Abstract
Primary and secondary CNS lymphomas are aggressive brain tumors that pose an immense challenge to define in terms of molecular pathogenesis, as well as to effectively treat. During the past 10 years improvements in survival have been achieved with the implementation of anti-CD20 immunotherapy and optimization of dose-intensive consolidation strategies. The applications of whole-exome sequencing, comparative genomic hybridization, transcriptional profiling, and examination of the tumor microenvironment, particularly in the context of clinical investigation, provide insights that create a roadmap for the development and implementation of novel targeted agents for this disease. A body of genetic evidence strongly suggested that primary CNS lymphomas (PCNSLs) are likely largely dependent on NF-κB prosurvival signals, with enrichment of mutations involving the B-cell receptor pathway, in particular myeloid differentiation primary response 88 and cluster of differentiation 79B. The first set of early-phase investigations that target NF-κB in PCNSL have now been completed and support the NF-κB hypothesis but at the same time reveal that much work needs to be done to translate these results into meaningful advances in survival for a large fraction of patients. Insights into secondary prosurvival pathways that mediate drug resistance is a priority for investigation. Similarly, further evaluation of the immune-suppressive mechanisms in the CNS lymphoma tumor microenvironment is requisite for progress. Combinatorial interventions that promote the antitumor immune response have significant potential. With increasing availability of targeted agents, there is also a need to develop more sensitive imaging tools, not only to detect this highly invasive brain neoplasm but also potentially to define an evolving molecular phenotype to facilitate precision medicine.
Learning Objectives
Provide an update on diagnostic strategies, including radiographic assessment of primary and secondary CNS lymphoma
Define the genomic landscape of CNS lymphomas and provide a roadmap for implementation of rationale therapies
Provide a review of the results from the first series of clinical studies that investigate small molecule–based strategies to inhibit key prosurvival pathways in CNS lymphomas, and the challenges that lay ahead
Introduction
The past 10 years have witnessed an acceleration in progress in central nervous system (CNS) lymphoma research. Key insights were made into its molecular pathophysiology, yielding advances in molecular and genetic diagnosis, prognostication, and implementation of innovative experimental approaches. Anti-CD20 antibody–based immunotherapy has become a cornerstone of treatment, and within a short time-frame the most important research questions no longer focus only on the dose and/or timing of high-dose methotrexate (HD-MTX), other antimetabolites, and whole-brain radiotherapy. Substantial gains in survival have been realized in a disease long considered to have a prognosis similar to glioblastoma. However, even with effective immuno-chemotherapy, strategies that include dose-intensive consolidation, such as the CALGB 50202 regimen, primary CNS lymphoma (PCNSL) is associated with a high rate of early refractory disease (20% to 30% rate of disease progression within the first 6 months1) and long-term survival remains elusive for many patients with secondary CNS lymphoma. Moreover, CNS lymphomas often have devastating long-term neurologic complications that are poorly understood and are compounded by therapeutic interventions such as whole-brain radiotherapy, underscoring the need for new therapeutic strategies and agents.
Diagnosis, pathogenesis, and radiographic assessment
PCNSL is an aggressive brain tumor that typically presents in brain parenchyma, leptomeninges, cerebrospinal fluid (CSF), or the vitreo-retinal compartment, without clinically evident systemic disease. Approximately 95% of PCNSL are large B-cell lymphomas; other histologies include T cell, Burkitt, lymphoblastic, and marginal zone lymphomas.2
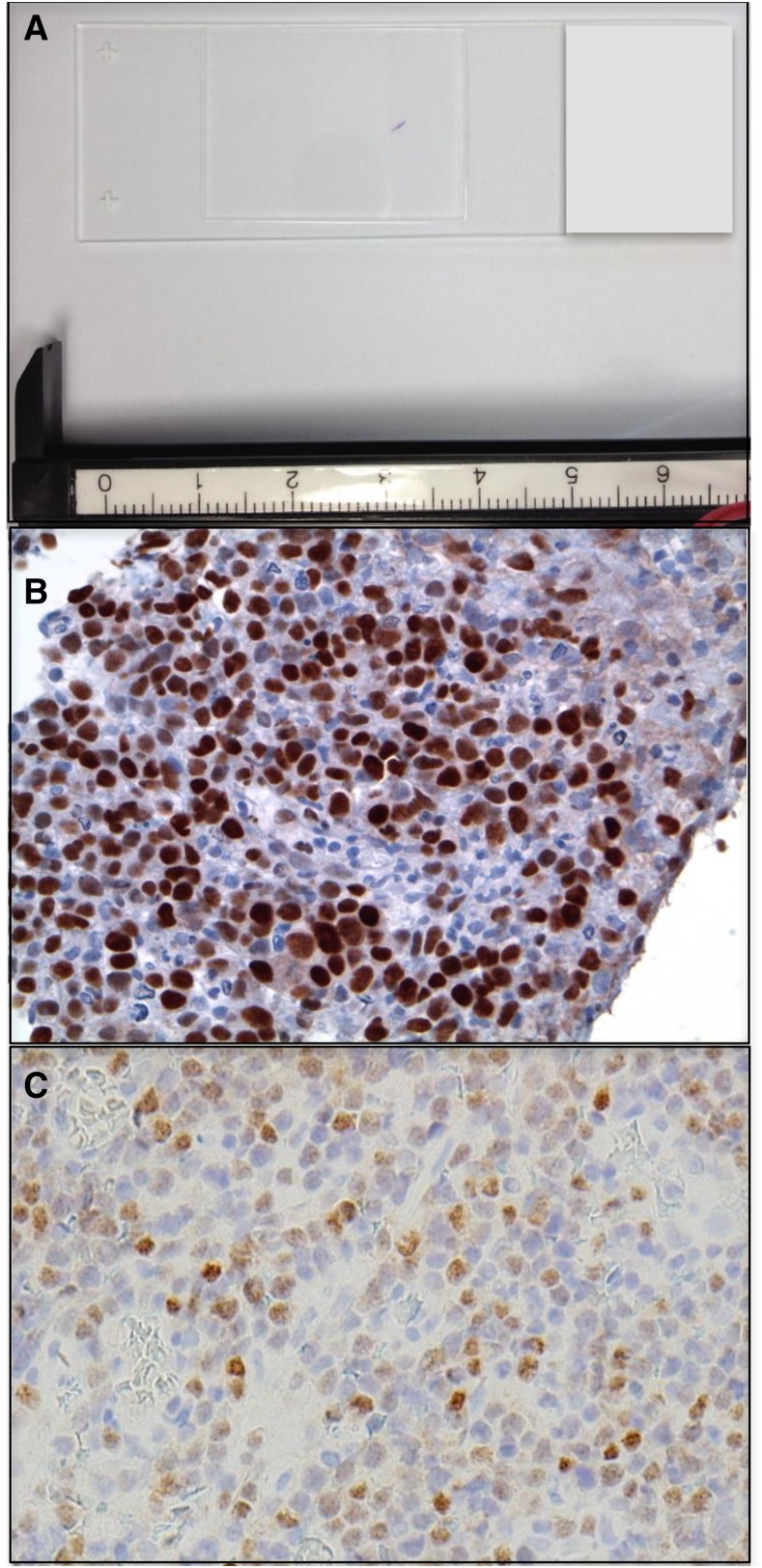
Determination of the unique genetic features of PCNSL poses a greater challenge than for systemic diffuse large B-cell (DLBCL), because of both the rarity of this neoplasm and the paucity of material available for investigational studies. Diagnostic specimens are typically obtained by stereotactic brain biopsy or via CSF. (Figure 1). The majority of research effort has focused on the elucidation of the properties of PCNSL, large-cell type. Between 50% and 80% of PCNSL express BCL-6 by immunohistochemistry,3,4 and 95% stain positive for MUM-1; therefore, the majority of PCNSL cases are classified as activated B-cell (ABC) immunophenotype.5 Immunohistochemical characterization of tumors from patients who participated in CALGB 50202 demonstrated that high BCL6 correlated with shorter survival, thus revealing a potentially useful molecular prognostic biomarker.1 Although the adverse prognostic significance of high BCL-6 in PCNSL was recently confirmed in an independent large prospective trial,6 several small retrospective studies provided a conflicting result: BCL-6 correlates with a better prognosis.3 This discrepancy raises the possibility that the prognostic significance of BCL-6 may be dependent on coexpression of MYC or BCL-2, or such treatment-related factors as whole-brain radiation or rituximab.
