Abstract
Objective
To implement a standardized approach to characterize neurologic outcomes among 12-month survivors in the Therapeutic Hypothermia after Pediatric Cardiac Arrest (THAPCA) trials.
Methods
Two multicenter trials enrolled children age 48 hours to 18 years who remained comatose after cardiac arrest (CA) occurring out-of-hospital (THAPCA-OH, NCT00878644) or in-hospital (THAPCA-IH, NCT00880087); patients were randomized to therapeutic hypothermia or therapeutic normothermia. The primary outcome, survival with favorable 12-month neurobehavioral outcome (Vineland Adaptive Behavior Scales [VABS-II]), did not differ between treatment groups in either trial. Neurologists examined 181 12-month survivors, described findings using the novel semi-quantitative Pediatric Resuscitation after Cardiac Arrest (PRCA) form, and rated findings in 6 domains; scores ranged from 0 (no deficits) to 21 (maximal deficits). PRCA scores were compared with 12-month VABS-II scores and cognitive scores.
Results
Neurologic outcome PRCA scores were classified as no/minimal impairment, PRCA 0–3, 81/179 (45%); mild impairment, PRCA 4–7, 24/179 (13%); moderate impairment, PRCA 8–11, 15/179 (8%); severe impairment, PRCA 12–16, 20/179 (11%); profound impairment, PRCA 17–21, 39/179 (21%) (2/181 incomplete). VABS-II scores correlated strongly with PRCA category (r = −0.88, p < 0.0001, Pearson correlation coefficient) and cognitive scores (r = −0.72, p < 0.0001). Factors associated with poor outcomes included out-of-hospital CA, seizure recognition in the early postarrest period, and poor neurologic status at hospital discharge.
Conclusion
The PRCA provides a robust method for depicting neurologic outcomes after acute encephalopathy caused by CA in children. It provides a global semiquantitative rating of neurologic impairment and domain-specific impairment. The strong correlation with well-established neurobehavioral outcome measures supports its validity over a broad age range and wide spectrum of outcomes.
Children who remain comatose after cardiac arrest (CA) resuscitation experience high rates of long-term functional impairment. Recent independent parallel randomized clinical trials compared 2 targeted temperature management interventions in pediatric CA survivors, one for patients with in-hospital CA and one for patients with out-of-hospital CA, in pediatric intensive care units at 37 hospitals in North America and Great Britain (Therapeutic Hypothermia After Pediatric Cardiac Arrest [THAPCA]–IH, NCT NCT00880087; THAPCA-OH, NCT00878644). The primary neurologic inclusion criterion was a Glasgow Coma Scale motor response below 5 within 6 hours after return of circulation; a score of 5 represents localizing pain or, if under age 2 years, withdrawing to touch. The primary efficacy outcome was 12-month survival with favorable neurobehavioral outcome, defined as composite score 70 or higher on the Vineland Adaptive Behavior Scales, Second Edition (VABS-II) (scaled from 20 to 160 with higher scores indicating better function). Only individuals with pre-CA VABS-II scores at least 70 were eligible for the primary outcomes. In both trials, the primary outcome did not differ between treatment groups.
A total of 179 surviving children, all eligible for the primary outcomes, aged 1–18 years, underwent neurologic examinations at 12 months to characterize their neurologic impairments and delineate the neurologic correlates of adaptive functional deficits measured with VABS-II. We developed a standardized semiquantitative format, the Pediatric Resuscitation after Cardiac Arrest (PRCA) form, similar to the Pediatric Stroke Outcome Measure (PSOM),1 to describe findings. The major adaptations in PRCA were expansion of the score range and addition of a domain to capture nonlateralizing or brainstem function deficits.
Methods
THAPCA study design
Details of study design, outcome selection, protocols, inclusion and exclusion criteria, and outcomes for the THAPCA trials have been published.2,3
The trials enrolled children older than 48 hours and younger than 18 years who sustained CA, required chest compressions for at least 2 minutes, and remained comatose and dependent on mechanical ventilation after return of circulation. Major exclusion criteria were traumatic brain injury; scores of 5 or 6 on the Glasgow Coma Scale motor response subscale (scored 1–6, low scores are worse; scores of 5–6 indicate age-appropriate lateralized response to pain); inability to randomize within 6 hours; life expectancy less than 12 months; and lack of commitment to aggressive care. THAPCA-OH included cases of out-of-hospital CA (with few preexisting medical conditions); THAPCA-IH included patients whose CA began within a hospital. In both trials, the primary outcome was survival with favorable neurobehavioral outcome at 12-month follow-up, defined as VABS-II score 70 or higher.
