Abstract
Background
The ideal timing for melanoma treatment, predominantly surgery, remains undetermined. Patient concern for receiving immediate treatment often exceeds surgeon or hospital availability, requiring establishment of a safe window for melanoma surgery.
Objective
To assess the impact of time to definitive melanoma surgery on overall survival.
Methods
Patients with stage I to III cutaneous melanoma and with available time to definitive surgery and overall survival were identified by using the National Cancer Database (N = 153,218). The t test and chi-square test were used to compare variables. Cox regression was used for multivariate analysis.
Results
In a multivariate analysis of patients in all stages who were treated between 90 and 119 days after biopsy (hazard ratio [HR], 1.09; 95% confidence interval [CI], 1.01–1.18) and more than 119 days (HR, 1.12; 95% CI, 1.02–1.22) had a higher risk for mortality compared with those treated within 30 days of biopsy. In a subgroup analysis of stage I, higher mortality risk was found in patients treated within 30 to 59 days (HR, 1.05; 95% CI, 1.01–1.1), 60 to 89 days (HR, 1.16; 95% CI, 1.07–1.25), 90 to 119 days (HR, 1.29; 95% CI, 1.12–1.48), and more than 119 days after biopsy (HR, 1.41; 95% CI, 1.21–1.65). Surgical timing did not affect survival in stages II and III.
Limitations
Melanoma-specific survival was not available.
Conclusion
Expeditious treatment of stage I melanoma is associated with improved outcomes.
Keywords: melanoma, National Cancer Database, stage I melanoma, survival, time to surgery, time to treatment
Cutaneous malignant melanoma (MM) is the fifth most common cancer among men and seventh among women.1,2 Rates of melanoma continue to rise in the United States and worldwide, and melanoma will be diagnosed in approximately 2.1% of the U.S. population in their lifetime.3–5 Furthermore, approximately 76,380 new cases of melanoma will be diagnosed in 2016, and 10,230 people will die of melanoma.2
The standard of care is surgery, which can include sentinel lymph node biopsy (SLNB) or lymphadenectomy depending on melanoma stage.3,6 On diagnosis, the majority of melanomas are confined to a primary site (84%), with occasional spread to regional lymph nodes (9%) and metastasis (4%).3 The estimated 5-year relative survival is 98.4% if confined to a primary site, 62.4% if lymph nodes are affected, and 17.9% if distantly metastatic.3
Intuitively, a more expeditious treatment after cancer diagnosis should lead to better outcome. The data analysis of the National Cancer Database (NCDB) showed a better overall survival (OS) if time to treatment initiation (TTI) was reduced for head and neck squamous cell carcinoma and breast cancers.7,8 Despite this likely relationship between TTI and survival, there are only a few small and predominantly retrospective studies examining the impact of TTI on melanoma survival.9–11
Although conclusive data regarding impact of time to definitive (ie, surgical) treatment on melanoma survival are lacking, there has been speculation regarding “ideal” TTI. The current recommendation for definitive melanoma treatment is within 3 to 4 weeks following diagnosis in the United States and 4 to 6 weeks in Europe.12,13 To address this issue, we used the NCDB to evaluate the effect of time from biopsy to definitive surgery on melanoma OS.
METHODS AND MATERIALS
The NCDB is a clinical oncology database established in 1989 as a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and American Cancer Society. A business associate agreement, including a data use agreement, between the American College of Surgeons and CoC accredited facilities is in place. The NCDB is a facility-based database, representing 48.4% of all melanomas diagnosed in the United States.14,15 This database contains cases of patients 18 or older who received all or part of their first course therapy at a reporting cancer program.16 These data were provided to the authors by the NCDB in a de-identified file, with prior approval from the Cleveland Clinic Institutional Review Board.14
Patients in whom malignant melanoma had been diagnosed from 2004 to 2012 were identified from the NCDB by using the International Classification of Diseases for Oncology codes. Included cases had follow-up time, living status, TNM stages I to III, and TTI longer than 0 but no longer than 365 days.
