Abstract
Although difficulties in nutrition research and formulating guidelines fuel ongoing debate, the complexities of dietary fats and overall diet are becoming better understood, argue Nita G Forouhi and colleagues
In past decades, dietary guidance has almost universally advocated reducing the intake of total and saturated fat, with the emphasis shifting more recently from total fat to the replacement of saturated fat with polyunsaturated fats and the elimination of trans fat. These recommendations and the link between fat consumption and the risk of cardiovascular disease have been among the most vexed issues in public health: are dietary fats “villains,” are they benign, or are they even “heroes” that could help us consume better overall diets and promote health? And, which dietary fats fit into which category?
The medical literature is still full of articles arguing opposing positions. For example, in 2017, after a review of the evidence, the American Heart Association Presidential Advisory strongly endorsed that “lowering intake of saturated fat and replacing it with unsaturated fats, especially polyunsaturated fats, will lower the incidence of CVD”.1Three months later, the 18-country observational Prospective Rural Urban Epidemiology (PURE) Study concluded much the opposite: “Total fat and types of fat were not associated with cardiovascular disease, myocardial infarction, or cardiovascular disease mortality”.2 The devil, as always, is in the detail, including the inherent complexity of human diets, methodological considerations, and the role of bias and confounding.
This article takes a critical look at the evolution of scientific understanding about dietary fats and health, the difficulties of establishing public health dietary guidelines, and what the current advice should be for dietary fat consumption. Although the focus is on cardiovascular disease, we also consider other outcomes, including weight gain and obesity, type 2 diabetes, and cancer.
History, evolution, and current understanding of dietary fat and health
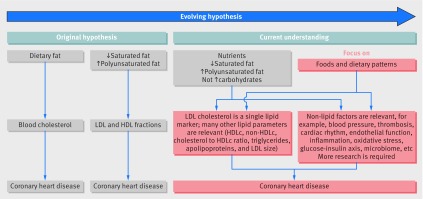
The public health debate about dietary fats and health has been ongoing for over 60 years. The “diet-heart hypothesis” (box 1) has been the focus of discussions on dietary fat and health because coronary heart disease has been and remains the main cause of death worldwide.
Box 1. Diet-heart hypothesis.
The seemingly simple diet-heart hypothesis was first proposed by nutritionist Ancel Keys in the early 1950s. The hypothesis outlined a sequence of relationships in which a fatty diet elevates serum cholesterol levels, leading to atherosclerosis and myocardial infarction. The focus of the hypothesis soon shifted from the total fat consumed in the diet to the more nuanced idea that saturated fats should be replaced by polyunsaturated fats and the benefits of replacing animal fats with vegetable fats were advocated. Total cholesterol as the atherogenic biomarker was later replaced by cholesterol subfractions: the cholesterol in low density lipoproteins and high density lipoproteins, and serum levels of triglycerides. That science, too, has continued to evolve in complexity with further research (fig 1).
Pathophysiological and genetic studies and randomised clinical trials with different cholesterol lowering drugs have led to a consensus that low density lipoprotein (LDL) particles are a cause of coronary heart disease.3 The effect of saturated fat on LDL cholesterol levels1 4 5 and the association of LDL with coronary heart disease1 3 have led to the inference that dietary saturated fat directly promotes the development of coronary heart disease. However, direct evidence of the benefits of lowering cholesterol or LDL cholesterol by changing the fat content of the diet is lacking. Meta-analyses and systematic reviews still place emphasis on the results of a few small trials done 40 to 50 years ago, supplemented by the observations of prospective epidemiological cohort studies.4 6 7 8 9 This evidence has not been sufficient to resolve controversies as both the randomised trials and the observational evidence have many methodological and interpretive problems. Moreover, as the science has evolved, fat consumption itself and its relation with health have become more complex.
Fat is not just fat
Despite decades of dietary advice that the lower the total fat content, the healthier the diet, researchers and public health authorities now agree that to consider the effect of total fat intake alone on health is meaningless; different types of fats must be considered (box 2).
Box 2. Dietary fats and their food sources.
Dietary fats are mostly triglycerides, with each triglyceride molecule containing three fatty acids on a glycerol backbone
The structure and function of dietary fatty acids can vary greatly depending on chain length (6-24 carbon units); number of double bonds—saturated (with no double bonds), monounsaturated, or polyunsaturated; and whether the double bonds are in a cis (same side) or trans (opposite side) position
Polyunsaturated fats with double bonds that are 3 carbon atoms or 6 carbons from the n-terminal end of the fatty acid (n-3 or n-6, respectively) are considered essential—that is, they must be obtained from the diet because they are not synthesised in the body. Both have important structural and physiological functions
Different fatty acids have distinct biochemical properties and can therefore produce different metabolic and physiological effects with different clinical manifestations, such as cardiovascular, neurological, or other
Food sources of dietary fats
Food sources of individual fatty acids vary within each class. For instance, within the omega 3 polyunsaturated fatty acid class, alpha linolenic acid comes from plants, including some nuts and seeds such as walnuts and linseed, whereas eicosapentaenoic acid and docosahexaenoic acid come mostly from fish and other marine sources
Many food sources contain different types of fatty acids. For example, olive oil is a good source of monounsaturated fatty acids but also contains saturated and polyunsaturated fatty acids in smaller proportions. Animal products are rich in saturated fats but some also contain large proportions of monounsaturated and polyunsaturated fatty acids
To produce public health guidelines on which foods to eat or avoid to reduce the risk of chronic disease is complicated because dietary fats are typically mixtures of different types of fatty acids. Animal fats, for instance, are the main sources of saturated fats in many modern diets, but some animal fats are higher in monounsaturated fats than saturated fats, and polyunsaturated fats in vegetable oils will typically contain both omega 3 and omega 6 fatty acids in different concentrations. Hence, conclusions about the health effects of saturated and polyunsaturated fatty acids are unlikely to consistently translate to the health effects of the fats, oils, and foods in which those fatty acids are present.
