Abstract
Objective
To determine whether spindle activity differs in young children with and without autism.
Methods
We investigated differences in spindle density, duration, and oscillatory features in 135 young children with autism, developmental delay without autism (DD), or typical development (TD) and secondarily assessed the dimensional relationship between spindle density and both cognitive ability and social functioning.
Results
Compared to TD, both spindle density (Cohen d 0.93, 95% confidence interval [CI] 0.49–1.37) and duration (Cohen d 0.58, 95% CI 0.15–1.01) were significantly decreased in autism. Spindle density was also significantly reduced in autism compared to DD (Cohen d 0.61, 95% CI 0.13–1.09). Decreased spindle frequency in autism compared to both TD (Cohen d 0.47, 95% CI 0.04–0.90) and DD (Cohen d 0.58, 95% CI 0.10–1.06) did not survive correction. The DD group did not differ significantly from the TD group on any spindle parameter. These results, suggesting a relationship between spindle density and autism but not DD, were further illustrated in exploratory analyses, wherein nonverbal ratio IQ (RIQ) and the Vineland Socialization domain standard score were strongly correlated with spindle density in the full sample (r = 0.33, p ≤ 001 and r = 0.41, p ≤ 001, respectively) but not within group. After nonverbal RIQ was accounted for, the relationship between spindle density and Vineland Socialization remained statistically significant (r = 0.23, p < 0.01). However, Vineland Socialization scores accounted for the relationship between spindle density and nonverbal RIQ (r = 0.04, p = 0.67).
Conclusion
In a large cohort of young children with autism, spindle density was reduced compared to groups of age-matched children with DD or TD. Alterations in the maturational trajectory of spindles may provide valuable insight into the neurophysiologic differences related to behavior in disorders of neurodevelopment.
Autism spectrum disorder is a neurodevelopmental disorder characterized by deficits in social communication and reciprocal interaction accompanied by restricted, repetitive, and stereotyped patterns of behavior.1 Symptoms are present from early childhood and are impairing to everyday function. The disorder is heterogeneous in presentation and pleiotropic in origin, making the prognostication of developmental trajectories difficult. Many aspects of sleep physiology such as spindle activity2 follow a distinct maturational developmental pattern, and deviations may offer clues to differences in neural organization in autism and other disorders.
Sleep spindles are measured by EEG as brief distinct bursts of activity in the frequency range of 10 to 15 Hz. They have a characteristic waxing and waning shape and can be defined by their oscillatory frequency, amplitude, and density (events per minute of N2 sleep) throughout the night. They are a key grapho-element of the EEG used to identify the onset of non-REM N2 sleep and represent a gating function that signals deepening disengagement from the surrounding environment. Sleep spindle characteristics are very stable from night to night within the individual,3 and the morphology and oscillatory frequency of spindles have been used as markers of the developing brain in infants4 because changes in spindle properties can be tracked over the lifespan.5–7 In addition, spindles are believed to play an important functional role in sleep-dependent synaptic plasticity and memory consolidation,8 although their relationship with cognition may be sexually dimorphic.9,10
We evaluated spindle density, duration, morphology, and oscillatory features during N2 in a cohort of children with autism and comparison groups with nonautism developmental delay (DD) and typical development (TD). We hypothesized that the spindle activity of children with autism differs from that of the comparison groups.
Methods
Study design and participants
Sleep spindle data were obtained from 135 children 2 to 6 years of age with DSM-IV-TR autistic disorder (referred to as the autism group; n = 85, 84% male, 69% white), DD (n = 21, 62% male, 60% white), or TD (n = 29, 72% male, 82% white). Other sleep/EEG parameters from subsets of children enrolled in this study are reported elsewhere.11–13
Recruitment for the autism group was based on parental concern about autism or a previous autism diagnosis, through community resources such as pediatrician's offices and flyers. Children were enrolled in the autism group if they met the DSM-IV-TR criteria for autistic disorder based on the Autism Diagnostic Interview–Revised,14 the Autism Diagnostic Observation Schedule,15 and clinical judgment. Cognitive ability was assessed with a ratio IQ (RIQ) from either the Mullen Scales of Early Learning16 or the Differential Abilities Scales, Second Edition.17
Children in the DD group served as an IQ-matched comparison group. Like the autism group, these children were evaluated with the Autism Diagnostic Interview–Revised and Autism Diagnostic Observation Schedule, but an autism diagnosis was unequivocally ruled out. The additional entry criterion for this group was based on cognitive scores (verbal and nonverbal, from the tests described above) at least 1.5 SDs below the mean. The TD group received the Autism Diagnostic Observation Schedule, a parent-report autism screener,18 and the cognitive evaluation. They were enrolled in the TD group if there was no evidence of personal or first-degree family history of autism or significant developmental delay.
The comparison groups in this study were selected to allow for the decomposition of differences between children with autism and TD. Necessarily, children in the autism group differed from children in the DD and TD groups in autism symptom severity. However, the autism group was enriched for cognitive deficit and minimal language. Thus, differences between autism and TD might be driven by symptoms of autism or by cognitive deficit, which is independent of the autism diagnosis. The DD group, which is matched to the autism group on cognitive impairment, allows us to explore whether a phenomenon is more strongly related to autism symptoms or cognitive ability. Thus, we may deduce that constructs on which autism differs from TD but not DD may be related to a general developmental abnormality, while those distinguishing autism from both DD and TD may be specially related to autism.
