Abstract
Clinical and basic science research have revealed persistent effects of early life injury on nociceptive processing and resulting pain sensitivity. While recent work has identified clear deficits in fast GABAA- and glycine receptor-mediated inhibition in the adult spinal dorsal horn after neonatal tissue damage, the effects of early injury on slow, metabotropic inhibition within spinal pain circuits are poorly understood. Here we provide evidence that neonatal surgical incision significantly enhances postsynaptic GABAB receptor signaling within the mature superficial dorsal horn (SDH) in a cell type-dependent manner. In vitro patch-clamp recordings were obtained from identified lamina I projection neurons and GABAergic interneurons in the SDH of adult female mice following hindpaw incision at postnatal day (P)3. Early tissue damage increased the density of the outward current evoked by baclofen, a selective GABAB receptor agonist, in projection neurons but not inhibitory interneurons. This could reflect enhanced postsynaptic expression of downstream G protein-coupled inward-rectifying potassium channels (GIRKs), as the response to the GIRK agonist ML297 was greater in projection neurons from neonatally incised mice compared to naive littermate controls. Meanwhile, presynaptic GABAB receptor-mediated reduction of spontaneous neurotransmitter release onto both neuronal populations was unaffected by early life injury. Collectively, our findings suggest that ascending nociceptive transmission to the adult brain is under stronger control by spinal metabotropic inhibition in the aftermath of neonatal tissue damage.
Keywords: Dorsal horn, pain, metabotropic, inhibition, synapse
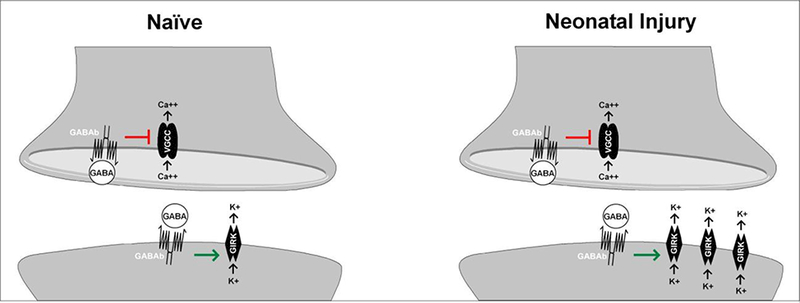
3. Introduction
Clinical studies report that infants in the neonatal intensive care unit (NICU) are subjected to an average of 14 invasive procedures per day in the first two weeks of life (Simons et al., 2003). Medically necessary procedures, such as heel lance, reliably evoke nociceptive responses (Cornelissen et al., 2013) and dramatically alter pain thresholds in neonates (Fitzgerald et al., 1989). While invasive procedures during the preterm period have been linked to reduced pain reactivity to modest stimuli (Oberlander et al., 2000), time spent in the NICU is correlated with increased activation of pain-related brain areas in response to moderately painful stimuli during adolescence (Hohmeister et al., 2010). Adolescents previously admitted to the NICU also exhibited greater perceptual sensitization and less habituation during repeated noxious stimulation (Hohmeister et al., 2010). Similarly, a single hindpaw injury in neonatal rats causes acute hyperalgesia followed by a generalized hypoalgesia, and yet exacerbates pain severity following reinjury (Ren et al., 2004; Walker et al., 2009). Unfortunately, the cellular and molecular mechanisms underlying these prolonged changes in pain processing after early life tissue damage remain poorly understood.
The spinal cord serves as a conduit between the periphery and the brain for sensory and motor functions. In the SDH, lamina I projection neurons receive primary afferent input and convey signals to supraspinal pain centers, while local inhibitory interneurons normally suppress nociceptive transmission and reduce the output of the SDH network (Todd, 2010). Given the ongoing reorganization of pain circuits in the CNS during the early postnatal period, aberrant sensory input during this sensitive period of development can exert dramatic and long lasting effects on circuit function (Fitzgerald, 2005). For example, GABAergic signaling is highly plastic in the neonatal spinal cord, with significant regulation occurring throughout early postnatal and adolescent life (Keller et al., 2004; Keller et al., 2001), raising the possibility that tissue damage during this critical period could interfere with the normal maturation of spinal inhibitory circuits. Indeed, early-life surgical incision persistently dampens fast inhibitory neurotransmission in the adult mouse SDH (Li et al., 2013; Li et al., 2015). However, although persistent deficits in GABAA receptor-mediated signaling occur after neonatal incision, little is known about the relationship between early life injury and metabotropic inhibitory signaling in the mature dorsal horn.
The GABAB receptor (GABABR) is a metabotropic G protein-coupled receptor that is highly expressed in laminae l-ll of the SDH (Yang Kun et al., 2001). GABABR acts to reduce presynaptic neurotransmitter release (Barrai et al., 2000; Bussieres and El Manira, 1999; Chen and van den Pol, 1998; Sakaba and Neher, 2003) and evoke postsynaptic membrane hyperpolarization via the activation of downstream GIRK channels (Fernández-Alacid et al., 2009). Significant evidence supports the importance of the GABABR for the modulation of spinal nociceptive processing. Intrathecal administration of GABABR antagonists causes allodynia and thermal hyperalgesia in rodents (Malan, Mata, & Porreca, 2002). Additionally, intrathecal, but not systemic, baclofen administration mitigates hyperalgesia in adult rats after hindpaw incision (Reichl, Augustin, Zahn, & Pogatzki-Zahn, 2012). This highlights the need to understand how neonatal tissue damage alters GABABR signaling in the mature SDH. Furthermore, given the rich heterogeneity of cell types that characterizes the SDH network (Peirs and Seal, 2016), it is important to elucidate the influence of early life injury on GABABR function in identified neuronal populations within the spinal pain circuit.
The present results demonstrate that neonatal surgical injury enhances postsynaptic GABABR-mediated signaling in ascending projection neurons, but not inhibitory interneurons, of the adult mouse SDH. This may reflect an elevated expression of GIRK channels, as the direct activation of these channels also evoked greater outward currents in mature projection neurons after neonatal hindpaw incision. Meanwhile, early tissue damage failed to affect GABABR- mediated presynaptic inhibition of spontaneous neurotransmitter release onto either neuronal population in the adult SDH. In summary, this work suggests that noxious sensory experience during early life may strengthen the ability of spinal GABABRs to restrict the flow of ascending nociceptive transmission to the brain.
4. Experimental Procedures
4.1. Animals
In order to identify inhibitory interneurons in the spinal cord dorsal horn, homozygous transgenic mice expressing enhanced green fluorescent protein (eGFP) under control of the glutamic acid decarboxylase 1 (GAD67) gene promoter (FVB-Tg(GadGFP)4570Swn/J; Jackson Laboratory, Bar Harbor, ME) were used in all experiments. We recorded from GFP-labeled laminae I and II interneurons located within 50 μΜ of the myelin border. We chose to initiate our study with adult (P56–77) female mice based on previous research demonstrating sex differences after neonatal injury. Although both sexes experience hypoalgesia in adulthood after neonatal inflammation, female rats show higher mechanical thresholds and the phenotype occurs earlier (LaPrairie and Murphy, 2007). Additionally, female but not male mice show an acute elevation in spontaneous excitatory signaling in the spinal dorsal horn after neonatal incision (Li and Baccei, 2011).
4.2. Neonatal hindpaw incision
On P3, pups were anesthetized via isoflurane inhalation (5%), and the left hindpaw was incised through the plantar skin and muscle as previously described (Brennan et al., 1996). The skin was sutured (7–0; Ethicon; Cincinnati, OH, USA) and the injury completely healed within two weeks.
