Abstract
The IKZF1 gene encodes the Ikaros protein, a zinc finger transcriptional factor that acts as a master regulator of hematopoiesis and a tumor suppressor in leukemia. Impaired activity of Ikaros is associated with the development of high-risk acute lymphoblastic leukemia (ALL) with a poor prognosis. The molecular mechanisms that regulate Ikaros’ function as a tumor suppressor and regulator of cellular proliferation are not well understood. We demonstrated that Ikaros is a substrate for Casein Kinase II (CK2), an oncogenic kinase that is overexpressed in ALL. Phosphorylation of Ikaros by CK2 impairs Ikaros’ DNA-binding ability, as well as Ikaros’ ability to regulate gene expression and function as a tumor suppressor in leukemia. Targeting CK2 with specific inhibitors restores Ikaros’ function as a transcriptional regulator and tumor suppressor resulting in a therapeutic, anti-leukemia effect in a preclinical model of ALL. Here, we review the genes and pathways that are regulated by Ikaros and the molecular mechanisms through which Ikaros and CK2 regulate cellular proliferation in leukemia.
Keywords: Ikaros, Casein Kinase II (CK2), Leukemia, CX4945, PP1, Protein phosphatase 1, Phosphorylation
1. Structure and biological function of Ikaros
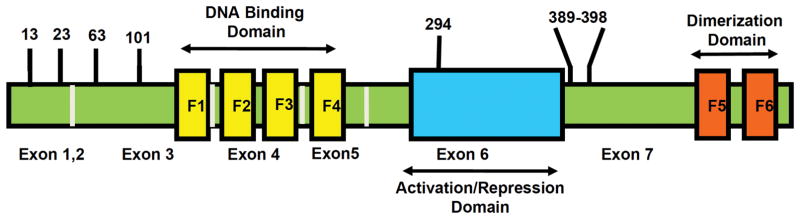
The Ikaros protein was independently discovered by two groups (Georgopoulos et al., 1992; Lo et al., 1991) as an early lymphoid-specific transcription factor that regulates gene expression in lymphocytes and mediates T cell differentiation. The gene that encodes Ikaros, IKZF1, undergoes alternative splicing to produce multiple, functionally diverse DNA binding zinc finger proteins (Molnár and Georgopoulos, 1994a). The largest of these proteins (Ik1 and Ik-H) (Molnár and Georgopoulos, 1994b; Ronni et al., 2007) contain six C2H2 zinc fingers organized into two major domains (Fig. 1). Four of the zinc finger domains (ZF1-4) are found in the N-terminal region while the remaining two zinc finger domains (ZF5-6) are located near the C-terminus of the protein. While the various Ikaros isoforms all have different combinations of ZF1-ZF4, all isoforms of Ikaros share the same C-terminal zinc finger domains (Georgopoulos et al., 1997a).
Fig. 1.
Structure of Ikaros and CK2 phosphorylation sites.
Ikaros’ ZF1-4 form a complex with the DNA double-stranded cognate sequence and are responsible for Ikaros’ DNA-binding ability (Molnár and Georgopoulos, 1994a). It is highly likely that Ikaros’ ZF2 and ZF3 bind according to the classical model described by Pabo et al., (Cobb et al., 2000). The biological role of ZF1 and ZF4 were determined using two Ikzf1-mutant mice that had deletions of the exons encoding either ZF1 or ZF4. This study demonstrated that ZF1 and ZF4 regulate different stages of lymphoid differentiation, and that ZF4 was selectively required for tumor suppression (Schjerven et al., 2013).
The C-terminal ZF5 and ZF6 are essential for dimerization with other Ikaros isoforms and/or other members of Ikaros family such as Helios (Kelley et al., 1998), Aiolos (Morgan et al., 1997), Eos, and Pegasus (Perdomo et al., 2000). Homo- and heterodimerization between Ikaros isoforms capable of binding DNA (i.e. those with at least three N-terminal zinc fingers) dramatically increases their DNA affinity and transcriptional activity. However, heterodimers comprised of Ikaros DNA binding isoforms and isoforms that lack an intact DNA binding domain are unable to bind DNA. In this way, Ikaros isoforms that do not bind DNA might inhibit Ikaros’ overall transcriptional activity (Georgopoulos et al., 1997b).
Ikaros primarily functions as a master regulator of hematopoiesis where it controls lymphoid development on at least two levels(Mullighan et al., 2007). The first level is in lymphoid-primed multipotent progenitors (LMPPs) where expression of the Flt3 receptor, a defining characteristic of LMPPs, is dependent on Ikaros activity. Not only do Ikaros-null LMPPs lack flt3, but they are also unable to upregulate the IL-7 receptor alpha chain (IL7R-α), which provides pro-lymphoid differentiation signals. Under conditions that normally support B cell production, Ikaros-null LMPPs readily differentiate into myeloid cells, but not B cells, and generate T cells with a ~10-fold reduced frequency (Yoshida et al., 2006).
The second level of Ikaros-mediated regulation occurs during T- and B-cell differentiation. Ikaros regulates two key steps in early B cell differentiation. First, Ikaros binds to the promoter region of the Igll1 gene that encodes Lambda 5. Lambda 5 is a component of the pre-B cell receptor (pre-BCR) (Thompson et al., 2007). For progression beyond the pre-B cell stage, expression of the pre-BCR is essential and Ikaros plays an important role in its regulation. Ikaros also plays a critical role in immunoglobulin heavy chain rearrangement. Ikaros binds to the promoter region of recombinase activating genes (rag) and upregulates expression of the RAG1 and RAG2 proteins thereby regulating another essential step in B cell differentiation (Reynaud et al., 2008). Ikaros plays a key role in normal T-cell development by regulating the expression of several important genes involved in T-cell differentiation, including terminal deoxyneucleotide transferase (TdT), CD4, CD8 and IL2 (Wang et al., 2014b).
Mice heterozygous for an inactivating Ikaros mutation are capable of producing mature T and B lymphocytes but have thymocytes with augmented proliferative responses and autoproliferative peripheral T cells. The same study linked the role of Ikaros in regulating normal hematopoiesis with lymphoproliferation and neoplastic transformation based on the distinct thresholds of Ikaros activity: mice heterozygous for inactivated Ikaros invariably developed T cell malignancies that arose from a hyperproliferative T cell clone that had undergone a secondary mutation targeting the remaining Ikaros wild type allele (Winandy et al., 1995).
Ikaros mediates its biological function by altering the transcription of its target genes through chromatin remodeling. Ikaros associates with both Mi-2 and histone deacetylases in T cells to form Ikaros-NuRD and Ikaros-HDAC1 complexes that actively remodel chromatin and deacetylate histones, respectively (Kim et al., 1999). Chromatin remodeling of promoter regions leads to transcriptional modulation in gene expression and is hypothesized to be the major mechanism through which Ikaros represses and activates its target genes (Koipally et al., 1999). A deacetylase-independent mechanism of gene repression involving a CtBP-independent interaction between Ikaros and CtIP has also been described (Koipally and Georgopoulos, 2002).
2. Clinical significance of Ikaros dysfunction in leukemia
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy(Pui and Evans, 2006). Despite an eighty percent cure rate, relapsed disease remains the leading cause of pediatric mortality (Mullighan, 2011, 2012; Mullighan et al., 2009). Relapse is multifactorial, however certain notable genes and transcriptional dysregulations are associated with chemotherapy resistance, higher risk ALL, and poorer prognosis (Dovat, 2011; Gowda and Dovat, 2013; Mullighan, 2012; Payne and Dovat, 2011). Alterations in IKZF1, a lymphoid transcription gene leading to various isoforms of Ikaros, is one of the hallmarks of developing B-cell ALL (B-ALL) with poor prognosis. Inactivating mutations in Ikaros have been associated with an increased relapse rate of up to 12-fold in B-ALL (Kuiper et al., 2010).
