Abstract
Active contraction of the diaphragm and other inspiratory pump muscles during swallow create a negative thoracic pressure to improve the movement of the bolus (food/liquid) into the esophagus. We tested the hypothesis that dorsomedial medullary inspiratory neurons, including the nucleus tractus solitarius (NTS, pre-motor to the phrenic) would be active during swallow induced by oral water infusion. We recorded neurons in the NTS and medial reticular formation in anesthetized spontaneously breathing cats, and induced swallow by injection of water into the oropharynx. Our results indicate that: 1) a majority of inspiratory cells in the dorsomedial medulla are active during swallow, 2) expiratory neurons are present in the medial reticular formation (deeper to the NTS) in unparalyzed cats and a majority of these cells decreased firing frequency during swallow. Our findings suggest that the dorsomedial medulla is a source of inspiratory motor drive during swallow and that a novel population of breathing-modulated neurons that also are modulated during swallowing exist in the medial reticular formation in unparalyzed animals.
Introduction
The “swallow-breath” (i.e. schluckatmung in German) was of great interest in the earliest experiments in deglutition [1, 2]. While there have been relatively few studies recently, Bosma [2] stated that the schluckatmung “has been studied extensively”, and references the work of several groups of European scientists. This issue was discussed by Marckwald [1] in Appendix I, with the ultimate conclusion that diaphragm movement is an active portion of swallowing produced by the swallow central pattern generator (CPG). Summarizing earlier work together with his studies in the rabbit he drew the following conclusions: a) the phrenic motor burst is approximately 200–300 ms after the burst of the mylohyoid; b) ablation of the phrenic nerve did not suppress all movement of the thorax during swallowing; c) central apnea (induced by barbiturate overdose) did not change the schluckatmung magnitude; and d) brainstem transection of the respiratory centers from the “swallow” centers abolished the schluckatmung.
During the 1960–70’s, with the use of esophageal manometry, the negative pressure during swallow was attributed to elongation of the esophagus termed “initial negative deflection” [3]. More recently, work by McConnel has termed it “hypopharyngeal suction pump” and reported it to be created by laryngeal elevation and the opening of the UES [4–9]. However, recordings during swallow in infants [10], adults [11], cats [12–15] and goats [16–18] demonstrate that electromyography (EMG) activity of inspiratory muscles occurs at the onset of swallow. More specifically, this diaphragmatic activity did not produce an inspiratory airflow, as measured with spirometry (i.e. airflow at the level of the mouth), but did produce a negative esophageal pressure deflection [11].
In general, it is understood that dorsal inspiratory neurons are active during swallow. Saito and colleagues [19] recorded from respiratory and non-respiratory neurons in the nucleus of the tractus solitarius (NTS) during fictive swallow in rats, and demonstrated that 19/53 inspiratory neurons were active during the hypoglossal burst. Additionally, Gestreau and colleagues [20] recorded from inspiratory neurons in the dorsal respiratory group of cats and reported that 28/33 neurons were active at the beginning of fictive swallow, however all displayed a peak discharge frequency lower than during breathing. Conversely, when we inspect examples from the presented figures the phrenic burst during swallow is very small compared to breathing-related activity. Of note, these studies used electrical stimulation of the superior laryngeal nerve to produce swallowing, which also produces profound suppression of phrenic motor neurons. Due to this, we believe that the phrenic activity during swallow (schluckatmung) has been classified as “unimportant” and not vital to appropriate behavioral execution. However, our working theory is that the functional contribution of the schluckatmung to swallow is to expand the thoracic cavity, creating a negative trans-diaphragmatic pressure, supporting bolus transition across the upper esophageal sphincter (UES) (i.e. similar to the movement of air during inspiration).
Bellingham and colleagues [21], during breathing, demonstrated that superior laryngeal nerve stimulation inhibits inspiratory output via a short latency (8ms) chlorine–dependent oligo-synaptic inhibition of phrenic motoneurons. Additionally, Sun, et al. [22] found that this inhibition is due to activation of expiratory-decrementing neurons in the rostral ventral respiratory group (Bӧtzinger Complex) via the NTS. In light of these studies, we hypothesized that the behavior of dorsal medullary respiratory neurons during swallowing induced by natural stimuli in the anesthetized cat model would be different than what was previously reported. Additionally we hypothesized that a higher number of dorsal inspiratory neurons would increase their activity during swallow relative to breathing. This information is vital to understand the regulation of the schluckatmung and its importance in the normal swallow motor pattern.
Methods
Experiments were performed on six spontaneously breathing adult male cats. The protocol was approved by the University of Florida and University of Louisville Intuitional Animal Care and Use Committee (IACUC). The animals were initially anesthetized with sodium pentobarbital (Lundbeck, Inc., Deerfield, IL) (35 mg/kg i.v.); supplementary doses were given as needed (3–5 mg/kg i.v.). The right femoral artery and vein were cannulated to monitor blood pressure and administer i.v. fluids, respectively. Physiologic levels of end-tidal CO2 (4–4.5%), body temperature, and arterial blood gas composition were continually maintained and monitored.
EMGs were recorded using bipolar insulated fine wire electrodes according to the technique of Basmajian and Stecko [23]. Eight muscles were used to evaluate swallow occurrence: mylohyoid, geniohyoid, thyrohyoid, thyropharyngeus, thyroarytenoid, cricopharyngeus, parasternal, and costal diaphragm. These muscles span the actions during the pharyngeal phase of swallow: a) mylohyoid, geniohyoid and thyrohyoid for hyolaryngeal elevation; b) thyropharyngeus for inferior pharyngeal constrictor; c) cricopharyngeus for upper esophageal sphincter regulation; d) thyroarytenoid for laryngeal adduction; and e) parasternal and costal diaphragm for inspiratory activity [12, 13, 15, 24].
The digastric muscles were blunt dissected away from the surface of the mylohyoid and electrodes were placed in the left mylohyoid. A small horizontal incision was made at the rostral end of the right mylohyoid followed by an incision down the midline for approximately 5 mm to reveal the geniohyoid muscle. Electrodes were placed 1 cm from the caudal insertion of the geniohyoid muscle. The thyroarytenoid muscle electrodes were inserted through the cricothyroid window into the anterior portion of the vocal folds, which were visually inspected post-mortem. Minor rotation of the larynx and pharynx counterclockwise revealed the superior laryngeal nerve, which facilitated placement of the thyropharyngeus muscle electrodes. The thyropharyngeus is a fan shaped muscle with the smallest portion attached to the thyroid cartilage; electrodes were placed in the ventral, caudal portion of the muscle overlaying thyroid cartilage within 5 mm of the rostral insertion of the muscle. To place electrodes within the cricopharyngeus muscle, the larynx and pharynx were rotated counterclockwise to reveal the posterior aspect of the larynx. The edge of the cricoid cartilage was located by palpation and electrodes were placed in the cricopharyngeus muscle just cranial to the edge of this structure. Thyrohyoid muscle electrodes were inserted approximately 5 mm rostral to the attachment to the thyroid cartilage; those for the parasternal muscle were placed in the third intercostal space, just adjacent to the sternum, and the costal diaphragm EMGs were placed through the skin just under the xiphoid process. The positions of all electrodes were confirmed by visual inspection (following electrode placement and post-mortem) and by EMG activity patterns during breathing and swallow.
