Abstract
Transdermal drug delivery systems that utilize transcutaneous patches of arrayed microneedles have attracted increasing interest in medical practice as an alternative method to hypodermic injection. Over the past ten years, research has focused on leveraging physiological signals associated with diseases or skin-specific tissues to create bioresponsive patches that release drug directly in response to an internally-generated stimulus. This review surveys the recent advances in the development and use of bioresponsive transcutaneous patches for on-demand smart and precise drug delivery, exploiting different physiological signals including pH, serum glucose levels, and enzyme activity. The clinical potential of these devices, including challenges and opportunities, is also discussed.
Introduction
Hypodermic injection is a widely used delivery technique for most biotherapeutics and represents a low-cost and rapid delivery approach [1]. However, injections are often associated with poor patient adherence and may lead to injection phobia and distress [2•,3–5]. An attractive alternative to hypodermic injection is to deliver therapeutics across the skin using transcutaneous patches [2•,3,6]. Typically, these transcutaneous patches incorporate arrays of microneedles (MNs) that are designed to penetrate skin’s outer stratum corneum layer to enhance delivery capabilities [2•,7s–9]. Since the needles are micron-size, they can deliver almost any drug or small particulate formulation as well as facilitate localized tissue delivery [2•]. Critically, transcutaneous patches are a more appealing approach to patients as this method of drug delivery is painless and can be self-administered [2•,3,6].
Recently, transdermal patch models that incorporate stimuli-responsive MNs which release drug in response to an internally-generated stimuli have been proposed for smart and precise drug release [10–13]. Compared to delivery systems triggered by external stimuli like electric field [14,15], light [16,17], or mechanical force [18], the MN patches activated by a physiological signal provide self-regulated delivery of drug in response to the abnormal physiological signals, thereby maximizing therapeutic efficiency and minimizing side effects or toxicity [19].
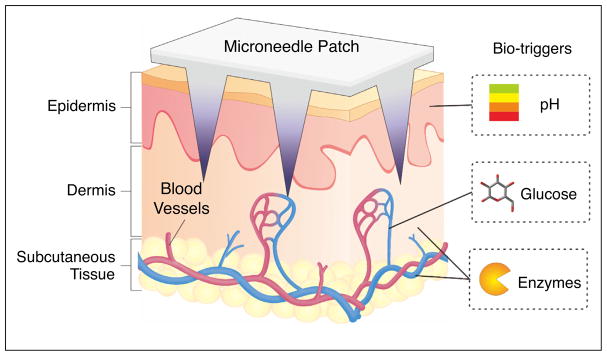
For instance, glucose-responsive MNs can be triggered to release insulin in response to abnormally high glucose levels in vascular and lymph capillary networks while showing basal insulin release in euglycemic conditions, achieving a smart closed-loop system for insulin delivery [20•]. Herein, we will summarize and classify recent advances in the development of bioresponsive transcutaneous patches, including pH-responsive, glucose-responsive, and enzyme-activated systems (Figure 1), and discuss the advantages, limitations of these current formulations. Future challenges and opportunities in terms of clinical translation will also be discussed.
Figure 1.
Typical physiological signals (bio-triggers) for bioresponsive transcutaneous patches.
