Abstract
Background
Acute kidney injury (AKI) occurs in one in four children admitted to the intensive care unit (ICU) and AKI severity is independently associated with increased patient morbidity and mortality. Early prediction of AKI has the potential to improve outcomes. In smaller, single center populations, we have previously derived and validated the performance of the renal angina index (RAI), a context driven risk stratification system, to predict severe AKI.
Methods
A prospective, observational study (AWARE1, January–December 2014) was conducted in intensive care units from 32 centers in 9 countries. The primary outcome was the presence of severe AKI (“AKIS”; Stage 2–3 AKI KDIGO guidelines) on the third day after ICU admission (). We compared the performance of the RAI to changes in serum creatinine relative to baseline (SCr/Base) for prediction of the primary outcome and secondary outcomes of interest. A RAI ≥ 8 defined fulfillment of renal angina (RA+); RA+ was compared to SCr increased relative to baseline (“SCr>Base”; using maximum SCr in first 12 hours of ICU admission).
Findings
The 1590 patients studied were 55% male and had median age of 54.5 months. 286 patients (17.9%) were RA+. AKIS occurred in 121 (42.3%) RA+ vs. 247 (18.9%) RA-patients (relative risk (RR) 2.23; 95% confidence interval (CI): 1.87–2.66, p<0.001). 368 (23.1%) patients with AKIS had increased renal replacement therapy utilization (10.9% vs. 1.5%, p<0.001) and increased mortality (7.6% vs. 4.3%, p=0.01) compared to patients without AKIS. RA+ demonstrated better prediction for AKIS than SCr>Base (RR: 1.61; (1.33–1.93), p<0.001) which was maintained on multivariate regression (independent odds ratio (OR): RA+ 3.21; 95% CI (2.20–4.67) vs. SCr>Base 0.68; 95% CI (0.49–4.94)).
Interpretation
Earlier, better prediction of severe AKI has the potential to improve AKI associated patient outcomes. Compared to isolated, context-free changes in SCr, renal angina risk assessment improved accuracy for prediction of severe AKI in critically ill children and young adults.
Introduction
Acute kidney injury (AKI) occurs frequently in critically ill patients and is associated with poor patient outcome. In both adults and children, increasing AKI severity is independently associated with incremental increases in mortality.1, 2 Current management guidelines for patients with AKI recommend augmentation of supportive care and are intended to limit AKI progression3 and recent reports suggest that early recognition of AKI risk can expedite both incorporation of the guidelines in critically ill patients and early employment of prevention strategies to reduce AKI severity.4-6 This current state of AKI management is implicitly reactive and employs strategies based on mitigation of established AKI. A proactive approach aimed at prevention of injury, requires the ability to reliably identify patients at-risk for AKI, or those with developing AKI early in their course.7,8 A broader appreciation and recognition of AKI risk factors is a cornerstone of international directives to reduce the global AKI burden.8,9
Adjudication of patient risk modulates decision making and management strategy. A clear example is the difference in management of fever in a previously healthy child versus in an immunocompromised child with an indwelling central venous catheter. Management of critically ill patients is also dependent on risk assessment. International consensus guidelines account for patient risk factors to guide corticosteroid therapy and multiple antimicrobial agents in treatment of severe sepsis.10-12 The time-dependent treatment algorithms for ischemic stroke are guided by both physical symptoms and risk factors. Recent evidence suggests the time to initial benzodiazepine administration to abort seizures should vary based on the presence or absence of a history of epilepsy syndrome with prior status epilepticus. Management of acute chest pain varies by the presence or absence of the Framingham risk factors for atherosclerotic heart disease. In each of these examples, patient context and risk is incorporated into the decision-making process for patient management. Ultimately, improved patient outcome occurs because of risk stratified patient management.
Risk stratification for AKI may be possible using the concept of renal angina,14 which combines risk factors and early signs of loss of function (increases in serum creatinine or degrees of fluid accumulation) to stratify patients for risk subsequent severe AKI (Stage 2 or 3 AKI by the KDIGO criteria3) (Figure 1, Supplement 1).7,15,16 We previously derived and validated a simple mathematical operationalization of renal angina, the renal angina index (RAI), in multiple relatively small, single-center pediatric populations. The predictive efficacy of the RAI for development of severe AKI three days after admission to the intensive care unit (ICU) was tested.17-19 The time point of three days was chosen because of the poor patient outcome associated with severe AKI occurring 48 hours after ICU admission and as a point to signify clinically significant AKI (termed “persistent AKI”).20 The RAI was highly sensitive as a screening tool for severe AKI risk; absence of renal angina, defined as an index value below the validated cut-off of 8, demonstrated high negative predictive (92–99%) value for severe AKI on day three. Furthermore, confirmatory biomarkers integrated into the RAI of patients with a RAI ≥ 8 improved positive prediction for AKI (i.e., higher pre-test probability increasing post-test probability).19
Figure 1. The Renal Angina Index.
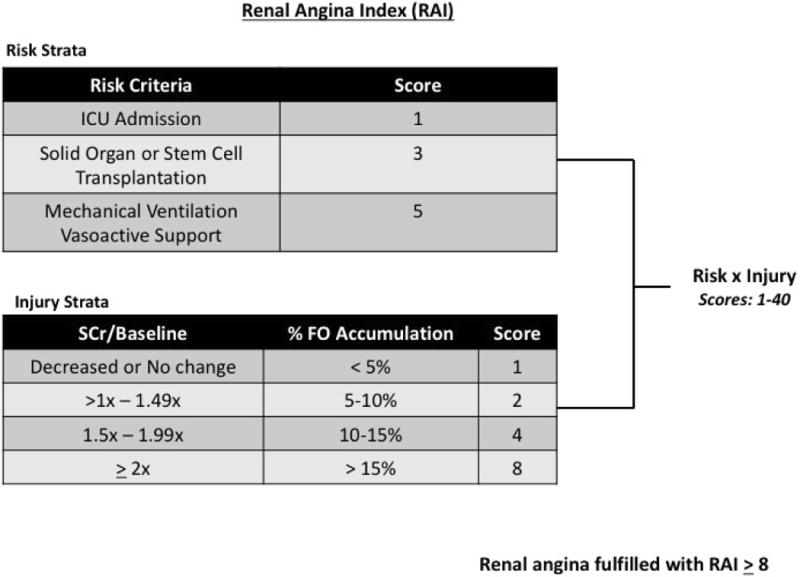
The index calculation for the fulfillment of renal angina is assessed 12 hours after a patient is admitted to the intensive care unit and used for prediction of severe AKI 72 hours (3 days) later. Risk factors are determined as described and assigned a point value (1, 3, and 5). Mechanical ventilation and vasoactive support should be used within the 12-hour time point but are not required to be simultaneously for a patient to be categorized as a “5”. Injury strata are described and assigned to a patient as appropriate. The index is a multiplication of the risk and injury scores assigned. SCr = serum creatinine/baseline = relationship to baseline serum creatinine, %FO = % fluid overload as described previously.27
We now present results from a large prospective, multi-national study of children assessing the ability of the RAI to predict development of severe AKI. This work expands our preliminary study findings and tests the RAI in a larger and more heterogeneous population of patients admitted to pediatric ICUs across the world. We hypothesized that compared to isolated, context-free elevation in serum creatinine (SCr), application of the RAI would increase the predictive accuracy for severe AKI.
