ABSTRACT
Information on the role of radiotherapy in anti-PD-1 monoclonal antibody-treated melanoma patients is limited. We report on a prospective cohort of advanced melanoma patients treated simultaneously with radiotherapy and anti-PD-1 therapy between 01/01/15 and 30/06/16. Tumor evaluations (RECIST 1.1) were performed every 3 months on radiated and non-radiated lesions. Twenty-five advanced melanoma patients (64% AJCC stage IV M1c, 64% on second-line treatment or more, 60% with elevated LDH serum levels) were included. Radiotherapy was performed early (median: 24 days) after the first anti-PD-1 dose in 15 patients with rapidly progressing symptomatic lesion(s) or later (median: 5.4 months) in 10 patients with progressive disease (PD) despite PD-1 blockade. Radiotherapy was limited to one organ in 24 patients and consisted mainly of hypo-fractioned radiotherapy (median dose 26 Gy in 3–5 fractions, 17 patients) or brain radiosurgery (5 patients). Median follow-up after first anti-PD-1 dose was 16.9 m (range 2.7-27.4), with 44% of patients alive at last follow-up. For radiated lesions, rates of complete (CR), partial (PR) responses, stable disease (SD) or PD were 24%, 12%, 24%, and 32%, respectively. For non-radiated lesions, rates of CR, PR, SD, and PD were 20%, 19%, 12%, and 40%, respectively. Responses achieved after radiotherapy for radiated and non-radiated areas were correlated (Pearson correlation r: 0.89, P<0.0001) suggesting an abscopal effect. Five patients with CR remained disease-free after discontinuation of anti-PD-1 for a median of 9.5 months. No unusual adverse event was recorded. Hypo-fractionated radiotherapy may enhance efficacy of anti-PD1 therapy in difficult-to-treat patients. Controlled studies are needed.
KEYWORDS: abscopal effect, melanoma, immunotherapy, anti-PD-1 monoclonal antibody, radiotherapy
Introduction
Immune checkpoint blockade with monoclonal antibodies (mAb) has considerably improved progression-free (PFS) and overall survivals (OS) in patients with various cancers, including those with inoperable advanced melanoma,1 namely American Joint Committee on Cancer (AJCC) stages IIIC and IV.2
Ipilimumab, a mAb directed against cytotoxic T–lymphocyte associated antigen 4 (CTLA-4), was first shown to induce long-lasting survivals in 20% of melanoma patients.1 Nivolumab and pembrolizumab, mAbs directed against programed death protein 1 (PD-1), were later shown to improve overall response rates (ORR), PFS and OS in previously untreated melanoma patients when compared to ipilimumab or chemotherapy.3,4 As anti-PD-1 mAbs are associated with ORR ranging from 20 to 40% and a median PFS of 4.1 to 6.9 months in melanoma patients, depending on the line of therapy,1 there is need for improvement. Moreover, contrary to the rapid onset of responses obtained with targeted therapies blocking BRAF+MEK proteins in BRAFV600-mutant melanoma,1 responses to anti-PD-1 mAb are rarely achieved before 3 months of treatment, which may be challenging in patients with very rapid disease kinetics or threatening metastases. Melanoma patients who have progressed on anti-PD-1 monotherapy also remain challenging, as only some patients treated beyond progression experience a delayed partial response.5
Thus, other therapeutic approaches are warranted, including various combinations of the above-mentioned drugs, which are unfortunately associated with frequent grade 3–4 toxicities.1 Several case-series reported improved efficacy of ipilimumab when combined with radiotherapy,6–8 including regression of non-radiated lesions, which is referred to as the abscopal effect.9 The association of anti-PD-1 mAb with radiotherapy was only investigated in small retrospective series,10–17 although it is a promising combination.18 Brain or extra-cranial radiotherapy was generally well tolerated, but the retrospective design, the diversity of clinical scenarios, the absence of a clear delineation between responses observed in radiated and non-radiated areas, and diversity of radiation protocols preclude any definitive conclusion on the best radiation protocol.
Using a single-center cohort of prospectively followed patients with stage IIIC-IV melanoma who received concomitant mainly hypo-fractioned radiotherapy to a limited number of sites and anti-PD-1 therapy, we aimed to study tumor responses on radiated and non-radiated sites in two clinical situations: 1/early on in patients with very rapid disease kinetics or with life-threatening locations (“emergency group”); 2/later, in patients experiencing late progression while on anti-PD-1 mAb and proceeding on anti-PD-1 mAb beyond progression (“late radiotherapy group”). We also aimed to report PFS and melanoma-specific survival (MSS) in these difficult-to-treat patients.
Results
Twenty-five patients were included (Table 1). Most had very severe disease, with 64% stage IV M1c melanoma, 60% elevated LDH serum level, 64% Eastern Cooperative Oncology Group (ECOG) performance status >0, and 24% with brain metastases at baseline. Only 36% of them received anti-PD-1 mAb as first-line treatment. Previous treatments included BRAF+MEK inhibition in all 7 patients with BRAFV600-mutant melanoma, ipilimumab in 5 patients, and cytotoxic chemotherapy in 8 patients (dacarbazine in 7 patients, fotemustine in 1 patient).
Table 1.