Swallowing was defined as a quiescence of cricopharyngeus (upper esophageal sphincter) activity with overlapping mylohyoid, geniohyoid, thyropharyngeus, thyrohyoid, thyroarytenoid, inspiratory muscle activity (parasternal/diaphragm). Swallow can be clearly differentiated from other behaviors (augmented breath, laryngeal elevation, cough, expiration reflex, and aspiration reflex) by this activity pattern [15, 25–28].
The animals were then placed prone in a stereotaxic frame and the dorsal surface of the medulla was exposed with removal of the cerebellum. The surface of the brain stem was covered with warm paraffin oil.
Medullary recordings
Extracellular recordings of single respiratory neurons were made with tungsten microelectrodes (10–15 MΏ) arranged in an 8- or 16- channel array. The electrodes were arranged either linearly or within a rectangular grid with tips averaging 200–500 microns apart and individually advanced in micron steps, allowing isolation of signals from single neurons. The array was placed using stereotaxic coordinates derived from Berman [29]. Coordinates for the NTS (i.e. dorsal respiratory group) neurons were from the level of the obex to 2000 μm rostral, 1500–2400 μm lateral to the midline, and <2500 μm deep (Fig 1). Electrodes were routinely driven deeper than these coordinates into the medial reticular formation (MRF) (as deep as 4600 μm from the surface of the medulla) because the frequency of encountering breathing modulated neurons did not decrease with depth (Fig 2). The neurons were identified as inspiratory or expiratory by their discharge relationship to diaphragm/parasternal EMG during breathing. Recording locations are demonstrated in Fig 3.
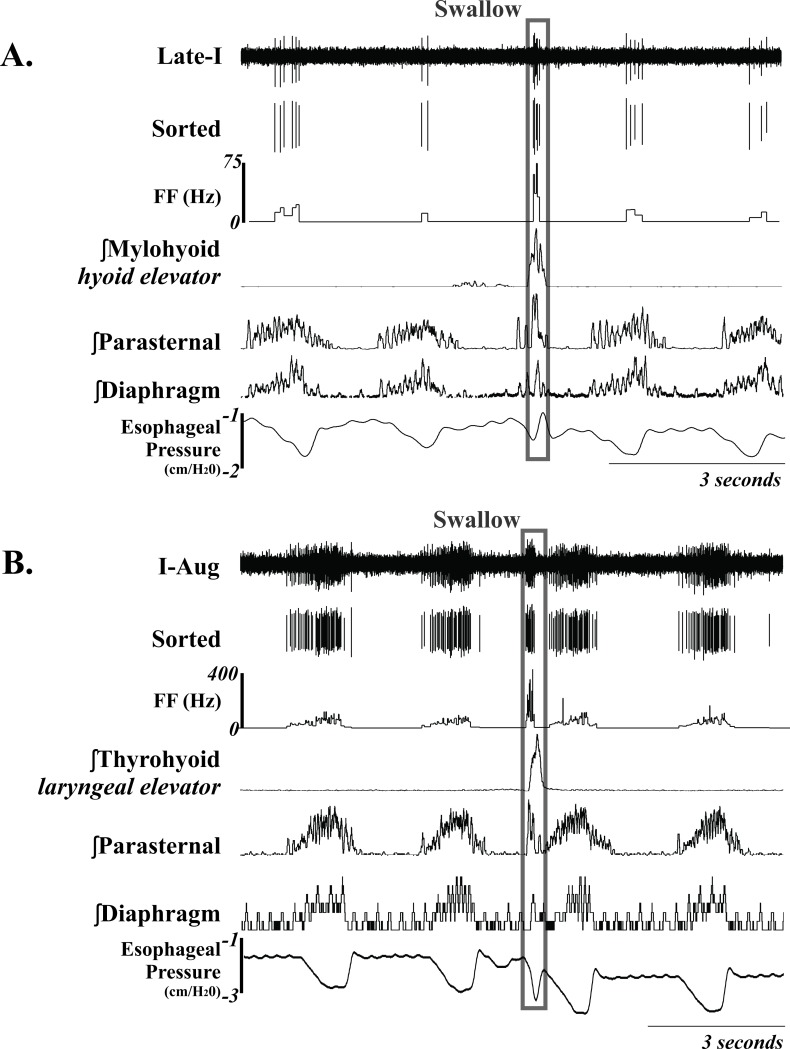
Fig 1. Example of two NTS Inspiratory neurons during breathing and swallow.
Unsorted and sorted spike trains, and instantaneous firing frequency (FF) (Hz) are displayed; demonstrating an increase in FF during swallow. The swallow is outlined in the gray box. See Table 2 for anatomical location and Table 1 for neuron discharge pattern definitions. Recordings of EMG moving averages from the mylohyoid, thyrohyoid, parasternal, diaphragm and esophageal pressure are also shown.
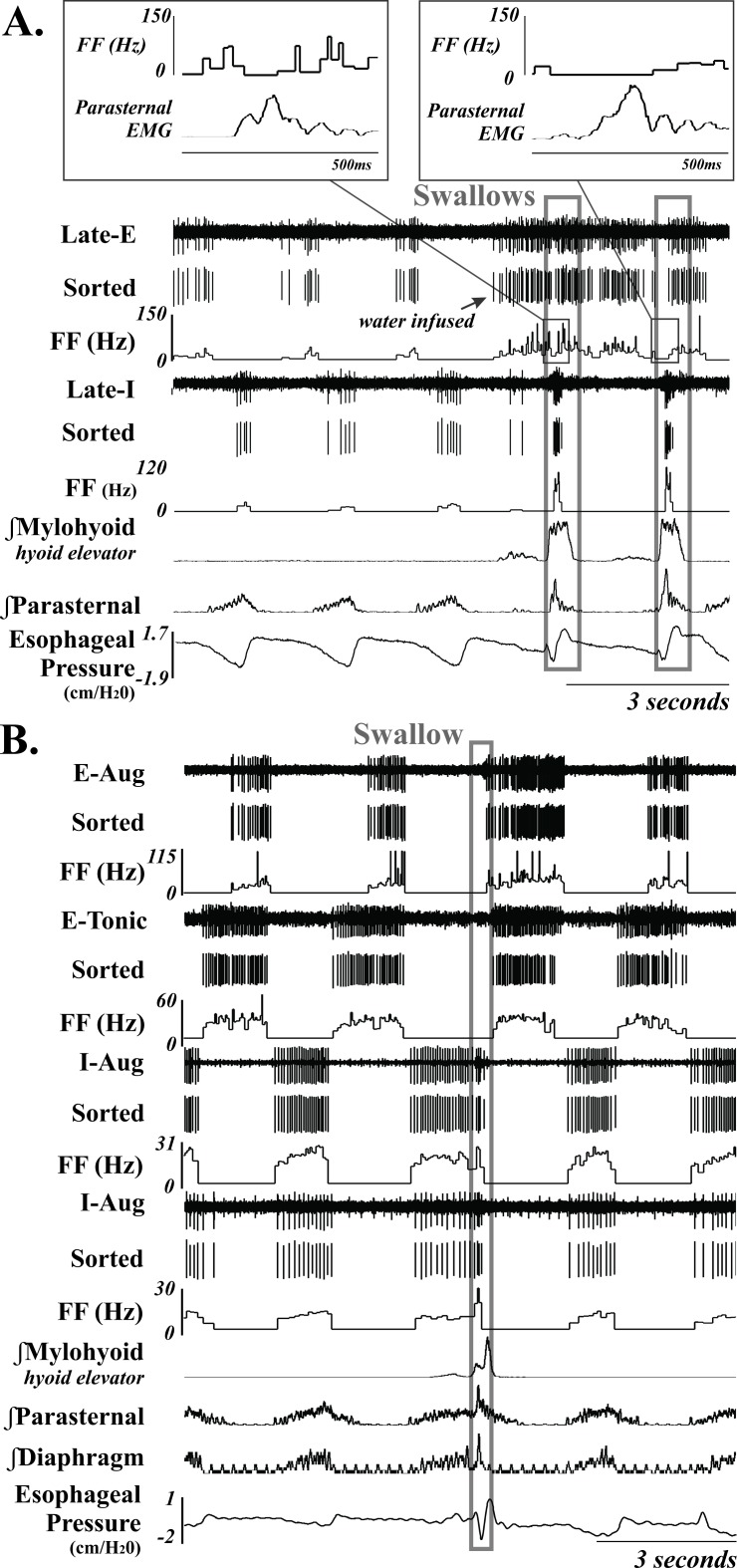
Fig 2. Example of 6 MRF neurons (3-I and 3-E) during breathing and swallow.