Figure 1.
PCNSL at diagnosis. (A) Example of a stereotactic brain biopsy used to diagnose DLBCL involving the corpus callosum in PCNSL. The diagnostic specimen is <2 mm in length. (B) Example of MYC protein expression, detected by immunohistochemistry in a diagnostic PCNSL brain biopsy (with diaminobenzidine detection). High expression of MYC is detected with increased frequency in PCNSL (∼50% of cases) compared with systemic DLBCL (original magnification ×40). (C) IRF-4/MUM-1 is expressed in >90% of PCNSL (original magnification ×40).
Primary CNS DLBCL in the setting of AIDS has a uniform ABC immunophenotype distinct from PCNSL in the immunocompetent in that BCL-6 coexpression is rare (<5% of cases). Epstein-Barr virus (EBV) is detected in virtually all cases of AIDS-related PCNSL, distinct from PCNSL arising in the immunocompetent, in which 5% to 15% of cases are EBV positive.7
Another challenging aspect of CNS lymphoma management and research is the radiographic assessment of disease prognosis, response, and progression. With advances in treatment, it is increasingly recognized that there is a need to improve upon the neuroimaging tools available to detect and quantify CNS lymphoma burden and aggressiveness. Although the standard radiographic method for response assessment in PCNSL has been validated, this metric is essentially based on principles proposed in 1990, that is, measurement of the dimensions of contrast enhancement on T1-based magnetic resonance imaging (MRI).8
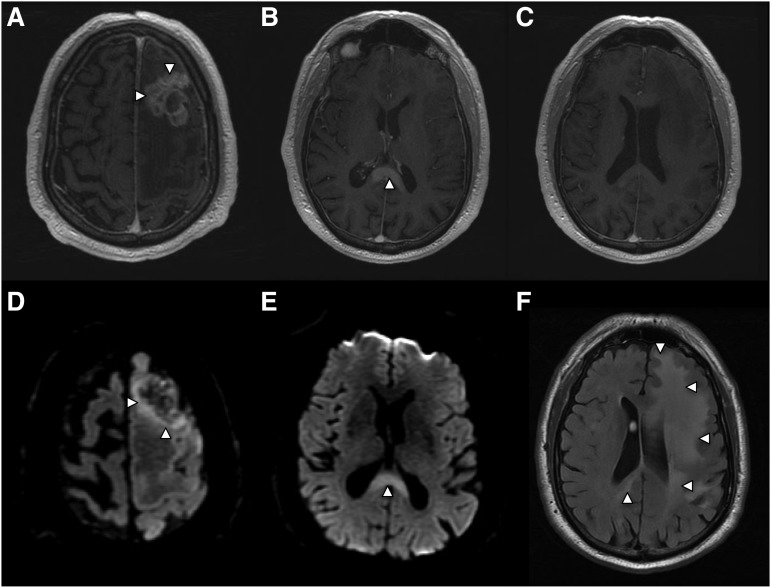
While approximately 50% of PCNSL tumors in immunocompetent patients present as a solitary enhancing brain lesion on T1-weighted MRI, ∼25% of tumors at baseline are associated with a separate, nonenhancing lesion that is hyperintense on T2/FLAIR (fluid attenuated inversion recovery)-weighted imaging at a locus that is distinct from the enhancing lesion(s).9 This observation is consistent with the fact that lymphomatous dissemination in the brain may occur in the absence of contrast enhancement on MRI (Figure 2).
