Abstract
The influence of adenosine receptors on blood pressure in salt-sensitive hypertension is unknown. Here we examined the effects of salt diets on arterial blood pressures (radiotelemetry) in female and male Dahl salt-sensitive wildtype versus female and male Dahl salt-sensitive A1, A2A, or A2B receptor knockouts (A1KOs, A2AKOs, and A2BKOs, respectively). At baseline all rats were on a 0.3% salt diet; then separate groups were switched to either 4% or 8% salt diet for two weeks. Compared to wildtypes, baseline pressures were not affected by knockout of A1 or A2B receptors; yet, mean, systolic, and diastolic pressures were significantly (P<0.01) higher in A2AKOs versus wildtypes, an effect independent of sex. During the second week on a 4% salt diet, mean, systolic, and diastolic blood pressures (mm Hg, mean±SEM) in female A1KOs (176±5, 209±5, and 147±4, respectively) and A2BKOs (166±8, 198±9, and 139±8, respectively) were significantly lower (P<0.001) than wildtype on a 4% salt diet (202±4, 240±5, 172±3, respectively). Male A1KOs and A2BKOs were not protected against 4% salt diet-induced hypertension. This female advantage was overwhelmed by an 8% salt diet. Female and male A2AKOs were more salt sensitive, a phenotype that was apparent in male A2AKOs on 4% and 8% salt diets and in females on 8% salt diet. Female A1KOs and A2BKOs, were less susceptible to salt-induced stroke and experienced improved survival. Adenosine receptors influence blood pressure and survival in salt-sensitive rats, and the impact of deleting adenosine receptors on blood pressure and survival depends on salt diet and sex.
Keywords: A1 receptors, A2A receptors, A2B receptors, Dahl SS rats, high salt diet, adenosine, hypertension
INTRODUCTION
Adenosine receptors may be involved in blood pressure regulation. A1 receptors are expressed in the renal microcirculation1 where they induce renal vasoconstriction, mediate tubuloglomerular feedback2–4, augment renal vasoconstriction induced by angiotensin II5, 6 and norepinephrine7, 8, and are essential for a full renovascular response to renal sympathetic nerve activation7, 8. A1 receptors also mediate vasoconstriction in the aorta9, 10 and mesenteric arteries11, 12, and A1 receptors in the nucleus tractus solitarii increase sympathetic ouflow13. In the renal proximal tubules, A1 receptors stimulate the reabsorption of sodium14–18. A2A receptors mediate vasodilation in many vascular beds including the kidneys19–22, heart23, mesentery24, 25, skeletal muscle26, 27, and aorta28. A2A receptors inhibit the activity of helper and cytotoxic T-lymphocytes29–33, and these effector T cells contribute to the pathophysiology of hypertension34–37. A2B receptors mediate vasodilation, for example in the kidneys38, 39, heart23, 40, 41, mesentery11, and aorta42. In the renal medullary circulation, A2 receptors may be critically important in regulating blood pressure by increasing medullary blood flow and thus enhancing sodium excretion43, 44. However, A2B receptors, by increasing production of endothelin-1 in the kidney may contribute to renal fibrosis and chronic renal failure45. Although there is theoretical evidence that adenosine receptors likely participate in blood pressure regulation, the extant literature on this question is sparse, inconsistent, and incomplete (for examples see: Brown et al.46; Kim et al.47,3; Gao et al.48; Lee et al.49; Sakata et al.50 ; and Nayak et al.51).
Motivated by the pervasive expression of adenosine A1, A2A, and A2B receptors in physiological systems related to blood pressure regulation and the paucity of information on whether or not adenosine receptors actually do contribute to blood pressure regulation, we embarked on a comprehensive project to examine the long-term effects of knocking out A1, A2A, and A2B receptors on hypertension in Dahl salt-senistive (SS) rats on a normal (0.3%), high (4%), or very high (8%) salt diet. The experiments reported herein were performed by long-term monitoring of blood pressure by radiotelementry in a large number of animals and included both female and male animals. Here we report that all three adenosine receptors are involved in salt-sensitive hypertension, that the degree of involvement depends not only on the level of salt intake but also on sex, and that knocking out adenosine receptors can affect susceptibility to salt-induced stroke.
METHODS
Note: For raw data and for additional information on analytic methods or study materials contact Edwin K. Jackson, PhD at edj@pitt.edu.
Production of Knockout Animals
The University of Pittsburgh Institutional Animal Care and Use Committee approved all procedures, and this investigation conforms to National Institutes of Health Guide for the Care and Use of Laboratory Animals. The knockout rats used in this investigation were generated by the MCW Gene Editing Rat Resource Program (Dr. Aron M. Geurts, Department of Physiology and Human Molecular Genetics Center, Medical College of Wisconsin, Milwaukee, WI). The A1 receptor knockout rats (A1KOs) and A2A receptor knockout rats (A2AKOs) were produced by injecting a CRIPSR targeting the sequence GGCTCTGCTCGCCATTGCTG for A1KOs and GATGTACACCGAGGAGCCCA for A2AKOs into Dahl SS/JrHsdMcwi rat embryos. The A2B receptor knockout rats (A2BKOs) were also generated in Dahl SS/JrHsdMcwi rat embryos. These rats, however, were produced using zinc-finger nuclease technology and were previously described and characterized by Nayak and coworkers51. For all three strains, founders were backcrossed into the same parental strain (Dahl SS/JrHsdMcwi). Heterozygous male and female rats of each strain were mated to produce colonies of homozygous knockouts and homozygous wildtype Dahl SS. Figures S1, S2, and S3 (online-only Data Supplement) provide the specific mutations in the Adora1, Adora2a, and Adora2b genes coding for the A1, A2A, and A2B adenosine receptors, respectively. Also shown are the PCR primers used for genotyping and the predicted sizes of the PCR amplicons. Figure S4 displays typical genotyping results for wildtype and A1KO Dahl SS rats using agarose gel electrophoresis of PCR amplicons stained with ethidium bromide and imaged by using a Bio-Rad Gel Doc XR+ System (Product number 1708195; Hercules, CA), and Figure S5 shows the same for wildtype, A2AKO, and A2BKO Dahl SS rats. Figures S4 and S5 demonstrate the unambiguous assignment of offspring to their respective genotypes using the indicated primer sets.
Western Blotting for Adenosine Receptors
See online-only Data Supplement for methods.
Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) for Assessment of Adenosine Receptor RNA Expression
See online-only Data Supplement for methods.
Acute Hemodynamic Effects of Intravenous Administration of Adenosine Receptor Agonists
See online-only Data Supplement for methods.
Effects of Selective A2B Receptor Agonist on Proliferation of Cardiac Fibroblasts from Wildtype, A1KO, A2AKO, and A2BKO Rats
See online-only Data Supplement for methods.
Long-term Measurement of Arterial Blood Pressure by Radiotelemetry
Breeding pairs and offspring were maintained on a 0.3% salt diet. Offspring were placed into radiotelemetry studies at approximately 12 weeks of age. Long-term arterial blood pressures were monitored by radiotelemetry using a Data Sciences International (DSI; St. Paul, MN) system consisting of the following components: Data Exchange Matrix 20CH; Receiver Model RPC-1 for plastic cages; HD-S10 Transmitter with suture rib; Ambient Pressure Reference Model APR-1; DataQuest A.R.T. Silver 4.31 Data Acquisition and Analysis System; and Dell Computer Windows7 Model 7010. Rats were briefly anesthetized with isoflurane, and the catheter from a HD-S10 transmitter was inserted into the femoral artery and advanced into the abdominal aorta. The transmitter was gently located to the lower body cavity and secured by suture, and the wound was closed by metal clips. Post-operative buprenorphine (0.1 mg/kg) was administered by subcutaneous injection twice daily for 3 days. Rats were housed in plastic cages that were placed on receivers, and arterial pressures were sampled for 15 seconds every 10 minutes. Animals were housed in a temperature (20ºC–24ºC), humidity (30%–70%), and light/dark cycle (7:00 AM – 7:00 PM) controlled room within the University of Pittsburgh Division of Laboratory Animal Resources. Three days after implantation of transmitters, baseline blood pressures were recorded for 20 days while the animals continued feeding on the basal 0.3% salt diet. Next, in some rats the rat chow was changed to provide a 4% salt diet, and in other rats (separate groups) the rat chow was changed to provide an 8% salt diet (i.e., each rat was placed on either a 4% or 8% salt diet, but not both). Blood pressures were then recorded for an additional two weeks. All 3 salt diets were the AIN-76A diet formulated by Research Diets, Inc. (New Brunswick, NJ) that contained either 0.3%, 4%, or 8% NaCl. We independently confirmed the sodium content of all the diets by flame photometry. The protein source in the AIN-76A salt diet is casein-based. We selected this diet because Geurts et al. have shown that in Dahl SS rats fed the AIN-76A diet baseline arterial blood pressures are much higher and more responsive to a high-salt diet compared to Dahl SS rats fed a grain-based diet52.
