Abstract
Noroviruses are a leading cause of gastroenteritis across the world in all age groups and are linked to increased hospitalization and mortality in children, the elderly and immunocompromised. The development of specific antiviral treatment for norovirus gastroenteritis is urgently needed. We explored in a mouse model whether an inhibitor of norovirus replication could be used therapeutically post murine norovirus (MNV)-infection of mice.
Using the MNV, we previously discovered that the viral polymerase inhibitor 2’-C-methylcytidine (2CMC) is able to protect against diarrhea and mortality in mice when used prophylactically and to block the transmission of MNV between mice. Here, we investigated whether 2CMC could be used therapeutically, starting treatment between 12 h and 3 days post-infection with 2CMC.
Post-exposure treatment of MNV-infected mice with 2CMC was efficient up to 2 days after infection, preventing norovirus-induced diarrhea, delaying and reducing MNV shedding in stool of treated mice. Rehydration of 2CMC-treated animals did not result in a further improvement of the disease evolution compared to antiviral treatment only. The presence of MNV antigens and inflammation in the small intestine of infected mice inversely correlated with the effectiveness of delayed antiviral treatment. Anti-MNV IgGs were detected in re-challenged mice 10 weeks after the first contact, these protected the mice from re-infection. We here demonstrate the benefit of antiviral treatment in ongoing norovirus infections.
Keywords: antiviral drugs, mouse model, diarrhea, outbreak, control and prevention
1. Introduction
Human noroviruses are a leading cause of gastroenteritis across the world in all age groups. Infections with these viruses can be particularly severe for children ˂ 5 years of age. They are second only to rotavirus as etiologic agents of childhood diarrhea in both low-/ middle- and high-income countries (Chhabra et al., 2014; Ramani and Kang, 2009; Walker et al., 2013; Yu et al., 2015). In those countries where routine vaccination against rotavirus has been implemented, human norovirus has become the more commonly detected agent of childhood diarrhea (Hemming et al., 2013; Payne et al., 2013). Whereas single episodes of diarrhea are typically self-limiting and of short duration, several episodes per year can lead to nutritional deficits and long-term consequences, such as growth stunting. This important sequela, which is associated with decreased cognitive function, could (in ~25% of cases) be attributed to five or more episodes of diarrhea before the age of 2 (Walker et al., 2013). Whereas there are two vaccines on the market to prevent rotavirus-induced diarrhea, there is no vaccine or specific antivirals to prevent or treat norovirus-induced gastroenteritis. Supportive care consists mostly of electrolyte replenishment of dehydrated individuals. Today highly effective and potent antivirals are available for the treatment of infections with herpesviruses, HIV, hepatitis B and C and influenza viruses. There is no doubt that it should be possible to develop highly potent and safe inhibitors of noroviruses, provided sufficient efforts. Such drugs should allow the treatment of severe and prolonged diarrhea not only in children, but also among the elderly population for whom norovirus infections account for the majority of gastroenteritis-associated hospitalization and deaths (Hall et al., 2012; Lopman et al., 2011; Trivedi et al., 2012; van Asten et al., 2011) and for immunocompromised patients.
Murine norovirus (MNV) is a genogroup V norovirus that has been widely used as a surrogate for human noroviruses (Karst et al., 2003; Wobus et al., 2004). The recent report that a human B cell line can support the replication of the human virus and the development of a mouse model for human norovirus infection may bring new possibilities for future studies (Jones et al., 2014; Taube et al., 2013). We recently reported that a small-molecule inhibitor of norovirus replication – 2’-C-methylcytidine (2CMC) – is able to protect against diarrhea and mortality in alpha/beta (IFN-α/β) and gamma interferon (IFN-γ) receptor knockout AG129 mice when given prophylactically (Rocha-Pereira et al., 2013). Furthermore, we used MNV to develop a transmission model and provided evidence that MNV is efficiently transmitted from infected animals to sentinel mice (Rocha-Pereira et al., 2015). In this model, we demonstrated for the first time that transmission of norovirus is efficiently blocked by prophylactic treatment of the sentinel mice with 2CMC. Here, we explore whether therapeutic use of an inhibitor of norovirus replication (i.e., 2CMC) also results in a beneficial effect on norovirus infection in the infected host.
2. Materials and methods
2.1. Cells, viruses and compound
2’-C-methylcytidine (2CMC) was synthesized as described (Pierra et al., 2006) and dissolved in sterile saline. Lactated Ringer’s Solution (LRS, Lactated Ringer’s Injection USP, BBraun, Belgium) was used for rehydration experiments. The MNV, strain MNV-1.CW3 (kindly provided by Dr. Herbert Virgin, Washington University, St. Louis, USA) was propagated in RAW 264.7 cells (ATCC, Barcelona, Spain) grown in DMEM (Life Technologies, Gent, Belgium) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 20 mM HEPES, 0.075 g/L sodium bicarbonate, 1 mM sodium pyruvate, 100 U penicillin/mL, 100 μg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2. Virus stocks were generated from their seventh passage in cell culture and viral titres were determined by endpoint titration. Cell lines were regularly tested for Mycoplasma contamination.
2.2. Effect of post-exposure treatment with 2CMC in a MNV mouse model
AG129 mice (129/Sv mice deficient in IFN-α/β and IFN-γ receptors) originally from BK Universal, UK, were bred and housed at the Rega Institute under specific-pathogen-free conditions. All experiments were performed under the guidelines and authorization of the Ethical Committee of the KU Leuven (P101/2012).
