Abstract
The relationship between the spatial variability of soil multifunctionality (i.e. the capacity of soils to conduct multiple functions; SVM) and major climatic drivers, such as temperature and aridity, has never been assessed globally in terrestrial ecosystems. We surveyed 236 dryland ecosystems from six continents to evaluate the relative importance of aridity and mean annual temperature, and of other abiotic (e.g., texture) and biotic (e.g., plant cover) variables as drivers of SVM, calculated as the averaged coefficient of variation for multiple soil variables linked to nutrient stocks and cycling. We found that increases in temperature and aridity were globally correlated to increases in SVM. Some of these climatic effects on SVM were direct, but others were indirectly driven through reductions in the number of vegetation patches and increases in soil sand content. The predictive capacity of our structural equation modelling was clearly higher for the spatial variability of N- than for C- and P- related soil variables. In the case of N cycling, the effects of temperature and aridity were both direct and indirect via changes in soil properties. For C and P, the effect of climate was mainly indirect via changes in plant attributes. These results suggest that future changes in climate may decouple the spatial availability of these elements for plants and microbes in dryland soils. Our findings significantly advance our understanding of the patterns and mechanisms driving SVM in drylands across the globe, which is critical for predicting changes in ecosystem functioning in response to climate change.
Keywords: Multifunctionality, carbon cycling, nitrogen cycling, phosphorous cycling, spatial heterogeneity, climate change
Introduction
The uneven distribution of soil characteristics is a ubiquitous feature of most terrestrial ecosystems, and is known to regulate a wide range of ecosystem processes including individual plant performance and competitive ability (Day et al. 2003), community-level productivity (Maestre and Reynolds 2006), trophic interactions (Tsunoda et al. 2014), and nutrient cycling (Schlesinger et al. 1990, Ochoa-Hueso et al. 2018). This spatial variability in soil variables (SV hereafter) is largely controlled by the interaction of multiple biological, chemical and physical processes acting simultaneously at multiple scales (Jackson and Caldwell 1993, Farley and Fitter 1999, Zuo et al. 2010). While the environmental drivers of SV have been studied on individual functions and at local scales (e.g. N availability), the role of environment in driving multiple ecosystem functions simultaneously still needs to be considered to achieve an integrative understanding on the drivers of within-site SV (Byrnes et al. 2014).
Given the ubiquitous nature of SV and its role as a modulator of plant and soil fauna responses to climate change (García-Palacios et al. 2012), a deeper understanding of the major drivers of the spatial variability in multiple soil functions (i.e. soil multifunctionality, SVM) across the globe is of paramount importance to anticipate changes in ecosystem functioning under global change scenarios (Fraterrigo and Rusak 2008, IPCC 2013). Remarkably, a relatively large body of literature has explored the effects of SV locally or in regional comparisons (Linstädter et al. 2014, Guuroh et al. 2018), but no study has yet assessed the major environmental drivers of SVM in terrestrial ecosystems across the globe, which remains largely unexplored and poorly understood. This is particularly relevant for dryland ecosystems (i.e. arid, semi-arid and dry-subhumid ecosystems), where SVM is a widespread phenomenon (Schlesinger et al. 1990, Ochoa-Hueso et al. 2018). In drylands, which typically have a patchy plant distribution, SVM likely arises from the strong functional differences between vegetated patches, where plants largely drive biological processes such as litter decomposition or N fixation, and unvegetated areas, with higher importance of physical processes such as erosion by wind or water (Li et al. 2007). Drylands cover about 45% of Earth’s land surface and support more than 38% of the global human population (Prăvălie 2016), and their global extent may increase by up to 23% by the end of this century due to forecasted increases in aridity (Huang et al. 2016). These areas are particularly sensitive to the effects of climate change, so expected increases in aridity and temperature (up to 2-4 ºC, depending on projections, IPCC 2013) will promote changes in vegetation and soil properties that could have significant consequences for SVM in drylands worldwide. For instance, recent field surveys have found that increases in aridity are linked to decreases in plant cover and to increases in the encroachment rate of woody vegetation across the globe (Dougill and Thomas 2004, Vicente-Serrano et al. 2012, Delgado-Baquerizo et al. 2013), phenomena that would likely lead to parallel increases in SVM. However, to date, no study has considered the multiple direct and indirect (e.g. via plant and soil features) effects of temperature or aridity on SVM. The likely influence of climate on SVM, together with the well-known influence of SVM on ecosystem functioning, anticipate changes in the ability of dryland ecosystems to provide goods and services under ongoing climate change. This, together with the global importance of drylands and their particular sensitivity to climatic changes, justify efforts to better understand the role of major climate change drivers, such as temperature and aridity, in determining SVM globally directly and indirectly via plant and soil attributes.
Here, we used a database including 236 dryland sites from six continents (Fig. S1) to evaluate the role and relative importance of aridity and mean annual temperature, together with other key environmental factors (soil properties and plant attributes), as drivers of SVM in drylands worldwide. We hypothesized that increases in aridity and temperature will promote SVM directly, but also indirectly via reductions in plant cover and shifts in soil properties, as plant community composition and structure are largely known to be major drivers of soil spatial variability in drylands (Schlesinger et al. 1990).
Methods
We used data from a global field survey conducted in 236 dryland sites from 19 countries (Fig. S1; Argentina, Australia, Botswana, Brazil, Burkina Faso, Chile, China, Ecuador, Ghana, Iran, Israel, Kenya, Mexico, Morocco, Peru, Spain, Tunisia, USA, and Venezuela). These sites include the 224 drylands used in Maestre et al. (2012) plus 12 additional sites from Botswana, Ghana and Burkina Faso surveyed in 2012 and 2013. All sites surveyed were restricted to dryland ecosystems [defined as regions with an aridity index (AI = mean precipitation/mean potential evapotranspiration) between 0.05 and 0.65 (Gao and Giorgi 2008)] and encompassed a wide variety of dryland vegetation types, including grasslands, shrublands, savannas, dry seasonal forests and open, tree-dominated woodlands. Mean annual precipitation and temperature of the study sites ranged from 66 mm to 1219 mm, and from -1.8 ºC to 27.8 ºC.
