Abstract
Objective
To evaluate the association between a frailty index (i.e., scale of accumulated deficits) and neurocognitive functioning among persons living with HIV/AIDS (PLWHA).
Methods
Observational, cross-sectional data were gathered from the University of California, San Diego, HIV Neurobehavioral Research Program from 2002 to 2016. Eight hundred eleven PLWHA aged 18 to 79 years completed comprehensive physical, neuropsychological, and neuromedical evaluations. The frailty index was composed of 26 general and HIV-specific health maintenance measures, and reflects the proportion of accumulated deficits from 0 (no deficits) to 1 (all 26 deficits). Multiple linear regression was used to examine the association between continuous frailty index scores and neurocognitive functioning.
Results
Participants had a mean age of 44.6 years (11.2), and were mostly male (86.9%) and white (60.2%) with a mean frailty index of 0.26 (0.11). Over the study period, prevalence of HIV-related components (e.g., low CD4) decreased, while non-HIV comorbidities (e.g., diabetes) increased. There were no changes in the frailty index by study year. Higher frailty index was associated with worse global neurocognitive functioning, even after adjusting for covariates (age, employment, and premorbid intellectual functioning; b = −0.007; 95% confidence interval [CI] = −0.0112 to −0.003; p < 0.001). The cognitive domains of verbal fluency (b = −0.004; 95% CI = −0.006 to −0.002), executive functioning (b = −0.004; 95% CI = −0.006 to −0.002), processing speed (b = −0.005; 95% CI = −0.007 to −0.003), and motor skills (b = −0.006; 95% CI = −0.007 to −0.005) also significantly predicted worse frailty index score (p values <0.001).
Conclusion
A frailty index can standardize how clinicians identify PLWHA who may be at higher risk of neurocognitive impairment.
With recent advances in antiretroviral therapy, persons living with HIV/AIDS (PLWHA) are now able to live into late life,1–3 but at an increased risk of early acquisition of age-related diseases4–7 and HIV-associated neurocognitive disorders (HAND).8,9
Furthermore, PLWHA have higher rates of frailty at a younger age.10,11 In geriatric medicine, frailty is used to quantify an individual's risk of acquiring multisystem vulnerability.12,13 Although frailty is correlated with aging, studies show a similar physiologic process to aging in younger PLWHA, which is theorized to be due to increased immune activation secondary to the disease process.14,15 Frailty has been shown to predict mortality independent of measures of HIV disease severity,16 suggesting that frailty is important to track in providing optimal care.
One means of quantifying frailty has been as an index,13 or a proportion of acquired age-related deficits. This model represents frailty as a potentially reversible state and allows for customization with disease- and age-related criteria. Studies have shown that although each deficit may not be individually predictive, cumulatively the index has a robust predictive value for overall morbidity and mortality.17
Among PLWHA, frailty has been associated with mortality, psychiatric factors (e.g., depression), and impaired everyday functioning (e.g., instrumental activities of daily living [IADL]).10,16,18–20 There are few studies of frailty and neurocognition among PLWHA21–23; however, these studies point toward a relationship that warrants further exploration. Therefore, the aim of our study was to determine the association between frailty and global neurocognitive functioning among PLWHA.
Methods
Participants
Participants consisted of 811 HIV+ adults enrolled in various NIH-funded research studies at the University of California, San Diego, HIV Neurobehavioral Research Program (HNRP) from 2002 to 2016. Baseline visit data were used for each participant.
Standard protocol approvals, registrations, and patient consents
All HNRP studies from which data were used received approval from the University of California, San Diego, institutional review board. All participants provided written informed consent.
Measures
Frailty index
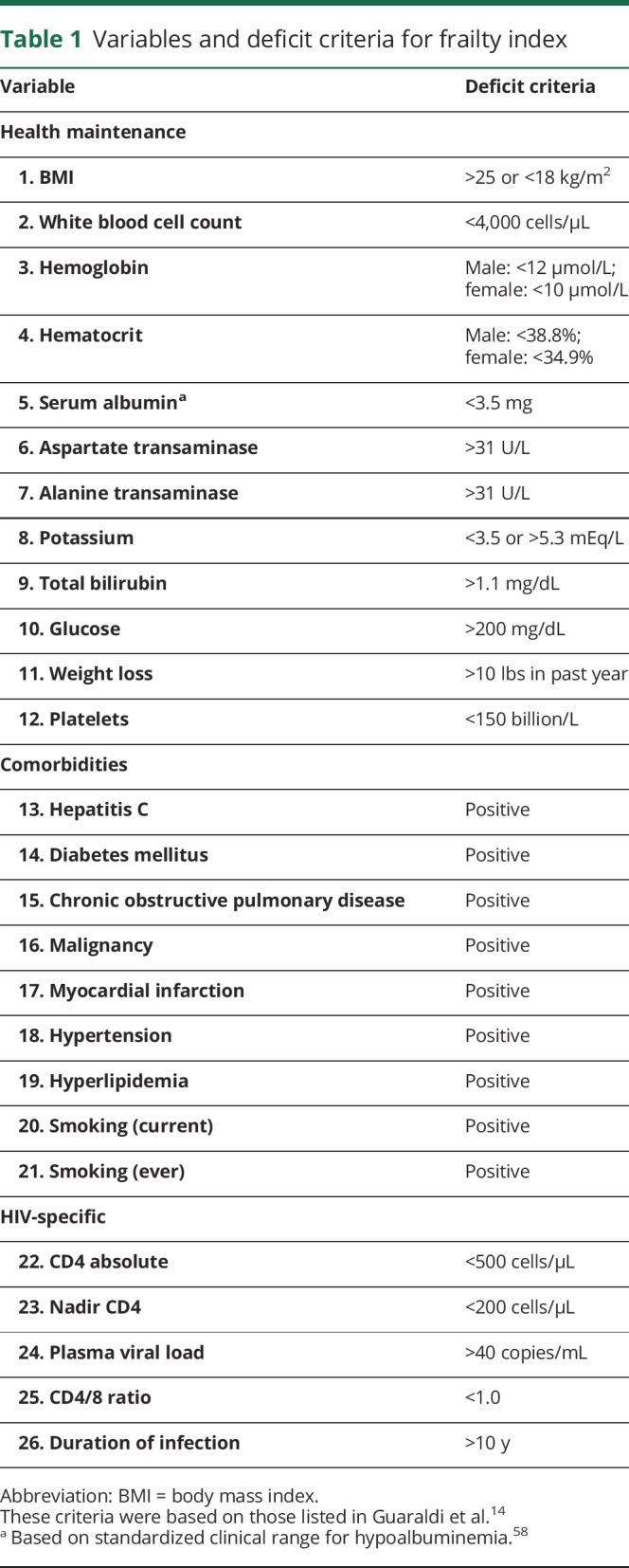