We defined TTI as time from biopsy to definitive surgery. A small percentage of the patients had excisional biopsy listed as their definitive surgery, resulting in a time of 0 days to biopsy, and these patients were excluded; this rate was similar between the stages, with 12.5% excluded from stage I, 12.5% from stage II, and 11.7% from stage III. In addition, patients treated after more than 365 days were excluded on account of probable existence of other factors affecting the decision to postpone surgery. SLNB data before 2012 were not provided to researchers; however, this information was available to the NCDB and used for staging.17 In addition, information on compliance with National Comprehensive Cancer Network guidelines was unavailable.
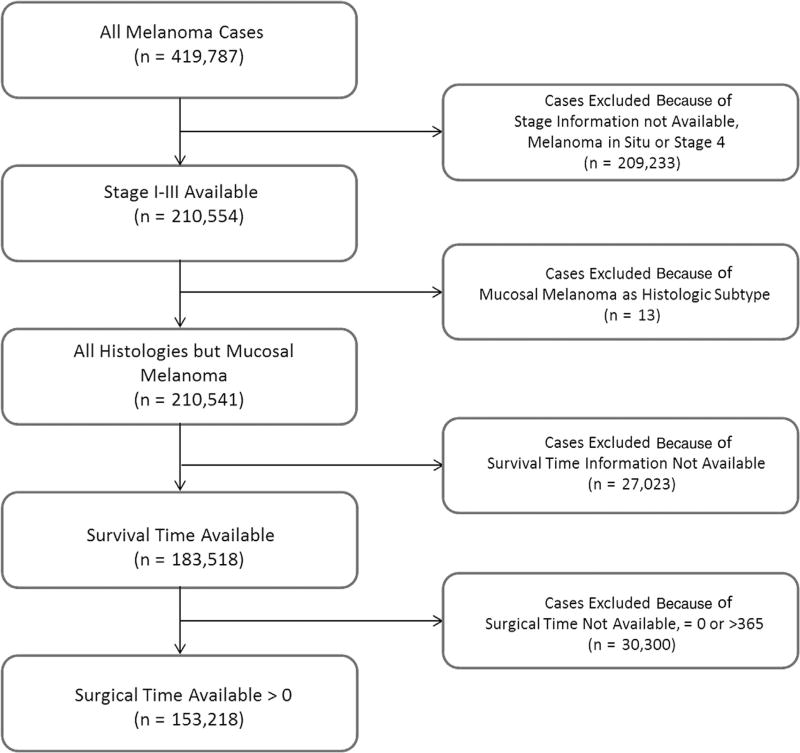
We identified 419,787 cases, of which 210,650 were TNM stage I to III (Fig 1). After exclusion of patients without follow-up, 183,622 cases remained. Lastly, patients without a time to definitive surgery or a time longer than 365 days were excluded, leaving 153,218 in our final cohort, which corresponded to 37% of the initial cohort.
Fig 1.
Flowchart presenting selection of patients from the National Cancer Database.
Variables that can influence melanoma treatment and outcomes, such as age, sex, comorbidity status, insurance type, treating facility type and location, primary site, laterality, histologic type, and melanoma stage were analyzed. Follow-up ranged between 3 and10 years, and patients who remained alive once their observation period ended were censored. Survival data are based on OS.
Statistical analysis
Available covariates included age, sex, race, insurance type, facility location, facility type, comorbidity score, tumor site, laterality, and cancer stage. Age at diagnosis was categorized as younger than 30, 30 to 39, 40 to 49, 50 to 59, 60 to 69, 70 to 79, and older than 79 years. Insurance type was classified as Medicare, Medicaid, private insurance, other type of government insurance, no insurance, or insurance status unknown. Facility type was classified as academic/research program, community cancer program, comprehensive community cancer program, integrated network cancer program, other, or unknown. The Charlson-Deyo comorbidity score was designated as 0 if patients had no comorbidities, 1 if there was 1 comorbidity, and 2 if there were 2 or more comorbidities, with comorbidities defined as previously published.18 Tumor site was designated as head and neck, upper extremities, trunk, lower extremities, overlapping, or not specified. Tumor laterality was left, right, midline, or unknown. Breslow depth was categorized as less than 1 mm, 1.01 to 2.0 mm, 2.01 to 4.0 mm, more than 4 mm, or not specified. Ulceration and lymphovascular invasion (LVI) were classified as present, not present, or unknown. TNM stages IA and IB were combined into stage I; stages IIA, IIB and IIC were combined into stage II; and stages IIIA, IIIB and IIIC were combined into stage III.
