Abstract
Alcohol consumption causes comprehensive liver disorders, designated as alcoholic liver disease (ALD). Because alcohol is detoxified by alcohol dehydrogenase (ADH), a major ethanol metabolism system, the development of ALD was initially believed to be due to malnutrition caused by alcohol metabolism in liver. The discovery of the microsomal ethanol oxidizing system (MEOS) changed this dogma. Cytochrome P450 enzymes (CYP) constitute the major components of MEOS. Cytochrome P450 2E1 (CYP2E1) in MEOS is one of the major ROS generators in liver and is recognized as a risk factor for ALD. Our labs have been studying the relationship between CYP2E1 and ALD. Recently we found that human CYP2A6 and its mouse analog CYP2A5 are also induced by alcohol, and the alcohol induction of CYP2A5 in mice is regulated by Nrf2 and is CYP2E1-dependent. Unlike CYP2E1, CYP2A5 protects against the development of ALD. The relationship of CYP2E1, CYP2A5, and ALD is a major focus of this review.
Keywords: Alcohol induction, antioxidants, CYP2A5, CYP2A6, CYP2E1, Nrf2, reactive oxygen species, interactions, pyrazole, alcoholic liver disease, liver fibrosis, nicotine, PPARα, FGF21, LPS, TNFα, MAPK, HIF-1α, autophagy, coumarin 7-hydroxylase
1. INTRODUCTION
The cytochrome P450 enzymes serve as terminal oxidases in the mixed-function oxidase system for metabolizing various endogenous substrates and xenobiotics, including steroids, fatty acids, drugs, toxins, and carcinogens [1]. Many different enzymes belong to this cytochrome P450 family. The enzymes are named CYP for cytochrome P450, followed by an Arabic number denoting the family, a letter designating the subfamily, and finally an Arabic numeral representing the individual gene in the subfamily [2, 3]. In this review, we focus on CYP2E1 and CYP2A6 in alcoholic liver disease (ALD).
CYP2E1 is expressed in multiple organs, but the highest level of CYP2E1 is expressed in liver. Hepatic CYP2E1 can be induced by many energy-related pathological conditions such as obesity, diabetes, starvation, high fat diet, fasting, ketone bodies, insulin, glucagon, and alcohol consumption (for details about CYP2E1, please refer to reviews [4, 5]). More than 15 million people in the U.S. abuse or overuse alcohol. Alcohol consumption can induce ALD, which is a spectrum of liver disorders ranging from fatty liver (steatosis), liver inflammation (steatohepatitis), to liver fibrosis and cirrhosis [5]. ALD is a significant clinical problem and a major cause of morbidity and mortality worldwide. In developed countries, ALD is a major cause of end-stage disease that requires transplantation. We have been focusing on hepatic CYP2E1 and ALD. By using CYP2E1 inducers, CYP2E1 inhibitor, CYP2E1-adenovirus, HepG2 cells over-expressing CYP2E1 (E47 cells) and control cells not expressing CYP2E1 (C34 cells), CYP2E1 knockout (cyp2e1−/−) mice, and cyp2e1−/− mice reconstituted with human CYP2E1 (humanized CYP2E1 transgenic mice, cyp2e1−/− Tg mice), we have confirmed that CYP2E1 is one of major risk factors for the development of ALD.
Among members of CYP2A subfamilies, mouse CYP2A5 and rat CYP2A3 are orthologs to human CYP2A6 [6, 7]. Human CYP2A6 and mouse CYP2A5 are mainly expressed in liver, but rat CYP2A3 is not expressed in the liver [6]. Therefore, human CYP2A6 can be reflected by mouse CYP2A5 but not rat CYP2A3. Coumarin, a plant alkaloid, is 7-hydroxylated by liver coumarin 7-hydroxylase (COH) which is encoded by the cyp2a5 gene in mouse and the cyp2a6 gene in human (for details about CYP2A5, please refer to recent reviews [8, 9]). COH (CYP2A6 in human and CYP2A5 in mice) is a major enzyme for nicotine metabolism in humans and mice [10, 11]. Recently, we found that mouse CYP2A5 can also be induced by alcohol feeding, and alcohol induction of CYP2A5 is CYP2E1-dependent [12]. Further studies show that CYP2E1-generated reactive oxygen species (ROS) up-regulates Nrf2, which in turn elevates CYP2A5 levels in liver [13]. Unlike CYP2E1, CYP2A5 does not promote but protects against the development of ALD because ALD found in CYP2A5 knockout mice (cyp2a5−/− mice) is more severe than that developed in wild type (WT) mice [14].