Data collection was carried out between February 2006 and December 2013, focusing on 30 m x 30 m plots representative of the vegetation at each site, and using a standardized sampling protocol (Maestre et al. 2012). Soils were sampled during the dry season in most of the sites using a stratified random procedure. At each plot, five 50 cm × 50 cm quadrats were randomly placed under the canopy of the dominant perennial species and in open areas devoid of perennial vegetation. A composite sample consisting of five 145 cm3 soil cores (0 - 7.5 cm depth) was collected from each quadrat, bulked, and homogenized in the field. When more than one dominant plant species was present, samples were also collected under the canopies of five randomly selected individuals of the co-dominant species. Thus, the number of soil samples varied between 10 and 15 per site. Soil samples were taken to the laboratory, sieved (2 mm mesh), air-dried for one month and stored for laboratory analyses. In drylands, soil properties remain largely similar after air-drying (the most common status for these soils), so we did not expect large changes in soil properties after air-drying (Zornoza et al. 2006, 2009). To facilitate the comparison of results across sites, dried soil samples from all sites were shipped to Spain for laboratory analyses.
We measured the cover and number of perennial plant patches at each site using the line-intercept method along four 30 m long transects separated 8 m from each other (Brun and Box 2006). At each transect, we also surveyed 20 contiguous 1.5 m × 1.5 m quadrats (80 quadrats per site). Within these quadrats, we counted the number of species present to estimate species richness. Soil pH was measured with a pH meter, in a 1:2.5 (mass:volume, soil:water) suspension. Soil sand content was estimated according to (Kettler et al. 2001). Mean annual temperature (MAT) and aridity (1-aridity index [ratio of precipitation to potential evapotranspiration]) were obtained from Zomer et al. (2008), who used interpolations from the Worldclim global database (Hijmans et al. 2005).
We measured 14 variables closely related to C, N and P cycling and storage. Total N was determined using a CN analyzer (Leco CHN628 Series, Leco Corporation, St Joseph, MI, USA). Total organic C was determined by colorimetry after oxidation with a mixture of potassium dichromate and sulfuric acid (Anderson and Ingram 1993). Soil pentoses and hexoses, as well as soil ammonium and nitrate were measured colorimetrically from soil extracts as described in Delgado-Baquerizo et al. (2015). Soil samples (2.5 g of soil) were extracted with 0.5 m K2SO4 in a ratio 1:5. Extracts were shaken in an orbital shaker at 200 r.p.m. for 1 h at 20°C and filtered to pass a 0.45-μm Millipore filter (Jones and Willett 2006). Potential net N mineralization rate was estimated as the difference between initial and final inorganic N (sum of ammonium and nitrate) before and after incubation under potential conditions (Allen et al. 1986). Phosphatase activity was measured by determination of the amount of p-nitrophenol released from 0.5 g soil after incubation at 37°C for 1 h with the substrate p-nitrophenyl phosphate in MUB buffer (Tabatabai and Bremner 1969). The activity of b-glucosidase was assayed following the procedure for phosphatase, but using p-nitrophenyl-b-D-glucopyranoside as substrate and trishydroxymethyl aminomethane instead of NaOH when preparing the buffer (Tabatabai 1982). Available phosphorus was determined colorimetrically from sodium bicarbonate extracts (Moir and Tiessen 2007). Soil proteins, phenols and aromatic compounds were evaluated as detailed in Maestre et al. (2012). These variables were selected because they are considered good proxies of key ecosystem processes linked to soil fertility, nutrient cycling, biological productivity, and the build-up of nutrient pools.
To estimate SVM, we first calculated the variability within each site of the different soil variables measured. To do so, we calculated the site-level coefficient of variation (CV) using the composite soil samples obtained at each site (n per site varied between 10 and 15; see above). The CV is a relative measure of heterogeneity that can accommodate variance-mean scaling, avoiding the tendency for variance to increase with the mean (Duarte 1991). Therefore, it has been shown to be more useful than absolute measures of variability such as the standard deviation for comparing variability within biological properties (Schlesinger et al. 1990, Fraterrigo and Rusak 2008). We calculated SVM as the arithmetic mean for all individual site-level CVs of all soil variables. We also calculated the SVM of C-, N-, and P- related variables separately (hereafter C-SVM, N-SVM, and P-SVM) by calculating the arithmetic mean of individual site-level CVs of soil variables related to the C (i.e. total organic C, activity of b-glucosidase, phenols, aromatic compounds, hexoses and pentoses), N (total nitrogen, ammonium, nitrate, proteins, potential nitrification rate, and amino acids), and P (available phosphorus, and phosphatase activity) cycles.
To evaluate the effects of biotic and abiotic factors on SVM, we first explored the relationships between MAT, aridity and SVM using regression analysis. Then, we used random forest modelling (Breiman 2001) to identify the most important predictors of SVM among the following: latitude, longitude, mean annual temperature, aridity, plant species richness, plant cover, number of plant patches, the ratio between woody and herbaceous cover, soil sand, and soil pH. Finally, to achieve a system-level understanding of the major drivers of SVM, we used structural equation modelling (SEM, (Grace 2006)). In particular, we used SEM to evaluate the multiple direct and indirect effects of biotic and abiotic drivers on SVM (our a priori model based on our current knowledge is available in Fig. S2). Structural equation modelling is particularly useful in large-scale correlative studies because it allows us to partition causal influences among multiple variables, and to separate the direct and indirect effects of the predictors included in the model (Grace 2006). Variables were log- or square root-transformed, when necessary, to improve linearity in the relationships in our SEM models. There is no single universally accepted test of overall goodness of fit for SEM, applicable in all situations regardless of sample size or data distribution. Here we used the chi-squared test (χ2; the model has a good fit when χ2 is low, i.e., c. ≤ 2, and P is high, traditionally > 0.05); and the root-mean-square error of approximation (RMSEA; the model has a good fit when RMSEA is low, i.e., c. ≤ 0.05, and P is high, traditionally > 0.05) (Schermelleh-Engel et al. 2003). After verifying the adequate fit of our model, we were free to interpret the path coefficients of the model and their associated P-values. A path coefficient is analogous to the partial correlation coefficient, and describes the strength and sign of the relationships between two variables (Grace 2006). The probability that a path coefficient differs from zero was tested using bootstrap tests (Schermelleh-Engel et al. 2003). We calculated the standardized total effects of all biotic and abiotic drivers on the selected heterogeneity metrics (Grace 2006). The net influence that one variable had upon another was calculated by summing all direct and indirect pathways (effects) between two variables. Then we parameterized the model using our dataset and tested its overall goodness of fit. All SEM and regression analyses were conducted using AMOS 20 (IBM SPSS Inc., Chicago, IL, USA) and Sigma Plot 12, respectively (Systat Software Inc., San José, CA, USA). Random forest modelling was performed with R 3.3.2 using the rfPermute package (R Core Team, Vienna, Austria).
