Abstract
Epstein-Barr virus (EBV) was the first human tumor virus discovered more than 50 years ago. EBV-associated lymphomagenesis is still a significant viral-associated disease as it involves a diverse range of pathologies, especially B-cell lymphomas. Recent development of high-throughput next-generation sequencing technologies and in vivo mouse models have significantly promoted our understanding of the fundamental molecular mechanisms which drive these cancers and allowed for the development of therapeutic intervention strategies. This review will highlight the current advances in EBV-associated B-cell lymphomas, focusing on transcriptional regulation, chromosome aberrations, in vivo studies of EBV-mediated lymphomagenesis, as well as the treatment strategies to target viral-associated lymphomas.
Keywords: Epstein-Barr virus, Latent infection, B-cell lymphomas
5.1 Introduction
Approximately two million new cases of cancer are annually attributed to infectious agents. 12% to 15% of human cancers are associated with oncogenic virus infection and are suspected to be major drivers [1, 2]. Uncovering the roles of infectious agents will help facilitate our understanding of the mechanism of cancer pathogenesis mediated by infectious agents and develop potential methods for therapeutic intervention. Epstein-Barr virus (EBV), also known as herpesvirus 4, was the first human tumor virus to attract significant attention since it was discovered associated with Burkitt’s lymphoma in 1964 [3]. EBV infects more than 95% of the world’s population and sustains lifelong asymptomatic infection. Its ability to induce oncogenesis is likely due to suppression of the immune system or a result of the uncontrolled proliferation. A recent study demonstrated that 1.8% of cancer deaths were related to EBV-attributable malignancies worldwide [4].
Initial infection of EBV is usually asymptomatic or can cause infectious mononucleosis (IM) [5]. The following lytic infection in epithelial cells results in the expression of the complete viral gene program. Previous studies clearly showed that EBV had the ability to transform human primary B lymphocytes into lymphoblastoid cell lines (LCLs) [6, 7]. To date, EBV is still the most efficient transforming virus in culture and can rapidly transform resting B cells in vitro [8, 9]. The persistence of EBV infection is mainly in B cells and leads to EBV associated B-cell lymphoma, typically in individuals with suppressed immune systems. Nasopharyngeal carcinoma (NPC) and EBV-associated gastric carcinoma (GC) are also related with EBV-infected epithelial cells, but whether or not the virus is a major contribution to the pathogenesis of these tumors is still unclear. Therefore, the presence and precise contributions of EBV to numerous human cancers is a challenge to explain. However, it also provides a great opportunity to the development of novel prophylactic or therapeutic methods.
5.2 EBV-Associated B-Cell Lymphomas
5.2.1 Burkitt’s Lymphoma (BL)
Burkitt’s lymphoma (BL) can be classified into three forms based on the geographic distribution: endemic BL (eBL), sporadic BL (sBL), and HIV-associated BL [10]. The discovery of EBV in BL tumors and the fact that almost 100% of endemic BL are EBV positive support the possibility that BL tumors are driven by EBV as a major contributor. Further sera-epidemiological studies have provided evidence that African BL tumors are positive for EBV [11]. One critical feature of BL tumors is the translocation and activation of MYC [10]. MYC overexpression in BL tumors results from a translocation event between the MYC gene and immunoglobulin locus which further regulates the downstream network and facilitates tumorigenesis [12, 13]. Most EBV-positive BL tumors consistently express latent antigen EBNA1 as the predominant latent antigen and are termed latency I [14]. Previous studies show that EBNA1 can play antiapoptotic roles which also contributes to increased tumorigenicity [15, 16]. In addition, and different from that observed in Africa, only 15–20% of BL tumors are EBV positive in other parts of the world [12]. The extremely uncommon observation is consistent with the fact that EBV together with malaria can increase the frequency of BL tumors. However, the mechanism of their interaction is not fully understood and needs further investigation [12, 17].
5.2.2 Diffuse Large B-Cell Lymphoma (DLBCL)
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma (NHL), accounting for 40% of adult NHL [18]. Two major subtypes of DLBCL, germinal center B cell (GCB) and activated B cell (ABC), were divided based on genomic signatures [19]. Approximately 10% DLBCL is EBV positive, which has been described in the World Health Organization (WHO) classification system [20]. EBV-positive DLBCL is mainly identified in the elderly because the median age of these patients is 71 years, although in younger patients can also be found [20, 21]. The incidence of EBV among DLBCL patients is less than 5% in the United States and European countries but 10–15% in Asian and Latin American countries [21–24]. EBV-positive DLBCL is associated with activation of NF-κB and JAK/STAT signaling pathways, but the detailed mechanisms of tumorigenesis will need to be further investigated [25].
5.2.3 Posttransplant Lymphoproliferative Disease (PTLD)
Posttransplant lymphoproliferative disease (PTLD) is mainly derived from B cells in transplant patients [26, 27]. It is often associated with EBV infection in the context of an impaired immune surveillance system. Furthermore, 60–80% of PTLDs are shown to be EBV positive [28]. EBV is the crucial driver of PTLDs development that is typically early-onset cases of posttransplantation [29]. Early-onset PTLDs that are associated with EBV-infected B cells are usually polyclonal or oligoclonal, while most late-onset PTLDs with or without EBV infection are monoclonal [30]. The transplant-associated immunosuppression in PTLDs leads to expression of EBNA3 family members in addition to all the latent antigens, which are characteristics of latency III-associated EBV infection [9]. The prevention and treatment of EBV-associated PTLDs rely on surgery with irradiation, immunotherapy with monoclonal antibodies (e.g., rituximab), and antiviral drugs [31]. The development of T-cell-based therapies has been very promising to treat EBV-driven PTLDs by transferring patient-derived ex vivo amplified EBV-specific cytotoxic T cells back to patients [32].
5.2.4 Hodgkin Lymphoma (HL)
Hodgkin lymphoma (HL) is characterized by the presence of Hodgkin-Reed-Sternberg (HRS) cells [33]. The direct link of EBV and HL is confirmed by the detection of EBER expression in HRS cells using EBER-specific in situ hybridization [34]. In addition, EBNA1, LMP1, and LMP2A are also expressed in EBV-infected HRS cells [35]. HL cells are B-cell originated and derived from the germinal center. They require the necessary signals to escape apoptosis as a result of the lack of functional BCRs [36]. Therefore, in EBV-infected HRS cells, LMP1 mimics the CD40 receptor, recruits TRAF family members, and further activates downstream NF-κB signaling pathways to promote cell survival by inhibiting cell apoptosis [37]. Meanwhile, LMP2A recruits cytoplasmic kinase to activate B-cell Ig receptors or activates the PI3K-AKT pathway in the absence of Ig receptors to promote B-cell survival and growth [9, 38].