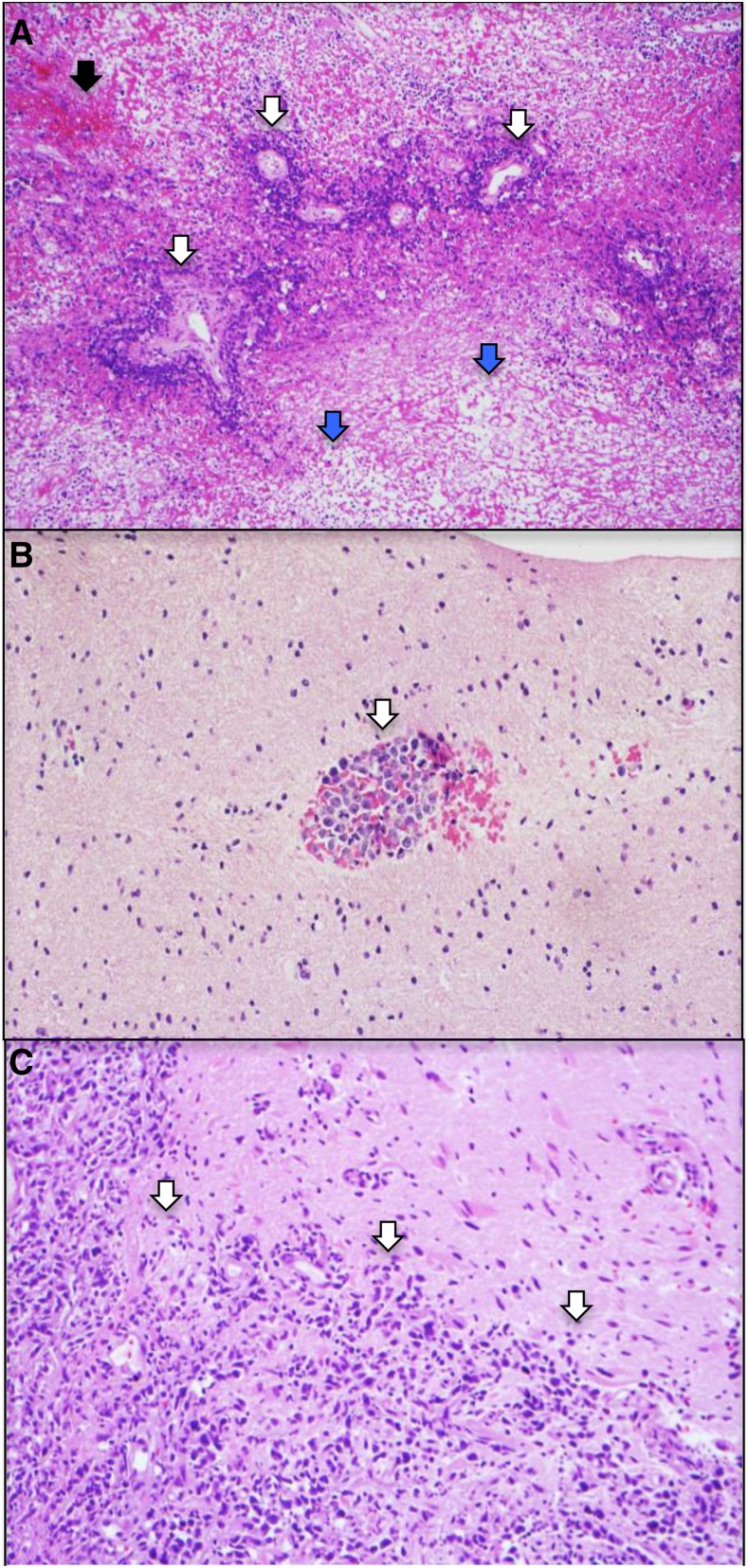
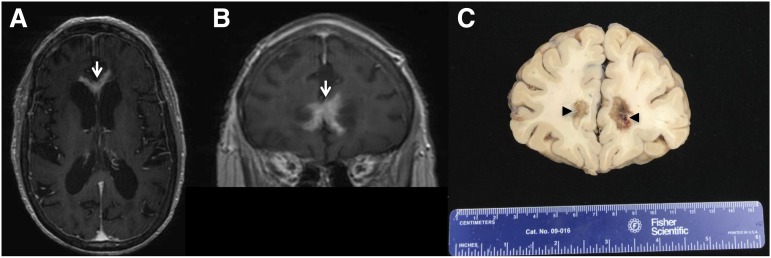
PCNSL is a highly infiltrative disease, particularly at relapse, whereupon the majority of lesions are multifocal and present at sites distinct from the focus of disease presentation. Histopathologic analysis of PCNSL supports at least 2 patterns of tumor dissemination. In one, an angiotropic growth pattern, lymphoma cells appear to coopt existing brain vasculature and migrate in the perivascular (Virchow-Robin) space. This invasive phenotype disrupts the blood-brain barrier and enables radiographic detection of lesions via pathologic contrast enhancement. A second pattern is direct invasion along nerve roots, such as the optic nerve, along white matter tracks such as corpus callosum, or into the meningeal space. (Figure 3). Common patterns of relapse occur within the vitreoretinal compartment and subependymal growth into the periventricular space, leading to spread within CSF pathways. (Figure 4).
Figure 2.
Radiographic presentation of multifocal PCNSL, characterized by distinct MRI sequences. (A) T1 postgadolinium image demonstrates an enhancing mass involving the left frontal lobe (arrows) with central necrosis and surrounding vasogenic edema. On spectroscopy the mass demonstrated increased tumor metabolites, lactate, and choline (not shown). (B) An apparently isolated, second homogeneously enhancing mass (arrow) is identified inferiorly, involving splenium of the corpus callosum. (C) No enhancing foci are detected in white matter between the 2 distinct lesions. (D) Diffusion-weighted imaging demonstrates foci of reduced or restricted diffusion (arrows), suggestive of dense cellularity, in the superior aspect of the frontal mass. (E) Restricted diffusion within the enhancing lesion involving the corpus callosum, strongly suggestive of lymphoma. (F) Extensive T2/FLAIR signal hyperintensity (arrows) extending from the frontal mass posteriorly to the corpus callosal lesion, consistent with nonenhancing lymphoma dissemination involving the cerebral hemisphere.
Figure 3.
Two patterns of tumor invasion identified in the diagnostic PCNSL specimen (subtotal resection) from the patient in Figure 2. Hematoxylin and eosin (×10). (A) In a background of necrosis (blue arrows), and hemorrhage (black arrow) angiotropic growth of viable DLBCL (white arrows), in which lymphoma cells appear to coopt brain vasculature. (B) In a separate area containing predominantly normal brain elements, angiotropic lymphoma cells (white arrow) are noted within the perivascular (Virchow-Robin) space. (C) A second pattern is direct invasion, for example, into the meninges (white arrows).
Figure 4.
PCNSL at relapse with periventricular and subependymal invasion. Common patterns of relapse are within the vitreoretinal compartment and subependymal growth into the periventricular space, leading to spread within CSF pathways. (A and B) T1 axial postgadolinium images of PCNSL that progressed after 3 cycles of HD-MTX: Contrast-enhancing disease is evident, involving the anterior commissure of the corpus callosum. (C) Brain autopsy, obtained 1 week after the MRI, confirmed periventricular DLBCL with subependymal spread.
While T2/FLAIR images may detect subclinical, nonenhancing dissemination of CNS lymphoma, confounding MRI abnormalities involving white matter are commonly elicited by the effects of chemotherapy, including HD-MTX, brain irradiation, and normal aging. For this reason there is interest in development of novel, advanced imaging approaches to noninvasively detect and provide prognostic insight into PCNSL. One approach is diffusion-weighted imaging, which measures restricted water diffusion in hypercellular tumors such as PCNSL, resulting in hyperintensity on MRI that can distinguish CNS lymphomas from less cellular tumors such as malignant gliomas. Foci of restricted diffusion may be useful to discriminate residual CNS lymphoma from benign processes, and very low diffusion coefficients have been associated with high-risk, aggressive CNS lymphoma (Figure 2).10 Other advanced imaging approaches include magnetic resonance spectroscopy and positron emission tomography (PET), each of which are capable of detecting tumor-associated Warburg metabolism.11
Molecular pathogenesis
A combination of genomic profiling studies have provided a basic understanding of the genomic landscape of PCNSL and yielded insights into key pathways and mediators of disease pathogenesis. In particular, whole-exome sequencing combined with comparative genomic hybridization and transcriptional profiling analyses have identified the molecular aberrations that both overlap with and distinguish PCNSL from systemic DLBCL.