For all experiments, age- and sex-matched mice, 8–12 weeks of age were randomly distributed per groups and infected by oral gavage with 6×104 CCID50 (50% cell culture infectious dose) of MNV. Treatment with 2CMC was initiated either one hour before infection (n=6), 12 h (n=10), 24 h (n=10), 48 h (n=9) or 72 h (n=3) post-infection (pi) with a dose of 100 mg/ kg/ day, divided in two daily treatments (2×50 mg/ kg) until day 7 pi, by the subcutaneous route. Saline was administered to untreated control mice following the same schedule as for 2CMC (n=6). Treated and untreated mice were kept separate in independently ventilated cages for all the experiments. Bedding was replaced once a week. Starting at day 0 pi, mice were weighed daily and stools were collected from each animal (except for the 72 h pi group) and scored for consistency (0, normal feces; 1, mixed stool samples containing both solid and pasty feces; 2, pasty feces; 3, semiliquid feces; 4, liquid feces). When animals were severely ill (weight loss of >20% from the start of the experiment or >15% in 2 days, lethargy, watery squinted eyes) they were humanely euthanized using pentobarbital (Nembutal®). Blood was collected from the tail vein either (i) before infection or (ii) at one time point after infection or by cardiac puncture (iii) one week after re-challenge.
2.3. Effect of rehydration in 2CMC-treated and untreated MNV-infected mice
To study whether a combination of 2CMC-treatment and rehydration could have a beneficial effect and impact on the survival of MNV-infected animals, groups of 4 mice were treated with: (i) 2CMC 100 mg/kg/day starting 48 h pi (n=4), (ii) LRS, for rehydration (n=4) or (iii) a combination of 2CMC 100 mg/kg/day starting 48h pi and LRS (n=4). To determine when to start the rehydration of a particular animal, the fluid homeostasis of each mouse was evaluated daily by scoring the loss of skin turgor and weight loss. When mice were clinically dehydrated, i.e. had ≥ 5% body weight loss and/ or loss of skin turgor, a volume equivalent to ~5% body weight of LRS at 37°C was administered subcutaneously (plus animals were allowed to drink water ad libitum) according to the standard recommendations (Devey, 2010; Gargiulo et al., 2012). Animals were monitored once daily for symptoms, weight variation, loss of skin turgor and stool samples were collected for quantification of viral RNA.
2.4. Study of MNV replication in the small intestine of mice
AG129 mice (n=18) were infected by oral gavage with 6×104 CCID50 of MNV. At 0, 8, 12, 24, 48 and 72 h pi (n=3 per time point), mice were euthanized for dissection of the small intestine which was processed through the Swiss roll technique (Moolenbeek and Ruitenberg, 1981) and fixed in 4% formaldehyde. For histological examination, 5 μm-thick intestinal tissue slides were embedded in paraffin, sectioned and stained with hematoxylin–eosin (H&E) or with a 1:5000 dilution of anti-MNV VLP (strain S99) rabbit polyclonal antibody or the corresponding pre-bleed rabbit serum. (Taube et al., 2013)
The H&E stained slides of small intestine where the Peyer’s Patches were present, were scored for the presence of MNV antigen as well as for the presence of inflammation and increased apoptosis of epithelial cells.
2.5. Re-challenge with MNV of 2CMC-treated infected mice
AG129 mice, which had been infected with MNV and treated with 2CMC starting one hour before or at 12 h post infection, were re-challenged with MNV 3 (n=7), 6 (n=7) or 10 (n=2) weeks after the first contact with the virus. During the week after re-challenge, mice were weighed daily and their general condition was assessed. Blood was collected from animals before re-challenge (at 3 weeks pi) and one week after re-challenge (week 7 pi and 11 pi).
2.6. Detection/ quantification of specific anti-MNV IgG by ELISA
For detection of IgG in the blood of 2CMC-treated re-challenged mice, the Recombivirus™ Mouse Anti-Norovirus (MNV-1/VP1) IgG kit (Alpha Diagnostics/ Gentaur, Belgium) was used according to the manufacturer’s instructions. The presence of anti-MNV mouse IgG antibodies in samples was determined relative to mouse anti-MNV IgG controls, by calculating the threshold index (TI) as follows: TI = ODsample/ODsc, where ODsample is the OD value obtained for each tested mouse serum and ODsc is the OD value of the sensitivity control, a low level mouse anti-MNV IgG intended to represent a threshold OD for most true positives, according to the manufacturer. A TI > 1 represents a positive serum sample for anti-MNV IgG.
2.7. RNA isolation and quantitative RT-PCR
To extract RNA from stool samples, the RNeasy minikit (Qiagen, The Netherlands) was used according to the manufacturer’s protocol. For the MNV RT-qPCR, primers and probe were used as described before (Rocha-Pereira et al., 2012). One-step RT-qPCR was performed in a 20 μL reaction mixture containing 10 μL iTaq Universal Probes reaction mix (Bio-Rad, Belgium), 0.5 μL of iScript advanced reverse transcriptase, 4 μL of template RNA, 900 nM of MNV primers and 200 nM of MNV probe. Cycling conditions were: reverse transcription at 50°C for 10 min, initial denaturation at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing and extension at 60°C for 30 s (Roche LightCycler 96, Roche Diagnostics, Belgium). For MNV absolute quantification, standard curves were generated using 10-fold dilutions of MNV template DNA of known concentration.
2.8. Statistical analysis
All data were analyzed using Prism 5 software (Graph-Pad Software, San Diego, CA). In all graphs, *** = p<0.001, ** = p<0.01, * = p<0.05, and ns = not significant (p > 0.05) were determined with the nonparametric Kruskal-Wallis test with Dunn’s post-test. The one sample t test was used to compare means to the limit of detection (LOD). All error bars depict standard error of the mean (SEM). Grubbs’ test was used to detect significant outliers (p< 0.01).