VABS-II provides age-corrected standard scores in 4 domains (communication, daily living, socialization, motor skills) and an overall adaptive behavior composite score (mean score 100, SD 15). VABS-II includes a parent/caregiver rating form and a survey interview (using caregiver as informant) that yield comparable scores.4
Pre-CA function was evaluated using the VABS-II parent–caregiver questionnaire, completed on-site within 24 hours of randomization. The 3- and 12-month outcome VABS-II data were collected centrally (Kennedy Krieger Institute, Baltimore, MD) via telephone by a trained interviewer unaware of treatment assignment. As prespecified, enrolled children with pre-CA VABS-II scores below 70 were excluded from the primary efficacy analyses, but remained in the studies. Patients with no baseline VABS-II data available were eligible for primary analyses if their baseline Pediatric Overall Performance Category (POPC) and Pediatric Cerebral Performance Category (PCPC) scores were in normal or mild disability categories (1 or 2). Scores range from 1 to 6 and lower scores represent less disability; PCPC measures neurologic functioning whereas POPC measures overall health.5 These clinician-rated scales have been used to report outcomes following pediatric CA.5–9 PCPC scoring guidelines for THAPCA10 and details of VABS-II 3- and 12-month scores for both trials have been reported.10–12
Neurologic assessments
The study protocols did not include early neurologic examinations, apart from GCS motor response assessment (by a pediatric critical care physician site investigator); nor was any neurodiagnostic testing specified. Clinical seizure recognition was documented daily during the initial 5-day intervention period.
Neurologic examinations were performed at 12-month follow-up (except at UK sites that joined THAPCA-IH late in its course). This report includes data on 12-month survivors who were eligible for the primary outcome (i.e., with broadly normal baseline function), for whom subsequent neurologic deficits were most likely attributable to CA. Outcomes in survivors who were ineligible for the primary outcome will be reported separately. Of 270 eligible THAPCA-OH patients, 87 survived for 1 year; 75/87 (86%) underwent neurologic examination. Of 257 eligible THAPCA-IH patients, 135 survived for 1 year; 104/135 (77%) underwent 12-month neurologic examination. Neurologic evaluations were scheduled after VABS-II assessments: cognitive testing (Mullen up to age 5 years 9 months,13 and Wechsler Abbreviated Scale of Intelligence [WASI] over age 6 years14) was scheduled on the same day. Composite cognitive scores have been reported.2,3,11,12
Each site investigator identified at least one pediatric neurologist who agreed to participate. Orientation meetings were held at the 2011 and 2012 Child Neurology Society meetings. Two pediatric neurologists (R.I., F.S.S.) were available for consultation regarding scoring. Neurologists were instructed to perform a conventional, detailed age-appropriate neurologic examination, and to record and score each element of the examination using a novel method we developed for this study, the Pediatric Resuscitation after Cardiac Arrest (PRCA) form (appendix e-1, links.lww.com/WNL/A571 and appendix e-2, links.lww.com/WNL/A572). This was developed as a modification of the PSOM instrument.1 Versions were developed for children under age 3 years, and for older children, reflecting age-related items for assessing language and cognition. As with the PSOM, they were also asked to complete global assessments and assign scores (from 0, normal, to 3, severe impairment) in 6 domains. Sensorimotor function was scored independently for each side of the body (so that scores ranged from 0 to 6); the 5 other scored domains included other nonlateralizing sensorimotor function (encompassing cranial nerve deficits, movement/tone disorder, global delays), language production, language comprehension, cognition, and behavior. Total PRCA scores ranged from 0 to 21, with 0 indicating no deficits and 21 indicating maximal deficits.
PRCA forms were submitted to the University of Utah Data Coordinating Center (DCC), and subsequently reviewed for legibility, completeness, and scoring consistency relative to findings described in neurologic examination elements (by R.I. and F.S.S.). When inconsistencies or lack of clarity was apparent, the site neurologist was contacted, revisions were made, and final adjudications were submitted to the DCC (by R.I., except examinations performed by R.I. were reviewed by F.S.S.).
Standard protocol approvals, registrations, and patient consents
Institutional review boards of all sites approved the protocol and informed consent documents.
Data analysis
Patient characteristics were compared between studies using the Wilcoxon rank-sum test, Fisher exact test, or the Cochran-Armitage test for trend. Pearson correlation coefficients were calculated to assess associations between PRCA and other assessments. To quantify association of factors with PRCA scores, standard linear regression models were evaluated first in univariate models; candidate predictors (p < 0.10) were then included in multivariable models. All analyses were completed using SAS software version 9.4 (Cary, NC).