Methods
Study Design and Ethics
We conducted the “Assessment of Worldwide AKI, Renal Angina and Epidemiology (AWARE)” study (NCT01987921) and have published the design21 and the initial epidemiologic results.1 Briefly, AWARE was a prospective observational study that recruited children and young adults from 32 pediatric ICUs across Asia, Australia, Europe, and North America (Supplement 2). Each center collected data for three consecutive months in 2014. All patients between three months and 25 years admitted to ICU ≥48 hours were eligible. Exclusion criteria were: a) history of stage 5 chronic kidney disease (i.e., estimated glomerular filtration rate (eGFR) < 15 ml/min/1.73m2 or on maintenance dialysis) and b) kidney transplantation in the preceding 90 days.
Eligible patients’ medical records were reviewed to collect data on the following timelines: up to three months before (SCr only), daily during the first 7 days and on day 28 after ICU admission. Day 28 outcome data were recorded whether the patient was still in ICU, discharged, or dead. All centers obtained health research ethics board approval prior to commencement with approval for a waiver of informed consent.
Metrics
Baseline SCr (Base) was defined as the lowest SCr in the 3 months prior to admission. When baseline SCr was unavailable, a baseline SCr was imputed by calculating a body surface area (m2) using patient’s height and weight and an estimated glomerular filtration (eGFR) of 120 ml/min/1.73m2, as validated in the literature. For this analysis, we implemented the Kidney Disease Improving Global Outcomes (KDIGO) staging criteria to define and classify AKI; however, renal replacement therapy (RRT) utilization was assessed as an outcome and not counted as Stage 3 AKI. We required measurement of ≥1 SCr or ≥12 hours of recorded UOP in the first seven days of admission to assess for AKI. Severe AKI (denoted “AKIS”) was defined as ≥ Stage 2 AKI as these stages are associated with worse outcomes in children. The worse AKI stage defined by the serum creatinine or UOP criteria (both data required for patient inclusion) was used to classify AKI stage. When explicitly specified, all-stage AKI (KDIGO stages 1–3) was also analyzed (“AKIa”). The RAI score was determined after 8–12 hours on the day of admission as previously described combining risk and injury scores (Figure 1).18 Determination of the “injury” component of the RAI utilized the worse of either percent fluid overload (% FO) from ICU admission or change of SCr from baseline (SCr/Base). The change from baseline was used to determine the binary definition of SCr>Base or SCr≤Base. For both RAI and SCr/Base determination, the maximum SCr measured between ICU admission and hour 12 on admission day was used. A RAI ≥ 8 was considered fulfillment of renal angina based on our previous derivation and validation studies (Supplement 1). Fulfillment or absence of renal angina was denoted “RA+” or “RA−”, respectively. Any elevation of Dayo-SCr over baseline was denoted as “SCr>Base”.
Outcomes
The primary outcome of interest was the presence of AKIS on ICU Day3 (AKIS). Patients with AKIS before Day3 or after Day3 were not included as positive for AKIS. The primary analyses tested the diagnostic utility of the RAI for the prediction of this outcome compared to SCr/Base. The primary outcome was chosen for multiple reasons. Our prior data and the data presented in this report verify the association between Day3-AKIS and poor patient outcomes. Additionally, this point in ICU course has been suggested by international consensus as beyond the point of rapid reversal of transient AKI.20 In fact, AKI prior to this point, as suggested by the consensus, should no longer be termed severe AKI. Secondary outcomes of interest included presence of all-stage AKI on Day3 (AKIA) and longer term outcomes assessed at 28 days: use of renal replacement therapy (RRT), duration of mechanical ventilation (days), ICU length of stay (LOS), and mortality.
Statistical Analyses
Continuous variables are reported as median with interquartile range and compared using the Mann-Whitney test. Categorical variables are summarized using frequency and proportion and compared by chi-square or Fisher’s exact tests. A RAI cutoff of ≥ 8 was used to define renal angina fulfillment [RA (+)] and this cut-off was used for diagnostic test evaluations. For tests of comparison, RAI was compared to SCr/Base. RA+ was compared to SCr>Base. Bivariate and multivariate logistic models were used to correct for significant covariate effects and identify independent associations with outcomes. All bivariate associations carrying associations with p<0.15 were included as multivariate model terms. Association between severity of illness (SOI) score and outcomes were only performed as bivariate analyses as the linearity of these scores cannot be assumed. PRISM-III – Pediatric Risk of Mortality Score III, PIM-2: Pediatric Index of Mortality-2, and PELOD: Pediatric Logistic Organ Dysfunction score were used based on center preference.16 Multivariate regression was also performed including both RA+ and SCr>Base as model terms to delineate the independent association with chosen outcomes for each predictor. Statistical analyses were performed using SigmaStat version 13.1 software (San Antonio, Texas). A p-value of <0.05 was considered significant.
Role of the Funding Source
This work was supported by a grant (NIH P50 DK096418, to Drs. Basu and Goldstein) from the Pediatric Nephrology center of Excellence at Cincinnati Children’s Hospital Medical Center. Dr. Kaddourah’s Pediatric Acute Care Nephrology and Dialysis Fellowship at Cincinnati Children’s Hospital Medical Center was supported by an educational grant from Gambro Renal Products.
