Abstract
Inflammatory bowel disease (IBD) is a chronic relapsing disorder of the intestine, with increasing incidence worldwide. At present, the management of IBD is an unmet medical need due to the ineffectiveness of currently available drugs in treating all patients, and there is strong demand for novel therapeutics. In this regard, vasoactive intestinal peptide, a potent anti-inflammatory endogenous hormone, has shown promise in managing multiple immune disorders in animal models. However, when administered in the free form, VIP undergoes rapid degradation in vivo, and with continuous infusion, it causes severe dose limiting side effects. To overcome these barriers, we have developed a superior mode to deliver VIP in its native form, using sterically stabilized micelles (VIP-SSM). Our previous studies demonstrated that, VIP, when administered in SSM, prevented joint damage and inflammation in a mouse model of rheumatoid arthritis at a significantly lower dose than the free peptide, completely abrogating the serious side effect of hypotension associated with VIP. In the current study, we demonstrate the therapeutic benefit of VIP-SSM over free peptide in reversing severe colitis associated with IBD. First, we conducted preliminary studies with dextran sulfate sodium (DSS) induced colitis in mice, to determine the effectiveness of VIP administered on alternate days in reducing disease severity. Thereafter, a single intra peritoneal injection of VIP-SSM or the free peptide was used to determine its therapeutic effect on the reversal of colitis and associated diarrhea. The results demonstrated that when administered on alternate days, both VIP-SSM and VIP were capable of alleviating DSS colitis in mice. However, when administered as a single dose, in a therapeutic setting, VIP-SSM showed superior benefits compared to the free peptide in ameliorating colitis phenotype. Namely, the loss of solid fecal pellets and increased fluid accumulation in colon resulting from DSS insult was abrogated in VIP-SSM treated mice and not with free VIP. Furthermore, reduced protein and mRNA levels of the major chloride bicarbonate exchanger, down regulated in adenoma (DRA), seen with DSS was reversed with VIP-SSM, but not with the free peptide. Similarly, VIP-SSM treatment significantly reduced the elevated mRNA levels of pro-inflammatory cytokines and showed significant histologic recovery when compared to mice treated with free VIP. Therefore, these results demonstrated that as a single dose, the anti-inflammatory and antidiarrheal effects of VIP can be achieved effectively when administered as a nanomedicine. Therefore, we propose VIP-SSM to be developed as a potential therapeutic tool for treating ulcerative colitis, a type of IBD.
Keywords: VIP-SSM, inflammatory bowel disease, ulcerative colitis, sterically stabilized micelles
Graphical Abstract
INTRODUCTION
Inflammatory bowel disease (IBD) is a broad term used to identify two types of chronic disorders of the gastrointestinal tract (GIT), Crohn’s disease (CD) and ulcerative colitis (UC).1,2 This disease is multifactorial in nature with no definitive cure and can significantly affect patient’s quality of life. Treatments currently available for IBD are ineffective in treating all patients and cause severe side effects.3 In this regard, vasoactive intestinal peptide (VIP), an endogenous neuropeptide has emerged as a potent anti-inflammatory and immunomodulatory agent affecting both innate and adaptive immune systems.4
The anti-inflammatory properties of VIP have been demonstrated in animal models of several immune disorders such as septic shock,5 multiple sclerosis,6 rheumatoid arthritis (RA),7,8 and CD.2,9 During severe inflammation in the intestine, levels of VIP and its receptors are known to be down-regulated.10,11 Furthermore, VIP knockout mice show profound abnormalities along the GI tract, indicating the importance of VIP in health and disease of the intestine.12 VIP has also been studied as a potential candidate for the management of CD in animal models and has shown promising results.4,9,13–16 However, the therapeutic role of VIP in managing UC is still not well established and conflicting findings have been reported.17,18 The labile peptide VIP undergoes rapid degradation once administered systemically; therefore, delivering the required amounts of the peptide to the target site becomes challenging.19 In order to be effective in vivo, the free VIP needs to be administered parenterally at higher doses and with increased frequency, which results in severe toxicities such as hypotension and tachycardia.15,20 Thus, one important reason for variability in the previous studies could be explained by potential variations in the administered VIP reaching the sites of disease. To overcome these delivery challenges, we have developed a biocompatible nanomedicine (VIP-SSM), which can successfully deliver active VIP to the target tissue.21,22
Sterically stabilized micelle (SSM) is a versatile, self-assembled lipid based nanocarrier, which is capable of incorporating water insoluble drugs as well as amphiphilic peptides such as VIP.22–24 Nanoparticles are ideal for drug delivery to inflamed tissues and are superior over free drugs due to their selective accumulation by enhanced permeation and retention (EPR) effect at the diseased site.25,26 In addition, the nanocarrier SSM can protect peptide drugs from enzymatic degradation and improve their half-life and bioavailability.21,27,28 In our previous studies, we have tested VIP-SSM on a RA model and showed superior therapeutic effects of the nanomedicine over the free peptide.21 VIP-SSM was effective at a significantly lower dose than the free peptide while eliminating the hypotensive toxicity by preventing the off-target effects of VIP.21
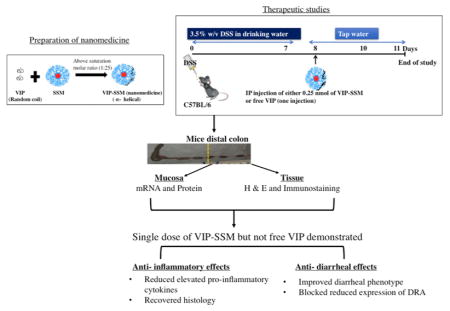
In the current study, we have explored the anti-inflammatory action of VIP in ameliorating the DSS colitis phenotype. Inflammation in the gastrointestinal tract gives rise to systemic effects and VIP is an important enteric hormone; thus, it is important to understand if VIP can be used to manage UC, a type of IBD.29,30 We initially determined the preventive capacity of VIP in ameliorating the disease severity of DSS colitis when given on alternate days. After confirming that both VIP and VIP-SSM can prevent severe inflammation in this model, another thorough study was undertaken to determine the therapeutic potential of a single dose of VIP in both forms. As a single dose of 0.25 nmol, VIP-SSM showed superior effects than the free peptide in reversing colitis associated inflammation and diarrhea and therefore, may serve as a potential new treatment option for IBD.
MATERIALS AND METHODS
Materials
Sodium salt of 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine-N-[methoxy(poly(ethylene glycol))-2000] (DSPE-PEG2000) was purchased from LIPOID GmbH (Ludwigshafen, Germany). Human vasoactive intestinal peptide was synthesized by Protein Research Laboratory, Research Resource Center, University of Illinois at Chicago (Chicago, IL). Sterile normal saline was purchased from Baxter (Deerfield, IL), RNeasy mini kits were purchased from Qiagen (Hilden, Germany), and all other chemicals were of analytical grade and purchased from Sigma-Aldrich (St.Louis, MO).