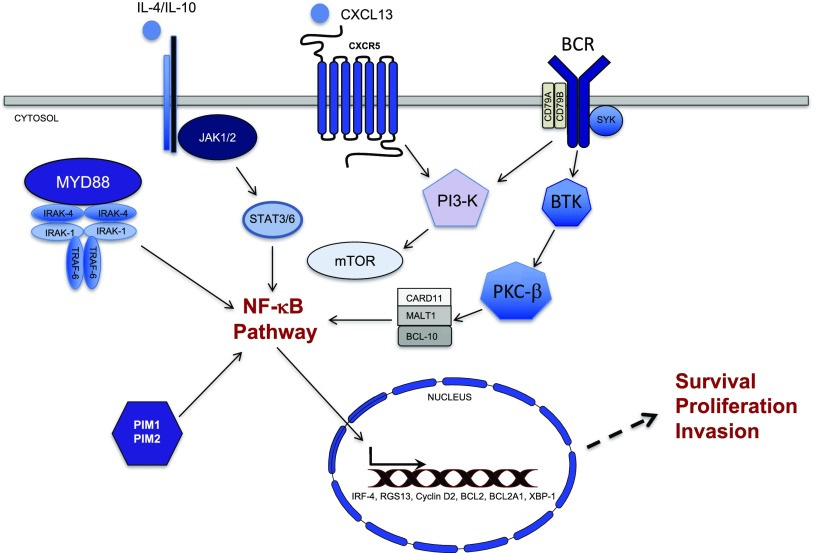
First, a number of whole-exome sequencing studies of PCNSL confirmed enrichment of protein coding mutations that involve mediators of NF-κB activation, the majority of which were identified in the ABC DLBCL subgroup. For example, mutations in the Toll/IL-1 receptor (TIR) domain of the cytosolic adapter protein myeloid differentiation primary response 88 (MYD88), in particular, the activating exchange of leucine to proline at position 265 (L265P), are detected in ∼55% of cases of PCNSL. Importantly, MYD88 is an adapter protein that is used by most Toll-like receptors to activate NF-κB via interleukin-1 receptor–associated kinases (IRAKs). The significance of this mutation was recently highlighted by the discovery that B-cell–specific conditional expression of MYD88 L265P promotes the development of DLBCL in mice.12 In addition, mutations involving the immunoreceptor tyrosine-based activation motif (ITAM) of the B-cell antigen receptor–associated protein cluster of differentiation 79B (CD79B) occur in ∼40% of PCNSL. Importantly, mutational frequencies for these genes are considerably higher in PCNSL compared with non-CNS, ABC-type large cell lymphoma, in which MYD88 and CD79B mutations are detected by resequencing in ∼39% and in ∼20% of cases, respectively.13 Interestingly, there is evidence that secondary CNS DLBCL, relapsing in the CNS, rarely contain the oncogenic MYD88 and CD79B mutations that are enriched in PCNSL, suggesting a distinct pathogenesis that, at least at the genetic level, may not be highly dependent on cooperative signals arising from mutations involving the B-cell receptor and MYD88 in the activation of NF-κB (Figure 5).14
Figure 5.
NF-κB Activation in primary CNS lymphoma. NF-κB transcriptional activation is regulated by multiple signals in PCNSL, including the MYD88/IRAK complex and the BCR complex consisting of CD79A and B. Activation of IRAK kinases via the oncogenic mutation of MYD88 at L265P impacts ∼55% of PCNSL cases. MYD88 is an adapter protein that mediates Toll-like receptor and interleukin-1 receptor signaling. Chronic active signaling via the BCR involving BTK likely cooperates with the MYD88/IRAK pathway in NF-κB activation. Other prosurvival signals include PIM kinases, the PI3K/mTOR, and JAK/STAT pathways. NF-κB target genes such as IRF-4, BCL-2, cyclin D2, RGS-13, and XBP-1 likely potentiate survival, proliferation, and invasion. IL, interleukin; MALT1, mucosa-associated lymphoid tissue; mTOR, mammalian target of rapamycin; PI3-K, phosphoinositide 3-kinase; PKC-β, protein kinase C beta; RGS, regulator of G-protein signaling; XBP-1, X-box binding protein-1.
Less frequent activating mutations that involve the NF-κB pathway include mutations involving CARD11 (caspase recruitment domain family member 11), which have been detected in up to 16% of PCNSL.15 Somatic mutations involving TNFAIP3 (tumor necrosis factor α–induced protein 3), also known as A20, a negative regulator of NF-κB, have been identified in ∼3% to 15% of PCNSL. An overview of the frequency of genetic alterations in individual PCNSL cases revealed recurrent patterns of aberrations that likely reinforce or amplify NF-κB activation, eg, concurrent mutations involving CD79B and MYD88.16,17
Transcriptional analyses of PCNSL identified several candidate mediators of disease pathogenesis, including upregulated expression of Pim kinases, activators of NF-κB. In addition, intratumoral JAK1 transcripts are upregulated in PCNSL.18 Finally, a number of NF-κB target genes are highly expressed in PCNSL, including IRF-4 and XBP-1.18
Several lines of evidence support a role for the JAK/STAT pathway as a mediator of prosurvival signals in PCNSL. Interleukin-4, a B-cell growth factor that signals via JAK/STAT, is upregulated at the transcript and protein level within the vascular microenvironment in PCNSL.18 Increased concentration of IL-10 (another JAK/STAT activator) is detectable in the vitreous and CSF in PCNSL and correlated with adverse prognosis. A recent study demonstrated upregulated IL-10 transcripts in PCNSL tumors compared with secondary CNSL (SCNSL) and nodal lymphomas, with concomitant upregulated IL-10 protein in CSF from cases of PCNSL. In addition, changes in CSF concentration of IL-10 correlated with tumor response and progression in patients treated with rituximab and MTX.19 Importantly, elevated IL-10 expression plus activation of JAK/STAT signaling in PCNSL are manifestations of aberrant activation of the MyD88 pathway (Figure 5).20
Gene expression profiling identified upregulated expression of MYC in PCNSL, and increased MYC protein in PCNSL was confirmed in the CALGB 50202 study.1 Upregulation of microRNAs (miRNAs) associated with MYC pathway (miR-17-5p, miR-20a, miR-9) has been demonstrated.21 PIM1, which cooperates with MYC in lymphomagenesis, is frequently identified as a target of aberrant somatic hypermutation in PCNSL.22
Evaluation of DNA copy number aberrations in PCNSL complements whole-exome sequencing in identification of prosurvival pathways that promote activation of NF-κB in this disease. For example, a frequent genomic aberrational hotspot in PCNSL involves broad deletions on chromosome 6q. In particular 6q21-23 deletions occur in ∼40% to 60% of PCNSL and are associated with adverse prognosis. Candidate tumor suppressors on 6q include A20 (TNFAIP3), PRDM1, regulator of B-cell differentiation, and PTPRK, a tyrosine phosphatase.13 Further evidence for aberrant activation of NF-κB in PCNSL is supported by a gain in DNA copy number for MALT1,23 a protease that inactivates A20, also promoting NF-κB activation.