Foods are not just nutrients
The scientific investigation of nutrients (both macro- and micronutrients) is essential for understanding pathophysiological mechanisms of disease. However, people do not consume isolated nutrients and the foods they eat are more than the sum of their nutrients.
Synergism and interactions between different components of foods together with the degree of processing and preparation or cooking methods lead to a “food matrix” effect which is not captured by considering single nutrients. Different types of food that are high in saturated fats are likely to have different effects on health. For example, dairy products and processed meats, both high in saturated fats, are differentially associated with many health outcomes in prospective epidemiological studies, often in opposite directions.10 One explanation for this divergence is that despite their similar fat content, other components of these two food groups are associated with different health effects. For example, dairy products contain minerals such as calcium and magnesium and have probiotic features if fermented, whereas processed red meat has a high salt and preservative content.
The potential for residual confounding from lifestyle, dietary, and socioeconomic factors and bias in observational research limits causal interpretation. However, it is also unlikely that randomised clinical trials of individual foods or food groups for disease endpoints will be possible, not least because of the problems of sample size, adherence, dose, duration, and cost. For this reason, dietary guidance is usually derived from analysis and synthesis of different types of evidence.
Nutrient replacement
Within overall diets, eating less of one macronutrient implies eating more of others to keep calorie intake the same. Dietary manipulations in clinical trials always involve multiple variables. If total fat reduction is the intervention in a randomised trial, any effect observed could depend on the replacement source of energy, whether it is mainly carbohydrate or protein or a combination of the two. The quality of the replacement macronutrient (for instance, highly refined grains versus whole grains) can also influence the observed effect. If reduction of saturated fat but not total fat is the intervention, the effect will differ depending on whether the replacement fat is a mostly polyunsaturated fat, in which case whether an omega 3 or omega 6 polyunsaturated fatty acid, or a mostly monounsaturated fat such as from olive oil.
The same principles apply to observational studies, when statistical models of associations between diet and disease need to adjust for total energy intake. In recent years, most observational studies have done this, but the comparison sources of energy (eg, carbohydrate or unsaturated fats) are often not specified.
In meta-analyses of observational studies that adjust for total calorie intake, higher intakes of polyunsaturated fatty acids in place of saturated fatty acids were associated with a lower risk of coronary heart disease.6 11 12Similar findings were seen for total and cardiovascular mortality in 128 000 men and women followed for up to 32 years with repeated measures of diet.13 Replacing saturated fatty acids with polyunsaturated fatty acids has been part of dietary guidelines since the 1970s and has led to modest reductions in saturated fat intake and an increase in plant oil consumption in the US (eg, polyunsaturated fatty acid intake increased from about 3% to 7% of energy intake) at a time when coronary heart disease mortality fell by nearly 75%.14 At a minimum, these findings support the safety of these dietary changes, although the benefits of changing the type of fat are difficult to quantify because of changes in other factors such as other aspects of diet, smoking, and emergency medical services.
A global perspective on the health effects of dietary fats and other macronutrients was provided by the PURE study, which included 135 335 individuals in 18 countries.2 Higher intakes of total fat and saturated, monounsaturated, and polyunsaturated fatty acids individually were associated with lower total mortality but not with cardiovascular disease mortality or incidence, except for inverse associations of saturated fatty acids with the incidence of stroke. In contrast, higher carbohydrate intake was non-linearly associated with increased total mortality; in nutrient substitution analyses, only the replacement of carbohydrate with polyunsaturated fats was associated with lower mortality. Although limited by the observational study design, these findings add to the concern about guidelines that focus on limiting the intake of total and saturated fats, particularly without considering the replacement nutrient.
Taken together, a single nutrient approach can be misleading both in the interpretation of research findings and in the health implications of translating results into dietary guidelines and public health programmes.
Importance of lipid components in the diet-heart hypothesis
As the diet-heart hypothesis evolved in the 1960s and 1970s, the focus shifted from the effect of dietary fat on total cholesterol to LDL cholesterol. However, changes in LDL cholesterol are not an actual measure of heart disease itself. Any dietary intervention might influence other, possibly unmeasured, causal factors that could affect the expected effect of the change in LDL cholesterol. This possibility is clearly shown by the failure of several categories of drugs to reduce cardiovascular events despite significant reductions in plasma LDL cholesterol levels.15 16 17 LDL cholesterol can also be reduced through diet in ways that do not reduce the risk of coronary heart disease; for instance, when saturated fat is replaced by carbohydrates, this lowers LDL cholesterol but also reduces HDL cholesterol and increases triglycerides.5 In the past, these effects were considered less important as researchers and the pharmaceutical industry focused on the effect of the reduction of LDL cholesterol.