Standard protocol approvals, registrations, and patient consents
The sample consisted of participants in an NIH natural history study of autism approved by the National Institutes of Health Institutional Review Board (NCT00298246). Consent was obtained from parents or guardians.
Sleep spindle quantification/qualification
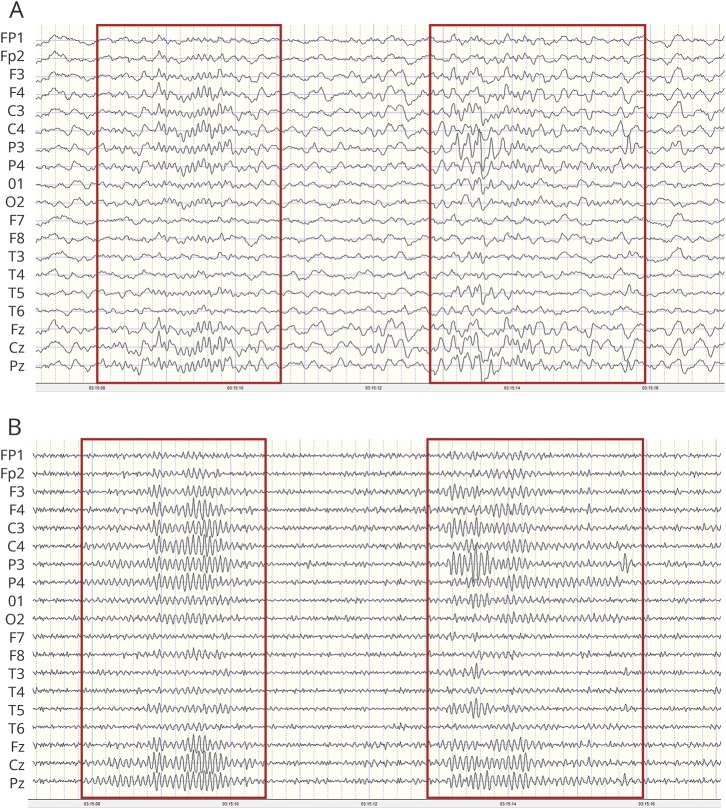
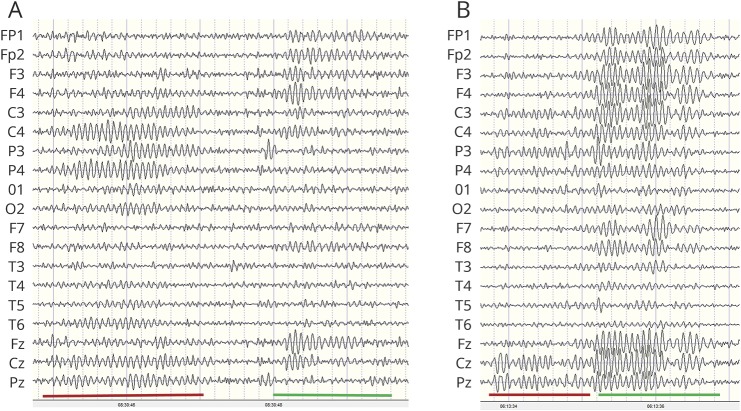
Overnight digital EEGs with video recordings were obtained for awake, drowsy, and sleep states. The 10-20 system for electrode placement was used; the high-pass filter was set at 1 Hz and the low-pass filter at 70 Hz. EEGs were analyzed with NeuroGuide (Applied Neuroscience, Inc, Largo, FL) using methods described elsewhere.13 Sleep spindles were designated as bursts of rhythmic, monomorphic oscillations, diphasic and symmetric in relationship to baseline, and characterized by progressively increasing and then decreasing amplitude activity with a frequency of 10 to 16 Hz and a duration of 0.5 to 5.0 seconds. We used manual counting, the gold standard for spindle quantification.19 While automated detection algorithms have been developed, they have yet to be demonstrated to be comparable or superior to manual counting19,20 (figure 1). Spindles were manually counted by an electroencephalographer (G.L.H.) for 5 minutes beginning with the first N2 stage followed by the next 3 consecutive N2 stages to produce what is referred to herein as spindle density. Spindle density was independently confirmed in a randomly selected subset of 10 participants by a second rater (P.C.), yielding an intraclass correlation of 0.986 (95% confidence interval [CI] 0.948–0.996). All records were masked, and no clinical information (such as group) was attached to the EEGs. Two types of spindles have been identified (slow spindles <12 Hz with a frontal maximum and fast spindles between 12 and 14 Hz with a centroparietal maximum).21 However, it is unclear whether the types have distinct generators. In our cohort of patients, there often was not a distinct difference in frequency between spindles with an anterior vs posterior voltage predominance, and propagation of centroparietal oscillations often spread to the anterior head region (figure 2). In addition, there is evidence from magnetoencephalography data that the generation of fast and slow spindles as observed is related to the propagation of spindle discharges from the posterior to anterior cortex and that analysis of central and frontal spindles as a separate phenomenon may lead to misinterpretation of the physiologic process of global spindle generation and pacing.2,22 For these reasons, no distinction was made between fast and slow spindles.
