Abstract
Incremental improvements in the treatment of children and adolescents with cancer have led to 5-year survival rates reaching nearly 85%. In the past decade, impressive progress has been made in understanding the biology of many pediatric cancers. With that understanding, multiple new agents have become available that offer the promise of more-effective and less-toxic treatment. These include agents that target various cell surface antigens and engage the adaptive immune system, as well as those that interfere with key signaling pathways involved in tumor development and growth. For local control, surgery and radiation techniques also have evolved, becoming less invasive or featuring new techniques and particles that more precisely target the tumor and limit the dose to normal tissue. Nevertheless, targeted agents, like conventional chemotherapy, radiotherapy, and surgery, may have off-target effects and deserve long-term follow-up of their safety and efficacy. These include injury to the endocrine, cardiovascular, and immunologic systems. New radiation and surgical techniques that theoretically reduce morbidity and improve long-term quality of life must also be validated with actual patient outcomes. Finally, with advances in genomics, information on host susceptibility to late effects is beginning to emerge. Such knowledge, coupled with improved metrics that better describe the spectrum of potential late effects across the entire lifespan, can lead to the development of decision models that project the potential long-term health outcomes associated with various treatment and follow-up strategies. These developments will help extend the current focus on precision medicine to precision survivorship, where clinicians, patients, and families will have a better grasp of the potential risks, benefits, and tradeoffs associated with the growing number of cancer treatment options.
INTRODUCTION
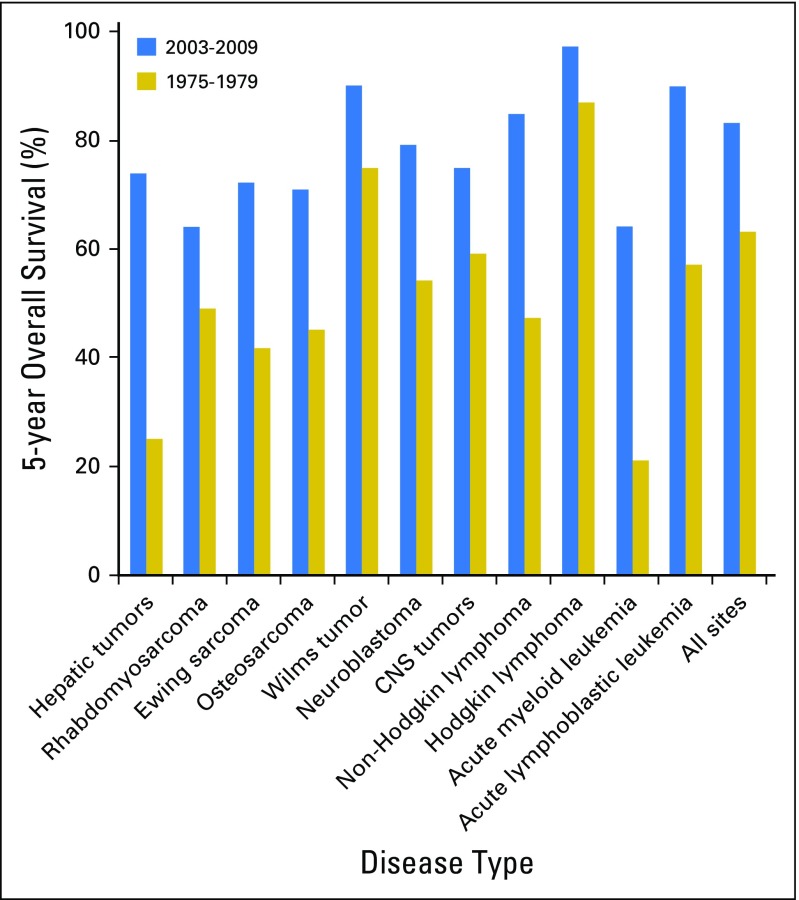
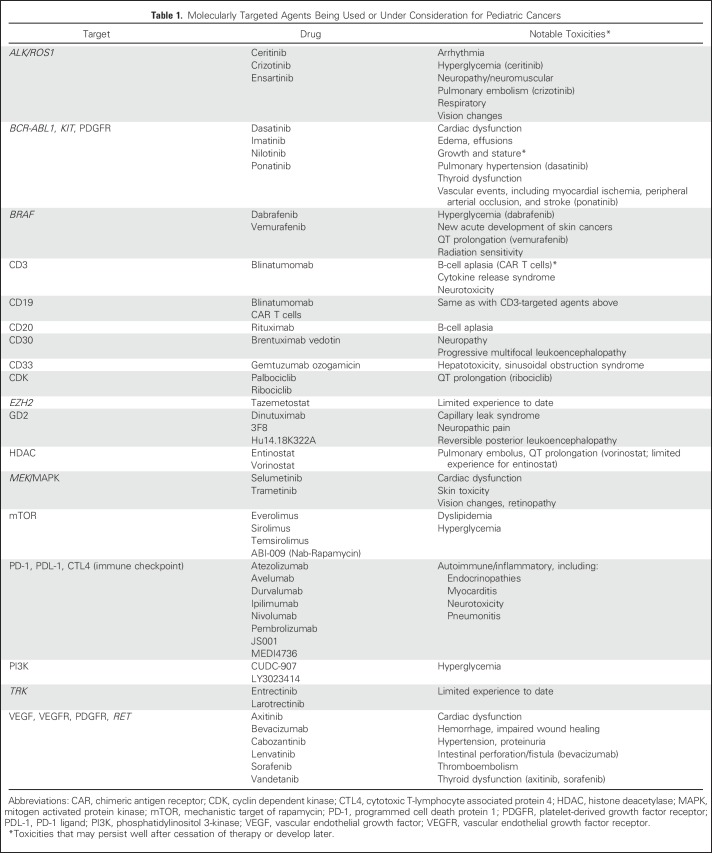
Almost 85% of children diagnosed with cancer will become long-term survivors.1 Nevertheless, many cancers remain difficult to treat, with survival rates < 70% (Fig 1). Other cancers are highly curable but still rely on treatments that cause significant long-term toxicities. In recent years, a better understanding of the biology of many pediatric cancers has led to the development of multiple new agents that offer the promise of more-effective and less-toxic treatment (Table 1). For local control, surgery and radiotherapy also have evolved, becoming less invasive or featuring new techniques and particles that more precisely target the tumor and limit the dose to normal tissues. Nevertheless, targeted agents, like conventional chemotherapy, radiotherapy, and surgery, may have off-target effects, and it will be important to follow children long-term for incidental late effects and to develop precision survivorship. This is survivorship that incorporates host genetics and statistical approaches that improve clinicians’ and patients’ understanding of the potential trade-offs between different treatment regimens with similar oncologic efficacy but varying toxicity profiles.