4.3. Projection neuron identification
The parabrachial nucleus (PB) was chosen as the site of injection since it is the target of the majority of lamina I projection neurons located on the contralateral side of the lumbar enlargement of the spinal cord (Cameron et al., 2015), and retrograde labeling would be most efficient at this site. At least three days before being euthanized, animals were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg) and secured in a stereotaxic apparatus (World Precision Instruments; Sarasota, FL, USA) with non-rupture ear bars (World Precision Instruments; Sarasota, FL, USA). An incision was made in the scalp to expose a skull area that included both lambda and bregma. The coordinates to target the PB were (in mm in relation to bregma): −0.47–0.49 rostrocaudal, −0.12 mediolateral, and −0.40–0.42 dorsoventral based on an atlas of the mouse brain (Paxinos, 2013); a hole was drilled in the skull using an OmniDrill35 (World Precision Instruments; Sarasota, FL, USA). Mice received an injection (150 nL at an infusion rate of 30 nL/min) of the retrograde tracer FAST Dil oil (2.5 mg/ml; Invitrogen; Carlsbad, CA, USA) into the PB contralateral to the P3 hindpaw incision using a Hamilton syringe (62RN; 2.5 μL; 33-gauge needle). The skin was closed using Vetbond (3M; Maplewood, MN, USA) and animals were returned to the home cage upon recovery.
4.4. Electrophysiology
4.4.1. Preparation of in vitro spinal cord slices
Adult female GAD67-GFP mice were euthanized with sodium pentobarbital (Fatal-Plus; Vortech Pharmaceuticals; Dearborn, Ml, USA) and transcardially perfused with cold, sucrose- substituted artificial cerebrospinal fluid (dissection solution; containing in mM: 250 sucrose, 2.5 KCI, 25 NaHCO3, 1.0 NaH2P04, 6 MgCI2, 0.5 CaCI2, and 25 glucose). All solutions were continuously bubbled with carbogen (95% oxygen, 5% carbon dioxide). After perfusion, animals were decapitated and the vertebral column was isolated and submerged in cold dissection solution. After laminectomy, the spinal cord was removed then stripped of the dura mater and nerve roots. The spinal cord was blocked to include the L3-L5 segments, and immersed in low- melting point agarose (3% in dissection solution; Invitrogen; Carlsbad, CA, USA). Parasagittal slices (250–300 μm) were cut from the dorsal horn ipsilateral to the incision with a vibrating microtome (7000smz-2; Campden Instruments; Lafayette, IN, USA). Slices were allowed to recover at room temperature for 15–20 minutes in a solution containing (in mM): 92 NMDG, 2.5 KCI, 1.2 NaH2P04, 30 NaHCO3, 20 HEPES, 25 glucose, 5 Na ascorbate, 2 thiourea, 3 Na pyruvate, 10 MgS04, and 0.5 CaCI2 (Ting et al., 2011) and then incubated for 45 minutes in artificial cerebrospinal fluid (aCSF; composition in mM: 125 NaCl, 2.5 KCI, 25 NaHCO3, 1.0 NaH2P04, 1.0 MgCI2, 2.0 CaCI2, and 25 glucose) at room temperature.
4.4.2. Patch-clamp recording of projection neurons and GAD67-GFP interneurons
Slices were anchored in a submersion-type recording chamber (RC-22; Warner Instruments, Hamden, CT, USA) then secured on the stage of an upright microscope (BX51WI; Olympus; Center Valley, PA, USA), while continuously perfused with room temperature aCSF. Dil-containing projection neurons in lamina I were identified by fluorescence and visualized using infrared illumination. Patch electrodes were made using a microelectrode puller (P-97; Sutter Instruments; Novato, CA, USA) and thin walled single-filament borosilicate glass (1.5 mm outer diameter; World Precision Instruments; Sarasota, FL, USA). Pipette resistances were 3 to 7 ΜΩ, and seal resistances were >1 GΩ Patch clamp recordings were obtained using a Multiclamp 700B amplifier (Molecular Devices; Sunnyvale, CA, USA). Membrane potentials were adjusted for liquid junction potentials (−14 mV), calculated using JPCalc software (P. Barry, University of New South Wales, Sydney, Australia; modified for Molecular Devices). Currents were filtered at 4 kHz through a low-pass Bessel filter, digitally sampled at 10 kHz, and stored using a commercially available data acquisition system (Digidata 1440A with pCIamp 10.4 software; Molecular Devices; Sunnyvale, CA, USA).
4.4.3. Postsynaptic GABABR activation
Postsynaptic GABABR-mediated outward currents in response to the selective agonist baclofen (100 μΜ; Hellobio; Princeton, NJ, USA) were measured in voltage clamp at a holding potential of −70 mV. A baseline measurement was taken for three minutes, and then baclofen was washed on continuously for six minutes. The patch electrode solution was composed of (in mM): 130 K-gluconate, 10 KCI, 10 HEPES, 1 EGTA, 0.1 CaCI2, 2 MgATP, 10 Na- phosphocreatine, and 0.3 Na2GTP, pH 7.2 (295–305 mOsm). Fast synaptic transmission was blocked via the bath application of selective antagonists for glycine (strychnine, 0.5 μM; Sigma; St. Louis, MO, USA), GABAA (gabazine, 10 μΜ; Hellobio; Princeton, NJ, USA), AMPA (NBQX, 10 μΜ; Hellobio; Princeton, NJ, USA) and NMDA (AP-5, 20 μΜ; Hellobio; Princeton, NJ, USA) receptors, while voltage-gated sodium channels were blocked with tetrodotoxin (TTX; 0.5 μΜ; Hellobio; Princeton, NJ, USA).
4.4.4. Postsynaptic GIRK activation
Postsynaptic activation of GIRK channels was measured in voltage clamp (at a holding potential of −70 mV) by recording outward currents, in response to the selective Kir 3.1/3.2 (GIRK1/2) agonist ML297 (100 μΜ; Tocris; Minneapolis, MN, USA). A baseline measurement was taken for three minutes, and then ML297 was washed on continuously for six minutes. We used the same patch electrode solution and pharmacological isolation method as described in Section 4.4.3.
4.4.5. Presynaptic GABABR activation
To characterize presynaptic GABABR-mediated inhibition of spontaneous neurotransmitter release, miniature postsynaptic currents (mPSCs) were recorded in the presence of TTX (0.5 μΜ; Hellobio; Princeton, NJ, USA) before and after bath application of the GABABR agonist baclofen (100 μΜ; Hellobio; Princeton, NJ, USA). A baseline measurement was taken for six minutes and then baclofen was washed on continuously for 12 minutes. Patch electrodes were filled with a solution containing the following (in mM): 130 Cs-gluconate, 10 CsCI, 10 HEPES, 11 EGTA, 1 CaCI2, and 2 MgATP, pH 7.2 (295–305 mOsm). The patch solution included 0.3 mM of GDPβS (Sigma; St. Louis, MO, USA) to block postsynaptic GABABR-evoked G protein-coupled signaling. In order to isolate and record miniature excitatory postsynaptic currents (mEPSCs), cells were voltage clamped at −70 mV. To record inhibitory mPSCs (mIPSCs), cells were held at 0 mV.