Results
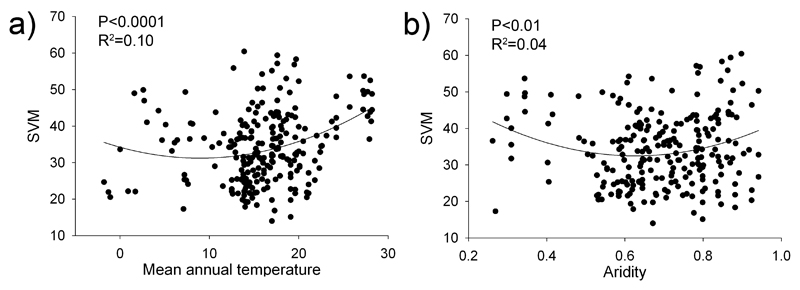
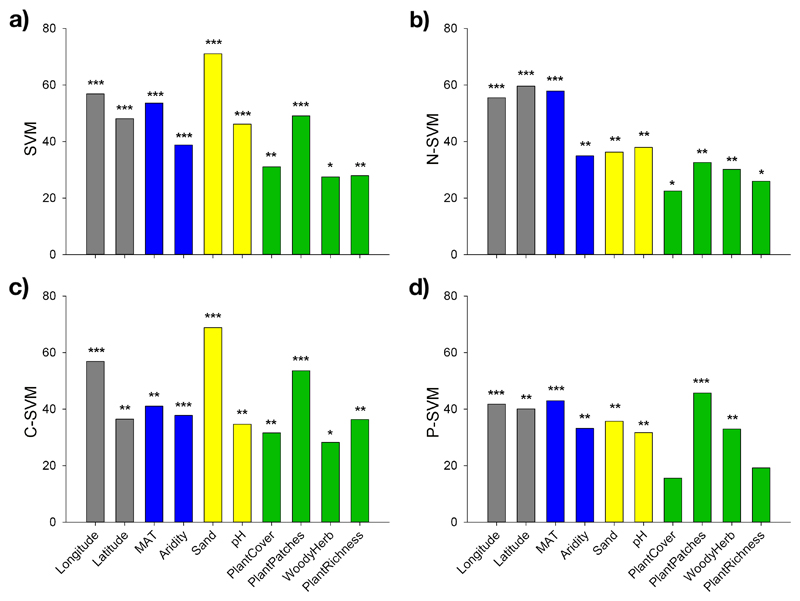
Regression analyses showed a positive quadratic relationship between SVM and mean annual temperature (MAT; Fig. 1a) and aridity (Fig. 1b). The random forest models indicated that all explored environmental factors were significant predictors of SVM, N-SVM, C-SVM, and P-SVM, except plant cover and plant richness in the case of P-SVM (Fig. 2). These models identified, in this order, (i) soil sand content, MAT, the number of plant patches per plot, soil pH, and aridity as the major individual predictors of SVM (Fig. 2a); (ii) MAT, soil pH and sand content, and aridity for N-SVM (Fig. 2b); (iii) soil sand content, the number of plant patches, MAT, and aridity for C-SVM (Fig. 2c); and (iv) the number of plant patches, MAT, soil sand content and aridity for P-SVM (Fig. 2d).
Figure 1.
Relationships between mean annual temperature and aridity [defined as 1-aridity index (ratio of precipitation to potential evapotranspiration)] and the variability (coefficient of variation) of soil multifunctionality (SVM). The solid lines represent the fitted regressions. R2 shows the proportion of variance explained.
Figure 2.
Random forest mean predictor importance (% of increase of MSE) of biotic and abiotic drivers on the spatial variability (coefficient of variation) of soil multifunctionality (SVM, a), N-related variables (SVM-N, b), C-related variables (SVM-C, c), and P-related variables (P-SVM, d). Significance levels of each predictor are as follows: *P<0.05, **P<0.01, and ***P<0.001.
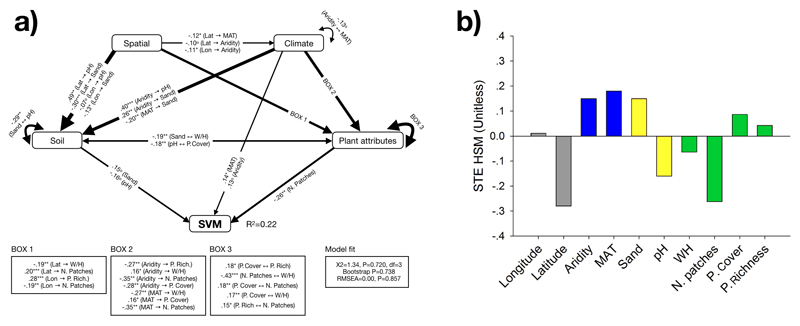
Our SEM explained around 22% of the variation in SVM (Fig. 3a). We found direct effects from both MAT and aridity on SVM (Fig. 3a). Moreover, we found multiple indirect effects of MAT and aridity on SVM via reductions in number of plant patches (Fig. 3a). Aridity also promoted SVM indirectly via positive effects on sand content. The standardized total effects (sum of direct and indirect effects from SEM) indicated that MAT and aridity were important predictors of SVM, with strong positive effects on SVM in both cases (Fig. 3b). Sand content (positive) and the number of plant patches and soil pH (negative) were also important drivers of SVM.