Patient characteristics at the beginning of anti-PD-1 therapy and type of radiotherapy.
| Group | Emergency radiotherapy N = 15 | Late radiotherapy N = 10 | Total N = 25 |
|---|---|---|---|
| Sex (F/M) | 5/10 | 6/4 | 11/14 |
| Median age in years (range) | 65 (36–88) | 65 (39–84) | 66 (36–88) |
| Mutational status | |||
| BRAFV600-mutant | 6 (40%) | 1 (10%) | 7 (28%) |
| NRASQ61-mutant | 4 (27%) | 6 (60%) | 10 (40%) |
| BRAFV600-WT & NRASQ61-WT | 5 (33%) | 3 (30%) | 8 (32%) |
| ECOG performance status | |||
| 0 | 9 (60%) | 7 (70%) | 16 (64%) |
| 1 | 6 (40%) | 2 (20%) | 8 (32%) |
| 2 | 0 | 1 (10%) | 1 (4%) |
| LDH above normal upper limit at first dose | 6 (40%) | 9 (90%) | 15 (60%) |
| Melanoma AJCC staging | |||
| IIIC | 4 (27%) | 1 (10%) | 5 (20%) |
| IV, M1a | 0 | 1 (10%) | 1 (4%) |
| IV, M1b | 0 | 3 (30%) | 3 (12%) |
| IV, M1c | 11 (73%) | 5 (50%) | 16 (64%) |
| Anti-PD-1 mAb used | |||
| Nivolumab | 13 (87%) | 7 (70%) | 20 (80%) |
| Pembrolizumab | 2 (13%) | 3 (30%) | 5 (20%) |
| Previous systemic therapy | |||
| 0 | 4 (27%) | 5 (50%) | 9 (36%) |
| 1 | 6 (40%) | 1 (10%) | 7 (28%) |
| 2 | 4 (27%) | 2 (20%) | 6 (24%) |
| 3 | 0 | 2 (20%) | 2 (8%) |
| 4 | 1 (6%) | 0 | 1 (4%) |
| Radiotherapy fields | |||
| Soft tissues & lymph nodes | 5 (33%) | 7 (70%) | 1 (48%) |
| Brain | 5 (33%) | 0 | 5 (20%) |
| Bone | 3 (20%) | 1 (10%) | 4 (16%) |
| Retroperitoneum or mediastinum | 1 (7%) | 2 (20%) | 3 (12%) |
| Brain + orbit | 1 (7%) | 0 | 1 (4%) |
WT (wild-type), mAb (monoclonal antibody).
Unless specified, data are numbers (percentage).
All patients received the scheduled FDA-approved regimen of nivolumab or pembrolizumab, without any dose reduction. The 15 patients of the “emergency group” had their first radiotherapy session after a median of 24 days (range -11-59) on anti-PD-1 mAb, including the 6 patients who received stereotactic radiosurgery (SRS) to the brain. Indications for emergency radiotherapy are summarized in Appendix 1. The 10 patients of the “late radiotherapy group” received radiotherapy for progressive disease (PD) after a median of 5.4 months (range 3.8-11.2) on PD-1-blockade.
All patients received one complete course of radiotherapy, and 5 (20%) received a second course at a median of 17 months (range 6–22) after the first session. Only one patient received multi-organ radiotherapy (SRS on 13 brain metastases and standard palliative radiotherapy of the orbit). Seventeen patients received hypo-fractioned radiotherapy, with a median total dose of 26 Gy (range 19.5-32.5) given in 3–5 fractions on a limited number of soft tissues & lymph nodes, bone, retroperitoneal or mediastinal targets (Table 1). Five patients received SRS on 1–5 brain metastases. The 2 remaining patients received standard palliative radiotherapy of 30 Gy delivered in 10 fractions.
Median follow-up after the first anti-PD-1 mAb infusion was 16.9 months (range 2.7-27.4). Eleven (44%) patients were still alive at the database-lock date, of whom 6 (24%, 4 and 2 from the “emergency” and “late radiotherapy” groups, respectively) achieved complete responses (CR). Of note, 4 of these 6 patients who reached CR had been radiated on lymph nodes/soft tissue targets, and the remaining 2 had received brain SRS.
Anti-PD-1 mAb treatment was withdrawn in all but one patient with CR: all remained disease-free after a median of 9.5 months (range 3–12) after anti-PD-1 cessation. Nineteen patients experienced progressive disease (PD). Eleven of them received subsequent systemic treatment (chemotherapy, ipilimumab, or BRAF+MEK inhibition in respectively 6, 3 and 2 patients) and 5 others were treated with a second course of hypo-fractioned radiotherapy on one additional site without discontinuing anti-PD-1 mAb. The 3 remaining patients received best supportive care.
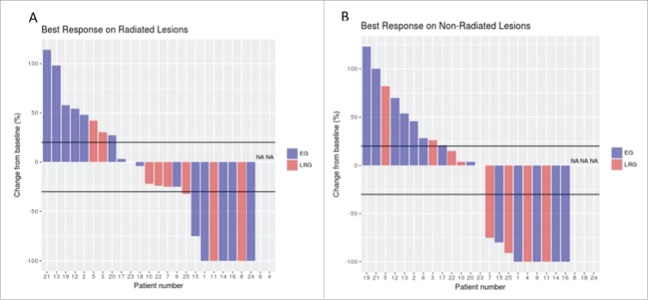
Responses in radiated fields could not be evaluated in patient #4 because of osteosclerotic bone metastases nor for technical reasons in patient #6 who rapidly progressed elsewhere. Three stage IIIC patients (#8, #18, #24) had no target lesion outside the radiotherapy field. Table 2, Fig. 1 and Appendix 2 show tumor responses in radiated and non-radiated areas. Best responses achieved for radiated and non-radiated sites after first radiotherapy were correlated (r: 0.89, P<0.0001) and of similar magnitude in the “emergency” and “late radiotherapy” groups of patients, with an overall response rate (CR+ partial response (PR)) of 36% in radiated and non-radiated areas. Among the 9 patients in the “late radiotherapy” group who had target lesions outside the radiation field, an abscopal effect was observed in 4 patients treated with PD-1 blockade for 134, 153, 190, and 335 days before radiotherapy, respectively. One CR, 1 PR and 1 PD were achieved among the 5 patients treated with a second course of radiotherapy (2 patients not evaluated at the database-lock date).