Time of surgery was categorized as 1 to 29, 30 to 59, 60 to 89, 90 to 119, and more than 119 days after biopsy on the basis of current clinical recommendations and knowledge. For univariate analysis, the chi-square test and t test were used. Data were presented as number and percent for categoric variables and as mean and standard deviation for continuous variables. Multivariate analysis was conducted by using a Cox proportional hazards model. Subgroup analysis was performed by stage because of its high impact on survival. Statistical significance was achieved with an α value less than 0.05. The data analysis was performed using R statistical software (version 3.2.4, R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
We identified 153,218 patients, of whom 71,950 were treated 30 days or more after biopsy. Specifically, 44.9% of stage I melanomas were treated 29 or more days after biopsy, as were 50.3% of stage II and 51.3% of stage III melanomas (Supplemental Fig 1; available at http://www.jaad.org). Furthermore 9% of stage I melanomas were treated 59 or more days after biopsy, as were 11.8% of stage II and 11.7% of stage III melanomas. Patients with a longer TTI tended to be older, be male, be using Medicare, have more comorbidities, have head and neck melanoma, have a higher Breslow thickness, be less likely to be ulcerated or have LVI, and be at a higher stage compared with those with a shorter TTI (Supplemental Table I; available at http://www.jaad.org).
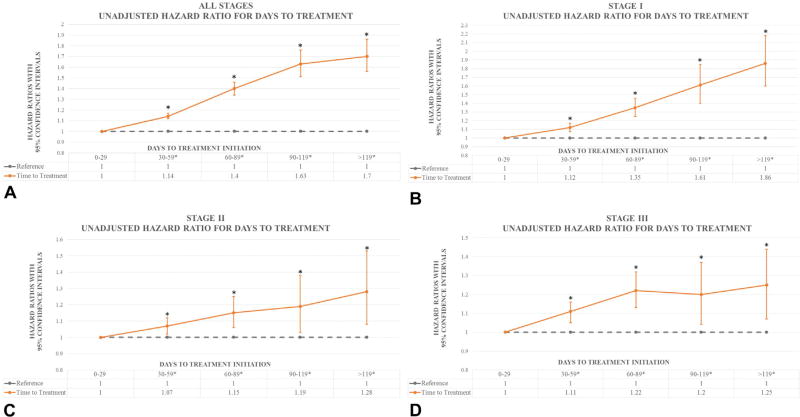
Unadjusted analysis of TTI showed improved survival for patients with shorter waiting times, with patients who were treated 30 to 59 days after biopsy having 14% worse OS, with 40% worse OS for those treated 60 to 89 days after biopsy, 63% worse OS for those treated 90 to 119 days after biopsy, and 70% worse OS for those treated more than 119 days after biopsy compared with patients treated within the first 29 days (Fig 2, A). In addition, unadjusted analysis of stage based on TTI showed worse prognosis with longer TTI, which was most apparent in stage I (Fig 2, B to D).
Fig 2.
Graphic representation of adjusted and unadjusted hazard ratios by time to treatment initiation. Point estimates represent hazard ratios. Bars represent 95% confidence intervals. A, Unadjusted hazard ratios by time to treatment initiation. B, Unadjusted hazard ratio for days to treatment in stage I. C, Unadjusted hazard ratio for days to treatment in stage II. D, Unadjusted hazard ratio for days to treatment in stage III. Asterisks denote statistical significance.
Multivariate adjustment for age, race, sex, comorbidities, year of diagnosis, hospital location, laterality, primary site, histologic type, Breslow depth, ulceration, LVI, insurance status, and stage was used for all models.