The funding source had no role in study design, collection of data or data analysis, data interpretation, or writing of the report. All primary authors (RKB, AK, SLB) had full access to all of the data. The corresponding author (RKB) had the final responsibility to submit for publication.
Results
Patients
5237 children and young adults were eligible in AWARE; in the 4683 patients with both Day28 and maximum AKI stage data available, 543 (11.6%) developed AKIS within the first 7 days.1 For this analysis, we studied the cohort of patients still admitted to ICU on Day3 (n=1590) (Figure 2). All patients had complete data for Days 0, 3, and 28. The current study cohort was sicker than the overall eligible patient population (5237), the 4683 patients reported in the primary epidemiological survey, and the population in AWARE not studied in this cohort (Supplement 2), as evidenced by higher severity of illness scores and increased morbidities such as use of extracorporeal therapy and renal replacement therapy (RRT). Mortality rates were higher in the study population studied than the remaining cohort from the reported AWARE dataset (5.1% vs. 2.6%, p<0.001).
Figure 2. CONSORT Diagram.
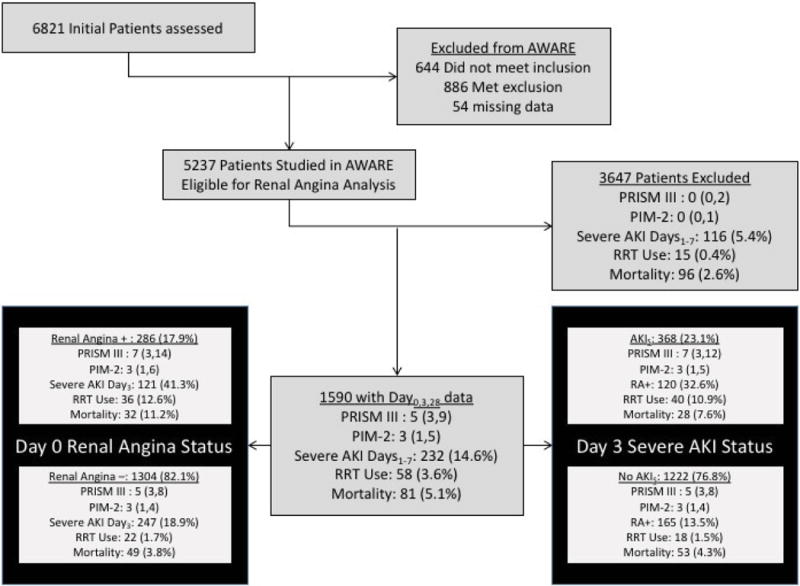
Inclusion in this analysis required full data on ICU Day 3 – inclusive of serum creatinine and urine output measurements. All patients included from the original AWARE study (1590/5237) had full data from the day of ICU admission, Day 3, and Day 28. Renal angina was fulfilled (RA+) on the day of admission in 17.9% of the studied patients. These patients were older and sicker and had worse outcome than patients without renal angina on admission (RA−). Although no different by multiple markers of severity of illness, patients with AKI on Day 3 (AKIS) were older and had worse outcome than patients without AKI. P values for comparison are listed in Supplement 2: Current patients (1590) vs. AWARE patients excluded for this study (3647), Table 1: Renal angina + vs. renal angina −, and Table 2: AKIS vs. No AKIS.
RA+ occurred in 286/1590 (17.9%) of patients. Compared to RA−, RA+ was associated with a higher incidence of diagnoses such as shock and cardiovascular illness and were less commonly diagnosed with trauma or central nervous system illness (Table 1). Additionally, RA+ was associated with both a greater risk of both AKIS (RR: 2.23; 95% CI 1.87–266, p < 0.001) and AKIA (RR: 1.87; 95% CI 1.65–2.14 p<0.001). RA+ was associated with worse outcomes including: increased utilization of renal replacement therapy (12.6% vs. 1.7%, p<0.001), prolonged duration of mechanical ventilation (median (IQR) 4 (0,9) vs. 0 (0,5) in days, p<0.001), and increased mortality (11.2% vs. 3.8%, p<0.001).
Table 1.
Renal Angina Fulfillment, Demographics and Outcomes
| Variable | RA− N=1304 (82.1) |
RA+ N=286 (17.9) |
P Value |
|---|---|---|---|
|
| |||
| Male | 738 (56.6) | 144 (50.3) | 0.05 |
|
| |||
| Age in months | 89 (27,167) | 94 (30–172) | 0.36 |
|
| |||
| BSA m2 | 0.88 (0.53, 1.42) | 0.94 (0.54, 1.47) | 0.22 |
|
| |||
| Diagnosis Group* | |||
| Shock | 337 (26.1) | 135 (52.2) | <0.001 |
| CV | 75 (4.3) | 20 (7.7) | 0.003 |
| Respiratory | 619 (35.1) | 129 (36.3) | 0.38 |
| Trauma | 263(27.9) | 64 (20.7) | 0.001 |
| CNS | 288 (21.2) | 39 (15.8) | 0.008 |
| Pain Management/Sedation | 44 (3.7) | 9(3.1) | 0.56 |
|
| |||
| History of Transplantation | 55 (4.2) | 57 (19.9) | <0.001 |
|
| |||
| Severity of Illness Score | |||
| PRISM-3 | 5 (3,8) | 7 (3, 14) | 0.02 |
| PIM-2 | 3 (1,4) | 3 (1, 6) | 0.16 |
| PELOD | 11 (1, 12) | 11 (1, 19) | 0.011 |
|
| |||
| Day 3 AKI Incidence | |||
| No AKI | 916 (70.3) | 121 (42.3) | <0.001 |
| Stage 1 | 141 (10.8) | 44 (15.4) | 0.03 |
| Stage 2 | 53 (4.1) | 41 (14.3) | <0.001 |
| Stage 3 | 194 (14.9) | 80 (28.0) | <0.001 |
| All-stage AKI (AKIA) | 388 (29.7) | 165 (57.7) | <0.001 |
| Severe AKI (AKIS) | 247 (18.9) | 121 (42.3) | <0.001 |
| Relative Risk (AKIS)^ | 2.23 (1.87–2.66) | <0.001 | |
|
| |||
| Ventricular Assist Device* | 4(0.1) | 0 (0) | nc |
|
| |||
| ECMO* | 14 (1.1) | 9(3.1) | 0.007 |
|
| |||
| Renal Replacement Therapy* | 22 (1.7) | 36 (12.6) | <0.001 |
|
| |||