Ikaros deletion or alteration is found in fifteen percent of all childhood B-ALL (Payne and Dovat, 2011). However, it is more commonly found in BCR-ABL1 positive ALL. IKZF1 Alterations are as high as eighty percent in pediatric Ph-positive ALL and greater than ninety percent in adult Ph-Positive ALL (Iacobucci et al., 2009; Martinelli et al., 2009; Payne and Dovat, 2011; Virely et al., 2010; Wang et al., 2014a). The BCR-ABL1 (Ph chromosome) translocation arises from t(9; 22)(q34; q11.2) and produces an activated BCR-ABL1 tyrosine kinase. BCR-ABL1-like ALL lacks the t(9; 22) translocation, however, it has a similar gene expression pattern (Den Boer et al., 2009) and tends to have a poor prognosis similar to Ph-positive ALL (Mullighan et al., 2009; Payne and Dovat, 2011). Ph-Positive ALL makes up five percent of all pediatric B-ALL and forty percent of all adult B-ALL. Event-free survival for BCR-ABL1-like ALL is 62.6% ± 6.9% compared to 85.8% ± 2% in non-BCR-ABL1-like cases as seen in the COG AALL0232 study (Mullighan, 2011, 2012; Mullighan et al., 2008, 2009).
Ikaros mutations are usually nonsense or frameshift mutations that result in loss of function (Payne and Dovat, 2011). The most common mutation is in the exon 3–6 region, which gives rise to dominant negative isoform IK6 (Iacobucci et al., 2009; Mullighan and Downing, 2008; Mullighan, 2011; Mullighan et al., 2008). This isoform is deficient in the DNA binding domain but has a functional C-terminal zinc finger (Iacobucci et al., 2009). The expression of IK6 reduces cellular apoptosis and enhances cellular survival. It also impairs lymphoid differentiation and B cell maturation contributing to leukemogenesis. Some studies have demonstrated that relapse risk is tripled due to deletion of Ikaros in BCR-ABL1-like ALL (Kuiper et al., 2010; Martinelli et al., 2009; Mullighan and Downing, 2008; Mullighan et al., 2008, 2009; Payne and Dovat, 2011; Tonnelle et al., 2001; Virely et al., 2010; Wang et al., 2014a). In general, ALL with Ikaros deletion has a poorer prognosis independent of other risk stratifications such as age at diagnosis, white cell count (WBC), sex, and minimal residual disease (MRD) (Mullighan, 2011, 2012; Mullighan et al., 2009). Ikaros alterations are also strongly associated with chemoresistant disease or detectible MRD at the end of induction (McCubrey et al., 2015; Mullighan, 2011, 2012; Mullighan et al., 2009).
Though Ikaros deletion and mutation is more often identified with B-ALL and accelerated chronic myeloid leukemia (CML) in blast crisis, it is also seen in five percent of T-cell ALL (T-ALL) (Kuiper et al., 2007; Maser et al., 2007; Mullighan et al., 2008; Nakayama et al., 1999; Payne and Dovat, 2011). The most common Ikaros isoform expressed in both T-ALL and CML in blast crisis the dominant negative (Ik6) isoform that is similar to what is seen in B-ALL (Nakase et al., 2000; Payne and Dovat, 2011; Ruiz et al., 2004). Though Ikaros mutation is less commonly seen in T-ALL (Marcais et al., 2010; Zhang et al., 2012), its absence leads to T cell proliferation and, as a result, current studies are investigating the role of Ikaros in other types of leukemia (Nakayama et al., 1999; Payne and Dovat, 2011; Ruiz et al., 2004). Ikaros deletion and mutation is seen in acute myeloid leukemia (AML) and myeloid dysplastic syndrome (MDS) (Iacobucci et al., 2009; Martinelli et al., 2009; Mullighan et al., 2008, 2009; Nakayama et al., 1999; Payne and Dovat, 2011; Wang et al., 2014a). Interestingly, mutations and/or deletions of Ikaros have not been observed in CML in chronic phase, which again suggests that Ikaros deletion and/or mutation supports leukemogenisis (Iacobucci et al., 2009; Maser et al., 2007; Mullighan et al., 2008; Nakase et al., 2000; Nakayama et al., 1999; Payne and Dovat, 2011; Ruiz et al., 2004).
Further studies are required to determine the exact mechanism by which Ikaros alters cellular maturation, differentiation, and leukemogenesis not only in B-ALL, but also T-ALL, AML, MDS and CML in blast crisis. Current data has established that Ikaros dysfunction is strongly associated with poor prognosis, chemoresistance, and increased risk of relapse. Development of a therapy that targets hematopoietic malignancies that have an Ikaros mutation is a high priority and will have a high impact on the overall survival rate in these diseases.
3. Structure, function and oncogenic activity of Casein Kinase II (CK2)
Casein kinase II (CK2) is a pro-oncogenic protein that has become a prominent target for research due to its role in tumorigenesis (Ahmad et al., 2005; Chon et al., 2015). High levels of CK2 have been associated with numerous malignancies and selective CK2 inhibitors have shown efficacy in the treatment of both solid tumors and hematologic malignancies (Buontempo et al., 2014; Song et al., 2015).
CK2 is a heterotetrameric protein kinase composed of 2 catalytic (α and/or α′) and 2 regulatory (β) subunits. Of the 4 genomic loci for CK2 in humans, one is not transcribed, and the remaining 3 are on chromosomes 6, 16, and 20, which correspond with the β, α′, and α subunits, respectively (Ackermann et al., 2005). Even though CK2 has low homology with other protein kinases, all domains of CK2α are highly conserved throughout evolution and its expression is ubiquitous (Ceglia et al., 2011; Pinna, 1990). CK2α can use either ATP or GTP as a phosphate donor and is the only protein kinase that contains more than 3 basic amino acids in a row, with a stretch of 6 basic amino acids just downstream of Val66 (Cochet and Chambaz, 1983; Jakobi and Traugh, 1995; Meisner et al., 1989; Pinna, 1990). This region is responsible for the binding of its regulatory subunit, CK2β (Jeffrey et al., 1995).
CK2α molecules cannot interact with each other and, as such, rely on CK2β molecules, which dimerize at their zinc-finger domains to hold the holoenzyme together (Boldyreff et al., 1996; Gietz et al., 1995). Binding of CK2β enhances the catalytic activity of CK2α in vitro by 5–20 fold. However, it should be noted that in the case of some substrates, such as calmodulin and MDM-2, CK2β inhibits phosphorylation by CK2α (Allende and Allende, 1998; Cochet and Chambaz, 1983; Meggio et al., 1992).
CK2 is a pleiotropic protein kinase with numerous physiological targets. It is constitutively active and has been detected in cell and tissue extracts in the absence of any stimuli (Litchfield et al., 1994). However, the levels of CK2 catalytic subunit do correspond to proliferation rate, as cells with higher rates of proliferation tend to exhibit higher levels of CK2 (Münstermann et al., 1990). Unlike most protein kinases, phosphorylation is not required for activation of CK2 even though a number of phosphorylation sites have been identified on both CK2α and CK2β (Bosc et al., 1995; Litchfield et al., 1991, 1992).
Because the activity of CK2 does not depend on small molecules typically involved in second messenger-dependent kinase activation, it has been classified as a messenger-independent kinase (Tuazon and Traugh, 1991). This classification, however, can be misleading as certain small molecules do participate in the regulation of CK2. Particularly, negatively charged compounds such as heparin inhibit CK2 while positively charged compounds such as polyamines activate CK2 (Shore et al., 1997). Although much progress has been made in the study of CK2 regulation, it continues to be an area of interest, especially with regards to cancer research.
In vitro studies have implicated CK2 in cell-cycle regulation, gene expression, DNA repair, and protein translation (Meggio and Pinna, 2003). Early experiments in transgenic mice revealed that overexpression of CK2α leads to accelerated early thymic lymphomas and T cell leukemia (Channavajhala and Seldin, 2002; Kelliher et al., 1996; Seldin and Leder, 1995). Since then, CK2 overexpression has been documented in prostate, breast, lung, head and neck, and colon carcinomas (Gray et al., 2014; Izeradjene et al., 2005; Wang et al., 2006b; Yu et al., 2006) as well as multiple hematologic malignancies, including TALL, B-ALL, CLL, Burkitt lymphoma, and AML (Bliesath et al., 2012; Kim et al., 2007; Martins et al., 2010; Pizzi et al., 2015; Quotti Tubi et al., 2013; Scaglioni et al., 2008). In most of these malignancies, elevated levels of CK2α correlate with meta-static potential, undifferentiated histologic grade, and poor clinical outcome (Martins et al., 2010). Additional studies have even shown that the degree of nuclear localization of CK2 can be used as a prognostic factor (Laramas et al., 2007; Piazza et al., 2006).