Table 2.
Response rates in radiated and non-radiated areas after first round of radiotherapy.
| RADIATED AREAS |
NON-RADIATED AREAS |
|||||
|---|---|---|---|---|---|---|
| Emergency radiotherapy group N = 15 | Late radiotherapy group N = 10 | Total N = 25 | Emergency radiotherapy group N = 15 | Late radio-therapy group N = 10 | Total N = 25 | |
| CRa | 4 (27%) | 2 (20%) | 6 (24%) | 4 (27%) | 1 (10%) | 5 (20%) |
| PR | 1 (7%) | 2 (20%) | 3 (12%) | 1 (7%) | 3 (30%) | 4 (16%) |
| SD | 3 (20%) | 3 (30%) | 6 (24%) | 0 | 3 (30%) | 3 (12%) |
| PD | 6 (40%) | 2 (20%) | 8 (32%) | 8 (53%) | 2 (20%) | 10 (40%) |
| NAb | 1 (7%) | 1 (10%) | 2 (4%) | 2 (13%) | 1 (10%) | 3 (12%) |
OR: objective response; CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; NA: not assessed.
Normal (18)F-labeled fluorodeoxyglucose-positron emission tomography (FDG-PET) scans were required to confirm CR.
Response in radiated areas could not be evaluated in a patient because of osteosclerotic bone metastases and in another patient for technical reasons. Three patients with IIIC disease had no target lesion outside the radiotherapy field.
Data are numbers (percentage).
Figure 1.
Target lesions changes in radiated and non-radiated areas. The waterfall plots show the maximum change from the baseline in the sum of the reference diameters of the target lesions on radiated (panel A) and non-radiated areas (panel B). Patients were divided in 2 groups: those with rapidly progressing symptomatic lesions or threatening location(s) who received radiotherapy within first 3 months of PD-1 blockade were in the “emergency” group (EG, blue bars); those who had progressive disease either slowly or after first response or stable disease on anti-PD-1 therapy were in the “late radiotherapy group” (LRG, red bars). Black lines in Panels A and B indicate a 20% increase or a 30% reduction in the sum of target lesions (cut-off for PD, PR and SD according to the RECIST 1.1. criteria).
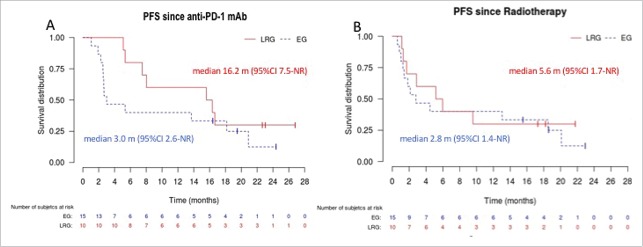
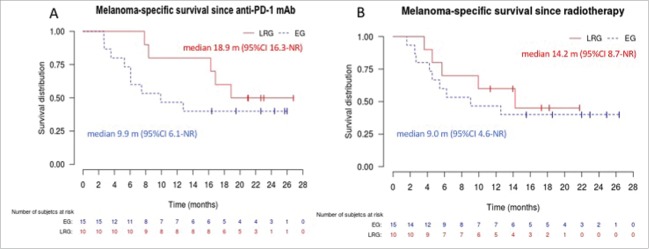
Median PFS (Fig. 2A) after initiation of anti-PD-1 mAb was 3.0 months (95%CI 2.6-not reached (NR)) in the “emergency” group and 16.2 months (95%CI 7.5-NR) in the “late radiotherapy” group. Median MSS (Fig. 3A) after initiation of anti-PD-1 mAb was 9.9 months (95%CI 6.1-NR) in the “emergency” group and 18.9 months (95%CI 16.3-NR) in the “late radiotherapy” group. When curves were plotted from the date of first radiotherapy session, differences between groups were less obvious (Fig. 2B &3B), with PFS and MSS of 2.8 (95%CI 1.4-NR) and 9.0 months (95%CI 4.6-NR) in the “emergency” group, and 5.6 (95%CI 1.7-NR) and 14.2 (95%CI 8.7-NR) in the “late radiotherapy” group, respectively. Fig. 4 shows an example of responses in radiated and non-radiated areas in a patient from the “late radiotherapy” group.
Figure 2.
Progression-free survival in the “emergency” (EG) and “late radiotherapy” (LRG) groups. Panel A shows the Kaplan–Meier curves for progression-free survival (PFS) plotted from the first dose of anti-PD-1 mAb. Panel B shows the Kaplan–Meier curves for PFS since the first day of radiotherapy. Curves for the EG and LRG groups are in blue and dotted, and in red and continuous, respectively. NR: not reached.
Figure 3.