The frailty index was constructed using 26 factors including general health maintenance, comorbidities, and HIV-specific measures (table 1). These criteria were based on those included in a previous study using a frailty index among PLWHA.14 Following the guidelines for creating a frailty index,15 we did not include factors that (1) had greater than 5% missing data, and (2) had less than 1% of participants meeting criteria for the deficit. Potential factors that were removed because more than 5% of data were missing included C-reactive protein, DSM-IV diagnosis of major depressive disorder, Beck Depression Inventory score, total cholesterol, high-density lipoprotein, low-density lipoprotein, phosphorus, triglycerides, hemoglobin A1C, and insulin. Potential factors that were removed because not enough participants (<1%) met the deficit criteria included sodium, renal disease, congestive heart failure, liver disease, and peripheral vascular disease. Using these criteria, we summated the count of fulfilled criteria for each individual and divided by 26 to calculate an individual's frailty index score.
Table 1.
Variables and deficit criteria for frailty index
Neurocognitive and everyday functioning
Neurocognitive functioning was measured using a well-validated, comprehensive neuropsychological (NP) battery designed to assess 7 domains of neurocognitive functioning commonly affected in HIV (i.e., verbal fluency, executive functioning, processing speed, learning, delayed recall, working memory, and motor skills).16 We utilized global and domain-specific NP mean scaled scores (SS), which are normalized composite scores (mean = 10, SD = 3); global NP mean SS are based on functioning across all neurocognitive measures, and domain-specific NP mean SS are based on functioning across neurocognitive measures within respective domains. SS are not corrected for demographics, allowing for accurate comparison to our similarly demographically uncorrected outcome measure (i.e., frailty index). We subsequently controlled for demographics in the regression model. Everyday functioning impairment was measured with self-reported IADL complaints used to determine functional dependence. Employment status was also obtained. Wide Range Achievement Test (WRAT)–Word Reading score was used as a proxy for level of education and cognitive reserve. The WRAT has been supported as a more accurate measure of education and cognitive reserve compared to years of education, particularly in racial/ethnic minorities living with HIV.24 DSM-IV diagnoses of current and lifetime major depressive disorder were identified via the Composite International Diagnostic Interview version 2.1.18
Statistical analysis
To ensure that the frailty index was not influenced by enrollment year (i.e., 2002–2016), Pearson correlational analyses were conducted to explore associations between (1) enrollment year and proportions of participants meeting criteria for each variable in the frailty index, and (2) enrollment year and frailty index.
To explore the relationship between the frailty index and neurocognitive functioning, the unadjusted associations between frailty index and global and domain-specific SS were assessed using Pearson correlations. Next, a multiple regression model was used to construct 8 total regression models: one regressing the frailty index on global NP mean SS and a model for each of the 7 cognitive domain scores as a predictor of frailty index, all adjusted for potential covariates (i.e., age, sex, ethnicity, WRAT score, employment status, and IADL dependence). Of note, only WRAT score was used (without years of education) to avoid multicollinearity; WRAT score and years of education were significantly correlated (Pearson r = 0.49, p < 0.0001). Potential covariates were added to the multivariable regression analyses if they were associated with frailty at p < 0.05. Backward model selection was applied and the final models were chosen to include only the predictor of interest and covariates with p values <0.05. Squared semipartial correlations were calculated to represent the effect size for each predictor variable in the multivariable regressions. These represent the unique proportion of variance explained in the outcome variable by each predictor variable above and beyond other predictor variables (i.e., removing the shared influence of other predictor variables). All analyses were performed using JMP Pro 12.0.118 and R version 3.2.2 statistical software.19
Data availability
HIV stigma persists in San Diego County and elsewhere. To maintain confidentiality, we do not make individual-level deidentified data publicly available, particularly since our data include unique individuals (e.g., older PLWHA of a rare race/ethnicity) that might be at higher risk of reidentification. Our deidentified data are maintained in a secure data resource and are available to qualified external investigators who agree to maintain the confidentiality of our participants. Requests for data should be submitted to the HNRP Data Resource Committee (hnrpresource@ucsd.edu).