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
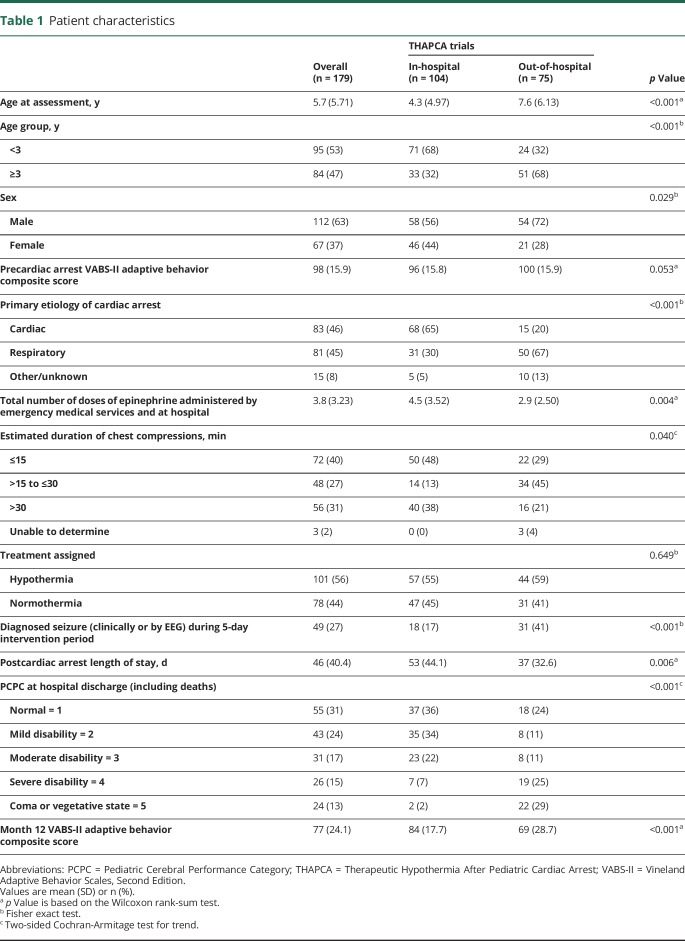
Table 1 describes CA survivors who were eligible for the THAPCA trial primary outcomes and underwent neurologic examinations at 1 year follow-up, 104 from THAPCA-IH and 75 from THAPCA-OH. There were many differences between these groups. In-hospital cases were younger (68% vs 32% <3 years old), had slightly lower baseline VABS-II scores (96 vs 100), included more with cardiac etiology of CA (65% vs 20%), received more epinephrine doses (4.5 vs 2.9), and differed from the out-of-hospital group with respect to durations of chest compression. Treatment group assignment did not differ. Seizure diagnosis during the intervention period was more common in the out-of-hospital group (41% vs 17%). PCPC score at hospital discharge differed significantly, with 69% classified as normal or mild disability in the in-hospital group, compared with 35% of out-of-hospital survivors. Similarly, 12-month VABS-II composite scores were higher in the in-hospital group (mean 84 vs 69 in the out-of-hospital group).
Table 1.
Patient characteristics
We also evaluated these characteristics in 41 survivors, eligible for the primary outcomes, who did not undergo neurologic examination, and compared them with the 181 cases who did (2 with partial PRCA data were excluded from subsequent analyses). The 2 cohorts did not differ in any measure (data not shown).
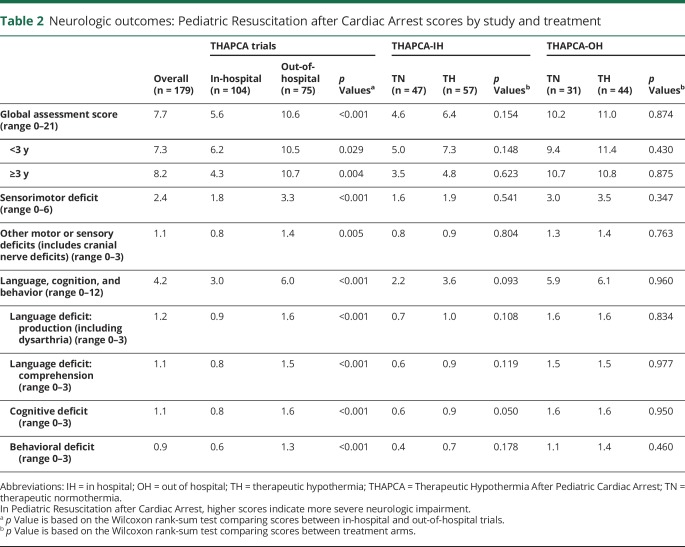
Table 2 provides detailed information about PRCA scores in all children evaluated. The mean PRCA global assessment score of 7.7 (out of a maximum of 21) reflected some degree of impairment in multiple domains in most children. Sensorimotor deficits were variable and generally global or nonlocalizing. Major findings discerned included consistently lower composite and domain scores (i.e., fewer abnormalities) in in-hospital than out-of-hospital cases, no significant differences between treatment arms in the out-of-hospital study group, and one very minor difference in cognitive deficit scores (0.6 vs 0.9, favoring therapeutic normothermia) in the in-hospital cohort.
Table 2.
Neurologic outcomes: Pediatric Resuscitation after Cardiac Arrest scores by study and treatment
The distribution of PRCA scores was stratified into 5 groups, shown in figure 1, and summarized as follows: no or minimal impairment, PRCA 0–3 in 81/179 (45%); mild impairment, PRCA 4–7 in 24/179 (13%); moderate impairment, PRCA 8–11 in 15/179 (8%); severe impairment, PRCA 12–16 in 20/179 (11%); and profound impairment, PRCA 17–21 in 39/179 (21%). Infants and young children with mild or moderate impairment commonly displayed diffuse hypotonia with mild delays in motor skills. Children of all ages in the mild and moderate impairment categories typically displayed mild or moderate deficits or delays in language and cognitive skills. Children with severe and profound impairment typically had spastic quadriplegia associated with bulbar dysfunction, and were nonverbal, nonambulatory, and completely dependent for activities of daily living.