Melanoma-specific survival in the “emergency” (EG) and “late radiotherapy” (LRG) groups. Panel A shows the Kaplan–Meier curves for disease-specific survival plotted from the first dose of anti-PD-1 mAb. Panel B shows the Kaplan–Meier curves for disease-specific survival since the first day of radiotherapy. Curves for the EG and LRG groups are in blue and dotted, and in red and continuous, respectively. NR: not reached.
Figure 4.
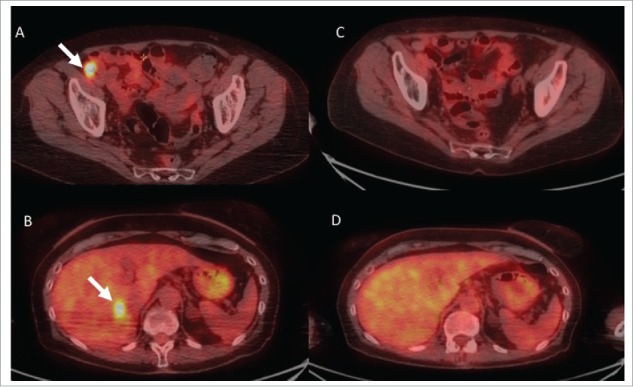
Example of responses in radiated and non-radiated zones in a patient of the “late radiotherapy” group. Fig. 4 shows representative images from (18)F-labeled fluorodeoxyglucose-positron emission tomography (FDG-PET) scans from patient #11. She had widespread in transit metastases on the right lower limb that had progressed on BRAF inhibitor monotherapy (vemurafenib). Despite switching treatment to nivolumab monotherapy for 4.5 months, lesions continued to progress on iliac nodes (panel A, arrow), lung and liver (panel B, arrow). Four sessions of 6 Gy were delivered to two adjacent metastatic iliac lymph nodes without withdrawing nivolumab treatment. All radiated (panel C) and non-radiated in-transit, lungs and liver (panel D) metastases had disappeared on PET-CT-scans performed 12 m after radiation. Nivolumab was discontinued, and the patient remained disease-free 6 months later.
Median duration of exposure to anti-PD-1 mAb was 11.5 months (range 0.7-26.8) and no treatment-related deaths were observed. Only one anti-PD-1-related grade 3 adverse event (diarrhea) was reported. Radiation-induced toxicities consisted of 1 case of grade 2 brain radiation necrosis, which resolved with steroids, and 1 case of minor radiation dermatitis (Table 3). One patient developed biopsy-proven cutaneous sarcoidosis-like granulomas.
Table 3.
Adverse events during treatment with radiotherapy and anti-PD-1 mAb.
| Number of patients N = 25 | Grade 1/2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Skin reactions | |||
| Bullous dermatosis, localized | 1 (4%) | 0 | 0 |
| Pruritus | 1 (4%) | 0 | 0 |
| Vitiligo-like depigmentation | 4 (16%) | 0 | 0 |
| Non-cutaneous adverse events: | |||
| Asthenia | 5 (20%) | 0 | 0 |
| Diarrhea | 5 (20%) | 1 (4%)* | 0 |
| Dyspnea | 1 (4%) | 0 | 0 |
| Reduced/elevated thyroid hormones | 4 (16%) | 0 | 0 |
| Elevated liver enzymes | 4 (16%) | 0 | 0 |
| Eosinophilia | 1 (4%) | 0 | 0 |
| Peripheral neuropathy | 2 (8%) | 0 | 0 |
| Hypotension | 1 (4%) | 0 | 0 |
| Vomiting | 1 (4%) | 0 | 0 |
| Weight loss | 1 (4%) | 0 | 0 |
| Radiotherapy toxicity | |||
| Radiation-induced dermatitis | 1 (4%) | 0 | 0 |
| Central nervous system necrosis | 1 (4%) | 0 | 0 |
Occurred in a patient with known Crohn disease.
Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The case of biopsy-proven local bullous pemphigoid was treated with local steroids without interruption of anti-PD-1. In addition to this table, one patient developed sarcoidosis-like cutaneous granulomas.
Data are numbers (percentage).
Discussion
We report herein, in a retrospective analysis of data prospectively collected in 25 difficult-to-treat advanced melanoma patients, that radiotherapy combined with anti-PD-1 mAb was well tolerated and induced long-lasting responses, with confirmed CR in 24% of patients. These CRs allowed us to discontinue anti-PD-1 mAb in all but one patient achieving CR, without recurrence to date. We also demonstrated a statistically significant correlation between responses in radiated and non-radiated areas, suggesting an abscopal effect.