4.5. Data Analysis
Statistical analyses were performed using GraphPad Prism 7.0 software (GraphPad Software; San Diego, CA). Outward current amplitude, decay, and area under the curve (AUC) data were quantified in Clampfit software (Molecular Devices; Sunnyvale, CA, USA). Outward current amplitude was calculated as the change in the mean holding current (in pA) during a 100 s period after drug application compared to the 100 sec period immediately prior to drug perfusion. The degree of current decay/desensitization was measured by analyzing the difference between the peak current amplitude and the mean amplitude of the current observed 180–280 sec after the peak (Fig. 1B, C). Given the slow rate of desensitization observed in some neurons during prolonged baclofen application (Fig. 1B), this period was chosen to better capture the full extent of the desensitization. Cell capacitance was calculated as the average capacitance of five membrane test measurements obtained with pCIamp 10.4 software. Postsynaptic GABABR activation experiments included both projection neurons and GAD67- GFP interneurons and were analyzed via two way ANOVAs with the factors: cell type X incision. If main effects were present, posthoc analyses with Sidak’s multiple comparison tests were performed. The amplitude and decay of outward currents evoked by GABABR and GIRK agonists in projection neurons were normalized to average cell capacitance to determine current density and analyzed with independent samples t-tests. Pearson correlation analysis was used to assess the relationship between GABABR-mediated current amplitude and decay. For studies examining presynaptic GABABR signaling, the amplitude and frequency of mPSCs were measured using MiniAnalysis 6.0.7 (Synaptosoft; Decatur, GA, USA), and the threshold for mEPSC and mIPSC detection was set at twice the average of the background noise. Results were analyzed via two-way ANOVAs with the factors: baclofen (repeated measures) X incision, followed by Sidak posthoc tests if main effects were present. Data on GABABR-mediated current amplitude, mPSC frequency, and GIRK-mediated current amplitude did not align with a Gaussian distribution (determined by the D’Agostino and Pearson normality test) and were transformed (with log or square root functions) or analyzed with nonparametric tests (Mann- Whitney U). All graphs of results present the untransformed data sets. Data are reported as mean ± SEM unless otherwise indicated.
Figure 1: Neonatal incision modulates postsynaptic GABABR currents in adult spino-PB neurons.
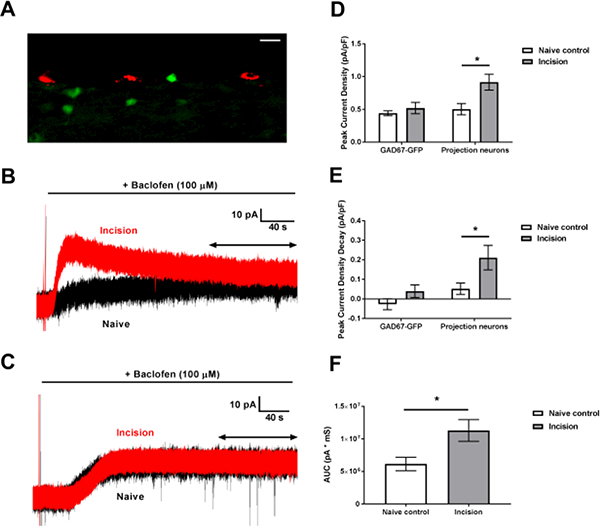
A: Image taken in a parasagittal section of the SDH containing Dil-labeled projection neurons in red and GAD67-GFP interneurons in green (scale bar = 20 μΜ). B: Representative traces of baclofen-evoked outward currents in adult projection neurons from P3 incision (red) and naïve littermate control (black) mice. Double arrow indicates the time period used to measure current desensitization (see Experimental Procedures). C: Representative traces of GABABR-mediated outward currents in GAD67-GFP interneurons from incision (red) and naïve (black) mice. Double arrow indicates the time period used to measure current desensitization. D: Neonatal incision increased the peak density of baclofen-evoked outward current in mature dorsal horn neurons, with a two-way ANOVA revealing main effects of cell type (p = 0.039) and incision (p = 0.026); posthoc analysis suggested this effect was exclusive to projection neurons (.n = 8; *p = 0.011; Sidak’s multiple comparisons test). E: Plot showing the degree of GABAbR- evoked current desensitization in GAD67-GFP inhibitory interneurons (n = 10) and projection neurons (n = 8). Two-way ANOVA revealed main effects of cell type (p = 0.0033) and incision (p = 0.0077). Outward currents showed greater decay exclusively in projection neurons from incision animals compared to naïve controls (*p = 0.019; Sidak’s multiple comparison tests). F: Mean AUC of baclofen-evoked currents. Neonatal injury increased the overall AUC of the outward current in projection neurons (n = 8; *p = 0.0194; Unpaired t-test).
5. Results
5.1. Neonatal incision enhances postsynaptic GABABR currents in adult projection neurons
We first investigated if neonatal hindpaw incision affects postsynaptic GABABR signaling in mature projection neurons, identified by labeling with Dil, and GABAergic interneurons labeled by GAD67-dependent GFP expression (Fig. 1A). Outward currents in response to the selective GABABR agonist baclofen (100 μΜ) were elicited from a holding potential of −70 mV (Fig. 1B, C). We found that neonatal incision increased the density of GABABR-mediated outward currents compared to naïve littermate controls (Fig. 1D), with a two-way ANOVA revealing main effects of cell type (F(1,34) = 4.61, p = 0.039) and incision (F(1,34) = 5.39, p = 0.026). Posthoc analysis revealed that incision enhanced GABABR-evoked currents in mature projection neurons (n = 8; p = 0.011; Sidak’s multiple comparisons test; Fig. 1B, D right), but not in GAD67-GFP interneurons (n = 10; p = 0.96; Fig. 1C, D left).
Interestingly, baclofen-induced outward currents also showed greater decay following neonatal tissue injury (Fig. 1E), with main effects of cell type (F(1,34)= 9.96, p = 0.0033) and incision (F(1,34) = 8.034, p = 0.0077). Heightened desensitization of the GABABR response was observed exclusively in projection neurons from incision animals compared to naïve controls (n = 8; p = 0.019; Sidak’s multiple comparison test; Fig. 1E right), as no such effect was seen in GABAergic interneurons (n =10; p = 0.41; Fig. 1E left). To examine the degree to which the increased amplitude of the GABABR-mediated outward currents might explain the enhanced current desensitization, we analyzed the relationship between outward current amplitude and decay. No correlation was found in neurons from incision (r(6) = 0.247, p = 0.56) or naïve animals (r(6) = −0.2, p = 0.63), suggesting that the rate of outward current decay occurred independently of the peak current amplitude.
Finally, despite the accelerated decay of the GABABR-evoked responses in adult projection neurons from neonatally incised mice, the net effect of early tissue damage was to enhance postsynaptic GABABR signaling in this population. Analysis of the mean AUC of the GABABR-mediated currents revealed significantly greater decay in projection neurons from animals that had previously undergone neonatal incision compared to littermate controls (n = 8; t = 2.64; p = 0.0194; unpaired t-test; Fig. 1F).
5.2. Neonatal incision persistently enhances the function of GIRK channels in mature projection neurons
The enhanced GABABR-evoked outward currents seen in adult projection neurons following early life injury could potentially stem from increased postsynaptic GIRK channel function, as these channels are a key downstream effector of GABABR signaling (Fernández-Alacid et al., 2009). To investigate this possibility, we measured the density of outward currents evoked by the selective GIRK1/2 agonist ML297 (10 μΜ, Fig. 2A) in identified lamina I projection neurons from naïve and neonatally injured adult mice. The density of ML297-evoked currents was significantly greater in the P3 Incision group compared to naïve littermate controls (n = 8; U = 9, p = 0.015, Mann-Whitney test; Fig. 2B). Importantly, since ML297 activates GIRK channels independently of G protein and GABABR signaling (Kaufmann et al., 2013; Wydeven et al., 2014), these results suggest that neonatal tissue damage directly elevates the function of GIRK channels in adult spinal projection neurons. Therefore, the enhanced GIRK channel function could contribute to the potentiation of postsynaptic GABABR signaling after early life injury.