Results
Sample characteristics
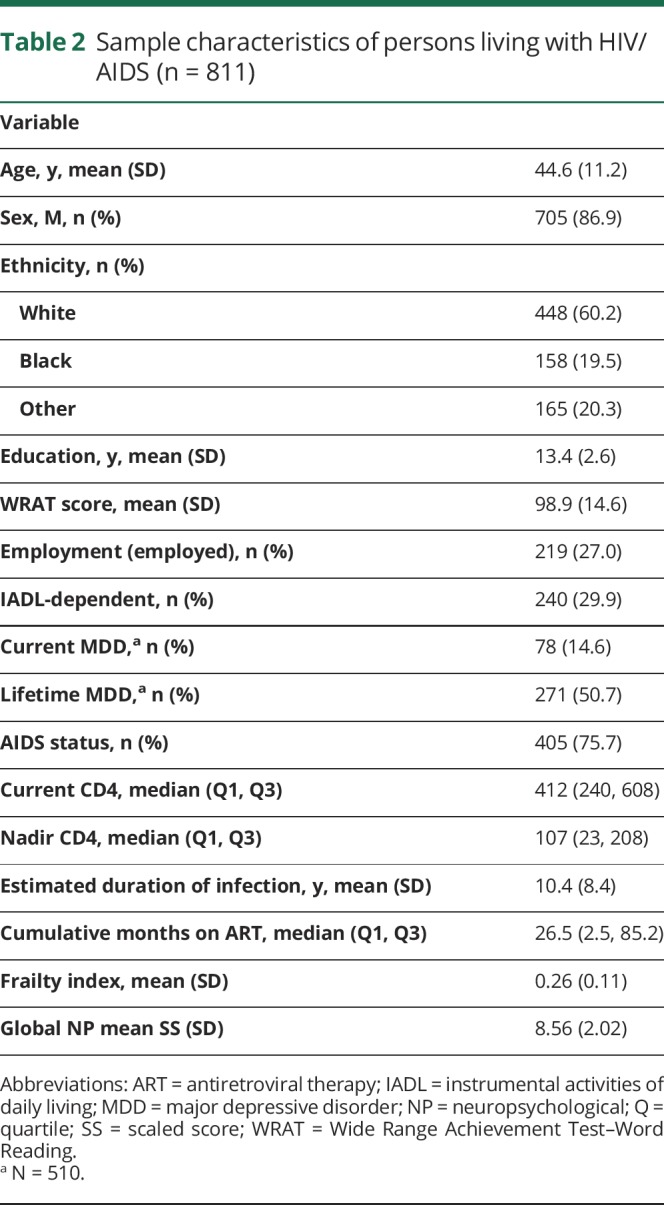
See table 2 for sample characteristics. The sample consisted of 705 (86.9%) men and 106 (13.1%) women between ages 18 and 79 years (mean = 44.6, SD = 11.2). At the time of visit, most participants (71.8%, n = 582) were prescribed combination antiretroviral therapy (cART), 3.3% (n = 27) were prescribed a non-cART HIV regimen, and 24.9% (n = 202) were not on any medication regimen. Of note, when examining differences in prescribing practices between the first (i.e., 2002–2009) and second half (i.e., 2010–2016) of the enrollment period, we found that the percent of participants on cART increased from 65.9% to 82.8% and the percent of participants not on any regimen decreased from 31.5% to 12.7% (χ2 = 40.9, p < 0.001). Approximately 60% (n = 489) of participants met criteria for AIDS, and median current CD4 count was 429 cells/mm3 (interquartile range 221–664). Using the standard global deficit score impairment cutoff of ≥0.5,20 47.8% (n = 388) of participants in our sample were considered cognitively impaired. The frailty index was approximately normally distributed (mean = 0.26, SD = 0.11, range = 0.00–0.65). Participant global NP mean SS was also approximately normally distributed (mean = 8.56, SD = 2.02, range = 1.70–14.26).
Table 2.