Unsorted and sorted spike trains, and instantaneous firing frequency (FF) (Hz) are displayed; demonstrating an increase in FF during swallow for the two I neurons. A also demonstrates more complicated swallow-related changes in the Late-E neurons firing frequency, with the second example having a longer suppression duration. B demonstrates a representative example of suppression of an E neuron during swallow. They often fire across the entire E duration, except during the execution of swallow. The swallows are outlined in a gray box. See Table 2 for anatomical location and Table 1 for neuron discharge pattern definitions. Recordings of EMG moving averages from the mylohyoid, parasternal, diaphragm (B only) are also shown.
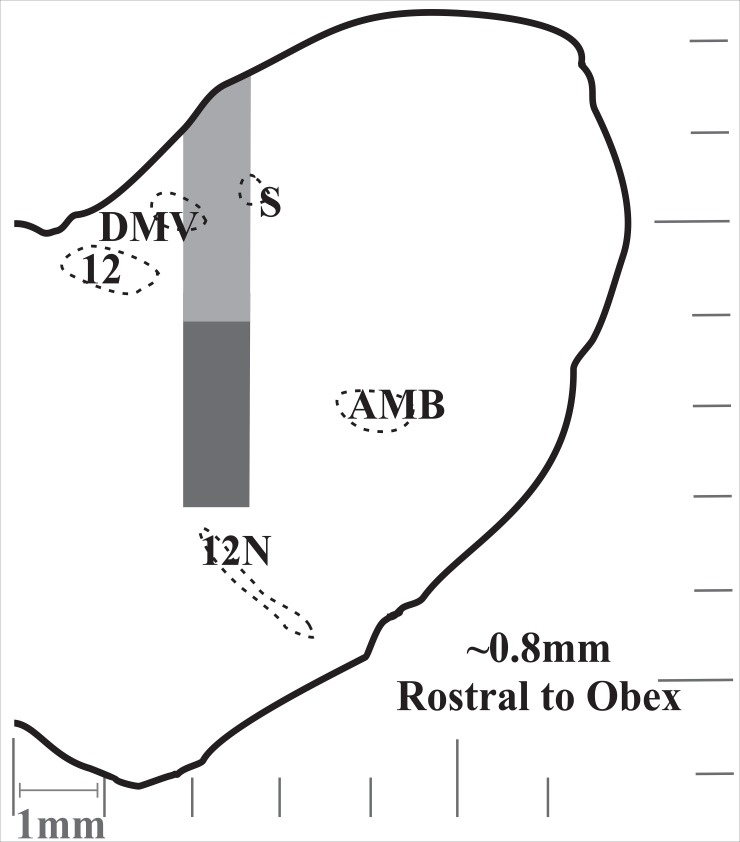
Fig 3. Display of recording location and depth at ~0.8mm rostral to obex.
Light gray represents the NTS and dark gray the medial reticular formation (MRF). Acronyms: S (solitary track; DMV (dorsal motor nucleus of the vagus); 12 (hypoglossal nucleus); AMB (nucleus ambiguus); and 12N (hypoglossal nerve). Fig was modified from Berman [29].
Elicitation of swallow
Following at least 5 breathing cycles swallow was elicited by infusion of 3ccs water into the oropharynx, via a one-inch long thin polyethylene catheter (diameter 0.5–1.0 mm), attached to a 6 ml syringe.
Data processing and statistical analysis
Spike trains were analyzed off-line with Spike 2 software (Cambridge Electronic Design, United Kingdom). Templates were created from action potentials on each channel and subjected to cluster analysis to differentiate each template from noise and other simultaneously recorded neurons. Typically, each recorded channel consisted of well isolated action potentials that were easily differentiated from spikes attributed to other neurons that were more distant from the electrode. Figs 1 and 2 demonstrates signals from the electrodes, sorted spike times, and firing frequency (FF) in Hz. Moving averages of EMGs were integrated with a 200 ms time constant. Table 1 provides the firing pattern descriptors, acronyms and definitions. If the swallow maximum FF was greater than or less than 1 standard deviation (SD) away from the frequency during breathing the neuron was marked as “changed” with an indication of the direction [increasing (↑) and decreasing (↓)] (Table 2).
Table 1. Terms used, acronyms, and definitions for neuron discharge patterns.
| Term | Acronym | Definition |
|---|---|---|
| Inspiratory | I | Maximum neuron firing rate during inspiration phase |
| Expiratory | E | Maximum neuron firing rate during expiration phase |
| Augmenting | -aug | Neuron whose firing rate increases throughout the phase |
| Decrementing | -dec | Neuron whose firing rate decreases throughout the phase |
| Tonic | -tonic | Neuron with no change in firing rate during the phase |
| Early | early- | Neuron with activity only at the beginning of the phase |
| Late | late- | Neuron with activity only at the end of the phase |
| Phase Span | -phase span | Neuron active across multi-phases of breathing |
Table 2. Summary of neuron discharge patterns and changes in peak firing frequency (FF) during swallow for all recorded neurons.