Statistical Analysis
For basal (ie., 0.3% salt diet) blood pressures and heart rates, data were analyzed by 2-factor analysis of variance (ANOVA) in which one factor was genotype and the second factor was sex. To determine the interaction of sex, genotype, and a high (4%) or very high (8%) salt diet on blood pressures and heart rates, data were analyzed by a repeated measures 3-factor ANOVA in which one factor was sex, the second factor was genotype, and the third factor was time on the high-salt diet (3 levels: 1 week on the basal 0.3% salt diet, 1st week on 4% or 8% salt diet, and 2nd week on 4% or 8% salt diet). Additional information was extracted by analyzing females and males separately using a repeated measures 2-factor ANOVA (genotype and time on the salt diet). Specific contrasts were performed by Bonferroni tests. ANOVAs and Bonferroni tests were conducted with NCSS 2004 software (Number Cruncher Statistical Systems; Kaysville, UT). Survival analysis was performed using a Gehan-Breslow-Wilcox test (Prism version 7.00 for Windows, GraphPad Software, La Jolla, CA).
RESULTS
To determine if the expression of A1, A2A, and A2B receptors was reduced in our respective knockout rats, we probed for these receptors by western blot in whole kidney tissue obtained from wildtype and knockout animals. As shown in Figure S6, in kidney tissue the western blot signal for the respective target receptor was much lower or absent in respective knockout rats compared with wildtype animals. We also isolated RNA from the kidneys and brains of 3 male and 3 female wildtype, A1KO, A2AKO, and A2BKO rats (total of 24 rats) and measured RNA expression of native A1, A2A, and A2B receptors using RT-qPCR. In kidney (Figure S7) and brain (Figure S8) tissue, RNA expression for a given adenosine receptor subtype was, for all intents and purposes, absent in knockout rats for that given receptor subtype, but was readily detectable in wildtype and knockout animals for the other adenosine receptor subtypes. With a few exceptions, adenosine receptor RNA expression was similar in males and females, and the RNA expression of a given receptor subtype was not altered by knockout of the other two receptor subtypes. However, in kidney tissue, A2B receptors were more highly (P<0.05) expressed in female wildtypes and A2AKOs compared with the corresponding males (Figure S7C). Also in brain tissue, there was a tendency (not significant) for increased A2A receptor RNA expression in male and female A1KOs and female A2BKOs compared with wildtype rats (Figure S8B).
To determine if receptor function was altered, we conducted 3 sets of experiments. First we injected intravenously a bolus (3 μmol/kg) of adenosine while monitoring heart rate, mean arterial blood pressure (MABP), and renal vascular resistance (RVR = MABP/RBF). Figures S9, S10, and S11 illustrate typical responses (heart rate, MABP, and RVR, respectively) to adenosine in wildtype, A1KO, A2AKO, and A2BKO Dahl SS rats. In wildtype, A2AKO, and A2BKO Dahl SS rats, adenosine elicited a profound, yet brief (<1 minute), reduction in heart rate and MABP and an increase in RVR. These responses are consistent with the well-known ability of cardiac A1 receptors to mediate adenosine-induced severe bradycardia53 and renovascular A1 receptors to mediate adenosine-induced renal vasoconstriction18. Consistent with the A1KO Dahl SS rats lacking functional A1 receptors, the effects of adenosine on heart rate (Figure S9) and RVR (Figure S11) were abolished, yet there was still a reduction in MABP (Figure S10).
To further characterize the A1KO, A2AKO, and A2BKO Dahl SS rats, we infused intravenously either 2-chloro-N6-cyclopentyladenosine (CCPA; selective A1 receptor agonist54; 1 μg/kg/min; Tocris, Minneapolis, MN) or CGS21680 (selective A2A receptor agonist54; 1 μg/kg/min; Tocris) while monitoring MABP, heart rate, RVR, and MVR (MVR = MABP/MBF). As illustrated in Figure S12, in A2AKO and A2BKO rats, CCPA reduced MABP and heart rate and increased RVR; and these responses were abolished in A1KO rats. In A1KO and A2BKO rats, CGS21680 profoundly decreased MABP, triggered a reflex tachycardia, and reduced MVR (Figure S13). As anticipated, these responses were abolished in A2AKO rats (Figure S13).
We attempted to confirm that A2BKO rats did not express functional A2B receptors by examining the cardiovascular responses to intravenous infusions of BAY60-6583 (selective A2B receptor agonist). However, we found that activation of A2B receptors by systemic administration of BAY60-6583 did not stimulate observable acute hemodynamic responses regardless of genotype. Therefore, we took an alternative approach. We previously discovered that A2B receptors mediate the ability of adenosine to inhibit FBS-induced proliferation of rat CFs55. Therefore, we isolated and cultured CFs from wildtype, A1KO, A2AKO, and A2BKO Dahl SS rats and determined the effects of BAY60-6583 on serum-induced proliferation of these cells. As summarized in Figure S14, BAY60-6583 inhibited serum-induced proliferation of CFs obtained from wildtype, A1KO, and A2AKO Dahl SS rats, but was inactive in this regard in CFs from A2BKO Dahl SS rats. Thus this assay system demonstrated that A2B receptors were non-functional in A2BKO Dahl SS rats.
Confident that the A1KO, A2AKO, and A2BKO Dahl SS rats had their respective adenosine receptors inactivated, we next proceeded to determine whether baseline blood pressures and heart rates were different in wildtype versus adenosine receptor knockout Dahl SS rats on a normal salt diet (0.3% NaCl). To achieve this, a large number (20 to 29) of each strain of rats was monitored by radiotelemetry for 20 days. Animals were approximately 12 weeks of age at the time of radiotelemetry implant. We had no a priori reason to rule out sex as a biological variable. Therefore, we included a balance of females and males; and we analyzed the data using 3-factor ANOVA in which one factor was sex.