Fig 1.
Five-year survival rates for two time periods for patients with pediatric cancer diagnosed from birth to 19 years old. Five-year survival is presented for all sites (International Classification of Childhood Cancers) and specific histologic subtypes contrasting outcome for children with cancer diagnosed between 1975 and 1979 with those with cancer diagnosed between 2003 and 2009. Data obtained from the National Cancer Institute SEER program from nine SEER registries on the basis of patient cases observed through 2010. Reprinted with permission.1 2014 American Society of Clinical Oncology. All rights reserved.
Table 1.
Molecularly Targeted Agents Being Used or Under Consideration for Pediatric Cancers
EVOLVING APPROACHES FOR PEDIATRIC CANCER TREATMENT
Hematologic Malignancies
Although outcomes for pediatric leukemia and lymphoma have improved substantially due in large part to combination chemotherapy, acute myeloid leukemia (AML) and relapsed disease in general remain difficult to cure. However, new targeted agents are leading to promising options for some of these patients. An initial success came in Philadelphia-positive acute lymphoblastic leukemia (Ph-positive ALL), where imatinib plus chemotherapy improved the 3-year event-free survival to 80% (v 35% historically).2 Many of these patients are now able to avoid hematopoietic cell transplantation. With the discovery of a large subset of patients with high-risk ALL with gene expression profiles similar to Ph-positive ALL, current studies are now incorporating tyrosine kinase inhibitors (TKI) with up-front chemotherapy.3 Combining TKIs with conventional chemotherapy is also occurring in AML and lymphoma treatments. On the basis of promising adult data,4 sorafenib is being tested in children with newly diagnosed AML with FLT3-internal tandem duplication mutations. Crizotinib, a TKI with specificity for anaplastic large-cell kinase, has demonstrated 90% response rate with durable remissions as a single agent in children with relapsed anaplastic large-cell lymphoma.5
Adding antibodies to chemotherapy also is improving the treatment of leukemias and lymphomas. In mature B-cell lymphoma, rituximab plus chemotherapy conferred a 1-year event-free survival rate of 94.2% versus 81.5% with chemotherapy alone.6 Brentuximab, an antibody drug conjugate targeting CD30, has shown high response rates in both anaplastic large-cell and Hodgkin lymphomas and has largely become standard of care for relapsed and refractory Hodgkin.7,8 It is now being tested as part of up-front treatment. Gemtuzumab ozogamicin (CD33 with calicheamicin) has been making a resurgence in the treatment of AML,9 and the development of inotuzumab ozogamicin (CD22 with calicheamicin) is showing promise in ALL.10 Antibody therapy targeting immune checkpoints may also become more commonplace. Pembrolizumab targets programmed cell death protein 1 (PD-1) and has been approved by the US Food and Drug Administration for refractory or relapsed Hodgkin lymphoma, while ongoing studies are investigating both PD-1 and cytotoxic T-lymphocyte associated protein 4 (CTLA4) inhibition.
T-cell–based immunotherapy is also undergoing intensive investigation. In phase II studies, blinatumomab, a bispecific antibody targeting both CD19 and CD3 (which facilitates T-cell–directed killing of CD19-positive leukemia cells), has demonstrated a 27% complete response.11 Alternatively, genetically engineered chimeric antigen receptor (CAR) T cells allow for the direct targeting of tumor cells, with the potential for lifelong activity through in vivo persistence of the CAR T cells. Trials in relapsed pediatric ALL have shown complete remission rates > 80%, and CAR T-cell technology is being applied to target other liquid tumors as well.12-14
Solid Tumors
Outcomes for pediatric solid tumors also have improved over the past decades. However, significant disparities remain, and treatment of patients with metastases or relapse remains challenging. Nevertheless, treatment of high-risk neuroblastoma exemplifies how a deeper understanding of tumor biology can improve survival. On a backbone of cytotoxic chemotherapy, radiotherapy, and surgery, dinutuximab, a monoclonal antibody targeting disialoganglioside GD2 expressed on neuroblastoma, combined with immunomodulatory (aldesleukin, sargramostim) and differentiating (isotretinoin) agents, have improved the 5-year overall survival of children with high-risk neuroblastoma from < 30% to approximately 50%.15,16 However, acute toxicities are extensive, including capillary leak and neuropathic pain.