Figure 2: Early life injury persistently enhances postsynaptic GIRK currents in lamina I projection neurons.
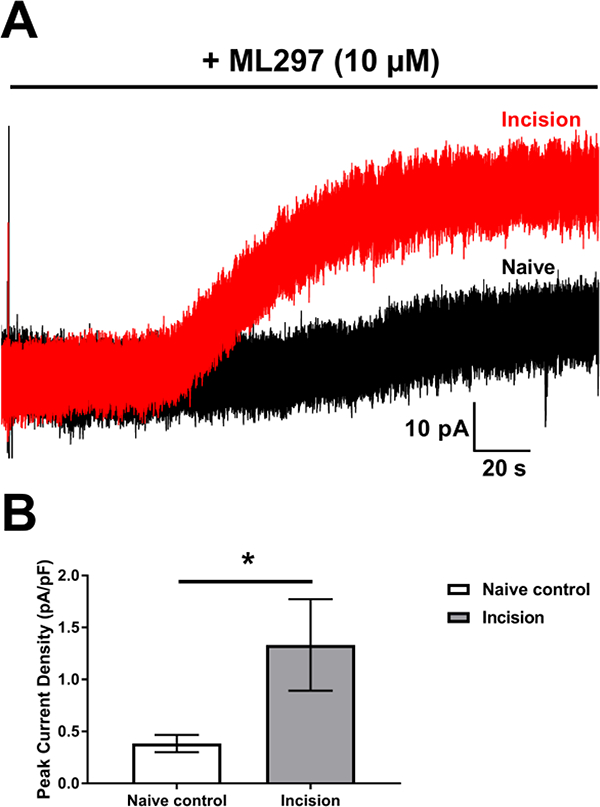
A: Representative traces of ML297-evoked outward currents in lamina I projection neurons from adult animals that received neonatal incision (red) or naïve controls (black). B: Neonatal incision increased the density of outward current in projection neurons following bath application of the GIRK agonist ML297 compared to naïve controls (n = 8; *p= 0.015; Mann-Whitney test).
To investigate the degree to which neonatal injury may evoke widespread changes in the function of K+ channels (including leak K+ channels) within adult projection neurons, we also measured membrane resistance in the spino-PB population in the absence or presence of early life tissue damage. We found no significant differences in the resting membrane resistance in projection neurons from incision (n = 9; 515.9 ± 76.46 ΜΩ) or naïve (n = 9; 736 ± 200.2 ΜΩ) animals (U = 37, p = 0.80, Mann-Whitney test). These results suggest that neonatal incision does not dramatically alter the properties of leak ion channels in mature projection neurons.
5.3. Presynaptic GABABR-mediated inhibition of spontaneous neurotransmitter release in the dorsal horn is unaffected by neonatal tissue damage
To determine the influence of neonatal surgical injury on GABABR-mediated presynaptic inhibition of spontaneous neurotransmitter release within the adult SDH (Barrai et al., 2000; Bussieres and El Manira, 1999; Chen and van den Pol, 1998; Sakaba and Neher, 2003), we measured the effect of bath-applied baclofen on the properties of mEPSCs and mIPSCs. We recorded from both projection neurons and GABAergic interneurons following the block of postsynaptic GABABR function (see Experimental Procedures). As expected, baclofen consistently reduced mPSC frequency (Figs. 3, 4, 5, and 6) without changing mPSC amplitude, indicating a presynaptic locus of action. In projection neurons (Fig. 3A), a repeated measures two-way ANOVA on the mEPSC frequency revealed a main effect of baclofen (F(1,18) = 61.55, p < 0.0001) but no effect of neonatal incision (F(1,18) = 0.08, p = 0.78; Fig. 3B). There were no differences in mEPSC amplitude after baclofen application (F(1,18) = 2.12, p = 0.16) in incision (n = 10; Baseline: 12.85 ± 1.11 pA; Baclofen: 12.33 ± 1.0 pA) or naïve animals (n = 10; Baseline: 11.79 ± 0.81 pA; Baclofen: 10.98 ± 1.0 pA). Similar results were observed when recording mIPSCs from projection neurons (n = 10; Fig. 4A), as a two-way ANOVA revealed a main effect of baclofen (F(1,18)= 92.03, p < 0.0001) and no effect of incision (F(1,18) = 0.86, p = 0.36; Fig. 4B) on event frequency. There was no effect of baclofen on mIPSC amplitude in this population (F(1,18) = 0.71, p = 0.41) in incision (n = 10; Baseline: 18.22 ± 2.54 pA; Baclofen: 18.68 ± 1.75 pA) or naïve animals (n = 10; Baseline: 16.07 ± 1.84 pA; Baclofen: 16.77 ± 1.48 pA). Additionally, baclofen (F(1,18) = 19.67, p = 0.0003) but not incision (F(1,18) = 0.74, p = 0.4) modulated mEPSC frequency in GAD67-GFP interneurons (n = 10; Fig. 5), yet baclofen had no effect on mEPSC amplitude (F(1,18) = 0.49, p = 0.5) in incision (n = 10; Baseline: 12.26 ± 1.38 pA; Baclofen: 10.89 ± 0.76 pA) or naïve animals (n = 10; Baseline: 12.16 ± 0.97 pA; Baclofen: 12.29 ± 1.55 pA). A two-way ANOVA on the mIPSC frequency data from GAD67-GFP interneurons (n = 10; Fig. 6A) again revealed a main effect of baclofen (F(1,18) = 17.92, p = 0.0006) without an effect of incision (F(1,18) = 0.72, p = 0.41; Fig. 6B). Finally, baclofen had no effect on the mIPSC amplitude in GAD67-GFP interneurons (F(1,18) = 0.83, p = 0.68) in incision (n = 10; Baseline: 11.46 ± 1.35 pA; Baclofen: 12.95 ± 1.32 pA) or naïve animals (n = 10; Baseline: 10.86 ±1.2 pA; Baclofen: 10.0 ± 0.82 pA). Collectively, these results strongly suggest that early life injury does not affect presynaptic GABABR-mediated suppression of spontaneous excitatory or inhibitory neurotransmitter release onto these two key neuronal populations within the mature spinal SDH.
Figure 3: Neonatal incision does not influence presynaptic GABABR-mediated inhibition of spontaneous glutamatergic signaling onto adult projection neurons.