Sample characteristics of persons living with HIV/AIDS (n = 811)
Associations between enrollment year and frailty index
To view a temporal snapshot of the potential effect of enrollment year (i.e., from 2002 to 2016) on variables included in our frailty index, we examined the proportion of participants who met criteria for a deficit in each variable for each enrollment year. Less than 10% of participants met criteria for elevated glucose, decreased serum albumin, and myocardial infarction across all enrollment years. Of the remaining 23 variables in the frailty index, 16 variables had significant changes in the proportion of participants meeting deficit criteria within each individual calendar year. For example, the (cross-sectional) prevalence of 9 of the frailty components decreased according to calendar year, including white blood cell count (r = −0.59, p = 0.021), hematocrit (r = −0.75, p = 0.001), aspartate transaminase (r = −0.073, p = 0.002), alanine transaminase (r = −0.062, p = 0.014), hepatitis C (r = −0.81, p < 0.001), nadir CD4 (r = −0.57, p = 0.026), absolute CD4 (r = −0.81, p < 0.001), CD4/8 ratio (r = −0.63, p = 0.011), and plasma viral load (r = −0.89, p < 0.001; table 1). The (cross-sectional) prevalence of 7 of the frailty components increased according to calendar year, including diabetes mellitus (r = 0.62, p = 0.014), malignancy (r = 0.63, p = 0.013), hypertension (r = 0.77, p = 0.001), hyperlipidemia (r = 0.90, p < 0.001), current smoking (r = 0.54, p = 0.037), smoking ever (r = 0.71, p = 0.003), and duration of HIV infection (r = 0.85, p < 0.001). To interpret these changes in the context of changes in antiretroviral therapy regimens over the study period, we also examined proportions of participants on certain regimen types from 2002 to 2016. Across all participants, the most prevalent regimen types (i.e., >5%) were protease inhibitors/nucleoside reverse transcriptase inhibitors (PI/NRTI; 45.6%), nonnucleoside reverse transcriptase inhibitors (NNRTI)/NRTI (27.7%), PI/NNRTI/NRTI (11.0%), and NRTI/integrase inhibitors (II; 7.7%). In later years, proportions of participants on PI/NRTI regimens decreased (r = −0.79, p < 0.001), proportions of participants on NRTI/II regimens increased (r = 0.82, p < 0.001), and proportions of participants on PI/NNRTI/NRTI and NNRTI/NRTI regimens remained stable (p values >0.05).
We then examined the association between frailty index and enrollment year to ensure that our frailty index is stable across calendar years, despite the temporal trends in specific variables. The frailty index displayed stability across enrollment years, with no significant correlation between frailty index and enrollment year (r = −0.06, p > 0.05).
Association between frailty and neurocognition
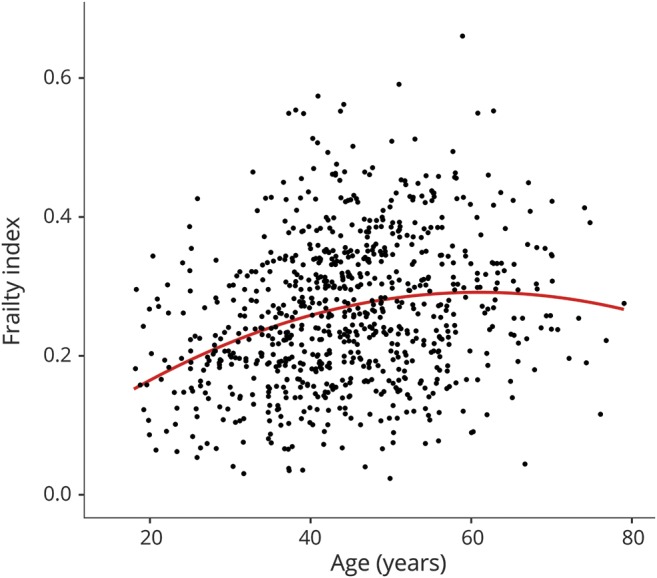
Table 3 shows univariate relationships between the frailty index and all potential covariates. Of note, age had significant linear and quadratic associations with frailty index, indicating an increase in frailty with increasing age only up to a certain age when the strength of the association decreases (figure). WRAT score, ethnicity, IADL dependence, and employment status also showed significant univariate relationships with frailty index.
Table 3.
Associations between frailty index and potential covariates

Figure. Quadratic relationship between age and frailty index.
