Abstract
Nonspecific target engagement by test compounds and purported chemical probes is a significant source of assay interference and promiscuous bioactivity in high-throughput screening (HTS) and chemical biology. Most counter-screens for thiol-reactive compounds utilize mass spectrometry or fluorescence detection, and non-proteinaceous reporters like glutathione that may not always approximate the reactivity of protein side-chains. By contrast, a La assay to detect reactive molecules by nuclear magnetic resonance (ALARM NMR) is an industry-developed protein-based [1H-13C]-heteronuclear multiple quantum coherence (HMQC) NMR counter-screen to identify nonspecific protein interactions by test compounds by reporting their tendencies to modulate the human La antigen conformation. This Current Protocol is a users-guide to the production of the 13C-labeled La antigen reporter protein, the reaction of test compounds with this reporter protein, as well as the collection and analysis of characteristic NMR spectra. Combined with other assay interference counter-screens, this assay will enhance chemical biology by helping researchers better prioritize chemical matter and which will increase the number of tractable HTS screening actives and aid in the development of better chemical probes.
Keywords: assay interference, bioassay promiscuity, chemical probes, chemical biology, drug discovery, high-throughput screening, NMR, thiol reactivity
Introduction
Many presumably active compounds found in high-throughput screening (HTS) are actually interfering with assay readouts or engaging in nonspecific compound-target interactions (Thorne et al., 2010). This can introduce significant confounding factors into effective HTS analysis and make it difficult to draw meaningful experimental conclusions in complex cellular systems that are composed of innumerable targets and system interactions. Compound-mediated assay interference and biological promiscuity originate from several sources, including nonspecific reactivity with biological thiols such as glutathione (GSH), coenzyme A (CoA), and protein cysteines (Dahlin et al., 2015a; Dahlin et al., 2015c). Additionally, nonspecific protein perturbations can result from test compounds forming aggregates (Coan et al., 2009; McGovern et al., 2003).
Conventional approaches in drug discovery for detecting thiol-reactive compounds often involve incubating a test compound with a thiol-containing reporter (e.g., cysteamine, GSH) and analyzing the products for direct compound-thiol adducts. However, non-proteinaceous reporters such as these may not approximate the reactivity of physiologically-relevant protein cysteine side-chains (Wilson et al., 1980). In one drug discovery setting, a notable subset of compounds (10/34, 29%) were negative by conventional GSH reactivity screens, but were flagged as thiol reactive in ALARM NMR. This suggests that reliance on GSH alone to rule-out thiol reactivity could provide scientists with an incomplete assessment of potential assay interference and nonspecific effects. There is a growing interest in utilizing biophysical techniques for compound triage, including NMR, which can provide insights into protein conformation (Zega, 2017).
In ALARM NMR, test compounds are incubated with a 13C-labeled La antigen reporter protein in physiological buffer. This reporter protein contains two electrophilic cysteines (C232 and C245) and several nearby leucine residues (L249, L294, and L296) amenable to detection by conventional 2-dimensional NMR (Figure 1A). In the absence of dithiothreitol (DTT), thiol-reactive test compounds can form covalent bonds with cysteine side chains on the La antigen. When analyzed by [1H-13C]-HMQC NMR, chemically modified cysteines cause characteristic decreases in peak intensities and occasionally peak shifts at several nearby leucine peaks (Huth et al., 2005). These perturbations are significantly attenuated when excess DTT is present in the reaction buffer (Figure 1B). In addition, known aggregators have been shown to cause DTT-independent perturbations in the La antigen conformation (Dahlin et al., 2015b). Compounds with cysteine-reactive readouts by ALARM NMR are highly liable to cause nonspecific thiol reactivity, and can be flagged for triage or additional work-up, depending on the experimental and overall scientific contexts.
Figure 1. Overview of ALARM NMR.
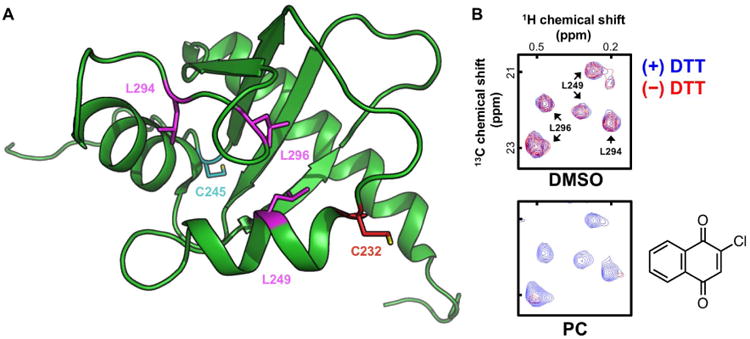
(A) The La antigen contains cysteine residues (C232, C245) sensitive to electrophiles. When covalently modified, these residues perturb nearby 13C-labeled leucine residues (L249, L294, L296). PDB ID: 1OWX (Jacks et al., 2003). (B) Portions of the [1H,13C]-HMQC spectra of the La antigen reporter protein in the presence and absence of DTT. Test compounds are incubated with the La antigen reporter protein in physiological buffer and compared to vehicle control (DMSO). With excess DTT present, thiol-reactive compounds (positive control, PC) are scavenged by DTT (blue contours); with DTT absent, thiol-reactive compounds can covalently modify cysteine thiols (red contours) which manifests as chemical shifts and/or attenuation of peak intensity. Z-axis, relative units.
This Current Protocol describes the process required to perform an ALARM NMR counter-screen from protein preparation to NMR spectra interpretation. Basic Protocol 1 describes the production of the 13C-labeled La antigen reporter protein. Using prototypical positive and negative control compounds as examples, Basic Protocol 2 details the testing of compounds for protein reactivity with the 13C-labeled La antigen reporter protein. When used as part of a comprehensive compound evaluation, ALARM NMR will help to identify potentially reactive screening and probe compounds, thereby assisting researchers in prioritizing more useful chemical matter.
Strategic Planning
The production and purification of the La antigen reporter protein should be performed well in advance of an ALARM NMR experiment. We recommend this approach for three reasons. First, approximately one week is required to produce and purify the protein (Basic Protocol 1). Producing the protein well in advance of the actual NMR experiment allows for troubleshooting and repeating the protein production if necessary. Second, the La antigen reporter protein is stable in storage at -80 °C for at least one year. Three, large-scale production of the protein minimizes potential batch-to-batch variation.
Another notable strategic factor with respect to ALARM NMR is its place in the post-HTS and chemical probe development/validation process. This assay can be used at multiple stages of a project pipeline. In our experience, the optimal time for performing ALARM NMR should balance the following factors:
Number of test compounds (up to 50 per run in standard configurations)
Chemical structures of test compounds (e.g., how many compounds are suspected thiol-reactive?)
Instrument time (e.g., if performing at an institutional core facility)
Additional counter-screens
Project timeline
One advantage of performing ALARM NMR at an early stage in a project is that it can guide additional counter-screens for reactivity or aggregation (if indicated by the experimental context). Depending on available resources, a potential drawback to performing ALARM NMR earlier in a project is that more compounds will be tested. To decrease the amount of time preparing La antigen reporter protein and to increase overall efficiency, we typically batch samples from multiple projects.
BASIC PROTOCOL 1 - Production and purification of ALARM NMR protein
ALARM NMR utilizes a [1H-13C]-HMQC readout. Due to the low natural abundance of NMR-active 13C nuclei in standard culture media, performing an HMQC experiment with the La antigen requires 13C-enrichment to attain a sufficient signal. Globally labeling proteins with 13C-glucose can be prohibitively expensive and can lead to signal reductions via 13C-13C coupling (Hajduk et al., 2000). However, a more economical approach involves the selective incorporation of 13C nuclei into methyl-bearing residues on protein side chains with the addition of 13C-labeled amino acid precursors such as [3-13C]-α-ketobutyrate (isoleucine precursor) and [3,3-13C]-α-ketoisovalerate (valine, leucine precursor) to the culture medium (Goto et al., 1999; Hajduk et al., 2000). These labeled amino acid precursors are then biosynthetically incorporated into the recombinant protein during induced bacterial protein production. While uniformly 13C-labeled amino acid precursors are available, like 13C-glucose strategies, they too are expensive and can also lead to 13C-13C coupling.
Aside from the timing of the addition of these amino acid precursors, the protein production and purification procedures follows otherwise standard methods familiar to most molecular biologists and scientists in related fields (Consortium et al., 2008). The La antigen reporter protein is cloned into the pET28b+ vector under the control of the T7 promoter and lac operator (Figure 2A). The 280 residue protein itself contains La antigen amino acids 100-324 and is flanked by N- and C-termini 6xHis tags (Figure 2B). These tags allow for facile purification by nickel (Ni2+) bead systems (Petty, 2001).
Figure 2. ALARM NMR reporter protein construct.
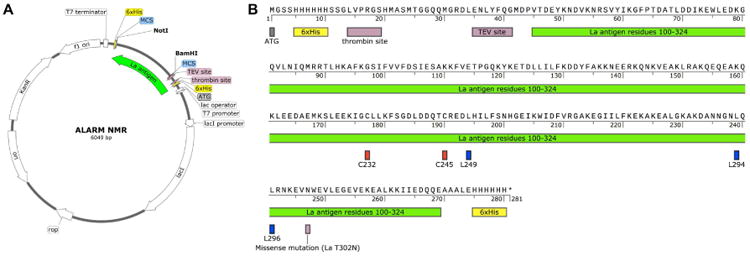
(A) ALARM NMR plasmid map. The human La antigen (amino acid residues 100-324) is located in the multiple cloning site (MCR) of the pET28b+ plasmid. Note this plasmid contains a kanamycin resistance gene (KanR). Sequencing data (Sanger and Next-Generation Sequencing) and plasmid available at addgene.org (plasmid ID 90209). (B) Primary protein sequence of La antigen reporter protein for use in the ALARM NMR assay. Final product: 280 amino acids, 32,260 Da calculated MW for unlabeled protein. The reactive cysteines (C232, C245) and key leucine residues (L249, L294, L296) are highlighted. The protein contains N- and C- terminal 6xHis tags.
This protocol enables the production and purification of the 13C-labeled La antigen reporter protein for use in the ALARM NMR assay. This protocol is written for a medium-sized protein production (4 L bacterial culture) suitable for most conventionally-equipped biochemistry laboratories. Briefly, bacteria competent cells are transfected with a plasmid containing the La antigen and flanking N- and C- termini 6xHis tags, then cultured in minimal liquid media. Shortly after adding 13C-labeled amino acid precursors during the bacterial growth log phase, protein expression is induced by isopropyl β-D-1-thiogalactopyranoside (IPTG). Harvested bacteria are lysed, the labeled La antigen reporter protein is purified by pre-charged Ni-NTA (“nitrilotriacetic acid”) beads, the target protein characterized by SDS-PAGE analysis, then the pooled pure protein fractions dialyzed in storage buffer.