Figure 1. Counting methods for sleep spindles.
(A) After identification of the N2 sleep stage, frequency (hertz) of spindles was determined with a linked-ears montage with the low-pass filter set at 40 Hz and the high pass filter at 10 Hz. Red boxes demarcate periods with spindles. (B) After identification of sleep spindles, the high-pass filter was set at 10 Hz to aid in quantifying spindle density and duration.
Figure 2. Centroparietal and frontal spindles.
(A) Example of similar frequency (12 Hz) of centroparietal (red underline) and frontal (green underline) spindles. (B) Note the onset of spindles in the centroparietal region (red underline) with spread after 1 second to the frontal region (green underline).
To characterize dynamic oscillatory features, we also analyzed the spindles for absolute spectral power, relative power, coherence, amplitude asymmetry, and cross-spectral power for the following 5 electrode pairs: C3-C4, Cz-Pz, F3-F4, Fz-Cz, and P3-P4. All spindles were analyzed with a fast Fourier transformation with the following parameters: epoch = 2 seconds at a sample rate of 128 samples per second = 256 digital time points and a frequency range from 0.5 to 40 Hz at a resolution of 0.5 Hz with a cosine taper window. Fast Fourier transformed absolute power (μV2) and relative power were calculated for each of the 10 electrodes referenced to linked ears for 10 to 15 Hz (or sigma), the frequency range for spindles used in this study. EEG amplitude asymmetry, a measure of the degree of association between amplitudes of the spindles, was computed as a ratio of differences in absolute power between 2 scalp locations or (A − B)/([A − B] × 200), where A and B are the absolute power recorded from 2 different electrode locations. When A = B, then the amplitude asymmetry = 0. Cross-spectral power, a frequency domain measure of the analysis of the cross-correlation of 2 time series, was calculated as the square root of the sums of squares of the real (cosine) and imaginary (sine) coefficients. EEG spectral coherence, a measure of the consistency of the phase between 2 EEG oscillations, serves as an estimate of the synchronization between the 2 signals and was measured for electrode pairs for sigma. Coherences vary from 0 (when there is no consistency between phases of 2 EEG signals) to 1 (when there is perfect alignment of phase). Coherence was defined as (Gxy[f]2)/(Gxx[f] × Gyy[f]) where Gxy(f) is the cross-power spectral density and Gxx(f) and Gyy(f) are the respective autopower spectral densities.
Statistical analysis
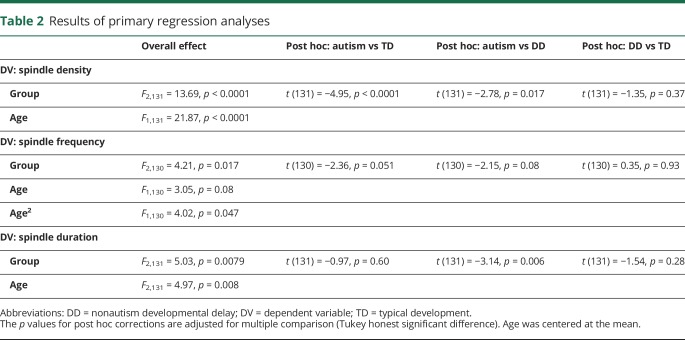
Data were analyzed with SAS/STAT version 9.3 (SAS Institute Inc, Cary, NC). For all analyses, α was set to 0.05. To accommodate distributional assumptions, coherence data were subjected to Fisher transformation before analysis, and absolute values of both amplitude asymmetry and cross-spectral power were used. Normality of residuals was assessed via visual inspection. Ordinary least-squares regression with post hoc Tukey honest significant difference tests was used to compare groups on spindle density, frequency, and duration and EEG coherence, controlling for the effects of age centered at the mean. A quadratic effect of age was tested and discarded if not supported. Kruskal-Wallis tests were used to compare amplitude asymmetry and cross-spectral power between groups.