See Table 1 for neuron discharge pattern definitions.
| Total Cells | Increase^ | Decrease^ | No Change | Cell Active* | Not active** | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NTS | 18 | 7 | 39 | % | 7 | 39 | % | 4 | 22 | % | 16 | 89 | % | 2 | 11 | % |
| I-Aug | 8 | 3 | 38 | % | 3 | 38 | % | 2 | 25 | % | 7 | 88 | % | 1 | 13 | % |
| I-Dec | 1 | 1 | 100 | % | 1 | 100 | % | |||||||||
| I-Tonic | 4 | 2 | 50 | % | 2 | 50 | % | 3 | 75 | % | 1 | 25 | % | |||
| Late-I | 2 | 1 | 50 | % | 1 | 50 | % | 2 | 100 | % | ||||||
| I-Phase Span | 2 | 2 | 100 | % | 2 | 100 | % | |||||||||
| E-Tonic | 1 | 1 | 100 | % | 1 | 100 | % | |||||||||
| MRF | 68 | 21 | 31 | % | 32 | 47 | % | 14 | 21 | % | 57 | 84 | % | 10 | 15 | % |
| I-Aug | 13 | 4 | 31 | % | 7 | 54 | % | 2 | 15 | % | 11 | 85 | % | 2 | 15 | |
| I-Dec | 7 | 5 | 71 | % | 1 | 14 | % | 1 | 14 | % | 6 | 86 | % | 1 | 14 | |
| I-Tonic | 16 | 9 | 56 | % | 3 | 19 | % | 4 | 25 | % | 16 | 100 | % | |||
| Early-I | 2 | 2 | 100 | % | 2 | 100 | % | |||||||||
| Late-I | 4 | 1 | 25 | % | 1 | 25 | % | 2 | 50 | % | 4 | 100 | % | |||
| I-Phase Span | 3 | 1 | 33 | % | 2 | 67 | 3 | 100 | % | |||||||
| E-Aug | 7 | 5 | 71 | % | 1 | 6 | 86 | % | 1 | 14 | % | |||||
| E-Dec | 3 | 1 | 33 | % | 2 | 67 | % | 1 | 33 | % | 2 | 67 | % | |||
| E-Tonic | 7 | 7 | 100 | % | 4 | 57 | % | 3 | 43 | % | ||||||
| Early-E | 1 | 1 | 100 | % | 1 | 100 | % | |||||||||
| Late-E | 4 | 2 | 50 | % | 2 | 50 | % | 4 | 100 | % | ||||||
| E-Phase Span | 1 | 1 | 100 | % | 1 | 100 | % | |||||||||
*At least one action potential during swallow
** No action potentials present during swallow
^ ± 1 SD change in FF from breathing
Data is represented as mean ± SD. For statistical analysis Pearson chi-square test of independence was used to examine associations. A relationship was considered significant if the p-value was less than 0.05.
Results
Eighty-six neurons were recorded from the dorsomedial medulla in the left brainstem. Infusion of water into the oropharynx resulted in a total of 297 swallows and 98% (291/297) occurred during the expiratory phase of breathing. Table 2 lists the recording site for each neuron with a peak-to-peak FF in Hz representation of its respiratory phase and peak FF change with swallow. Eighteen neurons were recorded within the region of the NTS (<2500μm), and 71 neurons within the MRF. Within the NTS 94% (17/18) were inspiratory (2 were phase spanning), 89% (16/18) were active during swallow, and 39% (7/18) increased in FF over that observed during breathing (Tables 2 and 3). The one E neuron in the NTS was active during swallow, but did not change its FF. Within the MRF 66% (45/68) of the recorded neurons were I (3 phase spanning), with the remaining 23 E (1 phase spanning). Of the MRF I cells 93% (42/45) were active during swallow, and 44% (20/45) increased their FF over that observed during breathing. While 65% (15/23) of the MRF E cells were active during swallow, 78% (18/23) decreased their FF during swallow (compared to breathing).
Table 3. Neurons recorded from the dorsomedial medulla described by neuron discharge identity; depth; coordinates for medial-lateral (ML); rostral-caudal (RC); the maximum peak-to-peak firing frequency (FF; in Hz) during breathing and swallow; and direction of change.
Neurons are arranged by discharge identity (see Table 1 for descriptions) and the NTS/dorsal respiratory group neurons (< 2500 μm depth) are italicized. Neurons displayed in Figs are noted under the identity column.
| M-L | R-C | Depth | Identity | Breathing | Swallow | Change* | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inspiratory- Increase FF | ||||||||||
| 2281 | 1713 | 1332 | I-Aug | 39 | ± | 9 | 146 | ± | 164 | ↑ |
| 2581 | 900 | 1432 | I-Aug | 43 | ± | 6 | 65 | ± | 38 | ↑ |
| 2000 | 350 | 2909 | I-Aug | 77 | ± | 6 | 90 | ± | 32 | ↑ |
| 2000 | 2688 | 4720 | I-Aug | 40 | ± | 2 | 51 | ± | 8 | ↑ |
| 2000 | 688 | 672 | I-Dec | 43 | ± | 11 | 211 | ± | 138 | ↑ |
| 2000 | 2300 | 3283 | I-Dec | 14 | ± | 8 | 204 | ± | 97 | ↑ |
| 2000 | 2300 | 3283 | I-Dec | 19 | ± | 15 | 92 | ± | 99 | ↑ |
| 2200 | 825 | 3488 | I-Dec | 83 | ± | 14 | 106 | ± | 194 | ↑ |
| 2000 | 725 | 3489 | I-Dec | 48 | ± | 3 | 146 | ± | 0 | ↑ |
| 2000 | 688 | 4505 | I-Dec | 47 | ± | 10 | 181 | ± | 139 | ↑ |
| 2150 | 2138 | 2462 | I-Tonic | 45 | ± | 3 | 64 | ± | 15 | ↑ |
| 1900 | 725 | 2467 | I-Tonic | 209 | ± | 113 | 393 | ± | 74 | ↑ |
| 2150 | 1875 | 2513 | I-Tonic | 33 | ± | 1 | 218 | ± | 84 | ↑ |
| 2100 | 1413 | 2922 | I-Tonic | 14 | ± | 1 | 36 | ± | 6 | ↑ |
| 2450 | 1413 | 2922 | I-Tonic | 13 | ± | 1 | 37 | ± | 1 | ↑ |
| 2255 | 750 | 3230 | I-Tonic | 18 | ± | 2 | 266 | ± | 233 | ↑ |
| 2000 | 2300 | 3283 | I-Tonic | 22 | ± | 14 | 218 | ± | 63 | ↑ |
| 2200 | 0 | 3347 | I-Tonic | 21 | ± | 2 | 114 | ± | 16 | ↑ |