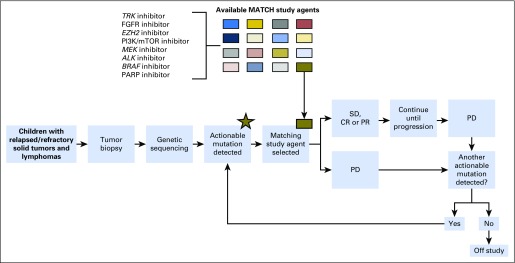
Clinical trials have recently demonstrated that identifying genomic alterations in pediatric solid tumors and selecting appropriately targeted therapy are feasible.17 The National Cancer Institute and the Children’s Oncology Group are conducting Pediatric MATCH (Molecular Analysis for Therapy Choice), a phase II basket trial, in which patients with relapsed solid tumors receive drugs paired to specific tumor molecular abnormalities (Fig 2).17 Other targeted agents being tested in children include larotrectinib for infants with fibrosarcoma or mesoblastic nephroma harboring NTRK fusions18; crizotinib for neuroblastoma, anaplastic large-cell lymphoma, or inflammatory myofibroblastic tumors associated with anaplastic lymphoma kinase aberrations5; and the RET inhibitor vandetanib for medullary thyroid cancers associated with multiple endocrine neoplasia 2B and germline RET mutations.19 The drug development paradigm for targeted agents encourages aggressive symptom management of on-target toxicity, because manifestation of these toxicities can sometimes correlate with increased drug efficacy.20
Fig 2.
National Cancer Institute–Pediatric MATCH (Molecular Analysis for Therapeutic Choice) trial schema. CR, complete response; FGFR, fibroblast growth factor receptor; mTOR, mechanistic target of rapamycin; PARP, polyadenosine diphosphate-ribose polymerase; PD, progressive disease; PI3K, phosphatidylinositol 3-kinase; PR, partial response; SD, stable disease. Reprinted with permission.17
Investigation of immune checkpoint inhibitors is ongoing.21 Thus far, serious immune-related toxicities observed in adults seem to be less common in children.22,23 However, duration of exposure of these inhibitors in children has been limited to date. New cytotoxic agents with novel mechanisms of action, such as selinexor,24 or new formulations of existing cytotoxic agents, such as doxorubicin,25 irinotecan,26 or tubulin-binding agents,27 are in clinical trials. Antibody drug conjugates that target highly potent cytotoxic drugs to cell surface receptors such as lorvotuzumab mertansine (linking an antimitotic agent, DM1, to an anti-CD56 antibody) also are in development.28 As new agents emerge for pediatric solid tumors, it will be essential to systematically follow patients long term to determine if there are significant expected and unexpected late effects. An example of this is the Children’s Oncology Group study ALTE15N2 (ClinicalTrials.gov identifier: NCT03057626), which seeks to determine the full spectrum of late effects in the emerging generation of high-risk survivors of neuroblastoma treated with contemporary therapy.
Radiotherapy
Although use of radiotherapy has declined, it remains an essential part of treatment of many pediatric cancers.29,30 Children treated with radiotherapy are most commonly given external beam radiation, although brachytherapy, radioisotopes, or combinations of these continue to be appropriate in select settings. The delivery of external beam radiation has been refined with conformal techniques that use modern imaging methods (primarily computed tomography) to provide three-dimensional definitions of the target and surrounding normal tissues and information about tissue density and depth from the skin. Radiation beams are then created that conform the dose distribution to the target.
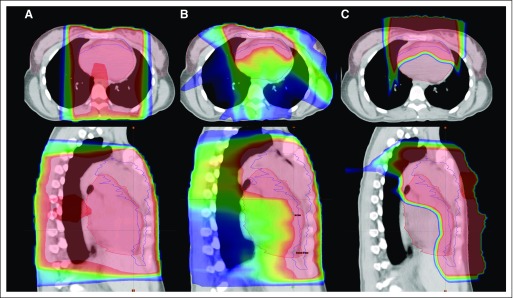
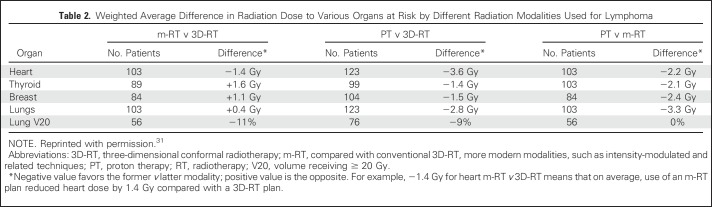
Intensity-modulated radiation therapy (IMRT) is a three-dimensional conformal radiation therapy planning and delivery tool that more precisely shapes the radiation dose distribution around the target, which can help further decrease the dose to adjacent normal structures (Fig 3). With IMRT, the physician determines radiation treatment parameters to maximize dose to the target and minimize dose to normal tissues, and the planning algorithm optimizes the adherence to these parameters by modifying the beam spatially and/or temporally. However, IMRT generally results in a greater deposition of low doses to normal tissues surrounding the target (because of the multiple beams used; Table 2).31 This conceptually could increase the risk of second malignancies. However, clinical data supporting this concern are lacking, whereas the general concept that decreasing dose to adjacent organs is beneficial has been validated.32,33
Fig 3.
Radiotherapy treatment plan for representative patient with Hodgkin lymphoma across different radiation modalities. (A) Three-dimensional conformal radiotherapy, (B) intensity-modulated radiotherapy, (C) proton therapy. The colorwash dose distributions are indicated by (from lower to higher doses) blue, green, yellow, and red shading. The thin colored lines outline the heart (red), breasts (pink), and the clinical target volume (blue). Top row shows axial views and bottom row shows sagittal views. Reprinted with permission.34
Table 2.
