi: 10.1007/s13361-014-1039-0. [DOI] [PubMed] [Google Scholar]
- 101.Li B, Bjarnholt N, Hansen H, Janfelt C. Characterization of barley leaf tissue using direct and indirect desorption electrospray ionization imaging mass spectrometry. J Mass Spectrom. 2011;46:1241–1246. doi: 10.1002/jms.2010. [DOI] [PubMed] [Google Scholar]
- 102.Li B, Hansen S H, Janfelt C. International Journal of Mass Spectrometry direct imaging of plant metabolites in leaves and petals by desorption electrospray ionization mass spectrometry . Int J Mass Spectrom. 2013;348:15–22. [Google Scholar]
- 103.Cabral E C, Mirabelli M F, Perez C J, Ifa D R. Blotting assisted by heating and solvent extraction for DESI-MS imaging. J Am Soc Mass Spectrom. 2013;24:956–965. doi: 10.1007/s13361-013-0616-y. [DOI] [PubMed] [Google Scholar]
- 104.Mohana Kumara P, Srimany A, Ravikanth G, Uma Shaanker R, Pradeep T. Ambient ionization mass spectrometry imaging of rohitukine, a chromone anti-cancer alkaloid, during seed development in Dysoxylum binectariferum Hook.f (Meliaceae) . Phytochemistry. 2015;116:104–110. doi: 10.1016/j.phytochem.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 105.Lane A L, Nyadong L, Galhena A S, Shearer T L, Stout E P, Parry R M, Kwasnik M, Wang M D, Hay M E, Fernandez F M, Kubanek J. Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proc Natl Acad Sci. 2009;106:7314–7319. doi: 10.1073/pnas.0812020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Andras T D, Alexander T S, Gahlena A, Parry R M, Fernandez F M, Kubanek J, Wang M D, Hay M E. Seaweed allelopathy against coral: surface distribution of a seaweed secondary metabolite by imaging mass spectrometry. J Chem Ecol. 2012;38:1203–1214. doi: 10.1007/s10886-012-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eberlin L S, Norton I, Dill A L, Golby A J, Ligon K L, Santagata S, Cooks R G, Agar N YR. Classifying human brain tumors by lipid imaging with mass spectrometry. Mol Cell Pathobiol. 2012;72:645–655. doi: 10.1158/0008-5472.CAN-11-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Banerjee S, Zare R N, Tibshirani R J, Kunder C A, Nolley R, Fan R, Brooks J D, Sonn G A. Diagnosis of prostate cancer by desorption electrospray ionization mass spectrometric imaging of small metabolites and lipids. PNAS. 2017;114:3334–3339. doi: 10.1073/pnas.1700677114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miyamoto S, Hsu C C, Hamm G, Darshi M, Diamond-Stanic M, Declèves A E, Slater L, Pennathur S, Stauber J, Dorrestein P C, Sharma K. Mass spectrometry imaging reveals elevated glomerular ATP/AMP in diabetes/obesity and identifies sphingomyelin as a possible mediator. EBioMedicine. 2016;7:121–134. doi: 10.1016/j.ebiom.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Naraoka H, Hashiguchi M. In-situ organic compound analysis of the meteorite surface by desorption electrospray ionization coupled with an orbitrap mass spectrometer. In: 79th Annual Meeting of the Meteoritical Society August 7–12, 2016. Meteorit Planet Sci. 2016;51:6169. doi: 10.1002/rcm.8121. [DOI] [PubMed] [Google Scholar]
- 111.Woolman M, Tata A, Bluemke E, Dara D, Ginsberg H J, Zarrine-Afsar A. An assessment of the utility of tissue smears in rapid cancer profiling with desorption electrospray ionization mass spectrometry (DESI-MS) J Am Soc Mass Spectrom. 2017;28:145–153. doi: 10.1007/s13361-016-1506-x. [DOI] [PubMed] [Google Scholar]
- 112.Eberlin L S, Norton I, Orringer D, Dunn I F, Liu X, Ide J L, Jarmusch A K, Ligon K L, Jolesz F A, Golby A J, Santagata S, Agar N YR. Ambient mass spectrometry for the intraoperative molecular diagnosis of human brain tumors. PNAS. 2013;110:1611–1616. doi: 10.1073/pnas.1215687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Woolman M, Tata A, Dara D, Meens J, DʼArcangelo E, Perez C, Prova S, Bluemke E, Ginsberg H, Ifa D, McGuigan A, Ailles L, Zarrine-Afsar A. Rapid determination of tumour stroma ratio in squamous cell carcinomas with desorption electrospray ionization mass spectrometry (DESI-MS): a proof-of-concept demonstration. Analyst. 2017;142:3250–3260. doi: 10.1039/c7an00830a. [DOI] [PubMed] [Google Scholar]
- 114.Lisboa A, Alves-júnior M, Vilczaki N, Nogueira M, Almeida A, Sussulini A. Lipid mapping by desorption electrospray ionization mass spectrometry in a murine breast DMBA carcinogenesis model. Int J Mass Spectrom. 2016;418:86–91. [Google Scholar]
- 115.Sans M, Gharpure K, Tibshirani R, Zhang J, Liang L, Liu J, Young J H, Dood R L, Sood A K, Eberlin L S. Metabolic markers and statistical prediction of serous ovarian cancer aggressiveness by ambient ionization mass spectrometry imaging. Cancer Res. 2017;77:2903–2913. doi: 10.1158/0008-5472.CAN-16-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cooks R G, Manicke N E, Dill A L, Ifa D R, Eberlin L S, Costa A B, Wang H, Guangming H, Ouyang Z. New ionization methods and miniature mass spectrometers for biomedicine: DESI imaging for cancer diagnostics and paper spray ionization for therapeutic drug monitoring. R Soc Chem. 2011;149:247–267. doi: 10.1039/c005327a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kauppila T J, Talaty N, Kuuranne T, Kotiaho T, Cooks R G. Rapid analysis of metabolites and drugs of abuse from urine samples by desorption electrospray ionization-mass spectrometry. Analyst. 2007;132:868–875. doi: 10.1039/b703524a. [DOI] [PubMed] [Google Scholar]
- 118.Zhang J, Feider C L, Nagi C, Yu W, Carter S A, Suliburk J, Cao H ST, Eberlin L S. Detection of metastatic breast and thyroid cancer in lymph. J Am Soc Mass Spectrom. 2017;28:1166–1174. doi: 10.1007/s13361-016-1570-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ferreira C R, Pirro V, Eberlin L S, Hallett J E, Cooks R G. Developmental phases of individual mouse preimplantation embryos characterized by lipid signatures using desorption electrospray ionization mass spectrometry. Anal Bioanal Chem. 2012;404:2915–2926. doi: 10.1007/s00216-012-6426-4. [DOI] [PubMed] [Google Scholar]
- 120.González-Serrano A F, Pirro V, Ferreira C R, Oliveri P, Eberlin L S, Heinzmann J, Lucas-Hahn A, Niemann H, Cooks R G. Desorption electrospray ionization mass spectrometry reveals lipid metabolism of individual oocytes and embryos. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0074981. [DOI] [PMC free article] [PubMed] [Google Scholar]