In addition, PCNSL reproducibly exhibits a high rate (∼70% to 80% of cases) of copy number loss at 9p21.3, involving the cell cycle regulator CDKN2A. Comparative genomic hybridization studies have identified several other genetic lesions in PCNSL, reflecting their genomic instability, including losses on 6p21 that harbor loci for human leukocyte antigens (∼50% of cases). Of particular interest is the recent identification of frequent 9p24.1/PDL1/PDL-2 copy number gains with associated increased PDL1/PDL2 protein expression. Importantly these genomic aberrations identify a mechanism of immune evasion that is shared with primary mediastinal B-cell lymphoma and suggest potential efficacy of programmed cell death 1 (PD-1) blockade in this disease.16
Tumor microenvironment
Under physiologic conditions, the brain is relatively immunologically quiescent, but diagnostic specimens of PCNSL often reveal a robust inflammatory response with infiltrating activated macrophages and reactive T cells. The presence of perivascular T-cell infiltrates in diagnostic specimens of PCNSL may be predictive of a favorable outcome, suggesting that immunotherapeutic strategies that potentiate T-cell-mediated immune surveillance may be effective.24 Tumor-infiltrating CD14+ macrophages constitute a source of complement and Fc receptors that are requisite for rituximab efficacy in the CNS lymphoma microenvironment.25
The molecular cues for tropism and the selective dissemination of DLBCL within the brain are problems fundamental to the pathogenesis of PCNSL. In vitro chemotactic responses by large B-cell lymphoma isolated from brain lesions were recently demonstrated in response to chemokines CXCL12 (SDF-1) and CXCL-13 (B-lymphocyte chemoattractant), providing evidence for their role as neurotropic factors for PCNSL and SCNSL. Moreover, high CXCL-13 concentration in CSF from CNS lymphoma patients correlates with adverse prognosis, supporting its role as a prosurvival factor in PCNSL. In addition, determination of the CSF concentration of both CXCL-13, as well as IL-10, facilitates the diagnosis of CNS lymphoma, in that bivariate expression of each molecule has diagnostic sensitivity at least twofold greater than cytology or flow cytometry. In a multicenter investigation, the positive predictive value of bivariate elevation of IL-10 plus CXCL-13 in CSF was 95% in the identification of untreated PCNSL.19 Circulating extracellular microRNAs in CSF, such as the miR-21 oncogene, were recently described in PCNSL, indicating their potential significance in disease pathogenesis as well as utility as clinical biomarkers.26
Establishing the diagnosis of primary CNS or vitreoretinal lymphoma often remains a significant challenge. Repeat CSF cytological or flow-cytometric studies infrequently improve diagnostic yield, supporting development of innovative diagnostic methods based on detection of genomic aberrations, such as the oncogenic alleles of MYD88.27 Such genetic approaches to diagnosis may also facilitate the rational implementation of precision-based approaches using targeted therapies.
The potential of novel agents in CNS lymphomas
Ibrutinib
Given the high proportion of mutations targeting B cell receptor (BCR) and MYD88 pathways in PCNSL and the results of the pioneering studies in targeting B cell-receptor signaling in systemic diffuse large B-cell lymphoma, there has been significant interest in ibrutinib in the CNS lymphomas. Ibrutinib is a first-in-class, oral inhibitor of Bruton’s tyrosine kinase (BTK) whereby it reduces malignant proliferation of B cells and induces apoptosis.4 Ibrutinib also potentiates T-cell responses via a mechanism independent of BTK.28
Recently, a few small case series and reports have documented activity of ibrutinib monotherapy in cases of relapsed SCNSL, including relapsed mantle cell lymphoma (N = 3 patients); 2 reports, as well, document ibrutinib monotherapy activity in CNS complications of chronic lymphocytic leukemia (CLL).29-31
A recent phase 1 investigation of ibrutinib monotherapy, conducted at Memorial Sloan-Kettering Cancer Center, evaluated its activity and safety in immunocompetent patients with relapsed, refractory PCNSL and SCNSL. CSF penetration of ibrutinib [molecular weight (MW) 440.5] was demonstrated, and frequent tumor responses in both relapsed PCNSL (10 of 13 patients) as well as in SCNSL (5 of 7 patients) were demonstrated (Table 1). Median progression-free survival (PFS) was 4.6 months. Ibrutinib monotherapy was well tolerated in general at the 560 mg and 840 mg dose levels with the exception of 1 case of pulmonary aspergillosis, an infectious complication that is uncommon in CNS lymphoma patients.22
Table 1.
Early-phase investigations in relapsed PCNSL/SCNSL
| Agent/study | Design | Response rate | PFS | Comments |
|---|---|---|---|---|
| Ibrutinib NCT02315326 | MSKCC phase 1 in relapsed PCNSL/SCNSL | 75% | 4.6 mo. | One case pulmonary aspergillosis; ibrutinib resistance with CARD11 mutation. |
| Ibrutinib NCT02542514 | French LOC network phase 2 in relapsed PCNSL and IOL | 56% | One case pulmonary aspergillosis; final analysis pending | |
| DA-TEDDi-R with ibrutinib monotherapy NCT02203526 | NCI phase 1 in newly diagnosed and relapsed PCNSL | 94% ibrutinib 86% CR rate with TEDDiR | 39% rate of invasive aspergillosis in lungs and brain; 28% treatment-related mortality. | |
| Temsirolimus NCT00942747 | Phase 1/2 in relapsed PCNSL | 54% | 2.1 mo. | 13.5% treatment-related mortality (pneumonia) |
| Nivolumab NCT02857426 | Phase 2 in recurrent, PCNSL and primary testicular NHL | In progress | ||
| Lenalidomide/rituximab NCT01542918 | UCSF phase 1 in relapsed PCNSL/SCNSL | 64% | 6 mo. | Independent, retrospective cohort: 12-fold increase in response duration in CR2-4 with lenalidomide maintenance compared with CR1 in relapsed PCNSL/SCNSL |
| Lenalidomide/rituximab NCT01956695 | French LOC network phase 2 in relapsed PCNSL/PVRL | 63% | 8.9 mo. | Final analysis pending |
| Pomalidomide/dex NCT01722305 | Phase 1 in relapsed PCNSL or newly diagnosed or relapsed IOL | In Progress |
IOL, intraocular lymphoma; NHL, non-Hodgkin lymphoma; PVRL, primary vitreoretinal lymphoma.
The overall response rate to ibrutinib in relapsed CNS lymphomas in this study was higher than the response rate observed in systemic DLBCL, in which ibrutinib produced complete or partial responses in 37% of those with ABC type DLBCL but in only 5% of those with germinal center type DLBCL.4 In an examination of candidate molecular determinants involving the BCR pathway and their relationship to response to ibrutinib in the phase 1 trial in relapsed CNS lymphoma, a relationship was observed between partial resistance to ibrutinib and mutations involving CARD11, CD79B, and/or A20 (TNFAIP3). Among the 10 patients for which this information was available, the only patient with complete resistance to ibrutinib had PCNSL with mutant CARD11 (R179Q), which was wild type for MYD88, CD79B, and A20. Two of the 5 patients who achieved CR with ibrutinib had tumors with mutant MYD88 (L265P) without other mutations in the BCR pathway and 1 tumor from the CR cohort was wild type at MYD88, CD79B, A20, and CARD11. Notably, in the phase 1/2 investigation of ibrutinib in systemic DLBCL, the highest number of responses occurred in ABC tumors that lacked BCR mutations, suggesting that oncogenic BCR signaling does not require mutations in the BCR pathway and might be initiated by nongenetic mechanisms. Another potential explanation is that signaling strength involving NF-κB activation pathways correlates with incomplete response as well as outright resistance to ibrutinib.