Methods
Preparation and Characterization of VIP-SSM Nanomedicine
VIP-SSM was prepared by a simple dissolution method as described earlier with some modifications.21 Briefly, a stock solution of DSPE-PEG2000 was prepared by dissolving a weighed amount of lipid into a clean glass vial in required amounts of normal saline. The solution was vortexed for 1 min, and the vial was saturated with argon gas to remove air. The resulting solution was kept in the dark at room temperature (RT) for an hour for micelles to form. Following incubation, the stock solution was mixed in a 1:10 dilution ratio with a freshly prepared 25 μM solution of VIP to obtain the nanomedicine, VIP-SSM, at 0.25 nmol in 100 μL of 1 mM SSM. As a control, 1 mM solution of SSM was used. All solutions were incubated in the dark at RT for nanomedicine to be formed by self-assembly and reach equilibrium. Free peptide solution of 0.25 nmol/100 μL was prepared in saline, just prior to injection to minimize aggregation and other instability. Final solutions were characterized for their size by dynamic light scattering (DLS) with the NICOMP particle sizer (Santa Barbara, CA).
DSS Induced Colitis Mouse Model
All animal experiments performed were approved by the animal care committees of the University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center (JBVAMC) (Chicago, IL). Mice were housed at the animal facility at the JBVAMC. Male C57Bl6 mice, 6–8 weeks old, were purchased from Jackson laboratories (Bar Harbor, ME). Following acclimation for 1 week, mice were randomly divided into 5 groups (n = 5). Groups included control, VIP-SSM, DSS + SSM, DSS + VIP-SSM, and DSS + VIP. Control and VIP-SSM groups were left on tap water throughout the study, and DSS containing groups received the stated amount of DSS mixed in drinking water for 7 days.
Preventive Studies
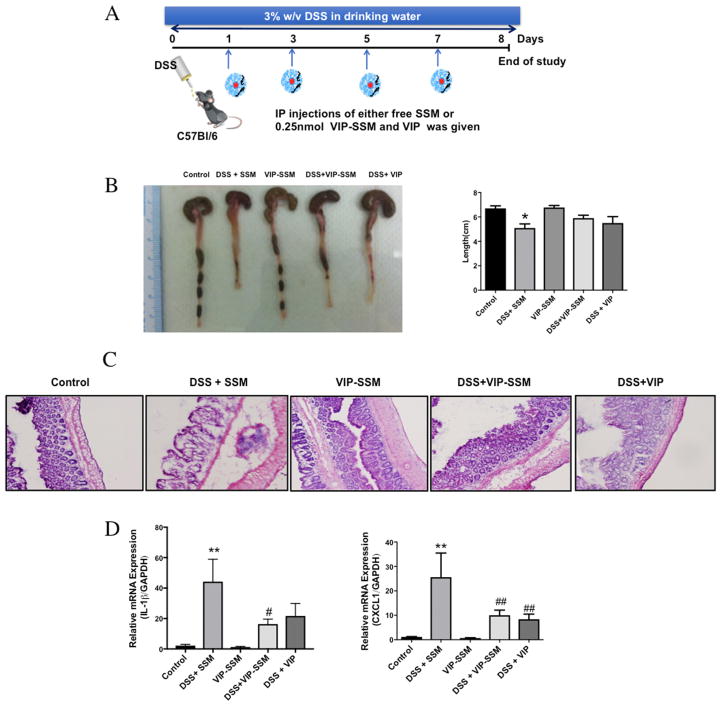
DSS (3% w/v, MP Biomedicals, Solon, OH), was dissolved in drinking water for 7 days (Figure 2A). Intraperitoneal (ip) route was chosen for nanomedicine administration after conducting preliminary studies with other systemic routes of administration (iv and sc), and determining its comparative efficacy. Furthermore, ip route was chosen over iv route for ease of administration and comparative efficacy with mice. Treatments were administered ip on alternate days starting at day 1. At the end of the study (day 8), mice were sacrificed, and tissues were used for analysis.
Figure 2.
Preventive action of VIP in DSS induced colitis. (A) Experimental design of the DSS study with arrows indicating time of injection. (B) Representative photographs and graphical presentation of average colonic length of mice in all treatment groups. (C) Representative micrographs of distal colonic tissues stained with H & E (magnification 20×). (D) Relative mRNA expression of pro-inflammatory cytokines in distal colonic mucosal scrapings. Values represent mean ± SEM, n = 5, *p < 0.05, **p < 0.005 vs control; #p < 0.05, ##p < 0.005 vs DSS.
Therapeutic Studies
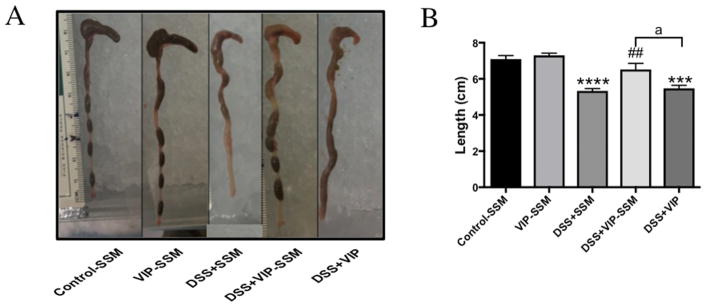
In order to establish a more severe inflammation, a higher concentration, that is, 3.5% w/v DSS (MP Biomedicals), was dissolved in drinking water for 7 days. Mice were switched to tap water at the end of day 7 and kept on tap water until day 11 (Figure 3A). Treatments were administered ip on the eighth day as a therapeutic agent, and on day 11 mice were sacrificed, and tissues were used for analysis.
Figure 3.
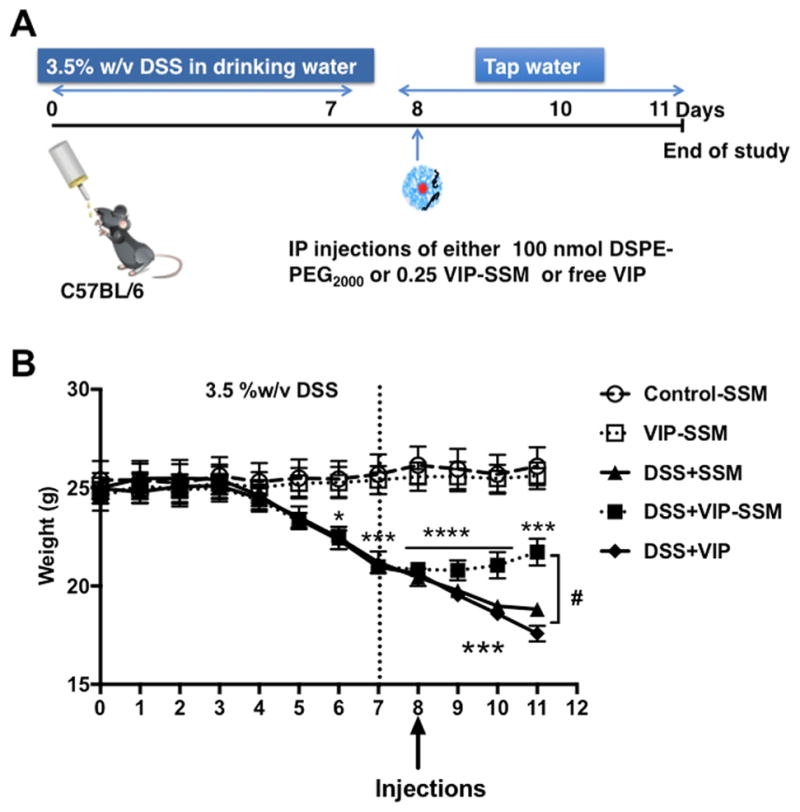
VIP-SSM increases recovery after DSS colitis insult by improving body weight in a therapeutic model of DSS colitis. (A) Experimental study design. (B) Body weight of mice during the study, vertical dotted line indicates switching from DSS to tap water. On day 8 indicated by arrowhead, all groups received a single 100 μL intraperitoneal (ip) injection of 1 mM SSM (control, DSS), 0.25 nmol of VIP in 1 mM SSM (VIP-SSM, DSS + VIP-SSM), or 0.25 nmol of VIP in saline (DSS + VIP). Values represent mean ± SEM, n = 5, *p < 0.05, ***p < 0.0005, ****p < 0.0001 vs control; #p < 0.05 vs DSS, DSS +VIP.