This series in which anti-PD-1 mAb was never discontinued for toxicity reasons confirms the tolerability of radiotherapy combined with anti-PD-1 mAb.10–17 We observed a brain radiation necrosis only in one radiated site, with a favorable outcome. It occurred in a patient with 13 brain metastases treated with SRS followed by palliative radiotherapy of the orbit. We cannot rule out that a localized accidental overdose induced by this complex radiotherapy occurred in this patient, who eventually experienced CR and discontinued anti-PD-1 mAb without recurrence to date. Only one other case of brain radiation necrosis associated with SRS and PD-1 blockade has been reported.16 Radiotherapy did not seem to induce unusual or severe anti-PD-1-related immune-related adverse events in our series, as all have been reported previously,19 including sarcoidosis20 and bullous pemphigoid.21 We also observed vitiligo-like depigmentation after radiotherapy in 16% of our patients, a feature associated with longer survival in advanced melanoma patients treated with immunotherapy.22
Responses to radiotherapy combined with PD-1 blockade in melanoma patients have been reported in small retrospective series, either mixed with responses to other new melanoma therapies,10,13,15,17 or focusing on PD-1 blockade efficacy.11,12,14,16 The information on extra-cerebral radiotherapy combined with anti PD-1 mAb is scarce, as most series mainly or exclusively were devoted to the association of anti PD-1 and brain stereotactic radiation, whole brain radiotherapy, or brain SRS.10–13,16,17 One series combined multi-site radiotherapy to CTLA-4 or PD-1 blockade, and showed tumor stabilization and/or response for 13 (93%) of 14 irradiated metastatic tumors.15 Aboudaram et al. reported on a smaller series treated with PD-1 blockade and standard palliative radiotherapy, with one occurrence of abscopal effect.14
We are doubtful that our results could be obtained with anti-PD-1 mAb alone. Indeed, we achieved favorable outcomes even in the second- or third-line setting, or in the presence of elevated LDH levels or brain metastases, situations with a low likelihood of anti-PD-1 monotherapy efficacy.1 Secondly, efficacy was observed in our “emergency” group, which consisted of very severe melanoma patients unable to await the usual average 3-month response time to anti-PD-1 mAbs.1 Thirdly, we also report responses in our “late radiotherapy” group, consisting of patients treated with anti-PD-1 mAb beyond progression, where responses occur infrequently and are rarely complete or almost complete.5 Finally, the tight correlation between responses achieved in radiated and non-radiated areas suggests an immune-mediated abscopal effect and is an argument for a synergic role of the combination. This is particularly obvious in the “late radiotherapy” group, where responses in non-radiated areas were achieved after >8 months of anti-PD-1 therapy, a delay after which responses are rarely observed on anti-PD-1 mAb alone.
Numerous preclinical data support the hypothesis that radiotherapy increases the response rates to anti-PD-1 mAb by stimulating accumulation and activation of CD8+ T cells in the tumor microenvironment.23 Localized radiotherapy induces interferon (IFN)-beta production, thereby elevating MHC class I expression on both parental and resistant tumor cells,24 enhances antigen cross-presentation in the draining lymph node, increases T-cell infiltration into tumors,25 enhances the diversity of the T-cell receptor repertoire of intratumoral T cells,26 and restores the responsiveness of resistant tumors to anti-PD-1 therapy.24 Radiotherapy may also be helpful in promoting inflammation, direct vascular damage and endothelial apoptosis, in disrupting the blood-brain barrier, and in creating greater antigen release.27
There are experimental arguments for using hypo-fractionated radiotherapy as we did. DNA exonuclease Trex1 is induced by radiation doses above 12–18 Gy in different cancer cells, and attenuates their immunogenicity by degrading DNA that accumulates in the cytosol upon radiation.28 Repeated irradiation at doses not inducing Trex1 amplifies IFN-beta production, resulting in recruitment and activation of Batf3-dependent dendritic cells. These are essential for priming of CD8+ T cells that mediate systemic tumor rejection in the context of immune checkpoint blockade.28 The most effective radiation protocol required fractions of 8 Gy, which is close to the fractions we delivered.
Limitations of this study include its retrospective nature and small size, the heterogeneity of the radiotherapy (dose, fractionation) and lack of comparison with a control group of patients treated with anti-PD-1 mAb alone. Thus, the significance of any potential survival benefit elicited by this combination is not formally established.
In conclusion, hypo-fractioned radiotherapy to a limited number of lesions combined with anti-PD-1 mAb showed encouraging long-lasting responses in difficult-to-treat melanoma patients and could be an alternative to potentially more toxic systemic treatments in patients with rapidly progressive disease or not responding to anti-PD-1 monotherapy. Future prospective trials are needed to determine the optimal radiotherapy dose, timing of checkpoint inhibition, and fractionation regimen.
Patients and methods
Data were prospectively collected in our skin cancer department between January 1, 2015 (date of early access programs and commercialization of anti-PD-1 mAb) and August 31, 2017, in melanoma patients treated with anti-PD-1 mAb and not included in industry-sponsored clinical trials. BRAFV600 and NRASQ61 mutational status were assessed as previously reported.29,30 Nivolumab was given intravenously every 2 weeks at a dose of 3 mg/kg and pembrolizumab at 2 mg/kg every 3 weeks, according to product labels, until unambiguous PD, unacceptable side effects, or clinician decision to discontinue treatment. However, anti-PD-1 mAb was continued beyond progression at first evaluation to allow for pseudo-progression31 or later if at least one lesion could be treated with a local treatment such as radiotherapy or surgery.
Patients were followed according to our standard procedures, which require a medical consultation with standardized questionnaire before each infusion and standardized blood tests before and at every other infusion of anti-PD-1 mAb. Efficacy was evaluated every 3 months using thoracic, abdominal and pelvic computed tomography (CT) scans, head CT scan or magnetic resonance imaging, carried out by radiologists experienced in melanoma. In addition, normal (18)F-labeled fluorodeoxyglucose-positron emission tomography (FDG-PET) scans were required to confirm CR. All images were analyzed on a weekly basis during a joint meeting with radiologists, with measuring of target lesions according to Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST 1.1) guidelines32 and evaluation of tumor response. All tumor evaluations were carried out blinded to characteristics of radiotherapy (except the site) and stored prospectively in a database. Adverse events were routinely graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Our specialized melanoma tumor board provided the indications for concomitant radiotherapy which could be performed either within the first 3 months of PD-1 blockade for rapidly progressing symptomatic or life-threatening lesion(s), or later, in patients with PD after first response or SD on anti-PD-1 therapy. The radiotherapy regimen was standardized: for extra-cranial lesions, patients received 3–5 doses; for cranial radiotherapy, patients received stereotactic radiosurgery (SRS) in one or two sessions delivered through a Gamma-knife.