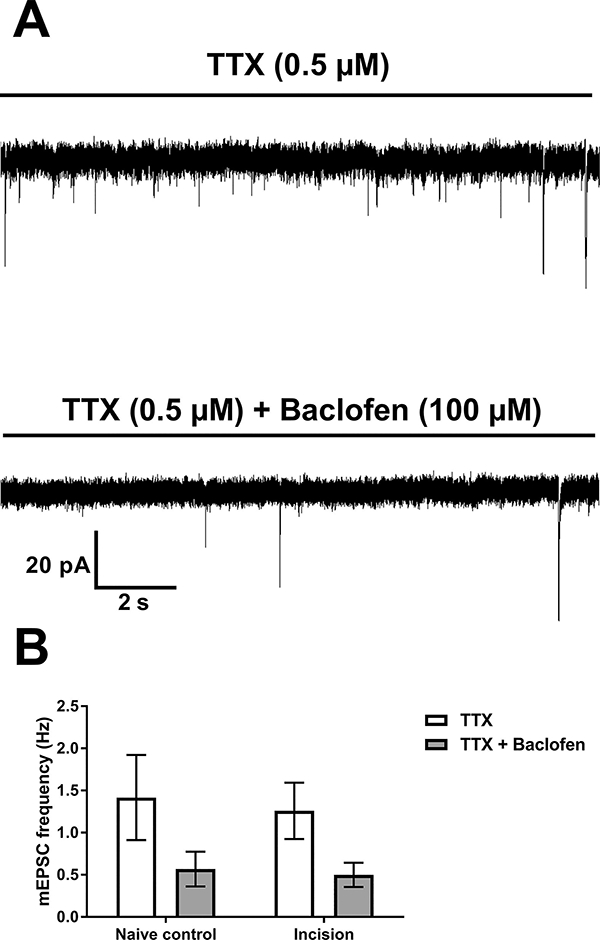
A: Representative traces of mEPSCs from a mature projection neuron following neonatal incision showing the efficacy of the GABABR agonist, baclofen, in reducing presynaptic glutamate release (top: baseline; bottom: after baclofen application). B: Baclofen-evoked depression of mEPSC frequency in projection neurons is unaffected by early incision (n = 10). Repeated measures two-way ANOVA revealed a main effect of baclofen (p < 0.0001) but no effect of incision (p = 0.78).
Figure 4: Early surgical injury does not persistently affect GABABR-mediated reduction of inhibitory neurotransmitter release onto spinal projection neurons.
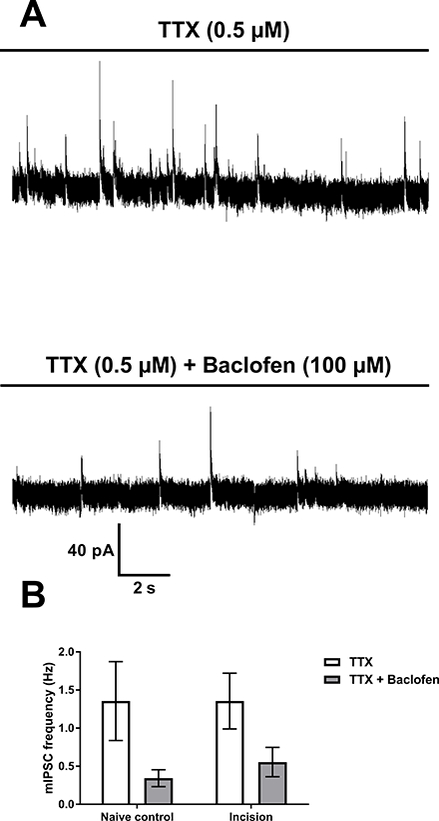
A: Recordings of mIPSCs in an adult projection neuron from a P3-incised mouse showing a baclofen-evoked reduction in mIPSC frequency (Top: baseline; bottom: after GABABR agonist application). B: Presynaptic GABABR-mediated reduction of mean mIPSC frequency in projection neurons (n = 10) is similar with or without neonatal incision. A repeated measures two-way ANOVA revealed a main effect of baclofen (p < 0.0001) but no effect of neonatal incision (p = 0.36).
Figure 5: Neonatal incision does not alter presynaptic GABABR inhibition at glutamatergic synapses onto adult GAD67-expressing interneurons.
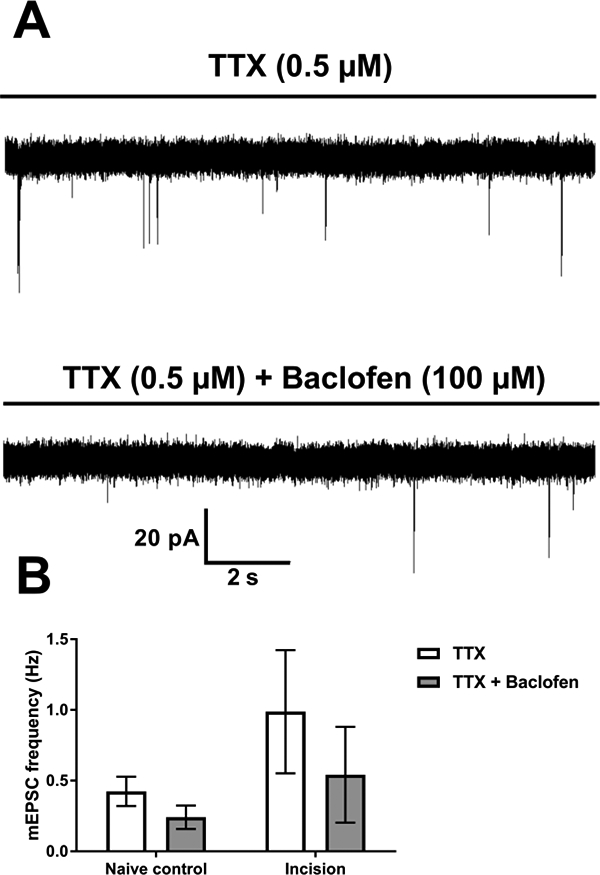
A: Representative traces in GAD67-GFP interneurons, from an adult animal with a prior P3 incision, showing isolated mEPSCs (top) and baclofen-induced depression of mEPSC frequency (bottom). B: Plots of mean mEPSC frequency in mature GAD67-expressing interneurons (n = 10) before and after baclofen application in neonatally injured and naïve mice. Repeated measures two-way ANOVA indicated a main effect of baclofen (p = 0.0003) and no effect of incision (p = 0.4).
Figure 6: Early tissue injury does not modulate spontaneous inhibitory signaling onto GABAergic neurons of the adult SDH.
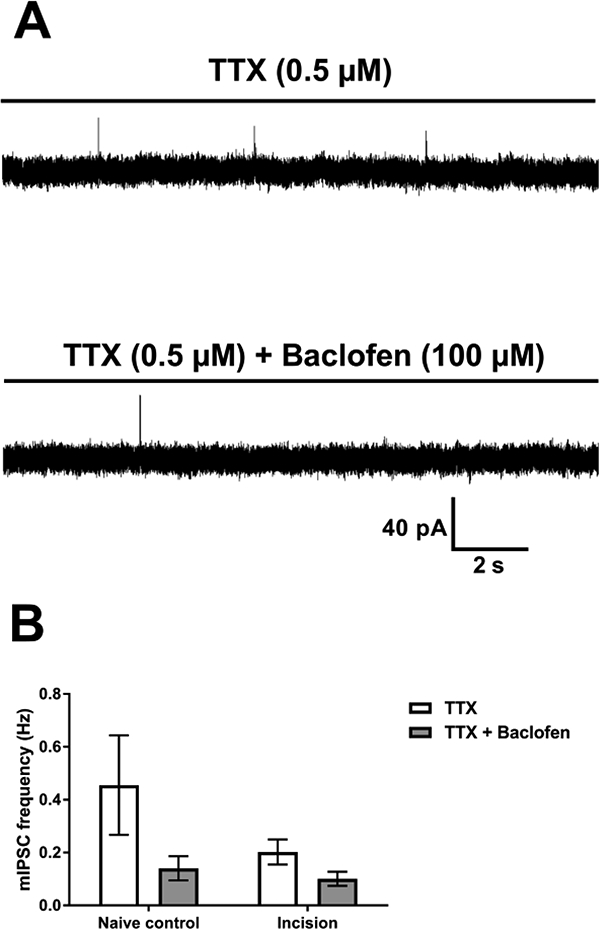
A: Recordings from a GAD67-GFP interneuron in an adult incision animal before (top) and after (bottom) baclofen-induced reduction of mIPSC frequency. B: Mean mIPSC frequency in GAD67-GFP interneurons (n = 10) before and after baclofen application, showing similar rates of depression in incision and naïve control animals. A repeated measures two-way ANOVA on the transformed data revealed a main effect of baclofen (p = 0.0006) but no effect of incision (p = 0.41).