| MV Duration | 0 (0,5 | 4(0,9) | <0.001 |
|
| |||
| ICU LOS | 5 (3,8) | 6 (3,10) | 0.21 |
|
| |||
| Mortality | 49 (3.8) | 32 (11.2) | <0.001 |
Categorical Variables expressed as n (% of cohort)
Continuous Variables expressed as medians with interquartile ranges
More than one diagnosis group is possible
Z-statistic = 8.96
RA = renal angina (+ = positive, − = negative) BSA = body surface area, IQR = interquartile range, CV = cardiovascular, CNS = central nervous system, PRISM-3 = Pediatric Risk of Mortality 3, PIM-2 =Pediatric Index of Mortality 2, PELOD = Pediatric Logistic Organ Dysfunction, KDIGO – Kidney Diseases Improving Global Outcomes, AKI = acute kidney injury, ICU = intensive care unit, ECMO = extracorporeal membrane oxygenation, MV = mechanical ventilation, LOS = length of stay
Primary Outcome
368/1590 (23.1%) patients developed AKIS. Patients with AKIS were older than patients without AKIS (p=0.002). Severity of illness scores trended toward patients with AKIS being sicker but did not demonstrate a consistently significant difference (Table 2). AKIS was associated with increased utilization of renal replacement therapy (p<0.001) and increased mortality (7.6% vs. 4.3%, p<0.01) (Table 2). RA+ demonstrated an increased relative risk for AKIS compared to SCr>Base (RR 1.61; 95% CI: 1.33–1.93, p<0.001) (Table 3), despite the lack of differences in severity of illness or age between these cohorts. A side-by-side comparison of multivariable regression demonstrated the persistence of the independent association between RA+ and AKIS after adjustment for all significant model terms from bivariate analyses. The independent association of SCr>Base for this outcome did not persist in this modeling (Table 4). Further, multivariable regression including both RA+ and SCr>Base as model terms also demonstrated a persistent independent association of RA+ with AKIS while a loss of independent association between SCr>Base and AKIS.
Table 2.
Day 3 AKI, Demographics and Outcomes
| Variable | No AKIS N=1222 (76.8) |
AKIS N=368 (23.1) |
P Value |
|---|---|---|---|
|
| |||
| Male | 683 (55.9) | 199 (54.1) | 0.54 |
|
| |||
| Age in months | 49.1 (16.3, 127.9) | 75.5 (17.9, 165.7) | 0.002 |
|
| |||
| BSA m2 | 0.67 (0.46, 1.18) | 0.84 (0.47, 1.36) | 0.005 |
|
| |||
| Diagnosis Group* | |||
| Shock | 343 (28.1) | 129 (35.1) | 0.01 |
| CV | 70 (5.7) | 29 (7.9) | 0.13 |
| Respiratory | 581 (47.5) | 167 (45.4) | 0.47 |
| Trauma | 237 (19.4) | 90 (24.5) | 0.04 |
| CNS | 263 (21.5) | 64 (17.4) | 0.09 |
| Pain Management or Sedation | 41 (3.4) | 12 (3.3) | 0.93 |
|
| |||
| History of Transplantation | 62 (5.1) | 50 (13.6) | <0.001 |
|
| |||
| Severity of Illness Score | |||
| PRISM-3 | 5 (3, 8) | 7 (3, 12) | 0.03 |
| PIM-2 | 3 (1, 4) | 3 (1, 5) | 0.09 |
| PELOD | 11 (1, 12) | 11 (1, 12) | 0.52 |
|
| |||
| RA+ | 165 (13.5) | 121 (32.9) | <0.001 |
|
| |||
| Day 0 KDIGO AKI | |||
| No AKI | 1040 (85.1) | 248 (67.4) | <0.001 |
| Stage 1 | 140 (11.5) | 34 (9.2) | 0.23 |
| Stage 2 | 55 (4.5) | 37 (10.0) | <0.001 |
| Stage 3 | 23 (1.9) | 49 (13.3) | <0.001 |
| All-stage AKI | 218 (17.8) | 120 (32.6) | <0.001 |
| Severe AKI | 78 (6.4) | 86 (23.4) | <0.001 |
|
| |||
| Ventricular Assist Device | 3 (0.2) | 1 (0.3) | 0.93 |
|
| |||
| ECMO | 14 (1.1) | 9 (2.4) | 0.07 |
|
| |||
| Renal Replacement Therapy | 18 (1.5) | 40 (10.9) | <0.001 |
|
| |||
| MV Duration | 1 (0, 6) | 1 (0, 5) | 0.57 |
|
| |||
| ICU LOS | 5 (4, 9) | 4 (3, 7.8) | <0.001 |
|
| |||
| Mortality | 53 (4.3) | 28 (7.6) | 0.01 |
Categorical Variables expressed as n (% of cohort)
Continuous Variables expressed as medians with interquartile ranges
Day 3 AKI = KDIGO ≥ Stage 2
More than one diagnosis group is possible
N = population, BSA = body surface area, IQR = interquartile range, CV = cardiovascular, CNS = central nervous system, PRISM-3 = Pediatric Risk of Mortality 3, PIM-2 =Pediatric Index of Mortality 2, PELOD = Pediatric Logistic Organ Dysfunction, KDIGO - Kidney Diseases Improving Global Outcomes, AKI = acute kidney injury, RA+ = renal angina positive, ICU = intensive care unit, ECMO = extracorporeal membrane oxygenation, MV = mechanical ventilation, LOS = length of stay
Table 3.