5.2.5 EBV-Associated B-Cell Lymphoma in the Context of HIV
The increased reports of EBV-associated lymphomas with the onset of acquired immunodeficiency syndrome (AIDS) imply a molecular connection between EBV and HIV in the infected hosts [39]. In HIV-associated lymphomas, EBV infection can be found in 80% of DLBCL and 80–100% of primary central nervous system lymphomas (PCNSL) [40]. BL can occur before HIV infection even if circulating CD4+ T-cell numbers are normal. DLBCL typically occurs only after HIV infection when circulating CD4+ T cells are exhausted [41]. AIDS-BLs involve the typical MYC translocation and are less frequently infected by EBV [42, 43]. These observations suggest that HIV may be a potential stimulator which leads to an increase in the risk of EBV-mediated MYC translocation and therefore lymphomagenesis. Most AIDS-associated lymphomas that are EBV positive do express broad expression of the latent antigens and are type III latency program. This is likely due to the suppressed immune system and so a loss of control of the EBV-positive cells.
5.3 Molecular Biology of EBV-Mediated B-Cell Lymphomas
EBV is an oncogenic herpesvirus because of its ability to immortalize human primary B lymphocytes in vitro. In general, EBV primary infection is asymptomatic, and the following persistent infection will be established in memory B cells after an early period of virus production [44]. Therefore, two typical EBV infections can be established in the host: lytic infection in epithelial cells and latent infection in memory B cells [45, 46]. The initial events of EBV primary infection are the focus of current studies, but the detailed mechanisms are still not completely understood. In latent infection, specific transcription programs are defined as latency I, II, and III according to the expression of the viral-encoded latent antigens, which are thought to be the critical drivers of EBV-associated lymphomagenesis.
5.3.1 EBV-Associated Transcription Regulatory Network
One hallmark of cancer is the dysregulation of gene expression [47]. The characteristic of effective in vitro transformation by EBV indicates its strong ability to regulate cellular transcriptional programs. With the rapid development of high-throughput sequencing technologies, more and more studies are focused on a complicated regulatory network during EBV-mediated B-cell transformation by utilizing the common database such as NCBI GEO, ENCODE, and TCGA project [48–50].
To determine the molecular mechanisms which drive lymphomagenesis, EBV-transformed lymphoblastoid cell lines (LCLs) are one of the best systems to perform in vitro studies. More recent studies have concentrated on EBV latent protein-mediated regulatory networks using next-generation high-seq analysis, of which the frequently used is ChIP-seq (Table 5.1). ChIP-seq analysis indicated that EBNA2 can convert B lymphocytes to LCLs by targeting H3K4me1 modified sites as well as noncoding regions to regulate cellular gene expression to drive proliferation of LCLs [51]. In addition, EBNA2 induces a new pattern of genome-wide binding through recruitment of RBPJκ and EBF1 to drive LCL survival [52]. EBNA2 recruits the SWI/SNF ATPase BRG1 to bind large-scale MYC enhancers activating its expression [53]. EBNA-LP binds with B-cell transcription factors (TFs), which are highly similar to EBNA2 including RBPJκ and EBF [54]. These high-seq data provides evidence to support the explanation that both EBNA2 and EBNA-LP are crucial for LCL outgrowth. EBNA3C, another EBV latent antigen essential for LCLs growth, is associated with cellular transcription factors. It binds to BATF/IRF4 and SPI1/IRF4 sites to repress CDKN2A transcription through the recruitment of Sin3A in LCLs [55]. EBV latent proteins EBNA3A and EBNA3C inhibit BCL2L11 transcription by recruiting the H3K27 methyltransferase EZH2 to silence long-range enhancers [53]. ChIP-seq analysis shows that EBNA2 and EBNA3s (EBNA3A, EBNA3B, and EBNA3C) can target multiple cellular genes through cell-specific regulation of long-range enhancer-promoter interactions [56]. Another study indicated that while these four latent antigens can competitively bind to RBPJκ at its repressive sites to control cellular genes expression, EBNA3s are more likely to interact with other transcription factors [57]. For example, IRF4 is essential for EBNA3C to associate with specific sites on viral and cellular DNA [16, 55, 57, 58]. A recent study identified a number of host dependency factors in BL and LCLs using CRISPR/Cas9 loss-of-function screen [59]. These specific genes, including PI3K/AKT, cFLIP, BATF/IRF4, and IRF2, are likely crucial in regulating downstream transcriptional network to facilitate cell growth and survival.
Table 5.1.
The transcription factors (TFs) identified in ChIP-seq analysis
| Associated TFsa | Targets | Cell lines | References |
|---|---|---|---|
| EBNA1 | Human genome, | Raji | [4] |
| EBV latent promoter | |||
| EBNA2, RBPJG, CTCF, EBF, RELA, H3K9ac, H3K4me1, Pol II, P300 | Human genome | GM12878 | [51] |
| EBNA2, EBF1, RBPJk | Human genome, | LCL, | [52] |
| EBV latent promoter | Mutu III | ||
| EBNA2, H3K27Ac | Human genome | GM12878, | [53] |
| Mutu III | |||
| EBNA-LP, EBNA2 | Human genome | GM12878 | [54] |
| EBNA3C, Sin3A, REST, EBNA2, RBPJG, IRF4, BATF, SPI1, RUNX3, p300, Pol II, H3K4me1, H3K4me3, H3K9ac | Human genome | GM12878 | [55] |
| EBNA2, RBPJG, H3K27ac, H3K4me1, BRD4, P300, Pol II, BATF, EBF1, PAX5, SPI1, Sp1, NFAT, STAT5, ETS1, IRF4, CTCF, RAD21, SMC3, YY1; EBNA3A, EBNA3C, EBNA-LP, RelA, RelB, cRel, p50, p52 | Human genome | GM12878 | [60] |
| Notch1, RBPJG, ZNF143 | Human genome | GM12878 | [109] |
| EBNA3A, EBNA3C, EBNA2, RBPJG, BATF, IRF4, SPI1, RUNX3, NF-GB, MEF2A, PAX5, POU2F2, MAX, MYC, POL2, SIN3A, H3K27ac | Human genome | GM12878 | [110] |
| CHD2, CFOS, BRCA, EGR1, PBX3, BCL3, GCN5, p300, TBP, TAF1, CTCF, Pol II, TCF12, EBF1, SP1, PU.1, PAX5, BATF, JUND, SMC3, RAD21, H3K27ac | Human genome, | GM12878 | [111] |
| EBV latent promoter | |||
| CTCF, RAD21, RPB1 | EBV genome | Raji | [112] |
| Cohesin, RNA Polymerase II | |||
| EBNA3A, EBNA3B, EBNA3C | Human genome | LCL | [113] |
EBV latent antigens are underlined
During EBV primary infection, the correlative latent antigens convert resting B cells to LCLs, and their dependent function may rely on super-enhancers to control B-cell growth [60]. EBV super-enhancers (ESEs) with higher H3K27c signals involve the oncogenes MYC and Bcl2 to promote LCL growth and survival, which provides new insights on EBV-induced lymphoproliferation [60]. EBNA2, EBNA3A, and EBNA3C can enhance RUNX3 expression via RBPJκ to regulate the upstream RUNX3 super-enhancer and meanwhile control the downstream RUNX1 expression [61]. Additionally, abundant enhancers (eRNAs) are also transcribed from ESEs and are regulated by the activity of ESEs [62]. For example, the inactivation of EBNA2 and bromodomain-containing protein 4 (BRD4) in ESEs will significantly reduce the expression of eRNAs and further the MYC protein, therefore affecting LCL growth [62].