6. Discussion
The above results demonstrate, for the first time, that neonatal tissue damage influences metabotropic GABAergic inhibition in the adult mouse SDH in a manner that depends on both cell type and synaptic localization. Surgical injury during early life potentiated postsynaptic GABABR signaling in ascending lamina I projection neurons, but not GAD67-expressing interneurons. This may reflect prolonged injury-evoked changes in the expression of the major downstream effectors of GABABR-mediated hyperpolarization, GIRK channels (Fernández-Alacid et al., 2009), in spinal projection neurons. Interestingly, these alterations in metabotropic signaling could be limited to the postsynaptic membrane, as we found no differences between the naïve and neonatally injured groups in presynaptic GABABR-mediated inhibition of spontaneous neurotransmitter release onto either projection neurons or GAD67- expressing interneurons in the mature SDH. Overall, these findings add to the growing body of evidence indicating that noxious sensory experience during early life can exert long-lasting effects on nociceptive processing in the CNS.
6.1. Cell type-selective effects of early injury on GABABR signaling in the adult SDH
While prior studies have documented GABABR immunoreactivity within spinal projection neurons targeting the caudal ventrolateral medulla and dorsal reticular nucleus (Castro et al., 2006), the present data provide the first direct evidence that GABABRs are also functionally expressed in lamina I spino-PB neurons. More importantly, the results demonstrate that neonatal surgical injury persistently enhances postsynaptic GABABR signaling in mature spino- PB neurons (Fig. 1B, D right, F). Notably, GABABR gene expression in the adult SDH is upregulated after prior inflammation at P3 but not P12 (Ren et al., 2005), raising the possibility that these receptors are modulated by trauma in an age-dependent manner within the SDH. It should be noted that the present studies exclusively employed the bath application of a selective GABABR agonist, and it will be important to elucidate the long-term effects of neonatal incision on the magnitude of GABABR-mediated hyperpolarization following primary afferent input to the SDH. A prolonged elevation in postsynaptic GABABR function may partially compensate for the weakening of feedforward GABAAR-mediated inhibition onto projection neurons after early tissue damage (Li et al., 2015). Interestingly, previous work suggests that GABABR activation can dampen GABAAR transmission (Shen et al., 2017; Xi et al., 1997), raising the possibility that the upregulation in GABABR function in projection neurons is mechanistically linked to the reduction in GABAAR-mediated inhibition onto this population. However, the significant elevation in afferent-evoked firing within mature projection neurons after early injury clearly suggests that the loss of fast synaptic inhibition, in conjunction with a strengthening of monosynaptic sensory inputs (Li et al., 2015), results in a persistent disruption in the normal balance of excitation vs. inhibition onto these output neurons of the SDH network. Importantly, the injury-evoked enhancement of postsynaptic GABABR signaling may be restricted to projection neurons, as the density of baclofen-evoked currents in adult GAD67-GFP interneurons was similar between groups (Fig. 1C, D left), although glutamatergic interneurons were not sampled in the present experiments. It is also important to note that GABAergic interneurons were sampled equally from laminae I and II in the present study, but the sample sizes were inadequate to rigorously analyze potential laminar differences in the density of baclofen-evoked current within this population. As a result, it remains feasible that the effects of neonatal injury on GABABR function may depend on laminar location and/or the pattern of sensory innervation rather than cell type. Nonetheless, the present results suggest that GABABRs may exert a tighter control over ascending nociceptive signaling to the mature brain following early tissue damage.
Although neonatal incision likely amplifies the magnitude of postsynaptic GABABR- mediated hyperpolarization, early life injury also induces a more pronounced desensitization of the outward current. While the functional implications of this change for spinal nociceptive processing remain unknown, it may influence the sensitivity of spinal projection neurons to ambient levels of GABA within the SDH. Indeed, tonic GABAergic signaling can exert profound effects on the intrinsic excitability of dorsal horn neurons (Takazawa and MacDermott, 2010) that may be stronger than those evoked by the phasic release of GABA (Ataka and Gu, 2006), although the relative contribution of the GABABR to the tonic inhibition of ascending projection neurons remains poorly understood. Alternatively, greater desensitization of the postsynaptic GABABR response could alter the inhibition of projection neurons under conditions of significantly elevated GABA release within the dorsal horn, such as that evoked by high- frequency sensory input under pathological conditions. Rapid GABABR desensitization is known to occur in the presence of intracellular potassium channel tetramerization domain- containing proteins (KCTDs) (Schwenk et al., 2010), as KCTD 8, 12, 12b, and 16 increase the amplitude and desensitization of baclofen-induced currents in cell culture (Schwenk et al., 2010). Clearly, additional studies are needed to elucidate the role of KCTD proteins in regulating GABAergic inhibition of spinal dorsal horn neurons, as well as their potential importance for shaping pain sensitivity.
The activation of GABABRs by baclofen can suppress the release of glutamate, GABA and glycine from synaptic terminals in the SDH (Choi et al., 2008; Fukuhara et al., 2013; lyadomi et al., 2000; Yang K and Ma, 2011). Our results demonstrate that surgical injury during early postnatal development fails to evoke widespread changes in spontaneous, presynaptic GABABR-mediated inhibition at synapses onto either projection neurons or GABAergic interneurons of the adult spinal nociceptive circuit (Figs. 4–6). Nonetheless, a limitation of the current study is that mPSCs, but not evoked synaptic responses, were used to characterize presynaptic inhibition. First, a localized effect of neonatal injury on GABABR-mediated inhibition of transmitter release from sensory neurons could be missed when analyzing mEPSCs, which predominantly reflect glutamate release from excitatory interneurons within the dorsal horn (Baccei et al., 2003). In addition, spontaneous and evoked neurotransmitter release involve distinct underlying mechanisms that could be differentially modulated by GABAB receptors. For example, at synapses onto CA3 hippocampal interneurons, baclofen reduces the probability of evoked glutamate release but has no effect on mEPSCs, due to the requirement that GABABRs inhibit presynaptic Ca2+ channels in order to reduce neurotransmitter release (Lei and McBain, 2003). Notably, baclofen is known to dampen primary afferent-evoked neurotransmission from the first days of life (Baccei and Fitzgerald, 2004). The magnitude of presynaptic GABABR inhibition also reportedly depends on the functional class of primary afferent, as baclofen suppresses C fiber-evoked EPSCs to a greater degree than Αδ fiber-mediated responses (Ataka et al., 2000). Taken together, these observations highlight the need to identify the consequences of early life injury for presynaptic GABABR-mediated inhibition of both spontaneous and evoked transmitter release at defined subtypes of synapses within the adult SDH.