Weighted Average Difference in Radiation Dose to Various Organs at Risk by Different Radiation Modalities Used for Lymphoma
Standard radiation is delivered with photons created by linear accelerators. Another approach is charged-particle irradiation (eg, protons). Charged particles stop abruptly in tissues, so there is less exit radiation dose through normal tissue. Although theoretically promising (Fig 3; Table 2),34 the benefits of protons in reducing radiation-associated malignancies and other late effects remain under study.31,35,36 Moreover, the energy deposition into normal tissues with the use of proton therapy is still being studied; increased energy deposition (or its biologic consequences) may be responsible for adverse effects.37
PENTEC (Pediatric Normal Tissue Effects in the Clinic) is a key initiative within the radiation oncology community to develop evidence-based guidelines for radiation dose-volume tolerances in multiple organ systems of children. These guidelines will consider the age-associated vulnerability of these normal tissues to the effects of radiation plus the impact of chemotherapy, surgery, and host genetics.38 Moreover, the possibility that radiation can augment systemic immune responses is being explored.39
Surgery
Surgical procedures performed for the diagnosis, staging, and treatment of childhood cancer can lead to long-term functional consequences, disfiguration with psychosocial impact, and late-occurring complications. A desire to minimize this impact and preserve long-term quality of life has been implicit within many modern surgical trends. These include advancements with laparoscopy and thoracoscopy, limb salvage procedures, and image-guided biopsy techniques. Relative to open operations, laparoscopy and thoracoscopy can minimize long-term effects by improving functional outcomes (eg, improved chest wall growth/function), limiting psychosocial impact (eg, smaller scars/less disfiguration), and minimizing the frequency of late complications (eg, late intestinal obstructions known to occur in 5% of children with abdominopelvic tumors).40,41 In addition, advancements in limb salvage techniques, now possible in > 80% of cases of osteosarcoma at specialized centers, may improve long-term function for children with extremity tumors.42 This includes use of expandable internal prostheses and novel operations such as rotationplasty.43 Finally, improvements in image-guided biopsy techniques and pathologic interpretation have reduced the need for large, morbid operations for diagnosis or staging, sparing children the potential long-term consequences of these procedures.44
However, as surgical techniques evolve to reduce long-term effects, it is critical to ensure that oncologic outcomes are not compromised. This includes studying the use of laparoscopy/thoracoscopy for Wilms tumor and osteosarcoma pulmonary metastases.41,45 Verification that long-term functional and psychosocial outcomes truly are superior (eg, limb-sparing techniques v amputation) also is necessary.43,46
POTENTIAL NEW LATE EFFECTS
Endocrine
Select endocrine late effects are beginning to emerge among children treated with targeted agents. TKIs have been associated with growth deceleration and alterations in bone mineral and thyroid metabolism. Children treated with imatinib for chronic myeloid leukemia (CML) have demonstrated varying degrees of growth restriction. Effects on growth seem to be more significant when treatment is initiated before puberty.47 The mechanism for the growth deceleration is unclear, but may be due to disrupted growth hormone signaling, inadequate signal transduction through the insulin-like growth factor 1 (IGF-1) receptor (a tyrosine kinase receptor), or inhibition of platelet-derived growth factor receptor signaling and disrupted chondrocyte recruitment at the growth plates.48
Off-target inhibition by TKIs of platelet-derived growth factor receptor and the macrophage colony-stimulating factor receptor c-fms may alter bone remodeling by affecting the differentiation and activity of osteoblasts and osteoclasts.48 Reduced serum calcium and phosphorus levels, vitamin D deficiency, and secondary hyperparathyroidism have been noted in children with CML receiving imatinib.49 However, these derangements do not always correlate with low bone mineral density.50 Further studies are needed to elucidate any loss of final adult height in children treated with imatinib. However, routine measurements of IGF-1 or formal growth hormone stimulation testing are not currently recommended, particularly given concerns about growth hormone exposure during active cancer treatment.
Although case series in children treated with imatinib have reported normal thyroid function, other TKIs may affect thyroid function. In adult studies, nilotinib, dasatinib, sunitinib, and sorafenib have been associated with de novo hypothyroidism, variably preceded by hyperthyroidism.51 Children receiving TKIs should have their thyroid function closely monitored.
At present, the effects of targeted agents on male and female reproductive function are largely unknown. In CML, pregnancy presents specific management and therapeutic challenges. There are limited data on the safety of TKIs in pregnancy and their effects on fertility. There have been some reports of congenital malformations and spontaneous abortions associated with imatinib therapy.52 There are also concerns that inhibitors of angiogenesis (eg, bevacizumab, a vascular endothelial growth factor [VEGF] inhibitor) may affect ovarian follicle development.53 It will be important to assess long-term gonadal function and fertility of patients receiving new targeted agents. Patients of childbearing age should be made aware of established fertility options that are available, including semen cryopreservation, ovarian or oocyte retrieval and storage, and embryo cryopreservation.54
Finally, late endocrine effects of immune checkpoint inhibitors in children are unknown, but these agents have been associated with hypophysitis and anterior pituitary deficiencies in adults.55 Thyrotropin deficiency has been the most common, followed by adrenocorticotropin and gonadotropin deficiencies. Although thyrotropin deficiency may resolve, adrenocorticotropin deficiency often persists and requires long-term steroid replacement.