pH-responsive transdermal patches
Normal skin is slightly acidic, with a pH ranging from 4.0 to 7.0, which provides a barrier to bacteria, viruses and other potential contaminants [21]. In particular, the acid mantle secreted by sebaceous glands maintains the epidermis pH at approximately 5.5 [22]. The acidic properties of skin enable the use of pH-sensitive patches for on-demand transdermal drug delivery. For example, MNs filled pH-responsive poly(lactic-co-glycolic acid) (PLGA) hollow microspheres were developed and reported to sequentially co-deliver multiple drugs to skin tissue by Ke et al. [23]. In this system, hollow PGLA microspheres encapsulated an aqueous core containing red-fluorescent dye Cy5 as a model drug and sodium bicarbonate (NaHCO3) loaded via a double-emulsion method. The Cy5-loaded microspheres and a second model drug, Alexa 488, were further encapsulated together in polyvinylpyrrolidone (PVP) MN arrays. Upon application to the skin, the PVP rapidly dissolved within minutes, simultaneously releasing the Alexa 488 dye. The acidic environment of the skin stimulated NaHCO3 in the PLGA microspheres to generate CO2 bubbles, thereby creating the channels in the PLGA shell and releasing the Cy5. Researchers demonstrated the sequential release of the two dyes into the porcine cadaver skin ex vivo using fluorescence microscopy. pH-sensitive surface modification was also reported in the fabrication of pH-sensitive microneedles. Here, MNs were coated with ovalbumin, a model antigen, and a pH-sensitive pyridine surface [24]. Upon insertion into the acidic skin conditions, reduced electrostatic interactions allowed the ovalbumin to be efficiently released. Layer-by-layer assembly of polyelectrolytes has also been shown to achieve pH-triggered drug release through weakened electrostatic binding that occurs between the negatively and positively charged layers in the physiological pH [25,26].
Glucose-responsive transdermal patches
Since MNs inserted into skin can directly contact the dermal microcirculation, these MNs can sense serum biomarker levels and changes thereof in a real-time manner [8,27]. For patients with diabetes who are tasked with frequent monitoring of blood glucose levels and timely injection of insulin as part of diabetes self-management [28,29], insulin-loaded MNs with glucose-responsive moieties are desirable for achieving closed-loop insulin delivery. Based on this concept, Yu et al. have developed a ‘smart insulin patch’ that effectively releases insulin in response to hyperglycemic conditions for diabetes treatment [20•]. In this study, glucose-responsive vesicles containing insulin and the glucose-specific enzyme (GOx) were loaded into the tips of MN arrays. These vesicles were formed from hypoxia-sensitive hyaluronic acid (HA) conjugated with a hydrophobic group that could be bio-reduced to hydrophilic under hypoxic conditions (2-nitroimidazole). In hyperglycemic conditions, oxygen consumption from the enzymatic conversion of glucose to gluconic acid generated a local hypoxic environment, which resulted in the reduction of 2-nitroimidazole to hydrophilic 2-aminoimidazole, disassembly of the vesicles, and subsequent insulin release. Researchers demonstrated this glucose-responsive insulin-delivery system was able to quickly ‘sense’ and correct elevated blood glucose levels of chemically induced type 1 diabetic mice to the normal state within 0.5 hour and maintain euglycemic conditions for several hours thereafter.
Furthermore, Gu group have also designed an MN patch integrated with insulin-secreting pancreatic beta-cells and loaded with glucose-signal amplifiers for glucose-responsive insulin delivery [30•]. Instead of direct insulin release from glucose-responsive vesicles, these vesicles were encapsulated with GOx, α-amylase as well as glucoamylase and acted as synthetic glucose-signal amplifiers. In high glucose concentrations, α-amylase and glucoamylase were released and hydrolyzed the α-amylose that loaded in MNs into glucose. This amplified glucose signal further diffused into the externally positioned beta-cell capsules on the base of MN patch, prompting secretion of insulin for diffusion into the vascular and lymph capillary networks. This model showed extended therapeutic efficacy compared the MNs without glucose-signal amplifiers, where one patch was shown to effective control on blood glucose levels for 6 hours in diabetic mouse.
Besides enzymatically-generated hypoxia, H2O2 produced during the enzymatic oxidation of glucose can also act as a trigger to facilitate insulin release from MNs. Hu et al. described a glucose-responsive insulin delivery device integrating H2O2-sensitive polymeric vesicles with a MNs-array patch [31•]. The insulin-loaded PVs were self-assembled from block copolymer incorporated with polyethylene glycol (PEG) and phenylboronic ester (PBE)-conjugated polyserine. In this system, the PBE pendant were degraded in a H2O2-mediated manner, leading to the disassociation of PVs. In vivo performance of the patch integrating with these PVs demonstrated the ability to correct hyperglycemia and self-regulate blood glucose levels in a diabetic mouse model.