6.2. Noxious sensory experience during early life influences GIRK activation in adult projection neurons
GIRK channels represent key downstream effectors of GABABR signaling (Fernández-Alacid et al., 2009) that are expressed throughout the spinal dorsal horn (Lyu et al., 2015) and appear to be instrumental for dampening nociceptive processing. For example, GIRK2 knockout mice display increased thermal pain sensitivity (Blednov et al., 2003), while inflammatory and neuropathic injury enhances the phosphorylation of Kir3.1 (at tyrosine 12) in the dorsal horn, which causes reduced K+ conductance and accelerated channel deactivation (Ippolito et al., 2005). Interestingly, the direct activation of GIRK1/2 channels (i.e. independent of G protein activity) produced significantly greater outward currents in mature projection neurons when preceded by early tissue injury (Fig. 2). Therefore, the enhanced postsynaptic response to baclofen seen in adult projection neurons from neonatally injured mice (Fig. 1) could reflect an elevation in the function of GIRK channels in this population, although we cannot exclude the possibility that the injury also evokes prolonged changes in the function of the GABABR itself.
It should be noted that GIRK channels are also essential components of other metabotropic signaling pathways that can evoke anti-nociceptive effects in the CNS, such as the opioidergic, somatostatinergic, M2 muscarinic, α2 adrenergic, and cannabinoidergic receptor families (Blednov et al., 2003). It is through these metabotropic systems that GIRK channels mediate the analgesic effects of morphine, oxotremorine, nicotine, clonidine, and cannabinoid receptor agonists (Blednov et al., 2003). As a result, the observation that adult projection neurons exhibit an elevated GIRK conductance after neonatal injury raises the interesting, yet untested, possibility that early tissue damage causes an increase in inhibitory G protein tone within developing nociceptive circuits in the CNS. Importantly, neonatal tissue damage has been linked to enhanced opioid signaling in the periaqueductal gray (Laprairie and Murphy, 2009) which could contribute to the stronger descending inhibition and baseline hypoalgesia seen after early tissue injury (Walker et al., 2015). Recent studies also indicate that tissue or nerve damage can initiate constitutive opioidergic inhibition in the spinal cord which may tonically suppress spinal nociceptive signaling via a process termed latent central sensitization (Corder et al., 2013; Walwyn et al., 2016). While this enhanced opioidergic signaling has been attributed to changes occurring at the level of the μ-opioid receptor (Corder et al., 2013; Walwyn et al., 2016), and/or the prolonged upregulation of endogenous ligands (Laprairie and Murphy, 2009), long-term elevations in the function of downstream GIRK channels could also potentially contribute to the greater opioidergic tone under pathological conditions. Further experiments are necessary to explore the degree to which neonatal surgical injury modulates the ability of spinal opioidergic and other metabotropic systems to inhibit nociceptive transmission in the adult spinal cord.
6.3. Conclusion
In summary, the results suggest that the metabotropic inhibition of spinal projection neurons by the GABABR-GIRK signaling pathway is strengthened by tissue damage during the neonatal period. Given the multitude of analgesic strategies that involve the activation of GIRK channels, as well as the clear importance of ascending projection neurons for the perception of pain under normal and pathological conditions, these findings suggest that early life injury may have important implications for the management of pain during adulthood.
Highlights.
Neonatal incision increases the amplitude of postsynaptic GABABR- and GIRK-mediated currents in adult projection neurons.
Early life injury enhances the desensitization of GABABR-mediated currents in mature spinal projection neurons.
Presynaptic GABABR-mediated inhibition of spontaneous neurotransmitter release in the SDH is unaffected by neonatal injury.
6.4. Acknowledgements
This work was funded by NIH grant NS080889 award to M.L.B. The authors would like to thank Dr. J. Li and E. Serafin for technical assistance.
Abbreviations
- SDH
Superficial dorsal horn
- P
Postnatal day
- GIRK
G protein-coupled inward-rectifying potassium channel
- NICU
Neonatal intensive care unit
- GABABR
GABAB receptor
- eGFP
Enhanced green fluorescent protein
- GAD67
Glutamic acid decarboxylase 67
- PB
Parabrachial
- aCSF
Artificial cerebrospinal fluid
- TTX
Tetrodotoxin
- mPSCs
Miniature postsynaptic currents
- mEPSCs
Miniature excitatory postsynaptic currents
- mIPSCs
Miniature inhibitory postsynaptic currents
- AUC
Area under the curve
- KCTD
Potassium channel tetramerization domain-containing protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ataka T, Gu JG (2006), Relationship between tonic inhibitory currents and phasic inhibitory activity in the spinal cord lamina II region of adult mice. Molecular pain 2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataka T, Kumamoto E, Shimoji K, Yoshimura M (2000), Baclofen inhibits more effectively C- afferent than Αδ-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain 86:273–282. [DOI] [PubMed] [Google Scholar]
- Baccei ML, Bardoni R, Fitzgerald M (2003), Development of nociceptive synaptic inputs to the neonatal rat dorsal horn: glutamate release by capsaicin and menthol. The Journal of physiology 549:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccei ML, Fitzgerald M (2004), Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. Journal of Neuroscience 24:4749–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrai J, Toro S, Galarraga E, Bargas J (2000), GABAergic presynaptic inhibition of rat neostriatal afferents is mediated by Q-type Ca(2+) channels. Neurosci Lett 283:33–36. [DOI] [PubMed] [Google Scholar]
- Blednov Y, Stoffel M, Alva H, Harris R (2003), A pervasive mechanism for analgesia: activation of GIRK2 channels. Proceedings of the National Academy of Sciences 100:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF (1996), Characterization of a rat model of incisional pain. Pain 64:493–501. [DOI] [PubMed] [Google Scholar]
- Bussieres N, El Manira A (1999), GABA(B) receptor activation inhibits N- and P/Q-type calcium channels in cultured lamprey sensory neurons. Brain Res 847:175–185. [DOI] [PubMed] [Google Scholar]
- Cameron D, Polgar E, Gutierrez-Mecinas M, Gomez-Lima M, Watanabe M, Todd AJ (2015), The organisation of spinoparabrachial neurons in the mouse. Pain 156:2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro AR, Morgado C, Lima D, Tavares I (2006), Differential expression of NK1 and GABAB receptors in spinal neurones projecting to antinociceptive or pronociceptive medullary centres. Brain Res Bull 69:266–275. [DOI] [PubMed] [Google Scholar]
- Chen G, van den Pol AN (1998), Presynaptic GABAB autoreceptor modulation of P/Q-type calcium channels and GABA release in rat suprachiasmatic nucleus neurons. J Neurosci 18:1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi l-S, Cho J-H, Jeong S-G, Hong J-S, Kim S-J, Kim J, Lee M-G, Choi B-J, et al. (2008), GABA B receptor-mediated presynaptic inhibition of glycinergic transmission onto substantia gelatinosa neurons in the rat spinal cord. Pain 138:330–342. [DOI] [PubMed] [Google Scholar]
- Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, et al. (2013), Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 341:1394–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen L, Fabrizi L, Patten D, Worley A, Meek J, Boyd S, Slater R, Fitzgerald M (2013), Postnatal temporal, spatial and modality tuning of nociceptive cutaneous flexion reflexes in human infants. PLoS One 8:e76470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Alacid L, Aguado C, Ciruela F, Martin R, Colón J, Cabañero MJ, Gassmann M, Watanabe M, et al. (2009), Subcellular compartment-specific molecular diversity of pre-and post-synaptic GABAB-activated GIRK channels in Purkinje cells. Journal of neurochemistry 110:1363–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M (2005), The development of nociceptive circuits. Nat Rev Neurosci 6:507–520. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Millard C, McIntosh N (1989), Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain 39:31–36. [DOI] [PubMed] [Google Scholar]