Creatinine Elevation versus Renal Angina Fulfillment
| Variable | SCr>Base N = 696 (43.8) |
RA+ N = 286 (17.9) |
P value |
|---|---|---|---|
|
| |||
| Male | 386 (55.4) | 144 (50.3) | 0.14 |
|
| |||
| Age in months | 45.6 (11.4, 143.8) | 49.9 (11.5,155.0) | 0.43 |
|
| |||
| Primary Diagnosis Group* | |||
| Shock | 226 (32.5) | 135 (47.2) | <0.001 |
| CV | 46 (6.6) | 20 (6.9) | 0.82 |
| Trauma | 133 (19.1) | 64 (22.4) | 0.25 |
| CNS | 134 (19.3) | 39 (13.6) | 0.04 |
|
| |||
| Severity of Illness Score | |||
| PRISM-3 | 5 (3,9) | 7(2,14) | 0.09 |
| PIM-2 | 2.5 (1,5) | 3 (1,6) | 0.16 |
| PELOD | 11 (1,12) | 11 (1,19) | 0.13 |
|
| |||
| History of Transplantation | 81 (11.6) | 57 (19.9) | <0.001 |
|
| |||
| Day 3 AKI Incidence | |||
| No AKI | 404 (58.0) | 121 (42.3) | <0.001 |
| Stage 1 | 109 (15.7) | 44 (15.4) | 0.91 |
| Stage 2 | 61 (8.7) | 41 (14.3) | 0.009 |
| Stage 3 | 122 (17.5) | 80 (27.9) | <0.001 |
| All-stage AKI (AKIA) | 292 (41.9) | 165 (57.7) | <0.001 |
| Severe AKI (AKIS) | 184 (26.4) | 121 (42.3) | <0.001 |
| Relative Risk (AKIS)# | 1.61 (1.33–1.93) | <0.001 | |
|
| |||
| AKIS Prediction | |||
| Sensitivity | 80 (56–94) | 67 (59–75) | |
| Specificity | 70 (62–77) | 87 (85–88) | |
| Positive Predictive Value | 25 (20–32) | 31(28–35) | |
| Negative Predictive Value | 96 (92–99) | 97 (96–97) | |
| Positive Likelihood Ratio | 2.6 (1.9–3.6) | 5.0 (4.2–5.9) | |
| AUC-ROC | 0.86 (0.82–0.89) | 0.83 (0.79–0.86) | |
|
| |||
| RRT Use | 41 (5.9) | 36 (12.6) | <0.001 |
|
| |||
| MV Duration | 1 (0,6) | 3.5 (0,9) | <0.001 |
|
| |||
| ECMO | 14 (2.0) | 9 (3.8) | 0.29 |
|
| |||
| ICU LOS | 5 (3,9) | 5 (3,10) | 0.96 |
|
| |||
| Mortality | 44 (6.3) | 32 (11.2) | 0.009 |
Categorical Variables expressed as n (% of cohort)
Continuous Variables expressed as medians with interquartile ranges
More than one diagnosis group is possible
Z-statistic: 5.07
SCr>Base = Serum creatinine greater than baseline creatinine, RA+ = renal angina fulfillment, AKI = acute kidney injury, BSA = body surface area, CV = cardiovascular, CNS = central nervous system, PRISM-III = Pediatric Risk of Mortality Score III, PIM-2 = Pediatric Index of Mortality-2, PELOD = Pediatric Logistic Organ Dysfunction, AUC-ROC = area under curve receiver operating characteristic, MV duration = mechanical ventilation duration, ICU = intensive care unit, ECMO = extracorporeal membrane oxygenation, RRT=Renal replacement therapy, LOS = length of stay
Table 4.
Multivariable Logistic Regression Comparison for AKIS Prediction
| Model Terms | Odds Ratio of Either Renal Angina+ or SCr>Base for Day3-AKIS | |
|---|---|---|
| RA+ | SCr>Base | |
| Age ≥ 75 months | 1.28 (0.73–2.23) | 1.37 (0.79–2.37) |
| BSA ≥ 0.84 m2 | 1.11 (0.63–1.94) | 1.02 (0.58–1.76) |
| Shock Dx | 1.21 (0.88–1.67) | 1.39 (1.02–1.90) |
| Cardiovascular Dx | 0.95 (0.53–1.68) | 0.98 (0.56–1.73) |
| Trauma Dx | 1.29 (0.93–1.82) | 1.29 (0.93–1.79) |
| Absence of CNS Dx | 0.96 (0.67–1.36) | 0.99 (0.70–1.40) |
| Transplant History | 1.87 (1.15–3.03) | 2.49 (1.56–3.97) |
| Renal Angina | 2.61 (1.88–3.63) | n/a |
| SCr>Base | n/a | 1.02 (0.78–1.35) |
| Model Terms | Inclusion of both Renal Angina+ and SCr>Base into Regression Model | |
| Age ≥ 75 months | 1.25 (0.71–2.19) | |
| BSA ≥ 0.84 m2 | 1.12 (0.64–1.97) | |
| Shock Dx | 1.20 (0.64–1.97) | |
| Cardiovascular Dx | 0.94 (0.53–1.67) | |
| Trauma Dx | 1.27 (0.91–1.78) | |
| Absence of CNS Dx | 0.95 (0.67–1.35) | |
| Transplant History | 1.97 (1.21–3.20) | |
| SCr>Base | 0.68 (0.49–0.94) | |
| Renal Angina | 3.21 (2.20–4.67) | |
N=368 patients
Results are expressed as odds ratios with 95% confidence intervals
AKI = acute kidney injury, CI = confidence interval, Dx = diagnosis, RA+=renal angina positive, SCr>Base = serum creatinine greater than baseline, BSA = body surface area, CNS = central nervous system
RA+ and SCr>Base demonstrated similar discrimination for AKIS (area under curve-receiver operating characteristics of 0.83 (95% CI; 0.79–0.86) versus 0.86 (95% CI; 0.82–0.89), respectively (p=ns)). However, the predictive accuracy for AKIS of RA+ was superior to SCr>Base: specificity (87% vs 70%), positive predictive value (31% vs. 25%), and positive likelihood ratio of RA+ (5.0 vs. 2.6) (Table 3).
Secondary Outcomes
RA+ and SCr>Base were both more common in patients with worse outcome (RRT utilization and mortality) (Supplements 3–4). For both RRT and 28-day mortality, RA+ carried an increased relative risk of outcome compared to SCr>Base for RRT utilization (2.14 (1.39–3.28), p<0.001) and mortality (1.77 (1.15–2.74), p=0.009). Similar to the primary outcome, multivariable regression analyses including both RA+ and SCr>Base as significant bivariate model terms demonstrated a persistent independent association of RA+ with RRT utilization and mortality with a concomitant loss of association between SCr>Base and these outcomes (Table 5).
Table 5.