3. Results
3.1. Post-exposure treatment of MNV-infected mice with 2CMC is efficient up to 2 days after infection
AG129 mice were infected orally with MNV and were treated with 2CMC whereby treatment was initiated either 12, 24, 48 or 72 h pi and was continued twice daily (2× 50 mg/kg) until day 7 pi. Control groups of (i) untreated MNV-infected animals and (ii) MNV-infected mice that were prophylactically treated with 2CMC (starting 1 h before infection) for 7 days were included in all experiments. All untreated mice showed first symptoms (such as weight loss, hunched posture, ruffled fur, and watery squinted eyes) at day 3 pi. This norovirus-induced disease progressed rapidly resulting in 100% of mice that had to be euthanized 4 days after infection (Fig. 1A). As demonstrated earlier (Rocha-Pereira et al., 2013), prophylaxis with 2CMC was able to completely protect against MNV-induced diarrhea and mortality (Fig. 1A). When treatment with 2CMC was initiated 12 h after MNV-infection, 9/10 animals (90%) survived the infection. Only one mouse in this group continued to lose weight after day 8 pi and had to be euthanized at day 13 pi; other mice showed no symptoms other than weight loss, akin to the prophylaxis control group (Fig. 1B). Mice in which treatment had been initiated at 12 h post infection lost at days 3 and 4 pi less weight than untreated mice (p˂0.01 in either case). When treatment was started either at 24 or 48 h pi, the survival rate was, respectively, 5/10 (50%) and 4/9 (44%) [Fig.1A]. We consider that some of the mice that had to be euthanized in the group in which treatment was initiated at 48 h pi could potentially have made a full recovery if they would not have been euthanized. This due to the fact that they had no symptoms other than weight loss. However, the current criteria for humane endpoints obliged us to euthanize animals with > 20% weight loss. No protection from disease was observed when 2CMC was first given 3 days after MNV-infection; since at the start of treatment mice suffered already from severe diarrhea (Fig. 1A). The day at which each group presented maximum weight loss ranged from day 4 pi in untreated mice and the 72 h pi group, to day 6, 7, and 8 pi in the 48 h pi, 24 h pi and 12 h pi groups, respectively. In the prophylaxis group, weight loss was most markedly delayed to day 9 pi (Fig. 1B). These data suggest that the inhibitory effect of 2CMC is delaying replication of MNV and consequently slowing down virus-induced disease progression. The earlier the antiviral treatment is initiated, the more potent the protective effect of 2CMC.
Fig. 1-.
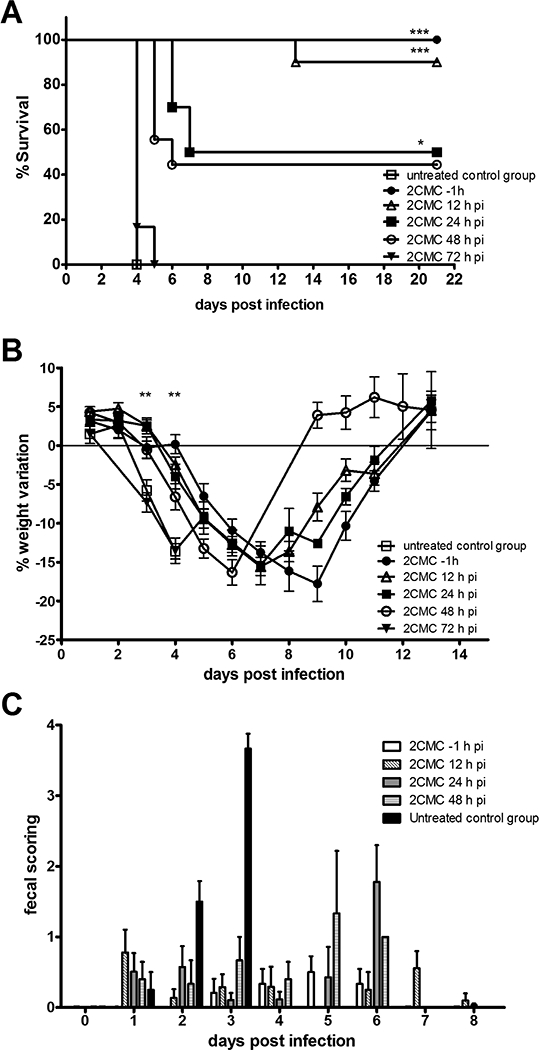
(A) Kaplan-Meier survival curve, (B) weight variation (mean ± SEM) of MNV-infected AG129 mice untreated (n=6) [open squares] or treated with 100 mg/ kg/ day of 2CMC subcutaneously, starting either 1 hour before infection (n=6) [filled circles], 12 h (n=10) [open triangles], 24 h (n=10) [filled squares], 48 h (n=9) [open circles] or 72 h (n=3) [filled inverted triangles] pi until day 7 pi. *** = p<0.001, ** = p<0.01, * = p<0.05 was determined with the Kruskal-Wallis test with Dunn’s post test, comparing (A) the median day of euthanasia of each treated group versus the untreated control group and (B) the weight variation of 2CMC-treated starting 1 hour before or 12 hours pi with untreated animals. (C) Fecal consistency (mean ± SEM) of MNV-infected AG129 mice untreated [solid black] or treated with 2CMC starting either one hour before infection (n=6) [solid white], 12 (n=10) [diagonally striped], 24 (n=10) [solid grey], or 48 (n=9) [horizontally striped] pi until day 7 pi. Scores for consistency of stool (0, normal feces; 1, mixed stool samples containing both solid and pasty feces; 2, pasty feces; 3, semiliquid feces; 4, liquid feces) versus time pi. Data are expressed as mean values ± SEM. In each group, individual stool samples were collected daily (with the exception of the untreated group at 4 days pi due to the severe dehydration of the animals).
3.2. Post-exposure treatment with 2CMC prevents norovirus-induced diarrhea, delays and reduces MNV shedding in stool
Every day after infection, a stool sample was individually collected from each infected mouse. It was scored for consistency and next used to quantify the viral RNA shed in the stool by each mouse. Untreated mice had a consistency score of ~2 (pasty feces) at day 2 pi, developed diarrhea at day 3 pi and became severely dehydrated (preventing collection of stool on day 4 pi) (Fig. 1C). In the groups that had been treated with 2CMC (either initiated at 12, 24 or 48 h pi) fecal consistency was mostly normal (score 0–1) up to day 4 pi. A predominance of pasty stool (score ~2) was observed in the groups for which treatment was initiated at 48 and 24 h pi group at days 5 and 6 pi, respectively (Fig. 1C). After day 6 pi, all the surviving mice in these groups produced normal stool and went on to fully recover from the MNV infection. MNV was detected in the stool as of day 3 pi in untreated animals. From the 2CMC-treated groups, those whose treatment was initiated at 48 h pi were shedding MNV RNA at days 3 and 4 pi (with titres of 2.0 ± 0.51 and 2.4 ± 1.4 log10 RNA copies/ g stool) (Fig. 2). The value at day 3 pi is ~2log10 lower than in the untreated controls (2.0 ± 0.51 versus 4.0 ± 0.75 log10 RNA copies/ g stool), which means that one single day of antiviral treatment had a significant impact in viral replication. In the group in which treatment was initiated at 24 h pi, the shedding of virus in the stool was first observed at day 5 pi and peaked at day 6 pi (with a titre of 3.1 ± 1.0 log10 RNA copies/ g stool). In mice for which treatment was initiated at 12 h pi, MNV shedding was detected in only 5/10 animals, and peaked between days 7 and 11 pi. Overall, viral titres in the stool were lower and appeared later, the earlier the treatment with 2CMC was initiated.