Since the 1980s, studies on the LDL cholesterol biomarker itself and the effects of dietary fats on other biomarkers of disease have revealed a more complicated situation. Researchers now widely recognise the existence of a range of LDL particles with different physicochemical characteristics, including size and density, and that these particles and their pathological properties are not accurately measured by the standard LDL cholesterol assay.18 Hence assessment of other atherogenic lipoprotein particles (either LDL alone, or non-HDL cholesterol including LDL, intermediate density lipoproteins, and very low density lipoproteins, and the ratio of serum apolipoprotein B to apolipoprotein A1) have been advocated as alternatives to LDL cholesterol in the assessment and management of cardiovascular disease risk.17 19 20 21 Moreover, blood levels of smaller, cholesterol depleted LDL particles appear more strongly associated with cardiovascular disease risk than larger cholesterol enriched LDL particles,22 while increases in saturated fat intake (with reduced consumption of carbohydrates) can raise plasma levels of larger LDL particles to a greater extent than smaller LDL particles.22 In that case, the effect of saturated fat consumption on serum LDL cholesterol may not accurately reflect its effect on cardiovascular disease risk. While polyunsaturated fats and monounsaturated fats reduce LDL cholesterol levels, their effects on cardiovascular disease risk factors that are associated with lipoprotein particles are less clear. Although uncertainty exists about the causal role, if any, of elevated triglycerides or low HDL cholesterol levels in coronary heart disease, there has been continued interest because of their association with insulin resistance and metabolic syndrome, and their relevance in global populations.19 23 Notably, these lipid markers improve—that is, triglycerides decrease or HDL cholesterol increases—when saturated, monounsaturated, or polyunsaturated fats replace carbohydrates.
Trans unsaturated fatty acids (trans fats) are an example of a fatty acid category whose effects on lipid biomarkers of cardiovascular disease risk are consistent with their association with cardiovascular disease events in prospective cohort studies. When substituted for other macronutrients, these fatty acids, such as those in industrially produced hydrogenated oils, have been shown to increase levels of LDL cholesterol and the number of atherogenic lipoproteins (LDL and very low density lipoproteins), while also increasing triglycerides and reducing HDL cholesterol and LDL particle size.24
To complicate the relation further, dietary fatty acid composition may affect the risk of cardiovascular disease independently of these lipid biomarkers, specifically through the effects on inflammation, endothelial function, thrombosis, ventricular arrhythmias, and blood pressure. However, as reviewed recently,25 insufficient evidence exists to draw firm conclusions about the effects of fatty acid type on these factors (fig 1).
Fig 1.
Diet-heart hypothesis and current understanding. HDL: high density lipoprotein, HDLc: high density lipoprotein cholesterol, LDL: low density lipoprotein
Finally, consideration must be given to the role of personal factors that may alter the effect of dietary fat on LDL cholesterol and other lipids. For example, evidence from clinical trials suggests that saturated fat may have little effect on LDL cholesterol levels in people with obesity.26 27 Moreover, the possibility of differing effects of dietary fats on lipids in racial and ethnic subgroups has not been systematically evaluated.
Beyond cardiovascular disease
Cancer
In the 1960s and 1970s, national per capita intakes of total and saturated fat correlated with rates of cancers of the breast, colon, prostate, and other common cancers in Western countries. Although the ecological findings were potentially confounded by many aspects of diet and lifestyle, and no clear biological basis was shown, these findings were used to support widespread dietary recommendations to reduce total fat intake.28 Later prospective cohort studies with follow-up of up to 20 years have not found positive associations between total fat intake and the incidence of these cancers,29 nor have large clinical trials, most notably, the Women’s Health Initiative.30 However, the Women’s Health Initiative may not be informative because the blood lipid fractions of participants randomised to a low fat diet did not change, which raises questions about adherence to the diet. In another trial in which participants did show the expected change in blood lipids with a low fat diet, no effect on breast cancer incidence was observed.31 A comprehensive review of the literature by the World Cancer Research Fund and the American Institute for Cancer Research concluded that no convincing or probable relation exists between intakes of total or saturated fat and risk of any form of cancer.32 An association between omega 6 intake and cancer incidence is also not supported.
Weight gain and obesity
The assumption that diets high in fat promote weight gain is based on the relative energy density of macronutrients—9 kcal/g for fat compared with 4 kcal/g for carbohydrate or protein. This assumption, however, ignores the important role of macronutrients in hunger and satiety and in pathways regulating fuel partitioning, fat storage, and fatty acid metabolism. Randomised trials of diet and weight loss are easier to conduct than trials that have chronic disease as the outcome because they can be shorter and require far fewer participants. However, several design issues are important in interpreting trial findings. For example, many people initially comply with the intervention diets when they start in a trial and lose weight but regain much of that weight by one year. Because long term weight control is of interest, trials should last a minimum of one year and ideally two years or longer. Moreover, dietary interventions such as counselling, monitoring, feedback, and support can help with weight loss independently of the specific dietary advice because of healthier eating, and this complicates interpretation. Control diet groups should receive a similar intervention.