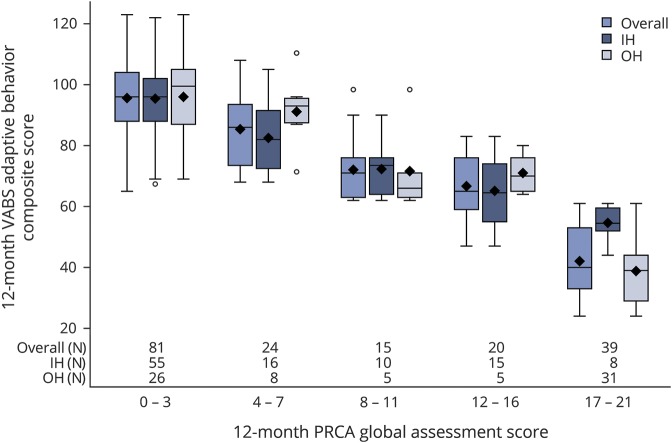
Figure 1. Relationship of 12-month Pediatric Resuscitation after Cardiac Arrest (PRCA) and Vineland Adaptive Behavior Scales, Second Edition (VABS-II) scores.
Data demonstrate a strong relationship between month 12 VABS-II composite score (mean 100, SD 15) on the Y axis and global PRCA score (range 0–21), divided into 5 strata of severity, on the X axis (r = −0.877, p < 0.0001, Pearson correlation coefficient). Data are presented for all cases (left bar), in-hospital (IH) (middle bar), and out-of-hospital (OH) (right bar) groups, as box and whisker plots. Number of patients is shown along the X axis in each group for each stratum. Each shaded box represents the interquartile range (IQR) (middle one-half of the data), the horizontal line within the box is the median, and symbols (◇) are means; upper and lower whiskers represent the maximum and minimum values, excluding outliers (defined as data points more than 1.5 IQRs from the box).
Figure 1 illustrates the distribution of VABS-II composite scores for each PRCA score category. Data are presented as box and whisker plots to illustrate the full range of VABS-II scores in each category. There was a strong relationship between the 2 measures (r = −0.88, p < 0.0001, Pearson correlation coefficient). The strength of this relationship was similarly robust when analyzed separately for children less than 3 years old, and for those 3 and older (not shown). We also evaluated the relationship between composite cognitive test scores (Mullen or Wechsler, available for 153 cases) and PRCA score category; trends were similar (r = −0.72, p < 0.0001, Pearson correlation coefficient) (figure e-1, links.lww.com/WNL/A573).
Data from 50 children with PRCA scores of 0 (i.e., classified as neurologically normal) were examined separately. In this distinct subpopulation (age 1–18 years; mean ± SD, 6.3 ± 6 years; 34/50 from in-hospital study), pre-CA VABS-2 scores were average (100 ± 17), and declined minimally (95 ± 13); cognitive composite scores were in a similar range (94 ± 20). Of note, estimated cardiopulmonary resuscitation duration was 30–40 minutes in 6 cases, and over 40 minutes in 10 cases.
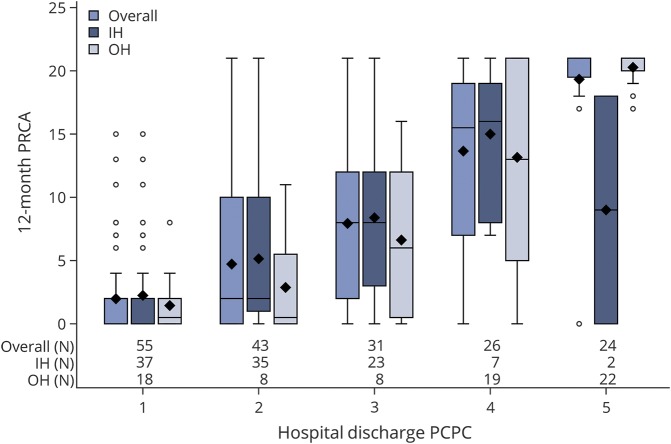
Figure 2 illustrates the relationship between early clinical assessments (PCPC scores, from 1 to 5, at hospital discharge) and subsequent 12-month neurologic outcome scores (0–21). Of note, the mean duration of hospitalization after CA until discharge, when this score was assigned, was 46 days (53 days for the in-hospital group and 37 days for the out-of-hospital group). Although hospital discharge PCPC strongly predicted 12-month PRCA scores (r = 0.74, p < 0.0001 Pearson correlation coefficient), individual patients had diverging trajectories. One young infant with a PCPC score of 5 at hospital discharge improved markedly and had a normal 12-month neurologic examination (PRCA = 0). Some individuals classified as normal or mildly impaired at hospital discharge had significant impairments at 12-month assessments. However, details of posthospital discharge medical complications that likely contributed to subsequent neurologic declines were not collected in the study protocols. Nor was any information about neurodiagnostic testing or rehabilitation services collected.