About 300 novel EBV transcripts have been predicted by combining multiple platform data from PacBio SMART Iso-Seq, RNA-Seq, and deep-CAGE, which illustrates the complex regulation of viral gene transcription during EBV infection [63]. Studies on miRNA targetome show that EBV miRNAs mainly target cellular transcription factors to manipulate the microenvironment during latent infection, suggesting the importance of EBV-expressed miRNAs in contributing to viral-mediated oncogenesis [64]. Furthermore, EBV miRNAs can modulate immune recognition to protect infected cells from killing by cytotoxic EBV-specific CD4+ T cells through repression of pro-inflammatory cytokine release, naïve CD4+ T-cell differentiation, and peptide presentation, which allow for establishment of latent infection and development of lymphomas [65]. Similarly, EBV miRNAs can use multiple pathways to evade immune surveillance and killing by EBV-specific CD8+ T cells [66].
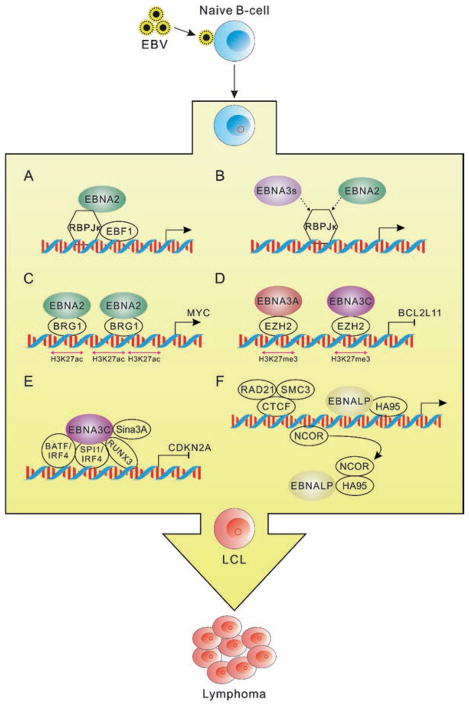
Although the development and manipulation of high-throughput sequencing technologies provide us a deeper and wider understanding of EBV-mediated transformation or lymphomagenesis (Fig. 5.1), the complicated regulatory network targeted by EBV latent infection is still being explored. Furthermore, systematic proteomic analyses can possibly validate some of the genomic observations and gain additional insights into EBV-host interactions [67]. In the future, more efficient systems and more advanced technologies with higher resolution, specificity, and sensitivity will be helpful in revealing the complex EBV-host interactions in associated lymphomas.
Fig. 5.1.
EBV latent antigen-associated cellular signaling pathways from the current high-throughput sequencing data during EBV-mediated lymphomagenesis. (a) EBNA2 regulates target genes expression through the recruitment of transcription factors RBPJκ and EBF1. (b) EBNA3s and EBNA2 bind with partially the same RBPJκ genomic sites. The interaction between RBPJκ and EBNA3s or EBNA2 will result in different effects of downstream gene expression, which are also associated with other EBNA-interacting cell transcription factors. (c) EBNA2 activates the three clusters of upstream enhancers of MYC promoter with increased H3K27Ac and BRG1 binding, and then EBNA2 mediates MYC activation through promoting the interaction of MYC promoter and the activated upstream enhancers. (d) EBNA3A and EBNA3C repress BCL2L11 expression by inactivating the upstream enhancers of its promoter. The inactivation is associated with increased H3K27me3 and EZH2 binding as well as the inhibition of interactions between BCL2L11 promoter and its enhancers. (e) EBNA3C binds to the promoters through BATF/IRF4, SPI1/IRF4, and RUNX and further recruits Sin3A to inhibit CDKN2A expression. (f) EBNA-LP regulates the derepression of target genes by removing NCOR repression complex from the promoters with the help of HA95 and further promotes the long-distance enhancer-promoter interaction through CTCF, RAD21, and SMC3 proteins. EBV latent antigens are highlighted by colorful patterns, while cellular factors are labeled with colorless patterns
5.3.2 Genomic Instability and Chromosome Aberrations
Genomic instability is a hallmark of cancer that increases the risks of oncogenic chromosome alterations [1, 9]. Previous studies have indicated that EBV persistent infection can result in chromosome aberrations in associated lymphomas [9, 26, 68]. EBV latent antigens play crucial roles in driving genomic instability. To be specific, EBNA1 may function to contribute to genomic instability through activation of the RAG gene or induction of reactive oxygen species (ROS) [17, 29]. EBNA3C can promote genomic instability by inhibiting BubR1 transcription and inactivating the mitotic spindle checkpoint [69]. Additionally, EBNA3C can compromise the mitotic spindle checkpoint and block caspase-mediated cell death, leading to abnormal mitosis and DNA damage accumulation [15, 70]. Although the detailed mechanism of EBNA3C-mediated genetic instability needs further investigation, multiple functions of EBNA3C may contribute to genetic instability directly or indirectly by binding with cell cycle or DNA damage checkpoint proteins, including cyclin A [71], Chk2 [72], cyclin D1 [73], p53 [74, 75], and the E2F family member E2F1/E2F6 [28, 76]. LMP1-associated genomic instability may also result from telomerase activation and DNA damage response (DDR) inhibition [69, 77]. Intriguingly, EBV tegument protein BNRF1 could also induce centrosome amplification and further chromosome instability during lytic infection, suggesting that EBV viral particles may be sufficient to modify host chromosome without the establishment of latent infection [78].
In addition, the EBV genome can frequently integrate into host cell chromosomes in persistently infected B cells [22, 79, 80]. This integration increases the possibility of lymphomagenesis when the constitutive regions release the viral genome which leads to loss of normal DNA or chromosome instability [81]. For instance, the integration of the EBV genome into chromosome 6q15 blocks the expression of the tumor repressor BACH2 in Burkitt lymphoma cell lines [80]. Using whole genome sequencing technology, a recent study reports that a comprehensive view of integration sites shows that they are randomly distributed across the entire host genome in EBV-positive Raji (Burkitt’s lymphoma cells), and C666-1 (nasopharyngeal carcinoma cells) and so may be contributing to lymphomagenesis [25]. The frequent chromosome recombination, involved in chromosome 8 and c-Myc activation, is also noted in Burkitt’s lymphoma cells after combined treatment with EBV and purified 4-deoxyphorbol ester [82].
5.3.3 In Vivo Models of EBV Infection
Host-range restriction is a major limitation of EBV research because humans are the exclusive natural host for EBV. Therefore, the development of a more efficient in vivo system to support the studies from in vitro results will provide additional information related to the complicated EBV-host interactions. An important achievement on in vivo system began with the development of scid-hu PBL mouse through the injection of human peripheral blood leukocytes (PBL) into C.B-17 scid mice that lack B and T cells because of the severe combined immunodeficiency (SCID) phenotype [83, 84]. Later, another scid-hu thy/liv mouse was generated by implanting fetal thymus, liver cells, and fetal lymph nodes into C.B-17 scid mice [85]. However, these mice have obvious shortcomings of generated graft versus host disease and transient immune responses [86]. Subsequently, a new series of mice models were generated to overcome the preceding disadvantages by transplanting human hematopoietic stem cells (HSCs) into various mice such as NOD/Shi-scid Il2rgnull (NOG) [87], BALB/c Rag2−/−Il2rg−/− (BRG) [88], and NOD/LtSz-scid Il2rg−/− (NSG) [89]. These transplanted HSCs reconstituted the human immune system by differentiating into diversified cells, including B cells, T cells, natural killer (NK) cells, dendritic cells (DCs), monocytes, and macrophages [86].