Most recently, Gu group have integrated hypoxia and H2O2 dual-sensitive vesicles to design MNs for enhanced glucose-responsive insulin delivery [32]. These dual-sensitive vesicles were prepared by diblock copolymer consisting of poly(ethylene glycol) (PEG) and polyserine modified with 2-nitroimidazole via a thioether moiety. Hydrophobic 2-nitroimidazole could be bio-reduced into hydrophilic 2-aminoimidazole under a hypoxic condition. In addition, the thioether acted as a H2O2-sensitive moiety that increased the aqueous solubility of the copolymer upon conversion to a sulfone by H2O2. When these vesicles encapsulating insulin and GOx were exposed to a high blood glucose level in the vascular and lymph capillaries, the quick oxygen consumption and H2O2 generation led to the increased water-solubility of copolymer, promoting the dissociation of the glucose-responsive vesicles and subsequent release of the insulin. Importantly, the undesirable H2O2 was eliminated during the conversion of thioether to sulfone, thereby mitigating free radical-induced damage to skin tissue and maintaining the activity of the GOx. Researchers demonstrated this integrated smart insulin patch could effectively regulate blood glucose levels in diabetic mice for 10 hours and was associated with insignificant inflammation during a longer two-week period of usage.
Aside from the use of hyperglycemia as a disease-associated trigger for the release of therapeutics such as insulin, normal blood glucose level can also be used as a physiological signal to achieve sustained drug release. For instance, Wang et al. reported an anti-PD-1 loaded MN patch for sustained drug delivery in a glucose-mediated degradation manner for the melanoma treatment [33]. The checkpoint inhibitor (anti-PD-1) that blocks the programmed death-1 (PD-1) pathway was encapsulated in glucose-responsive nanoparticles. With the GOx/Catalase enzymatic system immobilized inside the NPs, gluconic acid generated from the enzymatic oxidation of glucose facilitated the gradual self-dissociation of NPs, creating a sustained release of anti-PD-1 over a three-day administration period. In vivo studies demonstrated robust immune responses in a B16F10 mouse melanoma model treated with the aPD1 patches compared to control groups administrated with patches that cannot be triggered to degrade or intratumoral injection of free aPD1 with the same dose.
Enzymes-activated transdermal patches
Disease-associated enzymes have recently attracted remarkable attention as targets for precision medications [34,35]. For example, hyaluronidase (HAase) is overexpressed by various types of cancer cells and has become recognized as a tumor marker [36]. Recently, a HAase-activated drug delivery system was developed for synergistic transcutaneous immunotherapy to enhance antitumor immune responses [37]. In this study, 1-methyl-DL-tryptophan (1-MT), an inhibitor of immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO), was conjugated to hyaluronic acid (HA) to give an amphiphilic polymer that was self-assembled into therapeutic nanocapsule to encapsulate anti-PD1 antibody (aPD1), the inhibitor of the immunoinhibitory receptor programmed cell death protein 1 (PD1). Integrated with the MN arrays, this combination of therapeutics was shown to be readily transported across the stratum corneum and successfully reach the network of skin-resident dendritic cells (DCs) around the melanoma tumor. Drug release was facilitated by the high levels of HAase overexpressed in the tumor region, which activated by the enzymatic degradation of HA. This system exhibited enhanced local retention of the therapeutics and a potent antitumor effect in a B16F10 mouse melanoma model.
In another study, in order to achieve long-term auto-regulation of blood coagulation, Zhang et al. designed a thrombin-responsive patch for on-demand heparin delivery [38]. Heparin, a common anticoagulant, was conjugated to the main chain of hyaluronic acid through a thrombin-cleavage peptide (GGLVPR|GSGGC) to create a closed-loop device for sustained anticoagulant regulation. The MNs prepared from the heparin-HA conjugate were shown to quickly respond to an increased thrombin levels by releasing heparin, preventing the undesirable formation of blood clots by the thrombin-triggered cleavage of the linker. Under normal blood conditions, no drug was released. Subsequent in vivo thrombolytic challenge experiments revealed potential for this patch as an efficient, long-term protection against abnormal blood clotting and acute pulmonary thromboembolism.