Materials
Rosetta (DE3)pLysS cells (Novagen, Cat # 70956)
ALARM NMR plasmid (pET28b+, available from addgene.org (plasmid ID 90209); see also Figure 2)
LB agar petri plates
LB liquid media (see recipe)
M9 media (see recipe)
Kanamycyin stock solution (see recipe)
Chloramphenicol stock solution (see recipe)
[3-13C]-α-Ketobutyrate (Cambridge Isotope Laboratories, Cat # CLM-6820)
[3,3-13C]-α-Ketoisovalerate (Cambridge Isotope Laboratories, Cat # CLM-6821)
IPTG solution (see recipe)
Phosphate-buffered saline (PBS; see recipe)
Lysis buffer (see recipe)
Pre-charged Ni-NTA agarose beads (Qiagen)
Wash buffer (see recipe)
Elution buffer (see recipe)
Bradford reagent solution or microvolume spectrophotometer (e.g., ThermoFisher Nanodrop™)
SDS-PAGE gels (e.g., 10-15%)
5× SDS loading buffer
1× SDS running buffer
Coomassie brilliant blue solution
Purification dialysis buffer (see recipe)
SnakeSkin™ dialysis membrane 10 kDa MWCO (ThermoFisher, Cat # 68305)
Incubator oven
Temperature-controlled bacterial incubated shaker
Tabletop centrifuge (e.g., Beckman-Coulter Allegra X-12)
High-performance centrifuge (e.g., Beckman-Coulter Avanti J-26XP)
Compatible high-speed rotor (e.g., Beckman-Coulter JA-30.50 fixed-angle titanium rotor) with compatible 50 mL bottles (e.g., Beckman-Coulter Cat # 361694)
Compatible large-volume capacity rotor (e.g., Beckman-Coulter JLA-8.1000 fixed-angle rotor) with compatible 1 L bottles (e.g., Beckman-Coulter Cat # 366751)
French press or probe sonicator (e.g., Qsonica Q125)
Gravity drip columns (e.g., Bio-Rad Econo-Pac® or Econo-Column®)
Spectrophotometer
Gel electrophoresis apparatus with power supply
Rocker
Gel imaging system
Note: additional required reagents are listed under recipes.
Production and purification of La antigen reporter protein
-
Transform the plasmid containing human La protein into freshly thawed Rosetta™ (DE3) pLysS competent cells.
Note: This particular competent cell type contains a chloramphenicol resistance gene. Comparable bacterial strains designed for recombinant eukaryotic protein production can likely be substituted. If so, users must modify protocol accordingly to utilize the correct antibiotics.
Incubate the transformed bacteria overnight on a petri dish containing agar media (plus appropriate selection antibiotics) at 37 °C in an incubator oven.
-
Pick a single bacterial colony from the petri dish. Inoculate a sterile 3 mL liquid culture (e.g., LB media) at 25 °C at 150 rpm overnight. Include 3 μL each kanamycin and chloramphenicol stock solutions in the liquid culture.
Protein production protocol adapted from previous procedures (Gardner et al., 1998). See also kglab.asrc.cuny.edu for helpful protein labeling resources).
-
Centrifuge the bacterial liquid culture at 4 °C at 3000 g for 5 min using a tabletop centrifuge. Discard the resulting supernatant. Re-suspend the remaining cell pellet in 250 mL sterile, pre-warmed M9 media, with a starting OD600 of approximately 0.03-0.1. Include 250 μL each kanamycin and chloramphenicol stock solutions in the liquid culture.
Disinfect the supernatant with bleach (10% final volume) for 20 min before discarding.
-
Incubate the bacterial liquid culture for approximately 3-4 h at 37 °C at 150 rpm in an orbital shaker incubator until the culture is mid-log (OD600 = 0.5-0.8).
Typical doubling times are approximately 60-75 min for these culturing conditions. Actual times may vary.
-
Centrifuge the bacterial culture at 4 °C at 3000 g for 5 min using a tabletop centrifuge. Discard the resulting supernatant. Re-suspend the remaining cell pellet with 100 mL sterile, pre-warmed M9 media. Split the re-suspended liquid culture into four 1-L batches of sterile, pre-warmed M9 media. Include 1 mL each kanamycin and chloramphenicol stock solutions in each 1 L liquid culture.
Disinfect the supernatant with bleach (10% final volume) for 20 min before discarding. We typically prepare the ALARM NMR protein in 4 L batches, though this could be scaled-up in more protein product is desired. We perform cultures in 2 L Erlenmeyer flasks (1 L per flask) to ensure optimal media stirring while on orbital shakers. Other culture volumes are also compatible with this protocol.
-
Incubate the bacterial liquid culture for 2 h at 37 °C at 150 rpm in an orbital shaker incubator. Adjust the incubator temperature to 25 °C. Continue the incubation until the cultures are mid-log (OD600 = 0.5-0.8).
Typical doubling times are approximately 60-75 min for these culturing conditions. Actual times may vary. The temperature reduction step is intended to allow cultures time to equilibrate to the lower temperatures used for protein induction (Step 9).
-
Add 13C-labeled amino acid precursors to the bacterial liquid culture. Incubate for 30 min prior to induction with IPTG.
Suggested concentrations: [3-13C]-α-ketobutyrate: 200 mg/L culture; [3,3′-13C]-α-ketoisovalerate: 300 mg/L culture.
-
Induce La antigen protein expression by adding 1 mL of 1 M IPTG solution (1 mM final concentration) to each 1 L bacterial liquid culture 30 min after adding the amino acid precursors.
For troubleshooting, collect 1 mL culture media before IPTG induction, then pellet and freeze for later analysis of induction.
-
Incubate the bacterial liquid culture for 10 h at 22 °C at 150 rpm in an orbital shaker incubator.
Note that this incubation occurs at a lower temperature than previous incubations.
-
Harvest the bacteria from the liquid media culture by centrifugation at 4 °C at 5000 g for 15 min using a high-performance centrifuge with compatible large-volume bottles. Transfer the bacterial pellets to standard Falcon tubes by re-suspending the pellets in 5-10 mL cold phosphate-buffered saline (PBS).
Keep all solutions containing bacteria on ice when not in use. Disinfect the supernatant with bleach (10% final volume) for 20 min before discarding.
-
Centrifuge the bacterial suspension at 3000 g for 15 min at 4 °C using a tabletop centrifuge. Discard the resulting supernatant.
Disinfect the supernatant with bleach (10% final volume) for 20 min before discarding.
-
Wash the resulting pellet by re-suspending the pellet in cold PBS. Repeat centrifugation using a tabletop centrifuge, then discard the resulting supernatant.
At this point, the pellet can be frozen at -80 °C until commencing protein purification.
-
Re-suspend the bacterial pellet in 5-10 mL lysis buffer per 1 L original culture until the resulting suspension is homogenous.
Thaw the pellet on ice if previously frozen. The re-suspended solution can be gently vortexed to speed up the homogenization process. Prepare the lysis buffer fresh and keep on ice.
-
Lyse the bacterial suspension by French press or sonication.
For sonication, use 15 × 1 s pulses at medium power, repeat 3-5 times. Keep solutions and lysates on ice throughout purification process. Include lysozyme in lysis buffer when performing cell lysis by sonication.
Clear the cellular debris in the lysed bacterial suspension by centrifugation at 30,000 g for 30 min at 4 °C using a high-speed centrifuge with compatible bottles. Transfer the resulting supernatant to fresh centrifuge tubes.
-
Repeat the centrifugation of lysed bacterial suspension. Transfer the resulting supernatant to a clean container.
Keep the supernatant on ice when not in use.
-
Prepare the protein purification column (Petty, 2001). Load pre-charged Ni2+-beads into a gravity-drip column.
Protein purification should be done preferably in a cold room or 4 °C refrigerator. Use 1-2 mL pre-charged beads per 1 L bacterial culture. Wash the loaded column with 10 column equivalents (CE) wash buffer by gravity drip. Comparable Ni2+-based beads or columns can likely be substituted. This protocol can also be adapted for automated protein purification systems. The gravity drip method is described here for general applicability.
-
Load the purification column with the supernatant using gravity drip.
Aliquots of column run-off during the purification process can be saved for potential troubleshooting.
-
Wash the column with 10 CE wash buffer using gravity drip.
This step removes untagged proteins or other weakly adsorbing cellular debris.
-
Elute proteins from the column with elution buffer.
The hLa protein usually elutes with 100-250 mM imidazole. For manual elution, add elution buffers in 1-3 CE increments. Recommended elution buffers are: 50, 100, 250, and 500 mM imidazole final concentration. Collect 1-2 mL aliquots by gravity drip. To assess the progress of protein elution, periodically measure the protein concentrations of elution fractions using the Bradford method (Bradford, 1976) or microvolume spectrophotometer (e.g., NanoDrop™).
-
Perform SDS-PAGE on eluted fractions. Stain proteins with Coomassie Brilliant Blue or a comparable protein stain.
Utilize standard SDS-PAGE methods. One can include aliquots from the previous purification steps for troubleshooting if indicated.
-
Pool pure fractions containing the recombinant La antigen reporter protein.
The desired product should be approximately 32 kDa (Figure 3). Purity can be estimated by gel electrophoresis and other supporting methods (Mohan, 1992; Rhodes and Laue, 2009). The minimum purity should be 90%. If there are concerns about purity, additional purification strategies such as gel filtration may be useful.
-
Transfer the pooled protein to dialysis tubing (SnakeSkin™).
Other types of dialysis tubing are compatible, provided the MW cut-off (MWCO) is compatible with product size. We prefer Snakeskin™ due to ease of use. For smaller volume dialyses (less than 3 mL), Biovision DiaEasy™25 kDa MWCO (Cat # K1015) has also been validated.
Transfer the dialysis tubing containing reduced La antigen to 4 L pre-chilled purification dialysis buffer (25 mM sodium phosphate, pH 7.0, 5 mM DTT).
-
Dialyze the sample at 4 °C for 8-16 h. Gently stir the buffer while performing the dialysis.
This dialysis step reduces the La antigen reporter protein and removes residual imidazole from the protein purification process.
Exchange the purification dialysis buffer with 4 L fresh pre-chilled purification dialysis buffer. Continue the dialysis for 8-16 h.
Exchange the purification dialysis buffer with 4 L fresh pre-chilled purification dialysis buffer. Continue the dialysis for 8-16 h.
-
Remove the purified La antigen reporter protein from the dialysis tubing. Measure the protein concentrations of post-dialysis samples.
Protein quantification can be performed using the Bradford method or microvolume spectrophotometry. Concentrate the sample by protein concentrator ultrafiltration centrifugation device (10 kDa MWCO suggested) if necessary (we store protein at approximately 5 mg/mL protein concentrations). Determine the overall yield. The expected yield should be greater than 20 mg purified protein per L bacterial culture.
-
Flash-freeze 1 mL aliquots in liquid N2.
Use standard microcentrifuge tubes for aliquots. The protein aliquots can be stored at -80 °C until their intended use. The addition of cryoprotectants like glycerol is not required for storage.
Figure 3. Representative human La antigen purification.