Figure 2. Relationship between 12-month Pediatric Resuscitation after Cardiac Arrest (PRCA) score (range 0–21) and v Pediatric Cerebral Performance Category (PCPC) scores (range 0–5) at hospital discharge.
Data are presented for all cases (left bar), in-hospital (IH) (middle bar), and out-of-hospital (OH) (right bar) groups, as box and whisker plots; number of cases in each group are shown along the X axis. Each shaded box represents the interquartile range (IQR) (middle one-half of the data), the horizontal line within the box is the median, and symbols (◇) are means; upper and lower whiskers represent the maximum and minimum values, excluding outliers (defined as data points more than 1.5 IQRs from the box). Higher PRCA scores indicate more severe neurologic impairment. Overall there is a strong relationship between hospital discharge PCPC scores and 12-month PRCA scores (r = 0.74, Pearson correlation coefficient, p < 0.0001), but individual outlying cases are evident.
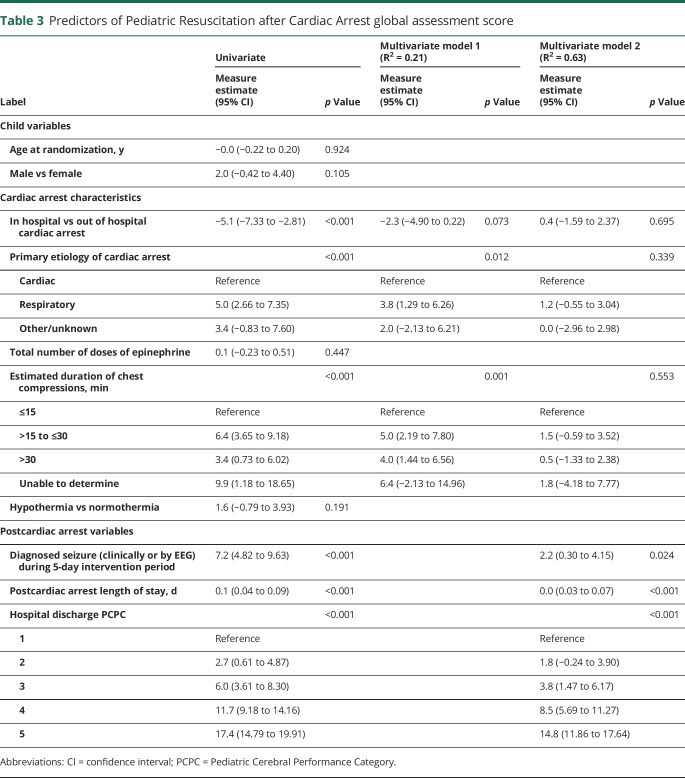
Table 3 describes the predictors of outcome measured by the 12-month PRCA score. In univariate analyses, CA characteristics that were associated with an increased risk of poor outcome included site (out-of-hospital worse than in-hospital), primary etiology (respiratory worse than cardiac), and longer duration of chest compressions. Post-CA variables that influenced outcome included seizure detection during the 5-day intervention period, greater length of hospital stay, and hospital discharge PCPC. Treatment group, age, and sex of the child and first lactate level did not predict 12-month outcome. However, in a model that included only CA characteristics (model 1) these variables, together, were very weak predictors of outcome (R2 = 0.21). A model that incorporated post-CA variables more robustly predicted PRCA scores (R2 = 0.63). Yet, as illustrated in figure 2, although neurologic status at hospital discharge was strongly correlated with 12-month neurologic examination scores, unidentified factors also contributed substantially to individual variation in 12-month PRCA scores.
Table 3.
Predictors of Pediatric Resuscitation after Cardiac Arrest global assessment score
Discussion
We evaluated a unique cohort of prospectively evaluated children, who survived for 1 year after cardiac arrest with initial postresuscitation coma. These children, ranging in age from 1 to 18 years, had a broad range of neurologic outcomes. The spectrum of outcomes was approximately evenly distributed among 3 broad categories of normal or near normal, mild to moderate impairment, and severe impairment. Those children with severe impairment typically had mixed severe motor, cognitive, and adaptive functional deficits.
Our study demonstrated that neurologic assessment using the PRCA is a powerful method for depicting neurologic outcomes after acute encephalopathy caused by cardiac arrest in children. It provides both a global semiquantitative rating of neurologic impairment as well as more specific characterization of localizing deficits and domain-specific impairment. The strong correlation with previously well-established measures of functional and cognitive outcomes (VABS-II, Mullen, WASI) provides support for the validity of the PRCA in children with CA from a range of ages, widely varying underlying diseases, and wide spectrum of outcomes in these trials. The PRCA represents a novel approach to standardizing assessment of neurologic outcome following an acute diffuse brain injury across a broad pediatric age range. It builds upon the strengths of the widely used PSOM for assessing long-term stroke outcomes in children.15–17 Both are based on detailed standard neurologic examinations, and standardized ratings in multiple key functional domains using a simple Likert scale for each domain, relative to age-specific expectations. By expanding total scores from a maximum of 10 in PSOM up to 21, the PRCA had a greater capacity to capture variability in neurologic status over the full range of outcomes seen in this population.