In response to a special issue “Current advances in pharmacotherapy and drug design against inflammatory-related pathologies”, in this review, we summarize the relationship of CYP2E1 and CYP2A5 and ALD development and the mechanisms involved. In addition to recent advances, some unpublished data in our labs are also shown in Figures 1, 2, 3, 5, and 7.
Figure 1.
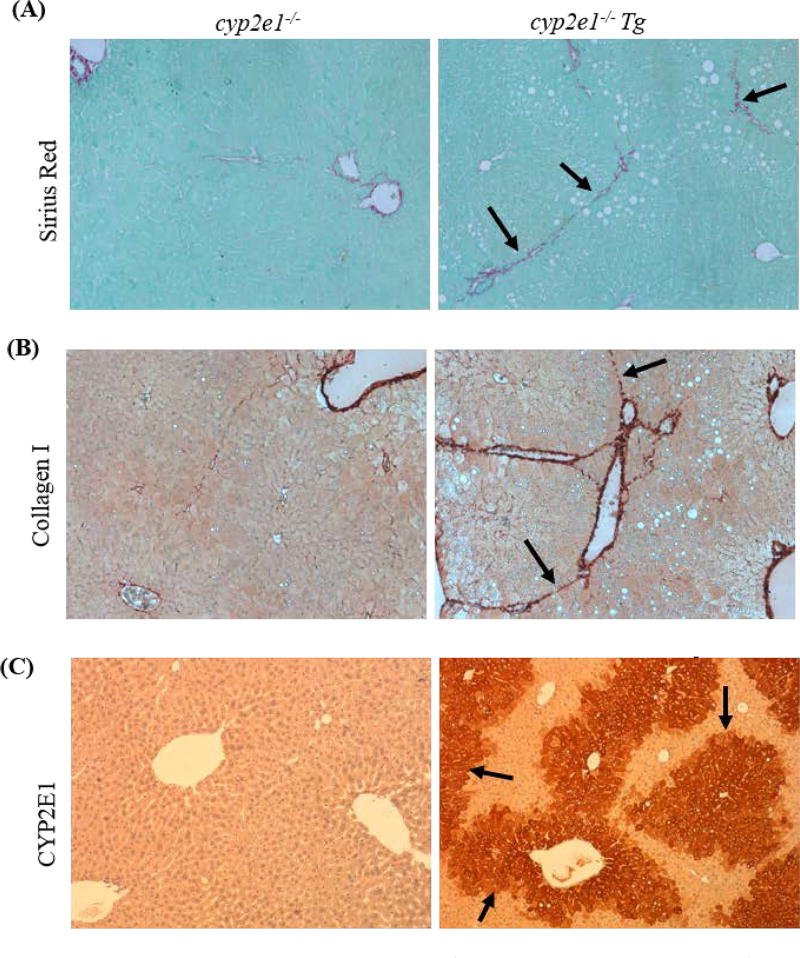
Liver fibrosis was induced in cyp2e1−/− Tg mice but not in cyp2e1−/− mice. The mice were fed ethanol diet (35% calorie derived from ethanol) for 5 weeks, and binge ethanol was orally administrated at 5 g/kg weekly for 5 times during the chronic ethanol feeding. (A) Sirius Red/Fast Green staining. Arrows show collagenous fibers. (B) Collagen I immunohistochemistry staining. Arrows show fibers with collagen I positive staining. (C) CYP2E1 immunohistochemistry staining. Arrows show positive CYP2E1 staining in cyp2e1−/− Tg mice but not in cyp2e1−/− mice.
Figure 2.