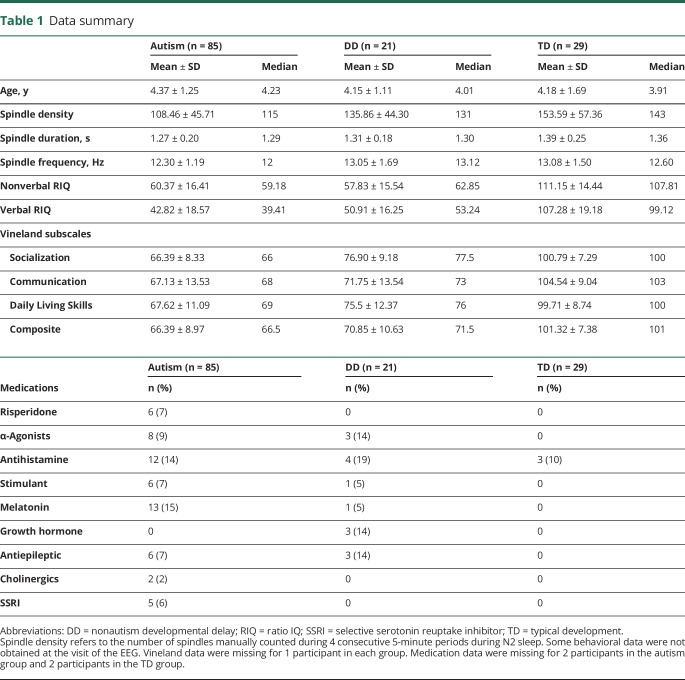
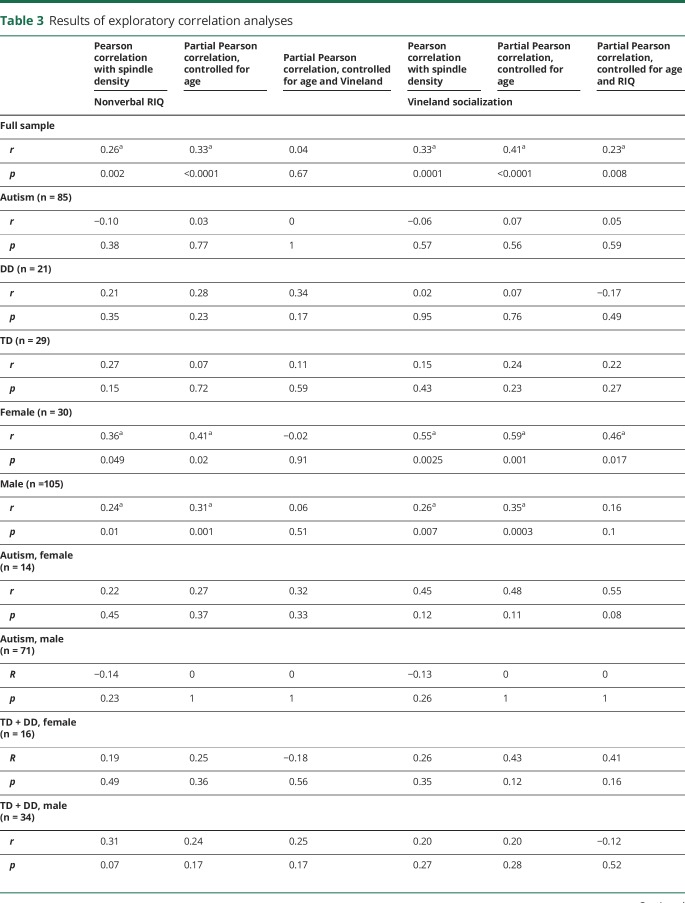
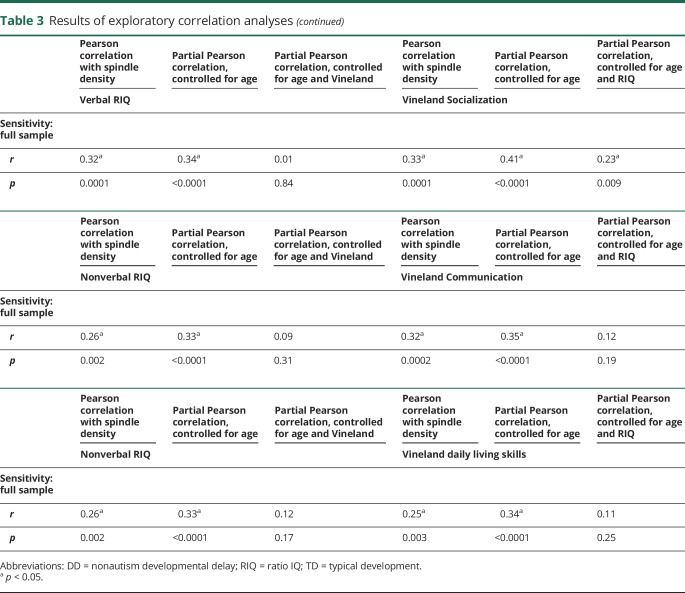
In exploratory analyses, we exploited the design of this study to evaluate the dimensional relationships between spindle density and the full spectrum of both cognitive ability (nonverbal RIQ) and social function (operationalized with the Vineland Adaptive Behavior Scales, Second Edition Socialization domain23; selected in lieu of a direct measure of autism symptoms such as the Autism Diagnostic Observation Schedule, which is not valid for such dimensional analysis in children who are not suspected of having autism24). With a series of partial Pearson correlations in the full sample, we explored whether controlling for either nonverbal RIQ or Vineland Socialization accounted for the correlation between the other variable and spindle density (i.e., partial Pearson correlation not different from zero). We performed sensitivity analyses with verbal IQ and with the other Vineland subscales (table 1).
Table 1.
Data summary
Data availability
Data are available from the National Institute of Mental Health Data Archive (Clinical and Immunologic Investigations of Subtypes of Autism No. 2368, ndar.nih.gov/edit_collection.html?id=2368) and on request from any qualified investigator.