An integral element of this study was the central review procedure. Orientation and central review of all PRCA data provided an important means to standardize the assessment and to clarify questions or discrepancies in administration and interpretation by site neurologists. Challenges with interpretation or scoring that were reported centered on the difficulties of scoring the cognitive subscale in children with motor impairment, and the subjective nature of differentiating degrees of severity in the subscale scores. These problems are inherent to any rating scale of this nature. Despite these challenges, our finding of robust correlations of the PRCA scores compared to other well-established functional endpoint assessments suggest that the PRCA, when administered with centralized, consistent oversight, is a valid method to measure neurologic outcome over a broad range of variation after diffuse brain injury in children.
Comparison of PRCA scores between in-hospital CA and out-of-hospital CA groups demonstrated substantial differences, congruent with findings of our prior retrospective study.18 There were major differences in underlying disease characteristics of these 2 populations, cardiac etiologies more common in in-hospital arrest and respiratory etiologies more common in out-of-hospital arrest, and neurologic outcomes were worse in out-of-hospital than in in-hospital CA survivors. In fact, awareness of these inherent differences justified the conduct of independent trials of therapeutic hypothermia for in-hospital and out-of-hospital pediatric CA.
Analysis of outcome predictors in surviving children who underwent 12-month neurologic examinations was challenging. As in the parent THAPCA trials, hypothermia did not confer any benefit, compared with normothermia. Although several contributing factors were identified in univariate analyses, in multivariate models postarrest factors, including seizure recognition in the first 5 days postresuscitation and poor neurologic status at hospital discharge, were more powerful than prearrest or intra-arrest factors.
In particular from a neurologic perspective, our data had significant limitations. No element of the neurologic examination apart from the Glasgow Motor Scale was consistently documented prior to enrollment. Although EEGs and neuroimaging were frequently performed in patients enrolled in these trials, no neurodiagnostic or biomarker testing was included in the THAPCA protocols, and no information about results of these tests was collected centrally. The data element that described presence or absence of seizures during the intervention period was broadly defined to encompass clinical or electrographic seizures, as recognized by the local clinical teams. The distribution of neurologic outcomes at 12 months in survivors must also be interpreted with caution, as an early clinical assessment of poor neurologic outcome led to redirection of goals of care for some children in each trial.
Our observations have important implications for clinical care as well as for the design of future studies of outcome in children surviving CA. Our findings suggest that attempts to prognosticate during the acute hospital phase should be undertaken with caution, and some children make significant improvements during the first year after their initial insult.
In future clinical trials that enroll children at substantial risk for neurologic impairment, standardized neurologic examinations have the potential to yield robust, clinically meaningful outcome measures.
Glossary
- CA
cardiac arrest
- DCC
Data Coordinating Center
- PCPC
Pediatric Cerebral Performance Category
- POPC
Pediatric Overall Performance Category
- PRCA
Pediatric Resuscitation after Cardiac Arrest
- PSOM
Pediatric Stroke Outcome Measure
- THAPCA
Therapeutic Hypothermia After Pediatric Cardiac Arrest
- VABS-II
Vineland Adaptive Behavior Scales, Second Edition
- WASI
Wechsler Abbreviated Scale of Intelligence
Footnotes
CME Course: NPub.org/cmelist
Contributor Information
Collaborators: THAPCA Trial Group, Frank W. Moler, J. Michael Dean, Richard Holubkov, James R. Christensen, Beth S. Slomine, Frank W. Moler, J. Michael Dean, Richard Holubkov, James R. Christensen, Beth S. Slomine, K. Meert, J. Hutchison, V. Nadkarni, S. Shankaran, F. Silverstein, V. Pemberton, C. Nicholson, J. Alten, S. Borasino, K. Hock, K. Sewell, L. Dure, H. J. Dalton, S. Buttram, K. Wai, A. La