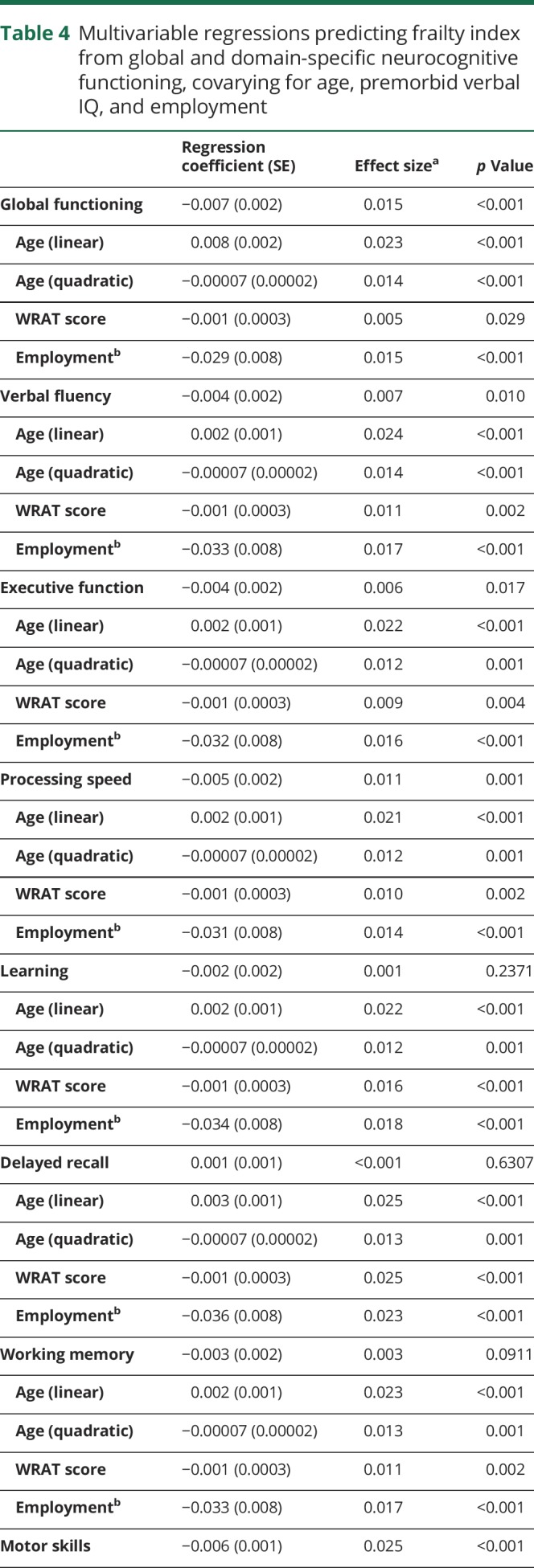
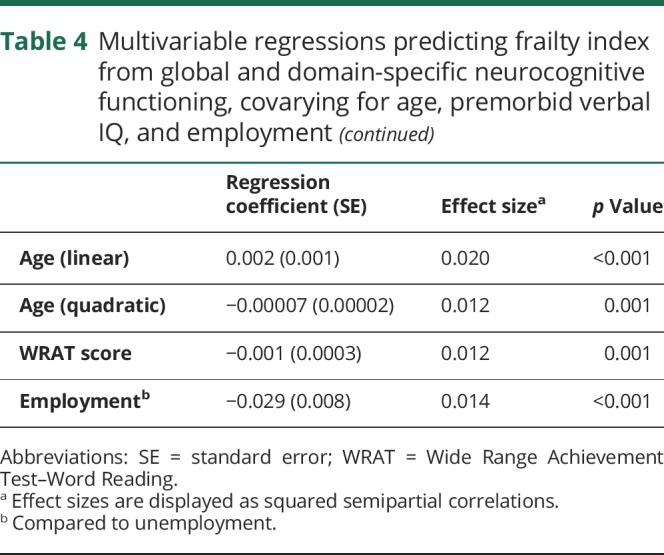
In the unadjusted analyses, global NP mean SS was negatively associated with the frailty index (r = −0.28, p < 0.001), indicating increased frailty with worsening global cognitive ability. Frailty index was also increased (univariately) with an increase in all neurocognitive domains (verbal fluency: r = −0.18, p < 0.001; executive functioning: r = −0.24, p < 0.001; processing speed: r = −0.23, p < 0.001; learning: r = −0.20, p < 0.001; delayed recall: r = −0.12, p < 0.001; working memory: r = −0.17, p < 0.001; motor skills: −0.29, p < 0.001). Covariates included in the multivariable model selection were age (linear and quadratic), WRAT score, ethnicity, IADL dependence, and employment status. For all final multivariable models, stepwise selection resulted in the inclusion of only the primary predictor variable and 4 covariates (i.e., linear age, quadratic age, WRAT score, and employment status). Table 4 displays results of all 8 multivariable models examining relationships between frailty and global and domain-specific neurocognitive functioning. Higher frailty index was associated with worse global neurocognitive functioning, verbal fluency, executive functioning, processing speed, and motor skills (p values <0.001). Each predictor included in the final models had a small to medium effect size. Of note, when the frailty index is modified to include all variables with <20% missing data, rather than the more restrictive <5% data, results do not change.
Table 4.
Multivariable regressions predicting frailty index from global and domain-specific neurocognitive functioning, covarying for age, premorbid verbal IQ, and employment
Lastly, to better understand the construct of frailty and its association with neurocognitive and everyday functioning across the lifespan among PLWHA, we reexamined the relationship between frailty and global neurocognitive functioning (controlling for linear age, quadratic age, WRAT score, and employment status) separately for younger (younger than 50 years; n = 553) and older (50 years or older; n = 258) participants in our sample. Results indicated a similar relationship between global neurocognitive functioning and frailty index in both the younger (b = −0.008; 95% CI = −0.011 to −0.005; p = 0.002) and older (b = −0.010; 95% CI = −0.014 to −0.006; p = 0.029) groups, such that frailty index increased as global NP mean SS decreased. Results also revealed, however, that global neurocognitive functioning was the only significant predictor of frailty index in the older group, whereas in the younger group, frailty index was also significantly predicted by linear age (b = 0.004; 95% CI = 0.003 to 0.005; p < 0.001) and employment status (b = −0.031 [unemployed vs employed]; 95% CI = −0.040 to −0.022; p = 0.001).
Discussion
Among PLWHA, we found that lower cognitive performance (specifically in domains of verbal fluency, executive functioning, processing speed, and motor skills) was significantly associated with increased frailty, even when controlling for other important covariates such as demographics. Although effect sizes were overall small, the clinical implications of our findings are important because the use of a frailty index may allow providers to identify PLWHA who are at risk of worse neurocognitive functioning (and vice versa).