Results
Group differences in spindle activity
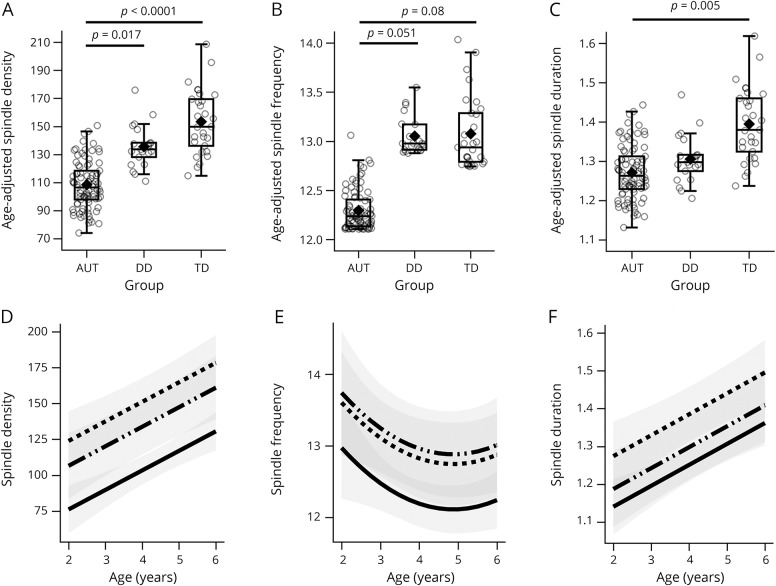
Controlling for age, spindle density was decreased in autism compared to both TD (age-adjusted mean difference 47.76, SE 9.65, Cohen d 0.93, 95% CI 0.49–1.37) and DD (age-adjusted mean difference 30.41 SE 10.94, Cohen d 0.61, 95% CI 0.13–1.09) groups (figure 3A and table 2). Spindle frequency was also reduced in the autism group compared to the DD (age-adjusted mean difference 0.77, SE 0.33, Cohen d 0.58, 95% CI 0.10–1.06) and TD (age-adjusted mean difference 0.63, SE 0.29, Cohen d 0.47, 95% CI 0.04–0.90) groups, but these differences failed to survive correction (figure 3B and table 2). Although the distribution of the spindle frequency residuals did not appear to upset assumptions, we also performed both nonparametric analysis of covariance25 and truncated regression to ensure consistency and obtained identical results. Spindle duration was also significantly shorter in the autism group compared to the TD group (age-adjusted mean difference 0.13, SE 0.04, Cohen d 0.58, 95% CI 0.15–1.01) (figure 3C and table 2).
Figure 3. Comparison of spindle parameters among groups.
(A–C) Primary between-group comparison, controlling for age (table 2 shows test statistics; p values are corrected for multiple comparison). (D–F) Effects of the covariate age in each of the models illustrated in panels A–C by group (solid = autism [AUT], dash-dot-dot = nonautism developmental delay [DD], dashes = typical development [TD]); estimated means were obtained across the age range and plotted with 95% confidence intervals (shaded regions). Interaction between age terms and group was evaluated in each model and discarded as nonsignificant. The amount of variance explained by each model (R2) was as follows: density 26%, frequency 10%, and duration 17%. Overall means not accounting for age are found in table 1.
Table 2.
Results of primary regression analyses
Relationship between spindle density and behavioral features
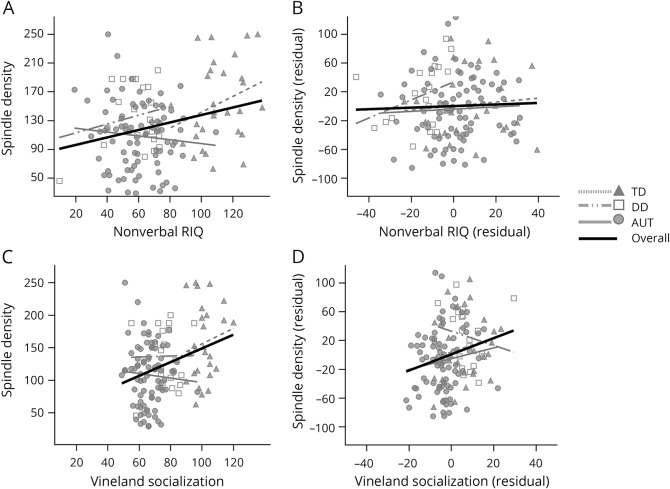
Given that we found differences between autism and both DD and TD in spindle density, we performed exploratory analyses to illustrate the relationship of spindle density to cognitive ability and social functioning. These were explored in the combined sample with a series of partial Pearson correlations. However, we note that these dimensional analyses are a recapitulation of the group differences and that the correlations between nonverbal RIQ/Vineland Socialization and spindle density do not hold within the group (figure 4 and table 3). Age was controlled in all models. Nonverbal RIQ and Vineland Socialization were strongly correlated with one another (r = 0.77, p < 0.001).
Figure 4. Exploratory correlation analyses.
(A and B) Correlation between nonverbal ratio IQ (RIQ) and spindle density and between residuals for nonverbal RIQ and spindle density after Vineland Socialization was partialled (i.e., correlation between residuals). (C and D) Correlation between Vineland Socialization and spindle density and between residuals for Vineland Socialization and spindle density after nonverbal RIQ was partialled (i.e., correlation between residuals). Correlations lines are shown for the full sample and within each diagnostic group. See table 3 for test statistics. AUT indicates autism; DD = nonautism developmental delay; and TD = typical development.
Table 3.