While overexpression of CK2 has been linked to cancer, a relatively small reduction in CK2 expression significantly induces apoptosis (Wang et al., 2001). Studies using antisense CK2, siRNA, and CK2-specific inhibitors such as 4,5,6,7-tetrabromobenzotriazole (TBB), Apigenin, and CX4945 have shown that downregulation of CK2 makes tumor cells vulnerable to death receptor ligands (Wang et al., 2005, 2006a). Of these, only one specific CK2 inhibitor, CX-4945, an ATP-competitive inhibitor of both CK2α than CK2α′, has been shown to significantly reduce cell-growth and survival in cancer cells (Chon et al., 2015; Pierre et al., 2011a, 2011b; Siddiqui-Jain et al., 2010). CX4945 is an orally bioavailable small molecule which is in Phase I clinical trial for solid tumors. The role of CK2 inhibitor as an anti-leukemic drug in high-risk leukemia are being explored. We will review the progress made on this front in the following sections.
4. Regulation of Ikaros function by CK2-mediated phosphorylation
CK2 is overexpressed in most malignancies, including lymphoblastic leukemia, and has several substrates notably Ikaros (Dovat et al., 2011). As a result of CK2 mediated phosphorylation at multiple sites during post translational modification, Ikaros losses its regulatory effects on cellular proliferation and differentiation in lymphoid cells (Gurel et al., 2008). Fig. 1 illustrates the structure of Ikaros along with several CK2 phosphorylation sites (amino acid sites 13, 23, 63, 101, 294 and 389 to 398). Studies by Popescu et al. and Gurel et al. showed that localization of Ikaros to the pericentromeric heterochromatin, a vital first step of the regulatory functions of Ikaros is impaired by its phosphorylation at specific amino acids (Gurel et al., 2008; Popescu et al., 2009; Wang et al., 2014b).
Studies have demonstrated that phosphorylation of Ikaros by CK2 results in the loss of essential functions of Ikaros. Phosphorylated Ikaros poorly binds to its target genes and cannot localize to subcellular regions in the nucleus (Song et al., 2011). This leads to ineffective regulation of cell cycle progression and T cell differentiation, which results in malignant transformation and the development of leukemia (Gowda et al., 2016; Li et al., 2012). Hyperphosphorylated Ikaros protein undergoes rapid ubiquitination and degradation in cells (Popescu et al., 2009). Below is a brief summary of the Ikaros functions that are regulated by CK2-mediated phosphorylation.
4.1. Regulation of the Ikaros function during cell cycle
During mitosis Ikaros binds to DNA of its target genes in a cell cycle dependent manner (Dovat et al., 2002; Thompson et al., 2007). Functional analyses using phosphomimetic and phosphoresistant Ikaros mutants showed that cell cycle specific phosphorylation of Ikaros during mitosis at a specific linker sequence in the DNA-binding zinc finger regulates its ability to bind to its target genes. Several amino acids in the Ikaros protein have been identified as CK2 phosphorylation sites (Li et al., 2012). In mice, CK2-mediated phosphorylation of Ikaros has been shown to impair its regulation of G1-to-S cell cycle progression (Li et al., 2012). In human leukemia cells, similar effects are seen during the S phase of the cell cycle where CK2 mediated Ikaros phosphorylation leads to impaired DNA binding of Ikaros (Gurel et al., 2008).
4.2. Regulation of Ikaros pericentromeric localization
Localization at pericentromeric heterochromatin (PC-HC) in the nucleus of hematopoietic cells is an important part of Ikaros’ function as a transcriptional regulator. This type of localization confers a punctate staining pattern to the nucleus when observed with confocal microscopy. In cells transduced with phosphomimetic Ikaros mutants (mutants with substituted amino acids that carry a charge that mimics phosphorylation at CK2 phosphosites) the localization was lost and a diffuse nuclear pattern was noted with confocal microscopy. However, phosphoresistant mutants (mutants with substituted non-charged amino acids that cannot be phosphorylated at CK2 phosphosites) showed no change in the localization pattern as compared to wild type Ikaros. In summary, the ability of Ikaros to bind DNA at PC-HC and its subcellular localization is impaired by phosphorylation at CK2 specific sites (Popescu et al., 2009).
4.3. Regulation of TdT expression during T cell differentiation
Binding to the upstream regulatory element (URE) of target genes and recruiting them to the PC-HC where their expression is either activated or repressed is one component of Ikaros’s transcriptional regulatory function. TdT, a DNA polymerase enzyme and a marker of immature lymphoid cell plays an important role in lymphoid development. During T cell differentiation, when Ikaros binds to the URE of the TdT gene, it decreases expression of TdT. Ikaros-mediated repression of TdT is an important part of normal T cell development. Phosphopeptide mapping of endogenous Ikaros in developing thymocytes that Ikaros undergoes dephosphorylation at CK2 phosphosites(Wang et al., 2014b). These data suggest that the DNA-binding affinity of Ikaros to the URE of TdT during thymocyte differentiation is controlled by phosphorylation at specific amino acids. Studies have demonstrated that phosphorylation of Ikaros by CK2 controls its ability to bind to the regulatory sequence of the TdT gene. Protein Phosphatase 1 (PP1) has the opposite effect on the phosphorylation status of Ikaros (Wang et al., 2014b).
4.4. Regulation of cell cycle and PI3K pathway genes in leukemia
Recently published studies show that Ikaros controls cellular proliferation by repressing genes that promote cell cycle progression and the phosphatidylinositol-3 kinase (PI3K) pathway (Follo et al., 2015; Fransecky et al., 2015; Neri et al., 2014; Payrastre and Cocco, 2015; Song et al., 2015). CK2, which phosphorylates Ikaros and impairs its tumor suppressor functions, was shown to be increased in B-ALL cells (Song et al., 2015). The ability of Ikaros to repress cell cycle control and PI3K pathway genes in B-ALL cells, could be increased by inhibiting CK2, either through molecular inhibition (CK2 shRNA) or pharmacological inhibition (using the specific CK2 inhibitor, CX4945). Results showed that the repression of Ikaros target genes observed under CK2 inhibition was similar to that which is seen after overexpression of Ikaros. The repression of PI3K and cell cycle control genes induced by the loss of CK2 activity did not occur when Ikaros was knocked down in the same cells. This confirms that the repression of cell cycle and PI3K genes observed with CK2 inhibition occurs via restoration of Ikaros function as a transcriptional repressor. The therapeutic efficacy of CK2 inhibitors was demonstrated using several patient-derived xenograft (PDX) mice generated using primary B-ALL patient samples. Treatment of PDX mice with CX-4945 resulted in decreased leukemia progression and prolonged survival (Song et al., 2015).
4.5. Regulation of chromatin remodeling in leukemia
Song et al. studied the genome wide occupancy of Ikaros and HDAC1 in a human B-ALL cell line (Nalm6) as well as in primary B-ALL cells. Binding of Ikaros and HDAC1 to their target genes was determined using chromatin immunoprecipitation followed by deep sequencing(CHIP Seq)(McCubrey et al., 2015; Pellagatti et al., 2016; Song et al., 2016). Changes in the epigenetic environment surrounding the Ikaros and/or HDAC1 peaks were analyzed in detail. These studies revealed that the binding of Ikaros and HDAC1, alone or together, to a target gene results in unique histone modifications that result in either induction or suppression of the target gene expression. Ikaros is known to exert its tumor suppressor function by repressing several genes involved in cell cycle progression like CDC7 and CDC2. As described above, studies by Song et al (Song et al., 2015). demonstrate that functions of Ikaros are severely impaired by CK2-mediated phosphorylation and that CK2 inhibition restores Ikaros’ regulatory functions and results in an anti-leukemic effect in high-risk B-ALL.
Their subsequent studies of Ikaros and HDAC1 described in detail the mechanism of chromatin remodeling via histone modifications that result in transcriptional regulation of target genes by Ikaros and HDAC1 and the regulation of these Ikaros/HDAC1-mediated epigenetic changes by CK2. The histone modifications that followed Ikaros binding to the promoter regions of its target genes were studied after molecular inhibition (shRNA) and pharmacological inhibition (CX4945) of CK2 in Nalm6 and in primary high risk B-ALL cells. The results confirmed that Ikaros-mediated chromatin remodeling and transcriptional regulation of target gene expression is impaired by CK2 mediated phosphorylation of Ikaros in high-risk B-ALL. Inhibition of CK2 using a specific inhibitor, CX4945, restored the epigenetic regulation of cell cycle progression by Ikaros (Song et al., 2016).