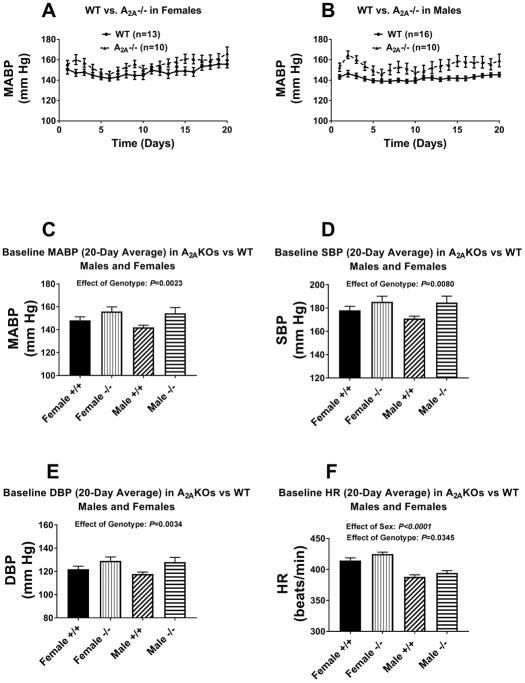
Baseline (i.e., on a 0.3% salt diet) MABPs, systolic blood pressures (SBPs), diastolic blood pressure (DBPs), and heart rates (HRs) in female and male A1KO and A2BKO Dahl SS rats are presented in Figures S15 and S16, respectively. Shown are both the day-to-day 24-hour averaged MABPs as well as the overall 20-day averaged MABPs, SBPs, DBPs, and HRs. Compared to wildtype Dahl SS rats, baseline MABPs, SBPs, DBPs, and HRs were not significantly affected by knockout of either A1 or A2B receptors; also, there were no significant interactions between sex and genotype in this regard. Unlike A1KO and A2BKO Dahl SS rats, baseline MABPs, SBPs, and DBPs, were significantly higher in A2AKOs versus wildtype rats (P-values by 2-factor ANOVA for effect of genotype were P=0.0023, P=0.0080, and P=0.0034, respectively), and this effect was independent of sex (Figure 1). Notably, regardless of genotype, baseline HRs were significantly higher in females compared to males [P-values by 2-factor ANOVA for effect of sex were P<0.0001, P=0.0345, and P<0.0001 for A1KOs (Figure S15, A2AKOs (Figure 1), and A2BKOs (Figure S16), respectively].
Figure 1.
Panels A and B illustrate the day-to-day profile for 20 days of baseline (i.e., on 0.3% salt diet) mean arterial blood pressure (MABP) in wildtype Dahl SS rats (WT) versus A2A knockout Dahl SS rats (A2A−/−). Panels A, B, C, and D summarize the 20-day average MABP (C), systolic blood pressure (SBP, D), diastolic blood pressure (DBP, E), and heart rate (HR, F) in female and male wildtype (+/+) versus A2A knockout (−/−) Dahl SS rats. P-values are from 2-factor analysis of variance. Values are means ± SEM.
Next, we examined in both female and male A1KO, A2AKO, and A2BKO Dahl SS rats the effects of 14 days of a either a 4% (high) or 8% (very high) salt diet. We limited the telemetry studies to 14 days of high-salt diet because after 14 days animals began to expire from sudden strokes. Over time, sudden strokes systematically removed the most severely hypertensive animals from the groups and therefore rendered statistical comparisons at time points past 14 days invalid.
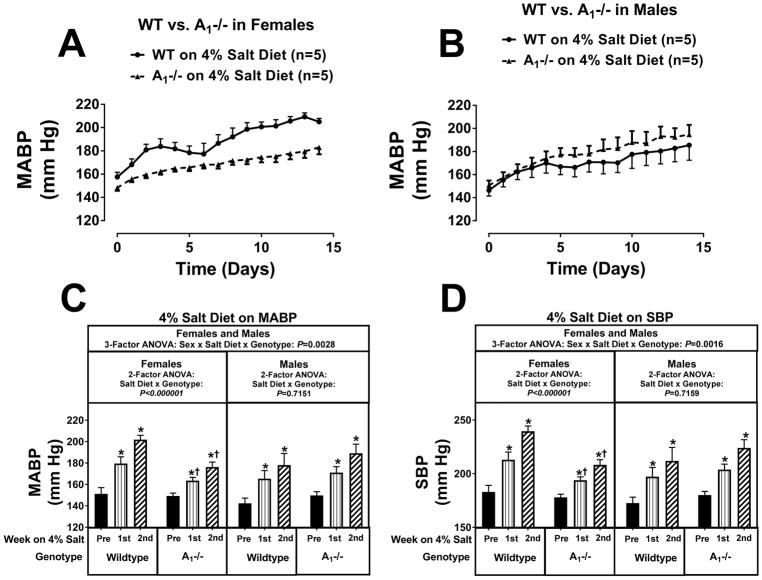
The increases in MABPs (Figure 2), SBPs (Figure 2), and DBPs (Figure S17) induced by a 4% salt diet were significantly attenuated by approximately 26, 32, and 25 mm Hg, respectively, by the 2nd week of a 4% salt diet in female A1KOs compared to female wildtype during the 2nd week of the 4% salt diet (P-value for the interaction between salt diet and genotype by 2-factor ANOVA was P<0.000001 for all three variables). In contrast to female A1KOs, male A1KOs were not protected against 4% salt diet-induced hypertension (see Figures 2, and S17). The sex-dependence on the effects of A1KO genotype on 4% salt diet-induced hypertension was confirmed by 3-factor ANOVA (P-values for the sex × salt diet × genotype interaction were P=0.0028, P=0.0016, and P=0.0034 for MABPs, SBPs, and DBPs, respectively). Notably, in wildtype females, HR increased during the 2nd week of a 4% salt diet, and this increase was abolished in female A1KO rats (Figure S17). In separate group of rats, we examined the effects of knocking out the A1 receptor on hypertension induced by an 8% salt diet. Although in female rats knocking out the A1 receptor attenuated 4% salt diet-induced hypertension, when the salt diet was 8% this protection was overwhelmed (see Figures S18 and S19; P-values for the salt diet × genotype interaction by 2-factor ANOVA were P=0.6848, P=0.8403, and P=0.8450 for MABPs, SBPs, and DBPs, respectively). In males, blood pressures tended to be higher in A1KOs on an 8% salt diet (Figures S18 and S19); however, this trend did not achieve statistical significance (P-values for the salt diet × genotype interaction by 2-factor ANOVA were P=0.2886, P=0.3144, and P=0.1552 for MABPs, SBPs, and DBPs, respectively).
Figure 2.
Panel A illustrates the effects of a 4% salt diet on daily mean arterial blood pressure (MABP) in female wildtype Dahl SS rats (WT) versus female A1 knockout Dahl SS rats (A1−/−); panel B reports the effects of 4% salt diet on daily MABP in male WT versus male A1−/− Dahl SS rats. Panel C summarizes for both females and males the weekly average MABP before (pre) starting the 4% salt diet and then during the 1st and 2nd weeks of the 4% salt diet. Panel D provides the same information and analysis as panel C for systolic blood pressure (SBP). Data were analyzed by 2-factor and 3-factor analysis of variance (ANOVA), with specific contrasts using Bonferroni tests (*indicates P<0.05 versus corresponding “pre” period; †indicates P<0.05 versus corresponding period in wildtype). Values are means ± SEM.