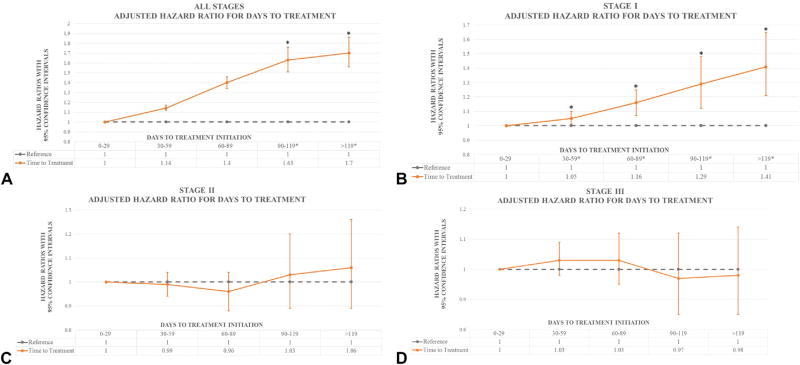
All-stage model
After adjustment, there were no differences in survival for patients with a TTI less than 90 days (Supplemental Table II [available at http://www.jaad.org] and Fig 3, A). Patients who were treated within 90 to 119 days and after 119 days were 9% and 12% more likely to die, respectively, than were those treated within 30 days. Patients with a Breslow depth of 1.01 to 2.00 mm, 2.01 to 4.00 mm, and more than 4.0 mm had a 12%, 25%, and 80% greater chance of death, respectively, than those with a Breslow depth less than 1.0 mm regardless of other factors. Worse outcomes were present with increasing age, male sex, head and neck melanoma, ulceration, LVI, and more comorbidities.
Fig 3.
Graphic representation of adjusted hazard ratios by time to treatment initiation. Point estimates represent hazard ratios. Bars represent 95% confidence intervals. A, Adjusted hazard ratios by time to treatment initiation. B, Adjusted hazard ratio for days to treatment in stage I. C, Adjusted hazard ratio for days to treatment in stage II. D, Adjusted hazard ratio for days to treatment in stage III. Asterisks denote statistical significance.
Stage I model
Following adjustment, patients who waited between 30 and 59 days after biopsy had 5% worse survival; those who waited 60 to 89 days had 16% worse survival; those who waited 90 to 119 days had 29% worse survival; and those who waited longer than 119 days had 41% worse survival (Supplemental Table III [available at http://www.jaad.org] and Fig 3, B). Patients with a Breslow depth of 1.01 to 2.0 mm had 35% worse survival than those with a Breslow depth less than 1.0 mm independent of other factors. Additional factors that negatively affected survival were older age, male sex, more comorbidities, nodular or acral melanoma, a diagnosis of head and neck melanoma, midline tumors, and ulceration or LVI. Factors that positively affected survival were having private insurance, lower extremity melanoma, and desmoplastic melanoma. In an effort to pinpoint the breakpoint at which TTI starts to significantly affect survival, we analyzed TTI in 2-week intervals, with the breakpoint being between 43 and 56 days. The exact day at which TTI becomes important for survival could not be identified, likely because of an insufficient number of events.
Stage 2 model
After adjustment, there were no differences in survival times between the 5 TTI categories (Supplemental Table IV [available at http://www.jaad.org] and Fig 3, C). Younger age, female sex, lack of comorbidities, desmoplastic or spindle histologic type of melanoma, upper or lower extremity melanoma, and lower Breslow depth favorably affected survival. Factors that negatively affected survival were Medicaid, acral melanoma, head and neck melanoma, and ulceration or LVI.
Stage 3 model
As in stage II, there were no differences in survival between the 5 TTI categories after adjustment (Supplemental Table V [available at http://www.jaad.org], Fig 3, D). Older age at diagnosis, male sex, Medicaid, acral melanoma, higher Breslow depth, and presence of ulceration or LVI negatively affected survival. Patients with fewer comorbidities, melanoma on the extremities, and desmoplastic melanoma had better survival.
DISCUSSION
In this study, we were, to the best of our knowledge, the first to show that OS decreases in patients waiting longer than 90 days for definitive surgical treatment of melanoma regardless of stage. Moreover, we believe that we are reporting for the first time that delay of surgery beyond the first 29 days for stage I melanoma negatively OS when 30-day intervals are used. Furthermore, when TTI was analyzed in 2-week intervals for stage I, we found that the critical time point at which TTI in the 30- to 59-day group leads to worse outcomes is between 43 and 56 days.