Bell, C. Bliss, M. Lavoie, V. Bordes-Edgar, A. Theodorou, K. Typpo, C. Wells, J. Deschenes, C. Newth, S. Rubin, R. Bart, T. Deakers, A. Bhalla, B. Markovitz, J. DiCarlo, P. Ross, C. Herrington, W. Wells, R. Kim, V. Wang, M. Villa, F. Fajardo, J. Kwok, J. Serrano, J. Valentine, A. Yamakawa, S. Briones, S. Cauley, A. Briseno, C. Young, M. Nyc, T. Rosser, J.I. Gold, R. Engilman, A. Schwarz, J. Haykawa, O. Vargas-Shiraishi, A. Galion, M. Mathur, J. Newcombe, A. Pinto, S. Ashwal, J. Pivonka-Jones, R. Harrison, R. Kelly, A. Madikians, M. Federman, M. Morgan, A. Yamakawa, M. Nyc, S. Briones, M. Villa, J. Kwok, J. Serrano, J. Valentine, T. Rosser, R. Engilman, J.I. Gold, P. McQuillen, L. Haeusslein, H. Glass, J. Hutchison, S. Schwartz, A. Guerguerian, K. Boutis, D. Clark, J. Van Huyse, K. Fusco, K. McBain, A. Krancevic, L. Toller, R. Gaiteiro, C. Hahn, R. Sananes, E. Dobyns, J. Albietz, T. Wilson, B. Wathen, T. Bernard, J. Dise-Lewis, N. Pham, N. Chanani, K. Walson, J. Sturm, W. Mahle, M. Wolf, C. Stone, A. Wellons, S. Meisner, E. Hoar, S. Gentry, L. Smitley, L. McMaster, P. Holt, B. Weissman, A. Alexander, R. Lutfi, D. Sokol, B. McDonald, D. Goodman, E. Powell, S. Shah, M. Porter, J. Sullivan, M. Ruppe, J. Berkenbosch, M. Thomas, L. Sears, U. Bhalala, J.K. Lee, S. Kudchadkar, D. Shaffner, M. Shackelford, P. Melvin, R. Felling, B. Slomine, J.R. Christensen, B.S. Slomine, E. DeMatt, M. Talley, C. Rodweller, K. Meert, S. Heidemann, J. Clark, A. Pawluszka, M. Lulic, L. Sivaswamy, F. Moler, M. Gaies, T. Cornell, M. Weber, J. Reske, L. Conlin, F. Silverstein, M. Carlson, S. Warschausky, T. Behnke, D. Poszywak, J. Nowak, H. Ortega, D. Milner, E. Zielinski, J. Pineda, M. Shoykhet, S. Friess, A. Gazit, K. Gilliams, D. Jaffe, T. Day, T. Hicks, L. Barganier, E. Fish, L. Toennies, P. Thurst, S. Blankenship, M. Noetzel, K. Guilliams, D. White, C. Schleien, N. Talathoti, C Aguilar, V. Hinton, E. W. van der Jagt, E. R. Taillie, E. B. Nazarian, L. E. Daugherty, C.O. Davis, H. R. Adams, G. Ofori-Amanfo, K. Rehder, S. Wrenn, T. Uhl, C. Milleson, E. Smith, K. Gustafson, S. Asbeck, W. Gallentine, E. Lloyd, M. Hall, N. Khan, J. Frazier, Daniel Cohen, J. Haines, K. Carter, L. Bird, W. Lo, D. Wheeler, G. Geis, E. Beckman, S. Banschbach, K. Krallman, K. Lidsky, S. Bergant, A. Browning, N. Bass, R. Tangen, NJ. Thomas, A. Shelly, J. Vallati, P. Carper, D. Spear, A. McMonagle, J. Stokes, H. Watts, W. Trescher, C. Flaherty-Craig, A. Topjian, R. Berg, V. Nadkarni, A. Zuppa, J. Fitzgerald, P. Meaney, M. DiLiberto, C. Twelves, S. McGowan, M. Sisko, B. Park, R. Ichord, K. Friedman, E. Fink, R. Hickey, A. Abraham, S. Shah, K. Anand, B. Moore, A. Nico West, M. Grandberry, N. Shah, A. DeCrow, O. King DeBerry, A. Huling, J. Koch, P. Okada, D. Miles, L. Raman, M. Green, E. Golson, A. Jones, D. Kelly, T. Plumb, K. Van de Bruinhorst, M. Dowling, P. Stavinoha, A. Hernandez, T. Wu, C. Bauerfeld, M. Rodkey, H. Dibrell, S. Atkinson, L. O'Donnell, J. Eubanks, J. M. Dean, R. Holubkov, B. Browning, M. Gildea, R. Kuhn, A. Webster, K. Page, R. Telford, L. Herrera, J. Yearley, J. Burr, K.S. Bennett, J. Sweney, R. Lane, J. Lilley, S. Bjerregaard, K. Jacobsen, A. Watson, D. Stephens, M. DelaCruz, K. Cooley, N. Kwendakwema, G. Jensen, R. Moore, M. Sweney, D. Morita, R. Burr, T. Bennett, J. Henricksen, E. Hirshberg, G. Larsen, M. Schober, R. Dixon, J. Workman, J. Zimmerman, J. McGuire, R. Farris, O. Yanay, J. Reid, L. Smith, S. Hamilton, C. Greeley, C. Cheng, S. Gospe, C. Amlie-Lefond, D. Breiger, A. Paolozzi, J. Berger, D. Wessel, M. Sharron, S. Basu, A. Wratney, N. Dean, J. Reardon, E. Tomanio, J. Carpenter, S. Swanson, T. Brennan, P. Glass, B. Malek, M. Mintz, M. T. Meyer, M. Wakeham, S. Hanson, K. Murkowski, P. Baines, N. Shetty, A. Wood, S. Siner, L. Walsh, B. Scholefield, A. Jones, T. Cameron, A. Mohammed Ali, K. Morris, I. Zafurallah, I A. Top, J. Menzies, S. Skellett, T. Thiruchelvam, A.J. Petros, M.J. Peters, D. Goodacre, A. Jones, J. Pappachan, R. Ensom, K. Morton, and V. Pemberton
Author contributions
Rebecca Ichord: design and conceptualization of the study, interpretation of data, drafting the manuscript, revising the manuscript for intellectual content. Faye Silverstein: design and conceptualization of the study, interpretation of data, drafting the manuscript, revising the manuscript for intellectual content. Beth S. Slomine: design and conceptualization of the study, oversaw all VABS-II data collection, interpretation of data, revising the manuscript for intellectual content. Russell Telford: performed all statistical analyses of the data. James Christensen: revising the manuscript for intellectual content. Richard Holubkov: oversaw all statistical analyses of the data. J. Michael Dean: design and conceptualization of the study; oversaw all data collection, served as principal investigator for the THAPCA data coordinating center. Frank Moler: design and conceptualization of the study and served as principal investigator for the THAPCA trials, interpretation of data, revising the manuscript for intellectual content.