In studies where the intensity of intervention was similar for the diets tested, participants randomised to low carbohydrate, high fat diets lost about 1.5 kg more weight than those in the low fat group.33 Diets higher in fat and restricted in carbohydrate seem to help weight loss28 but dietary adherence is a critical factor in the effectiveness of any diet.34 Another relevant factor for weight loss is diet quality. A trial comparing diets high in carbohydrates or fats showed an equal amount of weight loss by participants when consuming whole and natural food sources while avoiding sugar, sugary drinks, refined grains, trans fats, and processed foods in general.35
Maintaining a healthy weight to prevent obesity is also important. The typical weight gain in many Western countries is about 0.5 kg a year, which seems small but by age 50 this results in weight levels that are associated with important health risks.36 Few randomised trials have examined the effects of diet on long term weight gain in people who are initially not overweight. In prospective observational studies, a simple design assessing baseline diet in relation to long term weight change is not optimal because many people change their diet during follow-up; a longitudinal design examining the change in diet with change in weight is preferable. Evidence is sparse, but an analysis in the Nurses’ Health Study suggested that intakes of saturated and trans fat were positively associated with weight gain in women who were normal weight at the start, while intakes of mono and polyunsaturated fat (mainly omega 6) were not; total fat intake was only weakly associated with weight gain.37
Taken together, the evidence does not support a benefit of low fat diets for weight loss or prevention of overweight compared with low carbohydrate diets. Other aspects of diet may have a greater influence on long term weight control, including the quantity and quality of carbohydrate intake; thus any effect of fat is likely to depend on its food source and the overall dietary pattern.
Type 2 diabetes
A combination of insulin resistance and an inadequate capacity to secrete insulin leads to the development of type 2 diabetes, with adiposity a critical risk factor. Prospective studies report little association between total fat consumption and risk of diabetes but an association may exist, as with cardiovascular disease, for type of fat. The findings of a few short term feeding trials (usually lasting four weeks, with some up to 16 weeks) that assessed intermediate endpoints support this evidence. Evidence from randomised controlled trials suggests that industrially produced trans fats increase inflammatory factors and adversely affect lipid levels, but no or inconclusive evidence was found for an effect on markers of glucose homoeostasis.38 39 Evidence from prospective studies suggests that intake of industrially produced trans fats is positively associated with the incidence of type 2 diabetes, while the intake of polyunsaturated fatty acids is inversely associated.4 40 More specifically, a blood biomarker of the most abundant omega 6 fatty acid, linoleic acid, is inversely associated with the incidence of type 2 diabetes.41 42 Despite promising studies in animals, diets rich in marine omega 3 fatty acids have not been shown in humans to reduce insulin resistance or the incidence of type 2 diabetes. However, biomarker studies point to an inverse association between blood omega 3 fatty acids (alpha linolenic acid) derived from plants and type 2 diabetes.41 43 Because the type of dietary carbohydrate may also affect the risk of diabetes, any relation between dietary fat and type 2 diabetes may depend on the quantity and quality of carbohydrate as well.
Moving from controversy to consensus
Two related issues have caused the most controversy about the relative roles of saturated fats and polyunsaturated fats and the evidence for making public health recommendations.
Since the 1960s, the evidence has suggested that replacing saturated fats in the diet with polyunsaturated fats reduces the risk of chronic disease and premature death. In practice this means replacing red and processed meat and high fat dairy with fish, nuts, and seeds, and replacing animal fats such as butter and lard with vegetable oils such as corn, sunflower, soy, rapeseed, or olive oils. The PREDIMED study, a large primary prevention randomised controlled trial on people at high risk of cardiovascular disease, reported that increased intake of extra virgin olive oil or nuts with a Mediterranean diet significantly reduced the incidence of cardiovascular disease compared with a low fat dietary advice group.44 However, while the intake of unsaturated fats increased in both the olive oil and nut arms and may therefore have contributed to the clinical outcomes, there was no nutrient substitution for saturated fats and their intake was not reduced compared with the low fat dietary advice group. How this evidence should be interpreted and applied remains controversial.
Since 2000, clinical trials lasting up to two years have suggested that low carbohydrate diets in which total and saturated fat replaces the carbohydrate content of the diet have beneficial effects on overweight as well as on lipid risk factors such as HDL cholesterol and triglycerides (but not LDL cholesterol) and on risk factors for type 2 diabetes.45 46 Here, we ourselves disagree on the significance and interpretation of these trials because long term trial evidence is not available, definitions of low carbohydrate vary substantially across studies, and few clinical trial data exist on the incidence of clinical endpoints (see related article in this series on diet and management and prevention of type 2 diabetes).47
These controversies arise largely because existing research methods cannot resolve them. In the current scientific model, hypotheses are treated with scepticism until they survive rigorous and repeated tests. In medicine, randomised controlled trials are considered the gold standard in the hierarchy of evidence because randomisation minimises the number of confounding variables. Ideally, each dietary hypothesis would be evaluated by replicated randomised trials, as would be done for the introduction of any new drug. However, this is often not feasible for evaluating the role of diet and other behaviours in the prevention of non-communicable diseases.