For this study, the database was locked on September 30, 2017, and we searched for records of all patients with confirmed inoperable, AJCC stage IIIC-IV cutaneous or mucous membrane melanoma treated with pembrolizumab or nivolumab monotherapy, regardless of BRAF and NRAS mutational status and number and type of previous therapies, and who received concomitant radiotherapy between January 1, 2015, and August 30,, 2016. Key exclusion criteria were age <18 years, previous radiotherapy in the same field, association with ipilimumab, ECOG status >2. Patients were divided in 2 groups. Those with rapidly progressing symptomatic lesions or threatening location(s) received radiotherapy within the first 3 months of PD-1 blockade and were in the “emergency group”; those who had PD either slowly or after first response or SD on anti-PD-1 therapy were in the “late radiotherapy group”.
Primary endpoints were responses in radiated and non-radiated areas. The baseline images for evaluating radiotherapy and PD-1 blockade were those taken immediately prior to radiotherapy. According to the RECIST 1.1 criteria,32 CR was defined as the disappearance of all lesions (with lymph nodes having reached a dimension <10 mm in their smallest axis), PR as a decrease by at least 30% of the sum of the diameters of the target lesions, PD as an increase >20% of the sum of the diameters of the target lesions or occurrence of any new lesions, and SD as having neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD.
Secondary endpoints were MSS (time from the first dose of anti-PD1 mAb to death from melanoma), PFS (time from first dose to documented PD or death), and safety. We also calculated MSS and PFS from the first day of radiotherapy.
According to French Law, this study abided by standard medical practices and did not require a written informed consent. However, consent was obtained orally from all patients. In addition, patients gave written informed consent to participate in one or two national prospective cohorts of advanced melanoma (MelBase: NTC028228202, RIC-Mel: NCT03315468). Study was conducted according to the principles of the declaration of Helsinki.33 Quantitative data were expressed as median and range, qualitative data as frequency and percent. The Kaplan–Meier method was used to calculate estimates of PFS and MSS and the Pearson correlation coefficient and the Pearson correlation test to study relationships between the best responses on radiated and non-radiated areas. Statistical analysis was carried out using R software version 3.2.3 (https://www.r-project.org/).
Appendix 1.
Indications for emergency radiotherapy.
| Patient N | Indication for emergency radiotherapy |
|---|---|
| 1 | Multimetastatic melanoma with 4 brain metastases, the largest measuring 12 mm in largest diameter |
| 2 | Multimetastatic melanoma with fast-growing (from 0 to 9 cm in 3 months) painful left adrenal mass |
| 6 | Multimetastatic melanoma with rapidly growing (2 months) highly painful nodule of the right ankle, 5.7 cm in largest diameter, with inability to walk |
| 9 | Melanoma with rapidly (3 months) growing painful mass within right parotid + lymph nodes measuring 12 cm in largest diameter |
| 12 | Multimetastatic melanoma with 4 brain metastases |
| 13 | Multimetastatic melanoma with rapidly growing (2 months) painful 3.0x2.8 cm D9-D12 lytic corporeal vertebral lesions. |
| 14 | Multimetastatic melanoma with 13 rapidly growing (2 months) brain metastases and 1 intraorbital metastasis with exophtalmia. |
| 15 | Multimetastatic melanoma with 1 rapidly growing brain metastasis |
| 16 | Multiple in-transit painful metastases of left thigh appearing in less than 3 months, the largest measuring 11 cm in largest diameter |
| 17 | Multimetastatic melanoma with multiple D4-D8 lytic corporeal vertebral lesions, with D5-D7 epidural compression |
| 18 | Nasal mucous membrane melanoma, with nodal involvement, ethmoid destruction and invasion of the left orbit resulting in painful exophtalmia developing in 4 months |
| 19 | Multimetastatic melanoma with 2 rapidly growing brain metastases |
| 20 | Multimetastatic melanoma with rapidly growing (3 months) highly painful right groin mass measuring 10 cm in largest diameter, with major lymphedema |
| 21 | Multimetastic melanoma with rapidly growing painful left axillary mass measuring 36 cm in largest diameter |
| 24 | Multimetastic melanoma with rapidly growing painful soft tissue metastases of the neck, the largest measuring 5 cm in largest diameter |
Emergency radiotherapy was performed after a median delay of 24 days after first dose of anti-PD-1 mAb. None of these patients had a second imaging procedure performed between the first dose of anti-PD-1 mAb and first radiotherapy session. Melanoma was considered multimetastic if there was more than one organ involved: the more relevant ones are detailed in the table.
Data are numbers (percentage).
Appendix 2.