The finding that increasing TTI negatively affects survival of patients with earlier stages of cancer is similar to the findings of several nonmelanoma studies. In a recent report, Bleicher et al used the NCDB to examine the impact of TTI on breast cancer OS by stage, concluding that there is a 16%mortality increase for each 30-day increase in waiting time in stage I and a 9% increase in stage II but no effect on stage III.8 Similarly, an analysis of head and neck squamous cell carcinoma found that patients with stage I and II squamous cell carcinoma who waited 31 to 60 days had a 17% increase in mortality, whereas those waiting 61 to 90 days had a 54% increase in mortality; in comparison, there was no effect on mortality for those with stage III or IV disease who waited 31 to 60 days and an 8% increase for those who waited 61 to 90 days.7
Because of the much smaller cohort size, and thus lower statistical power, prior studies examining the impact of TTI on melanoma survival were not able to examine their data in the same level of granularity as ours, which explains why these outcomes have not been previously reported. For example, McKenna et al found no impact of TTI in OS or disease-specific survival regardless of melanoma stage, in a cohort of 986 patients.9 Similarly, Carpenter et al concluded that there was no difference in OS or disease-specific survival in 473 patients who waited less than or more than 28 days for treatment.19 Lastly, Parret et al found no difference in OS after analyzing TTI on the basis of groups of patients who waited less than or more than 40 days.20
We hypothesize that the negative effect of prolonging TTI on early-stage melanoma is visible as a result of the lower baseline mortality found in these patients. However in stages II and III, the potential benefits of a shorter TTI are likely overshadowed by a higher metastatic potential and baseline mortality. In other words, it appears that prompt initiation of definitive treatment is beneficial in stage I melanoma but likely not in stage II or III.
Stages II and III melanoma are likely influenced by time to diagnosis, the importance of which is illustrated by several national studies and programs created to increase patient suspicion and physician recognition, leading to a decreasing incidence and mortality.5,21–24 For example, the state of Israel had the second highest incidence and mortality due to melanoma in the world; however, following prevention and detection efforts, its incidence rate dropped to 13th place for men and 20th place for women, whereas mortality dropped to eight and 10th place, respectively.22,23,25 Furthermore, rates of melanoma in situ increased, indicating a higher awareness and increase in early detection.22,25 Next, in an effort to improve waiting time after identification of a suspicious lesion, the United Kingdom implemented a “2-week” rule, according to which patients needed to see a specialist within 2 weeks of being referred by their general practitioner for any suspicion of cancer. Following implementation of this rule, detected melanomas were lower in stage and OS was significantly improved.21 Finally, following implementation of a new systematic skin cancer screening program in Germany, rates of mortality due to melanoma decreased.5 The findings of these studies support our hypothesis and indicate that detection in early stages and prompt treatment initiation following identification likely result in decreased mortality.
Our study comes at a critical time in melanoma treatment. Stage I melanoma is typically treated by various specialties, including general practitioners, dermatologists, otorhinolaryngologists, general surgeons, surgical oncologists, and plastic surgeons, leading to high variability in approach and treatment.26–29 Furthermore, even in a single specialty, there is a high variability in physicians’ treatment approach for stage I melanoma; this includes controversy in performing SLNB.30 Finally, as a larger variety of physicians can treat stage I melanoma, a disproportionate number of cases may not be captured in the NCDB, which could have influenced our data. This fact may limit the applicability of our conclusions to CoC reporting facilities. However this concern is likely balanced by the fact that the database contains almost half of all melanomas treated in the United States. In contrast, stage II and III melanomas tend to be treated by specialists who perform SLNB and lymphadenectomy; given many guidelines such as those from the National Comprehensive Cancer Network, this coalesced group of surgeons likely deal with these advanced stages by using a more algorithmic approach. Our data highlight the need for stricter adherence to recommended TTI and standardization of the treatment approach for physicians who treat stage I melanoma.
Lastly, our data provide critical information for ensuring safety in managing and planning neoadjuvant trials for melanoma. Neoadjuvant treatment, in which patients are treated before definitive surgical treatment, is used primarily in stage II and III melanoma and may result in delay of surgery.31–33 Despite current controversies in neoadjuvant therapy, currently there are 26 active neoadjuvant trials for MM, indicating that neoadjuvant trials will continue as a scientific endeavor.34,35 According to our data, TTI does not affect OS in these stages, allowing temporal freedom to maximize preoperative/“neo” components.