Conclusions and outlook
Bioresponsive transcutaneous patches hold tremendous promise for on-demand drug delivery to enhance therapy efficacy while minimizing associated toxicity or side effects. In Table 1, we summarize typical responsive mechanisms triggered by a variety of the physiological stimuli described in this article, including changes in pH, serum glucose levels, and enzyme activity. Looking ahead, physiological signal-responsive devices may be highly desirable for the precision treatment of diseases that are associated with metabolic levels [39•].
Table 1.
Summary of recently developed bioresponsive transcutaneous patches
| Stimulus | Materials | Model therapeutics | Ref. |
|---|---|---|---|
| pH | PVP, pH-sensitive PLGA nanoparticles | Alexa488 and Cy5 | [23] |
| Pyridine modified silicon, N-trimethyl chitosan | Ovalbumin | [24,26] | |
| Metal, polydopamine, heparin, and albumin | DNA | [25] | |
| Glucose | HA, hypoxia-sensitive vesicles | Insulin | [20•,30•] |
| HA, H2O2-sensitive polymersomes | Insulin | [31•] | |
| HA, hypoxia and H2O2 dual-sensitive polymersomes | Insulin | [32] | |
| HA, pH-sensitive dextran nanoparticles | anti-PD-1 | [33] | |
| Enzyme | 1-MT conjugated HA | 1-MT and anti-PD-1 | [37] |
| Heparin conjugated HA | Heparin | [38] |
Despite remarkable achievements in this area, the field of bioresponsive transdermal delivery is still in its infancy, and the investigation of these systems remains centered in in vivo preclinical models or early clinical trials. How to achieve sufficient biocompatibility and complete safety is a critical issue to be solved in future research that is focused on translation from the bench to clinical use [40–42]. This work requires a closer investigation of the response rate of the patches, detailed characterization of the relevant physiological gradients required to achieve smart and precise release of cargoes, and thorough evaluation of the local and systemic side effect of such transcutaneous patches. In addition, most in vivo experiments use rodent animal models, and the potential for translation to human studies could be limited by the loading capacity of general patches. It is essential that the next-generation of transcutaneous devices are developed with sufficient loading capacity to match the long-term dosage of the specific therapeutics.
Acknowledgments
This work was supported by the grants from the American Diabetes Association (ADA) (1-15-ACE-21), the grant from JDRF (grant no. 3-SRA-2015-117-Q-R), and the grant from NC TraCS, NIH’s Clinical and Translational Science Awards (CTSA, NIH grant 1UL1TR001111) at UNC-CH.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
- 1.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. One of the most cited reviews on microneedle-mediated drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn HL, Kearney M-C, Courtenay AJ, McCrudden MT, Donnelly RF. The role of microneedles for drug and vaccine delivery. Expert Opin Drug Deliv. 2014;11:1769–1780. doi: 10.1517/17425247.2014.938635. [DOI] [PubMed] [Google Scholar]
- 4.Kim Y-C, Prausnitz MR. Enabling skin vaccination using new delivery technologies. Drug Deliv Transl Res. 2011;1:7–12. doi: 10.1007/s13346-010-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann C, Buchholz L, Schnitzler P. Reduction of needlestick injuries in healthcare personnel at a university hospital using safety devices. J Occup Med Toxicol. 2013;8:20. doi: 10.1186/1745-6673-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larrañeta E, McCrudden MT, Courtenay AJ, Donnelly RF. Microneedles: a new frontier in nanomedicine delivery. Pharm Res. 2016;33:1055–1073. doi: 10.1007/s11095-016-1885-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh H, Shin J, Kim Y-C. Microneedle patches for vaccine delivery. Clin Exp Vaccine Res. 2014;3:42–49. doi: 10.7774/cevr.2014.3.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnelly RF, Singh TRR, Morrow DI, Woolfson AD. Microneedle-mediated Transdermal and Intradermal Drug Delivery. John Wiley & Sons; 2012. [Google Scholar]