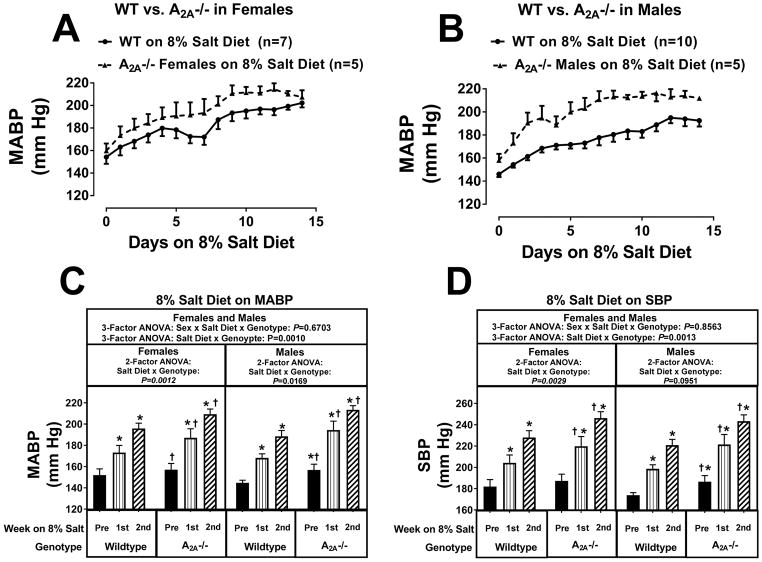
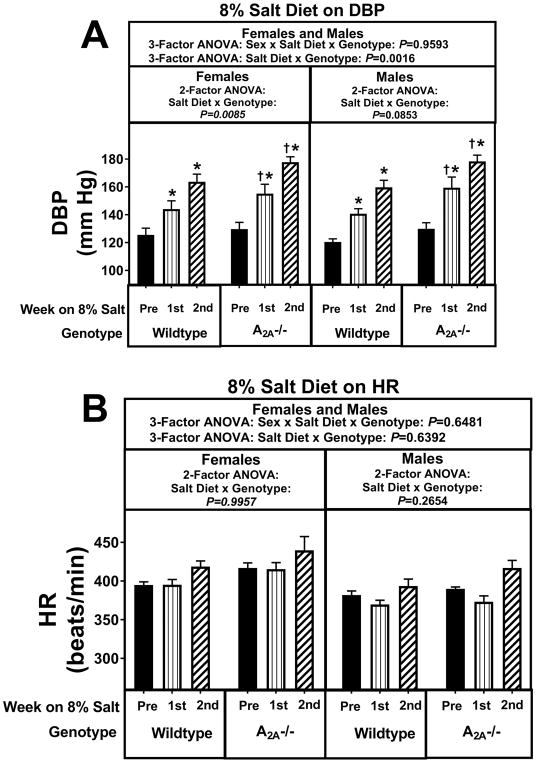
Knocking out A2A receptors did not increase the hypertensive response to the 4% salt diet in females (Figures S20 and S21). However, in male A2AKOs (Figures S20 and S21) there was a strong tendency for an increased hypertensive response to 4% salt diet (P-values for the salt diet × genotype interaction by 2-factor were P=0.0560, P=0.1070, and P=0.0706 for MABPs, SBPs, and DBPs, respectively). Although these 2-factor ANOVA P-values did not achieve P<0.05, by 3-factor ANOVA the sex × salt diet × genotype interaction was near significant for MABPs (P=0.0534; Figure S20) and reached significance for DBPs (P=0.0418; Figure S21). Taken together, these findings strongly suggest that male A2AKOs, but not female A2AKOs, were more sensitive to the pro-hypertensive effects of a 4% salt diet. The 8% salt diet revealed more clearly the detrimental effects of knocking out A2A receptors. As shown in Figures 3 and 4, there was a striking salt diet × genotype interaction (P-values by 3-factor ANOVA were P=0.0010, P=0.0013, and P=0.0016 for MABPs, SBPs, and DBPs, respectively) that was independent of sex (P-values for the sex × salt diet × genotype interaction were P=0.6703, P=0.8563, and P=0.9593 for MABPs, SBPs, and DBPs, respectively). In this regard, during the 2nd week of an 8% salt diet, MABPs, SBPs, and DBPs were approximately 13, 18, and 14 mm Hg, respectively, higher in A2AKO females and approximately 24, 22, and 18 mm Hg, respectively, higher in A2AKO males compared with the corresponding wildtype females and males during the 2nd week of an 8% salt diet.
Figure 3.
Panel A illustrates the effects of a 8% salt diet on daily mean arterial blood pressure (MABP) in female wild-type Dahl SS rats (WT) versus female A2A knockout Dahl SS rats (A2A−/−); panel B reports the effects of 8% salt diet on daily MABP in male WT versus male A2A−/− Dahl SS rats. Panel C summarizes for both females and males the weekly average MABP before (pre) starting the 8% salt diet and then during the 1st and 2nd weeks of the 8% salt diet. Panel D provides the same information and analysis as panel C for systolic blood pressure (SBP). Data were analyzed by 2-factor and 3-factor analysis of variance (ANOVA), with specific contrasts using Bonferroni tests (*indicates P<0.05 versus corresponding “pre” period; †indicates P<0.05 versus corresponding period in wildtype). Values are means ± SEM.
Figure 4.
Panel A summarizes for both female and male A2A knockout (−/−) and wildtype Dahl SS rats the weekly average diastolic blood pressure (DBP) before (pre) starting the 8% salt diet and then during the 1st and 2nd weeks of the 8% salt diet. Panel B provides the same information and analysis as panel A, but for heart rate (HR). Data were analyzed by 2-factor and 3-factor analysis of variance (ANOVA), with specific contrasts using Bonferroni tests (*indicates P<0.05 versus corresponding “pre” period; †indicates P<0.05 versus corresponding period in wildtype). Values are means ± SEM.
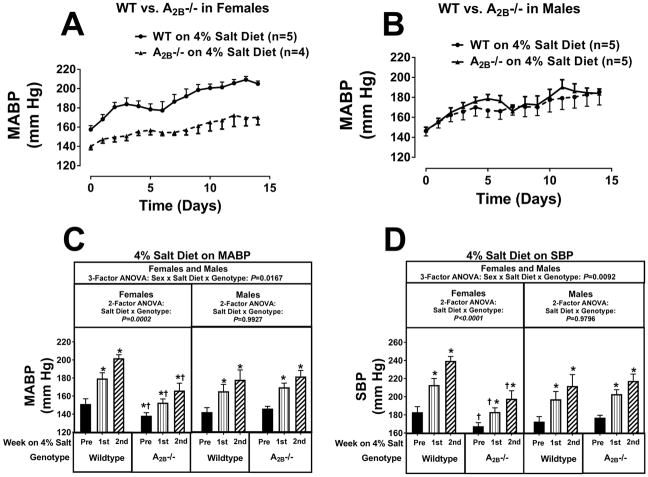
Knocking out A2B receptors yielded results similar to those observed in A1KO rats. During the 2nd week of a 4% salt diet, MABPs, SBPs, and DBPs were significantly lower (by approximately 36, 42, and 33 mm Hg) in female A2BKOs compared with female wildtype rats (Figures 5 and S22). In this regard, the P-values for the salt diet × genotype interaction by 2-factor ANOVA in females were P=0.0002, P<0.0001, and P=0.0003 for MABPs, SBPs, and DBPs, respectively. In contrast to female A2BKOs, male A2BKOs were not protected against 4% salt diet-induced hypertension (P-values by 2-factor ANOVA for the salt diet × genotype interaction in males were P=0.9927, P=0.9796, and P=0.9082 for MABPs, SBPs, and DBPs, respectively). The sex-dependence on the effects of A2BKO genotype on 4% salt diet-induced hypertension was confirmed by 3-factor ANOVA (the P-values for the sex × salt diet × genotype interaction were P=0.0167, P=0.0092, and P=0.0333 for MABPs, SBPs, and DBPs, respectively). In yet another group of rats, we examined the effects of knocking out the A2B receptor on hypertension induced by an 8% salt diet (Figures S23 and S24). Although in female rats knocking out the A2B receptor was able to attenuate 4% salt diet-induced hypertension, when the salt diet was 8% this protection was overwhelmed (P-values for the salt diet × genotype interaction by 2-factor ANOVA were P=0.7221, P=0.9383, and P=0.9532 for MABPs, SBPs, and DBPs, respectively). In males, MABPs, SBPs and DBPs were similar in A2BKOs versus wildtype on an 8% salt diet (Figure S23 and S24).
Figure 5.
Panel A illustrates the effects of a 4% salt diet on daily mean arterial blood pressure (MABP) in female wildtype Dahl SS rats (WT) versus female A2B knockout Dahl SS rats (A2B−/−); panel B reports the effects of 4% salt diet on daily MABP in male WT versus male A2B−/− Dahl SS rats. Panel C summarizes for both females and males the weekly average MABP before (pre) starting the 4% salt diet and then during the 1st and 2nd weeks of the 4% salt diet. Panel D provides the same information and analysis as panel C for systolic blood pressure (SBP). Data were analyzed by 2-factor and 3-factor analysis of variance (ANOVA), with specific contrasts using Bonferroni tests (*indicates P<0.05 versus corresponding “pre” period; †indicates P<0.05 versus corresponding period in wildtype). Values are means ± SEM.