4.6. Regulation of JARID1B/KDM5B histone demethylase
The histone H3K4 demethylase, JARID1B (KDM5B), is overexpressed in leukemia. Ikaros suppresses KDM5B resulting in increased global levels of H3K4 trimethylation. In leukemia, inhibition of KDM5B results in the arrest of cell growth. CK2-mediated phosphorylation of Ikaros impairs the regulation of KDM5B expression by Ikaros. Restoring Ikaros regulatory functions using a CK2 inhibitor results in an anti-leukemia effect and an Ikaros-mediated decrease in KDM5B expression. KDM5B is a potential therapeutic target in leukemia (Wang et al., 2016).
4.7. Regulation of IL-7R and SH2B3 expression in high-risk leukemia
A subset of adult B-ALL patients with high risk factors and poor prognosis have recently been noted to have high IL-7Rα (IL-7R) expression, low SH2B3 expression and Ikaros dysfunction (Ge et al., 2016a). Cell surface IL-7R is required for lymphoid development, but overexpression of IL-7R is considered pro-oncogenic. The SH2B adaptor protein 3 (SH2B3), also known as the lymphocyte adaptor protein (LNK), negatively regulates cytokine signaling. It plays a vital role in homeostasis of hematopoietic stem cells and lymphoid progenitors. Previous genome wide mapping of Ikaros binding using ChIP-Seq had shown that IL7R and SH2B3 are Ikaros target genes. Expression of IL-7R and SH2B3 were shown by Ge et al. to be regulated by Ikaros via chromatin remodeling (Ge et al., 2016a). Ikaros was shown to suppress the promoter activity of IL-7R, while it activated that of SH2B3. Results of this study demonstrated that Ikaros overexpression and CK2 inhibition (using TBB) leads to decreased IL-7R and increased SH2B3 expression, whereas CK2 inhibition after Ikaros silencing (using Ikaros shRNA) blocks changes in IL-7R and SH2B3 expression. These data demonstrate that CK2 regulates Ikaros ability to repress and to activate its target genes (IL-7R and SH2B3 respectively). These results demonstrate the interplay between the CK2-Ikaros axis and IL7R signaling pathways and provided a mechanistic rationale for the therapeutic efficacy of CK2 inhibitors for treatment of the IL7R-high/SH2B3-low subtype of high-risk B-ALL (Ge et al., 2016a).
4.8. Regulation of CRLF2 expression in B-ALL
Cytokine receptor-like factor 2 (CRLF2) over expression is found in a subgroup of ALL patients without CRLF2 rearrangement and is associated with Ikaros deletion and dysfunction. This study does not include patients with increased CRLF2 expression due to CRLF2 rearrangement (Hispanic population)(Chen et al., 2012). Studies by Ge et al. showed that Ikaros directly binds to the promoter region of CRLF2 and suppresses its transcription via chromatin remodeling in human B-ALL cell lines and in primary B-ALL. Similar to other subtypes of B-ALL, a high level of CK2 results in Ikaros hyperphosphorylation, which impairs its functions as a transcriptional repressor. Targeted inhibition of CK2 was able to restore Ikaros binding to the CRLF2 promoter, and repress transcription of the CRLF2 gene in primary B-ALL cells. These data further support the paradigm established in previous studies that: 1) The CK2-Ikaros axis regulates tumor suppression in high-risk B-ALL via transcriptional control of the genes that have a key role in leukemogeneis; and 2) Restoration of Ikaros’ tumor suppressor function occurs following treatment with CK2 inhibitors, which contributes to the therapeutic efficacy of CK2 inhibitors in leukemia (Ge et al., 2016b).
5. Conclusion
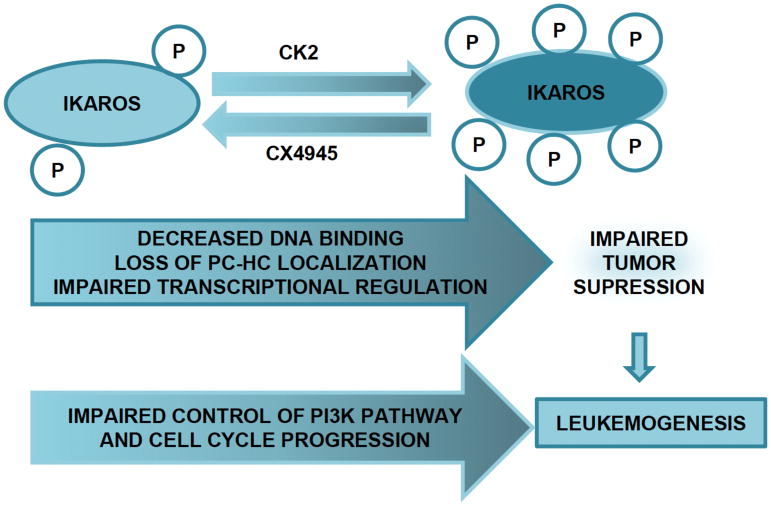
In summary, Ikaros is a master regulator of hematopoiesis and deletion or dysfunction of Ikaros leads to the development of leukemia. Ikaros regulates the expression of a large number of genes, and thus, has a crucial role in controlling multiple biological pathways. Increased activity of CK2 in leukemia results in hyperphosphorylation of Ikaros and impairs its function as a transcriptional regulator (Fig. 2). The use of CK2-specific inhibitor restores Ikaros activity and its tumor suppressor function, resulting in an anti-leukemic effect. Advancing knowledge of the role of the CK2-Ikaros signaling axis in leukemia will provide further insight into the regulation of cellular proliferation in hematopoietic malignancies.
Fig. 2.
Ikaros- CK2 axis in Leukemia and role of CK2 inhibitor (CX4945).
Acknowledgments
This work has been supported by NIH R01 CA209829, a Hyundai Hope on Wheels Scholar Grant Award, Alex’s Lemonade Stand Grant, Bear Necessities Pediatric Cancer Foundation, the Four Diamonds Fund of the Pennsylvania State University, College of Medicine, and the John Wawrynovic Leukemia Research Scholar Endowment (SD); by St. Baldrick’s Foundation Fellows Award and Hyundai Hope on Wheels Fellowship Grant Award (C.G.): by St. Baldrick’s Foundation Summer Fellows Award (JLP).