Multivariable Logistic Regression for Prediction of Secondary Outcomes Renal Replacement Therapy Utilization#
| Model Terms | Odds Ratio of Either Renal Angina+ or SCr>Base for AKIS | |
|---|---|---|
| RA+ | SCr>Base | |
| Age > 83 months | 2.03 (0.61–6.69) | 2.47 (0.78–7.77) |
| BSA > 0.87 m2 | 0.50 (0.15–1.69) | 0.40 (0.13–1.31) |
| Shock Dx | 2.52 (1.24–5.13) | 3.02 (1.52–6.03) |
| Cardiovascular Dx | 1.25 (0.36–4.34) | 1.26 (0.37–4.32) |
| Absence of Trauma Dx | 2.56 (0.83–7.89) | 2.45 (0.80–7.47) |
| Absence of CNS Dx | 1.16 (0.46–2.90) | 1.19 (0.48–2.97) |
| Transplant Hx | 3.32 (1.34–8.19) | 4.22 (1.75–10.15) |
| Renal Angina | 4.12 (2.08–8.17) | n/a |
| SCr>Base | n/a | 1.92 (0.97–3.82) |
| Model Terms | Inclusion of both Renal Angina+ and SCr>Base into Regression Model | |
| Age > 83 months | 2.03 (0.61–6.68) | |
| BSA > 0.87 m2 | 0.50 (0.15–1.69) | |
| Shock Dx | 2.52 (1.24–5.13) | |
| Cardiovascular Dx | 1.25 (0.36–4.34) | |
| Absence of Trauma Dx | 2.56 (0.83–7.89) | |
| Absence of CNS Dx | 1.16 (0.46–2.90) | |
| Transplant Hx | 3.30 (1.33–8.21) | |
| SCr>Base | 1.02 (0.46–2.31) | |
| Renal Angina | 4.07 (1.83–9.04) | |
| Mortality^ | ||
| Model Terms | Exclusion of Either Renal Angina+ or SCr>Base from Regression Model | |
| RA+ | SCr>Base | |
| Shock Dx | 1.12 (0.66–1.89) | 1.29 (0.78–2.15) |
| Cardiovascular Dx | 2.59 (1.25–5.42) | 2.64 (1.27–5.47) |
| Absence of Trauma Dx | 2.09 (0.91–4.78) | 2.02 (0.88–4.61) |
| CNS Dx | 2.53 (1.48–4.31) | 2.42 (1.43–4.11) |
| Transplant History | 1.32 (0.59–2.95) | 1.52 (0.69–3.37) |
| Renal Angina | 2.38 (1.36–4.15) | n/a |
| SCr>Base | n/a | 1.22 (0.75–1.99) |
| All-stage AKI | 0.99 (0.59–1.68) | 1.15 |
| ECMO | 2.56 (0.80–8.16) | 2.55 (0.81–8.06) |
| RRT Use | 6.05 (2.89–12.63) | 7.48 (3.65–15.31) |
| Model Terms | Inclusion of both Renal Angina+ and SCr>Base into Regression Model | |
| Odds Ratio | 95% CI | |
| Shock Dx | 1.16 | 0.66–1.88 |
| Cardiovascular Dx | 2.59 | 1.24–5.43 |
| Absence of Trauma Dx | 2.11 | 0.92–4.83 |
| CNS Dx | 2.52 | 1.48–4.30 |
| Transplant Hx | 1.34 | 0.60–2.99 |
| SCr>Base | 0.86 | 0.49–1.51 |
| Renal Angina | 2.58 | 1.36–4.89 |
| All stage AKI | 1.01 | 0.60–1.71 |
| ECMO | 2.59 | 0.81–8.23 |
| RRT Use | 6.04 | 2.89–12.59 |
58 patients,
81 patients
Model terms included demonstrated bivariate association with outcome p ≤ 0.15
BSA = body surface area, Dx = diagnosis, Hx = history, CNS = central nervous system, SCr>Base = Serum creatinine greater than baseline creatinine, RA+ = renal angina fulfillment, AKI = acute kidney injury, ICU = intensive care unit, ECMO = extracorporeal membrane oxygenation, RRT=Renal replacement therapy
An analysis of the accuracy of identification of AKI stage on Day3 patients was performed (Supplements 5–7). 171 patients (108%) with AKIS were negative for both RA+ and Day0-SCr>Base and conversely, 142 (8.9%) patients were positive for both on admission but negative for AKIS (Supplement 6). Despite being a smaller proportion of the studied cohort (17.9% vs. 43.8%), patients with RA+ were more likely to suffer AKIS than any patient with SCr>Base (121 (42.3%) vs. 184 (26.4%), respectively (p<0.001) (Supplement 7).
Discussion
The multi-national, multi-center AWARE and AKI-EPI epidemiologic studies describe strong associations between severe AKI and worse patient outcome in adults and children.1,2 Opportunities to improve predictive precision and accuracy for significant severe AKI can assist clinicians managing patients on the front-line, early in the ICU course. In this large, multi-center prospective observational study in critically ill children and young adults, we demonstrate assessment of renal angina, using the RAI risk stratification system early in ICU course, confers a distinct predictive advantage compared to serum creatinine increase for clinically significant AKI. Comparison of the test characteristics illustrates the basis of the important predictive improvement conferred by renal angina. Renal angina fulfillment improves the specificity (by ~ 20%) and likelihood (two-fold, with non-overlapping confidence intervals) for Day3-AKIS than context-free increases in creatinine. These improvements indicate an increase in the posttest probability for disease.
We confirm and broaden the findings of our previous single-center studies reporting the performance of the RAI for severe AKI prediction. This study is, however, distinct from our previous work. None of our previous studies compared the performance of the RAI to increases in creatinine concentrations. Most of our previous reports were retrospective analyses.17,18 The inclusion criteria for expected length of stay were less stringent between our prospective pilot study AKI-CHERUB (NCT01735162) and the AWARE study (72 hours vs. 48 hours) resulting in more patients included in AWARE with a lower severity of illness (assessed by severity of illness score comparisons). The inclusion of more patients and patients from a wider range of illness severity (i.e., inclusion of “less” sick patients), increases the generalizability and validity of our findings. Additionally, the previous work was primarily performed at only two institutions while the current study was performed at 32 separate, unique international institutions, which strengthens the validity of the model and findings.