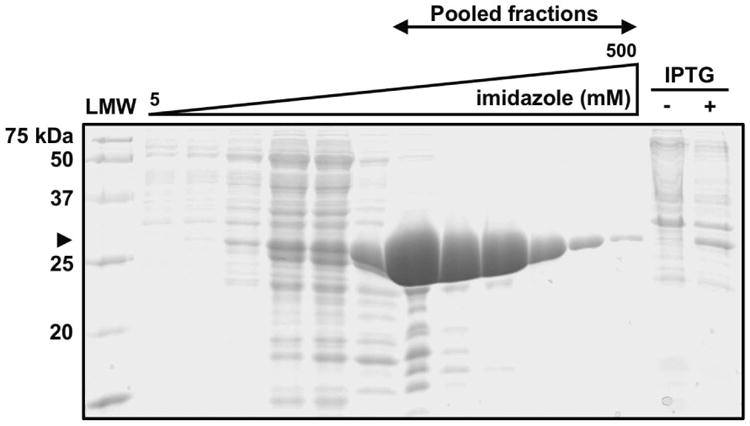
The 6xHis-tagged human La antigen (approximately 32 kDa, arrowhead) elutes from pre-charged Ni2+-beads using an imidazole gradient. Fractions can be analyzed by SDS-PAGE (shown here 15% SDS, Coomassie Brilliant Blue stain). The purest fractions containing the desired La antigen product are pooled for use in ALARM NMR. IPTG, bacterial culture aliquots before (–) and after (+) IPTG induction; LMW, low molecular weight protein ladder.
BASIC PROTOCOL 2 - ALARM NMR counter-screen
ALARM NMR is a protein-based 2D NMR experiment to identify thiol-reactive test compounds, which are not uncommon in HTS. Some commonly used molecular probes are also thiol-reactive (Baell, 2010; Baell et al., 2013; Dahlin et al., 2015c). The basic premise is that test compounds identified as modulators of unrelated proteins in HTS, or those compounds touted as specific chemical probes, should not react with the La antigen reporter protein. If covalent modification occurs, nonspecific protein and biological thiol reactivity may at least be partially responsible for the original bioactivity.
The assay utilizes a truncated human La antigen labeled with 13C at the δ-methyl groups of leucine, δ-methyl group of isoleucine, and γ-methyl groups of valine. Methyl groups can serve as excellent nuclear probes for molecular structure and dynamics (Gardner and Kay, 1997). In the case of the 13C-methyl-labeled La antigen, the protein conformation can then be sampled using either heteronuclear single quantum coherence (HSQC) or heteronuclear multiple quantum coherence (HMQC) NMR techniques (Figure 4) (Dahlin et al., 2015c; Huth et al., 2005). Notably, conventional HMQC pulse sequences of methyl groups take advantage of transverse relaxation-optimized spectroscopy (TROSY) for enhanced spectral sensitivity and resolution, though this effect is most pronounced at higher magnetic fields (Pervushin et al., 1997; Tugarinov et al., 2003; Tugarinov and Kay, 2003; Tugarinov and Kay, 2004). Note that SOFAST versions of HMQC are able to increase signal sensitivity up to 50%, making data acquisition even faster (Rossi et al., 2016; Schanda et al., 2005).
Figure 4. Representative ALARM NMR [1H-13C]-HMQC spectra with DMSO vehicle control.
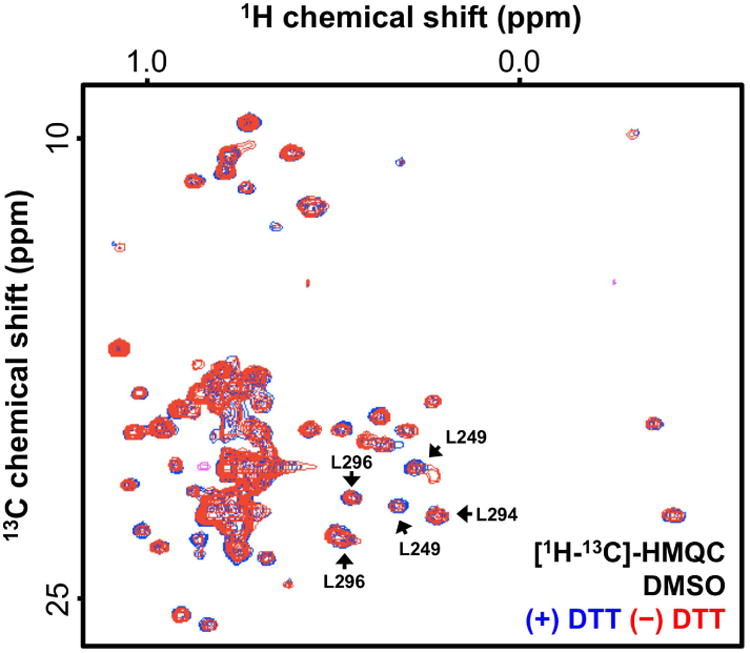
The observed La antigen conformation is independent of excess DTT (compare blue and red spectra). ALARM NMR analysis typically focuses on the spectral region for residues L249, L294, and L296. Z-axis, relative units.
Performing ALARM NMR is relatively straightforward. For a given compound, parallel samples are tested with or without excess DTT to control for nonreactive, nonspecific protein perturbation. Electrophilic test compounds such as CPM can react with C232 and/or C245 in the absence of excess DTT. This covalent modification produces characteristic chemical shifts and/or signal attenuation at nearby 13C-labeled leucine residues, most prominently L249, L294, and L296. This effect is significantly, if not completely, attenuated by including excess DTT, while nonreactive test compounds such as fluconazole will not perturb the La antigen conformation regardless of DTT. Additionally, nonspecific protein conformation disruptors will perturb the La antigen conformation independent of excess DTT.
This Basic Protocol 2 details how to perform an ALARM NMR experiment with the purified, 13C-labeled La antigen reporter protein synthesized from Basic Protocol 1. This includes the preparation of a reduced La antigen reporter protein, the reaction of control compounds with this protein reporter, the acquisition of NMR spectra, and data processing and analysis.
Materials
ALARM NMR protein (from Support Protocol 1)
SnakeSkin™ dialysis membrane, 10 kDa MWCO (ThermoFisher, Cat # 68305)
Regeneration dialysis buffer (see recipe)
Nitrogen (N2) gas
Bradford reagent or microvolume spectrophotometer (e.g., ThermoFisher NanoDrop™)
Reaction buffers (see recipes)
1.7 mm SampleJet NMR tubes (Bruker, Cat # Z106462)
384-well nonsterile, nonbinding surface, non-tissue culture treated microplate (e.g., Corning # 3640)
Plate seals (e.g., Corning # 07-200-683)
DMSO
CPM (Sigma-Aldrich, Cat # C1484) positive reactive control compound
2-Chloro-1,4-naphthoquinone (Santa Cruz Biotechnology, Cat # sc-209130) positive reactive control compound
Fluconazole (Sigma-Aldrich, Cat # PHR1160) negative reactive control compound
Additional test compounds (as 10 mM DMSO stocks)
D2O (Cambridge Isotope Labs)
Heating block
Refrigerator with N2 line
Stir plate with stir bar
Tabletop centrifuge (e.g., Beckman-Coulter Allegra X-12)
Oven incubator
NMR spectrometer (recommended 400 MHz or higher fields; e.g., Bruker Avance 700 MHz spectrometer)
Room temperature probe or CryoProbe™ (preferred)
NMR autosampler (preferred)
NMR analysis software (e.g., Bruker TopSpin®)
Note: additional required reagents are listed under recipes.
Reduction of ALARM NMR protein reporter
Thaw 2 mL of the La antigen reporter protein stock solution (from Basic Protocol 1) on ice.
-
Add 40 μL of 1 M DTT stock solution to the thawed La antigen stock solution to yield approximately 20 mM DTT final concentration. Incubate the solution at 37 °C for 1 h in a heating block.
This step reduces any oxidized cysteine thiols on the La antigen reporter protein, making them available to react with test compounds. Prepare the DTT solution fresh, as DTT has a limited half-life in aqueous solutions.
-
Transfer all of the reduced La antigen stock solution to dialysis tubing (SnakeSkin™).
Other dialysis tubes should also work in this step. Ensure dialysis tubing MWCO is appropriate for the La antigen.
Transfer the dialysis tubing containing reduced La antigen reporter protein to 4 L pre-chilled regeneration dialysis buffer.
-
Dialyze the sample at 4 °C for 8-16 h with constant gentle bubbling of N2 gas and gentle stirring while performing the dialysis.
The N2 bubbling (degassing) helps reduce oxidation of protein cysteines during the prolonged dialysis process. This setup requires a stir plate inside a refrigerator along with a nitrogen line.
Exchange the regeneration dialysis buffer with fresh 4 L pre-chilled dialysis buffer. Continue the dialysis with N2 bubbling for 8-16 h.
Exchange the regeneration dialysis buffer with fresh 4 L pre-chilled dialysis buffer. Continue the dialysis with N2 bubbling for 8-16 h.
-
Remove the reduced La antigen reporter protein from the dialysis tubing. Measure the protein concentrations of post-dialysis samples.
Concentrate the samples with protein concentrator ultrafiltration centrifugation device (10 kDa MWCO suggested) as needed. Expect minimal protein loss (typically less than 5%). The protein concentration should be 250-500 μM so that it can be diluted in reaction buffer. Keep the protein solutions on ice. The reduced La antigen reporter protein is remarkably stable, and can be stored at 4 °C for up to 1 week in this buffer.
Incubation of test compounds and ALARM NMR protein
-
9
Prepare/thaw the test compound stock solutions.
Typically, compounds are derived from 10 mM stocks in neat DMSO. Alternatively, compounds can be tested as mixtures, and ALARM NMR-positive results followed-up with testing individual compounds (Huth et al., 2005).
-
10Prepare the reaction solutions (default final concentrations for ALARM NMR are 50 μM protein in 25 mM sodium phosphate buffer, pH 7.0, 10% D2O, ± 20 mM DTT, and later 400 μM test compound delivered from DMSO stocks).
- Prepare the DTT reaction solution: To a microcentrifuge tube, add 100 μL D2O, 95 μL 10× ALARM NMR buffer, 735 μL H2O, 50 μL protein stock solution, 20 μL DTT.
-
Prepare the DTT-free reaction solution: To a microcentrifuge tube, add 100 μL D2O, 95 μL 10× ALARM NMR buffer, 755 μL H2O, 50 μL protein stock solution.Keep the solutions on ice. Remember to take into account that the protein solution already contains sodium phosphate. For this example, assume a 500 μM protein stock solution. The default reaction buffer pH is 7.0 for general applicability, and the La antigen reporter protein is grossly stable between pH 6.0 and 9.5 (Huth et al., 2005). In principle, the reaction pH can be modified, though users should note protein reactivity may be pH-sensitive due to changes in thiolate anion concentration.
-
11
Transfer 2 μL compounds and controls to reaction vessels or well using a liquid dispensing device (e.g., calibrated pipette, Echo acoustic dispenser).