Treatments given in both preventive and therapeutic studies were either 0.25 nmol of VIP in SSM or free VIP. Control and DSS + SSM mice received SSM intraperitoneally. Body weight of mice was recorded daily until the end of the study.
Histological Evaluations
Formalin fixed paraffin embedded distal colonic tissues were sectioned at 5 μm thickness using a microtome. The sections were then stained with hematoxylin and eosin (H & E) stain kit commercially available from ScyTek laboratories inc. (Logan, UT) per the manufacturers protocol. The slides were then mounted with permount (Fisher scientific, Waltham, MA). Images from each mouse distal colon were acquired with Zeiss Axiocam acc1 (Oberkochen, Germany). All slides were blinded, and pathological scores were assessed by a pathologist according to the following criteria: scores ranging from 0 to 3 were given where 0 indicated no change and 3 indicated maximal change. Parameters scored for include epithelial integrity, edema, chronic inflammatory infiltrate, crypt destruction, and erosion and ulceration.
Real Time PCR
RNA was isolated from mouse distal colon mucosal scrapings with Qiagen RNeasy kits. Equal amounts (50 ng) of RNA were reverse transcribed and amplified using Brilliant SYBR green qPCR master mix kit (Stratagene, La Jolla, CA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified as an internal control for each sample. Relative expression of genes was calculated according to the ΔΔCt method.31 Primers used are listed in Table 1.
Table 1.
Gene Specific Primer Sequences
| gene | sequence (5′ → 3′) |
|---|---|
| mouse IL-1β | F: GCAACTGTTCCTGAACTCAACT R: ATCTTTTGGGGTCCGTCAACT |
| mouse CXCL-1 | F: AAAGATGCTAAAAGGTGTCCCCA R: AATTGTATAGTGTTGTCAGAAGCCA |
| mouse CXCL-2 | F: CCAACCACCAGGCTACAGG R: GCGTCACACTCAAGCTCTG |
| mouse CCL3 | F: TTCTCTGTACCATGACACTCTGC R: CGTGGAATCTTCCGGCTGTAG |
| mouse occluding | F: CCTCCAATGGCAAAGTGAAT R: CTCCCCACCTGTCGTGTAGT |
| mouse DRA | F: TGGTGGGAGTTGTCGTTACA R: CCCAGGAGCAACTGAATGAT |
| mouse GAPDH | F: TGTGTCCGTCGTGGATCTGA R: CCTGCTTCACCACCTTCTTGAT |
Western Blotting
Protein was extracted from distal colonic mucosal scrapings into RIPA buffer (Cell signaling, Danvers, MA), supplemented with protease inhibitor cocktail (Roche) and phosphatase inhibitors (Sigma-Aldrich). Protein samples were prepared in Laemmli sample buffer (Bio-Rad, Hercules, CA) and boiled for 5 min. Samples containing 75 μg of protein were then run in 7.5% Mini-Protean precast gel (Bio-Rad) and transferred to nitrocellulose membranes. Membranes were processed after blocking for 1 h in 5% milk in phosphate buffered saline (PBS) for proteins of interest with corresponding primary antibodies at a ratio of 1:100 unless specified. Primary antibodies used include anti-rabbit-DRA (raised in house against peptide sequence),32 anti-rabbit-occludin (Invitrogen, Carlsbad, CA), and anti-rabbit-GAPDH at a ratio of 1:3000 (Sigma-Aldrich St. Louis, MO). Following overnight incubation with primary antibody at 4 °C, membranes were washed with PBS containing 0.1% tween 20 and incubated with 1:2000 horseradish peroxidase-conjugated anti-rabbit-IgG secondary antibody (Santa-Cruz, Santa Cruz, CA) followed by ECL detection (Biorad) per manufacturer’s instructions.
Immunofluorescence Staining of Occludin
Cryopreserved colon tissues in OCT (Fisher Scientific) were sectioned in a microtome cryostat at 5 μm thickness. The sections were stored at −80 °C until use. Before the staining procedure, the slides were kept at room temperature in a moistened dark container for OCT compound to dissolve. The tissues were then fixed in 4% paraformaldehyde for 15 min. Following fixation, the slides were washed a few times in PBS and then permeabilized with immersing in 0.3% NP-40 solution for 5 min. After a few washes in PBS the tissues on the slides were demarcated with a water repellant Pap pen (Sigma-Aldrich) and blocked with 5% normal goat serum (NGS) in PBS for 1 h. Afterward the blocking solution was decanted, and primary antibody solutions in 1% NGS was added to the tissue sections. Primary antibodies of antirabbit-occludin (Invitrogen) and anti-mouse-villin (Invitrogen) was used at a ratio of 1:100 and incubated in a dark moistened container for 2 h. The solutions were then decanted and washed several times with PBS, following which the samples were incubated with anti-goat secondary antibodies conjugated to either anti-rabbit Alexa fluor 568 (red) or anti-mouse Alexa fluor 488 (green) (Thermofisher) for 1 h at a ratio of 1:100 in wash buffer containing 1% NGS. After a few washes in PBS, the slides were mounted with prolong gold antifade/DAPI (Thermofisher) and sealed with clear nail polish. Slides were stored in −20 °C until use. Images were acquired with the use of the Olympus BX51 fluorescent microscope.
RESULTS
Characterization of Nanoparticles after Formulation
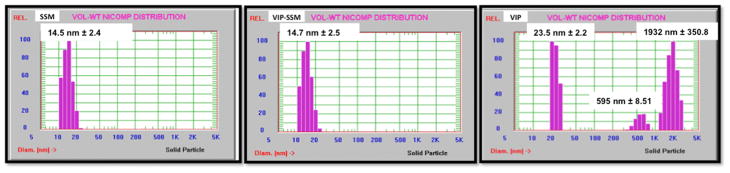
All nanoparticles were characterized as described before to confirm the formation of the nanoparticles.33 As shown in Figure 1, empty nanocarrier SSM and VIP associated SSM (VIP-SSM), both showed a similar mean particle size (~15 nm) and a narrow distribution. However, at the same conditions, VIP in saline showed aggregation and decomposition, giving rise to particle populations with different sizes.