| 2200 | 0 | 3347 | I-Tonic | 21 | ± | 2 | 222 | ± | 50 | ↑ |
| 2150 | 1875 | 3622 | I-Tonic | 278 | ± | 109 | 583 | ± | 273 | ↑ |
| 2000 | 688 | 4505 | I-Tonic | 3 | ± | 2 | 314 | ± | 112 | ↑ |
| 2255 | 375 | 3769 | I-Tonic Fig 2B | 84 | ± | 5 | 103 | ± | 50 | ↑ |
| 2481 | 1325 | 2568 | Late-I | 10 | ± | 2 | 243 | ± | 0 | ↑ |
| 2450 | 2588 | 3305 | Late-I | 12 | ± | 7 | 190 | ± | 215 | ↑ |
| 1880 | 1525 | 3914 | Late-I | 13 | ± | 2 | 87 | ± | 20 | ↑ |
| 2100 | 1825 | 4378 | Late-I | 10 | ± | 1 | 36 | ± | 15 | ↑ |
| 2000 | 1238 | 608 | Late-I Fig 1A | 22 | ± | 8 | 82 | ± | 10 | ↑ |
| 1880 | 1125 | 3926 | Late-I Fig 2A | 21 | ± | 6 | 108 | ± | 9 | ↑ |
| Inspiratory- No Change FF | ||||||||||
| 2300 | 2588 | 3145 | Early-I | 294 | ± | 252 | 276 | ± | 255 | — |
| 2000 | 1913 | 4607 | Early-I | 46 | ± | 14 | 45 | ± | 10 | — |
| 2268 | 375 | 1198 | I-Aug | 298 | ± | 121 | 173 | ± | 77 | — |
| 2250 | 325 | 2062 | I-Aug | 143 | ± | 48 | 160 | ± | 46 | — |
| 2150 | 1950 | 2807 | I-Aug | 152 | ± | 220 | 96 | ± | 64 | — |
| 2000 | 2300 | 4406 | I-Aug | 736 | ± | 246 | 713 | ± | 446 | — |
| 2150 | 775 | 2450 | I-Aug Fig 1B | 77 | ± | 24 | 90 | ± | 44 | — |
| 2000 | 725 | 3489 | I-Dec | 503 | ± | 185 | 478 | ± | 268 | — |
| 2255 | 375 | 2583 | I-Tonic | 576 | ± | 389 | 722 | ± | 347 | — |
| 2150 | 1950 | 2807 | I-Tonic | 83 | ± | 21 | 92 | ± | 126 | — |
| 2100 | 1950 | 2946 | I-Tonic | 346 | ± | 256 | 110 | ± | 66 | — |
| 2250 | 1113 | 3236 | I-Tonic | 23 | ± | 1 | 1 | ± | 0 | — |
| 2250 | 2138 | 4130 | I-Tonic | 11 | ± | 0 | 8 | ± | 11 | — |
| 2181 | 900 | 2260 | Late-I | 11 | ± | 3 | 12 | ± | 21 | — |
| 2150 | 2700 | 3681 | Late-I | 22 | ± | 19 | 34 | ± | 0 | — |
| 1880 | 375 | 3920 | Late-I | 51 | ± | 19 | 38 | ± | 54 | — |
| Inspiratory- Decrease FF | ||||||||||
| 2245 | 0 | 1943 | I-Aug | 247 | ± | 208 | 0 | ± | 0 | ↓ |
| 2250 | 325 | 2159 | I-Aug | 111 | ± | 16 | 80 | ± | 22 | ↓ |
| 2245 | 375 | 2258 | I-Aug | 342 | ± | 19 | 269 | ± | 124 | ↓ |
| 2000 | 2263 | 2579 | I-Aug | 35 | ± | 9 | 0 | ± | 0 | ↓ |
| 2400 | 2588 | 3305 | I-Aug | 41 | ± | 12 | 1 | ± | 0 | ↓ |
| 2481 | 1713 | 3375 | I-Aug | 51 | ± | 3 | 0 | ± | 0 | ↓ |
| 2275 | 750 | 3944 | I-Aug | 67 | ± | 10 | 0 | ± | 0 | ↓ |
| 1880 | 1950 | 4120 | I-Aug | 38 | ± | 3 | 18 | ± | 25 | ↓ |
| 2300 | 2213 | 4552 | I-Aug | 251 | ± | 92 | 83 | ± | 63 | ↓ |
| 2255 | 1413 | 4554 | I-Aug | 33 | ± | 1 | 24 | ± | 1 | ↓ |
| 2200 | 825 | 3043 | I-Dec | 273 | ± | 49 | 0 | ± | 0 | ↓ |
| 2150 | 0 | 1612 | I-Tonic | 91 | ± | 14 | 57 | ± | 21 | ↓ |
| 2245 | 0 | 1943 | I-Tonic | 407 | ± | 386 | 0 | ± | 0 | ↓ |
| 2250 | 325 | 2062 | I-Tonic | 43 | ± | 10 | 24 | ± | 19 | ↓ |
| 2150 | 325 | 2349 | I-Tonic | 805 | ± | 180 | 297 | ± | 212 | ↓ |
| 2100 | 1950 | 2946 | I-Tonic | 600 | ± | 424 | 29 | ± | 23 | ↓ |
| 2181 | 1713 | 3221 | I-Tonic | 54 | ± | 9 | 16 | ± | 19 | ↓ |
| 1880 | 1950 | 3639 | I-Tonic | 175 | ± | 34 | 11 | ± | 6 | ↓ |
| 2000 | 1550 | 3766 | I-Tonic | 46 | ± | 2 | 25 | ± | 21 | ↓ |
| 2250 | 2138 | 4130 | I-Tonic | 11 | ± | 1 | 8 | ± | 11 | ↓ |
| 2100 | 2700 | 3078 | Late-I | 34 | ± | 6 | 5 | ± | 4 | ↓ |
| Expiratory- Increase FF | ||||||||||
| 2000 | 725 | 4145 | E-Aug | 109 | ± | 0 | 126 | ± | 4 | ↑ |
| 1880 | 1125 | 3662 | E-Dec | 76 | ± | 4 | 87 | ± | 25 | ↑ |
| 2000 | 2300 | 4406 | E-Tonic | 34 | ± | 3 | 219 | ± | 315 | ↑ |
| Expiratory- No Change FF | ||||||||||
| 2255 | 750 | 3307 | E-Late Fig 2A | 51 | ± | 14 | 60 | ± | 85 | — |
| 2200 | 2438 | 803 | E-Tonic | 43 | ± | 16 | 56 | ± | 12 | — |
| 1900 | 1913 | 4269 | Late-E | 61 | ± | 18 | 71 | ± | 25 | — |
| Expiratory- Decrease FF | ||||||||||
| 1880 | 1525 | 3881 | Early-E | 10 | ± | 3 | 0 | ± | 0 | ↓ |
| 2250 | 1875 | 3438 | E-Aug | 41 | ± | 6 | 0 | ± | 0 | ↓ |
| 2000 | 1550 | 3570 | E-Aug | 108 | ± | 64 | 4 | ± | 0 | ↓ |
| 1900 | 2688 | 3662 | E-Aug | 411 | ± | 846 | 9 | ± | 13 | ↓ |
| 1900 | 1913 | 3699 | E-Aug | 61 | ± | 10 | 32 | ± | 49 | ↓ |
| 2000 | 2688 | 4137 | E-Aug | 36 | ± | 2 | 18 | ± | 22 | ↓ |
| 2255 | 1200 | 3532 | E-Dec | 22 | ± | 8 | 0 | ± | 0 | ↓ |
| 2255 | 1413 | 3621 | E-Dec Fig 2B | 23 | ± | 1 | 0 | ± | 0 | ↓ |
| 2000 | 750 | 3427 | E-Tonic | 18 | ± | 4 | 12 | ± | 9 | ↓ |
| 2481 | 2438 | 3525 | E-Tonic | 343 | ± | 477 | 31 | ± | 0 | ↓ |
| 2000 | 1125 | 3536 | E-Tonic | 31 | ± | 4 | 0 | ± | 0 | ↓ |
| 1880 | 1950 | 3639 | E-Tonic | 7 | ± | 1 | 0 | ± | 0 | ↓ |
| 1880 | 2300 | 4053 | E-Tonic | 30 | ± | 4 | 0 | ± | 0 | ↓ |
| 2000 | 1713 | 4084 | E-Tonic | 19 | ± | 8 | 1 | ± | 0 | ↓ |
| 2000 | 2300 | 4406 | E-Tonic | 43 | ± | 2 | 0 | ± | 0 | ↓ |
| 1900 | 2688 | 4572 | E-Tonic | 33 | ± | 19 | 4 | ± | 8 | ↓ |
| 1900 | 2688 | 3662 | Late-E | 23 | ± | 3 | 4 | ± | 8 | ↓ |
| 1900 | 2300 | 4246 | Late-E | 24 | ± | 6 | 4 | ± | 10 | ↓ |
NTS neurons are italicized (depth <2500)
*Greater than or less than 1 SD away from the mean FF during breathing.