We typically perform reactions at 50 μL volumes in standard 384-well HTS microplates. For each test compound, remember to set up two independent reactions (for ± DTT reactions). Note the reaction volume will ultimately depend on the size of the NMR tube. Conventional NMR tubes (3, 5 mm diameters) will require larger reaction volumes.
-
12
Add 48 μL ALARM NMR reaction solutions to the appropriate reaction vessel or well. Gently pipette the reaction mixture up and down several times.
For example, using our standard testing conditions, add 48 μL reaction solution (with DTT) to one compound sample, then add 48 μL reaction solution (without DTT) to the other compound sample.
-
13
Briefly centrifuge the samples at 4 °C for 5 min using tabletop centrifuge. Check for gross compound precipitation.
If using a microplate as a reaction vessel, seal it before centrifugation.
-
14
Incubate the reaction samples at 37 °C for 1 h in an oven incubator.
Protect from light in case of photosensitive compounds.
-
15
Incubate the reaction samples at RT for 16 h.
Consider protecting from light in case of photosensitive compounds.
-
16
Transfer the reaction samples to NMR tubes using a pipette or liquid handling system (e.g., Bruker Gilson 215). Label and seal the tubes with caps.
Collection of NMR spectra
-
17
Place the reaction samples into NMR spectrometer or autosampler (if available).
Samples can be stored at from 4-25 °C while in queue, depending on the expected length of sample collection.
-
18
Set-up the NMR instrument parameters: 1 s repetition delay, 310 K, 16 scans with 2048 complex points in F2 (1H), and 80 points in F1 (13C) dimensions using either SOFAST HMQC (preferred, pulse sequence: IBS_SOFAST of MNMR Center) or methyl-TROSY with 3919 for water suppression (pulse sequence hmqcgpph19.x of MNMR Center) or 1H-13C HSQC with watergate (pulse sequence: Chsqc of MNMR Center) or sensitivity enhancement (pulse sequence: hsqcetgpsisp2.2 of MNMR Center).
-
19
Collect the 1H spectra, then the [1H-13C]-HMQC spectra of samples using an NMR spectrometer. NMR spectra of multiple samples can be automatically acquired with Bruker IconNMR™. The automated procedure inserts and ejects each sample automatically, allows auto-tuning and matching (command: atma) of the 1H channel. The software can also perform auto-lock (command: lock), gradient shimming (command: topshim), and adjusting receiver gain (command: rga) prior to starting data acquisition (command: zg). This procedure (either automated or manually entered) is repeated for all queued experiments.
We use a Bruker Avance 700 MHz spectrometer equipped with a CryoProbe™ 1.7 mm TCI probe of Z-gradient. Lower field instruments (e.g., 400 MHz) and/or alternative spectrometer models should also be sufficient for this assay. A CryoProbe™ is recommended to increase instrument sensitivity. As an additional quality control, the La antigen conformation can be verified by comparing to reference spectra (BMRB ID: 5235) (Jacks et al., 2002).
-
20
Program the autosampler and collect the NMR spectra for remaining samples.
Using the above set-up, each sample takes approximately 25 min to acquire data. For a given test compound, we typically collect the DTT-containing sample followed by the DTT-free sample. Vehicle controls, then positive controls, then negative controls are tested first, followed by test compounds. To control for time-dependent effects, additional controls can be interspersed in the queue or re-scanned throughout the collection process.
Data analysis
-
21
Load the NMR spectra into TopSpin® NMR processing software.
Analyze vehicle controls first, followed by positive and negative controls, followed by test compounds. Data can be processed by the NMR manufacturer software Topspin®, as well as other NMR processing software such as NMRPIPE and NMRGLUE (nmrbox.org/registry).
-
22
Process the NMR spectra.
This processing can be done manually or automatically, depending on user preferences and the particular analysis software. The users should acquire 1D 1H and 13C spectra of DSS (4,4-dimethyl-4-silapentane-1-sulfonic acid) to be used as references for 1H and 13C 0.0 ppm.
-
23
Verify the NMR spectra quality.
Analyze overall spectrum quality, then focus analysis the 13C-labeled methyl region (approximately 0-0.6 ppm in F2, 20-24 ppm in F1). Data can be compared to reference spectra (Huth et al., 2005; Huth et al., 2007). Peak intensities and chemical shifts at L249, L294, and L296 are highly reproducible. Reject and troubleshoot samples with poor quality.
-
24
Adjust the peak contour scaling as necessary.
-
25
Repeat the analysis for each sample.
-
26
Overlay each spectrum (± DTT) for control and test compounds with their corresponding DMSO controls.
-
27
Qualitatively score each control and test compound compared to corresponding vehicle controls.
Determine the magnitude of spectral perturbation at L249, L294, and L296 relative to vehicle controls for the samples with and without DTT present. Score samples with major perturbations (greater than approximately 30% reductions in intensity) as positive. Also note any peak chemical shifts or appearance of new peaks. Score samples with significant changes in chemical shifts as positive (n.b. exact ppm will vary depending on dimension, instrumentation, experimental settings, protein, etc.). Score samples with misfolded protein (i.e., random coils only when entire spectrum analyzed) as positive. Score samples with mild perturbations (between approximately 10-30% reduction in intensity) as equivocal. Score samples with minor perturbations (less than 10% reduction in intensity) as negative.
-
28
Interpret the readouts for each compound.
Grade samples with negative scores independent of DTT as ALARM NMR-negative. Grade samples with positive scores dependent on DTT as ALARM NMR-positive (thiol-reactive). Grade samples with positive scores independent of DTT as ALARM NMR-positive (nonspecific perturbation).
Reagents and Solutions
Use deionized, distilled water in all recipes and protocol steps.
Culture Solutions
Glucose stock solution (20% w/v; to make 1 L)
200 g glucose (natural abundance)
Dissolve glucose in 800 mL H2O with vigorous stirring. Add additional H2O to reach 1 L final volume. Autoclave the resulting solution for use in bacterial cultures. Keep the solution sterile and store at room temperature for up to 1 yr.
Thiamine stock solution (10 mg/mL; to make 10 mL)
100 mg thiamine HCl
Dissolve thiamine in 10 mL H2O. Sterile filter the resulting solution through a 0.22 μm filter for use in bacterial cultures. Keep the solution sterile and at -20 °C for up to 1 yr.
Biotin stock solution (10 mg/mL; to make 10 mL)
100 mg biotin
Dissolve biotin in 10 mL sterile H2O. Note this concentration is above solubility limit, so do not sterile-filter. Keep the solution sterile and store at -20 °C for up to 1 yr.
CaCl2 stock solution (0.1 M; to make 100 mL)
1.11 g CaCl2 • 2 H2O
Dissolve CaCl2 in 80 mL H2O with vigorous stirring. Add additional H2O to reach 100 mL final volume. Autoclave the resulting solution for use in bacterial cultures. Keep the solution sterile and store at room temperature for up to 1 yr.
MgSO4 stock solution (1 M; to make 25 mL)
6.15 g MgSO4 • 7 H2O
Dissolve MgSO4 in 20 mL H2O with vigorous stirring. Add additional H2O to reach 25 mL final volume. Autoclave the resulting solution for use in bacterial cultures. Keep the solution sterile and store at room temperature for up to 1 yr.
Autoclaved H2O
Autoclave H2O in 1 L aliquots for use in bacterial cultures. Keep the solution sterile and store at room temperature for up to 6 mo.
IPTG stock solution (1 M; to make 10 mL)
2.38 g IPTG
Dissolve IPTG (MW = 238) in 10 mL H2O. Sterile filter the resulting solution through a 0.22 μm filter for use in bacterial cultures. Prepare fresh.
LB media (to make 1 L)
10 g tryptone
5 g yeast extract
10 g NaCl
Mix 10 g tryptone, 5 g yeast extract, 10 g NaCl in 800 mL H2O
Mix tryptone, yeast extract, NaCl in 800 mL H2O. Stir the resulting solution vigorously with stir bar at room temperature then dilute the solution with additional H2O until 1 L final volume. Stir for additional 5 min. Autoclave the solution for use in bacterial cultures. Keep the medium sterile and store at room temperature for up to 1 mo.
10× M9 salts (to make 1 L)
67.8 g Na2HPO4 (anhydrous)
30 g KH2PO4
5 g NaCl
Mix Na2HPO4, KH2PO4, NaCl in 800 mL H2O. Stir the resulting solution vigorously with a stir bar at room temperature then the solution dilute with additional H2O until 1 L final volume. Adjust to pH 7.4. Autoclave the solution for use in bacterial cultures. Keep the solution sterile and store at room temperature for up to 1 mo.
M9 media (to make 1 L)
100 mL 10× M9 stock solution
1 g NH4Cl (dissolved in 10 mL H2O and sterile-filtered)
15 mL glucose stock solution
1 mL CaCl2 stock solution
1 mL MgSO4 stock solution
1 mL thiamine stock solution
1 mL biotin stock solution
Add M9, NH4Cl, glucose, CaCl2, MgSO4, thiamine, biotin stock solutions to a sterile 2 L flask. Add autoclaved H2O to the flask to make 1 L media. Note: M9 media is used to minimize the amount of unlabeled amino acids incorporated into the recombinant protein. Make additional 1 L solutions as necessary for culture scale.
Kanamycin stock solution (1000×, 50 mg/mL; to make 10 mL)
500 mg kanamycin disulfate
Dissolve kanamycin disulfate in 10 mL H2O. Sterile filter the resulting solution through a 0.22 μm filter for use in bacterial cultures. Store the solution at -20 °C for up to 1 yr.
Chloramphenicol stock solution (1000X, 34 mg/mL; to make 10 mL)
340 mg chloramphenicol
Dissolve chloramphenicol in 10 mL ethanol. Sterile filter the resulting solution through a 0.22 μm filter for use in bacterial cultures. Store the solution at -20 °C for up to 1 yr.
Purification Solutions
BME stock solution (5 M; to make 10 mL)
3.50 mL BME (14.3 M pure solution)
Dissolve BME in 6.50 mL H2O. Handle the stock solution in fume hood. Keep the resulting solution frozen at -20 °C when not in use.
Sodium phosphate buffer stock solution (1 M, pH 7.0; to make 1 L)
138 g NaH2PO4•H2O
142 g Na2HPO4
Dissolve NaH2PO4•H2O (monobasic; MW = 138) in 800 mL H2O. Stir the resulting solution vigorously with a stir bar at room temperature then dilute the solution with additional H2O until 1 L final volume. Stir the solution for an additional 5 min. Dissolve Na2HPO4 (dibasic; MW 142) in H2O for 800 mL. Stir the resulting solution vigorously with a stir bar at room temperature then dilute the solution with additional H2O until 1 L final volume. Stir the solution for an additional 5 min. Combine 390 mL 1 M NaH2PO4 solution and 610 mL 1 M Na2HPO4 solution. Measure the buffer pH, adjusting as needed with 1 M solutions of NaH2PO4 or Na2HPO4. Store the buffer at room temperature for up to 1 yr. Occasionally, concentrated sodium phosphate crystallizes during storage, but this can be reversed by gentle heating in water bath.