Figure 1.
Characterization of formulations for its size using dynamic light scattering. Empty nanocarrier (SSM) and VIP in sterically stabilized micelles (VIP-SSM) both show similar particle size (~15 nm). VIP under the same conditions in saline showed aggregation and decomposition giving rise to multiple particles in solution.
Preventive Studies
VIP (0.25 nmol) Administered on Alternate Days Alleviated Disease Severity of DSS Colitis
DSS, when administered in drinking water to mice, is known to disrupt the distal colonic mucosa and give rise to clinical and pathological signs and symptoms of colitis.34,35 In first set of studies, we administered VIP as a free peptide or in SSM formulation on alternate days. The dose and administration regimen including route was based on the previously published data of the free peptide in another colitis mouse model2 (Figure 2A). During the preventive studies, we observed that both free VIP and VIP-SSM at a dose of 0.25 nmol given every other day was able to reduce the severity of colitis after 7 days of DSS. This was evident from improvement of the diarrheal phenotype and associated colon length shortening after DSS insult (Figure 2B), where untreated DSS mice had a significantly lower colon length than control mice. In addition, the damaged distal colonic histology (Figure 2C) and the increased mRNA expression of proinflammatory cytokines in the distal colonic tissues, associated with DSS (Figure 2D), were significantly reduced with VIP treatment. These results indicated to us that VIP is anti-inflammatory in the DSS colitis model and that when administered on alternate days via ip route free peptide was also as effective as the nanoparticle at the same dose. Next, we aimed at determining the effectiveness of a single dose of 0.25 nmol of VIP, which will be more clinically accepted, for its therapeutic effect in reversing severe inflammation associated with DSS colitis.
Therapeutic Studies
VIP-SSM Improved Overall Health of Mice after DSS Insult as Indicated by Improved Body Weight
In these studies, mice were given 3.5% w/v DSS for 7 days in drinking water to develop severe colitis and associated diarrhea (Figure 3A). The weight change of the mice was monitored during the course of the study. All groups receiving DSS (DSS, DSS + VIP-SSM, and DSS + VIP), started losing weight significantly from day 6 (p < 0.05 vs control) (Figure 3B). The treatments were administered on day 8, after mice were switched to tap water. The VIP-SSM treated mice group showed an improvement of average weight starting at day 9, and the weight increased significantly (p < 0.05 vs DSS), from the untreated (DSS) and the free VIP treated (DSS + VIP) mice at day 11, thus indicating a significant improvement of overall health in mice receiving VIP-SSM (Figure 3B).
VIP-SSM Alleviated Colitis Associated Diarrheal Phenotype and Increased Length of Mice Colon
DSS associated diarrheal phenotype demonstrates loss of solid fecal pellets in the colonic lumen and reduced colonic length.35 At the end of this study (day 11), when all mouse groups were analyzed for macroscopic differences in the colon, we observed a significant loss of solid fecal pellets and length of colon in DSS mice (p < 0.05 vs control). However, these pathological features were alleviated in the mice treated with VIP-SSM (Figure 4). Mice treated with free VIP did not show any significant improvement compared to DSS mice indicating better therapeutic effects of the VIP nanomedicine.
Figure 4.
DSS associated colonic diarrheal phenotype and length reduction is abrogated with VIP-SSM treatment. (A) Representative photographs of colons from mice in each group; (B) graphical representation of average colon length of mice from all groups. Values represent mean ±SEM, n = 5, ***p < 0.0005, ****p < 0.0001 vs control; ##p < 0.005 vs DSS; “a” indicates a significant difference between the groups at a p value of 0.05.
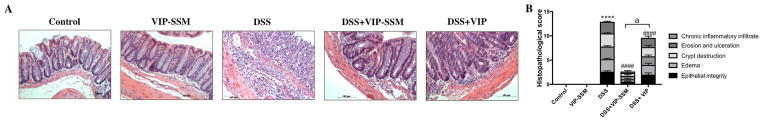
DSS Induced Distal Colonic Histologic Damage Assessed by Histopathological Score Was Improved by VIP-SSM
Since VIP is a known anti-inflammatory agent and we observed morphological improvements in the treated mice, we next evaluated the histology of all mouse distal colonic tissues. DSS mice showed severe histological damage and inflammation quantified by blinded histopathological scoring (Figure 5B). VIP-SSM treated mice showed remarkable recovery (p < 0.0001 vs DSS) as demonstrated by the improved histology and almost control level scores. Interestingly, the recovery was partial in the free peptide treated mice but was significantly different (p < 0.0001) from VIP-SSM treated mice, confirming the previous results of higher bioactivity of a given dose of VIP in SSM.
Figure 5.
VIP-SSM improves distal colonic histology assessed by blinded histopathological score. (A) Representative micrographs of H & E stained distal colonic tissues, scale bar = 100 μm. (B) Graphical representation of the average histopathological score across treatment groups. Values represent mean ± SEM, n = 5, ****p < 0.0001 vs control, ####p < 0.0001 vs DSS, and “a” indicates a significant difference between the groups at a p value of 0.0001.
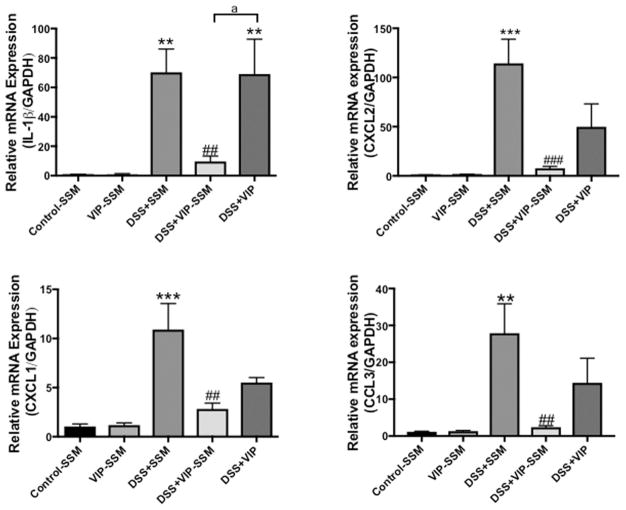
VIP-SSM Reduced the Increased Proinflammatory Cytokine mRNA Levels in Distal Colonic Mucosa
Distal colon is the most affected part of the intestine when insulted with DSS. Therefore, in order to quantify the relative mRNA expression of the secreted pro-inflammatory cytokines at the target tissue, qPCR analysis was employed. Cytokines investigated include interleukin-1 β (IL-1β), the chemokine (CXC motif) ligand-1 and 2 (CXCL-1,2), and chemokine (C–C motif) ligand 3 (CCL-3). Expression of all these proinflammatory cytokines and chemokines was significantly increased with DSS (Figure 6). There was a significant reduction of all the proinflammatory cytokines, to control levels, in mice treated with VIP-SSM but not with the free VIP, confirming better local anti-inflammatory activity of VIP-SSM over the free peptide.