Cardiovascular
Although left ventricular dysfunction/cardiomyopathy and accelerated atherosclerosis are known complications of anthracyclines and chest radiation, respectively,56 the late cardiovascular effects of novel agents described above remain largely unknown in survivors of childhood cancer. Most cardiovascular events reported in adult patients with cancer have typically occurred while receiving therapy or soon after. TKIs, particularly later-generation agents such as nilotinib, dasatinib, and ponatinib, have been associated with an increased risk of pulmonary hypertension (dasatinib) and vascular events (particularly ponatinib), including myocardial ischemia, peripheral arterial occlusive disease, and stroke.57 Crizotinib has been associated with arrhythmia in adults.58 VEGF inhibitors (eg, bevacizumab) and TKIs with anti-VEGF activity (eg, sunitinib, sorafenib) have been strongly associated with hypertension in adult patients and, less commonly, thromboembolic events and heart failure.58 Hypertension with these agents has only been occasionally reported in pediatric patients, but nowhere near the rates seen in adults.59 Mechanistic target of rapamycin inhibitors (eg, temsirolimus) can cause dyslipidemia and hyperglycemia.60 Finally, rare autoimmune myocarditis has been reported after immune checkpoint inhibitor therapy in adults.61
Increased emphasis also has been placed on developing less cardiotoxic versions of conventional cytotoxic agents and effective prevention strategies. Given data that traditional cardiovascular risk factors (eg, hypertension, dyslipidemia, diabetes) synergistically potentiate the cardiotoxic effects of anthracycline and radiation therapies in children,62 improved control of these conditions should be a focus of survivorship efforts.
Immunologic
B-cell depletion occurs with agents that target B-cell antigens (eg, rituximab, blinatumomab). Although B-cell aplasia is usually short term, there is a potential for long-term B-cell aplasia in patients who have CAR T-cell persistence.12-14 The health impact of prolonged B-cell aplasia is unclear but believed to be minimal so long as affected patients receive ongoing immunoglobulin replacement to minimize infectious risks. However, long-term financial costs may be important as these treatments become more widespread. To date, there have not been any reports of lymphoproliferative disorders or secondary malignancies directly related to CAR T-cell products. In addition, with gene therapy as a new field, it is possible new late effects will be uncovered. Finally, even conventional cytotoxic therapy may be associated with alterations in immunity that persist well after neutrophil recovery (eg, selective B- and T-cell subpopulations may remain depressed years after therapy).63 Adding immune-modulating therapies may further affect normal immune reconstitution.
Development of Precision Survivorship
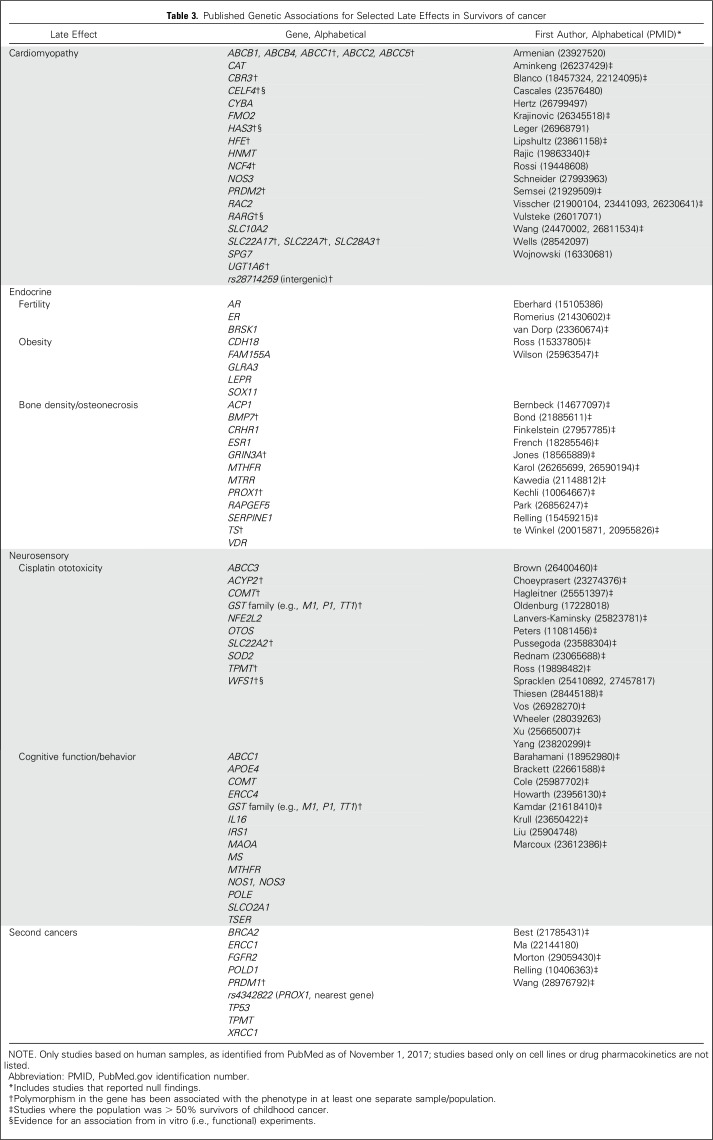
Five-year survival is a common oncology benchmark. However, with most children expected to survive decades after therapy, consideration of longer-term health quality should be emphasized. Metrics such as the cumulative burden of disease may provide a more complete picture of long-term health after cancer therapy.64 Given the interindividual variation in risks of late effects, further insights also may arise from genomics (Table 3). Large-scale genome-wide association studies have identified potential markers for multiple adverse outcomes, including cardiotoxicity,65,66 secondary cancers,67,68 ototoxicity,69 and reduced ovarian reserve.70 Using such genetic information may eventually help predict risk, individualize therapy, and reduce adverse outcomes among children with cancer. However, many genetic findings need to be replicated, and there needs to be alternative but similarly effective cancer treatments for those at increased genetic risk for toxicities associated with standard treatment.