Fig. 2-.
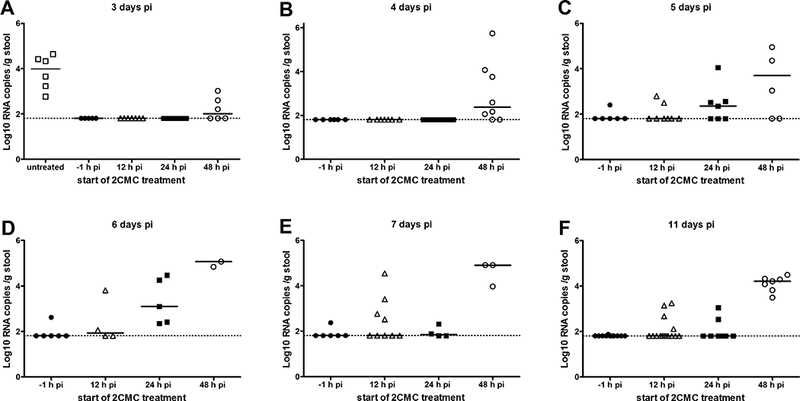
Viral RNA loads in stool samples of MNV-infected AG129 mice untreated [open squares] or treated with 2CMC starting either one hour before infection (n=6) [filled circles], 12 h (n=10) [open triangles], 24 h (n=10) [filled squares], 48 h (n=9) [open circles] pi until day 7 pi.. Data are presented as log10 RNA copy numbers per gram of stool at days 3 (A), 4 (B), 5 (C), 6 (D), 7 (E) and 11 (F) pi for each group. Each bar represents the median of each group; the dotted line represents the limit of detection. All mice in the untreated control group had to be euthanized at day 4 pi
3.3. Rehydration did not have an additional beneficial effect on the course of norovirus infection
To study whether loss of fluid homeostasis was in part responsible for the lower survival rate of MNV-infected mice for which antiviral treatment was initiated 2 days after infection, complementary rehydration was provided, by means of subcutaneous administration of LRS. Three groups of MNV-infected mice, either 2CMC-treated between days 2–7 pi (n=4), or rehydrated with LRS (n=4) or both 2CMC-treated and rehydrated (n=4), were monitored for their hydration status before, during and after complementary rehydration was provided (Table 1).
Table 1–
Effect of LRS or the combination of 2CMC starting 48h pi and LRS on the (re)hydration of MNV-infected mice
| Animal | Treatment with 2CMC (from day 2 to 7 pi) | Day of start of rehydration |
Condition upon start of rehydration | During rehydration | Outcome/ Last day of rehydration | ||
|---|---|---|---|---|---|---|---|
| Loss of skin turgor | Weight loss |
Loss of skin turgor | Maximum weight loss | ||||
| A | No | 4 pi | No | 11.5% | No | 16.6% at day 5 pi | Eut. at day 5 pi |
| B | No | 4 pi | No | 8.3% | No | 16.2% at day 5 pi | Eut. at day 5 pi |
| C | No | 3 pi | No | 4.9% | No | 11.2% at day 4 pi | Eut. at day 4 pi |
| D | No | 4 pi | No | 7.6% | No | 9.8% at day 5 pi | Eut. at day 5 pi |
| E | Yes | 4 pi | No | 10.5% | Yes, day 5–6 pi | 18.0% at day 6 pi | Full recovery/ day 8 pi |
| F | Yes | 4 pi | No | 11.1% | Yes, day 6 pi | 27.8% at day 7 pi | Eut. at day 7 pi |
| G | Yes | 3 pi | No | 5.4% | No | 18.1% at day 8 pi | Full recovery/ day 8 pi |
| H | Yes | 3 pi | No | 5.0% | Yes, day 4–6 pi | 21.5% at day 6 pi | Full recovery/ day 8 pi |
The sole administration of LRS did not result in a prolongation of the life of MNV-infected animals; all animals in the group were severely ill, presented profuse diarrhea and had to be euthanized on days 4 or 5 pi (Fig. 3A, 3C, Table 1). The combination of rehydration and 2CMC did not impact the disease symptoms and body weight loss as compared to mice that only received antiviral treatment. (Fig. 3A). Clinical dehydration (Table 1) was evident at day 3 or 4 pi in either treated or untreated MNV-infected mice. Additionally, the rehydration scheme used (5% of body weight in LRS, subcutaneously) had little effect on the hydration/ weight status and overall morbidity of the mice; the weight loss was equivalent in both groups and peaked at day 7 pi in 2CMC-treated mice and one day later in the treated and rehydrated group (Fig. 3B) and no differences in the consistency of the stool was observed between rehydrated and not rehydrated mice (Fig. 3C). Loss of skin turgor (one of the criteria used to score hydration status) was a late sign of dehydration, and only present when ≥ 18% of body weight was lost. Taken together, there was no beneficial impact of the complementary rehydration.
Fig. 3-.