20× Tris HCl (1 M, pH 7.6; to make 1 L)
121 g tris base
Dissolve tris base (MW = 121) in 800 mL H2O. Stir the resulting solution vigorously with a stir bar at room temperature then dilute with an additional H2O until 1 L final volume. Stir the solution for an additional 5 min. Adjust to pH 7.6 using concentrated HCl. Allow several hours for the temperature to equilibrate when determining the final pH. Store the solution at room temperature for up to 1 yr.
10× ALARM NMR buffer (250 mM sodium phosphate, pH 7.0; to make 1 L)
250 mL sodium phosphate buffer stock solution
Dissolve the sodium phosphate buffer stock solution in H2O for 1 L final volume. Store the resulting buffer at room temperature for up to 1 yr.
DTT stock solution (1 M; to make 5 mL)
770 mg DTT
Dissolve DTT (MW = 154) in 5 mL H2O. Prepare fresh; keep the resulting stock solution on ice or frozen at -20 °C when not in direct use.
Imidazole stock solution (2 M; to make 1 L)
136 g imidazole
Dissolve imidazole (MW = 68) in 800 mL H2O. Stir the resulting solution vigorously with a stir bar at room temperature, then dilute the solution with additional H2O until 1 L final volume. Stir the solution for an additional 5 min. Protect the solution from from light. Store at room temperature for up to 1 yr.
NaCl stock solution (3 M; to make 1 L)
174 g NaCl
Dissolve NaCl (MW = 58) in 800 mL H2O. Stir the resulting solution vigorously with a stir bar at room temperature then dilute the solution with additional H2O until 1 L final volume. Stir the solution for an additional 5 min. Store the solution at room temperature for up to 1 yr.
MgCl2 stock solution (1 M; to make 25 mL)
2.35 g MgCl2
Dissolve MgCl2 (MW 95.2) in 20 mL H2O with vigorous stirring. Add additional H2O to reach 25 mL final volume. Store the solution at room temperature for up to 1 yr.
Lysis buffer (50 mM Tris, pH 7.6; 300 mM NaCl, 10% glycerol, 5 mM β-mercaptoethanol (BME)
5 mM imidazole, 2 mM MgCl2, protease inhibitor cocktail, benzonase (25 U/mL lysate), 1 mg/mL lysozyme (include if sonicating); to make 100 mL)
5 mL tris stock solution
10 mL NaCl stock solution
10 mL glycerol
100 μL BME stock solution
250 μL imidazole stock solution
200 μL MgCl2 stock solution
Protease inhibitors (EDTA-free)
10 μL benzonase stock solution
100 mg bacterial lysozyme
Dissolve tris, NaCl, glycerol, BME, imidazole, MgCl2, protease inhibitors, and benzonase stock solutions in 50 mL H2O. Add lysozyme if performing cell lysis by sonication. Stir the resulting solution vigorously with a stir bar at room temperature, then dilute the solution with additional H2O until 100 mL final volume. Stir the solution for an additional 5 min. Prepare the solution fresh. Use commercial protease inhibitor cocktail tablets or concentrate (e.g., Pierce Protease Inhibitor tablets or Fisher Halt Protease Inhibitor cocktail), EDTA/chelator-free formulations to avoid interference with metal-based purification.
Wash buffer (50 mM Tris pH 7.6, 300 mM NaCl, 10% glycerol, 5 mM BME, imidazole 5 mM; to make 500 mL)
25 mL Tris stock solution
50 mL NaCl stock solution
50 mL glycerol
500 μL BME stock solution
1250 μL imidazole stock solution
Dissolve tris, NaCl, glycerol, BME, and imidazole stock solutions in 200 mL H2O. Stir the resulting solution vigorously with a stir bar at room temperature, then dilute the solution with additional H2O until 500 mL final volume. Stir the solution for additional 5 min. Prepare the solution fresh.
Elution solutions (to make 50 mL fractions)
Imidazole stock solution
BME stock solution
Dissolve the indicated imidazole and BME stock solutions in wash buffer up to 50 mL. Prepare the solutions fresh.
Elution solution 1 (5 mM imidazole): 50 μL BME stock solution. Elution solution 2 (50 mM imidazole): 1.25 mL imidazole stock solution, 50 μL BME stock solution. Elution solution 3 (125 mM imidazole): 3.125 mL imidazole stock solution, 50 μL BME stock solution. Elution solution 4 (250 mM imidazole): 6.25 mL imidazole stock solution, 50 μL BME stock solution. Elution solution 5 (500 mM imidazole): 12.5 mL imidazole stock solution, 50 μL BME stock solution.
Purification dialysis buffer (25 mM sodium phosphate, pH 7.0, 5 mM DTT; to make 4 L)
100 mL sodium phosphate stock solution
20 mL DTT stock solution
Dissolve sodium phosphate buffer stock solution in 3 L H2O final volume. Stir the resulting solution vigorously with a stir bar at room temperature, then dilute the solution with H2O until 4 L final volume. Stir the solution for an additional 5 min. Cool to 4 °C prior to use. Add fresh DTT stock solution immediately prior to beginning dialysis. Note the protocol dialysis requires three batches of the purification dialysis buffer.
Assay Solutions
Sodium phosphate buffer (1 M, pH 7.0; for 1 L; see above)
DTT stock solution (1 M; 5 mL; see above)
Regeneration dialysis buffer (25 mM sodium phosphate, pH 7.0, no DTT)
100 mL sodium phosphate stock solution
Dissolve sodium phosphate buffer stock solution in 3 L H2O final volume. Stir the resulting solution vigorously with a stir bar at room temperature, then dilute the solution with H2O until 4 L final volume. Stir the solution for an additional 5 min. Cool to 4 °C prior to use. Note the protocol dialysis requires three batches of the regeneration dialysis buffer.
Commentary
Background Information
Nonspecific thiol-reactive compounds represent a significant source of wasted resources in early drug discovery and chemical biology (Arrowsmith et al., 2015; Jadhav et al., 2010; Thorne et al., 2010). Illustrating the problem of nonspecific thiol reactivity, several HTS campaigns at a major pharmaceutical company were plagued by significant levels of ALARM NMR-positive hits, some approaching 50% (Huth et al., 2005).
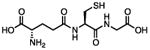
There are several assays for identifying thiol-reactive screening compounds, most of which rely on non-proteinaceous, thiol-containing reporters like GSH, coenzyme A (CoA), and cysteamine. Small-molecule thiol-based reporters have distinct advantages and disadvantages (Table 1). They are relatively inexpensive and easy to handle, and these reactions can be performed under assay-like or physiologically relevant conditions. However, a potential disadvantage of these reporters is that they may not fully approximate protein microenvironments (Wilson et al., 1980). To this point, we and others have encountered compounds that are negative by conventional GSH reactivity screens, but ALARM NMR-positive (Huth et al., 2005) (JL Dahlin and KM Nelson et al., in review). Lower-throughput methods include incubating a test compound with a specific protein target, and performing some type of protein MS to directly observe compound-protein adducts (Dahlin et al., 2015c). This approach may be highly useful for specific applications, but depends on the suitability of the target for proteomic analysis, and may be difficult to achieve large sample throughput. A “middle-ground” approach can utilize peptide-based reporters (Wei et al., 2014). Notably, most of these approaches, including ALARM NMR, do not lend themselves to identifying other sources of biological reactivity such as at lysine residues (Baeza et al., 2015).
Table 1. Summary of thiol-based reactivity reporters.
Adapted from NIH Assay Guidance Manual under Creative Commons AttrCoulter Avanti J-26XPibution-NonCommercial-ShareAlike 3.0 Unported license (CC BY-NC-SA 3.0) (Dahlin et al., 2015a).
| Reporter | Chemical structure | Potential advantages and formats | Other comments and potential disadvantages | References |
|---|---|---|---|---|
| Glutathione (GSH) |
|
|
|
(Dahlin et al., 2015c) |
| Coenzyme A (CoA) |
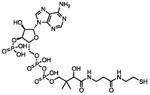
|
|
|
(Dahlin et al., 2015c) |
| Cysteamine |
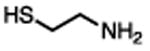
|
|
|
(Arsovska et al., 2014; Olson et al., 2013) |
| Dithiothreotol (DTT) |
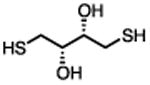
|
|
|
(Bowers et al., 2010) |
| L-cysteine |
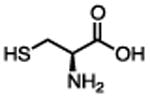
|
|
|
|
| MTSI |
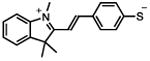
|
|
|
(McCallum et al., 2013) |
| Peptides | – |
|
|
(Wei et al., 2014) |
| Proteins | – |
|
|
(Dahlin et al., 2015c) |
| ALARM NMR | – |
|
|
(Dahlin et al., 2015b; Dahlin et al., 2015c; Huth et al., 2005; Huth et al., 2007) |
Thiol-based reactivity assays can utilize several detection technologies, the most common being MS and fluorescence. Most MS-based techniques involve a liquid chromatography separation step monitored by UV absorbance, followed by MS detection. An advantage of MS-based techniques is the direct detection of the compound-reporter adducts, and the m/z ratio and fragmentation patterns can provide valuable insights into adduct chemical structures. However, not all adducts are amenable to ionization. Fluorescence techniques utilize either a fluorescent thiol-based reporter such as MSTI (McCallum et al., 2013) or a competition between GSH or CoA with maleimide reporters (Epps and Taylor, 2001). These assays only require standard plate readers, but are subject to various light-based interferences that require additional assay-specific counter-screens.
ALARM NMR has several distinct advantages and disadvantages (Table 1). Notably, it utilizes a protein-based thiol reporter, which may better approximate the reactivity of proteins. The NMR-based readout does not require adduct ionization like MS-based methods, and provides information about protein conformation, including the identification of nonspecific compound-protein binding. It is not subject to light-based interferences.
Additionally, ALARM NMR can be successfully adapted to MS-based detection, so-called ALARM MS (Huth et al., 2005). ALARM MS can provide structural information about the compound-protein adduct through MW shifts, and does not require 13C-labelling. However, certain adducts may not be amenable or stable to MS ionization techniques. Nonspecific, noncovalent interactions may also not be readily observable by MS.
A notable disadvantage of ALARM NMR is the need for NMR instrumentation. Another drawback is the cost of the 13C-labeled amino acid precursors, which can be partially mitigated by first optimizing in-house protein production and purification with unlabeled proteins prior to using these specialized reagents. Lastly, ALARM NMR necessitates high concentrations of protein and compound, which can be problematic for some poorly soluble compounds (they may precipitate) or samples with trace impurities (insufficient stoichiometry to affect readout).
Critical parameters
Isotope labeling
The percentage of 13C-methyl incorporated into leucine, isoleucine and valine side chains is a critical determinant of assay signal intensity. Under optimal conditions, 13C-methyl incorporation can exceed 90% (Hajduk et al., 2000). Confirmation of 13C-methyl incorporation into the La antigen reporter protein can be performed using mass spectrometry. A second option is to acquire an FID of a 2D [1H-13C]-HSQC to get 13C-related 1H signal, then acquire another FID with 13C-filtered to get 12C-related 1H signal (n.b. the length of the second pulse sequence should be similar to the first HSQC so that they have similar signal decays). Comparing the signal strength in the two spectra should estimate the ratio of labeled (13C) and unlabeled (12C) methyl groups. A third option to assess labeling efficiency is to substitute NH4Cl in the M9 media with 15NH4Cl during the protein production, followed by comparing peak intensities obtained from 1H-13C and 1H-15N experiments (Dahlin et al., 2015c; Hajduk et al., 2000).