Within the general aging population, an increased frailty index has been shown to be a predictor of poor health outcomes, including increased emergency department visits, after-hours surgery visits, increased nursing home admissions, and increased mortality.21–23,25 Increased frailty has also shown to be associated with neurocognitive impairment among the general population.26,27 The current study represents a logical extension of these previous studies by examining PLWHA.
Our findings are consistent with recent research on aging PLWHA showing improved HIV disease outcomes over time that are belied by increasing prevalence of non–HIV-specific conditions.1–3,28–31 In formulating a frailty index that included both HANA diagnoses as well as specific medical parameters, we were able to evaluate the role of multiple comorbidities on neurocognition in HIV. In evaluating the proportion of participants that met the deficit criteria for each of our 26 frailty variables by enrollment year, we found significant temporal prevalence changes in 16 components of the frailty index. The 9 variables displaying decreased prevalence in our sample according to calendar year included HIV-related variables. This pattern is likely the result of continual improvement of HIV treatment and decreased side effect profiles, as supported by the changes in regimen type over time. In contrast, other factors on the rise included medical comorbidities (e.g., diabetes). These detrimental non-HIV conditions may denote downstream effects of HIV and antiretroviral treatment on overall health.7,32–34 Despite these temporal changes of specific components, the frailty index did not significantly change from 2002 to 2016, suggesting that while different variables contributed to frailty across years, the measure itself remained consistent.
Our study is one of the first to show an association between neurocognition and frailty measured as an index among PLWHA, which is consistent with literature on frailty and neurocognition in the general population. Among HIV-uninfected adults, proposed underlying mechanisms for the association between frailty and neurocognitive impairment include nutrition, chronic inflammation, cardiovascular risk, and mental health.27 Although the potential mechanisms linking frailty and neurocognition need further study among PLWHA, the aforementioned factors have also been examined as proposed mechanisms for the increased rates of HAND seen in older PLWHA.8,9,35 As such, it is not surprising to see a significant relationship between neurocognition and frailty among those living with HIV. Our findings importantly begin the process of disentangling relationships between factors that predict both neurocognitive functioning and frailty, as neurocognitive functioning independently predicts frailty in our model, even when controlling for important covariates. Furthermore, our novel results examining the relationship between global neurocognition and frailty separately by age group (i.e., younger than 50 years, 50 years and older) support 2 important conclusions regarding frailty across the lifespan among PLWHA: (1) that the construct of frailty in younger PLWHA is similar to that in geriatric literature, such that frailty increases with age; and (2) that the frailty index has important clinical relevance in younger PLWHA, as higher frailty index relates to worse global neurocognition and greater unemployment.
More specifically, of the 7 specific domains of neurocognition we investigated, verbal fluency, executive functioning, processing speed, and motor skills were the only neurocognitive domains that significantly predicted frailty, with processing speed and motor skills as the strongest predictors, as indicated by effect sizes. These domains do not map onto the neurocognitive domains known to be most affected in HAND in the cART era.16 In fact, although memory has shown greatest deficits in HAND compared to other domains, learning, delayed recall, and working memory were not significant predictors of frailty in the current study, suggesting that our frailty index is likely capturing more than just HIV-related complications as it is proposed to do. We plan on further examining this novel finding of HIV-specific markers effect on frailty and non–HIV-related neurocognitive decline using HIV-negative controls in future studies.
Accordingly, our findings also show that age, employment, and an estimate of verbal intelligence (e.g., WRAT–Word Reading score) were significant predictors of frailty. Age correlated with an increase in frailty up to a certain age when the strength of the association decreased. This quadratic relationship may represent a survival bias; that is, older PLWHA who have been living with HIV for an average of 10 years may be biologically, psychologically, or otherwise more fit than others who have not lived beyond a certain age with HIV. Resilience has been studied as a potential mechanism of successful aging in PLWHA and is defined as a capacity to thrive despite adverse circumstances.36 In many studies, individuals identified as having greater resilience have been shown to have better physical and mental health.37–39 Thus, the older participants in our study, having survived a long time with a chronic disease, may be more resilient, but this hypothesis remains to be explored.
Unemployment, serving as an indicator of everyday functioning ability, was associated with increased frailty. This finding is consistent with current research on frailty among PLWHA.16 Of note, IADL dependence, another measure of everyday functioning, was not a significant predictor of frailty when controlling for the other variables included in our final model. This suggests that employment may capture a unique facet of everyday functioning that is more relevant in the prediction of frailty compared to IADL dependence.
An estimate of verbal intelligence (e.g., WRAT–Word Reading score), serving as a proxy for education and cognitive reserve, decreased as frailty index scores increased. This suggests that cognitive reserve may be a protective factor for frailty. Cognitive reserve has been proposed as a neuroprotective factor that may explain the discrepancy observed between degree of neuropathology and the clinical manifestation of neurocognitive impairment in many individuals.40,41 Neuroplasticity facilitates alternate cognitive processing as neuropathology disrupts existing synaptic pathways, allowing an individual to adapt and delay the onset of clinical symptoms.42,43 Resultantly, though 2 individuals may have similar neuropathology, the individual with higher cognitive reserve shows less clinical impairment. Our novel finding relating cognitive reserve to frailty may suggest a similar mechanism at play in multisystem function among PLWHA and warrants ongoing research.