Inspiratory neurons that increased their firing frequency did so by an average of 820 ± 2115%. Inspiratory neurons who decreased their firing frequency did so by an average of 69 ± 30%, in contrast, expiratory neurons decreased their firing frequency by an average of 90 ± 16%.
A Pearson chi square test was performed to examine the relationship between respiratory phase and increase in firing frequency during swallow [X2 (1, n = 86) = 13.5, p < 0.001]; indicating inspiratory neurons in the dorsal medulla are more likely to increase firing frequency during swallow compared to expiratory neurons. Additionally, to examine the relationship between respiratory phase and the presence of neurons active during swallow [X2 (1, n = 86) = 8.49, p = 0.004]; indicating inspiratory neurons in the dorsal medulla are more likely to be active during swallow compared to expiratory neurons.
See S1 Text. NC3Rs ARRIVE Guidelines Checklist in Supporting Information for more information.
Discussion
This is the first study to report swallow-related firing frequency increases in inspiratory neurons in the dorsal medulla during swallow. The major findings of this study were that: of the recorded neurons in the dorsal medulla 73% of I neurons were active during swallow with 37% increasing in FF, and 67% of E neurons were active with 75% decreasing their FF. Additionally, we characterized a population of I and E neurons in the MRF during breathing and swallow which appears to be “anatomically” continuous with the NTS (Fig 3). Together this data expands our understanding of the overlapping regulation of breathing and swallow. Additionally, the increase in NTS I activity supports our hypothesis that the swallow pattern generator activates pre-motor neurons in the DRG for diaphragm recruitment during swallow (i.e. schluckatmung).
In freely breathing cats, I neurons in the region of the NTS that increased their FF during swallow relative to breathing did so by an average of 820%. The ventrolateral NTS contains a population of I neurons that has been termed the Dorsal Respiratory Group (DRG). Most of these I cells are bulbospinal and pre-motor to phrenic motoneurons [30–32]. Phrenic motoneurons innervate the diaphragm, and Figs 1 and 2 have examples of swallow demonstrating schluckatmung activity on parasternal and costal diaphragm EMG’s. However, FF of pre-motor neurons significantly contributes to motor drive, and our results demonstrate that it is possible for the pre-motor drive to inspiratory muscles to be higher during swallow when compared to breathing. Previously published recordings in decerebrate, decerebellate, paralyzed, ventilated cats, and using superior laryngeal nerve stimulation (SLN) to initiate swallow reported no I neurons increasing their FF during swallow [19, 20]. Of importance, neither of these papers discussed this I neuron activity relating to the schluckatmung as being important to the production of swallow. The lack of studies on I neuron activity during swallow is likely explained by two issues: a) the schluckatmung has not been universally accepted as a key feature in the swallow motor pattern; and b) the reported weak/reduced FF of I neurons during swallow lead to a theory that this activity is not “robust” enough to be important.
History of the research on inspiratory activity during swallow
Rosenthal [33] was the first to report on the diaphragm contractions during stimulation of the SLN, however Arloing [34] was the first to provide substantial evidence that these contractions are an active part of the swallow pattern, creating the negative deflection during swallow. It was hypothesized that the diaphragm activity produced a negative deflection in the thoracic cavity creating suction to pull the bolus (food/liquid) into the esophagus. Our results support the hypothesis that NTS I neurons contribute to I motor drive during swallow to generate this negative intrathoracic pressure. Additionally, we only recorded one E neuron with stereotaxic coordinates within the NTS and this finding is consistent with other reports i.e. Berger [30] and Wallois et al. [35].
McConnel and colleagues [4, 5, 36–40] postulated that the tongue force and negative esophageal deflection had the largest impact on movement of the bolus through the esophagus. It was further hypothesized that the “initial negative deflection” during the pharyngeal phase of swallow is achieved by the elevation of the larynx and opening of the upper esophageal sphincter (UES). We have strongly proposed that this function is by inspiratory muscle activity acting as an aspiration generating force (negative pressure), and it a vital part of the swallow motor pattern [12–15, 24].
Medial reticular formation (MRF)
We also recorded from a large population of respiratory neurons deeper than the NTS, in a region consistent with the medial reticular formation. One-third of this population discharged in phase with expiration. The occurrence of respiratory modulation of medial reticular formation neurons has been previously described in a series of papers from Lambertz, Langhorst, and Schultz in freely breathing dogs [41–44]. They also made extracellular recordings from a larger area (both rostral and caudal to obex) and reported that 30% had maximum activity during inspiration and 25% during expiration [41]. We propose that our experimental preparation (unparalyzed) increased the probability of encountering these neurons as paralyzed animals have historically been employed to investigate the locations of breathing-modulated neurons in the medulla.
The extent to which these neurons receive excitatory input from the central pattern generator for breathing in unknown. Neurons found in the MRF often receive feedback from somatic afferents via projections from spinoreticular neurons [45–47]. The source of respiratory drive to this population is less important than their function. Multiple anatomical studies have shown that neurons in this region of the medulla are members of pathways that are relevant to licking and chewing for example [48–50]. Further, some neurons in the medial reticular formation are pre-hypoglossal [51]. Our results show that some I neurons in this region increase their discharge rate during the schluckatmung, suggesting that they participate in swallow generation. In contrast to the activation of inspiratory cells, 83% of the expiratory cells had a decrease in firing frequency with 8/23 having no action potentials during swallow. As abdominal EMG suppression occurs during swallow [24], it is possible these expiratory neurons provide motor drive to spinal expiratory motor pathways, in addition to the caudal ventral respiratory column.