One of the hypotheses that requires rigorous testing is that changes in dietary fat consumption will reduce the risk of non-communicable diseases that take years or decades to manifest. Clinical trials that adequately test these hypotheses require thousands to tens of thousands of participants randomised to different dietary interventions and then followed for years or decades until significant differences in clinical endpoints are observed. As the experience of the Women’s Health Initiative suggests, maintaining sufficient adherence to assigned dietary changes over long periods (seven years in the Women’s Health Initiative) may be an insurmountable problem. For this reason, among others, when trials fail to confirm the hypotheses they were testing, it is impossible to determine whether the failure is in the hypothesis itself, or in the ability or willingness of participants to comply with the assigned dietary interventions. This uncertainty is also evident in diet trials that last as little as six months or a year.
In the absence of long term randomised controlled trials, the best available evidence on which to establish public health guidelines on diet often comes from the combination of relatively short term randomised trials with intermediate risk factors (such as blood lipids, blood pressure, or body weight) as outcomes and large observational cohort studies using reported intake or biomarkers of intake to establish associations between diet and disease.48 Although a controversial practice, many, if not most, public health interventions and dietary guidelines have relied on a synthesis of such evidence. Many factors need to be considered when using combined sources of evidence that individually are inadequate to formulate public health guidelines, including their consistency and the likelihood of confounding, the assessment of which is not shared universally. The level of evidence required for public health guidelines may differ depending on the nature of the guideline itself.
The controversies over the effects of replacing saturated fatty acids with polyunsaturated fatty acids—reduced consumption of animal fats, increased consumption of vegetable oils—and the significance of the evidence from trials of very low carbohydrate, high fat diets suggest both “additive” and “subtractive” nutritional approaches to prevention of cardiovascular disease (box 3). Both depend on assumptions about the nature of biological normality in human diets. The replacement of saturated fats with polyunsaturated fats implies that saturated fat as a nutrient causes disease and is being reduced and/or that the consumption of vegetable oils is healthy and without long term risks. Box 4 appraises the debate about the role of omega 6 and omega 3 polyunsaturated fatty acids and the use of plant oils. The consumption of very low carbohydrate, high fat diets assumes that high levels of dietary fat and saturated fat can be consumed for a lifetime without the risks outweighing the benefits. A point of controversy is whether such assumptions can be accepted without long term clinical trials of the kind that would be required for a pharmaceutical means of prevention. This controversy might be resolved by longer term clinical trials, but the cost and methodological and ethical challenges of such dietary trials suggest they may never be done.
Box 3. Prevention strategies.
Public health preventive measures can be divided into two main categories.
Subtractive—Rose described this as “the removal of an unnatural factor and the restoration of biological normality”.49 For coronary heart disease, this would include smoking prevention or cessation and, more recently, the near elimination of industrially produced trans fatty acids through a combination of information, advice, and regulation. Despite the absence of long term randomised controlled trials to support the benefits of these interventions, the evidence from observational studies and, in the case of trans fats, short term feeding trials is considered sufficiently consistent to support these public health interventions.
Additive—Rose described this as “not removing a supposed cause of disease but adding some other unnatural factor, in the hope of conferring protection”.49 Additive measures for coronary heart disease would include a high intake of polyunsaturated fats and long term medication. Rigorous testing would be required as for any pharmaceutical therapy to ensure long term safety and that harm does not outweigh benefit.
Box 4. Role of omega 6 and omega 3 polyunsaturated fatty acids in health.