Results of exploratory correlation analyses
In the full sample, both nonverbal RIQ and Vineland Socialization increased with spindle density (table 3). The correlation between Vineland Socialization and density remained significant after partialling of nonverbal RIQ (r = 0.23, p = 0.008). However, the correlation between nonverbal RIQ and density was rendered null after partialling of Vineland Socialization (r = 0.04, p = 0.67). Identical results were obtained when verbal RIQ was substituted for nonverbal RIQ (table 3). However, when other Vineland subscales were substituted for Socialization (i.e., Communication, Daily Living Skills), the partial correlations controlling for RIQ were nonsignificant, supporting the specificity of the result (table 3). Finally, similar patterns of results were observed when spindle duration was used as the dependent variable, while neither Vineland Socialization nor nonverbal RIQ was significantly correlated with spindle frequency (controlling for the effect of age) (data not shown).
No systematic differences among groups were observed in power, relative power, coherence, cross-spectral power, or amplitude asymmetry in the 10- to 16-Hz bandwidths for the electrodes and electrode pairs studied (data not shown).
Sex differences in spindle activity
Sex was entered as a covariate into the primary analyses, and it did not significantly predict or interact with group or age in any model (data available on request). However, as shown in table 3, the relationships observed in the full sample among nonverbal RIQ, Vineland Socialization, and spindle density appeared to be driven by female participants. While the trend of a significant partial correlation between Vineland Socialization and spindle density after accounting for nonverbal RIQ was observed in both male and female participants, only in female participants was the correlation statistically significant.
Discussion
Relative to children with TD and DD, children with autism exhibited reduced spindle density. Spindle activity is believed to promote synaptic plasticity critical to memory formation during sleep,26 mediating the overnight consolidation of both declarative and procedural memory.8,27 These oscillations, originating in the thalamic reticular nucleus28 and recorded on the EEG as a distinct grapho-element, reflect the health of the thalamocortical microcircuit. The thalamic reticular nucleus is composed of GABA neurons29 and projects to glutamatergic thalamic neurons that subsequently project to the cortex. Cortical neurons send glutamatergic inputs back to NMDA acid receptors on thalamic reticular nucleus neurons. Because sleep spindles are temporally correlated with hippocampal sharp-wave ripples,30 coherent spindles across the cortex could reflect the synchronous reactivation of recent memory traces. It has been suggested that such reactivation mediates the consolidation of newly acquired information into more enduring forms for long-term storage, integration, and retrieval.31
Despite the likelihood that spindle activity is important in memory consolidation, a well-defined relationship between measurable spindle metrics and behavior, including cognition, has yet to be firmly established. Some of the earliest work evaluating spindle activity in neurodevelopmental populations documented abnormal spindle morphology as a feature of “mental retardation,”4 but the supposition that other spindle metrics may be compromised in intellectual disability is not confirmed. While 2 studies in adults8,27 documented that higher IQ was associated with more spindles, the literature in children is scant. One study of children 9 to 12 years of age identified that, as nonverbal IQ increased, slow spindles decreased, but more spindles were observed in faster, centrally recorded frequencies.32 Another study in preschoolers found that the spindles recorded from frontal leads of preschool-aged children were increased among those with better processing speed.33
In addition to age-related changes, the relationship of spindle activity to behavior and cognition may be moderated by sex. One study of 24 healthy adolescents between 12 and 22 years of age demonstrated that only in the female participants was spindle density related to IQ.34 A study of 28 younger children between the ages of 4 and 8 years also showed that only in the female children did spindle metrics associate with cognition.10 These studies add important information to the understanding of age-dependent trajectories and underscore the need to better define the normal neuro-maturational course of behaviorally relevant microcircuits under study.
Three studies of spindle activity in autism are published, but only one of them is pediatric: among 13 typically developing children/adolescents and 13 children/adolescents with autism, the autism group had fewer spindles at the right frontopolar (Fp2) electrode.35 The study of 8 patients with Asperger syndrome (mean age 23 years, range 7–53 years), who by definition were people with autism without intellectual disability, also documented reduced spindles relative to typical controls.36 The largest available study of 27 adults with autism also revealed fewer spindles than in age- and sex-matched typical controls.37 Our study used a nonautism DD group to determine whether differences in spindle activity are uniquely associated with autism and separate from intellectual disability. Given that the symptoms of autism may be conceptualized as a conglomeration of dimensional social function, it was possible to explore the unique relationships between both social impairment and cognitive ability to spindle phenomena.
Our study confirms that spindle density is reduced in autism. Furthermore, we demonstrated that spindles are reduced relative not only to TD but also to DD and that there is evidence for sexual dimorphism. While spindle density was greater among children with higher IQ and those with higher social functioning, exploratory correlational analyses suggested that the relationship with social functioning was at least partially independent of cognitive ability in the full sample. Dynamic features of spindles such as frequency, spectral power, and coherence did not differ as dramatically or at all between groups, indicating that cognitive deficits and impaired social functioning were less strongly related to oscillatory features of the spindles. Our current findings indicate that the dynamic features of spindles may play a less critical role in cognitive and social function than spindle density, especially in girls.