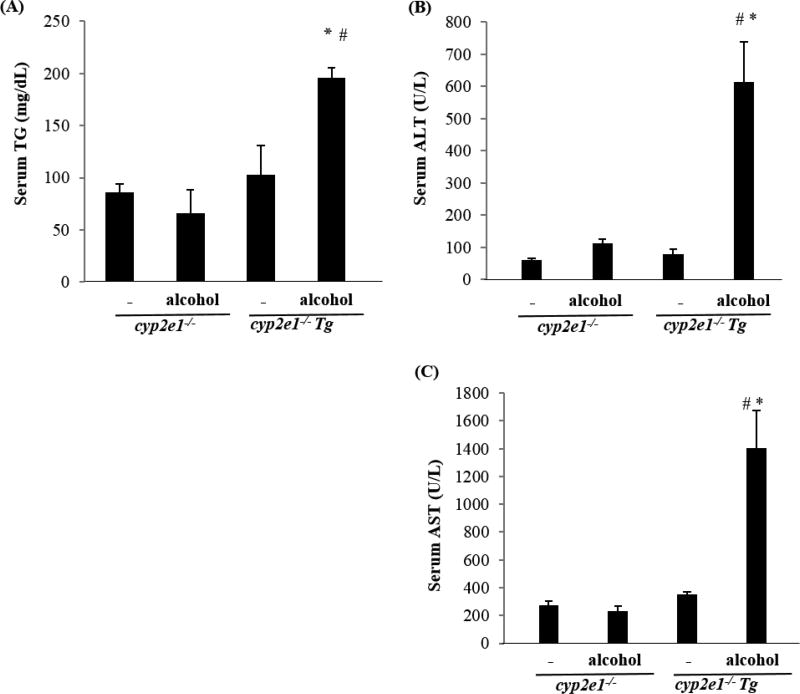
Binge alcohol induced liver injury in cyp2e1−/− Tg mice but not in cyp2e1−/− mice. The cyp2e1−/− mice and cyp2e1−/− Tg mice were treated with binge alcohol by gavage at 6 g/kg, 4 h later, blood was collected. (A) Serum TG; (B) Serum ALT; (C) Serum AST. * P<0.05, compared with cyp2e1−/− mice treated with binge alcohol; #P<0.05, compared with cyp2e1−/− Tg mice without alcohol treatment.
Figure 3.
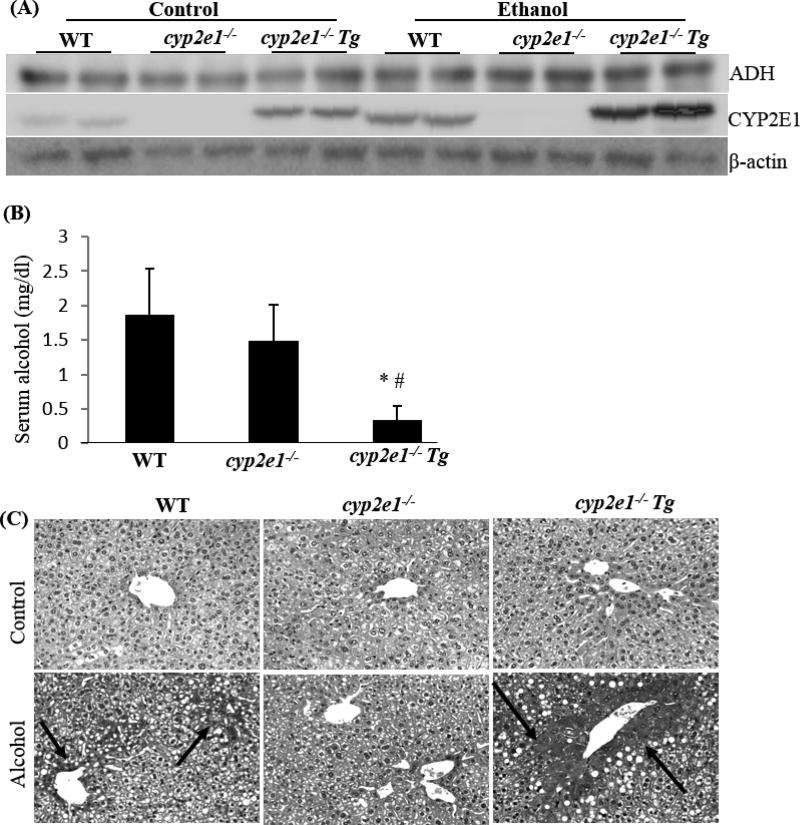
Alcohol induced hypoxia in cyp2e1−/− Tg mice to a greater extent than in cyp2e1−/− mice and WT mice. The mice were fed alcohol diet for 3 weeks. 30 min before being sacrificed, the mice were injected ip with pimonidazole, a hypoxia-specific marker. (A) Liver ADH and CYP2E1 expression; (B) Serum alocohol; * P<0.05, compared with cyp2e1−/− mice; #P<0.05, compared with WT mice. (C) Hypoxia detected by pimonidazole immunohistochemistry staining.
Figure 5.