Reaction conditions
Optimized reaction conditions are critical determinants of assay performance. In principle, longer reaction times and higher reaction temperatures may increase the detection of thiol-reactive test compounds. However, such adjustments may denature the protein or not be physiologically or assay relevant. The amount of test compound can also be adjusted. Our default assay settings utilize a 8:1 compound:protein ratio, so this may serve as a useful starting point for compound or protein titrations. Based on titrations of a prototypical thiol-reactive compound, approximately 50% of the total cysteines in an ALARM NMR solution must be covalently modified to trigger an ALARM NMR-positive readout (JL Dahlin and MA Walters, forthcoming results).
NMR settings
Optimized NMR settings (including pulse sequences, solvent suppression methods, shimming, collection time, and spectral width) are critical for a successful experiment. Both HSQC and HMQC pulse sequences can be used in ALAMR NMR, though the optimal pulse sequence will depend on the specific instrumentation. Parameters such as the length of sample acquisition can be adjusted depending on the required resolution and sensitivity.
Level of expertise needed to implement the protocol
The protocol can be performed by graduate students, doctoral-level researchers, as well as advanced research assistants and technicians. Prior experience in protein production is advised for ALARM NMR production and purification. Prior experience in small-molecule or protein NMR is advised. Support personal for troubleshooting instrumentation is highly advised (often through an institutional core facility).
Troubleshooting
There are several commonly encountered problems with the protein production and purification, compound-protein reactions, data collection, and data analysis procedures described in this article. Possible causes and suggested ways to overcome or avoid these problems are provided (Table 2).
Table 2. Troubleshooting commonly encountered problems.
| Problem | Possible cause | Potential solution |
|---|---|---|
| Low protein yield |
|
|
| Low protein purity |
|
|
| Poor signal |
|
|
| Positive control inactive |
|
|
| Negative control active |
|
|
Statistical considerations
We and others utilize ALARM NMR as a qualitative assay (i.e., positive, negative, equivocal, DTT-dependent, DTT-independent), which obviates the need for complex statistical analyses (Dahlin et al., 2015b; Dahlin et al., 2015c; Huth et al., 2005; Huth et al., 2007). In our experience, the assay readout is precise, with similar results for multiple positive and negative control compounds across several independent experiments and several batches of protein. At our institute, test compounds are assayed with and without DTT as duplicate technical replicates. Discordant results between replicates usually followed-up with additional replicate(s) and orthogonal counter-screens such as a UPLC-MS GSH assay for thiol reactivity and/or an AmpC β-lactamase activity for aggregation (Dahlin et al., 2015b; Dahlin et al., 2015c). Future modifications such as the inclusion of [1H-13C]-internal standards may enable semi-quantitative or even quantitative readouts, though assay precision would be limited by signal resolution.
Interpreting the results
The quality of the La antigen reporter protein in both the presence and absence of DTT should be assessed first in any ALARM NMR experiment while including vehicle control (e.g., DMSO in most cases). Representative NMR spectra for the La antigen reporter protein are provided (Figure 4). Overlays of DTT-present and DTT-free samples should show minimal differences in peak intensity and chemical shifts.
Thiol-reactive test compounds such as CPM should produce characteristic peak shifts and decreases in signal intensity in the absence of DTT, whereas minimal perturbations should be observed in the presence of excess DTT when normalized to DMSO vehicle controls (Figure 5). With particularly reactive compounds such as CPM, the difference between the DTT and DTT-free spectra can be highly pronounced. As another example of thiol-reactive chemical matter, consider compound 3a, which contains a well-characterized, nonspecific thiol-reactive 1,2,4-thiadiazole core that is generally unsuitable for an HTS lead or a chemical probe. This compound shows reproducible DTT-dependent activity versus the La antigen reporter protein (Figure 5) (Dahlin et al., 2015c).
Figure 5. Representative ALARM NMR results.
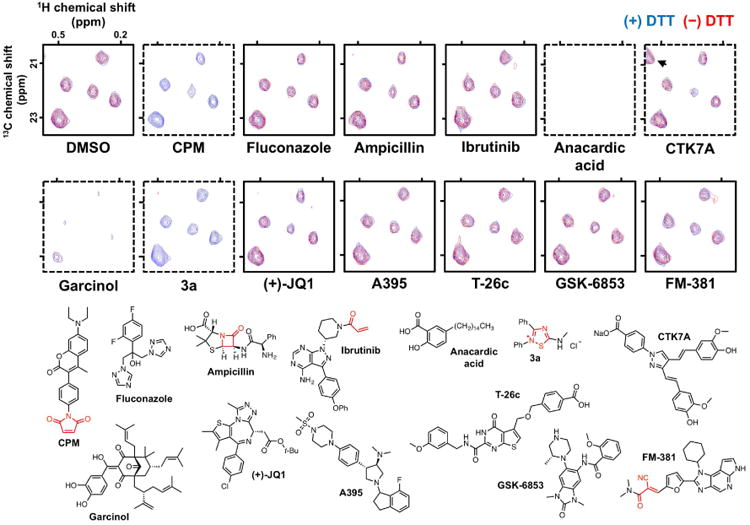
Shown are 2D [1H-13C]-HMQC spectral overlays of selected 13C-labeled methyl groups of the La antigen performed in the presence (blue) and absence (red) of excess DTT. DMSO is used as a solvent control. CPM is used as a thiol-reactive positive control compound. Fluconazole is used as a nonreactive and non-aggregating control compound. Ampicillin is used as a nonreactive control compound. Ibrutinib is a targeted covalent inhibitor of BTK. Anacardic acid, CTK7A, and garcinol are reported HAT inhibitors that aggregate, while compound 3a is a promiscuous thiol-reactive 1,2,4-thiadiazole. Note CTK7A generates a new peak (arrow) independent of DTT. (+)-JQ1, A395, T-26c, GSK-6853, and FM-381 are considered high-quality chemical probes. Bottom panel, chemical structures of test compounds with key reactive chemotypes highlighted in red. Spectra from test compounds were normalized to DMSO control. Dashed borders denote compounds that would be flagged as ALARM NMR-positive. Z-axis, relative units.
Occasionally, peak perturbations may occur at different rates. The likely explanation is that the C245 residue appears to be more reactive than C232, and chemical shifts nearest C245 (i.e., L249) may be more pronounced at lower compound concentrations (Huth et al., 2005).
ALARM NMR can also detect non-thiol-reactive protein binders, many of which are aggregators. These types of compounds form self-aggregates in common biological buffers and will appear to modulate the La antigen reporter protein conformation by nonspecific compound-protein interactions (McGovern et al., 2003). Nonreactive but promiscuous, nonspecific-binding compounds will perturb the La antigen reporter protein conformation independent of DTT. As an example, consider two reported HAT inhibitors anacardic acid and garcinol (Balasubramanyam et al., 2004; Balasubramanyam et al., 2003). Both compounds exhibit bioassay promiscuity and show detergent-sensitive activity in an AmpC aggregation counter-screen (JL Dahlin & KM Nelson et al., in review). Both compounds grossly perturb the La antigen reporter protein conformation independent of DTT (Figure 5, compare blue and red contours and also peak intensities to DMSO spectra). Rarely, some nonreactive, aggregating compounds like CTK7A can produce new spectral peaks independent of DTT (Figure 5) (Arif et al., 2010) (JL Dahlin & KM Nelson et al., in review).
Non-reactive and non-aggregating compounds such as fluconazole should produce similar readouts to the DMSO-only controls, with minimal peak shifts or changes in signal intensity (Figure 5). It is useful to include such inactive reference compounds to control for non-compound-mediated reporter effects such as suboptimal assay conditions (i.e., overly harsh reaction or storage conditions).
An uncommon result is a test compound that perturbs the La antigen conformation (i.e., peak shifts and line broadening) only in the presence of DTT. This readout pattern is strongly suggestive of redox-active compounds can produce H2O2 in situ and oxidize cysteine thiols in the presence of strong reducing agents like DTT (Johnston et al., 2008; Soares et al., 2010).
The ALARM NMR readout should be interpreted based on the chemical structure of the test compound and its experimental context. For example, certain targeted covalent modifiers have the potential to be flagged as thiol-reactive by ALARM NMR (Singh et al., 2011). But interestingly, some targeted covalent modulators are actually ALARM NMR-negative, suggesting they have more selective binding and reactivity profiles. For example, the β-lactam ampicillin and other penicillins, which covalently bind bacterial serine transpeptidases, does not react with the La antigen thiols (Figure 5) (Huth et al., 2007). More reactive β-lactam drugs, notably several cephalosporins, are ALARM NMR-positive (Huth et al., 2005).
Targeted covalent modulators, which often feature weakly reactive warheads, do not readily react with the La antigen reporter protein thiols. Ibrutinib, which contains a relatively weakly-reactive acrylamide warhead that covalently binds Bruton's tyrosine kinase C481, and FM-381, which contains a cyano-acrylamide warhead that inhibits JAK3 by reversible covalent binding to C909, do not react with the La antigen reporter protein (Figure 5) (Forster et al., 2016; Honigberg et al., 2010).
Of relevance to chemical biologists, high-quality (i.e., potent, selective) chemical probes should in principle be ALARM NMR-negative. For example, the chemical probes (+)-JQ1 (BRD4), A395 (EED), T-26c (MMP-13), and GSK-6853 (BRPF1) are all ALARM NMR-negative (Figure 5) (Bamborough et al., 2016; Filippakopoulos et al., 2010; He et al., 2017; Nara et al., 2014).
Additional representative ALARM NMR spectra are deposited at Figshare (10.6084/m9.figshare.5401837).
Compound-mediated assay interference is a significant obstacle in HTS and chemical biology. that should be considered even in counter-screens like ALARM NMR. An unlikely but theoretical source of interference are test compounds with chemical shifts in the spectrum analysis region, but this is unlikely given the natural abundance of 13C (1.1%). If interference is suspected, samples can be run in assay buffer without protein for comparison.
Screening compounds and purported tool compounds that are ALARM NMR-positive are usually deprioritized in HTS triage. Note that a negative ALARM NMR result does not conclusively exclude the possibility of a particular compound being thiol-reactive. It is possible that certain compounds may not react with either of the cysteines on the La antigen reporter protein, but may react with other thiols on different proteins due to differences in compound reactivity and target susceptibility (Backus et al., 2016; Jost et al., 2014). Similarly, with respect to aggregation, it is known (1) a given protein is not universally susceptible to all aggregators at a given concentration, and (2) a given aggregator can exhibit target-specific effects (Feng et al., 2007; Giannetti et al., 2008; McGovern et al., 2002). It is possible tractable compounds may still react with the La antigen reporter protein by ALARM NMR. However, our experience has been that ALARM NMR-positive compounds almost invariably exhibit significant assay interference and bioassay promiscuity.