Figure 3.
(a) Effects of mean annual temperature, aridity, soil variables (pH and sand content), biotic attributes (plant cover and richness, number of plant patches and woody to herbaceous cover ratio [W/H]) and geographical coordinates (longitude and latitude) on the spatial variability (coefficient of variation) of soil multifunctionality (SVM). Numbers adjacent to arrows are standardized path coefficients, analogous to relative regression weights, and indicative of the effect size of the relationship. *P<0.05 and **P<0.01. Only significant relationships (P<0.05) are shown. Arrow widths are proportional to the strength of the relationship. Squares are observable variables. The proportion of variance explained (R2) appears alongside the response variable in the model. Goodness-of-fit statistics for each model are shown in the bottom (d.f.= degrees of freedom; RMSEA = root mean squared error of approximation). The components within spatial geolocation, climate, soil (properties) and plant attributes are included as independent observable variables in the model, however we group them in the same box in the model for graphical simplicity. (b) Standardized total effects (direct plus indirect effects derived from the structural equation models) of SVM predictors.
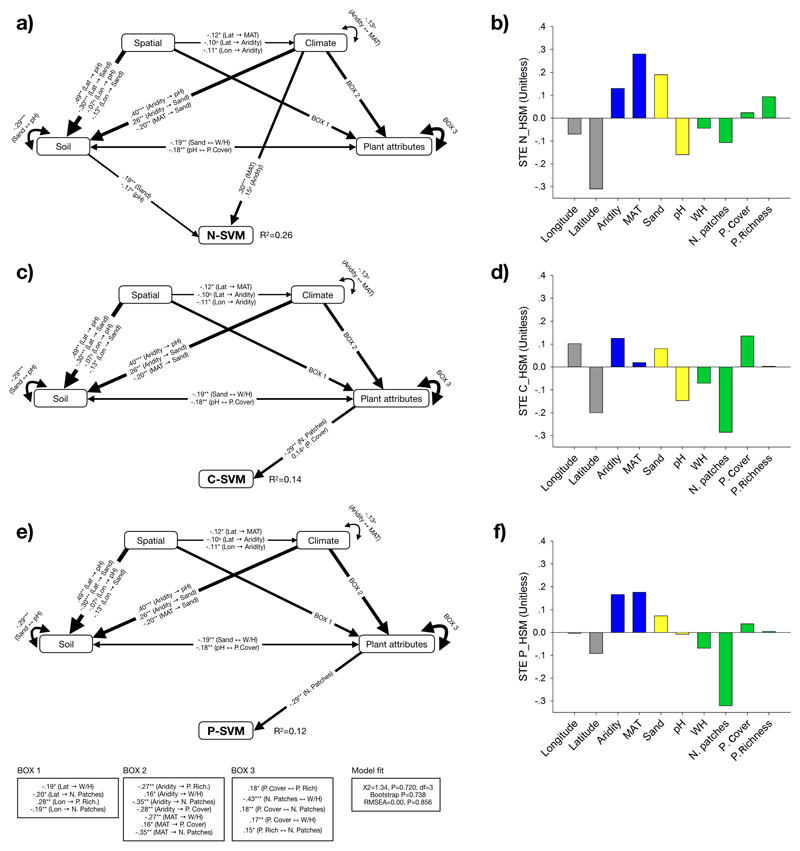
The element-specific SEMs explained 26%, 14% and 12% of the variation observed in N-, C-, and P-SVM, respectively (Fig. 4). The models showed that N-SVM was positively and significantly related to MAT and soil sand content, but negatively related to soil pH (Fig. 4a), whereas C-SVM and P-SVM were only negatively influenced by the number of plant patches (Figs. 4c, 4e). The standardized total effects indicated that N-SVM was positively influenced by MAT and soil sand content, but negatively by soil pH (Fig. 4b); C-SVM was positively regulated by aridity and plant cover, but negatively by the number of plant patches and soil pH (Fig. 4d); and P-SVM was mainly positively related to aridity and MAT, but strongly and negatively related to the number of plant patches (Fig. 4f).
Figure 4.
Effects of mean annual temperature, aridity, soil variables (pH and sand content), biotic attributes (plant cover and richness, number of plant patches, and woody to herbaceous cover ratio [W/H]) and geographical coordinates (longitude and latitude) on the spatial variability (coefficient of variation) of the soil multifunctionality of N- (a), C- (c), and P-(d) related variables (N-SVM, C-SVM, and P-SVM, respectively). Numbers adjacent to arrows are standardized path coefficients, analogous to relative regression weights, and indicative of the effect size of the relationship. *P<0.05 and **P<0.01. Only significant relationships (P<0.05) are shown. Arrow widths are proportional to the strength of the relationship. Squares are observable variables. The proportion of variance explained (R2) appears alongside the response variable in the model. Goodness-of-fit statistics for each model are shown in the bottom (d.f.= degrees of freedom; RMSEA = root mean squared error of approximation). The components within spatial geolocation, climate, soil (properties) and plant attributes are included as independent observable variables in the model, however we group them in the same box in the model for graphical simplicity. Plots (b), (d) and (f) show the standardized total effects (direct plus indirect effects derived from the structural equation models) of N-SVM, C-SVM, and P-SVM predictors, respectively.