Table 3.
Published Genetic Associations for Selected Late Effects in Survivors of cancer
Although randomized clinical trials have been the gold standard for the investigation of new therapeutic approaches, the rarity of childhood cancer and the long latency needed to observe late effects limit the feasibility of prospective trials to evaluate a precision-medicine approach to survivorship. By synthesizing evidence from all available sources, including randomized trials, observational studies, meta-analyses, and expert opinion, decision models can provide a valuable analytic framework for simulating health outcomes associated with various treatment and follow-up strategies.71 Decision models have been used by the US Preventive Services Task Force to inform evidence-based recommendations, including the screening of common adult cancers and chronic disease prevention approaches.72
Decision models may be particularly well-suited to estimate the benefits, risks and trade-offs associated with alternative treatment approaches for childhood cancer, where short-term efficacy and late toxicity risks are uncertain, but informative data from large survivor cohort studies are available to augment expert opinion. Such models are typically based on outcomes such as life-years or quality-adjusted life-years and could also incorporate genetic information that enhances prediction of individual late effects.
In conclusion, although the majority of new agents are still primarily being tested in patients with relapsed disease as part of phase I and II studies, over time some agents will demonstrate sufficient promise to become more widely used in patients with newly diagnosed disease. Drugs such as imatinib, blinatumomab, brentuximab, dinutuximab, gemtuzumab ozogamicin, rituximab, and sorafenib have already achieved this milestone in pediatric oncology. Similarly, growing numbers of children are being treated with IMRT and proton therapy and experiencing less-invasive surgeries. Although these new agents, surgical and radiation techniques, and their combinations offer the promise of improving cancer outcomes for children, they may have unanticipated late effects. Children tend to have fewer medical comorbidities but also developing bodies, which impact their vulnerability to therapy and can make extrapolations from adult data problematic. Therefore, it will be important to maintain long-term follow-up of children being treated with these new technologies, particularly because the numbers of children exposed to any single agent or modality may be more limited than those treated historically when there were fewer therapeutic options. Resources to enable long-term follow-up are critical, because most oncology clinical trials have limited follow-up and focus more on acute toxicities. Leveraging population-based cohorts and registries can also help. Given the rarity of pediatric cancers, international collaboration is essential, particularly as treatments become more refined and individualized on the basis of tumor and host biology.73 Development of classification systems explicitly for late effects may increase the comparability of outcomes among future studies.74 Finally, with the advances in precision medicine, the concurrent development of precision survivorship is the logical outgrowth of efforts to better understand the spectrum of late effects, genetic susceptibility, and individual family/patient preferences toward the potential risks, benefits, and tradeoffs associated with different cancer treatment choices.
Footnotes
Supported in part by the US National Institutes of Health Grants No. CA180886 and CA55727.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Eric J. Chow
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
New Agents, Emerging Late Effects, and the Development of Precision Survivorship
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Eric J. Chow
No relationship to disclose
Zoltan Antal
Speakers' Bureau: Novo Nordisk
Louis S. Constine
Honoraria: UpToDate, Springer, Lippincott
Travel, Accommodations, Expenses: IBA
Rebecca Gardner
Honoraria: Novartis, Medscape
W. Hamish Wallace
No relationship to disclose
Brent R. Weil
No relationship to disclose
Jennifer M. Yeh
No relationship to disclose
Elizabeth Fox
Travel, Accommodations, Expenses: Helsinn Therapeutics
REFERENCES
- 1.Hudson MM, Link MP, Simone JV: Milestones in the curability of pediatric cancers. J Clin Oncol 32:2391-2397, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz KR, Bowman WP, Aledo A, et al. : Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: A children’s oncology group study. J Clin Oncol 27:5175-5181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts KG, Li Y, Payne-Turner D, et al. : Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med 371:1005-1015, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Röllig C, Serve H, Hüttmann A, et al. : Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): A multicentre, phase 2, randomised controlled trial. Lancet Oncol 16:1691-1699, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Mossé YP, Voss SD, Lim MS, et al. : Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: A Children’s Oncology Group study. J Clin Oncol 35:3215-3221, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Minard-Colin V, Auperin A, Pillon M, et al: Results of the randomized Intergroup trial Inter-B-NHL Ritux 2010 for children and adolescents with high-risk B-cell non-Hodgkin lymphoma (B-NHL) and mature acute leukemia (B-AL): Evaluation of rituximab (R) efficacy in addition to standard LMB chemotherapy (CT) regimen. J Clin Oncol 34, 2016 (suppl; abstr 10507)