- 9.Shingade G. Review on: recent trend on transdermal drug delivery system. J Drug Deliv Ther. 2012:2. [Google Scholar]
- 10.Cheung K, Das DB. Microneedles for drug delivery: trends and progress. Drug Deliv. 2016;23:2338–2354. doi: 10.3109/10717544.2014.986309. [DOI] [PubMed] [Google Scholar]
- 11.Rejinold NS, Shin J-H, Seok HY, Kim Y-C. Biomedical applications of microneedles in therapeutics: recent advancements and implications in drug delivery. Expert Opin Drug Deliv. 2016;13:109–131. doi: 10.1517/17425247.2016.1115835. [DOI] [PubMed] [Google Scholar]
- 12.Cahill EM, O’Cearbhaill ED. Toward biofunctional microneedles for stimulus responsive drug delivery. Bioconjug Chem. 2015;26:1289–1296. doi: 10.1021/acs.bioconjchem.5b00211. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Wang N, Wang X, Zhen Y, Wang T. Microneedle arrays delivery of the conventional vaccines based on nonvirulent viruses. Drug Deliv. 2016;23:3234–3247. doi: 10.3109/10717544.2016.1165311. [DOI] [PubMed] [Google Scholar]
- 14.Yun J, Im JS, Lee Y-S, Kim H-I. Electro-responsive transdermal drug delivery behavior of PVA/PAA/MWCNT nanofibers. Eur Polym J. 2011;47:1893–1902. [Google Scholar]
- 15.Alexander A, Dwivedi S, Giri TK, Saraf S, Saraf S, Tripathi DK. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release. 2012;164:26–40. doi: 10.1016/j.jconrel.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Hardy JG, Larraneta E, Donnelly RF, McGoldrick N, Migalska K, McCrudden MT, Irwin NJ, Donnelly L, McCoy CP. Hydrogel-forming microneedle arrays made from light-responsive materials for on-demand transdermal drug delivery. Mol Pharm. 2016;13:907–914. doi: 10.1021/acs.molpharmaceut.5b00807. [DOI] [PubMed] [Google Scholar]
- 17.Chen M-C, Ling M-H, Wang K-W, Lin Z-W, Lai B-H, Chen D-H. Near-infrared light-responsive composite microneedles for on-demand transdermal drug delivery. Biomacromolecules. 2015;16:1598–1607. doi: 10.1021/acs.biomac.5b00185. [DOI] [PubMed] [Google Scholar]
- 18.Di J, Yao S, Ye Y, Cui Z, Yu J, Ghosh TK, Zhu Y, Gu Z. Stretch-triggered drug delivery from wearable elastomer films containing therapeutic depots. ACS Nano. 2015;9:9407–9415. doi: 10.1021/acsnano.5b03975. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y, Sun W, Gu Z. Stimuli-responsive nanomaterials for therapeutic protein delivery. J Control Release. 2014;194:1–19. doi: 10.1016/j.jconrel.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci U S A. 2015;112:8260–8265. doi: 10.1073/pnas.1505405112. This paper is the first demonstration of glucose-responsive microneedle array for controlled insulin delivery in a type 1 diabetic mouse model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmid-Wendtner M-H, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19:296–302. doi: 10.1159/000094670. [DOI] [PubMed] [Google Scholar]
- 22.Giusti F, Martella A, Bertoni L, Seidenari S. Skin barrier, hydration, and pH of the skin of infants under 2 years of age. Pediatr Dermatol. 2001;18:93–96. doi: 10.1046/j.1525-1470.2001.018002093.x. [DOI] [PubMed] [Google Scholar]
- 23.Ke C-J, Lin Y-J, Hu Y-C, Chiang W-L, Chen K-J, Yang W-C, Liu H-L, Fu C-C, Sung H-W. Multidrug release based on microneedle arrays filled with pH-responsive PLGA hollow microspheres. Biomaterials. 2012;33:5156–5165. doi: 10.1016/j.biomaterials.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 24.van der Maaden K, Yu H, Sliedregt K, Zwier R, Leboux R, Oguri M, Kros A, Jiskoot W, Bouwstra JA. Nanolayered chemical modification of silicon surfaces with ionizable surface groups for pH-triggered protein adsorption and release: application to microneedles. J Mater Chem B. 2013;1:4466–4477. doi: 10.1039/c3tb20786b. [DOI] [PubMed] [Google Scholar]