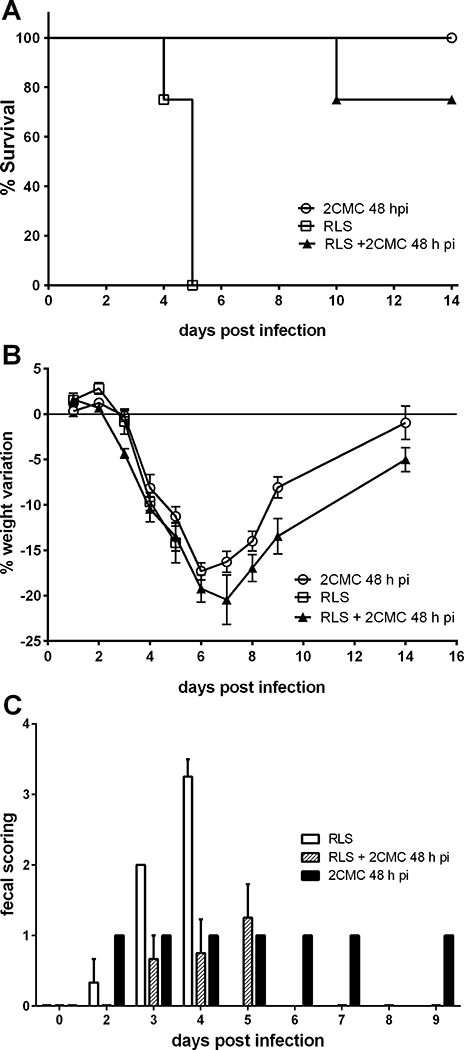
(A) Kaplan-Meier survival curve and (B) weight variation (mean ± SEM) of MNV-infected AG129 which were treated with: (i) 2CMC 100 mg/kg/day starting 48 h pi (n=4) [open circles], (ii) Lactated Ringer’s Solution (LRS), for rehydration (n=4) [open squares] or (iii) a combination of 2CMC 100 mg/kg/day starting 48h pi and LRS (n=4) [filled triangles]. (C) Fecal consistency (mean ± SEM) of MNV-infected AG129 treated with: (i) 2CMC 100 mg/kg/day starting 48 h pi (n=4) [solid black], (ii) Lactated Ringer’s Solution (LRS), for rehydration (n=4) [solid white] or (iii) a combination of 2CMC 100 mg/kg/day starting 48h pi and LRS (n=4) [diagonally striped].
3.4. Inflammation of the small intestine and viral antigens first detected 48 h after MNV-infection of AG129 mice
To better understand the early events following MNV-infection of AG129 mice and whether these may explain the lack of efficacy when post-exposure treatment is initiated 72 h pi, a histological study of the small intestine of MNV-infected mice was carried out at several time-points post infection. For that, the small intestine of infected mice was collected at 0, 8, 12, 24, 48 and 72 h pi (n=3 in each case). First signs of apoptotic epithelial cells and inflammation were observed at 48 h pi, those became more prominent at 72 h pi (Table 2 and Fig. 4). A few MNV-positive cells were detected at 48 h pi, while at 72 h pi cells expressing viral antigens were abundantly detected; no viral antigens were detected at earlier time points (Fig. 5). Hence, 2CMC loses its efficacy if the treatment is initiated when viral antigens are already widespread in the intestinal tissue.
Table 2–
Scoring of H&E staining of the small intestine of MNV-infected AG129 mice
| MNV-1.CW3 -infected AG129 mice / Time of organ harvest | Presence of Peyer’s Patches | Inflammation | Increased apoptosis in epithelial cells |
|---|---|---|---|
| 0 h pi | + | 0 | 0 |
| 8 h pi | + | 0 | 0 |
| 12 h pi | + | 0 | 0 |
| 24 h pi | + | 0 | 0 |
| 48 h pi | + | 0/1+ | 0/1+ |
| 72 h pi | + | 2+ | 1+ |
For each time point a total of 3 animals were scored, representative scoring for each group is given.
Fig 4.-.
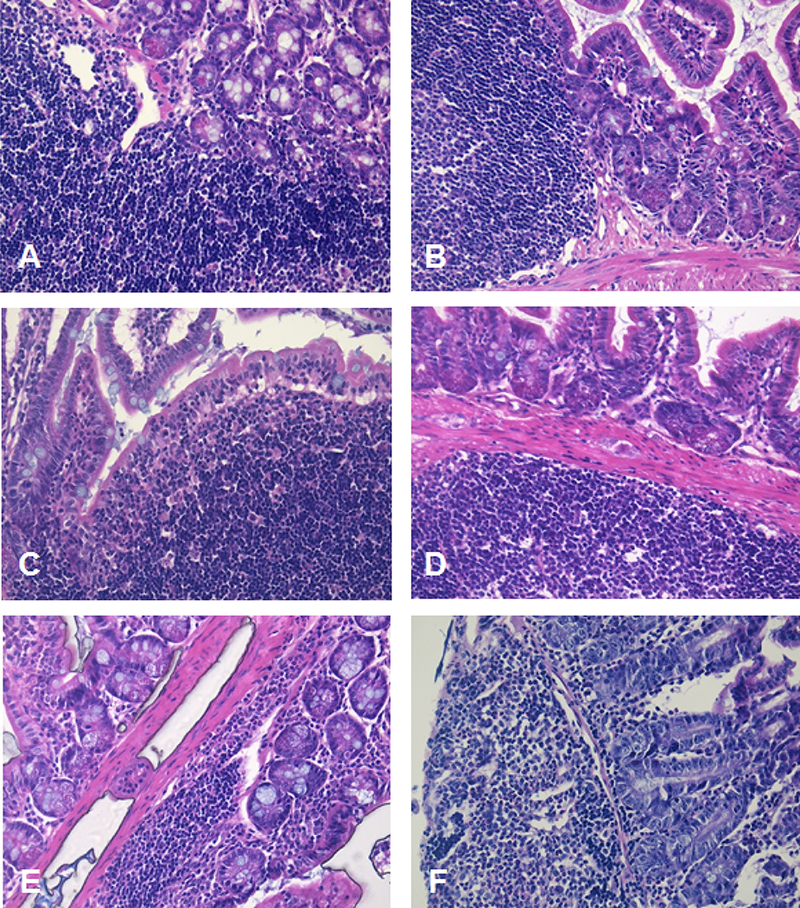
H&E staining (200x magnification) of the small intestine of AG129 mice (n=18) infected by oral gavage with 6×104 CCID50 of MNV and harvested (using the Swiss roll technique) at different times pi, namely at (A) 0 h, (B) 8 h, (C) 12 h, (D) 24 h, (E) 48 h and (F) 72 h pi (n=3 per time point)
Fig. 5-.