Nevertheless, given the activity of ibrutinib as monotherapy in this study, and its potential in chemosensitization, a successor trial is planned to investigate ibrutinib in combination with rituximab, HD-MTX, vincristine, and procarbazine in newly diagnosed PCNSL.
The French Lysa and network for oculocerebral lymphoma (LOC) conducted a phase 2 study of ibrutinib monotherapy in relapsed, refractory primary CNS and vitreoretinal lymphoma (NCT02542514), which at interim analysis (ASH, 2016) demonstrated a 56% rate of objective responses in patients treated with ibrutinib at the 560 mg/day dose level. Notably, 1 case of pulmonary aspergillosis was also detected in this prospective multicenter study at ten centers.
Given that the phase 1/2 studies involving ibrutinib in systemic DLBCL demonstrated a relatively short median PFS (2.02 months for ABC-type and 1.31 months for GCB-type DLBCL),4 a group of investigators at the National Cancer Institute tested the hypothesis that incorporation of ibrutinib within a novel chemotherapy regimen would take advantage of the proapoptotic potential of ibrutinib to mediate chemosensitization and yield longer term PFS.17 In addition, the investigators evaluated the hypothesis that inclusion of liposomal doxorubicin, a CNS penetrant formulation of this anthracycline, a class of agents important for the curative treatment of systemic DLBCL, would lead to improved outcomes for both newly diagnosed and relapsed patients. The experimental regimen DA-TEDDi-R included temozolomide, etoposide, liposomal doxorubicin, dexamethasone, and rituximab, in combination with ibrutinib. The rationale for exclusion of HD-MTX, a cornerstone of treatment in PCNSL, was the observation that ibrutinib antagonized the in vitro efficacy of antifolate agents in cell-line models of ABC DLBCL using an unbiased small-molecule combinatorial screen.
The phase 1 investigation of TEDDi-R included a 2-week window of ibrutinib monotherapy that evaluated dose levels of 560 mg, 700 mg, and 840 mg in 18 patients, 5 with newly diagnosed PCNSL. The partial response rate to ibrutinib monotherapy was 94%, and ultimately 86% achieved CR with addition of chemotherapy. Notably, among the 11 responding patients with refractory PCNSL, the responses in 6 exceeded 6 months in duration. However, the toxicity with TEDDi-R was highly significant: Invasive aspergillosis developed in the lungs and or brain of 39% of patients. Ninety-four percent developed grade 4 neutropenia and 28% of patients died of treatment-related toxicities, including 2 with newly diagnosed PCNSL. By comparison, the rate of treatment-related deaths for conventional high-dose chemotherapy–based strategies in newly diagnosed and/or relapsed CNS lymphoma is typically between 2% and 10%.32
The high rate of aspergillosis noted with the TEDDi-R regimen may be explained by antagonism by ibrutinib of wild-type BTK in myeloid cells that mediate the innate immune control of Aspergillus infection, as supported by a murine model comparing survival after Aspergillus fumigatus inoculation in wild-type vs BTK-deficient mice. The mechanistic role for BTK in this setting may be that Toll-like receptors (eg, TLR 2, 4, and 9), engaged in the response of myeloid cells to fungal spores, signal through BTK to induce expression of TNF-α, which mediates neutrophil recruitment. It is possible that glucocorticoids and/or other immunosuppressive mechanisms intrinsic to the pathogenesis of CNS lymphoma may also contribute to risk of aspergillosis.
Temsirolimus
The PI3-K/AKT/mTOR pathway is an important prosurvival pathway that integrates a variety of homeostatic signals to regulate protein synthesis and cell growth. Temsirolimus (MW 1030), an inhibitor of mTOR, has activity in DLBCL and was demonstrated to accumulate in resected glioma specimens in the setting of a phase 1/2 investigation.33 In a recent phase 2 investigation of intravenous temsirolimus monotherapy in relapsed PCNSL, an encouraging overall response rate of 54% was noted, exceeding the response rate of 20% to 30% to mTOR inhibitors in systemic DLBCL. Median PFS was 2.1 months.34 Toxicity with temsirolimus is a concern, and a treatment-related mortality rate of 13.5%, mainly due to pneumonia, was observed. A phase 2 trial involving the pan PI3K/MTOR inhibitor, PQR309, for patients with relapsed PCNSL, is now accruing.
Nivolumab
The recent demonstration of frequent 9p24.1/PDL1 (CD274) and PDL2 (PDCD1LG2) copy number gain with associated increased expression of the programmed cell death ligands PDL-1 and PDL2 in PCNSL and primary testicular lymphoma (PTL), motivated the pilot analysis of nivolumab, a human IgG4 antibody that targets PD-1 and blocks engagement of PDL-1 ligands in patients with relapsed PCNSL and SCNSL from PTL. In a case series of 5 patients, objective responses to treatment (nivolumab 3 mg/kg IV every 2 weeks) were noted with 4 complete responses; the median number of nivolumab treatments to objective response was 3. Therapy was well tolerated with the exception of worsening renal insufficiency in 1 patient, prompting discontinuation. Interpretation of responses in some of these patients perhaps should be made with some caution, however, given the fact that 2 received whole-brain or focal irradiation immediately prior to nivolumab.35 Nevertheless, these promising data, plus evidence that increased PDL-1/PD-1 expression in PCNSL may be associated with adverse prognosis,36 constitute the basis for a multicenter phase 2 study of nivolumab in recurrent refractory PCNSL and PTL (NCT02857426).