Study funding
Primary support for the conduct of the THAPCA-OH and THAPCA-IH Trials was funding from NIH U01HL094345 (F.W.M.) and U01HL094339 (J.M.D.). Additional support from the following federal grants contributed to the planning of the THAPCA Trials: NIH, Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), Bethesda, MD; HD044955 (F.W.M.) and HD050531 (F.W.M.). In part, support was from the participation of the following research networks: Pediatric Emergency Care Applied Research Network (PECARN) from cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008, and the Collaborative Pediatric Critical Care Research Network (CPCCRN) from cooperative agreements (U10HD500009, U10HD050096, U10HD049981, U10HD049945, U10HD049983, U10HD050012, and U01HD049934). At several centers, clinical research support was supplemented by the following grants or cooperative agreements: UL1TR000003, P30HD040677, P30HD062171, U07MC09174, UL1 RR 024986, and UL1 TR 000433.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Kitchen L, Westmacott R, Friefeld S, et al. The Pediatric Stroke Outcome Measure: a validation and reliability study. Stroke 2012;43:1602–1608. [DOI] [PubMed] [Google Scholar]
- 2.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after in-hospital cardiac arrest in children. N Engl J Med 2017;376:318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med 2015;372:1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparrow SCD, Balla D. Vineland Adaptive Behavior Scales: Survey Forms Manual. 2nd ed. Minneapolis: NCS Pearson; 2005. [Google Scholar]
- 5.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and Pediatric Cerebral Performance Category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med 2000;28:2616–2620. [DOI] [PubMed] [Google Scholar]
- 6.Zaritsky A, Nadkarni V, Hazinski MF, et al. Recommended guidelines for uniform reporting of pediatric advanced life support: the pediatric utstein style: a statement for healthcare professionals from a task force of the American Academy of Pediatrics, the American Heart Association, and the European Resuscitation Council: Writing Group. Circulation 1995;92:2006–2020. [DOI] [PubMed] [Google Scholar]
- 7.Horisberger T, Fischer E, Fanconi S. One-year survival and neurological outcome after pediatric cardiopulmonary resuscitation. Intensive Care Med 2002;28:365–368. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Herce J, Garcia C, Rodriguez-Nunez A, et al. Long-term outcome of paediatric cardiorespiratory arrest in Spain. Resuscitation 2005;64:79–85. [DOI] [PubMed] [Google Scholar]
- 9.Nishisaki A, Sullivan J III, Steger B, et al. Retrospective analysis of the prognostic value of electroencephalography patterns obtained in pediatric in-hospital cardiac arrest survivors during three years. Pediatr Crit Care Med 2007;8:10–17. [DOI] [PubMed] [Google Scholar]
- 10.Silverstein FS, Slomine BS, Christensen J, et al. Functional outcome trajectories after out-of-hospital pediatric cardiac arrest. Crit Care Med 2016;44:e1165–e1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slomine BS, Silverstein FS, Christensen JR, et al. Neurobehavioral outcomes in children after out-of-hospital cardiac arrest. Pediatrics 2016;137(4). pii e20153412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slomine BSSF, Christensen JR, Holubkov R, Telford R, Dean JM, Moler FW. Neurobehavioral outcomes in children after in-hospital cardiac arrest. Resuscitation 2018;124:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EM M. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- 14.Wechsler DW. Abbreviated Scale of Intelligence. New York: Psychological Corporation; 1999. [Google Scholar]
- 15.Beslow LA, Licht DJ, Smith SE, et al. Predictors of outcome in childhood intracerebral hemorrhage: a prospective consecutive cohort study. Stroke 2010;41:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol 2000;15:316–324. [DOI] [PubMed] [Google Scholar]
- 17.Kirton A, Shroff M, Visvanathan T, deVeber G. Quantified corticospinal tract diffusion restriction predicts neonatal stroke outcome. Stroke 2007;38:974–980. [DOI] [PubMed] [Google Scholar]
- 18.Moler FW, Meert K, Donaldson AE, et al. In-hospital versus out-of-hospital pediatric cardiac arrest: a multicenter cohort study. Crit Care Med 2009;37:2259–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.