If there is still concern that an ALARM NMR-negative compound may be thiol-reactive, we recommend testing by another thiol-reactivity counter-screen and appropriate mechanistic experiments (Dahlin et al., 2015a).
Time considerations
Basic Protocol 1
Production and purification of La antigen reporter protein can be completed in approximately five days, depending on the desired protein production scale. Plasmid containing sequence of La antigen reporter protein is transfected into bacterial cells overnight, followed by one to two days of bacterial culture. Protein purification can be completed within three days, which includes one day for cell lysis and protein purification, followed by two days of dialysis and protein characterization. The most time-intensive part of the procedure is undoubtedly the production and purification of the 13C-labeled La antigen reporter protein. To mitigate this impact, we have found it useful to scale-up the procedure to produce enough protein for multiple batches of ALARM NMR experiments, as the protein is remarkably stable in storage.
Basic Protocol 2
Regeneration of the La antigen reporter protein can be completed in three to four days, depending on the length of dialysis cycles. The first day involves reducing the La antigen reporter protein with excess DTT, followed by two days of dialysis to remove unreacted DTT. Incubation of test compounds with the La antigen reporter protein can be completed in approximately one day (or overnight). Collection of NMR spectra and data analysis takes approximately 45 min per compound (includes reaction with and without DTT), though this time can likely be decreased with additional optimization such as the use of SOFAST pulse sequences. In our hands, with appropriate instrumentation and experimental settings, ALARM NMR can be medium-throughput in practice, thereby allowing the testing of several hundred compounds per week.
Acknowledgments
The authors acknowledge: Drs. Sergei Gaidamakov and Richard Maraia for providing the original plasmid containing the full-length human La antigen; Hui Zhou and Dr. Zhiguo Zhang for assisting with the ALARM NMR plasmid construction and protein production; and Dr. Gunda Georg for providing (+)-JQ1. The chemical probes A395, T-26c, GSK-6853 and FM-381 were supplied by the Structural Genomics Consortium under an Open Science Trust Agreement (http://www.thesgc.org/click-trust).
Funding for NMR instrumentation was provided by the Office of the Vice President for Research, the University of Minnesota Medical School, the University of Minnesota College of Biological Science, the NIH, the NSF and the Minnesota Medical Foundation. GV was supported by the NIH GM100310.
Footnotes
Conflict of Interest Statement: The authors declare that they have no conflicts of interest.
Literature Cited
- Arif M, Vedamurthy BM, Choudhari R, Ostwal YB, Mantelingu K, Kodaganur GS, Kundu TK. Nitric oxide-mediated histone hyperacetylation in oral cancer: target for a water-soluble HAT inhibitor, CTK7A. Chem Biol. 2010;17:903–913. doi: 10.1016/j.chembiol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Arrowsmith CH, Audia JE, Austin C, Baell J, Bennett J, Blagg J, Bountra C, Brennan PE, Brown PJ, Bunnage ME, Buser-Doepner C, Campbell RM, Carter AJ, Cohen P, Copeland RA, Cravatt B, Dahlin JL, Dhanak D, Edwards AM, Frederiksen M, Frye SV, Gray N, Grimshaw CE, Hepworth D, Howe T, Huber KVM, Jin J, Knapp S, Kotz JD, Kruger RG, Lowe D, Mader MM, Marsden B, Mueller-Fahrnow A, Muller S, O'Hagan RC, Overington JP, Owen DR, Rosenberg SH, Ross R, Roth B, Schapira M, Schreiber SL, Shoichet B, Sundstrom M, Superti-Furga G, Taunton J, Toledo-Sherman L, Walpole C, Walters MA, Willson TM, Workman P, Young RN, Zuercher WJ. The promise and peril of chemical probes. Nat Chem Biol. 2015;11:536–541. doi: 10.1038/nchembio.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsovska E, Trontelj J, Zidar N, Tomasic T, Masic L, Kikelj D, Plavec J, Zega A. Evaluation of Michael-type acceptor reactivity of 5-benzylidenebarbiturates, 5-benzylidenerhodanines, and related heterocycles using NMR. Acta Chim Slov. 2014;61:637–644. [PubMed] [Google Scholar]
- Backus K, Correia B, Lum K, Forli S, Horning B, González-Paez G, Chatterjee S, Lanning B, Teijaro J, Olson A, Wolan D, Cravatt B. Proteome-wide covalent ligand discovery in native biological systems. Nature. 2016;534:570–574. doi: 10.1038/nature18002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baell JB. Observations on screening-based research and some concerning trends in the literature. Future Med Chem. 2010;2:1529–1546. doi: 10.4155/fmc.10.237. [DOI] [PubMed] [Google Scholar]
- Baell JB, Ferrins L, Falk H, Nikolakopoulos G. PAINS: relevance to tool compound discovery and fragment-based screening. Aust J Chem. 2013;66:1483–1494. [Google Scholar]
- Baeza J, Smallegan MJ, Denu JM. Site-specific reactivity of nonenzymatic lysine acetylation. ACS Chem Biol. 2015;10:122–128. doi: 10.1021/cb500848p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanyam K, Altaf M, Varier R, Swaminathan V, Ravindran A, Sadhale P, Kundu T. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem. 2004;279:33716–33726. doi: 10.1074/jbc.M402839200. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam K, Swaminathan V, Ranganathan A, Kundu TK. Small molecule modulators of histone acetyltransferase p300. J Biol Chem. 2003;278:19134–19140. doi: 10.1074/jbc.M301580200. [DOI] [PubMed] [Google Scholar]
- Bamborough P, Barnett HA, Becher I, Bird MJ, Chung Cw, Craggs PD, Demont EH, Diallo H, Fallon DJ, Gordon LJ, Grandi P, Hobbs CI, Hooper-Greenhill E, Jones EJ, Law RP, Le Gall A, Lugo D, Michon AM, Mitchell DJ, Prinjha RK, Sheppard RJ, Watson AJB, Watson RJ. GSK6853, a chemical probe for inhibition of the BRPF1 bromodomain. ACS Med Chem Lett. 2016;7:552–557. doi: 10.1021/acsmedchemlett.6b00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EM, Yan G, Mukherjee C, Orry A, Wang L, Holbert MA, Crump NT, Hazzalin CA, Liszczak G, Yuan H, Larocca C, Saldanha SA, Abagyan R, Sun Y, Meyers DJ, Marmorstein R, Mahadevan LC, Alani RM, Cole PA. Virtual ligand screening of the p300/CBP histone acetyltransferase: identification of a selective small molecule inhibitor. Chem Biol. 2010;17:471–482. doi: 10.1016/j.chembiol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Coan KED, Maltby DA, Burlingame AL, Shoichet BK. Promiscuous aggregate-based inhibitors promote enzyme unfolding. J Med Chem. 2009;52:2067–2075. doi: 10.1021/jm801605r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium SG, Consortium CSG, Consortium NSG, Gräslund S, Nordlund P, Weigelt J, Hallberg B, Bray J, Gileadi O, Knapp S, Oppermann U, Arrowsmith C, Hui R, Ming J, dhe-Paganon S, Park H, Savchenko A, Yee A, Edwards A, Vincentelli R, Cambillau C, Kim R, Kim S, Rao Z, Shi Y, Terwilliger T, Kim C, Hung L, Waldo G, Peleg Y, Albeck S, Unger T, Dym O, Prilusky J, Sussman J, Stevens R, Lesley S, Wilson I, Joachimiak A, Collart F, Dementieva I, Donnelly M, Eschenfeldt W, Kim Y, Stols L, Wu R, Zhou M, Burley S, Emtage J, Sauder J, Thompson D, Bain K, Luz J, Gheyi T, Zhang F, Atwell S, Almo S, Bonanno J, Fiser A, Swaminathan S, Studier F, Chance M, Sali A, Acton T, Xiao R, Zhao L, Ma L, Hunt J, Tong L, Cunningham K, Inouye M, Anderson S, Janjua H, Shastry R, Ho C, Wang D, Wang H, Jiang M, Montelione G, Stuart D, Owens R, Daenke S, Schütz A, Heinemann U, Yokoyama S, Bussow K, KC G. Protein production and purification. Nat Methods. 2008;5:135–146. doi: 10.1038/nmeth.f.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin JL, Baell JB, Walters MA. Assay interference by chemical reactivity. In: Sittampalam G, Coussens N, editors. Assay Guidance Manual. Eli Lilly and the National Center for Advancing Translational Science; Bethesda: 2015a. [PubMed] [Google Scholar]
- Dahlin JL, Nissink JWM, Francis S, Strasser J, John K, Zhang Z, Walters MA. Post-HTS case report and structural alert: promiscuous 4-aroyl-1,5-disubstituted-3-hydroxy-2H-pyrrol-2-one actives verified by ALARM NMR. Bioorg Med Chem Lett. 2015b;25:4740–4752. doi: 10.1016/j.bmcl.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin JL, Nissink JWM, Strasser JM, Francis S, Zhou H, Zhang Z, Walters MA. PAINS in the assay: chemical mechanisms of assay interference and promiscuous enzymatic inhibition observed during a sulfhydryl-scavenging HTS. J Med Chem. 2015c;58:2091–2113. doi: 10.1021/jm5019093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epps DE, Taylor BM. A competitive fluorescence assay to measure the reactivity of compounds. Anal Biochem. 2001;295:101–106. doi: 10.1006/abio.2001.5173. [DOI] [PubMed] [Google Scholar]
- Feng BY, Simeonov A, Jadhav A, Babaoglu K, Inglese J, Shoichet BK, Austin CP. A high-throughput screen for aggregation-based inhibition in a large compound library. J Med Chem. 2007;50:2385–2390. doi: 10.1021/jm061317y. [DOI] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith W, Fedorov O, Morse E, Keates T, Hickman T, Felletar I, Philpott M, Munro S, McKeown M, Wang Y, Christie A, West N, Cameron M, Schwartz B, Heightman T, La Thangue N, French C, Wiest O, Kung A, Knapp S, Bradner J. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster M, Chaikuad A, Bauer S, Holstein J, Robers M, Corona C, Gehringer M, Pfaffenrot E, Ghoreschi K, Knapp S, Laufer S. Selective JAK3 inhibitors with a covalent reversible binding mode targeting a new induced fit binding pocket. Cell Chem Biol. 2016;23:1335–1340. doi: 10.1016/j.chembiol.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KH, Kay LE. Production and incorporation of 15N, 13C, 2H (1H δ1 methyl) isoleucine into proteins for multidimensional NMR studies. J Am Chem Soc. 1997;119:7599–7600. [Google Scholar]