Given the great improvement in mouse models, it is possible to further study the mechanisms of EBV-associated lymphomagenesis in vivo using humanized mice. Previous studies have shown that EBV could infect humanized BALB/c Rag2−/−Il2rg−/− mice and induce specific T-cell response [88]. A mouse model for EBV infection was established by transplanting only CD34+-depleted human cord blood mononuclear cells into NOD/LtSz-scid Il2rg−/− (NSG) mice [90]. The results from this EBV-infected mouse model indicated that the PD-1/CTLA-4 blockade will induce strong specific T-cell responses and inhibit the outgrowth of EBV-associated lymphomas [90].
To further support human T cells which demonstrate HLA-restricted cytotoxic functions in mouse models, an immunodeficient NSG-HLA-A2/HHD mouse was created through the introduction of HLA-A2 allele into CD34+CD38− HSC- transplanted NSG mice [91]. The new mouse model showed a relatively complete immune system that expresses HLA class I heavy and light chains, promotes human T-cell development, and produces functional CD4+ and CD8+ T cells. In this mouse model, EBV infection will result in B-cell-associated lymphoproliferative diseases, which can be inhibited by HLA-restricted CTL cytotoxicity [91]. What’s more, NK cells are necessary to control infectious mononucleosis (IM) symptoms by targeting EBV lytic antigens and so control lytic infection [92]. Furthermore, NOD/SCID-hu BLT mice (or BLT mice) are developed by transplanting scid-hu thy/liv mice with autologous CD34+ cells which combines the advantages of scid-hu thy/liv mice model and CD34+ cell-transplanted NOD/SCID mice model [36]. BLT mice were shown to have a more complete human immune system, of which the T cells generate long-term, specific adaptive immune responses after EBV infection via human major histocompatibility complex (MHC) class I and II [36].
In 2011, an improved humanized mouse model was developed through the transplantation of human fetal CD34+ hematopoietic stem cells and thymus/liver tissue into NOD/LtSz-scid Il2rg−/− (NSG) mice [93]. The mouse model supports long-term EBV latent infection and lymphoma development. Further experiments showed that EBV lytic infection was critical for B-cell lymphomagenesis with limited help of the immune system [93]. The following application of this mouse model with wild-type EBV or LMP1-deficient EBV infection demonstrated that LMP1 may not be essential for EBV-mediated lymphomagenesis but that T cells may substitute LMP1 function for development of B-cell lymphomas [94].
Different from the application of humanized mouse model, a recent study reported establishment of a transgenic mouse model with conditional LMP1/2A coexpression in germinal center (GC) B cells [95]. In this mouse model, LMP1/2A showed very limited function in immunocompetent mice, while they promote B-cell lymphoproliferative diseases in the context of T-cell or NK-cell deficiency [95].
5.4 Treatment of B-Cell Lymphomas
Diffuse large B-cell lymphoma (DLBCL) continues to be one of the few lymphomas that remain curable due to advancements made over the last decade. More than half of the patients can be cured using treatments that include chemo-, radio-, or immunotherapeutic regimens [96]. However, approximately 30–40% of patients diagnosed will develop relapsed or refractory disease after being treated for DLBCL [97, 98]. Treatment of these patients has become extremely difficult due to the resistance that has grown with the disease [99]. The improved outcome in patients with DLBCL and relapsed-refractory DLBCL (RR-DLBCL) is largely attributed to the incorporation of rituximab into standard regimens [99, 100]. With further findings and introduction of novel specific anticancer agents and therapeutic approaches, treatment and survival of affected patients are likely to improve tremendously [101].
DLBCL is commonly treated with R-CHOP, a combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone, and it has shown great benefits for patients [102]. Tolerance in patients of all ages has been demonstrated, and survival rates have increased, specifically in patients diagnosed with non-Hodgkin’s lymphoma [103]. Recent findings indicate that in combination with rituximab or R-CHOP, drugs lenalidomide and epratuzumab could be effective in not only first-line treatment of DLBCL but also RR-DLBCL [96]. Other novel agents such as ibrutinib, bortezomib, CC-122, and pidilizumab have been shown to be successful in the first-line treatment of DLBCL as both single agents or in combination with rituximab-based chemotherapy [96]. Studies have also investigated the role of the NF-κB/Rel family, specifically nuclear factor kappa-B (NF-κB) and RelA (p65) in DLBCL. High p65 nuclear expression is a significant adverse biomarker in patients with early-stage (I/II) DLBCL [104]. Findings have shown that with p65 inactivation, cell growth and survival can be effectively inhibited. Furthermore, activation of the JAK-STAT and NF-κB pathways is characteristic of EBV-positive DLBCL [25]. Therefore, development of therapies targeting these pathways would be of potential benefit for these patients and lead to an improvement in their post-therapy outcomes.
Another major development in the treatment of DLBCL is CAR T-cell therapy. This therapy utilizes chimeric antigen receptor (CAR)-engineered T cells specifically engineered to recognize their target antigen through the scFv-binding domain [105]. This recognition results in the activation of T cells in a major histocompatibility complex (MHC)-independent manner [106]. Investigation of this therapy has demonstrated promising outcomes by targeting CD19, CD20, or CD30 which is significant for B-cell malignancies such as B-cell non-Hodgkin’s lymphoma (B-NHL) and Hodgkin’s lymphoma (HL) [106]. Though still in development, success has been shown in treatment of patients, and with a deeper understanding of its functional role, the future of this novel therapy will likely prove to be promising for many diseases.
Research has led to the discovery that B-aggressive lymphoma-1 protein and ADP-ribosyltransferase BAL1/ARTD9 may serve as a novel potential drug target for treatment [96, 107]. Combining a drug(s) targeting STAT1 or the macrodomains of BAL1/ARTD9 with common day therapeutic treatments might be a successful strategy toward increasing the sensitivity of HR-DLBCL to classic therapy [107]. Several other potential therapies have been identified through other ongoing investigations including the targeting of Deltex-3-like E3 ubiquitin ligase (DTX3L) and the BET Bromodomain Protein BRD4 [1, 96, 108]. Preliminary studies indicate that DTX3L controls CXCR4, a chemokine receptor [108]. Further studies would need to be done to identify the link, if any, of DTX3L via CRCX4 with DLBCL. However, a therapy involving this control mechanism shows great potential [108]. Regarding BRD4, studies have shown that the BET inhibitors have the ability to inhibit oncogenic NF-κB activity through decreased expression of the NF-κB target genes IL6 and IL10 [1]. These findings, along with the developments in understanding the functions of NF-κB and RelA (p65), highly support the need for further research into developing a therapeutic drug targeting NF-κB complex.