Figure 6.
VIP-SSM lowered the elevated proinflammatory cytokine mRNA levels in distal colonic mucosal scrapings from DSS colitis mice. Values represent mean ± SEM, n = 5, **p < 0.005, ***p < 0.0005 vs control, ##p < 0.005, ###p < 0.0005 vs DSS, and “a” indicates a significant difference between the groups at a p value of 0.005.
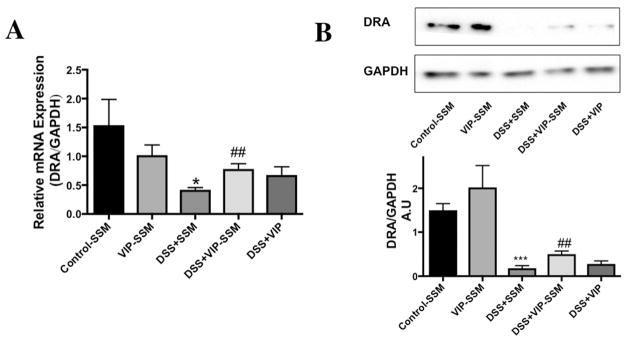
DSS Induced Reduction in DRA Expression Was Alleviated by VIP-SSM
Colitis induced diarrhea is a major health burden seen in IBD patients, which reduces their quality of life. One main reason for this pathology is the reduced function and expression of the major luminal membrane chloride bicarbonate exchanger present in the distal colon also known as down regulated in adenoma (DRA) or SLC26A3.36 To determine if VIP has an effect on the reduced mRNA and protein expression of DRA in distal colonic mucosa, tissues were analyzed with qPCR using gene specific primers. Interestingly, the significantly reduced DRA mRNA levels were improved with VIP-SSM treatment but not with free VIP (Figure 7A). Furthermore, the reduced protein levels were also recovered when mice were treated with VIP-SSM as compared to free VIP (Figure 7B). These results confirm the improvement of diarrheal phenotype observed (Figure 4) in the DSS mice treated with VIP-SSM.
Figure 7.
DSS induced reduction in chloride transporter DRA expression is alleviated by VIP-SSM treatment. (A) DRA mRNA and (B) protein expression in mice distal colonic mucosa. Values represent mean ± SEM, n = 5,*p < 0.05, ***p < 0.0005, vs control; ##p < 0.005, vs DSS.
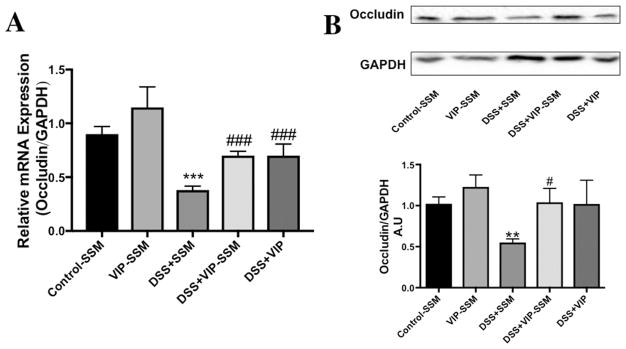
Impaired Epithelial Tight Junction Protein (Occludin) mRNA and Protein Levels Are Improved by VIP Administration in DSS Mice
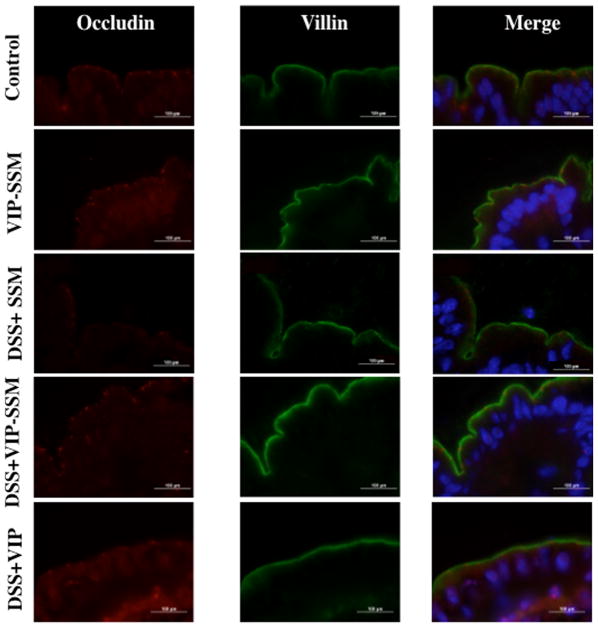
Epithelial integrity of the colonic mucosa plays a vital role in the pathogenesis of IBD, and one major defect observed in colitis is the loss of tight junction proteins, leading to loss of barrier functions.37 VIP is known to modulate the expression of tight junction proteins in epithelia including the intestinal epithelium.38 Therefore, to determine if VIP can inhibit the loss of barrier function we determined the mRNA and protein levels of the key tight junction protein, occludin. As depicted in Figure 8, mRNA and protein levels of occludin were drastically reduced with DSS. Interestingly, both VIP-SSM and the free peptide reversed the loss of mRNA and protein. However, this was the only beneficial effect that was observed so far with the administration of the free peptide. Therefore, to determine the localization of the tight junction protein occludin in the distal colonic epithelium, immunolocalization studies for occludin (red) were performed with the apical marker villin (green) (Figure 9). Significant reduction and redistribution of the total occludin levels were seen in DSS; however, the levels were increased with both VIP-SSM and VIP. In contrast to VIPSSM, mice treated with free VIP showed aberrant occludin localization and redistribution from the apical membranes of the epithelium. As shown in Figure 8, control mice have a punctate appearance of occludin, which is recovered with VIP-SSM treatment. When treated with VIP in free form, a subapical localization of occludin and redistribution in the epithelium, which is atypical from normal occludin expression pattern, was observed.
Figure 8.
Reduction in tight junction protein occludin observed in DSS colitis is blunted by VIP-SSM treatment. (A) mRNA and (B) protein expression of occludin in mouse distal colonic mucosa. Values represent mean ± SEM, n = 5, **p < 0.005, ***p < 0.0005, vs control; #p < 0.05, ###p < 0.0005 vs DSS.
Figure 9.
VIP-SSM improves the distribution of the tight junction (TJ) protein occludin in the distal colonic epithelium. Distal colonic tissues were stained with occludin (red) and villin (green) to determine the distribution of the TJ protein occludin in mouse distal colonic tissues, scale bar- 100 μm.
DISCUSSION
In our current studies, we demonstrate VIP in SSM as a potential therapeutic agent in preventing and ameliorating severe inflammation associated with DSS induced colitis. VIP-SSM reversed the increased proinflammatory cytokine expression, damaged distal colonic histology, and reduced tight junction and ion transporter protein expression associated with severe DSS colitis.