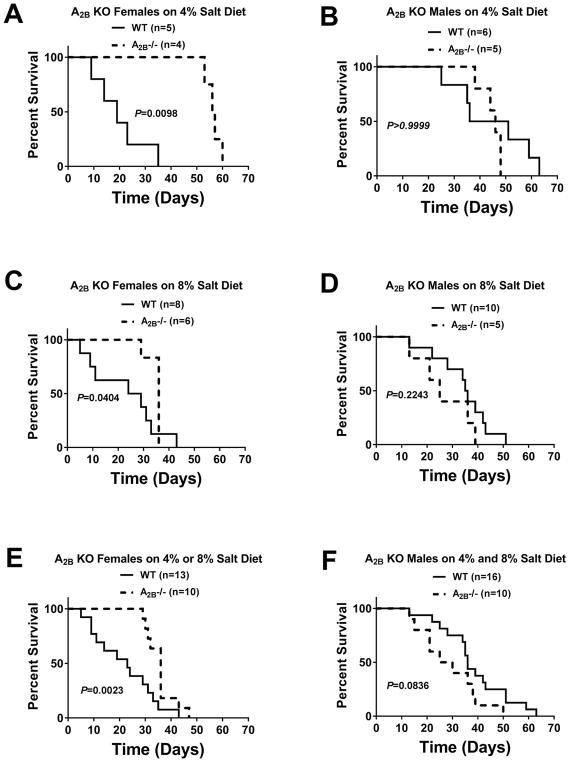
As mentioned, after 14 days of high-salt diet (either 4% or 8%), we observed that Dahl SS rats began to expire suddenly (i.e., animals were very active, feeding and grooming normally and in apparent good health; yet died suddenly). On occasion, an animal would develop partial paralysis in a limb or seizures, indicating that the likely cause of death was sudden stroke. In several rats, we confirmed stroke in conscious rats by magnetic resonance imaging. Therefore, we monitored all animals at least three times daily and immediately euthanized any rat that showed signs of limb paralysis or seizures. We noted the time of death or euthanasia and analyzed these data using the Gehan-Breslow-Wilcox test to compare survival curves because this test does not require a consistent hazard ratio. We analyzed survival curves following a 4% salt diet for each combination of sex and genotype, and did the same for the 8% salt diet. However, to increase sample size (and thus robustness) we also analyzed survival curves for each combination of sex and genotype but combined the survival data for both high-salt diets (4% + 8%). Knocking out A2A receptors had no consistent effect on survival. Female, but not male, A1KOs had increased survival on a 4% salt diet (P=0.0382), but not on an 8% salt diet (Figure S25). As shown in Figure 6, female A2BKOs had increased survival on 4% (P=0.0098), 8% (P=0.0404), and 4%+8% (P=0.0023) salt diets, whereas males did not.
Figure 6.
Figure summarizes survival curves for wildtype (WT) versus A2B knockout (KO, A2B−/−) Dahl SS rats for females (A, C, and E) and males (B, D, and F) on either a 4% (A, B) or 8% (C, D) salt diet. Panels E and F show the survival curves for females and males when the data from all high-salt diets (4% + 8%) were combined.
Diurnal variation of blood pressure is a well-recognized phenomenon in which blood pressure is higher during the active phase (dark for rats) versus the inactive phase (light for rats) of the 24-hour dark-light cycle. Since it is conceivable that salt diet, genotype, or sex affected diurnal variation of blood pressure, we calculated the 7-day average MABP for each rat during the dark phase (lights off 7:00 PM to 7:00 AM) and during the light phase (lights on 7:00 AM to 7:00 PM) for each week of the telemetry study (pre-high-salt diet week, 1st week of high-salt diet, and 2nd week of high-salt diet). We then calculated the diurnal variation of blood pressure as the dark phase minus light phase difference in MABP. As shown in Figures S26, S27, and S28, the expected diurnal variation of MABP was observed in all groups, regardless of sex or genotype. Also, in all groups a high-salt diet augmented the diurnal variation of MABP, particularly during the 1st week of a high-salt diet, and generally (but not always) less so during the 2nd week of a high-salt diet.
DISCUSSION
Here, we sought to provide a comprehensive blood pressure study to clarify the overall role of adenosine receptors in salt-induced hypertension. We selected salt diet as the pro-hypertensive stimulus because adenosine receptors are well-known to modulate renal excretory function. For example, using microdialysis, Siragy and Linden observed in rats that renal interstitial levels of adenosine increased ~18-fold in the cortex and medulla when the sodium diet was raised from 0.15% to 4%56. Similarly, Zou et al. reported that a 4% NaCl diet significantly elevated cortical and medullary adenosine levels in snap-frozen kidneys44. We established in 1993 that selective A1 receptor antagonism markedly increases sodium excretion in rats14, and Zou et al. discovered in 1999 that A2 receptors in the renal medulla dilate medullary vessels and thereby cause natriuresis43.
Because of the potential importance of adenosine in regulating sodium excretion, we selected as our experimental paradigm the Dahl SS rat. Until recently, generating knockout rats was problematic; however, with the introduction of ZFN and CRISPR technology, that barrier has been removed. Therefore, we used this opportunity to establish colonies of Dahl SS rats with three of the major adenosine receptor subtypes deleted. We did not include A3 receptor knockouts because there is little evidence for a role of this subtype in cardiovascular physiology. By using Dahl SS as the background strain and by placing these rats on an AIN-76A diet we now had the ability to induce large and reproducible increases in blood pressure by changing the salt diet.
To ensure that the target receptors were deleted in these novel rat strains, using western blotting we examined adenosine-receptor protein expression in the kidney medulla, juxtamedullary region, and cortex. Since the main purpose of these western blot experiments was to ensure that the target receptors were knocked out, any tissue that expresses these receptors would have been informative. However, we selected to examine kidney tissue because: 1) A1 receptors modulate preglomerular vascular tone, vascular responses to renal sympathetic neurotransmission, and tubular sodium reabsorption; 2) A2A receptors are involved in regulating blood flow to the medulla during salt loading; and 3) A2B receptors are known to cause renal fibrosis in response to angiotensin II-induced hypertension (see Introduction for references). The western blot results indicated that the target receptors were deleted by the knockout strategy, and this conclusion was further reinforced by four additional experiments. First, the RT-qPCR results showed that the RNA for the target receptor was deleted in the respective knockout animals. Second, adenosine and CCPA (a highly selective A1-receptor agonist) decreased heart rate and increased RVR in rats expressing A1 receptors, but not in A1KO rats. Third, CGS21680 (a highly selective A2A-receptor agonist) decreased blood pressure in rats expressing A2A-receptors, but not in A2AKO rats. Fourth, Bay60-6583 (a highly selective A2B-receptor agonist) decreased proliferation of cardiac fibroblasts obtained from rats expressing A2B-receptors, but did not affect proliferation of cardiac fibroblasts obtained from A2BKO rats. Taken together, these quality control experiments leave no doubt that the target receptors were deleted.
There are three important design aspects of the current study. First, we measured blood pressure in all rats using the gold-standard method of radiotelemetry. Second, we examined the effects of three different levels of salt diet on blood pressure. Third, we performed sufficient numbers of experiments in both females and males to permit inclusion of this critically important biological variable in a statistical approach designed to determine the effects of sex on outcomes. Inclusion of sex as a biological variable turned out to be essential for correct data interpretation.
There are three important conclusions that can be deduced from the present study. First, deletion of A1 or A2B receptors protects against salt-induced hypertension in females, but not males. However, this protection can be overcome when the salt diet reaches extremely high levels. Second, deletion of A1 and A2B receptors protects against salt-induced strokes in females, but not males, and this protection persists even when the salt diet is extremely high. Third, deletion of A2A receptors augments salt-induced hypertension, an effect which occurs in both females and males, yet is more pronounced in males.