Intraventricular immunotherapy
Because the blood-brain barrier limits brain and CSF penetration by therapeutic macromolecules such as monoclonal antibodies, the strategy of direct intraventricular administration as a means to achieve high concentrations of naked, recombinant immunoglobulins within the brain tumor microenvironment has been evaluated in early-phase investigation, initially with rituximab. Phase 1 studies of intraventricular rituximab monotherapy as well as intraventricular rituximab plus methotrexate have provided evidence for the safety, feasibility, and efficacy of this approach, particularly in the elimination of refractory lymphoma within the leptomeninges and cerebrospinal fluid. Combination intraventricular rituximab plus methotrexate yielded a 75% rate of complete cytologic response in relapsed, refractory leptomeningeal disease. This approach has subsequently been applied in other disease settings and demonstrated to be effective in a variety of inflammatory and neoplastic conditions within the CNS.25,37-45
Lenalidomide
Lenalidomide is a small-molecule immunomodulatory agent (MW 259.3) with pleiotropic antitumor effects, including stimulation of T-cell and natural-killer expansion. Lenalidomide also has cell-autonomous cytotoxic effects that are relevant to PCNSL, including antagonism of IRF4 and MYC prosurvival signals.46-48 Lenalidomide enhances antibody-dependent cell-mediated cytotoxicity and may overcome rituximab resistance.49 Single-agent activity of lenalidomide in refractory AIDS-related PCNSL, secondary CNS DLBCL, as well as mantle cell lymphoma involving CNS has been reported.50-52 Based on these data, and given evidence for synergy between lenalidomide and rituximab,49 UCSF conducted a phase 1 trial of lenalidomide plus combined intraventricular and intravenous rituximab in immunocompetent patients with recurrent/refractory CNS lymphomas with the phase 1 design enabling evaluation of response to and toxicity of lenalidomide first as monotherapy (NCT01542918). Interim results were presented at International Congress of Malignant Lymphoma 2015 and at ASCO, 2016. Nine out of 14 subjects with relapsed or refractory CNS non-Hodgkin lymphoma achieved ≥PR with lenalidomide monotherapy and 6 maintained response ≥9 months. Median PFS for lenalidomide/rituximab combination was 6 months. The CSF/plasma partition coefficient of lenalidomide was discovered to be ≥20% at 15- and 20-mg dose levels. Overall, toxicity was manageable, with bacterial infections and neutropenia as the principal toxicities, but there was no treatment-related mortality. The 15-mg dose level was determined to be the recommended dose for phase 2 evaluation. In addition, in an independent cohort of relapsed, refractory CNS lymphoma patients, response duration with lenalidomide maintenance, at modest doses of 5-10 mg/d, after confirmed CR 2-4, was 12 times longer than response duration in CR1 after standard therapy. Results with lenalidomide maintenance are relevant to management of elderly PCNSL patients in CR1 who are not candidates for dose-intensive chemotherapy consolidation. Notably, based on lenalidomide maintenance data presented in this phase 1 trial, the International Extranodal Lymphomas Study Group will conduct a randomized phase 2 trial to evaluate lenalidomide maintenance in older PCNSL patients after induction therapy, in lieu of intensive consolidation. In addition, given the results with lenalidomide as monotherapy and with rituximab, and the potential of PD1-checkpoint blockade in CNS lymphoma, a multicenter phase 2 trial is in development to evaluate lenalidomide/rituximab plus durvalumab, a PD-L1 antagonist.
The French LOC network is conducting a phase 2 study of rituximab plus lenalidomide (20 mg/day dose level) in relapsed, refractory PCNSL/primary vitreoretinal lymphoma. The overall response rate was 63% with median progression-free survival of 8.9 months as reported at ASH, 2016 (NCT01956695). Investigation of a related, second generation IMiD, pomalidomide, in combination with dexamethasone, in relapsed CNS lymphoma, is in progress (NCT01722305).
Conclusions and future directions
Insights into the molecular genetics and microenvironment of CNS lymphomas are providing a rational basis for the strategic evaluation of novel targeted agents in CNS lymphomas. While encouraging activity was demonstrated in these early phase investigations, significant toxicities and early resistance was encountered in a high fraction of patients. Insights into secondary prosurvival pathways that mediate drug resistance to these targeted agents is a priority for investigation. Similarly, further evaluation of the immune suppressive mechanisms in the CNS lymphoma brain-tumor microenvironment is requisite for further progress.
Acknowledgments
The author is grateful to colleagues at UCSF who contributed to the preparation of this manuscript: Soonmee Cha, Leo Sugrue, Kevin Connolly (UCSF Radiology) and Tarik Tihan, Walter Finkbeiner, Annie Hiniker (UCSF Pathology). The author is also grateful for the contribution of Eric Hsi (Pathology, Cleveland Clinic).
This work was supported by the Leukemia & Lymphoma Society, by National Institutes of Health Grant R01CA139-83-01A1, and by Sandler Program for Breakthrough Biomedical Research (J.L.R.).
References
- 1.Rubenstein JL, Hsi ED, Johnson JL, et al. . Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31(25):3061-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnevale J, Rubenstein JL. The Challenge of Primary Central Nervous System Lymphoma. Hematol Oncol Clin North Am. 2016;30(6):1293-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braaten KM, Betensky RA, de Leval L, et al. . BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003;9(3):1063-1069. [PubMed] [Google Scholar]
- 4.Wilson WH, Young RM, Schmitz R, et al. . Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri-Broët S, Crinière E, Broët P, et al. . A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107(1):190-196. [DOI] [PubMed] [Google Scholar]
- 6.Kreher S, Jöhrens K, Strehlow F, et al. . Prognostic impact of B-cell lymphoma 6 in primary CNS lymphoma. Neuro-oncol. 2015;17(7):1016-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta NK, Nolan A, Omuro A, et al. . Long-term survival in AIDS-related primary central nervous system lymphoma. Neuro-oncol. 2017;19(1):99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrey LE, Batchelor TT, Ferreri AJ, et al. ; International Primary CNS Lymphoma Collaborative Group. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034-5043. [DOI] [PubMed] [Google Scholar]
- 9.Tabouret E, Houillier C, Martin-Duverneuil N, et al. . Patterns of response and relapse in primary CNS lymphomas after first-line chemotherapy: imaging analysis of the ANOCEF-GOELAMS prospective randomized trial. Neuro-oncol. 2017;19(3):422-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wieduwilt MJ, Valles F, Issa S, et al. . Immunochemotherapy with intensive consolidation for primary CNS lymphoma: a pilot study and prognostic assessment by diffusion-weighted MRI. Clin Cancer Res. 2012;18(4):1146-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang YZ, Booth TC, Bhogal P, Malhotra A, Wilhelm T. Imaging of primary central nervous system lymphoma. Clin Radiol. 2011;66(8):768-777. [DOI] [PubMed] [Google Scholar]