References
- Ackermann K, Neidhart T, Gerber J, Waxmann A, Pyerin W. The catalytic subunit alpha’ gene of human protein kinase CK2 (CSNK2A2): genomic organization, promoter identification and determination of Ets1 as a key regulator. Mol Cell Biochem. 2005;274(1–2):91–101. doi: 10.1007/s11010-005-3076-2. [DOI] [PubMed] [Google Scholar]
- Ahmad KA, Wang G, Slaton J, Unger G, Ahmed K. Targeting CK2 for cancer therapy. Anticancer Drugs. 2005;16(10):1037–1043. doi: 10.1097/00001813-200511000-00001. [DOI] [PubMed] [Google Scholar]
- Allende CC, Allende JE. Promiscuous subunit interactions: a possible mechanism for the regulation of protein kinase CK2. J Cell Biochem Suppl. 1998;30–31:129–136. [PubMed] [Google Scholar]
- Bliesath J, Huser N, Omori M, Bunag D, Proffitt C, Streiner N, Ho C, Siddiqui-Jain A, O’Brien SE, Lim JK, Ryckman DM, Anderes K, Rice WG, Drygin D. Combined inhibition of EGFR and CK2 augments the attenuation of PI3K-Akt-mTOR signaling and the killing of cancer cells. Cancer Lett. 2012;322(1):113–118. doi: 10.1016/j.canlet.2012.02.032. [DOI] [PubMed] [Google Scholar]
- Boldyreff B, Mietens U, Issinger OG. Structure of protein kinase CK2: dimerization of the human beta-subunit. FEBS Lett. 1996;379(2):153–156. doi: 10.1016/0014-5793(95)01497-7. [DOI] [PubMed] [Google Scholar]
- Bosc DG, Slominski E, Sichler C, Litchfield DW. Phosphorylation of casein kinase II by p34cdc2. Identification of phosphorylation sites using phosphorylation site mutants in vitro. J Biol Chem. 1995;270(43):25872–25878. doi: 10.1074/jbc.270.43.25872. [DOI] [PubMed] [Google Scholar]
- Buontempo F, Orsini E, Martins LR, Antunes I, Lonetti A, Chiarini F, Tabellini G, Evangelisti C, Melchionda F, Pession A, Bertaina A, Locatelli F, McCubrey JA, Cappellini A, Barata JT, Martelli AM. Cytotoxic activity of the casein kinase 2 inhibitor CX-4945 against T-cell acute lymphoblastic leukemia: targeting the unfolded protein response signaling. Leukemia. 2014;28(3):543–553. doi: 10.1038/leu.2013.349. [DOI] [PubMed] [Google Scholar]
- Ceglia I, Flajolet M, Rebholz H. Predominance of CK2α over CK2α′ in the mammalian brain. Mol Cell Biochem. 2011;356(1–2):169–175. doi: 10.1007/s11010-011-0963-6. [DOI] [PubMed] [Google Scholar]
- Channavajhala P, Seldin DC. Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene. 2002;21(34):5280–5288. doi: 10.1038/sj.onc.1205640. [DOI] [PubMed] [Google Scholar]
- Chen IM, Harvey RC, Mullighan CG, Gastier-Foster J, Wharton W, Kang H, Borowitz MJ, Camitta BM, Carroll AJ, Devidas M, Pullen DJ, Payne-Turner D, Tasian SK, Reshmi S, Cottrell CE, Reaman GH, Bowman WP, Carroll WL, Loh ML, Winick NJ, Hunger SP, Willman CL. Outcome modeling with CRLF2, IKZF1, JAK, and minimal residual disease in pediatric acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2012;119(15):3512–3522. doi: 10.1182/blood-2011-11-394221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chon HJ, Bae KJ, Lee Y, Kim J. The casein kinase 2 inhibitor, CX-4945, as an anti-cancer drug in treatment of human hematological malignancies. Front Pharmacol. 2015;6:70. doi: 10.3389/fphar.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BS, Morales-Alcelay S, Kleiger G, Brown KE, Fisher AG, Smale ST. Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes & Dev. 2000;14:2146–2160. doi: 10.1101/gad.816400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet C, Chambaz EM. Oligomeric structure and catalytic activity of G type casein kinase. Isolation of the two subunits and renaturation experiments. J Biol Chem. 1983;258(3):1403–1406. [PubMed] [Google Scholar]
- Den Boer ML, van Slegtenhorst M, De Menezes RX, Cheok MH, Buijs-Gladdines JG, Peters ST, Van Zutven LJ, Beverloo HB, Van der Spek PJ, Escherich G, Horstmann MA, Janka-Schaub GE, Kamps WA, Evans WE, Pieters R. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. The Lancet Oncology. 2009;10(2):125–134. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovat S. Ikaros in hematopoiesis and leukemia. World J Biol Chem. 2011;2(6):105–107. doi: 10.4331/wjbc.v2.i6.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovat S, Ronni T, Russell D, Ferrini R, Cobb BS, Smale ST. A common mechanism for mitotic inactivation of C2H2 zinc finger DNA-binding domains. Genes Dev. 2002;16(23):2985–2990. doi: 10.1101/gad.1040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovat S, Song C, Payne KJ, Li Z. Ikaros, CK2 kinase, and the road to leukemia. Mol Cell Biochem. 2011;356(1–2):201–207. doi: 10.1007/s11010-011-0964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follo MY, Manzoli L, Poli A, McCubrey JA, Cocco L. PLC and PI3K/Akt/mTOR signalling in disease and cancer. Adv Biol Regul. 2015;57:10–16. doi: 10.1016/j.jbior.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Fransecky L, Mochmann LH, Baldus CD. Outlook on PI3K/AKT/mTOR inhibition in acute leukemia. Mol Cell Ther. 2015;3:2. doi: 10.1186/s40591-015-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Gu Y, Xiao L, Han Q, Li J, Chen B, Yu J, Kawasawa YI, Payne KJ, Dovat S, Song C. Co-existence of IL7R high and SH2B3 low expression distinguishes a novel high-risk acute lymphoblastic leukemia with Ikaros dysfunction. Oncotarget. 2016a Jun 14; doi: 10.18632/oncotarget.10014. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Ge Z, Gu Y, Zhao G, Li J, Chen B, Han Q, Guo X, Liu J, Li H, Yu MD, Olson J, Steffens S, Payne KJ, Song C, Dovat S. High CRLF2 expression associates with IKZF1 dysfunction in adult acute lymphoblastic leukemia without CRLF2 rearrangement. Oncotarget. 2016b Jul 6; doi: 10.18632/oncotarget.10437. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258(5083):808–812. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- Georgopoulos K, Winandy S, Avitahl N. The role of the Ikaros gene in lymphocyte development and homeostasis. Annu Rev Immunol. 1997a;15:155–176. doi: 10.1146/annurev.immunol.15.1.155. [DOI] [PubMed] [Google Scholar]
- Georgopoulos K, Winandy S, Avitahl N. The role of the Ikaros gene in lymphocyte development and homeostasis. Annu Rev Immunol. 1997b;15:155–176. doi: 10.1146/annurev.immunol.15.1.155. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Graham KC, Litchfield DW. Interactions between the subunits of casein kinase II. J Biol Chem. 1995;270(22):13017–13021. doi: 10.1074/jbc.270.22.13017. [DOI] [PubMed] [Google Scholar]
- Gowda C, Dovat S. Genetic targets in pediatric acute lymphoblastic leukemia. Adv Exp Med Biol. 2013;779:327–340. doi: 10.1007/978-1-4614-6176-0_15. [DOI] [PubMed] [Google Scholar]
- Gowda CS, Song C, Ding Y, Kapadia M, Dovat S. Protein signaling and regulation of gene transcription in leukemia: role of the Casein Kinase II-Ikaros axis. J Investig Med. 2016;64(3):735–739. doi: 10.1136/jim-2016-000075. [DOI] [PubMed] [Google Scholar]
- Gray GK, McFarland BC, Rowse AL, Gibson SA, Benveniste EN. Therapeutic CK2 inhibition attenuates diverse prosurvival signaling cascades and decreases cell viability in human breast cancer cells. Oncotarget. 2014;5(15):6484–6496. doi: 10.18632/oncotarget.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurel Z, Ronni T, Ho S, Kuchar J, Payne KJ, Turk CW, Dovat S. Recruitment of ikaros to pericentromeric heterochromatin is regulated by phosphorylation. J Biol Chem. 2008;283(13):8291–8300. doi: 10.1074/jbc.M707906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci I, Storlazzi CT, Cilloni D, Lonetti A, Ottaviani E, Soverini S, Astolfi A, Chiaretti S, Vitale A, Messa F, Impera L, Baldazzi C, D’Addabbo P, Papayannidis C, Lonoce A, Colarossi S, Vignetti M, Piccaluga PP, Paolini S, Russo D, Pane F, Saglio G, Baccarani M, Foa R, Martinelli G. Identification and molecular characterization of recurrent genomic deletions on 7p12 in the IKZF1 gene in a large cohort of BCR-ABL1-positive acute lymphoblastic leukemia patients: on behalf of Gruppo Italiano Malattie Ematologiche dell’Adulto Acute Leukemia Working Party (GIMEMA AL WP) Blood. 2009;114(10):2159–2167. doi: 10.1182/blood-2008-08-173963. [DOI] [PubMed] [Google Scholar]