Further investigation on these therapies, with or without standard immunochemotherapy, would provide major insights and pave the way to developing successful treatments for patients suffering from more aggressive types of DLBCL or RR-DLBCL or even different types of lymphomas. It is also believed that acquired drug resistance is mediated by a finite set of pathways. If these pathways can be identified and the targets that need to be suppressed or activated can be determined, sensitivity could be restored to drugs that were used successfully in a prior line of therapy or optimize the efficiency of the available therapeutic personalized regimens [13, 96].
5.5 Conclusions
EBV was discovered more than 50 years ago, but a large body of questions remain unanswered. Although EBV infects more than 90 % of the world’s population, only a subset of the related infections results in lymphomagenesis. The lifelong relationships between host and EBV suggest the importance of the immune system in normal individuals. For many immunodeficient patients, EBV-induced lymphomagenesis is a frequent occurrence. Although EBV-associated lymphomas have been studied for many years, the precise roles of EBV in these processes are still unclear. EBV can infect B cells and establish latent infection, further inducing them toward lymphomagenesis under specific conditions in the microenvironment. Although the in vitro model of EBV infection has been established for many years, the detailed strategies of EBV infection, which includes latent and lytic infection, are not completely understood. The complex regulatory network is associated with regulation of numerous transcription factors, viral lytic/latent antigens, and their associated relationships. In addition, the development of NPC or GC after EBV infection has not been completely investigated because of the limitation of an efficient in vitro and in vivo model system. It is anticipated that the combined application of high-throughput next-generation sequencing technologies and in vivo mouse models will significantly improve our understanding of EBV biology in the near future and the development of potential therapeutic intervention strategies.
Acknowledgments
This work was supported by National Cancer Institute at the National Institutes of Health public health service Grants P30-CA016520, P30-DK050306, R01-CA171979, P01-CA174439, and R01-CA177423 to ESR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We sincerely apologize to the authors whose important work could not be cited here due to space limitations.
References
- 1.Ceribelli M, Kelly PN, Shaffer AL, Wright GW, Xiao W, et al. Blockade of oncogenic IkappaB kinase activity in diffuse large B-cell lymphoma by bromodomain and extraterminal domain protein inhibitors. Proc Natl Acad Sci U S A. 2014;111:11365–11370. doi: 10.1073/pnas.1411701111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morini E, Dietrich P, Salani M, Downs HM, Wojtkiewicz GR, et al. Sensory and autonomic deficits in a new humanized mouse model of familial dysautonomia. Hum Mol Genet. 2016;25:1116–1128. doi: 10.1093/hmg/ddv634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein MA, Achong BG, Barr YM. Virus particles in cultured Lymphoblasts from Burkitt’s lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 4.Lu F, Wikramasinghe P, Norseen J, Tsai K, Wang P, et al. Genome-wide analysis of host-chromosome binding sites for Epstein-Barr Virus Nuclear Antigen 1 (EBNA1) Virol J. 2010;7:262. doi: 10.1186/1743-422X-7-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henle G, Henle W, Diehl V. Relation of Burkitt’s tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968;59:94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope JH. Establishment of cell lines from peripheral leucocytes in infectious mononucleosis. Nature. 1967;216:810–811. doi: 10.1038/216810a0. [DOI] [PubMed] [Google Scholar]
- 7.Henle W, Diehl V, Kohn G, Zur Hausen H, Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967;157:1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- 8.Cerchietti L, Damm-Welk C, Vater I, Klapper W, Harder L, et al. Inhibition of anaplastic lymphoma kinase (ALK) activity provides a therapeutic approach for CLTC-ALK-positive human diffuse large B cell lymphomas. PLoS One. 2011;6:e18436. doi: 10.1371/journal.pone.0018436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesri EA, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe. 2014;15:266–282. doi: 10.1016/j.chom.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rund D. Targeting drug resistance to close the gap in diffuse large B-cell lymphoma. Leuk Lymphoma. 2014;55:1966–1967. doi: 10.3109/10428194.2014.898762. [DOI] [PubMed] [Google Scholar]
- 11.Lv X, Feng L, Ge X, Lu K, Wang X. Interleukin-9 promotes cell survival and drug resistance in diffuse large B-cell lymphoma. J Exp Clin Cancer Res. 2016;35:106. doi: 10.1186/s13046-016-0374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang C, Lu P, Lee FY, Chadburn A, Barrientos JC, et al. Tyrosine kinase inhibition in diffuse large B-cell lymphoma: molecular basis for antitumor activity and drug resistance of dasatinib. Leukemia. 2008;22:1755–1766. doi: 10.1038/leu.2008.163. [DOI] [PubMed] [Google Scholar]
- 13.Wilson WH. Drug resistance in diffuse large B-cell lymphoma. Semin Hematol. 2006;43:230–239. doi: 10.1053/j.seminhematol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Yu X, Li Z. New insights into MicroRNAs involves in drug resistance in diffuse large B cell lymphoma. Am J Transl Res. 2015;7:2536–2542. [PMC free article] [PubMed] [Google Scholar]
- 15.Parker GA, Touitou R, Allday MJ. Epstein-Barr virus EBNA3C can disrupt multiple cell cycle checkpoints and induce nuclear division divorced from cytokinesis. Oncogene. 2000;19:700–709. doi: 10.1038/sj.onc.1203327. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee S, Lu J, Cai Q, Saha A, Jha HC, et al. The EBV latent antigen 3C inhibits apoptosis through targeted regulation of interferon regulatory factors 4 and 8. PLoS Pathog. 2013;9:e1003314. doi: 10.1371/journal.ppat.1003314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsimbouri P, Drotar ME, Coy JL, Wilson JB. bcl-xL and RAG genes are induced and the response to IL-2 enhanced in EmuEBNA-1 transgenic mouse lymphocytes. Oncogene. 2002;21:5182–5187. doi: 10.1038/sj.onc.1205490. [DOI] [PubMed] [Google Scholar]
- 18.De Paepe P, De Wolf-Peeters C. Diffuse large B-cell lymphoma: a heterogeneous group of non-Hodgkin lymphomas comprising several distinct clinicopathological entities. Leukemia. 2007;21:37–43. doi: 10.1038/sj.leu.2404449. [DOI] [PubMed] [Google Scholar]
- 19.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 20.Heslop HE. Biology and treatment of Epstein-Barr virus-associated non-Hodgkin lymphomas. Hematol Am Soc Hematol Educ Program. 2005:260–266. doi: 10.1182/asheducation-2005.1.260. [DOI] [PubMed] [Google Scholar]
- 21.Oyama T, Yamamoto K, Asano N, Oshiro A, Suzuki R, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res. 2007;13:5124–5132. doi: 10.1158/1078-0432.CCR-06-2823. [DOI] [PubMed] [Google Scholar]