Discussion
Our work represents the first global assessment of the major environmental predictors of the spatial variability of multiple surrogates of ecosystem functions (SVM). More importantly, we provide new mechanistic insights indicating that increases in aridity and mean annual temperature are linked to overall increases in the SVM of drylands across the globe both directly and indirectly (via reductions in plant patches and increases in sand content). This study builds on the seminal article from Schlesinger et al. (1990) that illustrated how the loss of vegetation cover due to human-driven disturbances can lead to changes in the heterogeneity of soil resources at the local scale. Although the amount of variance explained by our models is relatively low (R2<0.25), this is a common outcome in global surveys in which the variability of sampled sites is inevitably high (Moles et al. 2009). Furthermore, it is inherently challenging to characterize the heterogeneity of soil resources, as it can be affected simultaneously by different sources of variability that can operate and affect SVM at different temporal and spatial scales (Fraterrigo and Rusak 2008).
Our results demonstrate that changes in temperature and aridity are not only directly related to SVM, but also emerge from the influence of these climatic variables on vegetation and/or soil features. For instance, increases in aridity enhanced SVM mainly through increases in soil sand content and through reductions in the number of plant patches. On the other hand, increases in MAT were negatively related to SVM through decreases in soil sand content. However, this effect was clearly overcome by both the negative effect of MAT on the number of plant patches (which further decreases SVM) and the direct effects of temperature on SVM, resulting in a net positive and strong effect of increasing temperature on SVM. Results from previous studies conducted at the local scale suggest that any disturbance leading to decreases in vegetation cover (e.g. overgrazing) and increases in woody plant encroachment should increase SV (Schlesinger et al. 1990, Dougill and Thomas 2004) through the development of high fertility areas under and around plant canopies (characterized by higher production of above- and below-ground litter, leachates, and exudates and lower erosion rates), and low fertility areas in the zones devoid of perennial vascular vegetation (Schlesinger et al. 1990, Hook et al. 1991, Ochoa-Hueso et al. 2018). Our results provide partial empirical support for this hypothesis at a global scale in dryland ecosystems, and confirm that any impact of climatic variation on vegetation spatial variability might also have significant consequences for SVM globally.
Our mechanistic model suggests that total plant cover has limited direct effects on SVM (Fig. 3). However, a higher number of plant patches in our plots was strongly and directly linked to lower levels of SVM. Thus, it seems that more even inputs of litter and a higher capacity to redistribute soil nutrients spatially, as a result of a more homogeneous distribution of plant patches, are more important than the total area covered by plants to maintain lower levels of soil spatial variability, at least at the scale evaluated in this study. Previous studies show that increases in aridity, or other disturbances such as overgrazing, are tightly linked to plant cover losses and to increases in the percentage of sand-sized particles in the soil (Schlesinger et al. 1990, Li et al. 2007). Here, we show that increases in temperature and aridity such as those forecasted with climate change, and the likely concomitant decreases in total plant cover and increases in the amount of sand in the soil might result in a general increase in the spatial variability of soil resources and functionality in drylands worldwide (Vicente-Serrano et al. 2012, Delgado-Baquerizo et al. 2013). Perennial plants modify soil texture by decreasing losses (or increasing the accumulation) of fine soil particles, thus decreasing the relative abundance of sand-sized grains in the soil (Linstädter and Baumann 2013). This process is likely to increase the functional differences between vegetated areas (where plants typically drive soil biological processes and promote soil nutrient redistribution via roots and microorganisms; Schlesinger et al. 1990, Hook et al. 1991), and open areas between plant patches dominated by physical processes such as wind and water erosion (Li et al. 2007).
Soil heterogeneity drives many key ecosystem processes (Farley and Fitter 1999, Day et al. 2003, Maestre and Reynolds 2006, Zuo et al. 2010, Tsunoda et al. 2014), but predicting the effects of the observed changes in SVM is not trivial. According to the traditional view of spatial heterogeneity as a driver of species diversity, increased SVM should promote plant and soil biota diversity (Bakker et al. 2003, Davies et al. 2005). Recent studies suggest that, more likely, SVM can both increase and decrease not only diversity, but also ecosystem function, depending on factors such as the scale of the heterogeneity, the environmental conditions, as well as species identity and composition (Hutchings et al. 2003). Increases in SVM in low productivity systems such as drylands could change the size symmetry of belowground competition, favouring larger (in the case of plants) or more mobile (in the case of soil biota) species, which are more capable of rapidly exploiting nutrient patches than smaller species (Rajaniemi 2007, Reynolds and Haubensak 2009). On the other hand, changes in SVM typically promote multiple plant morphological and physiological responses, such as changes in nutrient uptake kinetics, biomass allocation, and root production and morphology (Robinson 1994, García-Palacios et al. 2012). Thus, the ability of individual species (and individuals within species) to adapt to the forecasted increase (with climate change) in soil spatial variability will likely determine their establishment (Maestre et al. 2003), competitive ability (Robinson et al. 1999, Hodge 2004), productivity (Dougill and Thomas 2004), and survival rate (Wijesinghe et al. 2005).
Of particular interest were the different responses of the element-specific N-, C-, and P-SVM to the environmental predictors. The predictive capacity of our model was clearly higher for N-SVM than for C- and P-SVM. While we also found strong direct effects of aridity on N-SVM, the fate of C- and P-SVM was mainly indirectly driven by reductions in number of plant patches. Different ecosystem compartments or processes may have different sensitivities to the direct or indirect effects of aridity (Evans and Burke 2012), and past studies have shown asymmetrical responses of N, C, and P cycles to climate change, with N cycling being consistently the most susceptible among them (Durán et al. 2013). Several mechanisms linked to increases in aridity and temperature could be behind this different sensitivity of the spatial variability of N, C and P in soils. For instance, different microbial communities have different sensitivities to warming and drought, leading to the accumulation of different soil nutrient pools (Sheik et al. 2011). Also, unlike P and C, whose availabilities are principally linked to the parent material and the decomposition of litter from plant communities, respectively, soil N is fixed from the atmosphere by soil microbial communities (e.g., most cyanobacteria), which are common in many dryland soils (Schlesinger and Bernhardt 2012). Increases in aridity will increase the amount of potential habitat available for biocrust communities, which spread in the open areas between plant patches and form mosaics of multiple species (Delgado-Baquerizo et al. 2016a). Similarly, increases in aridity promote the abundance of autotrophic communities tightly linked to the N cycle (e.g. archaeal nitrifiers; Delgado-Baquerizo et al. 2016b). Thus, the strong links between aridity and the spatial distribution of N cycling-related microbial communities might help explain the strong direct effect of climate on N-SVM, not observed for P- or C-SVM. On the contrary, the strong indirect effects of aridity on C- and P-SVM via reductions in number of plant patches might be related to reduced litter decomposition, as our C- and P-SVM indexes include the activities of enzymes, such as beta-glucosidase and phosphatase, that are involved in the degradation of organic matter into simpler C and P components.