There are three implications of the above conclusions. One is that dual adenosine receptor antagonists that block both A1 and A2B receptors, but not A2A receptors, may be useful antihypertensive and anti-stroke medications in females. In fact, it is conceivable that even males may derive benefit from dual blockade of A1 and A2B receptors. Indeed, recently we reported that administration of BG9928 for 24 weeks to male, obese ZSF1 rats significantly improved left ventricular diastolic function in association with a reduction in cardiac vasculitis and cardiac necrosis57. BG9928 also significantly reduced focal segmental glomerulosclerosis, tubular atrophy, tubular dilation, and deposition of proteinaceous material in the tubules57. Although BG9928 is a highly potent A1 receptor antagonist (Ki, 7.4 nM), it has some affinity for A2B receptors (Ki, 90 nM)58; however, BG9928 binds poorly to A2A receptors (Ki, 6410 nM)58. We are currently developing a colony of dual A1KO/A2BKO Dahl rats to rigorously test the prediction that dual blockade of A1 and A2B receptors would provide increased benefit in females and perhaps some benefit in males. A second implication of the above conclusions is that a deficiency in A2A receptor number or receptor-effector coupling or a deficiency in the levels of adenosine in the biophase of A2A receptors could contribute to the pathophysiology of hypertension. A third implication is that drugs that increase adenosine levels in key biophases may be beneficial. For example, as mentioned salt diet increases adenosine levels in the renal medulla, an effect that dilates the renal medullary microvessels and promotes natriuresis. Therefore, agents that promote adenosine levels in the renal medulla may prove to be efficacious antihypertensives in both females and males.
Our motivation for examining the role of adenosine receptors in Dahl SS rats over a wide range of dietary salt levels (0.3%, 4%, and 8% salt diets) is that in humans there is a “dose-response” curve for dietary sodium intake versus arterial blood pressure that extends over a wide range (0.1 to 10 grams per day) of dietary sodium intake (see Figure 5 of article by Pettinger59). The current study indicates that the protection against salt-diet induced hypertension afforded to females by knocking out A1 or A2B receptors is lost at very high levels (8%) of dietary salt. The clinical implications of this finding is that any benefit of blocking A1 and/or A2B receptors can be overridden by extremely high dietary sodium levels.
The main limitation of the present study is that we did not attempt to dissect the mechanisms by which deleting adenosine receptors affects blood pressure. This omission was by design. In our experience, manipulating animals perturbs blood pressure and compromises the quality, robustness, and rigor of the blood pressure data. Since our focus was on blood pressure, we decided against conducting any additional procedures. Also, we noted that Dahl SS rats on a high-salt diet were very susceptible to stroke. For example, on occasion merely the entry of animal care personnel into the telemetry room was associated with onset of strokes in some Dahl SS rats. We therefore decided to avoid all stressors and maintain animals in a quiet, highly controlled environment.
The fact that the beneficial effects of knocking out A1 and A2B receptors were limited to females is quite interesting and deserves comment. Our RT-qPCR experiments in kidneys and brains indicated that as a general rule mRNAs coding for adenosine receptor subtypes were similarly expression in females and males, and mRNA expression for a given adenosine receptor subtype was as a general rule not influenced by knockout of the other two adenosine receptor subtypes. However, we noted two exceptions to these general rules. First, A2B-receptor mRNA was approximately 3-fold higher in the kidneys of female, compared to male, A2AKOs (Figure S7C). Second, there was a strong trend for increased expression of A2A-receptor mRNA in the brains of female, compared to male, A2BKOs (Figure S8B). It is conceivable that deleting A2AKOs in females was less pro-hypertensive because of compensation by increased expression of A2B receptors in the renal microcirculation. Also increased A2A receptors in the brain in A2BKOs could have contributed to the antihypertensive effects of deleting A2B receptors in females. Future studies focusing on these hypotheses are warranted.
Perspectives
An important aspect of this research is that it establishes beyond a reasonable doubt that A1, A2A, and A2B receptors play an important role in salt-sensitive hypertension. The present study therefore implies pharmacological approaches that may be used to prevent and treat hypertension. Finally, we should point out that a priori we had no strong rationale for including both females and males in this study. As it turned, the decision to do so was critically important. Therefore, the present study validates the recent recommendation by the National Institutes of Health to include sex as a biological variable whenever possible.
Supplementary Material
Novelty and Significance.
What Is New?
Deletion of A1 or A2B receptors protects against salt-induced hypertension in females, but not males.
Deletion of A1 and A2B receptors protects against salt-induced strokes in females, but not males.
Deletion of A2A receptors augments salt-induced hypertension, an effect which occurs in both females and males, yet is more pronounced in males.
What Is Relevant?
This study establishes beyond reasonable doubt that A1, A2A, and A2B receptors play a role in Dahl rat salt-sensitive hypertension; a finding that may apply to humans.
This study implies that dual blockade of A1 and A2B receptors may be an important pharmacological strategy to treat hypertension and prevent stroke, particularly in females.
The present study validates the recent recommendation by the National Institutes of Health to include sex as a biological variable whenever possible.
Summary.
The present study used three novel strains of Dahl SS rats to investigate the long-term influence of adenosine receptor subtypes in salt sensitive hypertension. The protocol design and statistical analysis allowed assessment of interactions among genotype, salt diet, and sex. Blood pressure was measured in all rats using the gold-standard method of radiotelemetry. The results indicated that: 1) deletion of A1 or A2B receptors protects against salt-induced hypertension in females, but not males; 2) deletion of A1 and A2B receptors protects against salt-induced strokes in females, but not males; and 3) deletion of A2A receptors augments salt-induced hypertension, an effect which occurs in both females and males, yet is more pronounced in males. These findings have important implications for: 1) the pathophysiology of salt sensitive hypertension; 2) the pharmacological treatment of salt sensitive hypertension; and 3) the importance of including sex as a biological variable in protocol design.
Acknowledgments
SOURCES OF FUNDING
The work was supported by the National Institutes of Health [DK091190, HL069846, DK068575, HL109002 and DK079307].