- Izeradjene K, Douglas L, Delaney A, Houghton JA. Casein kinase II (CK2) enhances death-inducing signaling complex (DISC) activity in TRAIL-induced apoptosis in human colon carcinoma cell lines. Oncogene. 2005;24(12):2050–2058. doi: 10.1038/sj.onc.1208397. [DOI] [PubMed] [Google Scholar]
- Jakobi R, Traugh JA. Site-directed mutagenesis and structure/function studies of casein kinase II correlate stimulation of activity by the beta subunit with changes in conformation and ATP/GTP utilization. Eur J Biochem. 1995;230(3):1111–1117. doi: 10.1111/j.1432-1033.1995.tb20662.x. [DOI] [PubMed] [Google Scholar]
- Jeffrey PD, Russo AA, Polyak K, Gibbs E, Hurwitz J, Massagué J, Pavletich NP. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376(6538):313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- Kelley CM, Ikeda T, Koipally J, Avitahl N, Wu L, Georgopoulos K, Morgan BA. Helios, a novel dimerization partner of Ikaros expressed in the earliest hematopoietic progenitors. Curr Biol. 1998;8:508–S501. doi: 10.1016/s0960-9822(98)70202-7. [DOI] [PubMed] [Google Scholar]
- Kelliher MA, Seldin DC, Leder P. Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase IIalpha. EMBO J. 1996;15(19):5160–5166. [PMC free article] [PubMed] [Google Scholar]
- Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E, Winandy S, Viel A, Sawyer A, Ikeda T, Kingston R, Georgopoulos K. Ikaros dna-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10:345–355. doi: 10.1016/s1074-7613(00)80034-5. [DOI] [PubMed] [Google Scholar]
- Kim JS, Eom JI, Cheong JW, Choi AJ, Lee JK, Yang WI, Min YH. Protein kinase CK2alpha as an unfavorable prognostic marker and novel therapeutic target in acute myeloid leukemia. Clin Cancer Res. 2007;13(3):1019–1028. doi: 10.1158/1078-0432.CCR-06-1602. [DOI] [PubMed] [Google Scholar]
- Koipally J, Georgopoulos K, Jun 28. Ikaros-CtIP interactions do not require CtBP and participate in a deacetylase-independent mode of repression. J Biol Chem. 2002;277(26):23143–23149. doi: 10.1074/jbc.M202079200. [DOI] [PubMed] [Google Scholar]
- Koipally J, Renold A, Kim J, Georgopoulos K. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. Embo J. 1999;18(11):3090–3100. doi: 10.1093/emboj/18.11.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper RP, Schoenmakers EF, van Reijmersdal SV, Hehir-Kwa JY, van Kessel AG, van Leeuwen FN, Hoogerbrugge PM. High-resolution genomic profiling of childhood ALL reveals novel recurrent genetic lesions affecting pathways involved in lymphocyte differentiation and cell cycle progression. Leukemia. 2007;21(6):1258–1266. doi: 10.1038/sj.leu.2404691. [DOI] [PubMed] [Google Scholar]
- Kuiper RP, Waanders E, van der Velden VH, van Reijmersdal SV, Venkatachalam R, Scheijen B, Sonneveld E, van Dongen JJ, Veerman AJ, van Leeuwen FN, van Kessel AG, Hoogerbrugge PM. IKZF1 deletions predict relapse in uniformly treated pediatric precursor B-ALL. Leukemia. 2010;24(7):1258–1264. doi: 10.1038/leu.2010.87. [DOI] [PubMed] [Google Scholar]
- Laramas M, Pasquier D, Filhol O, Ringeisen F, Descotes JL, Cochet C. Nuclear localization of protein kinase CK2 catalytic subunit (CK2alpha) is associated with poor prognostic factors in human prostate cancer. Eur J Cancer. 2007;43(5):928–934. doi: 10.1016/j.ejca.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Li Z, Song C, Ouyang H, Lai L, Payne KJ, Dovat S. Cell cycle-specific function of Ikaros in human leukemia. Pediatr Blood Cancer. 2012;59(1):69–76. doi: 10.1002/pbc.23406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield DW, Dobrowolska G, Krebs EG. Regulation of casein kinase II by growth factors: a reevaluation. Cell Mol Biol Res. 1994;40(5–6):373–381. [PubMed] [Google Scholar]
- Litchfield DW, Lozeman FJ, Cicirelli MF, Harrylock M, Ericsson LH, Piening CJ, Krebs EG. Phosphorylation of the beta subunit of casein kinase II in human A431 cells. Identification of the autophosphorylation site and a site phosphorylated by p34cdc2. J Biol Chem. 1991;266(30):20380–20389. [PubMed] [Google Scholar]
- Litchfield DW, Lüscher B, Lozeman FJ, Eisenman RN, Krebs EG. Phosphorylation of casein kinase II by p34cdc2 in vitro and at mitosis. J Biol Chem. 1992;267(20):13943–13951. [PubMed] [Google Scholar]
- Lo K, Landau NR, Smale ST. LyF-1, a transcriptional regulator that interactswith a novel class of promoters for lymphocyte-specific genes. Mollecular Cell Biol. 1991;11:5229–5243. doi: 10.1128/mcb.11.10.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcais A, Jeannet R, Hernandez L, Soulier J, Sigaux F, Chan S, Kastner P. Genetic inactivation of Ikaros is a rare event in human T-ALL. Leuk Res. 2010;34(4):426–429. doi: 10.1016/j.leukres.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Martinelli G, Iacobucci I, Storlazzi CT, Vignetti M, Paoloni F, Cilloni D, Soverini S, Vitale A, Chiaretti S, Cimino G, Papayannidis C, Paolini S, Elia L, Fazi P, Meloni G, Amadori S, Saglio G, Pane F, Baccarani M, Foa R. IKZF1 (Ikaros) deletions in BCR-ABL1-positive acute lymphoblastic leukemia are associated with short disease-free survival and high rate of cumulative incidence of relapse: a GIMEMA AL WP report. J Clin Oncol. 2009;27(31):5202–5207. doi: 10.1200/JCO.2008.21.6408. [DOI] [PubMed] [Google Scholar]
- Martins LR, Lúcio P, Silva MC, Anderes KL, Gameiro P, Silva MG, Barata JT. Targeting CK2 overexpression and hyperactivation as a novel therapeutic tool in chronic lymphocytic leukemia. Blood. 2010;116(15):2724–2731. doi: 10.1182/blood-2010-04-277947. [DOI] [PubMed] [Google Scholar]
- Maser RS, Choudhury B, Campbell PJ, Feng B, Wong KK, Protopopov A, O’Neil J, Gutierrez A, Ivanova E, Perna I, Lin E, Mani V, Jiang S, McNamara K, Zaghlul S, Edkins S, Stevens C, Brennan C, Martin ES, Wiedemeyer R, Kabbarah O, Nogueira C, Histen G, Aster J, Mansour M, Duke V, Foroni L, Fielding AK, Goldstone AH, Rowe JM, Wang YA, Look AT, Stratton MR, Chin L, Futreal PA, DePinho RA. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007;447(7147):966–971. doi: 10.1038/nature05886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Abrams SL, Fitzgerald TL, Cocco L, Martelli AM, Montalto G, Cervello M, Scalisi A, Candido S, Libra M, Steelman LS. Roles of signaling pathways in drug resistance, cancer initiating cells and cancer progression and metastasis. Adv Biol Regul. 2015;57:75–101. doi: 10.1016/j.jbior.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Meggio F, Boldyreff B, Marin O, Pinna LA, Issinger OG. Role of the beta subunit of casein kinase-2 on the stability and specificity of the recombinant reconstituted holoenzyme. Eur J Biochem. 1992;204(1):293–297. doi: 10.1111/j.1432-1033.1992.tb16636.x. [DOI] [PubMed] [Google Scholar]
- Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17(3):349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- Meisner H, Heller-Harrison R, Buxton J, Czech MP. Molecular cloning of the human casein kinase II alpha subunit. Biochemistry. 1989;28(9):4072–4076. doi: 10.1021/bi00435a066. [DOI] [PubMed] [Google Scholar]