The specific role of sleep in the proper maturation of the developing brain is an area of intense interest. While the implications of a relative reduction in sleep spindles in young children with autism are not yet clear, that spindle activity may also be implicated in specific behavioral valences and impairments, separate from IQ, is an idea beginning to take hold in other disorders. For example, spindle deficits can be reliably established in cohorts of adults and adolescents with schizophrenia,38–40 for whom the body of work strongly implicates diffuse abnormalities in the thalamic reticular circuits.41–43 In addition, 1 study reported reduced spindle activity in children and adolescents who either had a diagnosis of early-onset major depressive disorder or were at risk for one.44 This latter study is illustrative of the value of the continued exploration of sleep-mediated processes in the developing brain in that the approach offers a practical, noninvasive avenue for the potential early identification and stratification of objective, proximal, neuropsychiatric/neurodevelopmental biomarkers.
This study is an evaluation of cross-sectional data from 3 study groups within a cohort and was not specifically designed to evaluate spindle activity. We did not exclude children who were on medications that potentially alter sleep physiology (detailed in table 1). To the best of our knowledge, none of the medications listed decrease spindle activity, and in fact, the only evidence we found for altering spindle metrics was that certain medications increase activity.45 We performed sensitivity analyses excluding children with the most common medications (melatonin and antihistamines) and obtained the same results. While our exploratory analyses complemented the group-based analyses in suggesting a unique relationship between spindle density and socialization, we note that these relationships were not observed at the group level (e.g., figure 4). Thus, interpretation cannot be extended to the individual, and future prospective research should attempt to identify confounded variables that may explain the relationship observed in this study. We note that our findings, especially sex-related differences, can be explored more fully in the future with comprehensive, longitudinal studies of electrophysiologic sleep phenomenon conducted in both typically developing and developmentally delayed or otherwise at-risk children. These studies will greatly add to our understanding of the relationship between microcircuits such as the thalamocortical feedback loop discussed herein, cognitive processes, and behavior.
This study confirmed others' findings of reduced spindle density in children with an autism diagnosis. This study extends current knowledge by using a larger and younger sample than others, with 2 control cohorts comprising IQ-matched children with DD and TD. Our results suggest a relationship between decreased spindle density and impaired social functioning, which warrants prospective study of spindle activity related to specific domains of impairment rather than the general cognitive impairment currently described in the literature.
Acknowledgment
The authors thank the many families who participated in this research.
Glossary
- CI
confidence interval
- DD
nonautism developmental delay
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision
- RIQ
ratio IQ
- TD
typical development
Author contributions
A.E.T., S.E.S., A.W.B., and G.L.H. conceived and designed the study. A.E.T, S.E.S., A.W.B., G.L.H., and P.C. performed the data acquisition. C.A.F., A.W.B., and G.L.H. analyzed and interpreted the data. A.E.T., S.E.S., A.W.B., G.L.H., P.C., and C.A.F. drafted and wrote the manuscript.
Study funding
This work was supported by the Intramural Program of the National Institute of Mental Health of the NIH (ZIAMH002914, protocol NCT00298246) and R03MH108915 (G.L.H.). The views expressed in this article do not necessarily represent the views of the National Institute of Mental Health, NIH, US Department of Health and Human Services, or US government. Protocol number 06-M-010.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Clawson BC, Durkin J, Aton SJ. Form and function of sleep spindles across the lifespan. Neural Plast 2016;2016:6936381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werth E, Achermann P, Dijk DJ, Borbély AA. Spindle frequency activity in the sleep EEG: individual differences and topographical distribution. Electroencephalogr Clin Neurophysiol 1997;103:535–542. [DOI] [PubMed] [Google Scholar]
- 4.Shibagaki M, Kiyono S, Watanabe K. Spindle evolution in normal and mentally retarded children: a review. Sleep 1982;5:47–57. [DOI] [PubMed] [Google Scholar]
- 5.Crowley K, Trinder J, Kim Y, Carrington M, Colrain IM. The effects of normal aging on sleep spindle and K-complex production. Clin Neurophysiol 2002;113:1615–1622. [DOI] [PubMed] [Google Scholar]
- 6.Nicolas A, Petit D, Rompre S, Montplaisir J. Sleep spindle characteristics in healthy subjects of different age groups. Clin Neurophysiol 2001;112:521–527. [DOI] [PubMed] [Google Scholar]
- 7.Martin N, Lafortune M, Godbout J, et al. Topography of age-related changes in sleep spindles. Neurobiol Aging 2013;34:468–476. [DOI] [PubMed] [Google Scholar]
- 8.Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehavioral Rev 2011;35:1154–1165. [DOI] [PubMed] [Google Scholar]
- 9.Ujma PP, Sándor P, Szakadát S, Gombos F, Bódizs R. Sleep spindles and intelligence in early childhood: developmental and trait-dependent aspects. Dev Psychol 2016;52:2118. [DOI] [PubMed] [Google Scholar]