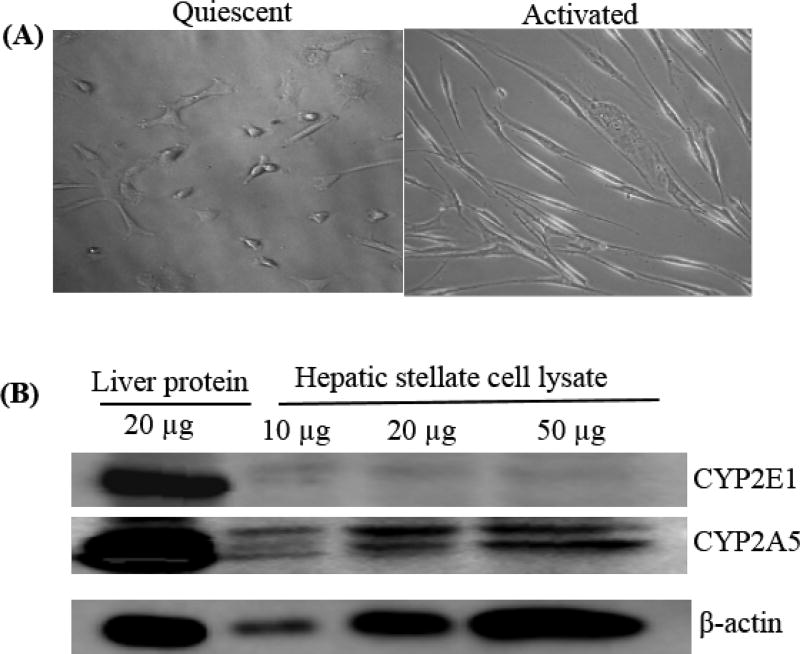
Hepatic stellate cells express CYP2A5 but do not express CYP2E1. (A) Morphology of isolated HSC. (B) HSC isolated from WT mice were cultured for 10 days, and then were collected for CYP2E1 and CYP2A5 analysis by Western blotting. Liver tissue homogenate was used as positive controls.
Figure 7.
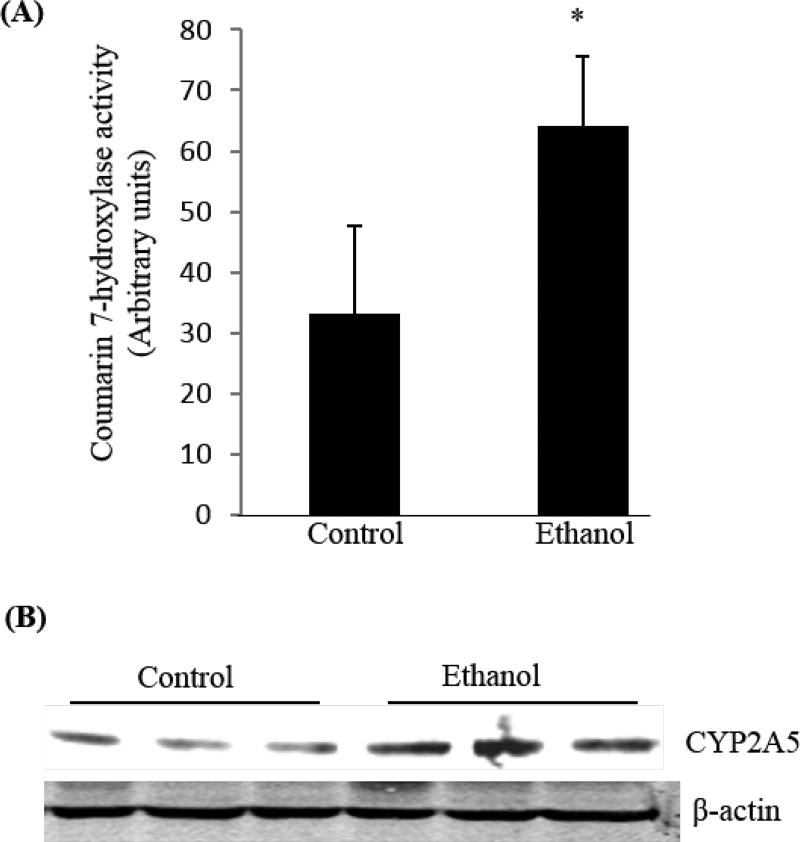
Alcohol feeding induced COH activity in lung. Male C57BL/6 mice were fed alcohol diet for 3 weeks. Microsomes were isolated from the lungs. (A) COH activity. *P<0.05, compared with WT mice. (B) CYP2A5 expression in lung was measured by Western blotting analysis.