- 22.Gibson SE, Hsi ED. Epstein-Barr virus-positive B-cell lymphoma of the elderly at a United States tertiary medical center: an uncommon aggressive lymphoma with a nongerminal center B-cell phenotype. Hum Pathol. 2009;40:653–661. doi: 10.1016/j.humpath.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Hoeller S, Tzankov A, Pileri SA, Went P, Dirnhofer S. Epstein-Barr virus-positive diffuse large B-cell lymphoma in elderly patients is rare in western populations. Hum Pathol. 2010;41:352–357. doi: 10.1016/j.humpath.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Park S, Lee J, Ko YH, Han A, Jun HJ, et al. The impact of Epstein-Barr virus status on clinical outcome in diffuse large B-cell lymphoma. Blood. 2007;110:972–978. doi: 10.1182/blood-2007-01-067769. [DOI] [PubMed] [Google Scholar]
- 25.Kato H, Karube K, Yamamoto K, Takizawa J, Tsuzuki S, et al. Gene expression profiling of Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly reveals alterations of characteristic oncogenetic pathways. Cancer Sci. 2014;105:537–544. doi: 10.1111/cas.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabik JF., 3rd Why coronary artery bypass grafting remains the standard of care for patients with complex, multivessel coronary artery disease. J Thorac Cardiovasc Surg. 2016;152:1227–1228. doi: 10.1016/j.jtcvs.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Del Sorbo L, Ranieri VM, Keshavjee S. Extracorporeal membrane oxygenation as “bridge” to lung transplantation: what remains in order to make it standard of care? Am J Respir Crit Care Med. 2012;185:699–701. doi: 10.1164/rccm.201202-0193ED. [DOI] [PubMed] [Google Scholar]
- 28.Schurko B, Oh WK. Docetaxel chemotherapy remains the standard of care in castration-resistant prostate cancer. Nat Clin Pract Oncol. 2008;5:506–507. doi: 10.1038/ncponc1201. [DOI] [PubMed] [Google Scholar]
- 29.Gruhne B, Sompallae R, Marescotti D, Kamranvar SA, Gastaldello S, et al. The Epstein-Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proc Natl Acad Sci U S A. 2009;106:2313–2318. doi: 10.1073/pnas.0810619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson MA. Completion lymphadenectomy for melanoma patients with a positive sentinel node biopsy remains standard of care. Ann Surg Oncol. 2006;13:761–763. doi: 10.1245/ASO.2006.09.915. [DOI] [PubMed] [Google Scholar]
- 31.Boules TN, Proctor MC, Aref A, Upchurch GR, Jr, Stanley JC, et al. Carotid endarterectomy remains the standard of care, even in high-risk surgical patients. Ann Surg. 2005;241:356–363. doi: 10.1097/01.sla.0000150270.86267.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bollard CM, Rooney CM, Heslop HE. T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat Rev Clin Oncol. 2012;9:510–519. doi: 10.1038/nrclinonc.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuppers R, Rajewsky K. The origin of Hodgkin and Reed/Sternberg cells in Hodgkin’s disease. Annu Rev Immunol. 1998;16:471–493. doi: 10.1146/annurev.immunol.16.1.471. [DOI] [PubMed] [Google Scholar]
- 34.Choudhary SK, Rezk NL, Ince WL, Cheema M, Zhang L, et al. Suppression of human immunodeficiency virus type 1 (HIV-1) viremia with reverse transcriptase and integrase inhibitors, CD4+ T-cell recovery, and viral rebound upon interruption of therapy in a new model for HIV treatment in the humanized Rag2−/−{gamma}c−/− mouse. J Virol. 2009;83:8254–8258. doi: 10.1128/JVI.00580-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yajima M, Imadome K, Nakagawa A, Watanabe S, Terashima K, et al. A new humanized mouse model of Epstein-Barr virus infection that reproduces persistent infection, lymphoproliferative disorder, and cell-mediated and humoral immune responses. J Infect Dis. 2008;198:673–682. doi: 10.1086/590502. [DOI] [PubMed] [Google Scholar]
- 36.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 37.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, et al. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 38.Merchant M, Caldwell RG, Longnecker R. The LMP2A ITAM is essential for providing B cells with development and survival signals in vivo. J Virol. 2000;74:9115–9124. doi: 10.1128/jvi.74.19.9115-9124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doll DC, List AF. Burkitt’s lymphoma in a homosexual. Lancet. 1982;1:1026–1027. doi: 10.1016/s0140-6736(82)92031-1. [DOI] [PubMed] [Google Scholar]
- 40.Gloghini A, Dolcetti R, Carbone A. Lymphomas occurring specifically in HIV-infected patients: from pathogenesis to pathology. Semin Cancer Biol. 2013;23:457–467. doi: 10.1016/j.semcancer.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Moir S, Fauci AS. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunol Rev. 2013;254:207–224. doi: 10.1111/imr.12067. [DOI] [PubMed] [Google Scholar]
- 42.Klein G. Specific chromosomal translocations and the genesis of B-cell-derived tumors in mice and men. Cell. 1983;32:311–315. doi: 10.1016/0092-8674(83)90449-x. [DOI] [PubMed] [Google Scholar]
- 43.Ziegler JL, Drew WL, Miner RC, Mintz L, Rosenbaum E, et al. Outbreak of Burkitt’s-like lymphoma in homosexual men. Lancet. 1982;2:631–633. doi: 10.1016/s0140-6736(82)92740-4. [DOI] [PubMed] [Google Scholar]
- 44.Jha HC, Pei Y, Robertson ES. Epstein-Barr virus: diseases linked to infection and transformation. Front Microbiol. 2016;7:1602. doi: 10.3389/fmicb.2016.01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsurumi T, Fujita M, Kudoh A. Latent and lytic Epstein-Barr virus replication strategies. Rev Med Virol. 2005;15:3–15. doi: 10.1002/rmv.441. [DOI] [PubMed] [Google Scholar]
- 46.Amon W, Farrell PJ. Reactivation of Epstein-Barr virus from latency. Rev Med Virol. 2005;15:149–156. doi: 10.1002/rmv.456. [DOI] [PubMed] [Google Scholar]
- 47.Bradner JE, Hnisz D, Young RA. Transcriptional addiction in cancer. Cell. 2017;168:629–643. doi: 10.1016/j.cell.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, et al. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41:D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Consortium EP. The ENCODE (ENCyclopedia of DNA elements) project. Science. 2004;306:636–640. doi: 10.1126/science.1105136. [DOI] [PubMed] [Google Scholar]