- Molnár A, Georgopoulos K. The Ikaros gene encodes a family of functionally diverse zinc finger DNA-binding proteins. Mol Cell Biol. 1994a;14:8292–8303. doi: 10.1128/mcb.14.12.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár A, Georgopoulos K. The Ikaros gene encodes a family of functionally diverse zinc finger DNA-binding proteins. Mol Cell Biol. 1994b;14(12):8292–8303. doi: 10.1128/mcb.14.12.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E, Wu P, Neben S, Georgopoulos K. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J. 1997;16:2004–2013. doi: 10.1093/emboj/16.8.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullighan C, Downing J. Ikaros and acute leukemia. Leuk Lymphoma. 2008;49(5):847–849. doi: 10.1080/10428190801947500. [DOI] [PubMed] [Google Scholar]
- Mullighan CG. Genomic profiling of B-progenitor acute lymphoblastic leukemia. Best Pract Res Clin Haematol. 2011;24(4):489–503. doi: 10.1016/j.beha.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullighan CG. The molecular genetic makeup of acute lymphoblastic leukemia. Hematol Am Soc Hematol Educ Program. 2012;2012:389–396. doi: 10.1182/asheducation-2012.1.389. [DOI] [PubMed] [Google Scholar]
- Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, Girtman K, Mathew S, Ma J, Pounds SB, Su X, Pui CH, Relling MV, Evans WE, Shurtleff SA, Downing JR. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446(7137):758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- Mullighan CG, Miller CB, Radtke I, Phillips LA, Dalton J, Ma J, White D, Hughes TP, Le Beau MM, Pui CH, Relling MV, Shurtleff SA, Downing JR. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110–114. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Miller CB, Ma J, Liu W, Cheng C, Schulman BA, Harvey RC, Chen IM, Clifford RJ, Carroll WL, Reaman G, Bowman WP, Devidas M, Gerhard DS, Yang W, Relling MV, Shurtleff SA, Campana D, Borowitz MJ, Pui CH, Smith M, Hunger SP, Willman CL, Downing JR CsO Group. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münstermann U, Fritz G, Seitz G, Lu YP, Schneider HR, Issinger OG. Casein kinase II is elevated in solid human tumours and rapidly proliferating non-neoplastic tissue. Eur J Biochem. 1990;189(2):251–257. doi: 10.1111/j.1432-1033.1990.tb15484.x. [DOI] [PubMed] [Google Scholar]
- Nakase K, Ishimaru F, Avitahl N, Dansako H, Matsuo K, Fujii K, Sezaki N, Nakayama H, Yano T, Fukuda S, Imajoh K, Takeuchi M, Miyata A, Hara M, Yasukawa M, Takahashi I, Taguchi H, Matsue K, Nakao S, Niho Y, Takenaka K, Shinagawa K, Ikeda K, Niiya K, Harada M. Dominant negative isoform of the Ikaros gene in patients with adult B-cell acute lymphoblastic leukemia. Cancer Res. 2000;60(15):4062–4065. [PubMed] [Google Scholar]
- Nakayama H, Ishimaru F, Avitahl N, Sezaki N, Fujii N, Nakase K, Ninomiya Y, Harashima A, Minowada J, Tsuchiyama J, Imajoh K, Tsubota T, Fukuda S, Sezaki T, Kojima K, Hara M, Takimoto H, Yorimitsu S, Takahashi I, Miyata A, Taniguchi S, Tokunaga Y, Gondo H, Niho Y, Harada M, et al. Decreases in Ikaros activity correlate with blast crisis in patients with chronic myelogenous leukemia. Cancer Res. 1999;59(16):3931–3934. [PubMed] [Google Scholar]
- Neri LM, Cani A, Martelli AM, Simioni C, Junghanss C, Tabellini G, Ricci F, Tazzari PL, Pagliaro P, McCubrey JA, Capitani S. Targeting the PI3K/Akt/mTOR signaling pathway in B-precursor acute lymphoblastic leukemia and its therapeutic potential. Leukemia. 2014;28(4):739–748. doi: 10.1038/leu.2013.226. [DOI] [PubMed] [Google Scholar]
- Payne KJ, Dovat S. Ikaros and tumor suppression in acute lymphoblastic leukemia. Crit Rev Oncog. 2011;16(1–2):3–12. doi: 10.1615/critrevoncog.v16.i1-2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payrastre B, Cocco L. Foreword: “The PI3-kinase/Akt pathway: from signaling to diseases”. Adv Biol Regul. 2015;59:1–3. doi: 10.1016/j.jbior.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Pellagatti A, Dolatshad H, Yip BH, Valletta S, Boultwood J. Application of genome editing technologies to the study and treatment of hematological disease. Adv Biol Regul. 2016;60:122–134. doi: 10.1016/j.jbior.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Perdomo J, Holmes M, Chong B, Crossley M. Eos and Pegasus, two members of the ikaros family of proteins with distinct DNA binding activities. J Biol Chem. 2000;275:38347–38354. doi: 10.1074/jbc.M005457200. [DOI] [PubMed] [Google Scholar]
- Piazza FA, Ruzzene M, Gurrieri C, Montini B, Bonanni L, Chioetto G, Di Maira G, Barbon F, Cabrelle A, Zambello R, Adami F, Trentin L, Pinna LA, Semenzato G. Multiple myeloma cell survival relies on high activity of protein kinase CK2. Blood. 2006;108(5):1698–1707. doi: 10.1182/blood-2005-11-013672. [DOI] [PubMed] [Google Scholar]
- Pierre F, Chua PC, O’Brien SE, Siddiqui-Jain A, Bourbon P, Haddach M, Michaux J, Nagasawa J, Schwaebe MK, Stefan E, Vialettes A, Whitten JP, Chen TK, Darjania L, Stansfield R, Anderes K, Bliesath J, Drygin D, Ho C, Omori M, Proffitt C, Streiner N, Trent K, Rice WG, Ryckman DM. Discovery and SAR of 5-(3-chlorophenylamino)benzo[c][2,6]naphthyridine-8-carboxylic acid (CX-4945), the first clinical stage inhibitor of protein kinase CK2 for the treatment of cancer. J Med Chem. 2011a;54(2):635–654. doi: 10.1021/jm101251q. [DOI] [PubMed] [Google Scholar]
- Pierre F, Chua PC, O’Brien SE, Siddiqui-Jain A, Bourbon P, Haddach M, Michaux J, Nagasawa J, Schwaebe MK, Stefan E, Vialettes A, Whitten JP, Chen TK, Darjania L, Stansfield R, Bliesath J, Drygin D, Ho C, Omori M, Proffitt C, Streiner N, Rice WG, Ryckman DM, Anderes K. Preclinical characterization of CX-4945, a potent and selective small molecule inhibitor of CK2 for the treatment of cancer. Mol Cell Biochem. 2011b;356(1–2):37–43. doi: 10.1007/s11010-011-0956-5. [DOI] [PubMed] [Google Scholar]
- Pinna LA. Casein kinase 2: an ‘eminence grise’ in cellular regulation? Biochim Biophys Acta. 1990;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Piazza F, Agostinelli C, Fuligni F, Benvenuti P, Mandato E, Casellato A, Rugge M, Semenzato G, Pileri SA. Protein kinase CK2 is widely expressed in follicular, Burkitt and diffuse large B-cell lymphomas and propels malignant B-cell growth. Oncotarget. 2015;6(9):6544–6552. doi: 10.18632/oncotarget.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu M, Gurel Z, Ronni T, Song C, Hung KY, Payne KJ, Dovat S. Ikaros stability and pericentromeric localization are regulated by protein phosphatase 1. J Biol Chem. 2009;284(20):13869–13880. doi: 10.1074/jbc.M900209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- Quotti Tubi L, Gurrieri C, Brancalion A, Bonaldi L, Bertorelle R, Manni S, Pavan L, Lessi F, Zambello R, Trentin L, Adami F, Ruzzene M, Pinna LA, Semenzato G, Piazza F. Inhibition of protein kinase CK2 with the clinical-grade small ATP-competitive compound CX-4945 or by RNA interference unveils its role in acute myeloid leukemia cell survival, p53-dependent apoptosis and daunorubicin-induced cytotoxicity. J Hematol Oncol. 2013;6:78. doi: 10.1186/1756-8722-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud D, Demarco IA, Reddy KL, Schjerven H, Bertolino E, Chen Z, Smale ST, Winandy S, Singh H. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat Immunol. 2008;9(8):927–936. doi: 10.1038/ni.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronni T, Payne KJ, Ho S, Bradley MN, Dorsam G, Dovat S. Human Ikaros function in activated T cells is regulated by coordinated expression of its largest isoforms. J Biol Chem. 2007;282(4):2538–2547. doi: 10.1074/jbc.M605627200. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Jiang J, Kempski H, Brady HJ. Overexpression of the Ikaros 6 isoform is restricted to t(4;11) acute lymphoblastic leukaemia in children and infants and has a role in B-cell survival. Br J Haematol. 2004;125(1):31–37. doi: 10.1111/j.1365-2141.2004.04854.x. [DOI] [PubMed] [Google Scholar]