The DSS colitis model is a widely used preclinical mouse model demonstrating pathological features similar to human UC.39 When treatment was given on alternate days, both VIP and VIP-SSM showed similar benefits in ameliorating colitis (Figure 2), most probably due to the repetitive dose providing sufficient active VIP at the site thus negating the protective effects of the nanocarrier. Furthermore, repetitive administration is not preferable and practical in clinical use. However, these preventive studies confirmed that VIP was capable of reducing DSS associated colitis and showed benefit in this model, which resembles human UC. Next, to determine the effectiveness of VIP in DSS colitis as a nanomedicine, in a therapeutic manner, we used a single dose of VIP with or without SSM. DSS concentration used was also increased from 3 to 3.5% w/v to ensure the development of more severe colitis in mice. Since DSS was removed from mice drinking water after the seventh day, it was considered that a higher DSS concentration would maintain a higher inflammatory status in mice for a prolonged period of time.40 In this model, when administered as a single therapeutic dose, VIP-SSM reduced the inflammation and tissue damage and improved the overall health of mice. Furthermore, VIP-SSM demonstrated other beneficial effects such as increased ion transporter and tight junction protein expression, which are significantly downregulated with DSS insult. However, these effects were not observed when the free peptide was administered as a single dose, indicating the in vivo instability of free peptide. A dose of 0.25 nmol in the nanomedicine form was capable of alleviating all disease associated signs and symptoms, thus demonstrating the effectiveness of VIP nanomedicine in managing UC.
The pathogenesis of DSS colitis resembles many features of human UC, including the increased proinflammatory cytokines and chemokine secretion and tissue damage.41,42 Previously, we have demonstrated the preventive capacity of another neuropeptide (glucagon like peptide-1) in SSM, in ameliorating DSS induced colitis.43 In line with that, in this study, we moved to investigate the benefit of the potent immunomodulatory peptide hormone VIP in DSS colitis. Loss of VIP levels in the intestine has been implicated to play an important role during the pathogenesis of human IBD and in rodent models of colitis.18,44 Furthermore, anti-inflammatory activity of other neuropeptides such as GLP-2 (glucagon like peptide-2) have also been shown to be mediated through the enteric nervous system, which activates VIP secretion.45 Therefore, VIP serves as an ideal candidate molecule to be developed as a therapeutic agent for better management of IBD.
When administered frequently, VIP-SSM and VIP, both demonstrated similar benefits in alleviation of colitis and associated symptoms (Figure 2). However, it should be noted that VIP is a well-known hypotensive agent, and when administered frequently in the free form, it can give rise to dose limiting toxic side-effects. This unfavorable side effect can be completely eliminated when administered in the nanomedicine form, VIP-SSM.21 Furthermore, it is important to study the potential of a drug molecule in a therapeutic setting, where results can be employed to gain information for clinical use. Moreover, frequent drug administration for a chronic condition such as IBD will be unrealistic to be translated to patient care, due to low compliance associated with it. To determine the potential of using VIP in reversing well established colitis we next determined in detail the benefit of a single dose of VIP.
The anti-inflammatory mechanisms of VIP are broadly studied, and in the context of IBD, it is known to have multiple actions including inhibition of neutrophil and macrophage migration and activation.4 In addition, it is also known to play a role in modulating the expression of pathogen recognition toll-like receptors (TLR) in the intestinal epithelial cells.13 Previously, studies conducted with the use of the free peptide VIP in the trinitrobenzenesulfonic acid (TNBS) induced colitis mouse model have demonstrated that clinical symptoms improved with administering VIP on alternate days at a doses of 1 or 5 nmol.2,13 However, when another group attempted to reproduce the same result, they did not see all parameters improving with treatment, and the reason for these conflicting results were speculated to be due to the differences in the amounts of TNBS used for disease induction and possibly the differences in the animal facilities resulting in differences in gut microbiome.15 In addition, apart from studies undertaken in VIP knock out mice, there have been no studies conducted to-date, to determine the effectiveness of VIP in DSS colitis.18,46 Therefore, the current study provides for the first time successful delivery of VIP in a stable form for therapeutic use in DSS induced colitis in mice.
Additionally, in DSS colitis, the disruption of intestinal epithelial integrity is identified as one of the initial causes leading to the pathogenesis of colitis.34,47 With a single dose of VIP-SSM (0.25 nmol), we were able to demonstrate a significant improvement in mRNA and protein expression of one of the key tight junction proteins, occludin (Figures 8, 9), in mouse distal colonic mucosa. An interesting observation was the comparative improvement of the tight junction protein expression with the treatment with free VIP (Figure 8). This was in contrast to other observed effects, where no anti-inflammatory activity as determined by proinflammatory cytokines and no histology improvement were observed with the free peptide (Figures 5, 6). This indicates that VIP mediates additional effects on tight junction proteins, independent from its anti-inflammatory actions. VIP-SSM was able to perform both these functions presumably due to its higher bioavailability at the target tissues compared to the free peptide.
Diarrhea is an important pathological hallmark of IBD, which significantly reduces patient quality of life. SLC26A3 or DRA is an important luminal membrane intestinal ion transporter, abundantly expressed in the distal colon of mice and in the human colon.48 It is the major transporter involved in chloride absorption in the intestine, and the loss of function and expression of DRA is seen in diarrheal diseases including IBD.49 After 7 days of DSS administration, we observe a significant reduction in the mRNA and protein expression of DRA (Figure 7). This was significantly improved with VIP-SSM but not with free VIP, demonstrating comparative results to improvement in mouse colonic diarrheal phenotype (Figure 4). We have observed in vitro, using differentiated Caco2 monolayers, that VIP is capable of increasing both mRNA and protein levels of DRA (unpublished data). Thus, under inflammatory conditions, VIP may improve the expression of DRA in two ways; by reducing overall inflammation associated damage and by having a direct effect on DRA mRNA and protein expression. However, further studies need to be undertaken in order to understand the exact molecular mechanisms involved. Although, VIP is a known prosecretory agent in the small intestine, under inflammatory conditions in the distal colon, we observed that it aids in reversing secretory effects and improving diarrheal phenotype, which are novel mechanisms of VIP that could aid in reversing the severity of colitis.
Being a disease of the gastrointestinal tract, IBD can be better managed if therapies can be dispensed in an oral formulation. We have recently demonstrated the predominant existence of the major VIP receptor, VPAC1, in the luminal membrane of the colon of mice and humans.50 Therefore, it maybe desirable if therapies containing VIP can be selectively delivered to the colon by oral route to manage UC without the use of invasive modes of administration. Oral delivery of nanomedicines of small molecules to target inflamed tissues of the intestine have shown better therapeutic benefit over conventional products.51 Currently, we are in the process of developing the first oral nanomedicine of a peptide (VIP-SSM) that can be delivered locally to the colon for the treatment of UC. Therefore, we propose VIP-SSM to be developed as a safe and novel nanomedicine for managing inflammation and associated diarrhea in UC, a type of IBD.
Acknowledgments
Special acknowledgement goes out to Dr. Anoop Kumar and Dr. Shubha Priyamvada at the University of Illinois at Chicago for their assistance during animal sacrifice and tissue harvesting. These studies were supported by the NIDDK Grants R01 DK54016, R01 DK81858, and R01 DK92441 (P.K.D.), the Department of Veterans Affairs Grant BX 002011 (P.K.D.), VA SRCS Award (P.K.D.), TUBITAK Award (H.O.), and UIC Deans scholar fellowship (D.J.).