- 25.Kim NW, Lee MS, Kim KR, Lee JE, Lee K, Park JS, Matsumoto Y, Jo D-G, Lee H, Lee DS, et al. Polyplex-releasing microneedles for enhanced cutaneous delivery of DNA vaccine. J Control Release. 2014;179:11–17. doi: 10.1016/j.jconrel.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 26.van der Maaden K, Sekerdag E, Schipper P, Kersten G, Jiskoot W, Bouwstra J. Layer-by-layer assembly of inactivated poliovirus and N-trimethyl chitosan on pH-sensitive microneedles for dermal vaccination. Langmuir. 2015;31:8654–8660. doi: 10.1021/acs.langmuir.5b01262. [DOI] [PubMed] [Google Scholar]
- 27.Harvey AJ, Kaestner SA, Sutter DE, Harvey NG, Mikszta JA, Pettis RJ. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharm Res. 2011;28:107–116. doi: 10.1007/s11095-010-0123-9. [DOI] [PubMed] [Google Scholar]
- 28.Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R. Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov. 2015;14:45–57. doi: 10.1038/nrd4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mo R, Jiang T, Di J, Tai W, Gu Z. Emerging micro-and nanotechnology based synthetic approaches for insulin delivery. Chem Soc Rev. 2014;43:3595–3629. doi: 10.1039/c3cs60436e. [DOI] [PubMed] [Google Scholar]
- 30•.Ye Y, Yu J, Wang C, Nguyen N-Y, Walker GM, Buse JB, Gu Z. Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery. Adv Mater. 2016;28:3115–3121. doi: 10.1002/adma.201506025. This study integrates live (cell-based) and synthetic glucose-responsive systems for glycemia regulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Hu X, Yu J, Qian C, Lu Y, Kahkoska AR, Xie Z, Jing X, Buse JB, Gu Z. H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano. 2017;11:620–621. doi: 10.1021/acsnano.6b06892. This paper is the first published study using H2O2-sensitive material for controlled insulin delivey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Qian C, Zhang Y, Cui Z, Zhu Y, Shen Q, Ligler FS, Buse JB, Gu Z. Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. Nano Lett. 2017;17:733–739. doi: 10.1021/acs.nanolett.6b03848. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16:2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- 34.Hu Q, Katti PS, Gu Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale. 2014;6:12273–12286. doi: 10.1039/c4nr04249b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De La Rica R, Aili D, Stevens MM. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv Drug Deliv Rev. 2012;64:967–978. doi: 10.1016/j.addr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Stern R. Hyaluronan metabolism: a major paradox in cancer biology. Pathol Biol (Paris) 2005;53:372–382. doi: 10.1016/j.patbio.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Ye Y, Wang J, Hu Q, Hochu GM, Xin H, Wang C, Gu Z. Synergistic transcutaneous immunotherapy enhances antitumor immune responses through delivery of checkpoint inhibitors. ACS Nano. 2016;10:8956–8963. doi: 10.1021/acsnano.6b04989. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Yu J, Wang J, Hanne NJ, Cui Z, Qian C, Wang C, Xin H, Cole JH, Gallippi CM. Thrombin-responsive transcutaneous patch for auto-anticoagulant regulation. Adv Mater. 2017;29:1604043. doi: 10.1002/adma.201604043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Lu Y, Aimetti AA, Langer R, Gu Z. Bioresponsive materials. Nat Rev Mater. 2016;1:16075. This review focuses on ‘smart’ bioresponsive materials that are sensitive to biological signals or pathological abnormalities. [Google Scholar]
- 40.Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of therapeutic proteins. J Pharm Sci. 2010;99:2557–2575. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du AW, Stenzel MH. Drug carriers for the delivery of therapeutic peptides. Biomacromolecules. 2014;15:1097–1114. doi: 10.1021/bm500169p. [DOI] [PubMed] [Google Scholar]
- 42.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13:813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]