- Fukuhara K, Katafuchi T, Yoshimura M (2013), Effects of baclofen on mechanical noxious and innocuous transmission in the spinal dorsal horn of the adult rat: in vivo patch-clamp analysis. European Journal of Neuroscience 38:3398–3407. [DOI] [PubMed] [Google Scholar]
- Hohmeister J, Kroll A, Wollgarten-Hadamek I, Zohsel K, Demirakca S, Flor H, Hermann C (2010), Cerebral processing of pain in school-aged children with neonatal nociceptive input: an exploratory fMRI study. Pain 150:257–267. [DOI] [PubMed] [Google Scholar]
- Ippolito DL, Xu M, Bruchas MR, Wickman K, Chavkin C (2005), Tyrosine phosphorylation of Kir3. 1 in spinal cord is induced by acute inflammation, chronic neuropathic pain, and behavioral stress. Journal of Biological Chemistry 280:41683–41693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- lyadomi M, lyadomi I, Kumamoto E, Tomokuni K, Yoshimura M (2000), Presynaptic inhibition by baclofen of miniature EPSCs and IPSCs in substantia gelatinosa neurons of the adult rat spinal dorsal horn. Pain 85:385–393. [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Romaine I, Days E, Pascual C, Malik A, Yang L, Zou B, Du Y, et al. (2013), ML297 (VU0456810), the first potent and selective activator of the GIRK potassium channel, displays antiepileptic properties in mice. ACS chemical neuroscience 4:1278–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AF, Breton J-D, Schlichter R, Poisbeau P (2004), Production of 5α-reduced neurosteroids is developmentally regulated and shapes GABAA miniature IPSCs in lamina II of the spinal cord. Journal of Neuroscience 24:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AF, Coull JA, Chéry N, Poisbeau P, De Koninck Y (2001), Region-specific developmental specialization of GABA-glycine cosynapses in laminas l-ll of the rat spinal dorsal horn. Journal of Neuroscience 21:7871–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPrairie JL, Murphy AZ (2007), Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury. Pain 132 Suppl 1:S124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie JL, Murphy AZ (2009), Neonatal injury alters adult pain sensitivity by increasing opioid tone in the periaqueductal gray. Front Behav Neurosci 3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, McBain CJ (2003), GABAB receptor modulation of excitatory and inhibitory synaptic transmission onto rat CA3 hippocampal interneurons. The Journal of physiology 546:439–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Baccei ML (2011), Neonatal tissue damage facilitates nociceptive synaptic input to the developing superficial dorsal horn via NGF-dependent mechanisms. Pain 152:1846–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Blankenship ML, Baccei ML (2013), Deficits in glycinergic inhibition within adult spinal nociceptive circuits after neonatal tissue damage. Pain 154:1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kritzer E, Craig PE, Baccei ML (2015), Aberrant synaptic integration in adult lamina I projection neurons following neonatal tissue damage. J Neurosci 35:2438–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu C, Mulder J, Barde S, Sahlholm K, Zeberg H, Nilsson J, Ârhem P, Hökfelt T, et al. (2015), G protein-gated inwardly rectifying potassium channel subunits 1 and 2 are down-regulated in rat dorsal root ganglion neurons and spinal cord after peripheral axotomy. Molecular pain 11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TP, Mata HP, Porreca F (2002), Spinal GABAAand GABABReceptor Pharmacology in a Rat Model of Neuropathic Pain. The Journal of the American Society of Anesthesiologists 96:1161–1167. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Grunau RE, Whitfield MF, Fitzgerald C, Pitfield S, Saul JP (2000), Biobehavioral pain responses in former extremely low birth weight infants at four months’ corrected age. Pediatrics 105:e6. [DOI] [PubMed] [Google Scholar]
- Paxinos G (2013) Paxinos and Franklin’s the mouse brain in stereotaxic coordinates Academic Press. [Google Scholar]
- Peirs C, Seal RP (2016), Neural circuits for pain: Recent advances and current views. Science 354:578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Anseloni V, Zou S-P, Wade E, Novikova S, Ennis M, Traub R, Gold M, et al. (2004), Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflamatory insult. Pain 110:588–596. [DOI] [PubMed] [Google Scholar]
- Ren K, Novikova SI, He F, Dubner R, Lidow MS (2005), Neonatal local noxious insult affects gene expression in the spinal dorsal horn of adult rats. Molecular Pain 1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Neher E (2003), Direct modulation of synaptic vesicle priming by GABAB receptor activation at a glutamatergic synapse. Nature 424:775. [DOI] [PubMed] [Google Scholar]
- Schwenk J, Metz M, Zolles G, Turecek R, Fritzius T, Bildl W, Tarusawa E, Kulik A, et al. (2010), Native GABAΛ sub ΒΛ receptors are heteromultimers with a family of auxiliary subunits. Nature 465:231. [DOI] [PubMed] [Google Scholar]
- Shen W, Nan C, Nelson PT, Ripps H, Slaughter MM (2017), GABAB receptor attenuation of GABAA currents in neurons of the mammalian central nervous system. Physiol Rep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons SH, van Dijk M, Anand KS, Roofthooft D, van Lingen RA, Tibboel D (2003), Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatr Adolesc Med 157:1058–1064. [DOI] [PubMed] [Google Scholar]
- Takazawa T, MacDermott AB (2010), Glycinergic and GABAergic tonic inhibition fine tune inhibitory control in regionally distinct subpopulations of dorsal horn neurons. J Physiol 588:2571–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JT, Chen Q, Feng G, Improved methods for acute brain slice preparation from adult and aging animals, Soc Neurosci Abstracts, 2011. [Google Scholar]
- Todd AJ (2010), Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11:823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SM, Fitzgerald M, Hathway GJ (2015), Surgical injury in the neonatal rat alters the adult pattern of descending modulation from the rostroventral medulla. Anesthesiology 122:1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SM, Tochiki KK, Fitzgerald M (2009), Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: critical period and dependence on initial afferent activity. Pain 147:99–106. [DOI] [PubMed] [Google Scholar]
- Walwyn WM, Chen W, Kim H, Minasyan A, Ennes HS, McRoberts JA, Marvizon JC (2016), Sustained Suppression of Hyperalgesia during Latent Sensitization by mu-, delta-, and kappa-opioid receptors and alpha2A Adrenergic Receptors: Role of Constitutive Activity. J Neurosci 36:204–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydeven N, de Velasco EMF, Du Y, Benneyworth MA, Hearing MC, Fischer RA, Thomas MJ, Weaver CD, et al. (2014), Mechanisms underlying the activation of G-protein-gated inwardly rectifying K+ (GIRK) channels by the novel anxiolytic drug, ML297. Proceedings of the National Academy of Sciences 111:10755–10760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Yamada K, Tsurusaki M, Akasu T (1997), Baclofen reduces GABAA receptor responses in acutely dissociated neurons of bullfrog dorsal root ganglia. Synapse 26:165–174. [DOI] [PubMed] [Google Scholar]
- Yang K, Ma H (2011), Blockade of GABA B receptors facilitates evoked neurotransmitter release at spinal dorsal horn synapse. Neuroscience 193:411–420. [DOI] [PubMed] [Google Scholar]
- Yang K, Wang D, Li Y-Q (2001), Distribution and depression of the GABA B receptor in the spinal dorsal horn of adult rat. Brain research bulletin 55:479–485. [DOI] [PubMed] [Google Scholar]