ABBREVIATIONS
- DSS
dextran sulfate sodium
- IBD
inflammatory bowel disease
- VIP
vasoactive intestinal peptide
- SSM
sterically stabilized micelles
- DRA
down regulated in adenoma
- GIT
gastrointestinal tract
- UC
ulcerative colitis
- CD
Crohn’s disease
- RA
rheumatoid arthritis
- DSPE PEG2000
1,2-distearoyl-sn-glycero-3- phosphatidylethanolamine-N-[methoxy(poly(ethylene glycol))- 2000]
- DLS
dynamic light scattering
- PBS
phosphate buffered saline
- NGS
normal goat serum
- qPCR
quantitative polymerase chain reaction
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- TNBS
trinitrobenzenesulfonic acid
- IL-1β
interleukin 1-β
- CXCL
chemokine (CXC motif) ligand
- CCL
chemokine (C–C motif) ligand
- DAPI
4′,6-diamidino-2-phenylindole
- GLP
glucagon like peptide
- VPAC1
VIP receptor 1
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 1991;325(14):1008–1016. doi: 10.1056/NEJM199110033251406. [DOI] [PubMed] [Google Scholar]
- 2.Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, Gomariz RP. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn’s disease. Gastroenterology. 2003;124(4):961–971. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- 3.Rogler G. Gastrointestinal and liver adverse effects of drugs used for treating IBD. Best Pract Res Clin Gastroenterol. 2010;24(2):157–65. doi: 10.1016/j.bpg.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Abad C, Gomariz R, Waschek J, Leceta J, Martinez C, Juarranz Y, Arranz A. VIP in inflammatory bowel disease: state of the art. Endocr, Metab Immune Disord: Drug Targets. 2012;12(4):316–322. doi: 10.2174/187153012803832576. [DOI] [PubMed] [Google Scholar]
- 5.Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. VIP and PACAP inhibit IL-12 production in LPS-stimulated macrophages. Subsequent effect on IFNgamma synthesis by T cells. J Neuroimmunol. 1999;96(2):167–81. doi: 10.1016/s0165-5728(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 6.FERNANDEZ-MARTIN A, GONZALEZ-REY E, Chorny A, Martin J, Pozo D, Ganea D, Delgado M. VIP prevents experimental multiple sclerosis by downregulating both inflammatory and autoimmune components of the disease. Ann N Y Acad Sci. 2006;1070(1):276–281. doi: 10.1196/annals.1317.026. [DOI] [PubMed] [Google Scholar]
- 7.Foey AD, Field S, Ahmed S, Jain A, Feldmann M, Brennan FM, Williams R. Impact of VIP and cAMP on the regulation of TNF-α and IL-10 production: implications for rheumatoid arthritis. Arthritis Res Ther. 2003;5(6):R317. doi: 10.1186/ar999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arranz A, Gutierrez-Canas I, Carrion M, Juarranz Y, Pablos JL, Martinez C, Gomariz RP. VIP reverses the expression profiling of TLR4-stimulated signaling pathway in rheumatoid arthritis synovial fibroblasts. Mol Immunol. 2008;45(11):306573. doi: 10.1016/j.molimm.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Abad C, Juarranz Y, Martinez C, Arranz A, Rosignoli F, García-Gómez M, Leceta J, Gomariz RP. cDNA array analysis of cytokines, chemokines, and receptors involved in the development of TNBS-induced colitis: homeostatic role of VIP. Inflammatory bowel diseases. 2005;11(7):674–684. doi: 10.1097/01.mib.0000171872.70738.58. [DOI] [PubMed] [Google Scholar]
- 10.Todorovic V, Janic B, Koko V, Micev M, Nikolic J, Ratkovic M, Leposavic G, Jankovic T, Knezevic-Usaj S, Milicevic Z. Colonic vasoactive intestinal polypeptide (VIP) in ulcerative colitis–a radioimmunoassay and immunohistochemical study. Hepato-gastroenterology. 1995;43(9):483–488. [PubMed] [Google Scholar]
- 11.Belai A, Boulos P, Robson T, Burnstock G. Neurochemical coding in the small intestine of patients with Crohn’s disease. Gut. 1997;40(6):767–774. doi: 10.1136/gut.40.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favrais G, Couvineau A, Laburthe M, Gressens P, Lelievre V. Involvement of VIP and PACAP in neonatal brain lesions generated by a combined excitotoxic/inflammatory challenge. Peptides. 2007;28(9):1727–37. doi: 10.1016/j.peptides.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Gomariz RP, Arranz A, Abad C, Torroba M, Martinez C, Rosignoli F, Garcia-Gómez M, Leceta J, Juarranz Y. Time-course expression of Toll-like receptors 2 and 4 in inflammatory bowel disease and homeostatic effect of VIP. J Leukocyte Biol. 2005;78(2):491–502. doi: 10.1189/jlb.1004564. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Rey E, Delgado M. Therapeutic treatment of experimental colitis with regulatory dendritic cells generated with vasoactive intestinal peptide. Gastroenterology. 2006;131(6):1799–1811. doi: 10.1053/j.gastro.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Newman R, Cuan N, Hampartzoumian T, Connor SJ, Lloyd AR, Grimm MC. Vasoactive intestinal peptide impairs leucocyte migration but fails to modify experimental murine colitis. Clin Exp Immunol. 2005;139(3):411–20. doi: 10.1111/j.1365-2249.2005.02673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conlin VS, Wu X, Nguyen C, Dai C, Vallance BA, Buchan A, Boyer L, Jacobson K. Vasoactive intestinal peptide ameliorates intestinal barrier disruption associated with Citrobacter rodentium-induced colitis. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;297(4):G735–G750. doi: 10.1152/ajpgi.90551.2008. [DOI] [PubMed] [Google Scholar]
- 17.Vukovic A, Markovic D, Petrovic B, Apostolovic M, Golijanin R, Kanjevac T, Stojkovic B, Peric T, Blagojevic D. Traumatic dental injuries in Serbian children–epidemiological study. Srp Arh Celok Lek. 2013;141(11–12):744–9. doi: 10.2298/sarh1312744v. [DOI] [PubMed] [Google Scholar]
- 18.Wu X, Conlin VS, Morampudi V, Ryz NR, Nasser Y, Bhinder G, Bergstrom KS, Hong BY, Waterhouse CC, Buchan AM. Vasoactive intestinal polypeptide promotes intestinal barrier homeostasis and protection against colitis in mice. PLoS One. 2015;10(5):e0125225. doi: 10.1371/journal.pone.0125225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domschke S, Domschke W, Bloom S, Mitznegg P, Mitchell S, Lux G, Strunz U. Vasoactive intestinal peptide in man: pharmacokinetics, metabolic and circulatory effects. Gut. 1978;19(11):1049–1053. doi: 10.1136/gut.19.11.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashur-Fabian O, Perl O, Lilling G, Fridkin M, Gozes I. SNV, a lipophilic superactive VIP analog, acts through cGMP to promote neuronal survival. Peptides. 1999;20(5):629–33. doi: 10.1016/s0196-9781(99)00017-0. [DOI] [PubMed] [Google Scholar]