- Gardner KH, Zhang X, Gehring K, Kay LE. Solution NMR studies of a 42 kDa Escherichia coli maltose binding protein/β-cyclodextrin complex: chemical shift assignments and analysis. J Am Chem Soc. 1998;120:11738–11748. [Google Scholar]
- Giannetti AM, Koch BD, Browner MF. Surface plasmon resonance based assay for the detection and characterization of promiscuous inhibitors. J Med Chem. 2008;51:574–580. doi: 10.1021/jm700952v. [DOI] [PubMed] [Google Scholar]
- Goto NK, Gardner KH, Mueller GA, Willis RC, Kay LE. A robust and cost-effective method for the production of Val, Leu, Ile (δ1) methyl-protonated 15N-, 13C-, 2H-labeled proteins. J Biomol NMR. 1999;13:369–374. doi: 10.1023/a:1008393201236. [DOI] [PubMed] [Google Scholar]
- Hajduk PJ, Augeri DJ, Mack J, Mendoza R, Yang J, Betz SF, Fesik SW. NMR-based screening of proteins containing 13C-labeled methyl groups. J Am Chem Soc. 2000;122:7898–7904. [Google Scholar]
- He Y, Selvaraju S, Curtin M, Jakob C, Zhu H, Comess K, Shaw B, The J, Lima-Fernandes E, Szewczyk M, Cheng D, Klinge K, Li H, Pliushchev M, Algire M, Maag D, Guo J, Dietrich J, Panchal S, Petros A, Sweis R, Torrent M, Bigelow L, Senisterra G, Li F, Kennedy S, Wu Q, Osterling D, Lindley D, Gao W, Galasinski S, Barsyte-Lovejoy D, Vedadi M, Buchanan F, Arrowsmith C, Chiang G, Sun C, Pappano W. The EED protein-protein interaction inhibitor A-395 inactivates the PRC2 complex. Nat Chem Biol. 2017;13:389–395. doi: 10.1038/nchembio.2306. [DOI] [PubMed] [Google Scholar]
- Honigberg L, Smith A, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm D, Miller R, Buggy J. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Nat Acad Sci USA. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth JR, Mendoza R, Olejniczak ET, Johnson RW, Cothron DA, Liu Y, Lerner CG, Chen J, Hajduk PJ. ALARM NMR: a rapid and robust experimental method to detect reactive false positives in biochemical screens. J Am Chem Soc. 2005;127:217–224. doi: 10.1021/ja0455547. [DOI] [PubMed] [Google Scholar]
- Huth JR, Song D, Mendoza RR, Black-Schaefer CL, Mack JC, Dorwin SA, Ladror US, Severin JM, Walter KA, Bartley DM, Hajduk PJ. Toxicological evaluation of thiol-reactive compounds identified using a la assay to detect reactive molecules by nuclear magnetic resonance. Chem Res Toxicol. 2007;20:1752–1759. doi: 10.1021/tx700319t. [DOI] [PubMed] [Google Scholar]
- Jacks A, Babon J, Kelly G, Manolaridis I, Cary PD, Curry S, Conte MR. Structure of the C-terminal domain of human la protein reveals a novel RNA recognition motif coupled to a helical nuclear retention element. Structure. 2003;11:833–843. doi: 10.1016/s0969-2126(03)00121-7. [DOI] [PubMed] [Google Scholar]
- Jacks A, Kelly G, Curry S, Conte M. Resonance assignment and secondary structure determination of a C-terminal fragment of the Lupus autoantigen (La) protein containing a putative RNA recognition motif (RRM) J Biomol NMR. 2002;22:387–388. doi: 10.1023/a:1014928117895. [DOI] [PubMed] [Google Scholar]
- Jadhav A, Ferreira RS, Klumpp C, Mott BT, Austin CP, Inglese J, Thomas CJ, Maloney DJ, Shoichet BK, Simeonov A. Quantitative analyses of aggregation, autofluorescence, and reactivity artifacts in a screen for inhibitors of a thiol protease. J Med Chem. 2010;53:37–51. doi: 10.1021/jm901070c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston PA, Soares KM, Shinde SN, Foster CA, Shun TY, Takyi HK, Wipf P, Lazo J. Development of a 384-well colorimetric assay to quantify hydrogen peroxide generated by the redox cycling of compounds in the presence of reducing agents. ASSAY Drug Dev Technol. 2008;6:505–518. doi: 10.1089/adt.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost C, Nitsche C, Scholz T, Roux L, Klein CD. Promiscuity and selectivity in covalent enzyme inhibition: a systematic study of electrophilic fragments. J Med Chem. 2014;57:7590–7599. doi: 10.1021/jm5006918. [DOI] [PubMed] [Google Scholar]
- McCallum MM, Nandhikonda P, Temmer JJ, Eyermann C, Simeonov A, Jadhav A, Yasgar A, Maloney D, Arnold LA. High-throughput identification of promiscuous inhibitors from screening libraries with the use of a thiol-containing fluorescent probe. J Biomol Screen. 2013;18:705–713. doi: 10.1177/1087057113476090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern S, Helfand B, Feng B, Shoichet BK. A specific mechanism of nonspecific inhibition. J Med Chem. 2003;46:4265–4272. doi: 10.1021/jm030266r. [DOI] [PubMed] [Google Scholar]
- McGovern SL, Caselli E, Grigorieff N, Shoichet BK. A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening. J Med Chem. 2002;45:1712–1722. doi: 10.1021/jm010533y. [DOI] [PubMed] [Google Scholar]
- Mohan S. Determination of purity and yield. In: Kenney A, Fowell S, editors. Methods in molecular biology. Vol. 11. Humana Press; Clifton, N.J: 1992. pp. 307–323. [DOI] [PubMed] [Google Scholar]
- Nara H, Sato K, Naito T, Mototani H, Oki H, Yamamoto Y, Kuno H, Santou T, Kanzaki N, Terauchi J, Uchikawa O, Kori M. Thieno[2,3-d]pyrimidine-2-carboxamides bearing a carboxybenzene group at 5-position: highly potent, selective, and orally available MMP-13 inhibitors interacting with the S1″ binding site. Bioorg Med Chem. 2014;22:5487–5505. doi: 10.1016/j.bmc.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Olson ME, Li M, Harris RS, Harki DA. Small-molecule APOBEC3G DNA cytosine deaminase inhibitors based on a 4-amino-1,2,4-triazole-3-thiol scaffold. ChemMedChem. 2013;8:112–117. doi: 10.1002/cmdc.201200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervushin K, Riek R, Wider G, Wuthrich K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Nat Acad Sci USA. 1997;94:12366–12371. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty K. Metal-chelate affinity chromatography. Curr Prot Protein Sci. 2001;4 doi: 10.1002/0471140864.ps0904s04. [DOI] [PubMed] [Google Scholar]
- Rhodes DG, Laue TM. Determination of protein purity. In: Burgess RR, Deutscher MP, editors. Methods in enzymology. Vol. 463. Elsevier; 2009. pp. 677–689. [DOI] [PubMed] [Google Scholar]
- Rossi P, Xia Y, Khanra N, Veglia G, Kalodimos C. 15N and 13C- SOFAST-HMQC editing enhances 3D-NOESY sensitivity in highly deuterated, selectively [1H,13C]-labeled proteins. J Biomol NMR. 2016;66:259–271. doi: 10.1007/s10858-016-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanda P, Kupce E, Brutscher B. SOFAST-HMQC experiments for recording two-dimensional heteronuclear correlation spectra of proteins within a few seconds. J Biomol NMR. 2005;33:199–211. doi: 10.1007/s10858-005-4425-x. [DOI] [PubMed] [Google Scholar]
- Singh J, Petter R, Baillie T, Whitty A. The resurgence of covalent drugs. Nat Rev Drug Discov. 2011;10:307–317. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- Soares KM, Blackmon N, Shun TY, Shinde SN, Takyi HK, Wipf P, Lazo J, Johnston P. Profiling the NIH Small Molecule Repository for compounds that generate H2O2 by redox cycling in reducing environments. ASSAY Drug Dev Technol. 2010;8:152–174. doi: 10.1089/adt.2009.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Auld DS, Inglese J. Apparent activity in high-throughput screening: origins of compound-dependent assay interference. Curr Opin Chem Biol. 2010;14:315–324. doi: 10.1016/j.cbpa.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugarinov V, Hwang P, Ollerenshaw J, Kay L. Cross-correlated relaxation enhanced 1H[bond]13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J Am Chem Soc. 2003;125:10420–10428. doi: 10.1021/ja030153x. [DOI] [PubMed] [Google Scholar]
- Tugarinov V, Kay L. Ile, Leu, and Val methyl assignments of the 723-residue malate synthase G using a new labeling strategy and novel NMR methods. J Am Chem Soc. 2003;125:13868–13878. doi: 10.1021/ja030345s. [DOI] [PubMed] [Google Scholar]
- Tugarinov V, Kay L. An isotope labeling strategy for methyl TROSY spectroscopy. J Biomol NMR. 2004;28:165–172. doi: 10.1023/B:JNMR.0000013824.93994.1f. [DOI] [PubMed] [Google Scholar]
- Wei C, Chupak L, Philip T, Johnson B, Gentles R, Drexler D. Screening and characterization of reactive compounds with in vitro peptide-trapping and liquid chromatography/high-resolution accurate mass spectrometry. J Biomol Screen. 2014;19:297–307. doi: 10.1177/1087057113492852. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Wu D, Motiu-DeGrood R, Hupe DJ. A spectrophotometric method for studying the rates of reaction of disulfides with protein thiol groups applied to bovine serum albumin. J Am Chem Soc. 1980;102:359–363. [Google Scholar]
- Zega A. NMR methods for identification of false positives in biochemical screens. J Med Chem. 2017 doi: 10.1021/acs.jmedchem.6b01520. [DOI] [PubMed] [Google Scholar]
Key References
- 1.Huth JR, Mendoza R, Olejniczak ET, Johnson RW, Cothron DA, Liu Y, Lerner CG, Chen J, Hajduk PJ. ALARM NMR: a rapid and robust experimental method to detect reactive false positives in biochemical screens. J Am Chem Soc. 2005;127:217–224. doi: 10.1021/ja0455547. Original report of ALARM NMR. Highlights differences in GSH and cysteine reactivities. [DOI] [PubMed] [Google Scholar]
- 2.Dahlin JL, Nissink JWM, Strasser JM, Francis S, Zhou H, Zhang Z, Walters MA. PAINS in the assay: chemical mechanisms of assay interference and promiscuous enzymatic inhibition observed during a sulfhydryl-scavenging HTS. J Med Chem. 2015;58:2091–2113. doi: 10.1021/jm5019093. Describes utility of ALARM NMR in an HTS triage of nonselective, thiol-reactive screening compounds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahlin JL, Nissink JWM, Francis S, Strasser J, John K, Zhang Z, Walters MA. Post-HTS case report and structural alert: promiscuous 4-aroyl-1,5-disubstituted-3-hydroxy-2H-pyrrol-2-one actives verified by ALARM NMR. Bioorg Med Chem Lett. 2015b;25:4740–4752. doi: 10.1016/j.bmcl.2015.08.020. Describes use of ALARM NMR to help triage a nonselective, nonreactive, detergent-sensitive chemotype. [DOI] [PMC free article] [PubMed] [Google Scholar]