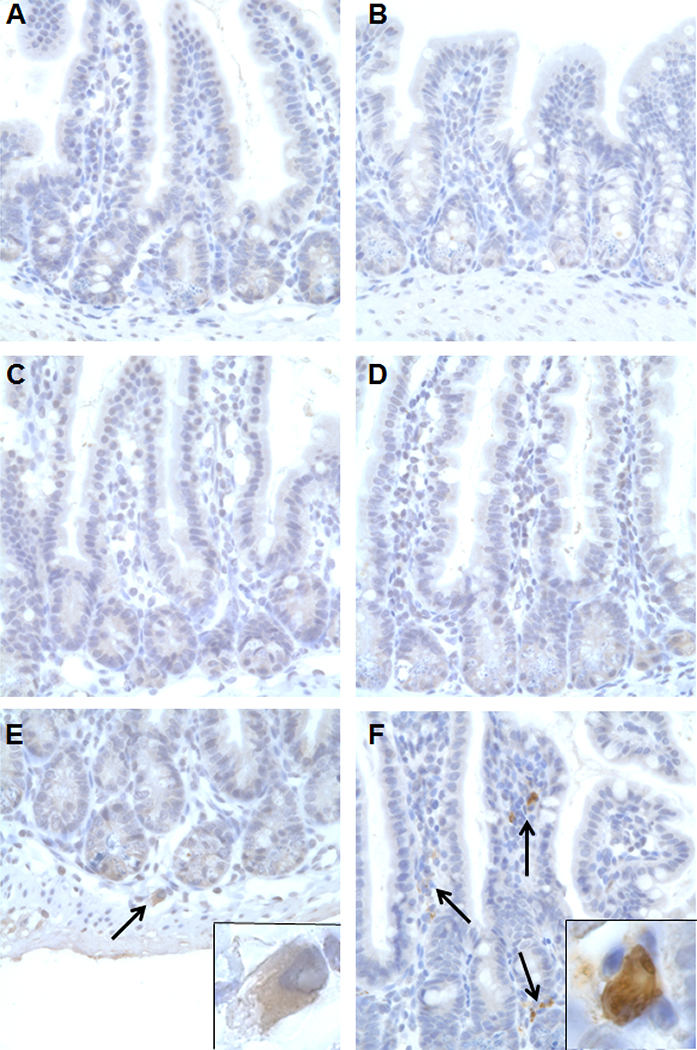
Staining with an anti-MNV capsid antibody (600x magnification) of the small intestine of AG129 mice (n=18) infected by oral gavage with 6×104 CCID50 of MNV and harvested at 0 (A), 8 (B), 12 (C), 24 (D), 48 (E) and 72 (F) h pi (n=3 per time point). Representative images are shown. Inset images (E) and (F) depict positive cells at 1000x magnification
3.5. Post-exposure treatment with 2CMC results in the production of high levels of specific IgGs that protect against re-infection
MNV-infected mice that had been treated with 2CMC did not develop virus-induced disease when re-challenged with the virus 3 weeks after infection (Rocha-Pereira et al., 2013). To study whether the protective immunity lasted for longer times, we re-challenged MNV-infected mice that had been efficiently protected by 2CMC-treatment at 6 weeks (n=7) and 10 weeks pi (n=2) [a 3 week pi (n=7) was included as control] and monitored the animals for one week after re-challenge (Fig 6A). All re-challenged mice remained healthy. The IgG titres in the blood of these animals were determined either before re-challenge – at 3 weeks pi – or one week after re-challenge – which was at 7 weeks pi (in mice re-challenged at 6 weeks pi) and at 11 weeks pi (in those re-challenged at 10 weeks pi) (Fig. 6B). High levels of anti-MNV IgG were detected in mice at 3 weeks pi, prior to re-challenge. Among the animals re-challenged at 6 and 10 weeks pi, all were positive for IgG in their serum, with the exception of one animal in the 6 week re-challenge group.
Fig. 6-.
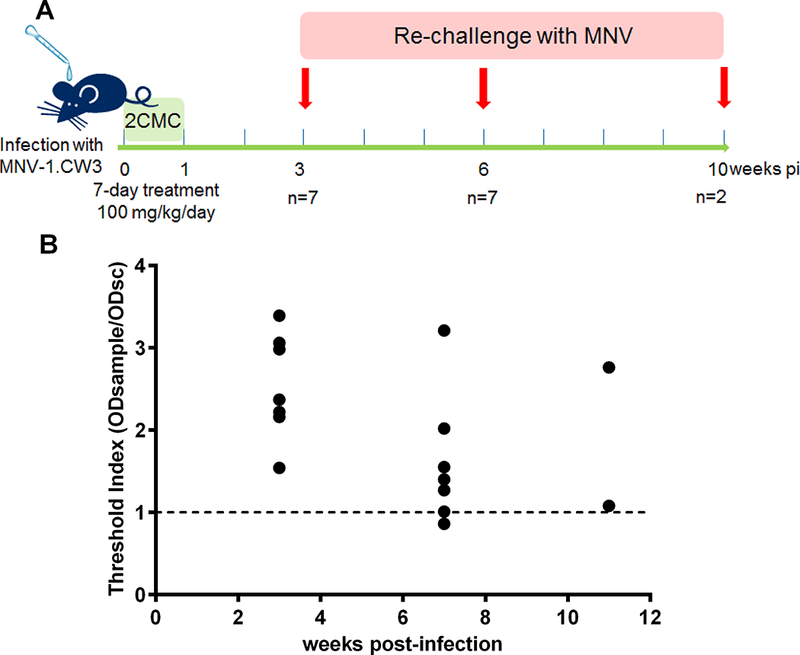
(A) Scheme of the experimental layout for the study of duration of immunity and IgG quantification in AG129 mice re-challenged with MNV at different time points. (B) Detection of anti-MNV IgG detected in AG129 mice [filled circles] before re-challenge (at 3 weeks pi, n=7) and one week after re-challenge [week 7 (n=7) and 11 (n=2) pi]. The presence of anti-MNV mouse IgG antibody in serum samples was determined relative to mouse anti-MNV IgG Controls, by calculating the threshold index (TI) for each serum sample. A TI > 1 represents a positive serum sample for anti-MNV IgG.