Clinical context drives management of hospitalized patients. A clinician adjudicates patient context to provide clinical decision support for both the optimal intervention and the urgency of the intervention. For example, time to antibiotic therapy in the management of sepsis is directly related to mortality23. Both timing and type of antimicrobial therapy are adjudicated by patient risk in the 2016 Surviving Sepsis Guidelines. Risk assessment in a patient with physical symptoms consistent with a stroke directs immediate management options (e.g., thrombolytic therapy). The most apt example for how risk adjudication modulates management resulting in improved patient outcome is for acute coronary syndrome (ACS). In ACS, the clinical context of risk factors for heart disease (Framingham) combined with physical signs of coronary vasospasm (Prinzmetal’s Angina) increase the pre-test probability of myocardial infarction. In this context, a clinician directs electrographic and confirmatory biomarker testing, ultimately optimizing prediction and/or identification of a heart attack. In the 1960s and 1970s, the adoption of this construct into clinical care provided a framework for clinicians and researchers to discover new therapeutic options for a disease process with high mortality. The construct continues to provide clinical decision support to this day and thanks to coronary bypass surgery, thrombolytic therapy, and small vessel stenting, more patients have an increased probability of survival.
The RAI may help differentiate reversible (functional) AKI from persistent (structural) AKI.24 Compared to context free increases in SCr, the relative risk for severe AKI at Day 3 is two-fold greater for patients fulfilling renal angina. Using creatinine alone, prediction of any stage of AKI would be correct only 40% of the time and only 25% of the time for severe AKI. By comparison, correct prediction by RAI occurs more frequently for any stage AKI (60%) and for severe AKI (40%). RAI increases predictive success (accuracy) and reduces predictive error (cost), with a potential increase in value.
Early prediction of severe AKI may improve patient outcomes. The majority of therapeutic trials to date have studied patients with established and severe AKI, enrolled well into ICU course. Even though on admission they were no sicker compared to those without AKI, patients with severe AKI three days after hospital admission had worse outcome by every measure assessed. Taken together, this means the RAI may provide a way to study treatment strategies earlier in the course of AKI25 Also, the RAI may enable targeted therapeutic trial design based on degree of AKI risk.19 Finally, RAI-directed biomarker assessment should bolster positive predictive for AKI and refine targeted testing strategies (biomarker work and analysis currently in process).
Our study has several strengths and limitations. The large population and multinational size studied is rare for any prospective pediatric study. The heterogeneity between units and countries could have varied based on practice style. This is simultaneously a strength and a weakness – it further supports the pragmatic nature of this study, but does not offer ready conclusion on steps to take or follow to mitigate the problematic sequelae of AKI. The study did not account for patient management differences amongst providers or between institutions, nor did it place any a priori management protocols on patients in the ICU. Again, as we did not change local practice, our findings are more generalizable. Future work will study biomarkers and directed confirmatory testing (urine was collected in AWARE from over 600 patients). A statistical limitation was that the model term of AKIS loses independent association with mortality in multivariate analysis when renal angina fulfillment is also included, presumably because of patients deceased by Day 3. Renal angina has inherent strengths and weaknesses. The index calculation, based on existing pediatric epidemiology and associated outcomes (Figure 1, Supplement 1)16 – relies upon metrics used for early signs of injury known to be suboptimal for rapid response to injury. Even though the positive predictive value was higher than SCr>Base, 57.7% RA+ patients were negative for AKIS on Day 3. Additionally, 18.9% of patients were negative for RA yet positive for AKIS on Day 3. The reasons for these predictive errors are multifactorial. A limitation of the index itself is the reliance on increases in serum creatinine 12 hours into ICU course. The rate of rise in creatinine in response to injury is a known limitation. Fidelity of data entry by bedside staff relating to urine output or other laboratory indices can vary depending on institution and integration of electronic medical record systems. Finally, we admit application of RAI across patients with multifactorial disease and reliability of creatinine as it relates to patient volume status,26 as well as other adjustments based on patient population, will be required and will ultimately enhance refinement of the RAI. Still, the RAI is easily calculable and quick (not reliant on sophisticated calculation or derivation methods) and based on universally accessible, standard collected vitals and laboratory data (even if imperfect).
Conclusions
Fulfillment of renal angina is associated with the development of poor renal function and overall poor outcome. The renal angina index is superior to context-free changes in creatinine and gives a provider an early window to recognize the potential for severe AKI that will occur at a meaningful time for a critically ill patient. Renal angina supplies the context needed to increase the pre-test probability and accuracy of AKI prediction in critically ill patients with a heterogeneous mix of AKI risk.
Supplementary Material
Research in Context.
Evidence Before This Study
We searched PubMed and OViD databases between Jan 1 1997 and Oct 1 2017 for studies in critically ill children and adults using the following individual or grouped search terms: “risk stratification”, “acute kidney injury” (or “acute renal failure”), “pediatric”, and “critical illness”. In available results, analysis of the references used per source was conducted. Additionally, the expertise of the Prospective Pediatric AKI (ppAKI: www.ppaki.org) was leveraged. Finally, this manuscript was the principal analysis after the epidemiologic report of the AWARE Study Investigators (Assessment of Worldwide Acute Kidney Injury, Renal Angina, and Epidemiology). The investigators represent a global cross-section of acute care nephrology practitioners (nephrologists and pediatric intensivists) providing expertise on relevant data sources and references related to risk stratification and AKI. A majority of the 128 studies identified in our comprehensive search were excluded from in-depth analysis as they were small case reports or small size (< 50 patients), non-human, or not studied in critically ill patients. Of the 29 reports examined closely, 17 were reviews or commentaries, 4 were our own reports, and 9 were focused on different biomarker-based risk stratification assessments (neutrophil gelatinase associated lipocalin, cell-cycle arrest markers, and the furosemide stress test). The overriding conclusion was a paucity of an objective assessment system based on risk factors for AKI, allowing a clinician to properly adjudicate a marker of injury (creatinine or a novel biomarker). Many studies of the biomarkers or functional tests identify good to excellent discrimination for prediction of AKI (area under curve receiver operating characteristic > 0.80), but they are tested in single-center, extremely sick populations.