- Scaglioni PP, Yung TM, Choi S, Choi SC, Baldini C, Konstantinidou G, Pandolfi PP. CK2 mediates phosphorylation and ubiquitin-mediated degradation of the PML tumor suppressor. Mol Cell Biochem. 2008;316(1–2):149–154. doi: 10.1007/s11010-008-9812-7. [DOI] [PubMed] [Google Scholar]
- Schjerven H, McLaughlin J, Arenzana TL, Frietze S, Cheng D, Wadsworth SE, Lawson GW, Bensinger SJ, Farnham PJ, Witte ON, Smale ST. Selective regulation of lymphopoiesis and leukemogenesis by individual zinc fingers of Ikaros. Nat Immunol. 2013;14(10):1073–1083. doi: 10.1038/ni.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin DC, Leder P. Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science. 1995;267(5199):894–897. doi: 10.1126/science.7846532. [DOI] [PubMed] [Google Scholar]
- Shore LJ, Soler AP, Gilmour SK. Ornithine decarboxylase expression leads to translocation and activation of protein kinase CK2 in vivo. J Biol Chem. 1997;272(19):12536–12543. doi: 10.1074/jbc.272.19.12536. [DOI] [PubMed] [Google Scholar]
- Siddiqui-Jain A, Drygin D, Streiner N, Chua P, Pierre F, O’Brien SE, Bliesath J, Omori M, Huser N, Ho C, Proffitt C, Schwaebe MK, Ryckman DM, Rice WG, Anderes K. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res. 2010;70(24):10288–10298. doi: 10.1158/0008-5472.CAN-10-1893. [DOI] [PubMed] [Google Scholar]
- Song C, Gowda C, Pan X, Ding Y, Tong Y, Tan BH, Wang H, Muthusami S, Ge Z, Sachdev M, Amin SG, Desai D, Gowda K, Gowda R, Robertson GP, Schjerven H, Muschen M, Payne KJ, Dovat S. Targeting casein kinase II restores Ikaros tumor suppressor activity and demonstrates therapeutic efficacy in high-risk leukemia. Blood. 2015;126(15):1813–1822. doi: 10.1182/blood-2015-06-651505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Li Z, Erbe AK, Savic A, Dovat S. Regulation of Ikaros function by casein kinase 2 and protein phosphatase 1. World J Biol Chem. 2011;2(6):126–131. doi: 10.4331/wjbc.v2.i6.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Pan X, Ge Z, Gowda C, Ding Y, Li H, Li Z, Yochum G, Muschen M, Li Q, Payne KJ, Dovat S. Epigenetic regulation of gene expression by Ikaros, HDAC1 and Casein Kinase II in leukemia. Leukemia. 2016;30(6):1436–1440. doi: 10.1038/leu.2015.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EC, Cobb BS, Sabbattini P, Meixlsperger S, Parelho V, Liberg D, Taylor B, Dillon N, Georgopoulos K, Jumaa H, Smale ST, Fisher AG, Merkenschlager M. Ikaros DNA-binding proteins as integral components of B cell developmental-stage-specific regulatory circuits. Immunity. 2007;26(3):335–344. doi: 10.1016/j.immuni.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Tonnelle C, Bardin F, Maroc C, Imbert AM, Campa F, Dalloul A, Schmitt C, Chabannon C. Forced expression of the Ikaros 6 isoform in human placental blood CD34(+) cells impairs their ability to differentiate toward the B-lymphoid lineage. Blood. 2001;98(9):2673–2680. doi: 10.1182/blood.v98.9.2673. [DOI] [PubMed] [Google Scholar]
- Tuazon PT, Traugh JA. Casein kinase I and II–multipotential serine protein kinases: structure, function, and regulation. Adv Second Messenger Phosphoprot Res. 1991;23:123–164. [PubMed] [Google Scholar]
- Virely C, Moulin S, Cobaleda C, Lasgi C, Alberdi A, Soulier J, Sigaux F, Chan S, Kastner P, Ghysdael J. Haploinsufficiency of the IKZF1 (IKAROS) tumor suppressor gene cooperates with BCR-ABL in a transgenic model of acute lymphoblastic leukemia. Leukemia. 2010;24(6):1200–1204. doi: 10.1038/leu.2010.63. [DOI] [PubMed] [Google Scholar]
- Wang G, Ahmad KA, Ahmed K. Modulation of death receptor-mediated apoptosis by CK2. Mol Cell Biochem. 2005;274(1–2):201–205. doi: 10.1007/s11010-005-2952-0. [DOI] [PubMed] [Google Scholar]
- Wang G, Ahmad KA, Ahmed K. Role of protein kinase CK2 in the regulation of tumor necrosis factor-related apoptosis inducing ligand-induced apoptosis in prostate cancer cells. Cancer Res. 2006a;66(4):2242–2249. doi: 10.1158/0008-5472.CAN-05-2772. [DOI] [PubMed] [Google Scholar]
- Wang G, Ahmad KA, Unger G, Slaton JW, Ahmed K. CK2 signaling in androgen-dependent and -independent prostate cancer. J Cell Biochem. 2006b;99(2):382–391. doi: 10.1002/jcb.20847. [DOI] [PubMed] [Google Scholar]
- Wang H, Davis A, Yu S, Ahmed K. Response of cancer cells to molecular interruption of the CK2 signal. Mol Cell Biochem. 2001;227(1–2):167–174. [PubMed] [Google Scholar]
- Wang H, Ouyang H, Lai L, Petrovic-Dovat L, Stankov K, Bogdanovic G, Dovat S. Pathogenesis and regulation of cellular proliferation in acute lymphoblastic leukemia - the role of Ikaros. J BUON. 2014a;19(1):22–28. [PubMed] [Google Scholar]
- Wang H, Song C, Ding Y, Pan X, Ge Z, Tan BH, Gowda C, Sachdev M, Muthusami S, Ouyang H, Lai L, Francis OL, Morris CL, Abdel-Azim H, Dorsam G, Xiang M, Payne KJ, Dovat S. Transcriptional regulation of JARID1B/KDM5B histone demethylase by ikaros, histone deacetylase 1 (HDAC1), and casein kinase 2 (CK2) in B-cell acute lymphoblastic leukemia. J Biol Chem. 2016;291(8):4004–4018. doi: 10.1074/jbc.M115.679332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Song C, Gurel Z, Song N, Ma J, Ouyang H, Lai L, Payne KJ, Dovat S. Protein phosphatase 1 (PP1) and casein kinase II (CK2) regulate ikaros-mediated repression of TdT in thymocytes and t-cell leukemia. Pediatr Blood Cancer. 2014b;61(12):2230–2235. doi: 10.1002/pbc.25221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83(2):289–299. doi: 10.1016/0092-8674(95)90170-1. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yao-Ming Ng S, Zuniga-Pflucker JC, Georgopoulos K. Early hematopoietic lineage restrictions directed by Ikaros. Nat Immunol. 2006;7:382–391. doi: 10.1038/ni1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Yeh J, Van Waes C. Protein kinase casein kinase 2 mediates inhibitor-kappaB kinase and aberrant nuclear factor-kappaB activation by serum factor(s) in head and neck squamous carcinoma cells. Cancer Res. 2006;66(13):6722–6731. doi: 10.1158/0008-5472.CAN-05-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, Easton J, Chen X, Wang J, Rusch M, Lu C, Chen SC, Wei L, Collins-Underwood JR, Ma J, Roberts KG, Pounds SB, Ulyanov A, Becksfort J, Gupta P, Huether R, Kriwacki RW, Parker M, McGoldrick DJ, Zhao D, Alford D, Espy S, Bobba KC, Song G, Pei D, Cheng C, Roberts S, Barbato MI, Campana D, Coustan-Smith E, Shurtleff SA, Raimondi SC, Kleppe M, Cools J, Shimano KA, Hermiston ML, Doulatov S, Eppert K, Laurenti E, Notta F, Dick JE, Basso G, Hunger SP, Loh ML, Devidas M, Wood B, Winter S, Dunsmore KP, Fulton RS, Fulton LL, Hong X, Harris CC, Dooling DJ, Ochoa K, Johnson KJ, Obenauer JC, Evans WE, Pui CH, Naeve CW, Ley TJ, Mardis ER, Wilson RK, Downing JR, Mullighan CG. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157–163. doi: 10.1038/nature10725. [DOI] [PMC free article] [PubMed] [Google Scholar]