Radiated and non-radiated areas: responses for each patient.
| Patient | Radiated Zone(s) | Best response in radiated zone(s) | Non-radiated Zone(s) with metastasis(es) | Best response in non-radiated zone(s) |
|---|---|---|---|---|
| 1 | Brain | −100%, CR | LN (parotid) | −100%, CR |
| 2 | Retroperitoneum | 48%, PD | Heart, LN, lung, peritoneal carcinomatosis | 46%, PD |
| 3 | LN | 30%, PD | Liver, LN, | 26%, PD |
| 4 | Bone | NA (osteosclerotic bone) | LN, lung | −100%, CR |
| 5 | LN | 42%, PD | Brain, LN | 82%, PD |
| 6 | Bone | NA | Brain, LN, lung, retroperitoneum | 28%, PD |
| 7 | Mediastinum | −25%, PR | Brain, retroperitoneum | −75%, PR |
| 8 | LN (parotid) | −100%, CR | / | NA, / |
| 9 | LN (parotid) | −25%, SD | LN | −100%, CR |
| 10 | Mediastinum | −22%, SD | Bone, LN | 4%, SD |
| 11 | LN | −100%, CR | Liver, LN, lung | −100% CR |
| 12 | Brain | 54%, PD | LN | 70%, PD |
| 13 | Bone | 98%, PD | Brain, LN, retroperitoneum | 54%, PD |
| 14 | Brain + orbit | −100%, CR | LN, lung, brain, mediastinum, retroperitoneum | −100%, CR |
| 15 | Brain | −75%, PR | Lung, retroperitoneum | −80%, PR |
| 16 | LN | −100%, CR | LN | −100%, CR |
| 17 | Bone | 3%, PD | Liver, LN, retroperitoneum | 21%, PD |
| 18 | Brain | −4%, SD | / | NA, / |
| 19 | Brain | 58%, PD | Brain, LN, lung | 123%, PD |
| 20 | LN | 27%, PD | Brain | 4%, SD |
| 21 | LN | 114%, PD | Bone, brain, LN, lung, retroperitoneum | 100%, PD |
| 22 | LN | −24%, SD | Liver, lung, retroperitoneum | 15%, SD |
| 23 | LN | 0%, SD | LN | 0%, SD |
| 24 | LN | −100%, CR | / | NA, / |
| 25 | LN | −32%, PR | LN, mediastinum | −91%, PR |
CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; NA: not assessed; /: no target; LN: Lymph nodes or soft tissue mass(es).
Data are numbers (percentage).
Disclosure of potential conflicts of interest
PS has received personal fees from Amgen, Bristol-Myers Squibb, MSD, Merck-Serono, Pfizer, Roche-Genentech, Pierre Fabre, and Novartis; has received nonfinancial support from Bristol-Myers Squibb, MSD, Roche-Genentech, and Novartis; and has received a funding grant from Roche-Genentech.
All remaining authors have declared no conflicts of interest.
Author contributions
PS, BB, AF, YO, PM participated in the conception and design of the study. All authors except AB contributed to the recruitment of the patients and to the acquisition and review of the data. AB analyzed the data. PS, AF, BB, YO, AB performed or supervised the analysis and contributed to the interpretation of the data. All authors contributed to the drafting of the manuscript. All authors critically reviewed each draft, provided important intellectual content and approved the version to be published.
References
- 1.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;18:463–82. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, et al.. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206. doi: 10.1200/JCO.2009.23.4799. PMID:19917835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al.. Nivolumab in previously untreated melanoma without braf mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. PMID:25399552. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al.. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. PMID:25891173. [DOI] [PubMed] [Google Scholar]
- 5.Long GV, Weber JS, Larkin J, Atkinson V, Grob JJ, Schadendorf D, Dummer R, Robert C, Marquez-Rodas I, McNeil C, et al.. Nivolumab for patients with advanced melanoma treated beyond progression: analysis of 2 phase 3 clinical trials. JAMA Oncol. 2017;3:1511–9. doi: 10.1001/jamaoncol.2017.1588. PMID:28662232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimaldi AM, Simeone E, Giannarelli D, Muto P, Falivene S, Borzillo V, Giugliano FM, Sandomenico F, Petrillo A, Curvietto M, et al.. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. doi: 10.4161/onci.28780. PMID:25083318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandra RA, Wilhite TJ, Balboni TA, Alexander BM, Spektor A, Ott PA, Ng AK, Hodi FS, Schoenfeld JD. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology. 2015;4:e1046028. doi: 10.1080/2162402X.2015.1046028. PMID:26451318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kropp LM, De Los Santos JF McKee SB, Conry RM. Radiotherapy to control limited melanoma progression following ipilimumab. J Immunother. 2016;39:373–8. doi: 10.1097/CJI.0000000000000142. PMID:27662339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al.. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. PMID:22397654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choong ES, Lo S, Drummond M, Fogarty GB, Menzies AM, Guminski A, Shivalingam B, Clarke K, Long GV, Hong AM. Survival of patients with melanoma brain metastasis treated with stereotactic radiosurgery and active systemic drug therapies. Eur J Cancer. 2017;75:169–78. doi: 10.1016/j.ejca.2017.01.007. PMID:28236768. [DOI] [PubMed] [Google Scholar]
- 11.Parakh S, Park JJ, Mendis S, Rai R, Xu W, Lo S, Drummond M, Rowe C, Wong A, McArthur G, et al.. Efficacy of anti-PD-1 therapy in patients with melanoma brain metastases. Br J Cancer. 2017;116:1558–63. doi: 10.1038/bjc.2017.142. PMID:28524161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed KA, Stallworth DG, Kim Y, Johnstone PA, Harrison LB, Caudell JJ, Yu HH, Etame AB, Weber JS, Gibney GT. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol. 2016;27:434–41. doi: 10.1093/annonc/mdv622. PMID:26712903. [DOI] [PubMed] [Google Scholar]