The biogeochemical cycles of N, C, and P are tightly interlinked in terrestrial ecosystems by processes such as photosynthesis, atmospheric N fixation, respiration, decomposition and microbial mineralization (Vitousek 2004, Schlesinger and Bernhardt 2012). However, as these processes are likely to be altered, perhaps in different ways, by anthropogenic disturbances such as climate change, it has been suggested that N, C, and P cycles can become decoupled (Schlesinger et al. 1990, Peñuelas et al. 2012, Vicente-Serrano et al. 2012, Delgado-Baquerizo et al. 2013). Here we show that increasing aridity and temperature had stronger effects on the heterogeneity of N- than C-, and P-related variables. These results suggest expected changes in climate, by compromising the essential co-occurrence of areas with similar N, C and P contents, might lead to a decoupling of the spatial availability of these elements for plants and microbes in dryland soils across the globe. Heterogeneity is rarely employed as a response variable to assess the effects of human impacts on ecosystems, but recent studies indicate that it can be a sensitive metric per se, capturing effects and differences sometimes not detected by averaging (Underwood 1991, Callaghan and Holloway 1999, Fraterrigo and Rusak 2008). Indeed, whereas a recent study using our same database showed that C and N cycles are likely to become uncoupled from the P cycle in coming decades due to increasing aridity (Delgado-Baquerizo et al. 2013), our explicit consideration of SVM unveiled an additional (spatial) C-N-P decoupling mechanism that could have important consequences for ecosystem functioning (Schimel 2010, Finzi et al. 2011, Peñuelas et al. 2012, Delgado-Baquerizo et al. 2013).
Together, our work provides the first empirical evidence that changes in temperature and aridity are linked to alterations of the SVM in drylands across the globe. Our results also confirm that the direction of these effects is maintained when analyzing the spatial variability of N-, C-, and P-variables independently. However, the spatial variability of N-cycling processes was more sensitive to changes in temperature and aridity than that of C-, and P-cycling. Whereas the effects of aridity and temperature on N-SVM were mainly direct, in the case of C-SVM and P-SVM these effects were indirectly driven by reductions in the number of plant patches promoted by aridity. These findings significantly advance our understanding of the patterns and mechanisms driving the spatial heterogeneity of soil multifunctionality across the globe, which is critical for understanding the responses of terrestrial ecosystems to ongoing climate change.
Supplementary Material
Acknowledgements
This research was supported by the European Research Council (ERC) under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 242658 (BIOCOM), and by the Spanish Ministry of Economy and Competitiveness (BIOMOD, ref. CGL2013-44661-R). J.D. acknowledges support from the Fundação para Ciência e Tecnologia (IF/00950/2014). M.D-B. acknowledges support from the Marie Sklodowska-Curie Actions of the Horizon 2020 Framework Program H2020-MSCA-IF-2016 under REA grant agreement n° 702057. A.L. and R.T.G acknowledge support of the German Federal Ministry of Education and Research (BMBF) through WASCAL (grant 01LG1202-A). F.T.M. acknowledges support from the BIODESERT project (ERC grant agreement no. 647038).
References
- Allen SE, Grimshaw HM, Rowland AP. Chemical analysis. In: Moore PD, editor. Methods in Plant Ecology. Blackwell Scientific Publications; Oxford: 1986. pp. 285–344. [Google Scholar]
- Anderson JM, Ingram JS. Tropical Soil Biology and Fertility: A Handbook of Methods. 2nd edition. CABI Publishing; 1993. [Google Scholar]
- Bakker C, Blair JM, Knapp AK. Does resource availability, resource heterogeneity or species turnover mediate changes in plant species richness in grazed grasslands? Oecologia. 2003;137:385–391. doi: 10.1007/s00442-003-1360-y. [DOI] [PubMed] [Google Scholar]
- Breiman L. Random Forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- Brun JM, Box TW. A Comparison of Line Intercepts and Random Point Frames for Sampling Desert Shrub Vegetation. Rangeland Ecology & Management / Journal of Range Management Archives. 2006;16:21–25. [Google Scholar]
- Byrnes JEK, Gamfeldt L, Isbell F, Lefcheck JS, Griffin JN, Hector A, Cardinale BJ, Hooper DU, Dee LE, Emmett Duffy J. Investigating the relationship between biodiversity and ecosystem multifunctionality: challenges and solutions. Methods in Ecology and Evolution. 2014;5:111–124. [Google Scholar]
- Callaghan A, Holloway GJ. The Relationship between Environmental Stress and Variance. Ecological Applications. 1999;9:456–462. [Google Scholar]
- Davies KF, Chesson P, Harrison S, Inouye BD, Melbourne BA, Rice KJ. Spatial Heterogeneity Explains the Scale Dependence of the Native-Exotic Diversity Relationship. Ecology. 2005;86:1602–1610. [Google Scholar]
- Day KJ, Hutchings MJ, John EA. The effects of spatial pattern of nutrient supply on yield, structure and mortality in plant populations. Journal of Ecology. 2003;91:541–553. [Google Scholar]
- Delgado-Baquerizo M, García-Palacios P, Milla R, Gallardo A, Maestre FT. Soil characteristics determine soil carbon and nitrogen availability during leaf litter decomposition regardless of litter quality. Soil Biology and Biochemistry. 2015;81:134–142. [Google Scholar]