- 7.Younes A, Gopal AK, Smith SE, et al. : Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 30:2183-2189, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pro B, Advani R, Brice P, et al. : Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: Results of a phase II study. J Clin Oncol 30:2190-2196, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Pollard JA, Loken M, Gerbing RB, et al. : CD33 expression and its association with gemtuzumab ozogamicin response: Results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol 34:747-755, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. : Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med 375:740-753, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Stackelberg A, Locatelli F, Zugmaier G, et al. : Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol 34:4381-4389, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Maude SL, Frey N, Shaw PA, et al. : Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507-1517, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. : T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 385:517-528, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner RA, Finney O, Annesley C, et al. : Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129:3322-3331, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu AL, Gilman AL, Ozkaynak MF, et al. : Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363:1324-1334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinto NR, Applebaum MA, Volchenboum SL, et al. : Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol 33:3008-3017, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seibel NL, Janeway K, Allen CE, et al. : Pediatric oncology enters an era of precision medicine. Curr Probl Cancer 41:194-200, 2017 [DOI] [PubMed] [Google Scholar]
- 18. Laetsch TW, DuBois SG, Nagasubramanian R, et al: A pediatric phase 1 study of larotrectinib, a highly selective inhibitor of the tropomyosin receptor kinase (TRK) family. J Clin Oncol 35, 2017 (suppl; abstr 10510)
- 19.Fox E, Widemann BC, Chuk MK, et al. : Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res 19:4239-4248, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rini BI, Cohen DP, Lu DR, et al. : Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 103:763-773, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto N, Park JR, Murphy E, et al. : Patterns of PD-1, PD-L1, and PD-L2 expression in pediatric solid tumors. Pediatr Blood Cancer 64:e26613, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Ciccarese C, Iacovelli R, Bria E, et al. : The incidence and relative risk of pulmonary toxicity in patients treated with anti-PD1/PD-L1 therapy for solid tumors: A meta-analysis of current studies. Immunotherapy 9:579-587, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Spain L, Walls G, Julve M, et al. : Neurotoxicity from immune-checkpoint inhibition in the treatment of melanoma: A single centre experience and review of the literature. Ann Oncol 28:377-385, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Gounder MM, Zer A, Tap WD, et al. : Phase IB study of selinexor, a first-in-class inhibitor of nuclear export, in patients with advanced refractory bone or soft tissue sarcoma. J Clin Oncol 34:3166-3174, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chawla SP, Papai Z, Mukhametshina G, et al. : First-line aldoxorubicin vs doxorubicin in metastatic or locally advanced unresectable soft-tissue sarcoma: A phase 2b randomized clinical trial. JAMA Oncol 1:1272-1280, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Norris RE, Shusterman S, Gore L, et al. : Phase 1 evaluation of EZN-2208, a polyethylene glycol conjugate of SN38, in children adolescents and young adults with relapsed or refractory solid tumors. Pediatr Blood Cancer 61:1792-1797, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Emambux S, Kind M, Le Loarer F, et al. : Clinical activity of eribulin in advanced desmoplastic small round-cell tumor. Anticancer Drugs 28:1053-1055, 2017 [DOI] [PubMed] [Google Scholar]
- 28. Geller JI, Pressey JG, Smith MA, et al: ADVl1522: a phase 2 study of IMGN901 (lorvotuzumab mertansine) in children with relaped or refractory Wilms tumor, rhabdomyosarcoma, neuroblastoma, leuropulomnary blastoma, malignant peripheral nerve sheath tumor (MPNST), and synovial sarcoma: A Children’s Oncology Group study. J Clin Oncol 35, 2017 (suppl; abstr 10537) [DOI] [PMC free article] [PubMed]
- 29.Jairam V, Roberts KB, Yu JB: Historical trends in the use of radiation therapy for pediatric cancers: 1973-2008. Int J Radiat Oncol Biol Phys 85:e151-e155, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haas-Kogan DA, Devine CA, Liu KX, et al. : A cautionary tale: Risks of radiation therapy de-escalation in pediatric malignancies. J Clin Oncol 35:2471-2472, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Tseng YD, Cutter DJ, Plastaras JP, et al. : Evidence-based review on the use of proton therapy in lymphoma from the Particle Therapy Cooperative Group (PTCOG) Lymphoma Subcommittee. Int J Radiat Oncol Biol Phys 99:825-842, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Tukenova M, Guibout C, Oberlin O, et al. : Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol 28:1308-1315, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Turcotte LM, Liu Q, Yasui Y, et al. : Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970-2015. JAMA 317:814-824, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. doi: 10.1016/j.ijrobp.2011.06.1959. Hoppe BS, Flampouri S, Su Z, et al: Consolidative involved-node proton therapy for stage IA-IIIB mediastinal Hodgkin lymphoma: Preliminary dosimetric outcomes from a phase II study. Int J Radiat Oncol Biol Phys 83:260-267, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Indelicato DJ, Merchant T, Laperriere N, et al. : Consensus report from the Stockholm pediatric proton therapy conference. Int J Radiat Oncol Biol Phys 96:387-392, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Yock TI, Yeap BY, Ebb DH, et al. : Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol 17:287-298, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Yock TI, Constine LS, Mahajan A: Protons, the brainstem, and toxicity: Ingredients for an emerging dialectic. Acta Oncol 53:1279-1282, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Paulino AC, Constine LS, Rubin P, et al. : Normal tissue development, homeostasis, senescence, and the sensitivity to radiation injury across the age spectrum. Semin Radiat Oncol 20:12-20, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Demaria S, Golden EB, Formenti SC: Role of local radiation therapy in cancer immunotherapy. JAMA Oncol 1:1325-1332, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Madenci AL, Fisher S, Diller LR, et al. : Intestinal obstruction in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol 33:2893-2900, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuchs J: The role of minimally invasive surgery in pediatric solid tumors. Pediatr Surg Int 31:213-228, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Allison DC, Carney SC, Ahlmann ER, et al. : A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012:704872, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayerson JL: Living with rotationplasty--quality of life in rotationplasty patients from childhood to adulthood. J Surg Oncol 105:743-744, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Lobeck I, Rymeski B, Burns K, et al. : Long-term morbidity after staging laparotomy for Hodgkin lymphoma. J Pediatr Surg 52:1430-1432, 2017 [DOI] [PubMed] [Google Scholar]