- 12.Knittel G, Liedgens P, Korovkina D, et al. ; German International Cancer Genome Consortium Molecular Mechanisms in Malignant Lymphoma by Sequencing Project Consortium. B-cell-specific conditional expression of Myd88p.L252P leads to the development of diffuse large B-cell lymphoma in mice. Blood. 2016;127(22):2732-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braggio E, Van Wier S, Ojha J, et al. . Genome-wide analysis uncovers novel recurrent alterations in primary central nervous system lymphomas. Clin Cancer Res. 2015;21(17):3986-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kersten MJ, Kraan W, Doorduijn J, et al. . Diffuse large B cell lymphomas relapsing in the CNS lack oncogenic MYD88 and CD79B mutations. Blood Cancer J. 2014;4:e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montesinos-Rongen M, Schmitz R, Brunn A, et al. . Mutations of CARD11 but not TNFAIP3 may activate the NF-kappaB pathway in primary CNS lymphoma. Acta Neuropathol. 2010;120(4):529-535. [DOI] [PubMed] [Google Scholar]
- 16.Chapuy B, Roemer MG, Stewart C, et al. . Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lionakis M.S., et al. , Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31(6): p. 833-843 e5. [DOI] [PMC free article] [PubMed]
- 18.Rubenstein JL, Fridlyand J, Shen A, et al. . Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107(9):3716-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubenstein JL, Wong VS, Kadoch C, et al. . CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood. 2013;121(23):4740-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ngo VN, Young RM, Schmitz R, et al. . Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer L, Hummel M, Korfel A, Lenze D, Joehrens K, Thiel E. Differential micro-RNA expression in primary CNS and nodal diffuse large B-cell lymphomas. Neuro-oncol. 2011;13(10):1090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grommes C, Pastore A, Palaskas N, et al. . Ibrutinib unmasks critical role of Bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwindt H, Vater I, Kreuz M, et al. . Chromosomal imbalances and partial uniparental disomies in primary central nervous system lymphoma. Leukemia. 2009;23(10):1875-1884. [DOI] [PubMed] [Google Scholar]
- 24.Ponzoni M, Berger F, Chassagne-Clement C, et al. ; International Extranodal Lymphoma Study Group. Reactive perivascular T-cell infiltrate predicts survival in primary central nervous system B-cell lymphomas. Br J Haematol. 2007;138(3):316-323. [DOI] [PubMed] [Google Scholar]
- 25.Kadoch C, Li J, Wong VS, et al. . Complement activation and intraventricular rituximab distribution in recurrent central nervous system lymphoma. Clin Cancer Res. 2014;20(4):1029-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baraniskin A, Kuhnhenn J, Schlegel U, et al. . Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood. 2011;117(11):3140-3146. [DOI] [PubMed] [Google Scholar]
- 27.Bonzheim I, Giese S, Deuter C, et al. . High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: a valuable tool to improve diagnostic yield of vitreous aspirates. Blood. 2015;126(1):76-79. [DOI] [PubMed] [Google Scholar]
- 28.Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci USA. 2015;112(9):E966-E972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernard S, Goldwirt L, Amorim S, et al. . Activity of ibrutinib in mantle cell lymphoma patients with central nervous system relapse. Blood. 2015;126(14):1695-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tam CS, Kimber T, Seymour JF. Ibrutinib monotherapy as effective treatment of central nervous system involvement by chronic lymphocytic leukaemia. Br J Haematol. 2017;176(5):829-831. [DOI] [PubMed] [Google Scholar]
- 31.Wanquet A, Birsen R, Lemal R, Hunault M, Leblond V, Aurran-Schleinitz T. Ibrutinib responsive central nervous system involvement in chronic lymphocytic leukemia. Blood. 2016;127(19):2356-2358. [DOI] [PubMed] [Google Scholar]
- 32.Grommes C, Younes A. Ibrutinib in PCNSL: The Curious Cases of Clinical Responses and Aspergillosis. Cancer Cell. 2017;31(6):731-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhn JG, Chang SM, Wen PY, et al. ; North American Brain Tumor Consortium and the National Cancer Institute. Pharmacokinetic and tumor distribution characteristics of temsirolimus in patients with recurrent malignant glioma. Clin Cancer Res. 2007;13(24):7401-7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korfel A, Schlegel U, Herrlinger U, et al. . Phase II Trial of Temsirolimus for Relapsed/Refractory Primary CNS Lymphoma. J Clin Oncol. 2016;34(15):1757-1763. [DOI] [PubMed] [Google Scholar]
- 35.Nayak L, Iwamoto FM, LaCasce A, et al. . PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Four M, Cacheux V, Tempier A, et al. . PD1 and PDL1 expression in primary central nervous system diffuse large B-cell lymphoma are frequent and expression of PD1 predicts poor survival. Hematol Oncol. 2016. [DOI] [PubMed] [Google Scholar]
- 37.Rubenstein JL, Li J, Chen L, et al. . Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood. 2013;121(5):745-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubenstein JL, Fridlyand J, Abrey L, et al. . Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol. 2007;25(11):1350-1356. [DOI] [PubMed] [Google Scholar]
- 39.Della-Torre E, Campochiaro C, Cassione EB, et al. . Intrathecal rituximab for IgG4-related hypertrophic pachymeningitis. J Neurol Neurosurg Psychiatry. 2017;jnnp-2017-316519. [DOI] [PubMed] [Google Scholar]
- 40.Topping J, Dobson R, Lapin S, et al. . The effects of intrathecal rituximab on biomarkers in multiple sclerosis. Mult Scler Relat Disord. 2016;6:49-53. [DOI] [PubMed] [Google Scholar]
- 41.Ceppi F, Weitzman S, Woessmann W, et al. . Safety and efficacy of intrathecal rituximab in children with B cell lymphoid CD20+ malignancies: An international retrospective study. Am J Hematol. 2016;91(5):486-491. [DOI] [PubMed] [Google Scholar]
- 42.Wu M, Sun J, Zhang Y, et al. . Intrathecal rituximab for EBV-associated post-transplant lymphoproliferative disorder with central nervous system involvement unresponsive to intravenous rituximab-based treatments: a prospective study. Bone Marrow Transplant. 2016;51(3):456-458. [DOI] [PubMed] [Google Scholar]
- 43.Czyzewski K, Styczynski J, Krenska A, et al. . Intrathecal therapy with rituximab in central nervous system involvement of post-transplant lymphoproliferative disorder. Leuk Lymphoma. 2013;54(3):503-506. [DOI] [PubMed] [Google Scholar]
- 44.Lu NT, Raizer J, Gabor EP, et al. . Intrathecal trastuzumab: immunotherapy improves the prognosis of leptomeningeal metastases in HER-2+ breast cancer patient. J Immunother Cancer. 2015;3:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubenstein JL, Combs D, Rosenberg J, et al. . Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101(2):466-468. [DOI] [PubMed] [Google Scholar]
- 46.Krönke J, Udeshi ND, Narla A, et al. . Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu G, Middleton RE, Sun H, et al. . The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343(6168):305-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez-Girona A, Mendy D, Ito T, et al. . Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26(11):2326-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu L, Adams M, Carter T, et al. . lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14(14):4650-4657. [DOI] [PubMed] [Google Scholar]
- 50.Rubenstein JL, Treseler PA, Stewart PJ. Regression of refractory intraocular large B-cell lymphoma with lenalidomide monotherapy. J Clin Oncol. 2011;29(20):e595-e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cox MC, Mannino G, Lionetto L, Naso V, Simmaco M, Spiriti MA. Lenalidomide for aggressive B-cell lymphoma involving the central nervous system? Am J Hematol. 2011;86(11):957. [DOI] [PubMed] [Google Scholar]
- 52.Gupta NK, Wang CC, Mannis GN, Yu JJ, Rubenstein JL. Regression of methotrexate-resistant AIDS-related primary central nervous system lymphoma with lenalidomide plus combination anti-retroviral therapy. Leuk Lymphoma. 2017;58(11):2748-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]