Critique of methods
This study had several limitations. The first is an inflation test was not performed to separate NTS I cells that are pre-motor to the diaphragm versus pump cells (i.e. responding to lung inflation). Secondarily, there are known effects of anesthesia on respiratory drive (i.e. reduction of central excitability and increase in CO2 tolerance) and possible suppression of the schluckatmung. However, we felt it was important to test the earlier work described by Marckwald [1], and determine if the experimental preparation may bias appreciation of the schluckatmung as an important part of the swallow pattern.
Conclusion
Inspiratory neurons in the dorsomedial medulla (including the NTS) contribute to the production of the of schluckatmung by increasing motor drive to inspiratory muscles. The schluckatmung is a reliable and robust portion of the swallow pattern in anesthetized animals. A novel population of expiratory neurons was identified in the medial reticular formation, deep to the NTS, that decreases activity during swallow, consistent with depression of expiratory motor drive that has been identified during this behavior. In conclusion, these results support the existence of an expanded neural network for the generation of swallowing that includes the medial medulla deep to the NTS.
Supporting information
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Research reported in this publication was supported by the Kentucky Spinal Cord and Head Injury Trust, The Commonwealth of Kentucky Challenge for Excellence, and the National Institute of Heart Lung and Blood of the National Institutes of Health under award R00HL111215; R01HL103415, R01HL109025, and R01HL131716. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Marckwald M. The movements of respiration and their innervation in the rabbit1888.
- 2.Bosma JF. Deglutition: Pharyngeal Stage. 1957. 10.1152/physrev.1957.37.3.275 [DOI] [PubMed] [Google Scholar]
- 3.Vantrappen G, Hellemans J. Studies on the normal deglutition complex. Digestive Diseases and Sciences. 1967;12(3):255–66. [DOI] [PubMed] [Google Scholar]
- 4.McConnel FM. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope. 1988;98(1):71–8. Epub 1988/01/01. 10.1288/00005537-198801000-00015 . [DOI] [PubMed] [Google Scholar]
- 5.McConnel FM, Cerenko D, Hersh T, Weil LJ. Evaluation of pharyngeal dysphagia with manofluorography. Dysphagia. 1988;2(4):187–95. Epub 1988/01/01. . [DOI] [PubMed] [Google Scholar]
- 6.McConnel FM, Cerenko D, Jackson RT, Guffin TN Jr. Timing of major events of pharyngeal swallowing. Arch Otolaryngol Head Neck Surg. 1988;114(12):1413–8. Epub 1988/12/01. . [DOI] [PubMed] [Google Scholar]
- 7.McConnel FM, Cerenko D, Jackson RT, Hersh T. Clinical application of the manofluorogram. Laryngoscope. 1988;98(7):705–11. Epub 1988/07/01. 10.1288/00005537-198807000-00003 . [DOI] [PubMed] [Google Scholar]
- 8.McConnel FM, Cerenko D, Mendelsohn MS. Manofluorographic analysis of swallowing. Otolaryngol Clin North Am. 1988;21(4):625–35. Epub 1988/11/01. . [PubMed] [Google Scholar]
- 9.McConnel FM, Mendelsohn MS, Logemann JA. Manofluorography of deglutition after supraglottic laryngectomy. Head & neck surgery. 1987;9(3):142–50. Epub 1987/01/01. . [DOI] [PubMed] [Google Scholar]
- 10.Wilson S, Thach B, Brouillette R, Abu-Osba Y. Coordination of breathing and swallowing in human infants. Journal of Applied Physiology. 1981;50(4):851 10.1152/jappl.1981.50.4.851 [DOI] [PubMed] [Google Scholar]
- 11.Hårdemark Cedborg AI, Sundman E, Bodén K, Hedström HW, Kuylenstierna R, Ekberg O, et al. Co‐ordination of spontaneous swallowing with respiratory airflow and diaphragmatic and abdominal muscle activity in healthy adult humans. Experimental Physiology. 2009;94(4):459–68. 10.1113/expphysiol.2008.045724 [DOI] [PubMed] [Google Scholar]
- 12.Pitts T, Rose MJ, Poliacek I, Condrey J, Davenport PW, Bolser DC. Effect of Laparotomy on the Swallow–Breathing Relationship in the Cat. Lung. 2015;193(1):129–33. 10.1007/s00408-014-9662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spearman DG, Poliacek I, Rose MJ, Bolser DC, Pitts T. Variability of the Pharyngeal Phase of Swallow in the Cat. 2014. 10.1371/journal.pone.0106121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitts T, Morris K, Lindsey B, Davenport P, Poliacek I, Bolser D. Co-ordination of cough and swallow in vivo and in silico. Experimental Physiology. 2012;97(4):469–73. 10.1113/expphysiol.2011.063362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitts T, Rose MJ, Mortensen AN, Poliaček I, Sapienza CM, Lindsey BG, et al. Coordination of cough and swallow: a meta-behavioral response to aspiration. Respiratory physiology & neurobiology. 2013;189(3):543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonis J, Neumueller S, Marshall B, Krause K, Qian B, Pan L, et al. The effects of lesions in the dorsolateral pons on the coordination of swallowing and breathing in awake goats. Respiratory Physiology and Neurobiology. 2011;175(2):272–82. 10.1016/j.resp.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feroah TR, Forster H, Fuentes CG, Lang IM, Beste D, Martino P, et al. Effects of spontaneous swallows on breathing in awake goats. Journal of Applied Physiology. 2002;92(5):1923–35. 10.1152/japplphysiol.01079.2000 [DOI] [PubMed] [Google Scholar]
- 18.Feroah TR, Forster H, Fuentes CG, Wenninger J, Martino P, Hodges M, et al. Contributions from rostral medullary nuclei to coordination of swallowing and breathing in awake goats. Journal of Applied Physiology. 2002;93(2):581–91. 10.1152/japplphysiol.01268.2001 [DOI] [PubMed] [Google Scholar]
- 19.Saito Y, Ezure K, Tanaka I. Swallowing-related activities of respiratory and non-respiratory neurons in the nucleus of solitary tract in the rat. Journal of Physiology. 2002;540(Pt 3):1047–60. Epub 2002/05/03. 10.1113/jphysiol.2001.014985 ; PubMed Central PMCID: PMCPMC2290262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gestreau C, Milano S, Bianchi AL, Grelot L. Activity of dorsal respiratory group inspiratory neurons during laryngeal-induced fictive coughing and swallowing in decerebrate cats. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1996;108(2):247–56. Epub 1996/03/01. . [DOI] [PubMed] [Google Scholar]
- 21.Bellingham M, Lipski J, Voss M. Synaptic inhibition of phrenic motoneurons evoked by stimulation of the superior laryngeal nerve. Brain research. 1989;486(2):391–5. [DOI] [PubMed] [Google Scholar]