Footnotes
Disclosures: None
References
- 1.Jackson EK, Zhu C, Tofovic SP. Expression of adenosine receptors in the preglomerular microcirculation. Am J Physiol Renal Physiol. 2002;283:F41–51. doi: 10.1152/ajprenal.00232.2001. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Lai EY, Huang Y, Eisner C, Mizel D, Wilcox CS, Schnermann J. Renal afferent arteriolar and tubuloglomerular feedback reactivity in mice with conditional deletions of adenosine 1 receptors. Am J Physiol Renal Physiol. 2012;303:F1166–1175. doi: 10.1152/ajprenal.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SM, Mizel D, Qin Y, Huang Y, Schnermann J. Blood pressure, heart rate and tubuloglomerular feedback in A1AR-deficient mice with different genetic backgrounds. Acta Physiol. 2015;213:259–267. doi: 10.1111/apha.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oppermann M, Qin Y, Lai EY, Eisner C, Li L, Huang Y, Mizel D, Fryc J, Wilcox CS, Briggs J, Schnermann J, Castrop H. Enhanced tubuloglomerular feedback in mice with vascular overexpression of A1 adenosine receptors. Am J Physiol Renal Physiol. 2009;297:F1256–1264. doi: 10.1152/ajprenal.00264.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traynor T, Yang T, Huang YG, Arend L, Oliverio MI. Inhibition of adenosine-1 receptor-mediated preglomerular vasoconstriction in AT1A receptor-deficient mice. Am J Physiol Renal Physiol. 1998;275:F922. doi: 10.1152/ajprenal.1998.275.6.F922. [DOI] [PubMed] [Google Scholar]
- 6.Lai EY, Patzak A, Steege A, Mrowka R, Brown R, Spielmann N, Persson PB, Fredholm BB, Persson AEG. Contribution of adenosine receptors in the control of arteriolar tone and adenosine-angiotensin II interaction. Kidney Int. 2006;70:690–698. doi: 10.1038/sj.ki.5001650. [DOI] [PubMed] [Google Scholar]
- 7.Jackson EK, Cheng D, Mi Z, Verrier JD, Janesko-Feldman K, Kochanek PM. Role of A1 receptors in renal sympathetic neurotransmission in the mouse kidney. Am J Physiol Renal Physiol. 2012;303:F1000–1005. doi: 10.1152/ajprenal.00363.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson EK, Cheng D, Tofovic SP, Mi Z. Endogenous adenosine contributes to renal sympathetic neurotransmission via postjunctional A1 receptor-mediated coincident signaling. Am J Physiol Renal Physiol. 2012;302:F466–476. doi: 10.1152/ajprenal.00495.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Yang JN, Arner A, Boels PJ, Fredholm BB. Adenosine A1 receptors and vascular reactivity. Acta Physiol. 2010;199:211–220. doi: 10.1111/j.1748-1716.2010.02093.x. [DOI] [PubMed] [Google Scholar]
- 10.Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2005;288:H1411–1416. doi: 10.1152/ajpheart.00684.2004. [DOI] [PubMed] [Google Scholar]
- 11.Teng B, Fil D, Tilley SL, Ledent C, Krahn T, Mustafa SJ. Functional and RNA expression profile of adenosine receptor subtypes in mouse mesenteric arteries. J Cardiovasc Pharmacol. 2013;61:70–76. doi: 10.1097/FJC.0b013e318278575e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav VR, Hong KL, Zeldin DC, Nayeem MA. Vascular endothelial over-expression of soluble epoxide hydrolase (Tie2-sEH) enhances adenosine A1 receptor-dependent contraction in mouse mesenteric arteries: role of ATP-sensitive K+ channels. Mol Cell Biochem. 2016;422:197–206. doi: 10.1007/s11010-016-2821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santiago FE, Fior-Chadi DR, Carrettiero DC. Alpha2-adrenoceptor and adenosine A1 receptor within the nucleus tractus solitarii in hypertension development. Auton Neurosci. 2015;187:36–44. doi: 10.1016/j.autneu.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Kuan CJ, Herzer WA, Jackson EK. Cardiovascular and renal effects of blocking A1 adenosine receptors. J Cardiovasc Pharmacol. 1993;21:822. doi: 10.1097/00005344-199305000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Kost CK, Herzer WA, Rominski BR, Mi Z, Jackson EK. Diuretic response to adenosine A1 receptor blockade in normotensive and spontaneously hypertensive rats: role of pertussis toxin-sensitive G-proteins. J Pharmacol Exp Ther. 2000;292:752. [PubMed] [Google Scholar]
- 16.Rieg T, Steigele H, Schnermann J, Richter K, Osswald H, Vallon V. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J Pharmacol Exp Ther. 2005;313:403–409. doi: 10.1124/jpet.104.080432. [DOI] [PubMed] [Google Scholar]
- 17.Modlinger PS, Welch WJ. Adenosine A1 receptor antagonists and the kidney. Curr Opin Nephrol Hypertens. 2003;12:497–502. doi: 10.1097/00041552-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 19.Tang L, Parker M, Fei Q, Loutzenhiser R. Afferent arteriolar adenosine A2a receptors are coupled to KATP in in vitro perfused hydronephrotic rat kidney. Am J Physiol. 1999;277:F926–933. doi: 10.1152/ajprenal.1999.277.6.F926. [DOI] [PubMed] [Google Scholar]
- 20.Liclican EL, McGiff JC, Pedraza PL, Ferreri NR, Falck JR, Carroll MA. Exaggerated response to adenosine in kidneys from high salt-fed rats: role of epoxyeicosatrienoic acids. Am J Physiol Renal Physiol. 2005;289:F386–392. doi: 10.1152/ajprenal.00421.2004. [DOI] [PubMed] [Google Scholar]
- 21.Carroll MA, Cheng MK, Liclican EL, Li J, Doumad AB, McGiff JC. Purinoceptors in renal microvessels: adenosine-activated and cytochrome P450 monooxygenase-derived arachidonate metabolites. Pharmacol Rep. 2005;57(Suppl):191–195. [PubMed] [Google Scholar]
- 22.Carlstrom M, Wilcox CS, Welch WJ. Adenosine A2A receptor activation attenuates tubuloglomerular feedback responses by stimulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol. 2011;300:F457–464. doi: 10.1152/ajprenal.00567.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talukder MA, Morrison RR, Ledent C, Mustafa SJ. Endogenous adenosine increases coronary flow by activation of both A2A and A2B receptors in mice. J Cardiovasc Pharmacol. 2003;41:562–570. doi: 10.1097/00005344-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 24.de Brito MT, Canto A, Correia JH, Cunha RA, Marques MC. Adenosine A2A receptors in portal hypertension: their role in the abnormal response to adenosine of the cranial mesenteric artery in rabbits. Br J Pharmacol. 2002;135:1324–1330. doi: 10.1038/sj.bjp.0704575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao G, Linke A, Xu X, Ochoa M, Belloni F, Belardinelli L, Hintze TH. Comparative profile of vasodilation by CVT-3146, a novel A2A receptor agonist, and adenosine in conscious dogs. J Pharmacol Exp Ther. 2003;307:182–189. doi: 10.1124/jpet.103.053306. [DOI] [PubMed] [Google Scholar]
- 26.Maimon N, Titus PA, Sarelius IH. Pre-exposure to adenosine, acting via A2A receptors on endothelial cells, alters the protein kinase A dependence of adenosine-induced dilation in skeletal muscle resistance arterioles. J Physiol. 2014;592:2575–2590. doi: 10.1113/jphysiol.2013.265835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray CJ, Marshall JM. Elucidation in the rat of the role of adenosine and A2A-receptors in the hyperaemia of twitch and tetanic contractions. J Physiol. 2009;587:1565–1578. doi: 10.1113/jphysiol.2008.163683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponnoth DS, Sanjani MS, Ledent C, Roush K, Krahn T, Mustafa SJ. Absence of adenosine-mediated aortic relaxation in A2A adenosine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2009;297:H1655–1660. doi: 10.1152/ajpheart.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su Y, Jackson EK, Gorelik E. Receptor desensitization and blockade of the suppressive effects of prostaglandin E(2) and adenosine on the cytotoxic activity of human melanoma-infiltrating T lymphocytes. Cancer Immunology, Immunotherapy. 2011;60:111–122. doi: 10.1007/s00262-010-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raskovalova T, Lokshin A, Huang X, Su Y, Mandic M, Zarour HM, Jackson EK, Gorelik E. Inhibition of cytokine production and cytotoxic activity of human antimelanoma specific CD8+ and CD4+ T lymphocytes by adenosine-protein kinase A type I signaling. Cancer Research. 2007;67:5949–5956. doi: 10.1158/0008-5472.CAN-06-4249. [DOI] [PubMed] [Google Scholar]