- 50.Cancer Genome Atlas Research N. Weinstein JN, Collisson EA, Mills GB, Shaw KR, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45:1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao B, Zou J, Wang H, Johannsen E, Peng CW, et al. Epstein-Barr virus exploits intrinsic B-lymphocyte transcription programs to achieve immortal cell growth. Proc Natl Acad Sci U S A. 2011;108:14902–14907. doi: 10.1073/pnas.1108892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu F, Chen HS, Kossenkov AV, DeWispeleare K, Won KJ, et al. EBNA2 drives formation of new chromosome binding sites and target genes for B-cell master regulatory transcription factors RBP-jkappa and EBF1. PLoS Pathog. 2016;12:e1005339. doi: 10.1371/journal.ppat.1005339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wood CD, Veenstra H, Khasnis S, Gunnell A, Webb HM, et al. MYC activation and BCL2L11 silencing by a tumour virus through the large-scale reconfiguration of enhancer-promoter hubs. Elife. 2016:5. doi: 10.7554/eLife.18270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Portal D, Zhou H, Zhao B, Kharchenko PV, Lowry E, et al. Epstein-Barr virus nuclear antigen leader protein localizes to promoters and enhancers with cell transcription factors and EBNA2. Proc Natl Acad Sci U S A. 2013;110:18537–18542. doi: 10.1073/pnas.1317608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang S, Willox B, Zhou H, Holthaus AM, Wang A, et al. Epstein-Barr virus nuclear antigen 3C binds to BATF/IRF4 or SPI1/IRF4 composite sites and recruits Sin3A to repress CDKN2A. Proc Natl Acad Sci U S A. 2014;111:421–426. doi: 10.1073/pnas.1321704111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McClellan MJ, Wood CD, Ojeniyi O, Cooper TJ, Kanhere A, et al. Modulation of enhancer looping and differential gene targeting by Epstein-Barr virus transcription factors directs cellular reprogramming. PLoS Pathog. 2013;9:e1003636. doi: 10.1371/journal.ppat.1003636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang A, Welch R, Zhao B, Ta T, Keles S, et al. Epstein-Barr virus nuclear antigen 3 (EBNA3) proteins regulate EBNA2 binding to distinct RBPJ genomic sites. J Virol. 2015;90:2906–2919. doi: 10.1128/JVI.02737-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pei Y, Banerjee S, Jha HC, Sun Z, Robertson ES. An essential EBV latent antigen 3C binds Bcl6 for targeted degradation and cell proliferation. PLoS Pathog. 2017;13:e1006500. doi: 10.1371/journal.ppat.1006500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Y, Walsh MJ, Bernhardt K, Ashbaugh CW, Trudeau SJ, et al. CRISPR/Cas9 screens reveal Epstein-Barr virus-transformed B cell host dependency factors. Cell Host Microbe. 2017;21(580–591):e587. doi: 10.1016/j.chom.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou H, Schmidt SC, Jiang S, Willox B, Bernhardt K, et al. Epstein-Barr virus oncoprotein super-enhancers control B cell growth. Cell Host Microbe. 2015;17:205–216. doi: 10.1016/j.chom.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gunnell A, Webb HM, Wood CD, McClellan MJ, Wichaidit B, et al. RUNX super-enhancer control through the notch pathway by Epstein-Barr virus transcription factors regulates B cell growth. Nucleic Acids Res. 2016;44:4636–4650. doi: 10.1093/nar/gkw085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang J, Zhou H, Gerdt C, Tan M, Colson T, et al. Epstein-Barr virus super-enhancer eRNAs are essential for MYC oncogene expression and lymphoblast proliferation. Proc Natl Acad Sci U S A. 2016;113(49):14121–14126. doi: 10.1073/pnas.1616697113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Grady T, Wang X, Honer Zu Bentrup K, Baddoo M, Concha M, et al. Global transcript structure resolution of high gene density genomes through multi-platform data integration. Nucleic Acids Res. 2016;44:e145. doi: 10.1093/nar/gkw629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skalsky RL, Corcoran DL, Gottwein E, Frank CL, Kang D, et al. The viral and cellular microRNA targetome in lymphoblastoid cell lines. PLoS Pathog. 2012;8:e1002484. doi: 10.1371/journal.ppat.1002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tagawa T, Albanese M, Bouvet M, Moosmann A, Mautner J, et al. Epstein-Barr viral miRNAs inhibit antiviral CD4+ T cell responses targeting IL-12 and peptide processing. J Exp Med. 2016;213:2065–2080. doi: 10.1084/jem.20160248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Albanese M, Tagawa T, Bouvet M, Maliqi L, Lutter D, et al. Epstein-Barr virus microRNAs reduce immune surveillance by virus-specific CD8+ T cells. Proc Natl Acad Sci U S A. 2016;113:E6467–e6475. doi: 10.1073/pnas.1605884113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ersing I, Nobre L, Wang LW, Soday L, Ma Y, et al. A temporal proteomic map of Epstein-Barr virus lytic replication in B cells. Cell Rep. 2017;19:1479–1493. doi: 10.1016/j.celrep.2017.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Etoh T, Baba H, Taketomi A, Nakashima H, Kohnoe S, et al. Sequential methothrextate and 5-fruororacil therapy for diffuse bone metastasis from gastric cancer. Anticancer Res. 1998;18:2085–2088. [PubMed] [Google Scholar]
- 69.Gray NA, Kapojos JJ, Burke MT, Sammartino C, Clark CJ. Patient kidney disease knowledge remains inadequate with standard nephrology outpatient care. Clin Kidney J. 2016;9:113–118. doi: 10.1093/ckj/sfv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meissner MH. Conventional anticoagulant therapy remains the current standard of care for the treatment of iliofemoral deep venous thrombosis. Dis Mon. 2010;56:642–652. doi: 10.1016/j.disamonth.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 71.Knight JS, Sharma N, Kalman DE, Robertson ES. A cyclin-binding motif within the amino-terminal homology domain of EBNA3C binds cyclin A and modulates cyclin A-dependent kinase activity in Epstein-Barr virus-infected cells. J Virol. 2004;78:12857–12867. doi: 10.1128/JVI.78.23.12857-12867.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choudhuri T, Verma SC, Lan K, Murakami M, Robertson ES. The ATM/ATR signaling effector Chk2 is targeted by Epstein-Barr virus nuclear antigen 3C to release the G2/M cell cycle block. J Virol. 2007;81:6718–6730. doi: 10.1128/JVI.00053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saha A, Halder S, Upadhyay SK, Lu J, Kumar P, et al. Epstein-Barr virus nuclear antigen 3C facilitates G1-S transition by stabilizing and enhancing the function of cyclin D1. PLoS Pathog. 2011;7:e1001275. doi: 10.1371/journal.ppat.1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yi F, Saha A, Murakami M, Kumar P, Knight JS, et al. Epstein-Barr virus nuclear antigen 3C targets p53 and modulates its transcriptional and apoptotic activities. Virology. 2009;388:236–247. doi: 10.1016/j.virol.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saha A, Bamidele A, Murakami M, Robertson ES. EBNA3C attenuates the function of p53 through interaction with inhibitor of growth family proteins 4 and 5. J Virol. 2011;85:2079–2088. doi: 10.1128/JVI.02279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pei Y, Banerjee S, Sun Z, Jha HC, Saha A, et al. EBV nuclear antigen 3C mediates regulation of E2F6 to inhibit E2F1 transcription and promote cell proliferation. PLoS Pathog. 2016;12:e1005844. doi: 10.1371/journal.ppat.1005844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeLong WB, Polissar NL, Neradilek MB, Laam LA. To the Editor: Re: Controversy: acute cauda equina syndrome caused by a disk lesion: is emergent surgery the correct option? In: Mahadevappa, et al., editors. Spine (Phila Pa 1976) Vol. 40. 2015. p. E1120. Surgical decompression remains the standard of care, by McLain et al. [DOI] [PubMed] [Google Scholar]