- Delgado-Baquerizo M, Maestre FT, Eldridge DJ, Bowker MA, Ochoa V, Gozalo B, Berdugo M, Val J, Singh BK. Biocrust-forming mosses mitigate the negative impacts of increasing aridity on ecosystem multifunctionality in drylands. New Phytologist. 2016a;209:1540–1552. doi: 10.1111/nph.13688. [DOI] [PubMed] [Google Scholar]
- Delgado-Baquerizo M, Maestre FT, Eldridge DJ, Singh BK. Microsite Differentiation Drives the Abundance of Soil Ammonia Oxidizing Bacteria along Aridity Gradients. Frontiers in Microbiology. 2016b:7. doi: 10.3389/fmicb.2016.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Baquerizo M, Maestre FT, Gallardo A, Bowker MA, Wallenstein MD, Quero JL, Ochoa V, Gozalo B, García-Gómez M, Soliveres S, García-Palacios P, et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature. 2013;502:672–676. doi: 10.1038/nature12670. [DOI] [PubMed] [Google Scholar]
- Dougill AJ, Thomas AD. Kalahari sand soils: Spatial heterogeneity, biological soil crusts and land degradation. Land Degradation & Development. 2004;15:233–242. [Google Scholar]
- Duarte CM. Variance and the description of nature. In: Cole J, Lovett GM, Findlay S, editors. Comparative Analyses of Ecosystems. Springer-Verlag; New York: 1991. pp. 301–318. [Google Scholar]
- Durán J, Rodríguez A, Morse JL, Groffman PM. Winter climate change effects on soil C and N cycles in urban grasslands. Global change biology. 2013;19:2826–2837. doi: 10.1111/gcb.12238. [DOI] [PubMed] [Google Scholar]
- Evans SE, Burke IC. Carbon and Nitrogen Decoupling Under an 11-Year Drought in the Shortgrass Steppe. Ecosystems. 2012;16:20–33. [Google Scholar]
- Farley RA, Fitter AH. Temporal and spatial variation in soil resources in a deciduous woodland. Journal of Ecology. 1999;87:688–696. [Google Scholar]
- Finzi AC, Austin AT, Cleland EE, Frey SD, Houlton BZ, Wallenstein MD. Responses and feedbacks of coupled biogeochemical cycles to climate change: examples from terrestrial ecosystems. Frontiers in Ecology and the Environment. 2011;9:61–67. [Google Scholar]
- Fraterrigo JM, Rusak JA. Disturbance-driven changes in the variability of ecological patterns and processes. Ecology Letters. 2008;11:756–770. doi: 10.1111/j.1461-0248.2008.01191.x. [DOI] [PubMed] [Google Scholar]
- Gao X, Giorgi F. Increased aridity in the Mediterranean region under greenhouse gas forcing estimated from high resolution simulations with a regional climate model. Global and Planetary Change. 2008;62:195–209. [Google Scholar]
- García-Palacios P, Maestre FT, Bardgett RD, de Kroon H. Plant responses to soil heterogeneity and global environmental change. The Journal of ecology. 2012;100:1303–1314. doi: 10.1111/j.1365-2745.2012.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace JB. Structural Equation Modeling and Natural Systems. Cambridge University Press; 2006. [Google Scholar]
- Guuroh RT, Ruppert JC, Ferner J, Čanak K, Schmidtlein S, Linstädter A. Drivers of forage provision and erosion control in West African savannas—A macroecological perspective. Agriculture, Ecosystems & Environment. 2018;251:257–267. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist. 2004;162:9–24. [Google Scholar]
- Hook PB, Burke IC, Lauenroth WK. Heterogeneity of soil and plant N and C associated with individual plants and openings in North American shortgrass steppe. Plant and Soil. 1991;138:247–256. [Google Scholar]
- Huang J, Yu H, Guan X, Wang G, Guo R. Accelerated dryland expansion under climate change. Nature Climate Change. 2016;6:166–171. [Google Scholar]
- Hutchings MJ, John EA, Wijesinghe DK. Toward understanding the consequences of soil heterogeneity for plant populations and communities. Ecology. 2003;84:2322–2334. [Google Scholar]
- IPCC. Summary for Policymakers. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, United Kingdom and New York, NY, USA: 2013. pp. 1–30. [Google Scholar]
- Jackson RB, Caldwell MM. The Scale of Nutrient Heterogeneity Around Individual Plants and Its Quantification with Geostatistics. Ecology. 1993;74:612–614. [Google Scholar]
- Jones DL, Willett VB. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biology and Biochemistry. 2006;38:991–999. [Google Scholar]
- Kettler TA, Doran JW, Gilbert TL. Simplified Method for Soil Particle-Size Determination to Accompany Soil-Quality Analyses. Soil Science Society of America Journal. 2001;65:849–852. [Google Scholar]
- Li J, Okin GS, Alvarez L, Epstein H. Quantitative effects of vegetation cover on wind erosion and soil nutrient loss in a desert grassland of southern New Mexico, USA. Biogeochemistry. 2007;85:317–332. [Google Scholar]
- Linstädter A, Baumann G. Abiotic and biotic recovery pathways of arid rangelands: Lessons from the High Atlas Mountains, Morocco. CATENA. 2013;103:3–15. [Google Scholar]
- Linstädter A, Schellberg J, Brüser K, García CAM, Oomen RJ, du Preez CC, Ruppert JC, Ewert F. Are There Consistent Grazing Indicators in Drylands? Testing Plant Functional Types of Various Complexity in South Africa’s Grassland and Savanna Biomes. PLOS ONE. 2014;9:e104672. doi: 10.1371/journal.pone.0104672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Cortina J, Bautista S, Bellot J, Vallejo R. Small-scale Environmental Heterogeneity and Spatiotemporal Dynamics of Seedling Establishment in a Semiarid Degraded Ecosystem. Ecosystems. 2003;6:630–643. [Google Scholar]