- 21.Sethi V, Rubinstein I, Kuzmis A, Kastrissios H, Artwohl J, Onyuksel H. Novel, biocompatible, disease modifying nanomedicine of VIP for rheumatoid arthritis. Mol Pharmaceutics. 2013;10(2):728. doi: 10.1021/mp300539f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vuković L, Madriaga A, Kuzmis A, Banerjee A, Tang A, Tao K, Shah N, Král P, Onyuksel H. Solubilization of therapeutic agents in micellar nanomedicines. Langmuir. 2013;29(51):15747–15754. doi: 10.1021/la403264w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim SB, Banerjee A, Önyüksel H. Improvement of drug safety by the use of lipid-based nanocarriers. J Controlled Release. 2012;163(1):34–45. doi: 10.1016/j.jconrel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee A, Onyuksel H. Peptide delivery using phospholipid micelles. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2012;4(5):562–574. doi: 10.1002/wnan.1185. [DOI] [PubMed] [Google Scholar]
- 25.Lamprecht A. IBD: selective nanoparticle adhesion can enhance colitis therapy. Nat Rev Gastroenterol Hepatol. 2010;7(6):311–2. doi: 10.1038/nrgastro.2010.66. [DOI] [PubMed] [Google Scholar]
- 26.Laroui H, Wilson DS, Dalmasso G, Salaita K, Murthy N, Sitaraman SV, Merlin D. Nanomedicine in GI. Am J Physiol Gastrointest Liver Physiol. 2011;300(3):G371–83. doi: 10.1152/ajpgi.00466.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim SB, Rubinstein I, Sadikot RT, Artwohl JE, Onyuksel H. A novel peptide nanomedicine against acute lung injury: GLP-1 in phospholipid micelles. Pharm Res. 2011;28(3):662–72. doi: 10.1007/s11095-010-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee A, Onyuksel H. A novel peptide nanomedicine for treatment of pancreatogenic diabetes. Nanomedicine. 2013;9(6):722–728. doi: 10.1016/j.nano.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ott C, Schölmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. 2013;10(10):585–595. doi: 10.1038/nrgastro.2013.117. [DOI] [PubMed] [Google Scholar]
- 30.Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169(3951):1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- 31.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 32.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl–/OH– exchange following infection with enteropathogenic E. coli. J Clin Invest. 2007;117(2):428–437. doi: 10.1172/JCI29625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim SB, Rubinstein I, Önyüksel H. Freeze drying of peptide drugs self-associated with long-circulating, biocompatible and biodegradable sterically stabilized phospholipid nanomicelles. Int J Pharm. 2008;356(1):345–350. doi: 10.1016/j.ijpharm.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon L, Mansor S, Mallon P, Donnelly E, Hoper M, Loughrey M, Kirk S, Gardiner K. The dextran sulphate sodium (DSS) model of colitis: an overview. Comp Clin Pathol. 2010;19(3):235–239. [Google Scholar]
- 35.Cooper HS, Murthy S, Shah R, Sedergran D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Laboratory investigation; a journal of technical methods and pathology. 1993;69(2):238–249. [PubMed] [Google Scholar]
- 36.Priyamvada S, Gomes R, Gill RK, Saksena S, Alrefai WA, Dudeja PK. Mechanisms Underlying Dysregulation of Electrolyte Absorption in IBD Associated Diarrhea. Inflammatory bowel diseases. 2015;21(12):2926. doi: 10.1097/MIB.0000000000000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hering NA, Fromm M, Schulzke JD. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol. 2012;590(5):1035–1044. doi: 10.1113/jphysiol.2011.224568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche J-P, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. American Journal of Physiology- Gastrointestinal and Liver Physiology. 2003;285(5):G1028–G1036. doi: 10.1152/ajpgi.00066.2003. [DOI] [PubMed] [Google Scholar]
- 39.Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflammatory bowel diseases. 2009;15(3):341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2(3):541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 41.Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.te Velde AA, de Kort F, Sterrenburg E, Pronk I, ten Kate FJ, Hommes DW, van Deventer SJ. Comparative analysis of colonic gene expression of three experimental colitis models mimicking inflammatory bowel disease. Inflammatory bowel diseases. 2007;13(3):325–330. doi: 10.1002/ibd.20079. [DOI] [PubMed] [Google Scholar]
- 43.Anbazhagan AN, Thaqi M, Priyamvada S, Jayawardena D, Kumar A, Gujral T, Chatterjee I, Mugarza E, Saksena S, Onyuksel H, Dudeja PK. GLP-1 nanomedicine alleviates gut inflammation. Nanomedicine. 2017;13(2):659–665. doi: 10.1016/j.nano.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimura M, Masuda T, Hiwatashi N, Toyota T, Nagura H. Changes in neuropeptide-containing nerves in human colonic mucosa with inflammatory bowel disease. Pathol Int. 1994;44(8):624–634. doi: 10.1111/j.1440-1827.1994.tb01723.x. [DOI] [PubMed] [Google Scholar]
- 45.Sigalet DL, Wallace LE, Holst JJ, Martin GR, Kaji T, Tanaka H, Sharkey KA. Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2007;293(1):G211–G221. doi: 10.1152/ajpgi.00530.2006. [DOI] [PubMed] [Google Scholar]
- 46.Vu JP, Million M, Larauche M, Luong L, Norris J, Waschek JA, Pothoulakis C, Pisegna JR, Germano PM. Inhibition of vasoactive intestinal polypeptide (VIP) induces resistance to dextran sodium sulfate (DSS)-induced colitis in mice. J Mol Neurosci. 2014;52(1):37–47. doi: 10.1007/s12031-013-0205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140(1):12–19. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 48.Talbot C, Lytle C. Segregation of Na/H exchanger-3 and Cl/HCO3 exchanger SLC26A3 (DRA) in rodent cecum and colon. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2010;299(2):G358–G367. doi: 10.1152/ajpgi.00151.2010. [DOI] [PubMed] [Google Scholar]
- 49.Yang H, Jiang W, Furth EE, Wen X, Katz JP, Sellon RK, Silberg DG, Antalis TM, Schweinfest CW, Wu GD. Intestinal inflammation reduces expression of DRA, a transporter responsible for congenital chloride diarrhea. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1998;275(6):G1445–G1453. doi: 10.1152/ajpgi.1998.275.6.G1445. [DOI] [PubMed] [Google Scholar]
- 50.Jayawardena D, Guzman G, Gill RK, Alrefai WA, Onyuksel H, Dudeja PK. Expression and localization of VPAC1, the major receptor of vasoactive intestinal peptide along the length of the intestine. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2017;313:G16. doi: 10.1152/ajpgi.00081.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hua S, Marks E, Schneider JJ, Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: selective targeting to diseased versus healthy tissue. Nanomedicine. 2015;11(5):1117–1132. doi: 10.1016/j.nano.2015.02.018. [DOI] [PubMed] [Google Scholar]