- 78.Shumilov A, Tsai MH, Schlosser YT, Kratz AS, Bernhardt K, et al. Epstein-Barr virus particles induce centrosome amplification and chromosomal instability. Nat Commun. 2017;8:14257. doi: 10.1038/ncomms14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crowther M, Donadini MP. Hematology/oncology clinics of North America. Hypercoagulable states and new anticoagulants. Preface. Hematol Oncol Clin North Am. 2010;24:xiii–xxiv. doi: 10.1016/j.hoc.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 80.Brown JR. Hematology/oncology clinics of North America. Chronic lymphocytic leukemia. Preface. Hematol Oncol Clin North Am. 2013;27:xiii–xxiv. doi: 10.1016/j.hoc.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 81.Jox A, Rohen C, Belge G, Bartnitzke S, Pawlita M, et al. Integration of Epstein-Barr virus in Burkitt’s lymphoma cells leads to a region of enhanced chromosome instability. Ann Oncol. 1997;8(Suppl 2):131–135. [PubMed] [Google Scholar]
- 82.O’Donnell MT, Greer LT, Nelson J, Shriver C, Vertrees A. Diversion remains the standard of care for modern management of war-related rectal injuries. Mil Med. 2014;179:778–782. doi: 10.7205/MILMED-D-13-00533. [DOI] [PubMed] [Google Scholar]
- 83.Kessinger A. Consensus conference on high-dose therapy with hematopoietic stem cell transplantation in diffuse large-cell lymphoma. Type of cells, optimal mobilization of stem cells – positive and negative selection. Ann Oncol. 1998;9(Suppl 1):S23–S30. doi: 10.1093/annonc/9.suppl_1.s23. [DOI] [PubMed] [Google Scholar]
- 84.Masip J, Vecilla F, Paez J. Diffuse pulmonary hemorrhage after fibrinolytic therapy for acute myocardial infarction. Int J Cardiol. 1998;63:95–97. doi: 10.1016/s0167-5273(97)00285-4. [DOI] [PubMed] [Google Scholar]
- 85.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, et al. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 86.Fujiwara S, Matsuda G, Imadome K. Humanized mouse models of epstein-barr virus infection and associated diseases. Pathogens. 2013;2:153–176. doi: 10.3390/pathogens2010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, et al. NOD/SCID/gamma(c) (null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 88.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 89.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma SD, Xu X, Jones R, Delecluse HJ, Zumwalde NA, et al. PD-1/CTLA-4 blockade inhibits Epstein-Barr virus-induced lymphoma growth in a cord blood humanized-mouse model. PLoS Pathog. 2016;12:e1005642. doi: 10.1371/journal.ppat.1005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma (null) humanized mice. Proc Natl Acad Sci U S A. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chijioke O, Muller A, Feederle R, Barros MH, Krieg C, et al. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep. 2013;5:1489–1498. doi: 10.1016/j.celrep.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma SD, Hegde S, Young KH, Sullivan R, Rajesh D, et al. A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J Virol. 2011;85:165–177. doi: 10.1128/JVI.01512-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ma SD, Xu X, Plowshay J, Ranheim EA, Burlingham WJ, et al. LMP1-deficient Epstein-Barr virus mutant requires T cells for lymphomagenesis. J Clin Invest. 2015;125:304–315. doi: 10.1172/JCI76357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Minamitani T, Ma Y, Zhou H, Kida H, Tsai CY, et al. Mouse model of Epstein-Barr virus LMP1- and LMP2A-driven germinal center B-cell lymphoproliferative disease. Proc Natl Acad Sci U S A. 2017;114:4751–4756. doi: 10.1073/pnas.1701836114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Camicia R, Winkler HC, Hassa PO. Novel drug targets for personalized precision medicine in relapsed/refractory diffuse large B-cell lymphoma: a comprehensive review. Mol Cancer. 2015;14:207. doi: 10.1186/s12943-015-0474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perry AR, Goldstone AH. High-dose therapy for diffuse large-cell lymphoma in first remission. Ann Oncol. 1998;9(Suppl 1):S9–14. doi: 10.1093/annonc/9.suppl_1.s9. [DOI] [PubMed] [Google Scholar]
- 98.Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 99.Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22(941–952):ix. doi: 10.1016/j.hoc.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raut LS, Chakrabarti PP. Management of relapsed-refractory diffuse large B cell lymphoma. South Asian J Cancer. 2014;3:66–70. doi: 10.4103/2278-330X.126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zinzani PL, Pellegrini C, Argnani L, Broccoli A. Prolonged disease-free survival in elderly relapsed diffuse large B-cell lymphoma patients treated with lenalidomide plus rituximab. Haematologica. 2016;101:e385–e386. doi: 10.3324/haematol.2016.147256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 103.Dotan E, Aggarwal C, Smith MR. Impact of rituximab (Rituxan) on the treatment of B-cell non-Hodgkin’s lymphoma. P T. 2010;35:148–157. [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang M, Xu-Monette ZY, Li L, Manyam GC, Visco C, et al. RelA NF-kappaB subunit activation as a therapeutic target in diffuse large B-cell lymphoma. Aging (Albany NY) 2016;8:3321–3340. doi: 10.18632/aging.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramos CA, Heslop HE, Brenner MK. CAR-T cell therapy for lymphoma. Annu Rev Med. 2016;67:165–183. doi: 10.1146/annurev-med-051914-021702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Z, Wu Z, Liu Y, Han W. New development in CAR-T cell therapy. J Hematol Oncol. 2017;10:53. doi: 10.1186/s13045-017-0423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Camicia R, Bachmann SB, Winkler HC, Beer M, Tinguely M, et al. BAL1/ARTD9 represses the anti-proliferative and pro-apoptotic IFNgamma-STAT1-IRF1-p53 axis in diffuse large B-cell lymphoma. J Cell Sci. 2013;126:1969–1980. doi: 10.1242/jcs.118174. [DOI] [PubMed] [Google Scholar]
- 108.Holleman J, Marchese A. The ubiquitin ligase deltex-3l regulates endosomal sorting of the G protein-coupled receptor CXCR4. Mol Biol Cell. 2014;25:1892–1904. doi: 10.1091/mbc.E13-10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang H, Zou J, Zhao B, Johannsen E, Ashworth T, et al. Genome-wide analysis reveals conserved and divergent features of Notch1/RBPJ binding in human and murine T-lymphoblastic leukemia cells. Proc Natl Acad Sci U S A. 2011;108:14908–14913. doi: 10.1073/pnas.1109023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schmidt SC, Jiang S, Zhou H, Willox B, Holthaus AM, et al. Epstein-Barr virus nuclear antigen 3A partially coincides with EBNA3C genome-wide and is tethered to DNA through BATF complexes. Proc Natl Acad Sci U S A. 2015;112:554–559. doi: 10.1073/pnas.1422580112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arvey A, Tempera I, Tsai K, Chen HS, Tikhmyanova N, et al. An atlas of the Epstein-Barr virus transcriptome and epigenome reveals host-virus regulatory interactions. Cell Host Microbe. 2012;12:233–245. doi: 10.1016/j.chom.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holdorf MM, Cooper SB, Yamamoto KR, Miranda JJ. Occupancy of chromatin organizers in the Epstein-Barr virus genome. Virology. 2011;415:1–5. doi: 10.1016/j.virol.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paschos K, Bazot Q, Ho G, Parker GA, Lees J, et al. Core binding factor (CBF) is required for Epstein-Barr virus EBNA3 proteins to regulate target gene expression. Nucleic Acids Res. 2016;45:2368–2383. doi: 10.1093/nar/gkw1167. [DOI] [PMC free article] [PubMed] [Google Scholar]