- 31.Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285:7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohta A, Lukashev D, Jackson EK, Fredholm BB, Sitkovsky M. 1,3,7-trimethylxanthine (caffeine) may exacerbate acute inflammatory liver injury by weakening the physiological immunosuppressive mechanism. J Immunol. 2007;179:7431–7438. doi: 10.4049/jimmunol.179.11.7431. [DOI] [PubMed] [Google Scholar]
- 33.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caillon A, Mian MOR, Fraulob-Aquino JC, Huo KG, Barhoumi T, Ouerd S, Sinnaeve PR, Paradis P, Schiffrin EL. gammadelta T Cells Mediate Angiotensin II-Induced Hypertension and Vascular Injury. Circulation. 2017;135:2155–2162. doi: 10.1161/CIRCULATIONAHA.116.027058. [DOI] [PubMed] [Google Scholar]
- 35.Sun XN, Li C, Liu Y, Du LJ, Zeng MR, Zheng XJ, Zhang WC, Liu Y, Zhu M, Kong D, Zhou L, Lu L, Shen ZX, Yi Y, Du L, Qin M, Liu X, Hua Z, Sun S, Yin H, Zhou B, Yu Y, Zhang Z, Duan SZ. T-Cell mineralocorticoid receptor controls blood pressure by regulating interferon-gamma. Circ Res. 2017;120:1584–1597. doi: 10.1161/CIRCRESAHA.116.310480. [DOI] [PubMed] [Google Scholar]
- 36.Wade B, Abais-Battad JM, Mattson DL. Role of immune cells in salt-sensitive hypertension and renal injury. Curr Opin Nephrol Hypertens. 2016;25:22–27. doi: 10.1097/MNH.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW., Jr Increased Perfusion Pressure Drives Renal T-Cell Infiltration in the Dahl Salt-Sensitive Rat. Hypertension. 2017;70:543–551. doi: 10.1161/HYPERTENSIONAHA.117.09208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng MG, Navar LG. Afferent arteriolar vasodilator effect of adenosine predominantly involves adenosine A2B receptor activation. Am J Physiol Renal Physiol. 2010;299:F310–315. doi: 10.1152/ajprenal.00149.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Gowelli HM, El-Gowilly SM, Elsalakawy LK, El-Mas MM. Nitric oxide synthase/K+ channel cascade triggers the adenosine A2B receptor-sensitive renal vasodilation in female rats. Eur J Pharmacol. 2013;702:116–125. doi: 10.1016/j.ejphar.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 40.Morrison RR, Talukder MA, Ledent C, Mustafa SJ. Cardiac effects of adenosine in A2A receptor knockout hearts: uncovering A2B receptors. Am J Physiol Heart Circ Physiol. 2002;282:H437–444. doi: 10.1152/ajpheart.00723.2001. [DOI] [PubMed] [Google Scholar]
- 41.Sanjani MS, Teng B, Krahn T, Tilley S, Ledent C, Mustafa SJ. Contributions of A2A and A2B adenosine receptors in coronary flow responses in relation to the KATP channel using A2B and A2A/2B double-knockout mice. Am J Physiol Heart Circ Physiol. 2011;301:H2322–2333. doi: 10.1152/ajpheart.00052.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ansari HR, Nadeem A, Talukder MA, Sakhalkar S, Mustafa SJ. Evidence for the involvement of nitric oxide in A2B receptor-mediated vasorelaxation of mouse aorta. Am J Physiol Heart Circ Physiol. 2007;292:H719–725. doi: 10.1152/ajpheart.00593.2006. [DOI] [PubMed] [Google Scholar]
- 43.Zou AP, Nithipatikom K, Li PL, Cowley AW. Role of renal medullary adenosine in the control of blood flow and sodium excretion. Am J Physiol Regul Integr Comp Physiol. 1999;276:R790. doi: 10.1152/ajpregu.1999.276.3.R790. [DOI] [PubMed] [Google Scholar]
- 44.Zou AP, Wu F, Li PL, Cowley AW. Effect of chronic salt loading on adenosine metabolism and receptor expression in renal cortex and medulla in rats. Hypertension. 1999;33:511. doi: 10.1161/01.hyp.33.1.511. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R, Sun K, Glover L, Grenz A, Sun H, Tao L, Zhang W, Colgan SP, Blackburn MR, Eltzschig HK, Kellems RE, Xia Y. Elevated ecto-5′-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ Res. 2013;112:1466–1478. doi: 10.1161/CIRCRESAHA.111.300166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown RD, Thoren P, Steege A, Mrowka R, Sallstrom J, Skott O, Fredholm BB, Persson AE. Influence of the adenosine A1 receptor on blood pressure regulation and renin release. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1324–1329. doi: 10.1152/ajpregu.00313.2005. [DOI] [PubMed] [Google Scholar]
- 47.Kim SM, Mizel D, Huang YG, Briggs JP, Schnermann J. Adenosine as a mediator of macula densa-dependent inhibition of renin secretion. Am J Physiol Renal Physiol. 2006;290:F1016–1023. doi: 10.1152/ajprenal.00367.2005. [DOI] [PubMed] [Google Scholar]
- 48.Gao X, Patzak A, Sendeski M, Scheffer PG, Teerlink T, Sallstrom J, Fredholm BB, Persson AE, Carlstrom M. Adenosine A1-receptor deficiency diminishes afferent arteriolar and blood pressure responses during nitric oxide inhibition and angiotensin II treatment. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1669–1681. doi: 10.1152/ajpregu.00268.2011. [DOI] [PubMed] [Google Scholar]
- 49.Lee DL, Bell TD, Bhupatkar J, Solis G, Welch WJ. Adenosine A1-receptor knockout mice have a decreased blood pressure response to low-dose ANG II infusion. Am J Physiol Regul Integr Comp Physiol. 2012;303:R683–688. doi: 10.1152/ajpregu.00116.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakata M, Sei H, Eguchi N, Morita Y, Urade Y. Arterial pressure and heart rate increase during REM sleep in adenosine A2A-receptor knockout mice, but not in wild-type mice. Neuropsychopharmacology. 2005;30:1856–1860. doi: 10.1038/sj.npp.1300727. [DOI] [PubMed] [Google Scholar]
- 51.Nayak S, Khan MA, Wan TC, Pei H, Linden J, Dwinell MR, Geurts AM, Imig JD, Auchampach JA. Characterization of Dahl salt-sensitive rats with genetic disruption of the A2B adenosine receptor gene: implications for A2B adenosine receptor signaling during hypertension. Purinergic Signal. 2015;11:519–531. doi: 10.1007/s11302-015-9470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geurts AM, Mattson DL, Liu P, Cabacungan E, Skelton MM, Kurth TM, Yang C, Endres BT, Klotz J, Liang M, Cowley AW., Jr Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Hypertension. 2015;65:447–455. doi: 10.1161/HYPERTENSIONAHA.114.04179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shryock JC, Belardinelli L. Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. Am J Cardiol. 1997;79:2–10. doi: 10.1016/s0002-9149(97)00256-7. [DOI] [PubMed] [Google Scholar]
- 54.Jacobson KA, Knutsen LJS. P1 and P2 purine and pyrimidine receptor ligands. In: Abbracchio MP, Williams M, editors. Purinergic and Pyrmidinergic Signalling I. Berlin: Springer-Verlag; 2001. pp. 129–175. [Google Scholar]
- 55.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Exogenous and endogenous adenosine inhibits fetal calf serum–induced growth of rat cardiac fibroblasts: role of A2B receptors. Circulation. 1997;96:2656–2666. doi: 10.1161/01.cir.96.8.2656. [DOI] [PubMed] [Google Scholar]
- 56.Siragy HM, Linden J. Sodium intake markedly alters renal interstitial fluid adenosine. Hypertension. 1996;27:404. doi: 10.1161/01.hyp.27.3.404. [DOI] [PubMed] [Google Scholar]
- 57.Tofovic SP, Salah EM, Smits GJ, Whalley ET, Ticho B, Deykin A, Jackson EK. Dual A1/A2B receptor blockade improves cardiac and renal outcomes in a rat model of heart failure with preserved ejection fraction. J Pharmacol Exp Ther. 2016;356:333–340. doi: 10.1124/jpet.115.228841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiesman WF, Zhao J, Conlon PR, Dowling JE, Petter RC, Lutterodt F, Jin X, Smits G, Fure M, Jayaraj A, Kim J, Sullivan G, Linden J. Potent and orally bioavailable 8-bicyclo[2.2.2]octylxanthines as adenosine A1 receptor antagonists. J Med Chem. 2006;49:7119–7131. doi: 10.1021/jm0605381. [DOI] [PubMed] [Google Scholar]
- 59.Pettinger WA. Hypertension’s 3 Dilemmas and 3 Solutions: Pharmacology of the Kidney in Hypertension. J Cardiovasc Pharmacol. 2017;69:129–139. doi: 10.1097/FJC.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.