- 45.Warmann SW, Godzinski J, van Tinteren H, et al. : Minimally invasive nephrectomy for Wilms tumors in children - data from SIOP 2001. J Pediatr Surg 49:1544-1548, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Aksnes LH, Bauer HC, Jebsen NL, et al. : Limb-sparing surgery preserves more function than amputation: A Scandinavian sarcoma group study of 118 patients. J Bone Joint Surg Br 90:786-794, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Shima H, Tokuyama M, Tanizawa A, et al. : Distinct impact of imatinib on growth at prepubertal and pubertal ages of children with chronic myeloid leukemia. J Pediatr 159:676-681, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Tauer JT, Hofbauer LC, Jung R, et al. : Impact of long-term exposure to the tyrosine kinase inhibitor imatinib on the skeleton of growing rats. PLoS One 10:e0131192, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaeger BA, Tauer JT, Ulmer A, et al. : Changes in bone metabolic parameters in children with chronic myeloid leukemia on imatinib treatment. Med Sci Monit 18:CR721-CR728, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mariani S, Giona F, Basciani S, et al. : Low bone density and decreased inhibin-B/FSH ratio in a boy treated with imatinib during puberty. Lancet 372:111-112, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Illouz F, Braun D, Briet C, et al. : Endocrine side-effects of anti-cancer drugs: Thyroid effects of tyrosine kinase inhibitors. Eur J Endocrinol 171:R91-R99, 2014 [DOI] [PubMed] [Google Scholar]
- 52.Palani R, Milojkovic D, Apperley JF: Managing pregnancy in chronic myeloid leukaemia. Ann Hematol 94, S167-S176, 2015. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 53.Imai A, Ichigo S, Matsunami K, et al. : Ovarian function following targeted anti-angiogenic therapy with bevacizumab. Mol Clin Oncol 6:807-810, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loren AW, Mangu PB, Beck LN, et al. : Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 31:2500-2510, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torino F, Corsello SM, Salvatori R: Endocrinological side-effects of immune checkpoint inhibitors. Curr Opin Oncol 28:278-287, 2016 [DOI] [PubMed] [Google Scholar]
- 56. doi: 10.1161/CIR.0b013e3182a88099. Lipshultz SE, Adams MJ, Colan SD, et al: Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation 128:1927-1995, 2013 [Erratum: Circulation 128:e394, 2013] [DOI] [PubMed] [Google Scholar]
- 57.Moslehi JJ, Deininger M: Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol 33:4210-4218, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moslehi JJ: Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 375:1457-1467, 2016 [DOI] [PubMed] [Google Scholar]
- 59.Paoletti X, Geoerger B, Doz F, et al. : A comparative analysis of paediatric dose-finding trials of molecularly targeted agent with adults’ trials. Eur J Cancer 49:2392-2402, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Sivendran S, Agarwal N, Gartrell B, et al. : Metabolic complications with the use of mTOR inhibitors for cancer therapy. Cancer Treat Rev 40:190-196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang DY, Okoye GD, Neilan TG, et al. : Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep 19:21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armstrong GT, Oeffinger KC, Chen Y, et al. : Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 31:3673-3680, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Tilburg CM, van Gent R, Bierings MB, et al. : Immune reconstitution in children following chemotherapy for haematological malignancies: A long-term follow-up. Br J Haematol 152:201-210, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Dong H, Robison LL, Leisenring WM, et al. : Estimating the burden of recurrent events in the presence of competing risks: The method of mean cumulative count. Am J Epidemiol 181:532-540, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aminkeng F, Bhavsar AP, Visscher H, et al. : A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet 47:1079-1084, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X, Sun CL, Quiñones-Lombraña A, et al. : CELF4 variant and anthracycline-related cardiomyopathy: A Children’s Oncology Group genome-wide association study. J Clin Oncol 34:863-870, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Best T, Li D, Skol AD, et al. : Variants at 6q21 implicate PRDM1 in the etiology of therapy-induced second malignancies after Hodgkin’s lymphoma. Nat Med 17:941-943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morton LM, Sampson JN, Armstrong GT, et al. : Genome-wide association study to identify susceptibility loci that modify radiation-related risk for breast cancer after childhood cancer. J Natl Cancer Inst 109: 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. doi: 10.1038/ng.478. Ross CJ, Katzov-Eckert H, Dubé MP, et al: Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet 41:1345-1349, 2009 [Erratum: Nat Genet 45:578, 2013] [DOI] [PubMed] [Google Scholar]
- 70.van Dorp W, van den Heuvel-Eibrink MM, Stolk L, et al. : Genetic variation may modify ovarian reserve in female childhood cancer survivors. Hum Reprod 28:1069-1076, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Hunink MG, Weinstein MC, Wittenberg E, et al. : Decision Making in Health and Medicine: Integrating Evidence and Values (ed 2). Cambridge, United Kingdom, Cambridge University Press, 2014 [Google Scholar]
- 72.Owens DK, Whitlock EP, Henderson J, et al. : Use of decision models in the development of evidence-based clinical preventive services recommendations: Methods of the U.S. Preventive Services Task Force. Ann Intern Med 165:501-508, 2016 [DOI] [PubMed] [Google Scholar]
- 73.Kremer LC, Mulder RL, Oeffinger KC, et al. : A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer 60:543-549, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hudson MM, Ehrhardt MJ, Bhakta N, et al. : Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev 26:666-674, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]