This novel study has many strengths, including a large and diverse sample of PLWHA who have varying levels of neurocognitive and medical impairments; however, our study also has limitations worth addressing. First, our sample was compiled from multiple studies across a wide time span. Although this allowed for a large and diverse sample, it also forced us to remove multiple potential variables from our frailty index with greater than 5% missing data. These missing data were attributed to differences in the data collected in each study. As a result, our frailty index had 26 variables, which is slightly short of the number of variables recommended to best capture associations with health risks.44 In addition, consequently from the wide time frame in which data were collected for our cohort, we did not have the data required to use a cognitive reserve index, which has been shown to be a more complete representation of cognitive reserve.5,9 However, we believe that the WRAT index, representing premorbid literacy, has been established as an adequate proxy of cognitive reserve, especially in a diverse population.31 Also, because of the large time frame in which the different studies were conducted, there are some participants who were not on antiretroviral medications or were on antiretroviral regimens other than cART. To limit the variability in antiretroviral treatment, we set a cutoff of 2002, after which a majority of individuals were on cART. The results of our study may also be influenced by selection bias; that is, those with greater health status may have opted to participate in these research studies, and those with the lowest health status may have been incapable of participating. In addition, with a cohort consisting solely of PLWHA, we were unable to evaluate the effect of HIV itself on frailty and its association with neurocognitive performance. We plan to compare PLWHA to HIV-uninfected individuals in the future to study this relationship as well as further evaluate the potential for survival bias in PLWHA. Finally, because the correlations between frailty index and cognition were small, it will be important for future studies to replicate these findings in support of the current conclusions.
Among PLWHA, there is an emerging need to identify individuals at greater risk of multiple organ system dysfunction, who may present with more frequent hospitalization, a greater risk of mortality, and resultantly more complexity in clinical management.45 Older PLWHA are at greater risk of multisystem dysfunction, HAND, and frailty.4,9,10,46 Our study adds to this literature by demonstrating an association between increased frailty and worse neurocognitive functioning among PLWHA. These findings are clinically important because they provide a simple and reliable method for providers to identify persons at risk of concurrent frailty and neurocognitive impairment, and who may require more complex care. For example, similar to the Framingham cardiovascular risk index, the frailty index could be translated into an online tool that would provide clinicians with a convenient way to stratify patients regarding the need for further testing or a referral to a neuropsychologist or neurologist. Inversely, it also suggests that neurocognitive function may have a role in the poor health outcomes seen with frailty. Continuing investigation to understand these complex relationships is clearly needed in order to provide optimal care to persons aging with HIV.
Glossary
- cART
combination antiretroviral therapy
- CI
confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition)
- HAND
HIV-associated neurocognitive disorders
- HNRP
HIV Neurobehavioral Research Program
- IADL
instrumental activities of daily living
- NNRTI
nonnucleoside reverse transcriptase inhibitor
- NP
neuropsychological
- NRTI
nucleoside reverse transcriptase inhibitor
- PI
protease inhibitor
- PLWHA
persons living with HIV/AIDS
- SS
scaled score
- WRAT
Wide Range Achievement Test
Author contributions
Hannah Oppenheim: contributed to all aspects of the study including study design, data interpretation, and writing. Emily W. Paolillo: contributed to all aspects of the study including study design, data analysis and interpretation, and writing. Raeanne C. Moore: contributed to all aspects of the study including study design, data interpretation, and writing. Ronald J. Ellis: contributed to study design, data collection, data interpretation, and writing. Scott L. Letendre: contributed to study design, data collection, and data interpretation. Dilip V. Jeste: contributed to data interpretation and writing. Igor Grant: contributed to data collection and interpretation. David J. Moore: contributed to all aspects of the study including study design, data interpretation, and writing.