- 13.Gaudy-Marqueste C, Dussouil AS, Carron R, Troin L, Malissen N, Loundou A, Monestier S, Mallet S, Richard MA, Regis JM, et al.. Survival of melanoma patients treated with targeted therapy and immunotherapy after systematic upfront control of brain metastases by radiosurgery. Eur J Cancer. 2017;84:44–54. doi: 10.1016/j.ejca.2017.07.017. PMID:28783540. [DOI] [PubMed] [Google Scholar]
- 14.Aboudaram A, Modesto A, Chaltiel L, Gomez-Roca C, Boulinguez S, Sibaud V, Delord JP, Chira C, Delannes M, Moyal E, et al.. Concurrent radiotherapy for patients with metastatic melanoma and receiving anti-programmed-death 1 therapy: A safe and effective combination. Melanoma Res. 2017;27:485–91. doi: 10.1097/CMR.0000000000000386. PMID:28858075. [DOI] [PubMed] [Google Scholar]
- 15.Doyen J, Picard A, Naghavi AO, Thyss A, Passeron T, Lacour JP, Montaudie H. Clinical outcomes of metastatic melanoma treated with checkpoint inhibitors and multisite radiotherapy. JAMA Dermatol. 2017;153:1056–59. doi: 10.1001/jamadermatol.2017.2222. PMID:28746710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liniker E, Menzies AM, Kong BY, Cooper A, Ramanujam S, Lo S, Kefford RF, Fogarty GB, Guminski A, Wang TW, et al.. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. Oncoimmunology. 2016;5:e1214788. doi: 10.1080/2162402X.2016.1214788. PMID:27757312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian JM, Yu JB, Kluger HM, Chiang VL. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer. 2016;122:3051–8. doi: 10.1002/cncr.30138. PMID:27285122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo T, Kim IA. Radiotherapy and immune checkpoint blockades: a snapshot in 2016. Radiat Oncol J. 2016;34:250–9. doi: 10.3857/roj.2016.02033. PMID:28030901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, Izzeddine H, Marabelle A, Champiat S, Berdelou A, et al.. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13:473–86. doi: 10.1038/nrclinonc.2016.58. PMID:27141885. [DOI] [PubMed] [Google Scholar]
- 20.Reddy SB, Possick JD, Kluger HM, Galan A, Han D. Sarcoidosis following anti-PD-1 and anti-CTLA-4 therapy for metastatic melanoma. J Immunother. 2017;40:307–11. doi: 10.1097/CJI.0000000000000181. PMID:28737620. [DOI] [PubMed] [Google Scholar]
- 21.Naidoo J, Schindler K, Querfeld C, Busam K, Cunningham J, Page DB, Postow MA, Weinstein A, Lucas AS, Ciccolini KT, et al.. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PDL-1. Cancer Immunol Res. 2016;4:383–9. doi: 10.1158/2326-6066.CIR-15-0123. PMID:26928461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, Luiten RM. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773–81. doi: 10.1200/JCO.2014.57.4756. PMID:25605840. [DOI] [PubMed] [Google Scholar]
- 23.De Wolf K, Kruse V, Sundahl N, van Gele M, Chevolet I, Speeckaert R, Brochez L, Ost P. A phase II trial of stereotactic body radiotherapy with concurrent anti-PD1 treatment in metastatic melanoma: evaluation of clinical and immunologic response. J Transl Med. 2017;15:21. doi: 10.1186/s12967-017-1123-x. PMID:28137295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Schoenhals JE, Li A, Valdecanas DR, Ye H, Zang F, Tang C, Tang M, Liu CG, Liu X, et al.. Suppression of type I IFN signaling in tumors mediates resistance to anti-PD-1 treatment that can be overcome by radiotherapy. Cancer Res. 2017;77:839–50. doi: 10.1158/0008-5472.CAN-15-3142. PMID:27821490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3:345–55. doi: 10.1158/2326-6066.CIR-14-0196. PMID:25527358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twyman-Saint\sVictor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, et al.. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7. doi: 10.1038/nature14292. PMID:25754329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakin N, Rulach R, Nowicki S, Kurian KM. Current advances in checkpoint inhibitors: Lessons from non-central nervous system cancers and potential for glioblastoma. Front Oncol. 2017;7:141. doi: 10.3389/fonc.2017.00141. PMID:28730140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. PMID:28598415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreau S, Saiag P, Aegerter P, Bosset D, Longvert C, Helias-Rodzewicz Z, Marin C, Peschaud F, Chagnon S, Zimmermann U, et al.. Prognostic value of BRAF (V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol. 2012;19:4314–21. doi: 10.1245/s10434-012-2457-5. PMID:22772867. [DOI] [PubMed] [Google Scholar]
- 30.Helias-Rodzewicz Z, Funck-Brentano E, Terrones N, Beauchet A, Zimmermann U, Marin C, Saiag P, Emile JF. Variation of mutant allele frequency in NRAS Q61 mutated melanomas. BMC Dermatol. 2017;17:9. doi: 10.1186/s12895-017-0061-x. PMID:28668077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, et al.. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–7. doi: 10.1200/JCO.2015.64.0391. PMID:26951310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al.. New response evaluation criteria in solid tumours: revised recist guideline (version 1.1). Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. PMID:19097774. [DOI] [PubMed] [Google Scholar]
- 33.World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. PMID:24141714. [DOI] [PubMed] [Google Scholar]