Added Value of this Study
The Assessment of Worldwide Acute Kidney Injury, Renal Angina and Epidemiology (AWARE) study is the largest continuously collected prospective observational study of critically ill children and young adults to date. Our multi-centre and multi-national registry included patients of significant heterogeneity (past medical history and comorbidities) and severity of illness. The study was designed with the express purpose of being able to assess the renal angina index methodology – purposely collecting specific data and at certain time points to expand our initial validation studies – demonstrating that across a wide swath of patients, risk stratification and provision of context to early signs of kidney injury is not only feasible and simple, but practical as well. Our findings of the predictive advantage using the objective-data based renal angina index fits squarely within the new consensus statements from the Acute Dialysis Quality Initiative (ADQI) for the definition of “acute kidney injury” (injury determined after 48 hours).
Additionally, the data provide a methodology to direct confirmatory biomarker testing, optimizing their use for prediction of severe AKI (the next step of our research).
Implications of all the Available Evidence
Of primary importance is that this report again emphasizes the negative associations between the presence of severe AKI and clinical outcomes in critically ill children and adults. The significantly worse outcomes of patients suffering AKI at Day 3 after admission highlights important opportunities: 1) prediction of Day 3 – AKI may be the first step in mitigation of AKI severity, 2) Screening out those patients on Day 0 with very low risk of Day 3 AKI can direct and target resources and potentially direct novel therapeutics on the population of greatest benefit, and 3) leverage of the electronic medical record – to provide support for decision making (i.e., the renal angina index can be integrated into existing EMR systems) is the next step in getting ahead of the AKI epidemic in children. Additional and next studies will leverage our existing data and also continue to grow the AWARE investigator network (e.g., the recently published AWAKEN – Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates works together with AWARE). Together, our previous reports and this report will continue building the foundation needed to attract the attention, resources, and personnel needed to make progress and improve outcomes for children who suffer AKI.
Acknowledgments
Funding/Trial Registration: NIH P50 DK096418 (Basu and Goldstein), NCT01987921
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none
Author Contributions
Author contributions were as follows:
RKB - Participated in literature search, figures, study design, data collection, data analysis, data interpretation, and writing the manuscript.
AK – Participated in designing the study, managing the data, data analysis and writing the manuscript.
SLG - Participated in the design, data collection, and writing of the manuscript.
References
- 1.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, Investigators A Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med. 2017;376(1):11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive care medicine. 2015;41(8):1411–23. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;(suppl (2)):1–138. [Google Scholar]
- 4.Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery- associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive care medicine. 2017 doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizo-Topete LM, Rosner MH, Ronco C. Acute Kidney Injury Risk Assessment and the Nephrology Rapid Response Team. Blood Purif. 2017;43(1–3):82–8. doi: 10.1159/000452402. [DOI] [PubMed] [Google Scholar]
- 6.Peters E, Mehta RL, Murray PT, et al. Study protocol for a multicentre randomised controlled trial: Safety, Tolerability, efficacy and quality of life Of a human recombinant alkaline Phosphatase in patients with sepsis-associated Acute Kidney Injury (STOP-AKI) BMJopen. 2016;6(9):e012371. doi: 10.1136/bmjopen-2016-012371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellum JA, Bellomo R, Ronco C. Kidney attack. Jama. 2012;307(21):2265–6. doi: 10.1001/jama.2012.4315. [DOI] [PubMed] [Google Scholar]
- 8.Mehta RL, Cerda J, Burdmann EA, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385(9987):2616–43. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 9.REGISTRY NRA-UR. THINK KIDNEYS. 2017 https://www.thinkidneys.nhs.uk/
- 10.Workman JK, Ames SG, Reeder RW, et al. Treatment of Pediatric Septic Shock With the Surviving Sepsis Campaign Guidelines and PICU Patient Outcomes. Pediatr Crit Care Med. 2016;17(10):e451–e8. doi: 10.1097/PCC.0000000000000906. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45(3):486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 12.Cvijanovich NZ, Anas N, Allen GL, et al. Glucocorticoid Receptor Polymorphisms and Outcomes in Pediatric Septic Shock. Pediatr Crit Care Med. 2017;18(4):299–303. doi: 10.1097/PCC.0000000000001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez Fernandez I, Jackson MC, Abend NS, et al. Refractory status epilepticus in children with and without prior epilepsy or status epilepticus. Neurology. 2017;88(4):386–94. doi: 10.1212/WNL.0000000000003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein SL, Chawla LS. Renal angina. Clin J Am Soc Nephrol. 2010;5(5):9439. doi: 10.2215/CJN.07201009. [DOI] [PubMed] [Google Scholar]
- 15.Chawla LS, Goldstein SL, Kellum JA, Ronco C. Renal angina: concept and development of pretest probability assessment in acute kidney injury. Crit Care. 2015;19:93. doi: 10.1186/s13054-015-0779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu RK, Chawla LS, Wheeler DS, Goldstein SL. Renal angina: an emerging paradigm to identify children at risk for acute kidney injury. Pediatr Nephrol. 2012;27(7):1067–78. doi: 10.1007/s00467-011-2024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu RK, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL. Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin JAm Soc Nephrol. 2014;9(4):654–62. doi: 10.2215/CJN.09720913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basu RK, Zappitelli M, Brunner L, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85(3):659–67. doi: 10.1038/ki.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menon S, Goldstein SL, Mottes T, et al. Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol Dial Transplant. 2016;31(4):586–94. doi: 10.1093/ndt/gfv457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–57. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 21.Basu RK, Kaddourah A, Terrell T, et al. Assessment of Worldwide Acute Kidney Injury, Renal Angina and Epidemiology in critically ill children (AWARE): study protocol for a prospective observational study. BMC Nephrol. 2015;16:24. doi: 10.1186/s12882-015-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3(4):948–54. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014;42(11):2409–17. doi: 10.1097/CCM.0000000000000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray PT, Mehta RL, Shaw A, et al. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int. 2014;85(3):513–21. doi: 10.1038/ki.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med. 2007;167(3):276–81. doi: 10.1001/archinte.167.3.276. [DOI] [PubMed] [Google Scholar]
- 26.Basu RK, Andrews A, Krawczeski C, Manning P, Wheeler DS, Goldstein SL. Acute kidney injury based on corrected serum creatinine is associated with increased morbidity in children following the arterial switch operation. Pediatr Crit Care Med. 2013;14(5):e218–24. doi: 10.1097/PCC.0b013e3182772f61. [DOI] [PubMed] [Google Scholar]
- 27.Goldstein SL, Currier H, Graf C, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107(6):130912. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