Both omega 6 and omega 3 are essential fatty acids and are intrinsic to cell membranes and the structure of the central nervous system. They are precursors of eicosanoids, which are involved in inflammation, cardiac rhythm, thrombosis, vascular function, and many other processes. Evidence suggests, but is inconsistent, that adequate intake of omega 3 fatty acids reduces cardiac arrhythmias and sudden cardiac death. Concerns have been raised that omega 6 polyunsaturated fats are pro-inflammatory but this is not supported by controlled feeding studies and large cross sectional observations.50 In a follow-up study of over 128 000 men and women for up to 32 years, higher intake of linoleic acid (the most abundant omega 6 fatty acid) was associated with lower risks of coronary heart disease, cancer, and total mortality.13 The inverse association between linoleic acid intake and risk of cardiovascular disease and overall death is approximately linear up to about 8% of energy, beyond which data are lacking.13 Notably, linoleic acid levels in blood, a direct marker of dietary intake, were inversely associated, with the incidence of type 2 diabetes in prospective studies but arachidonic acid was not.41 51 In contrast, blood omega 3 polyunsaturated fatty acids were modestly inversely associated with coronary heart disease52 but the association with type 2 diabetes varied by subtype: plant origin omega 3 fatty acid (alpha linolenic acid) was inversely associated while marine origin omega 3 fatty acids were not.41
Use of plant oils to replace saturated fatty acids
With the exception of the cardiovascular benefit44 of extra virgin olive oil (comprised predominantly of oleic acid, a monounsaturated fat), which has been used for thousands of years in Mediterranean countries, most of the literature on the effects of plant oils on the risk of cardiovascular disease and other outcomes has examined intakes of specific fatty acids. These oils contain a combination of saturated, monounsaturated, and omega 6 and omega 3 fatty acids but the proportions vary greatly. Plant oils also contain other minor constituents, including polyphenols and antioxidants, which may influence the effect of oil consumption on disease risk. A reduction in cardiovascular mortality was observed in the older randomised trials that used plant oils containing both omega 6 and omega 3 fatty acids to replace saturated fat.53 Recent publications from the Sydney Diet Heart Study and the Minnesota Coronary Trial raise questions about very high intakes of plant oils containing only omega 6 fatty acids.53 54 The concerns raised are complex and discussed elsewhere.1 The safety of long term use of polyunsaturated plant oils was supported by a significant inverse association between the intake of polyunsaturated plant oils and all-cause mortality at 32 years of follow-up; no increase in risk was seen for any outcome.13 Evidence also exists that rapeseed (canola) oil reduces the risk of coronary heart disease: most notably, rapeseed oil was the primary intervention in the Lyon Heart Study of secondary prevention of coronary heart disease, which reduced recurrent cardiovascular disease or death by about 70%.55 Other specific types of oil, including corn, sunflower, coconut oil, and palm oils, have not been well studied. Although a recent report suggests that coconut oil compared with butter results in a more favourable lipid profile (lower LDL, higher HDL cholesterol), and compared with olive oil was equivalent in lipid effects,56 further research is needed in large long term trials and current recommendations on caution about use should be upheld.1 Some plant oils, including corn and sunflower oil, have little omega 3 content. If these are the primary oils consumed and intake of omega 3 fatty acids from fish and other sources is low, this could result in inadequate intake of these essential fatty acids with possible adverse effects on cardiovascular disease and other outcomes. Also, to benefit from the use of unsaturated oils assumes that they are not partially hydrogenated as this (and some other industrial processes such as deodorisation if done improperly) produces trans fats.
In summary, evidence exists of the long term safety and benefit of many of the commonly consumed unsaturated plant oils. Further research is needed to define more precisely the long term effects and optimal intakes of specific fatty acids and plant oils, and their interactions with genetic and other dietary factors, including the amount and type of carbohydrate intake.
Although authorities still disagree, most consider that public health decisions should be made on the weight of the available evidence, acknowledging its limitations, and seeking to obtain further, better evidence when indicated. Equally important is to acknowledge when evidence is insufficient to formulate any guidance, in which case all the relevant options should be clearly outlined to enable informed choice.
Key messages.
For cardiovascular health, substantial evidence supports the importance of the type of fat consumed, not total fat intake, and the elimination of industrially produced trans fats
Much of the evidence suggests that the risk of coronary heart disease is reduced by replacing saturated fat with polyunsaturated fats (including plant oils) but not when carbohydrate is the replacement nutrient
Controversies remain about long term health effects of specific plant oils and of high fat, low carbohydrate diets, and research is needed to resolve these
The focus of dietary advice must be on the consumption of foods and overall dietary patterns, not on single nutrients
Contributors and sources: The author group spans a wide range of expertise from nutritional epidemiology and public health (NF, WW), to science journalism (GT), to cardiology and lipidology (RK), and all have contributed to past dialogue on dietary fats and health. Sources of information for this article included systematic reviews and primary research articles based on randomised clinical trials, or prospective observational study designs as well as dietary guidelines. All authors contributed to drafting this manuscript, with NGF taking a lead role and she is also the guarantor of the manuscript. All authors gave intellectual input to improve the manuscript and have read and approved the final version.
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following: NGF receives funding from the Medical Research Council Epidemiology Unit (MC_UU_12015/5). She is a member (unpaid) of the Joint SACN/NHS-England/Diabetes-UK Working Group to review the evidence on lower carbohydrate diets compared with current government advice for adults with type 2 diabetes and is a member (unpaid) of ILSI-Europe Qualitative Fat Intake Task Force Expert Group on update on health effects of different saturated fats. RK has held grants from the National Institutes of Health, Dairy Management, Inc., Almond Board of California, and Quest Diagnostics. He also licensed a patent for ion mobility analysis of lipoprotein particles. He is on the scientific advisory board of Virta Health and has received honoraria from Quest Diagnostics and Crossfit Foundation. GT is a director at Nutrition Science Initiative, senior editor at Crossfit Health, and author of several books and blogs on diet and health. WCW has no competing interests to declare.
Provenance and peer review: Commissioned; externally peer reviewed.
This article is one of a series commissioned by The BMJ. Open access fees for the series were funded by SwissRe, which had no input into the commissioning or peer review of the articles. The BMJ thanks the series advisers, Nita Forouhi and Dariush Mozaffarian, for valuable advice and guiding selection of topics in the series.