4. Discussion
We recently reported on the in vivo efficacy of the nucleoside analogue 2’-C-methylcytidine (2CMC) when used prophylactically in AG129 mice, not only to prevent norovirus-induced diarrhea and mortality but also to block the transmission of MNV from mouse to mouse. Norovirus drugs are also needed for the treatment of those with severe and prolonged diarrhea, for which electrolyte replenishment is currently the only treatment available. Scarce attempts to use ribavirin to treat severe and prolonged norovirus disease (Woodward et al., 2015) attest for the pressing need to develop better, safe and specific antiviral therapy for norovirus infections.
Here we explored the use of 2CMC to therapeutically treat AG129 mice infected with MNV to study whether such antiviral could help reduce the morbidity and even mortality associated with severe norovirus-induced diarrhea in mice. 2CMC was able to have a positive impact in outcome of infection when used during the first 2 days after infection, reducing the mortality in ~50 to 90% of such animals. Even when initiated late, one single day of antiviral treatment caused a ~2 log10 reduction in viral shedding in the stool. However, when 2CMC-treatment was initiated after the onset of symptoms (i.e. day 3 pi), this antiviral was not able to delay the course of infection or reduce mortality when compared to untreated mice. These observations suggest that the current paradigm should be revised: antiviral treatment is possible and can be effective and useful against acute viral infections. Even though an early start of treatment after contact/exposure to the viral pathogen can be challenging, due to the absence of symptoms and/ or of definitive laboratorial diagnostics, a reduction of disease burden, hospitalization risk and transmission could still be achieved with a late treatment. We here demonstrate this with a molecule whose structure was not designed nor optimized to treat norovirus infections – if proper efforts are made, a highly effective, potent and safe norovirus antiviral can be developed and thus would perform significantly better.
Within a household or in a hospital ward, the probability that other individuals in the same setting would have been or would be exposed is quite high, hence treatment/ prophylaxis could be beneficial to everyone. In larger settings, e.g. cruise ships, determining exposure before or just at onset of symptoms would present a greater challenge; on the other hand the fact that the mean age of cruise ship passengers is ≥55 years of age and those have greater risk of hospitalization would be an argument of favor of generalized prophylaxis. Additional studies with mice inoculated with lower inoculums will allow a more detailed assessment of the window of treatment/ prophylaxis. Later on, challenge studies in human volunteers will be necessary to determine exactly who should receive prophylaxis/ treatment with a safe and highly potent antiviral and until which time point this is advantageous.
Another aspect to consider is that while MNV infection of mice (and in particular the mouse model selected for this work) causes a systemic infection with the virus spreading through the lymph nodes, spleen, liver and causing intense viremia and mortality (Rocha-Pereira et al., 2014; Wobus et al., 2006), this is not the case for human infections, in which norovirus is thought to replicate locally in the intestine and viremia has only scarcely been reported (Green, 2013; Ito et al., 2006). We chose to use this aggressive model for the study of the in vivo efficacy of small molecule inhibitors because it has important features that such models require: it recapitulates the weight loss, diarrhea and intense shedding of virus in the stool observed in humans. Plus, being a mortality model it sets a high standard, necessary to select only those compounds that potently inhibit norovirus replication in the host. Therefore, the fact that 2 days post-exposure treatment is still effective with such a high barrier mouse model, strongly suggests an even stronger inhibitory effect would be observed in humans with a localized infection. Moreover, the achieved reduction of viral shedding in the stool, even when antiviral treatment is initiated late after infection, suggests that also in patients, a late start of treatment could reduce shedding and hence reduce the risk of transmission. This study adds to the body of work produced demonstrating that there is ground for the use of antiviral drugs for the control of human norovirus infections.
Considering that complications derived from severe human norovirus infection are often related to the dehydrated status of the infected individuals, we studied whether the ~50% mortality observed in animals in which 2CMC-treatment was initiated late could be partially due to an incapacity to recover from that severe dehydrated condition. Subcutaneous electrolyte replenishment had however no beneficial impact on disease evolution in these animals. Thus even though electrolyte replenishment is necessary and desirable in humans, antiviral treatment seems to be the defining factor that determines the reduction of symptoms and ultimately the resolution of the infection in this aggressive infection model. The loss of efficacy of the antiviral treatment coincided with the spread of the virus throughout the intestinal tissue, which started 2 days or later after infection; when treatment was started earlier an efficient control of viral replication as well as inflammation and tissue damage was achieved.
We showed earlier that efficient treatment of MNV-infected mice with 2CMC resulted in the development of protective immunity against the virus. Here we extended these findings by demonstrating that such protective IgG response was also evident as late as 10 weeks after the first contact with the virus.
5. Conclusions
In conclusion, we demonstrate that inhibiting norovirus replication two days after inoculation of mice with MNV still results in partial protection, in an aggressive infection model, for which hydration does not offer any beneficial clinical effect. The marked reduction of viral shedding and absence of diarrhea in those animals treated late emphasizes the potential benefit of using antivirals to manage norovirus infections. Put together, this highlights the potential of antiviral drugs in the control of norovirus infections.
6. Acknowledgements
This work was funded by EU FP7 project SILVER (260644) and KU Leuven IOF project HB/14/031 to JN and by National Institutes of Health grants R01 AI080611 and R21 AI110907 to CEW. JRP is a Marie Curie COFUND William Harvey International Translational Research Academy (WHRI-ACADEMY #343) Postdoctoral Fellow.
We gratefully acknowledge Herbert W. Virgin (Washington University, St. Louis, USA) for providing MNV and the University of Michigan Pathology Core for Animal Research (PCAR) for help with slide staining and reading. We thank Erby Wilkinson, Carolien De Keyzer, Jasper Rymenants and Charlotte Vanderheydt for excellent technical assistance and Dominique Brabants for fine editorial help.