CONCLUSION
In conclusion, we found that definitive surgical treatment for stage I melanoma should be expeditious, whereas definitive surgical treatment for stage II and III may not affect OS in the current milieu of melanoma. Furthermore, expeditious patient identification and biopsy could migrate would-be stage II and III patients to stage I, where TTI optimization can be used to further improve their OS. On the basis of the available literature combined with our data, it is likely that these times are underoptimized and greater efforts to improve the entire process from suspicion of melanoma to its ultimate treatment should be implemented.
Supplementary Material
CAPSULE SUMMARY.
Impact of time to treatment on survival in melanoma remains uncertain.
Prolonging time to treatment of melanoma negatively affects stage I but not stage II and III survival.
Expeditious treatment of stage I melanoma could improve survival.
Acknowledgments
Funding sources: None.
We thank Katherine Glass for assistance with accessing data.
Abbreviations used
- CoC
Commission on Cancer
- LVI
lymphovascular invasion
- MM
malignant melanoma
- NCDB
National Cancer Database
- OS
overall survival
- SLNB
sentinel lymph node biopsy
- TTI
time to treatment initiation
Footnotes
Conflicts of interest: None declared.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2016. Atlanta, Georgia: American Cancer Society; 2016. [Accessed July 6, 2016]. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2016/cancer-facts-and-figures-2016.pdf. [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975–2013. [Accessed June 10, 2016]. Available at: https://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 4.Nikolaou V, Stratigos AJ. Emerging trends in the epidemiology of melanoma. Br J Dermatol. 2014;170:11–19. doi: 10.1111/bjd.12492. [DOI] [PubMed] [Google Scholar]
- 5.Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in northern Germany. J Am Acad Dermatol. 2012;66:201–211. doi: 10.1016/j.jaad.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy CT, Galloway TJ, Handorf EA, et al. Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. J Clin Oncol. 2016;34:169–178. doi: 10.1200/JCO.2015.61.5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2:330–339. doi: 10.1001/jamaoncol.2015.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKenna DB, Lee RJ, Prescott RJ, Doherty VR. The time from diagnostic excision biopsy to wide local excision for primary cutaneous malignant melanoma may not affect patient survival. Br J Dermatol. 2002;147:48–54. doi: 10.1046/j.1365-2133.2002.04815.x. [DOI] [PubMed] [Google Scholar]
- 10.Baade PD, English DR, Youl PH, McPherson M, Elwood JM, Aitken JF. The relationship between melanoma thickness and time to diagnosis in a large population-based study. Arch Dermatol. 2006;142:1422–1427. doi: 10.1001/archderm.142.11.1422. [DOI] [PubMed] [Google Scholar]
- 11.Torring ML, Frydenberg M, Hansen RP, Olesen F, Vedsted P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: a cohort study in primary care. Eur J Cancer. 2013;49:2187–2198. doi: 10.1016/j.ejca.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Hajdarevic S, Hornsten A, Sundbom E, Isaksson U, Schmitt-Egenolf M. Health-care delay in malignant melanoma: various pathways to diagnosis and treatment. Dermatol Res Pract. 2014;2014:294287. doi: 10.1155/2014/294287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline—update 2016. Eur J Cancer. 2016;63:201–217. doi: 10.1016/j.ejca.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112(suppl 1):S92–S107. doi: 10.1038/bjc.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Survival NSCB. [Accessed January 7, 2017];NCDB survival reports. Available at: https://www.facs.org/~/media/files/quality%20programs/cancer/ncdb/survival_help_07172015.ashx.
- 17. [Accessed January 7, 2017];Scope of regional lymph node surgery. Available at: http://ncdbpuf.facs.org/sites/default/files/FORDS%20Scope%20of%20Regional%20LN%20Surgery.pdf.