- 10.Ujma PP, Konrad BN, Genzel L, et al. Sleep spindles and intelligence: evidence for a sexual dimorphism. J Neurosci 2014;34:16358–16368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckley A, Wingert K, Swedo S, et al. First night effect analysis in a cohort of young children with autism spectrum disorder. J Clin Sleep Med 2013;9:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckley AW, Rodriguez A, Jennison K, et al. Rapid eye movement sleep percentage in children with autism compared with children with developmental delay and typical development. Arch Pediatr Adolesc Med 2010;164:1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckley AW, Scott R, Tyler A, et al. State-dependent differences in functional connectivity in young children with autism spectrum disorder. EBioMedicine 2015;2:1905–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview–Revised. Los Angeles: Western Psychological Services; 2003:30. [Google Scholar]
- 15.Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule–generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 2000;30:205–223. [PubMed] [Google Scholar]
- 16.Mullen EM. Mullen Scales of Early Learning. Circle Pines: AGS Publishing: 1995. [Google Scholar]
- 17.Elliott CD. Differential Ability Scales.2nd ed. San Antonio: Psychological Corp; 2007. [Google Scholar]
- 18.Rutter M, LeCouteur A, Lord C. Social Communication Questionnaire (SCQ). Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 19.Warby SC, Wendt SL, Welinder P, et al. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat Methods 2014;11:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ujma PP, Gombos F, Genzel L, et al. A comparison of two sleep spindle detection methods based on all night averages: individually adjusted vs. fixed frequencies. Front Hum Neurosci 2015;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jankel W, Niedermeyer E. Sleep spindles. J Clin Neurophysiol 1985;2:1–36. [DOI] [PubMed] [Google Scholar]
- 22.Dehghani N, Cash SS, Chen CC, et al. Divergent cortical generators of MEG and EEG during human sleep spindles suggested by distributed source modeling. PLoS One 2010;5:e11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparrow SS, Balla DA, Cicchetti DV. Vineland-II Adaptive Behavior Scales: Circle Pines: AGS Publishing; 2005. [Google Scholar]
- 24.Kuhfeld M, Sturm A. An examination of the precision of the autism diagnostic observation Schedule using item response theory. Psychol Assess 2018;30:656–668. [DOI] [PubMed] [Google Scholar]
- 25.Zink RC, Koch GG. NParCov3: a SAS/IML macro for nonparametric randomization-based analysis of covariance. J Stat Softw 2012;50:1–17.25317082 [Google Scholar]
- 26.Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci 2005;25:9398–9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schabus M, Hödlmoser K, Gruber G, et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci 2006;23:1738–1746. [DOI] [PubMed] [Google Scholar]
- 28.Contreras D, Steriade M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J Physiol 1996;490:159–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houser CR, Vaughn JE, Barber RP, Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res 1980;200:341–354. [DOI] [PubMed] [Google Scholar]
- 30.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 1998;21:1123–1128. [DOI] [PubMed] [Google Scholar]
- 31.Buszsaki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res 1998;7:17–23. [DOI] [PubMed] [Google Scholar]
- 32.Geiger A, Huber R, Kurth S, Ringli M, Jenni OG, Achermann P. The sleep EEG as a marker of intellectual ability in school age children. Sleep 2011;34:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doucette MR, Kurth S, Chevalier N, Munakata Y, LeBourgeois MK. Topography of slow sigma power during sleep is associated with processing speed in preschool children. Brain Sci 2015;5:494–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bódizs R, Gombos F, Ujma PP, Kovács I. Sleep spindling and fluid intelligence across adolescent development: sex matters. Front Hum Neurosci 2014;8:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tessier S, Lambert A, Chicoine M, Scherzer P, Soulières I, Godbout R. Intelligence measures and stage 2 sleep in typically-developing and autistic children. Int J Psychophysiol 2015;97:58–65. [DOI] [PubMed] [Google Scholar]
- 36.Godbout R, Bergeron C, Limoges E, Stip E, Mottron L. A laboratory study of sleep in Asperger's syndrome. Neuroreport 2000;11:127–130. [DOI] [PubMed] [Google Scholar]
- 37.Limoges E, Mottron L, Bolduc C, Berthiaume C, Godbout R. Atypical sleep architecture and the autism phenotype. Brain 2005;128:1049–1061. [DOI] [PubMed] [Google Scholar]
- 38.Göder R, Graf A, Ballhausen F, et al. Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med 2015;16:564–569. [DOI] [PubMed] [Google Scholar]
- 39.Manoach DS, Pan JQ, Purcell SM, Stickgold R. Reduced sleep spindles in schizophrenia: a treatable endophenotype that links risk genes to impaired cognition? Biol Psychiatry 2016;80:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wamsley EJ, Tucker MA, Shinn AK, et al. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry 2012;71:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibrahim HM, Hogg AJ Jr, Healy DJ, Haroutunian V, Davis KL, Meador-Woodruff JH. Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am J Psychiatry 2000;157:1811–1823. [DOI] [PubMed] [Google Scholar]
- 42.Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry 2001;158:1393–1399. [DOI] [PubMed] [Google Scholar]
- 43.Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase 67 mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res 2009;43:970–977. [DOI] [PubMed] [Google Scholar]
- 44.Lopez J, Hoffmann R, Armitage R. Reduced sleep spindle activity in early-onset and elevated risk for depression. J Am Acad Child Adolesc Psychiatry 2010;49:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dijk DJ, Cajochen C. Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. J Biol Rhythms 1997;12:627–635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the National Institute of Mental Health Data Archive (Clinical and Immunologic Investigations of Subtypes of Autism No. 2368, ndar.nih.gov/edit_collection.html?id=2368) and on request from any qualified investigator.