References
- 1. Sacks FM, Lichtenstein AH, Wu JHY, et al. American Heart Association dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation 2017;136:e1-23. 10.1161/CIR.0000000000000510 [DOI] [PubMed] [Google Scholar]
- 2. Dehghan M, Mente A, Zhang X, et al. Prospective Urban Rural Epidemiology (PURE) study investigators Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050-62. 10.1016/S0140-6736(17)32252-3 [DOI] [PubMed] [Google Scholar]
- 3. Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459-72. 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids 2010;45:893-905. 10.1007/s11745-010-3393-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146-55. 10.1093/ajcn/77.5.1146 [DOI] [PubMed] [Google Scholar]
- 6. Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 2010;7:e1000252. 10.1371/journal.pmed.1000252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev 2015;(6):CD011737. [DOI] [PubMed] [Google Scholar]
- 8. Chowdhury R, Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 2014;160:398-406. 10.7326/M13-1788 [DOI] [PubMed] [Google Scholar]
- 9. de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 2015;351:h3978. 10.1136/bmj.h3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation 2016;133:187-225. 10.1161/CIRCULATIONAHA.115.018585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jakobsen MU, O’Reilly EJ, Heitmann BL, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 2009;89:1425-32. 10.3945/ajcn.2008.27124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farvid MS, Ding M, Pan A, et al. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation 2014;130:1568-78. 10.1161/CIRCULATIONAHA.114.010236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang DD, Li Y, Chiuve SE, et al. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med 2016;176:1134-45. 10.1001/jamainternmed.2016.2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health, National Heart Lung, and Blood Institute. Morbidity & mortality: 2012 chart book on cardiovascular, lung, and blood diseases. NIH, 2012.
- 15. Manson JE, Hsia J, Johnson KC, et al. Women’s Health Initiative Investigators Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003;349:523-34. 10.1056/NEJMoa030808 [DOI] [PubMed] [Google Scholar]
- 16. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. ACCELERATE Investigators Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med 2017;376:1933-42. 10.1056/NEJMoa1609581 [DOI] [PubMed] [Google Scholar]
- 17. Boden WE, Probstfield JL, Anderson T, et al. AIM-HIGH Investigators Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255-67. 10.1056/NEJMoa1107579 [DOI] [PubMed] [Google Scholar]
- 18. Krauss RM. All low-density lipoprotein particles are not created equal. Arterioscler Thromb Vasc Biol 2014;34:959-61. 10.1161/ATVBAHA.114.303458 [DOI] [PubMed] [Google Scholar]
- 19. Mente A, Dehghan M, Rangarajan S, et al. Prospective Urban Rural Epidemiology (PURE) study investigators Association of dietary nutrients with blood lipids and blood pressure in 18 countries: a cross-sectional analysis from the PURE study. Lancet Diabetes Endocrinol 2017;5:774-87. 10.1016/S2213-8587(17)30283-8 [DOI] [PubMed] [Google Scholar]
- 20. Di Angelantonio E, Gao P, Pennells L, et al. Emerging Risk Factors Collaboration Lipid-related markers and cardiovascular disease prediction. JAMA 2012;307:2499-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di Angelantonio E, Sarwar N, Perry P, et al. Emerging Risk Factors Collaboration Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993-2000. 10.1001/jama.2009.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr 2006;83:1025-31, quiz 1205. 10.1093/ajcn/83.5.1025 [DOI] [PubMed] [Google Scholar]
- 23. Kohli A, Siddhu A, Pandey RM, Reddy KS. Relevance of the triglyceride-to-high-density lipoprotein cholesterol ratio as an important lipid fraction in apparently healthy, young, and middle-aged Indian men. Indian J Endocrinol Metab 2017;21:113-8. 10.4103/2230-8210.196020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gebauer SK, Destaillats F, Dionisi F, Krauss RM, Baer DJ. Vaccenic acid and trans fatty acid isomers from partially hydrogenated oil both adversely affect LDL cholesterol: a double-blind, randomized controlled trial. Am J Clin Nutr 2015;102:1339-46. 10.3945/ajcn.115.116129 [DOI] [PubMed] [Google Scholar]
- 25. Siri-Tarino PW, Chiu S, Bergeron N, Krauss RM. Saturated fats versus polyunsaturated fats versus carbohydrates for cardiovascular disease prevention and treatment. Annu Rev Nutr 2015;35:517-43. 10.1146/annurev-nutr-071714-034449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hannon BA, Thompson SV, An R, Teran-Garcia M. Clinical outcomes of dietary replacement of saturated fatty acids with unsaturated fat sources in adults with overweight and obesity: a systematic review and meta-analysis of randomized control trials. Ann Nutr Metab 2017;71:107-17. 10.1159/000477216 [DOI] [PubMed] [Google Scholar]
- 27. Chiu S, Williams PT, Dawson T, et al. Diets high in protein or saturated fat do not affect insulin sensitivity or plasma concentrations of lipids and lipoproteins in overweight and obese adults. J Nutr 2014;144:1753-9. 10.3945/jn.114.197624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Research Council. Recommended dietary allowances: 10th ed. NRC, 1989.