- 18.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter S, Pockaj B, Dueck A, et al. Factors influencing time between biopsy and definitive surgery for malignant melanoma: do they impact clinical outcome? Am J Surg. 2008;196:834–842. doi: 10.1016/j.amjsurg.2008.07.044. [discussion 842–843] [DOI] [PubMed] [Google Scholar]
- 20.Parrett BM, Accortt NA, Li R, et al. The effect of delay time between primary melanoma biopsy and sentinel lymph node dissection on sentinel node status, recurrence, and survival. Melanoma Res. 2012;22:386–391. doi: 10.1097/CMR.0b013e32835861f6. [DOI] [PubMed] [Google Scholar]
- 21.Pacifico MD, Pearl RA, Grover R. The UK government two-week rule and its impact on melanoma prognosis: an evidence-based study. Ann R Coll Surg Engl. 2007;89:609–615. doi: 10.1308/003588407X205459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Israel Cancer Association. [Accessed August 16, 2016];2015 Skin Cancer Prevention and Detection Awareness Week. Available at: http://en.cancer.org.il/template_e/default.aspx?PageId=8925.
- 23.Haaretz. [Accessed September 28, 2016];Israel’s skin cancer rate second highest in the world. Available at: http://www.haaretz.com/israel-s-skin-cancer-rate-second-highest-in-the-world-1.10250.
- 24.Watts CG, Cust AE, Menzies SW, Coates E, Mann GJ, Morton RL. Specialized surveillance for individuals at high risk for melanoma: a cost analysis of a high-risk clinic. JAMA Dermatol. 2015;151:178–186. doi: 10.1001/jamadermatol.2014.1952. [DOI] [PubMed] [Google Scholar]
- 25.Sella T, Goren I, Shalev V, et al. Incidence trends of keratinocytic skin cancers and melanoma in Israel 2006–11. Br J Dermatol. 2015;172:202–207. doi: 10.1111/bjd.13213. [DOI] [PubMed] [Google Scholar]
- 26.McKenna DB, Marioni JC, Lee RJ, Prescott RJ, Doherty VR. A comparison of dermatologists’, surgeons’ and general practitioners’ surgical management of cutaneous melanoma. Br J Dermatol. 2004;151:636–644. doi: 10.1111/j.1365-2133.2004.06065.x. [DOI] [PubMed] [Google Scholar]
- 27.DeFazio JL, Marghoob AA, Pan Y, Dusza SW, Khokhar A, Halpern A. Variation in the depth of excision of melanoma: a survey of US physicians. Arch Dermatol. 2010;146:995–999. doi: 10.1001/archdermatol.2010.156. [DOI] [PubMed] [Google Scholar]
- 28.Corbo MD, Vender R, Wismer J. Comparison of dermatologists’ and nondermatologists’ diagnostic accuracy for malignant melanoma. J Cutan Med Surg. 2012;16:272–280. doi: 10.1177/120347541201600410. [DOI] [PubMed] [Google Scholar]
- 29.Martinka MJ, Crawford RI, Humphrey S. Clinical recognition of melanoma in dermatologists and nondermatologists. J Cutan Med Surg. 2016;20:532–535. doi: 10.1177/1203475415623513. [DOI] [PubMed] [Google Scholar]
- 30.Charles CA, Yee VS, Dusza SW, et al. Variation in the diagnosis, treatment, and management of melanoma in situ: a survey of US dermatologists. Arch Dermatol. 2005;141:723–729. doi: 10.1001/archderm.141.6.723. [DOI] [PubMed] [Google Scholar]
- 31.van Zeijl MC, van den Eertwegh AJ, Haanen JB, Wouters MW. (Neo)adjuvant systemic therapy for melanoma. Eur J Surg Oncol. 2017;43:534–543. doi: 10.1016/j.ejso.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Davar D, Tarhini AA, Kirkwood JM. Adjuvant immunotherapy of melanoma and development of new approaches using the neoadjuvant approach. Clin Dermatol. 2013;31:237–250. doi: 10.1016/j.clindermatol.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moschos SJ, Edington HD, Land SR, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol. 2006;24:3164–3171. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 34.Raigani S, Cohen S, Boland GM. The role of surgery for melanoma in an era of effective systemic therapy. Curr Oncol Rep. 2017;19:17. doi: 10.1007/s11912-017-0575-8. [DOI] [PubMed] [Google Scholar]
- 35.Clinical Trials.gov. [Accessed February 28, 2017];Search results, 24 studies found for neoadjuvant trials on melanoma. Available at: https://clinicaltrials.gov/ct2/results?term=neoadjuvant+trials+on+melanoma&pg=2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.