- 22.Sun QJ, Bautista TG, Berkowitz RG, Zhao WJ, Pilowsky PM. The temporal relationship between non‐respiratory burst activity of expiratory laryngeal motoneurons and phrenic apnoea during stimulation of the superior laryngeal nerve in rat. The Journal of physiology. 2011;589(7):1819–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basmajian J, Stecko G. A new bipolar electrode for electromyography. Journal of Applied Physiology. 1962;17(5):849–. [Google Scholar]
- 24.Pitts T, Gayagoy A, Rose M, Poliacek I, Condrey J, Musslewhite M, et al. Suppression of Abdominal Motor Activity during Swallowing in Cats and Humans. PloS one. 2015;10(5):e0128245–e. 10.1371/journal.pone.0128245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol. 2009;102(2):1017–25. Epub 2009/06/12. doi: 00100.2009 [pii] 10.1152/jn.00100.2009 ; PubMed Central PMCID: PMC2724334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol. 2007;102(2):587–600. Epub 2006/11/04. doi: 00456.2006 [pii] 10.1152/japplphysiol.00456.2006 . [DOI] [PubMed] [Google Scholar]
- 27.Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Impact of rhythmic oral activity on the timing of muscle activation in the swallow of the decerebrate pig. Journal of Neurophysiology. 2009;101(3):1386–93. Epub 2008/12/17. doi: 90847.2008 [pii] 10.1152/jn.90847.2008 ; PubMed Central PMCID: PMC2666403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller F, Sherrington C. Some observations on the bucco-pharyngeal stage of reflex deglutition in the cat. Experimental Physiology. 1915;9(2):147–86. [Google Scholar]
- 29.Berman AL. The brain stem of the cat: A cytoarchitectonic atlas with stereotaxic coordinates. 1968. [Google Scholar]
- 30.Berger AJ. Dorsal respiratory group neurons in the medulla of cat: spinal projections, responses to lung inflation and superior laryngeal nerve stimulation. Brain Res. 1977;135(2):231–54. Epub 1977/10/28. . [DOI] [PubMed] [Google Scholar]
- 31.Fedorko L, Merrill EG, Lipski J. Two descending medullary inspiratory pathways to phrenic motoneurones. Neurosci Lett. 1983;43(2–3):285–91. Epub 1983/12/30. . [DOI] [PubMed] [Google Scholar]
- 32.Averill DB, Cameron WE, Berger AJ. Neural elements subserving pulmonary stretch receptor-mediated facilitation of phrenic motoneurons. Brain Res. 1985;346(2):378–82. Epub 1985/11/04. . [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal J. De l’influence du nerf pneumogastrique et du nerf laryngé supérieur sur les movements du diaphragm. Comptes rendus. 1861;T. 52:764–56. [Google Scholar]
- 34.Arloing S. Application de la méthode graphique à l’étude du mécanisme de la déglutition1874 2 Nov 1874.
- 35.Wallois F, Bodineau L, Macron J, Marlot D, Duron B. Role of respiratory and non-respiratory neurones in the region of the NTS in the elaboration of the sneeze reflex in cat. Brain research. 1997;768(1):71–85. [DOI] [PubMed] [Google Scholar]
- 36.Cerenko D, McConnel FM, Jackson RT. Quantitative assessment of pharyngeal bolus driving forces. Otolaryngol Head Neck Surg. 1989;100(1):57–63. Epub 1989/01/01. 10.1177/019459988910000109 . [DOI] [PubMed] [Google Scholar]
- 37.McConnel FM, Guffin TN Jr, Cerenko D. The effect of asymmetric pharyngoesophageal pressures on manofluorographic measurements. Laryngoscope. 1991;101(5):510–5. Epub 1991/05/01. 10.1288/00005537-199105000-00012 . [DOI] [PubMed] [Google Scholar]
- 38.McConnel FM, Guffin TN Jr., Cerenko D, Ko AS. The effects of bolus flow on vertical pharyngeal pressure measurement in the pharyngoesophageal segment: clinical significance. Otolaryngol Head Neck Surg. 1992;106(2):169–74. Epub 1992/02/11. . [PubMed] [Google Scholar]
- 39.McConnel FM, Hood D, Jackson K, O'Connor A. Analysis of intrabolus forces in patients with Zenker's diverticulum. Laryngoscope. 1994;104(5 Pt 1):571–81. Epub 1994/05/01. . [DOI] [PubMed] [Google Scholar]
- 40.Mendelsohn MS, McConnel FM. Function in the pharyngoesophageal segment. Laryngoscope. 1987;97(4):483–9. Epub 1987/04/01. . [DOI] [PubMed] [Google Scholar]
- 41.Langhorst P, Schulz B, Schulz G, Lambertz M, Krienke B. Reticular formation of the lower brainstem. A common system for cardiorespiratory and somatomotor functions: discharge patterns of neighboring neurons influenced by cardiovascular and respiratory afferents. Journal of the autonomic nervous system. 1983;9(2):411–32. [DOI] [PubMed] [Google Scholar]
- 42.Schulz B, Lambertz M, Schulz G, Langhorst P, Krienke B. Reticular formation of the lower brainstem. A common system for cardiorespiratory and somatomotor functions: discharge patterns of neighboring neurons influenced by somatosensory afferents. Journal of the autonomic nervous system. 1983;9(2):433–49. [DOI] [PubMed] [Google Scholar]
- 43.Schulz G, Lambertz M, Schulz B, Langhorst P, Krienke B. Reticular formation of the lower brainstem. A common system for cardio-respiratory and somatomotor functions. Cross-correlation analysis of discharge patterns of neighbouring neurones. Journal of the autonomic nervous system. 1985;12(1):35–62. [DOI] [PubMed] [Google Scholar]
- 44.Langhorst P, Schulz B, Seller H, Koepchen H. Convergence of visceral and somatic afferents on single neurones in the reticular formation of the lower brain stem in dogs. Journal of the autonomic nervous system. 1996;57(3):149–57. [DOI] [PubMed] [Google Scholar]
- 45.Fields H, Clanton C, Anderson S. Somatosensory properties of spinoreticular neurons in the cat. Brain research. 1977;120(1):49–66. [DOI] [PubMed] [Google Scholar]
- 46.Haber L, Moore B, Willis W. Electrophysiological response properties of spinoreticular neurons in the monkey. Journal of Comparative Neurology. 1982;207(1):75–84. 10.1002/cne.902070107 [DOI] [PubMed] [Google Scholar]
- 47.Peterson BW. Reticulospinal projections to spinal motor nuclei. Annual Review of Physiology. 1979;41(1):127–40. [DOI] [PubMed] [Google Scholar]
- 48.Chen Z, Travers JB. Inactivation of amino acid receptors in medullary reticular formation modulates and suppresses ingestion and rejection responses in the awake rat. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2003;285(1):R68–R83. 10.1152/ajpregu.00054.2003 [DOI] [PubMed] [Google Scholar]
- 49.Jordan WP, Leaton RN. Effects of mesencephalic reticular formation lesions on habituation of startle and lick suppression responses in the rat. Journal of Comparative and Physiological Psychology. 1982;96(2):170 [DOI] [PubMed] [Google Scholar]
- 50.Venugopal S. Role of inhibition and hyperpolarization-activated membrane properties in a lick/gape central pattern generator: The Ohio State University; 2008. [Google Scholar]
- 51.Nasse JS. A novel slice preparation to study medullary oromotor and autonomic circuits in vitro. Journal of neuroscience methods. 2014;237:41–53. 10.1016/j.jneumeth.2014.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.