Study funding
This research was supported by (1) the National Institute of Mental Health (California NeuroAIDS Tissue Network [CNTN; U24 MH100928, U01 MH083506, R24 MH59745], HIV Neurobehavioral Research Center [HNRC; P30 MH062512], Multi-Dimensional Successful Aging Among HIV-Infected Adults [R01 MH099987], and K23 MH107260 [salary support to R.C.M.]); (2) the National Institute on Drug Abuse (Translational Methamphetamine AIDS Research Center [TMARC; P50 DA026306], and NeuroAIDS: Effects of Methamphetamine and HCV [P01 DA12065]); and (3) the National Institute on Alcohol Abuse and Alcoholism (T32 AA013525 [stipend support to E.W.P.]). None of the authors have a conflict of interest. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, NIH, or the United States Government.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998;338:853–860. [DOI] [PubMed] [Google Scholar]
- 2.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008;372:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison KM, Song R, Zhang X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J Acquir Immune Defic Syndr 2010;53:124–130. [DOI] [PubMed] [Google Scholar]
- 4.High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research: a report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012;60(suppl 1):S1–S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep 2010;7:69–76. [DOI] [PubMed] [Google Scholar]
- 6.Shah S, Mildvan D. HIV and aging. Curr Infect Dis Rep 2006;8:241–247. [DOI] [PubMed] [Google Scholar]
- 7.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 2009;338:a3172. [DOI] [PubMed] [Google Scholar]
- 8.Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology 2004;63:822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS 2004;18(suppl 1):S79–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Althoff KN, Jacobson LP, Cranston RD, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014;69:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci 2007;62:1279–1286. [DOI] [PubMed] [Google Scholar]
- 12.Guaraldi G, Palella FJ Jr. Clinical implications of aging with HIV infection: perspectives and the future medical care agenda. AIDS 2017;31(suppl 2):S129–S135. [DOI] [PubMed] [Google Scholar]
- 13.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001;1:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guaraldi G, Brothers TD, Zona S, et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS 2015;29:1633–1641. [DOI] [PubMed] [Google Scholar]
- 15.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011;17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Composite Diagnostic International Interview (CIDI core Version 2.1). Geneva: World Health Organization; 1998. [Google Scholar]
- 18.SAS Institute Inc. [computer program]. Cary, NC: SAS Institute Inc; 1989–2007. [Google Scholar]
- 19.R: A Language and Environment for Statistical Computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 20.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 2004;26:307–319. [DOI] [PubMed] [Google Scholar]
- 21.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drubbel I, de Wit NJ, Bleijenberg N, Eijkemans RJ, Schuurmans MJ, Numans ME. Prediction of adverse health outcomes in older people using a frailty index based on routine primary care data. J Gerontol A Biol Sci Med Sci 2013;68:301–308. [DOI] [PubMed] [Google Scholar]
- 23.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohit M, Levine A, Hinkin C, et al. Education correction using years in school or reading grade-level equivalent? Comparing the accuracy of two methods in diagnosing HIV-associated neurocognitive impairment. J Int Neuropsychol Soc 2007;13:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saum KU, Dieffenbach AK, Muller H, Holleczek B, Hauer K, Brenner H. Frailty prevalence and 10-year survival in community-dwelling older adults: results from the ESTHER cohort study. Eur J Epidemiol 2014;29:171–179. [DOI] [PubMed] [Google Scholar]
- 26.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc 2010;58:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment: a review of the evidence and causal mechanisms. Ageing Res Rev 2013;12:840–851. [DOI] [PubMed] [Google Scholar]
- 28.Rodger AJ, Lodwick R, Schechter M, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS 2013;27:973–979. [DOI] [PubMed] [Google Scholar]
- 29.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber R, Ruppik M, Rickenbach M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med 2013;14:195–207. [DOI] [PubMed] [Google Scholar]
- 31.Wing EJ. HIV and aging. Int J Infect Dis 2016;53:61–68. [DOI] [PubMed] [Google Scholar]
- 32.Bastard JP, Fellahi S, Couffignal C, et al. Increased systemic immune activation and inflammatory profile of long-term HIV-infected ART-controlled patients is related to personal factors, but not to markers of HIV infection severity. J Antimicrob Chemother 2015;70:1816–1824. [DOI] [PubMed] [Google Scholar]
- 33.D:A:D Study Group, Sabin CA, Worm SW, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 2008;371:1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuhaus J, Jacobs DR Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010;201:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011;62:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masten AS, Best KM, Garmezy N. Resilience and development: contributions from the study of children who overcome adversity. Dev Psychopathol 1990;2:425–444. [Google Scholar]
- 37.Emlet CA, Fredriksen-Goldsen KI, Kim HJ. Risk and protective factors associated with health-related quality of life among older gay and bisexual men living with HIV disease. Gerontologist 2013;53:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farber EW, Schwartz JA, Schaper PE, Moonen DJ, McDaniel JS. Resilience factors associated with adaptation to HIV disease. Psychosomatics 2000;41:140–146. [DOI] [PubMed] [Google Scholar]
- 39.Vance DE, Struzick TC, Masten J. Hardiness, successful aging, and HIV: implications for social work. J Gerontol Soc Work 2008;51:260–283. [DOI] [PubMed] [Google Scholar]
- 40.Morgan EE, Woods SP, Smith C, et al. Lower cognitive reserve among individuals with syndromic HIV-associated neurocognitive disorders (HAND). AIDS Behav 2012;16:2279–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vazquez-Justo E, Blanco AP, Vergara-Moragues E, Gestoso CG, Perez-Garcia M. Cognitive reserve during neuropsychological performance in HIV intravenous drug users. Appl Neuropsychol Adult 2014;21:288–296. [DOI] [PubMed] [Google Scholar]
- 42.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–460. [PubMed] [Google Scholar]
- 43.Stern Y. Cognitive reserve. Neuropsychologia 2009;47:2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007;62:722–727. [DOI] [PubMed] [Google Scholar]
- 45.Thurn M, Gustafson DR. Faces of frailty in aging with HIV infection. Curr HIV/AIDS Rep 2017;14:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wendelken LA, Valcour V. Impact of HIV and aging on neuropsychological function. J Neurovirol 2012;18:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
HIV stigma persists in San Diego County and elsewhere. To maintain confidentiality, we do not make individual-level deidentified data publicly available, particularly since our data include unique individuals (e.g., older PLWHA of a rare race/ethnicity) that might be at higher risk of reidentification. Our deidentified data are maintained in a secure data resource and are available to qualified external investigators who agree to maintain the confidentiality of our participants. Requests for data should be submitted to the HNRP Data Resource Committee (hnrpresource@ucsd.edu).