7. References
- Chhabra P, Samoilovich E, Yermalovich M, Chernyshova L, Gheorghita S, Cojocaru R, Shugayev N, Sahakyan G, Lashkarashvili M, Chubinidze M, Zakhashvili K, Videbaek D, Wasley A, Vinje J, 2014. Viral gastroenteritis in rotavirus negative hospitalized children <5 years of age from the independent states of the former Soviet Union. Infect Genet Evol 28, 283–288. [DOI] [PubMed] [Google Scholar]
- Devey JJ, 2010. Crystalloid and Colloid Fluid Therapy in: Ettinger S, Feldman E (Eds.), Textbook of Veterinary Internal Medicine Expert Consult, 7th Edition ed. Elsevier - Health Sciences Division. [Google Scholar]
- Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G, 2012. Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. Ilar j 53, E55–69. [DOI] [PubMed] [Google Scholar]
- Green KY, 2013. Caliciviridae: The Noroviruses, in: Knipe DM, Howley PM (Eds.), Fields Virology, 6th Edition ed. Lippincott Williams & Wilkins, Philadelphia, PA., pp. 583–609. [Google Scholar]
- Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA, 2012. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis 55, 216–223. [DOI] [PubMed] [Google Scholar]
- Hemming M, Rasanen S, Huhti L, Paloniemi M, Salminen M, Vesikari T, 2013. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. European journal of pediatrics 172, 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Takeshita S, Nezu A, Aihara Y, Usuku S, Noguchi Y, Yokota S, 2006. Norovirus-associated encephalopathy. Pediatr Infect Dis J 25, 651–652. [DOI] [PubMed] [Google Scholar]
- Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinje J, Tibbetts SA, Wallet SM, Karst SM, 2014. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 346, 755–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst SM, Wobus CE, Lay M, Davidson J, Virgin H.W.t., 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299, 1575–1578. [DOI] [PubMed] [Google Scholar]
- Lopman BA, Hall AJ, Curns AT, Parashar UD, 2011. Increasing rates of gastroenteritis hospital discharges in US adults and the contribution of norovirus, 1996–2007. Clin Infect Dis 52, 466–474. [DOI] [PubMed] [Google Scholar]
- Moolenbeek C, Ruitenberg EJ, 1981. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim 15, 57–59. [DOI] [PubMed] [Google Scholar]
- Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, Wikswo M, Shirley SH, Hall AJ, Lopman B, Parashar UD, 2013. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 368, 1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierra C, Amador A, Benzaria S, Cretton-Scott E, D’Amours M, Mao J, Mathieu S, Moussa A, Bridges EG, Standring DN, Sommadossi JP, Storer R, Gosselin G, 2006. Synthesis and pharmacokinetics of valopicitabine (NM283), an efficient prodrug of the potent anti-HCV agent 2’-C-methylcytidine. J Med Chem 49, 6614–6620. [DOI] [PubMed] [Google Scholar]
- Ramani S, Kang G, 2009. Viruses causing childhood diarrhoea in the developing world. Curr Opin Infect Dis 22, 477–482. [DOI] [PubMed] [Google Scholar]
- Rocha-Pereira J, Jochmans D, Dallmeier K, Leyssen P, Nascimento MS, Neyts J, 2012. Favipiravir (T-705) inhibits in vitro norovirus replication. Biochem Biophys Res Commun 424, 777–780. [DOI] [PubMed] [Google Scholar]
- Rocha-Pereira J, Jochmans D, Debing Y, Verbeken E, Nascimento MS, Neyts J, 2013. The viral polymerase inhibitor 2’-C-methylcytidine inhibits Norwalk virus replication and protects against norovirus-induced diarrhea and mortality in a mouse model. J Virol 87, 11798–11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Pereira J, Jochmans D, Neyts J, 2015. Prophylactic treatment with the nucleoside analogue 2’-C-methylcytidine completely prevents transmission of norovirus. J Antimicrob Chemother 70, 190–197. [DOI] [PubMed] [Google Scholar]
- Rocha-Pereira J, Neyts J, Jochmans D, 2014. Norovirus: targets and tools in antiviral drug discovery. Biochem Pharmacol 91, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube S, Kolawole AO, Hohne M, Wilkinson JE, Handley SA, Perry JW, Thackray LB, Akkina R, Wobus CE, 2013. A mouse model for human norovirus. mBio 4, e00450–00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi TK, DeSalvo T, Lee L, Palumbo A, Moll M, Curns A, Hall AJ, Patel M, Parashar UD, Lopman BA, 2012. Hospitalizations and mortality associated with norovirus outbreaks in nursing homes, 2009–2010. JAMA 308, 1668–1675. [DOI] [PubMed] [Google Scholar]
- van Asten L, Siebenga J, van den Wijngaard C, Verheij R, van Vliet H, Kretzschmar M, Boshuizen H, van Pelt W, Koopmans M, 2011. Unspecified gastroenteritis illness and deaths in the elderly associated with norovirus epidemics. Epidemiology 22, 336–343. [DOI] [PubMed] [Google Scholar]
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE, 2013. Global burden of childhood pneumonia and diarrhoea. Lancet 381, 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW, 2004. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol 2, e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus CE, Thackray LB, Virgin HW, 2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol 80, 5104–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JM, Gkrania-Klotsas E, Cordero-Ng AY, Aravinthan A, Bandoh BN, Liu H, Davies S, Zhang H, Stevenson P, Curran MD, Kumararatne D, 2015. The role of chronic norovirus infection in the enteropathy associated with common variable immunodeficiency. The American journal of gastroenterology 110, 320–327. [DOI] [PubMed] [Google Scholar]
- Yu J, Jing H, Lai S, Xu W, Li M, Wu J, Liu W, Yuan Z, Chen Y, Zhao S, Wang X, Zhao Z, Ran L, Wu S, Klena JD, Feng L, Li F, Ye X, Qiu Y, Wang X, Yu H, Li Z, Yang W, 2015. Etiology of diarrhea among children under the age five in China: Results from a five-year surveillance. J Infect. [DOI] [PMC free article] [PubMed] [Google Scholar]