- 29. Kim EH, Willett WC, Colditz GA, et al. Dietary fat and risk of postmenopausal breast cancer in a 20-year follow-up. Am J Epidemiol 2006;164:990-7. 10.1093/aje/kwj309 [DOI] [PubMed] [Google Scholar]
- 30. Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:629-42. 10.1001/jama.295.6.629 [DOI] [PubMed] [Google Scholar]
- 31. Martin LJ, Li Q, Melnichouk O, et al. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res 2011;71:123-33. 10.1158/0008-5472.CAN-10-1436 [DOI] [PubMed] [Google Scholar]
- 32.World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. WCRF/AICR, 2007.
- 33. Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2015;3:968-79. 10.1016/S2213-8587(15)00367-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA 2014;312:923-33. 10.1001/jama.2014.10397 [DOI] [PubMed] [Google Scholar]
- 35. Gardner CD, Trepanowski JF, Del Gobbo LC, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS Randomized Clinical Trial. JAMA 2018;319:667-79. 10.1001/jama.2018.0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng Y, Manson JE, Yuan C, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA 2017;318:255-69. 10.1001/jama.2017.7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Field AE, Willett WC, Lissner L, Colditz GA. Dietary fat and weight gain among women in the Nurses’ Health Study. Obesity (Silver Spring) 2007;15:967-76. 10.1038/oby.2007.616 [DOI] [PubMed] [Google Scholar]
- 38. Wallace SK, Mozaffarian D. Trans-fatty acids and nonlipid risk factors. Curr Atheroscler Rep 2009;11:423-33. 10.1007/s11883-009-0064-0 [DOI] [PubMed] [Google Scholar]
- 39. Aronis KN, Khan SM, Mantzoros CS. Effects of trans fatty acids on glucose homeostasis: a meta-analysis of randomized, placebo-controlled clinical trials. Am J Clin Nutr 2012;96:1093-9. 10.3945/ajcn.112.040576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salmerón J, Hu FB, Manson JE, et al. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr 2001;73:1019-26. 10.1093/ajcn/73.6.1019 [DOI] [PubMed] [Google Scholar]
- 41. Forouhi NG, Imamura F, Sharp SJ, et al. Association of plasma phospholipid n-3 and n-6 polyunsaturated fatty acids with type 2 diabetes: the EPIC-InterAct Case-Cohort Study. PLoS Med 2016;13:e1002094. 10.1371/journal.pmed.1002094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu JHY, Marklund M, Imamura F, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol 2017;5:965-74. 10.1016/S2213-8587(17)30307-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu JH, Micha R, Imamura F, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr 2012;107(Suppl 2):S214-27. 10.1017/S0007114512001602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Estruch R, Ros E, Salas-Salvadó J, et al. PREDIMED Study Investigators Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279-90. 10.1056/NEJMoa1200303 [DOI] [PubMed] [Google Scholar]
- 45. Mansoor N, Vinknes KJ, Veierød MB, Retterstøl K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr 2016;115:466-79. 10.1017/S0007114515004699 [DOI] [PubMed] [Google Scholar]
- 46. Meng Y, Bai H, Wang S, Li Z, Wang Q, Chen L. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2017;131:124-31. 10.1016/j.diabres.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 47. Forouhi NG, Misra A, Mohan V, Taylor R, Yancy W. Nutritional management and prevention of type 2 diabetes. BMJ 2018;361:k2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mozaffarian D, Forouhi NG. Dietary guidelines and health—is nutrition science up to the task? BMJ 2018;360:k822. 10.1136/bmj.k822 [DOI] [PubMed] [Google Scholar]
- 49. Rose G. Strategy of prevention: lessons from cardiovascular disease. Br Med J (Clin Res Ed) 1981;282:1847-51. 10.1136/bmj.282.6279.1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Su H, Liu R, Chang M, Huang J, Wang X. Dietary linoleic acid intake and blood inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Food Funct 2017;8:3091-103. 10.1039/C7FO00433H [DOI] [PubMed] [Google Scholar]
- 51. Wu JHY, Marklund M, Imamura F, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol 2017;5:965-74. 10.1016/S2213-8587(17)30307-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Del Gobbo LC, Imamura F, Aslibekyan S, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe) ω-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med 2016;176:1155-66. 10.1001/jamainternmed.2016.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ramsden CE, Zamora D, Leelarthaepin B, et al. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013;346:e8707. 10.1136/bmj.e8707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ramsden CE, Zamora D, Majchrzak-Hong S, et al. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ 2016;353:i1246. 10.1136/bmj.i1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. de Lorgeril M, Renaud S, Mamelle N, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994;343:1454-9. 10.1016/S0140-6736(94)92580-1 [DOI] [PubMed] [Google Scholar]
- 56. Khaw KT, Sharp SJ, Finikarides L, et al. Randomised trial of coconut oil, olive oil or butter on blood lipids and other cardiovascular risk factors in healthy men and women. BMJ Open 2018;8:e020167. 10.1136/bmjopen-2017-020167 [DOI] [PMC free article] [PubMed] [Google Scholar]