- Maestre FT, Quero JL, Gotelli NJ, Escudero A, Ochoa V, Delgado-Baquerizo M, García-Gómez M, Bowker MA, Soliveres S, Escolar C, García-Palacios P, et al. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science. 2012;335:214–218. doi: 10.1126/science.1215442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Reynolds JF. Biomass responses to elevated CO2, soil heterogeneity and diversity: an experimental assessment with grassland assemblages. Oecologia. 2006;151:512–520. doi: 10.1007/s00442-006-0577-y. [DOI] [PubMed] [Google Scholar]
- Moir J, Tiessen H. Page Soil Sampling and Methods of Analysis. Second Edition. CRC Press; 2007. Characterization of Available P by Sequential Extraction. [Google Scholar]
- Moles AT, Warton DI, Warman L, Swenson NG, Laffan SW, Zanne AE, Pitman A, Hemmings FA, Leishman MR. Global patterns in plant height. Journal of Ecology. 2009;97:923–932. [Google Scholar]
- Ochoa-Hueso R, Eldridge DJ, Delgado-Baquerizo M, Soliveres S, Bowker MA, Gross N, Bagousse-Pinguet YL, Quero JL, García-Gómez M, Valencia E, Arredondo T, et al. Soil fungal abundance and plant functional traits drive fertile island formation in global drylands. Journal of Ecology. 2018;106:242–253. [Google Scholar]
- Peñuelas J, Sardans J, Rivas-ubach A, Janssens IA. The human-induced imbalance between C, N and P in Earth’s life system. Global Change Biology. 2012;18:3–6. [Google Scholar]
- Prăvălie R. Drylands extent and environmental issues. A global approach. Earth-Science Reviews. 2016;161:259–278. [Google Scholar]
- Rajaniemi TK. Root foraging traits and competitive ability in heterogeneous soils. Oecologia. 2007;153:145–152. doi: 10.1007/s00442-007-0706-2. [DOI] [PubMed] [Google Scholar]
- Reynolds HL, Haubensak KA. Soil fertility, heterogeneity, and microbes: towards an integrated understanding of grassland structure and dynamics. Applied Vegetation Science. 2009;12:33–44. [Google Scholar]
- Robinson D. The responses of plants to non-uniform supplies of nutrients. New Phytologist. 1994;127:635–674. doi: 10.1111/j.1469-8137.1994.tb02969.x. [DOI] [PubMed] [Google Scholar]
- Robinson D, Hodge A, Griffiths BS, Fitter AH. Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proceedings of the Royal Society B: Biological Sciences. 1999;266:431. [Google Scholar]
- Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. Methods of Psychological Research-Online. 2003;8:23–74. [Google Scholar]
- Schimel DS. Drylands in the Earth System. Science. 2010;327:418–419. doi: 10.1126/science.1184946. [DOI] [PubMed] [Google Scholar]
- Schlesinger WH, Bernhardt ES. Biogeochemistry an analysis of global change. 2012. [Google Scholar]
- Schlesinger WH, Reynolds JF, Cunningham GL, Huenneke LF, Jarrell WM, Virginia RA, Whitford WG. Biological Feedbacks in Global Desertification. Science. 1990;247:1043–1048. doi: 10.1126/science.247.4946.1043. [DOI] [PubMed] [Google Scholar]
- Sheik CS, Beasley WH, Elshahed MS, Zhou X, Luo Y, Krumholz LR. Effect of warming and drought on grassland microbial communities. The ISME Journal. 2011;5:1692–1700. doi: 10.1038/ismej.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai MA. Methods in soil analyses. Part 2. American Society of Agronomy; Madison, WI (USA): 1982. [Google Scholar]
- Tabatabai MA, Bremner JM. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biology and Biochemistry. 1969;1:301–307. [Google Scholar]
- Tsunoda T, Kachi N, Suzuki J-I. Interactive effects of soil nutrient heterogeneity and belowground herbivory on the growth of plants with different root foraging traits. Plant and Soil. 2014;384:327–334. [Google Scholar]
- Underwood AJ. Beyond BACI: Experimental designs for detecting human environmental impacts on temporal variations in natural populations. 1991:42–569. 7583 Monitoring: Essential Foundation for Ecological Risk Assessment - DRAFT Page 11 of 11 Res. [Google Scholar]
- Vicente-Serrano SM, Zouber A, Lasanta T, Pueyo Y. Dryness is accelerating degradation of vulnerable shrublands in semiarid Mediterranean environments. Ecological Monographs. 2012;82:407–428. [Google Scholar]
- Vitousek PM. Nutrient Cycling and Limitation: Hawai’i as a Model System. Princeton University Press; 2004. [Google Scholar]
- Wijesinghe DK, John EA, Hutchings MJ. Does pattern of soil resource heterogeneity determine plant community structure? An experimental investigation. Journal of Ecology. 2005;93:99–112. [Google Scholar]
- Zomer RJ, Trabucco A, Verchot LV, Muys B. Land Area Eligible for Afforestation and Reforestation within the Clean Development Mechanism: A Global Analysis of the Impact of Forest Definition. Mitigation and Adaptation Strategies for Global Change. 2008;13:219–239. [Google Scholar]
- Zornoza R, Guerrero C, Mataix-Solera J, Arcenegui V, García-Orenes F, Mataix-Beneyto J. Assessing air-drying and rewetting pre-treatment effect on some soil enzyme activities under Mediterranean conditions. Soil Biology and Biochemistry. 2006;38:2125–2134. [Google Scholar]
- Zornoza R, Mataix-Solera J, Guerrero C, Arcenegui V, Mataix-Beneyto J. Storage Effects on Biochemical Properties of Air-Dried Soil Samples from Southeastern Spain. Arid Land Research and Management. 2009;23:213–222. [Google Scholar]
- Zuo XA, Zhao XY, Zhao HL, Guo YR, Zhang TH, Cui JY. Spatial pattern and heterogeneity of soil organic carbon and nitrogen in sand dunes related to vegetation change and geomorphic position in Horqin Sandy Land, Northern China. Environmental Monitoring and Assessment. 2010;164:29–42. doi: 10.1007/s10661-009-0872-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.