2. CYP2E1 INDUCTION BY ALCOHOL
The most of toxic effects of ethanol are associated with its metabolism. Absorbed ethanol is oxidized principally in the liver. The well-known major pathway for ethanol disposition in the liver is the alcohol dehydrogenase (ADH) system [15, 16]. ADH oxidizes ethanol to acetaldehyde in the cytosol; the resulting acetaldehyde enters mitochondria and is subsequently metabolized by aldehyde dehydrogenase (ALDH) to acetate/acetyl CoA for energy production in mitochondria [15]. In addition to ADH, microsomes, which are mainly derived from endoplasmic reticulum (ER), can also oxidize alcohol to acetaldehyde by a pathway designated as the microsomal ethanol oxidizing system (MEOS). Cytochrome P450 enzymes (CYP), which are mainly located in smooth endoplasmic reticulum (SER), constitute the major components of MEOS. Chronic alcohol consumption results in a proliferation of SER [15, 16], and accordingly striking induction of certain CYPs. Three CYP isoenzymes are reported to be able to oxidize ethanol: CYP2E1, CYP1A2, and CYP3A4; CYP2E1 was reported to play the major role in the ethanol oxidation by microsomes and has been studied extensively in the field of ALD [15].
CYP2E1 has a relatively high Km for ethanol (8–10 mM for CYP2E1 vs 0.2–2 mM for hepatic ADH), so CYP2E1 is believed to only accounts for a small amount (about 10%) of total ethanol metabolism but it is more important after chronic alcohol consumption [5, 15]. Unlike the ADH system that requires NAD+ to serve as a co-factor to accept the hydrogens/ reducing equivalents from alcohol, CYP2E1 efficiently activates molecular oxygen (O2) and consumes NADPH provided by the NADPH-cytochrome P450 reductase, which results in the production of ROS [5, 15]. Microsomes from ethanol-treated animals displayed elevated rates of production of reactive oxygen species (ROS) and lipid peroxidation (LPO). At the microsomal level, increased generation of ROS by ethanol was thought to be through the induction of CYP2E1, because CYP2E1 exhibits enhanced NADPH oxidase activity as it is poorly coupled with NADPH-cytochrome P450 reductase [17, 18]. Microsomes from ethanol treated animals displayed elevated rates of generation of hydrogen peroxide and superoxide radical [5]. Increases in production of ROS after ethanol treatment are prevented by anti-CYP2E1 IgG, thus linking generation of ROS to the ethanol induction of CYP2E1 [5].
While CYP2E1 plays an important role in the oxidative stress produced by acute and chronic ethanol treatment, CYP2E1 was found to promote an initial upregulation of antioxidant defense in liver cells. Levels of GSH, content and catalytic activities of catalase, glutathione transferases (GST) and heme oxygenase 1 (HO-1) were elevated in HepG2 E47 cells which express CYP2E1 compared to the control HepG2 C34 cells which do not express CYP2E1 [19–21]. The increase in GSH was accompanied by a two-fold increase in mRNA levels of γ-glutamyl cysteine synthase, a rate limiting enzyme for GSH synthesis, both catalytic subunit (GCSC) and regulatory subunit (GCSR) [19–21]. The upregulation of these antioxidant enzymes in the E47 cells was blunted by N-acetyl-L-cysteine (NAC) and other antioxidants as well as by inhibitors of CYP2E1 leading to the proposal that ROS generated from CYP2E1 in the E47 cells led to the upregulation of these antioxidants [21–23]. Similar results were obtained with hepatocytes isolated from rats with elevated levels of CYP2E1 [21]. The significance of this upregulation was apparent from observations that removal of GSH or inhibition of these enzymes e.g. catalase, or HO-1, sensitized the E47 cells to the CYP2E1-generated oxidative stress and the toxicity of pro-oxidants [23]. These studies suggested that upregulation of antioxidant enzymes was an initial adaptive response of liver cells to the oxidative stress and toxicity promoted by CYP2E1; when the cells are subjected to further or other oxidative damage, these antioxidant enzymes can’t further be elevated to protect against the oxidative damage and thus cytotoxicity was enhanced. Therefore, alcohol induction of CYP2E1 make livers vulnerable to oxidative stress and longer term of alcohol consumption may contribute to the development of ALD.
3. CYP2E1 AND PATHOGENESIS OF ALCOHOLIC LIVER DISEASE
Oxidative stress contributes to ALD including alcoholic steatosis, alcoholic steatohepatitis, and alcoholic liver fibrosis and cirrhosis [5]. CYP2E1-mediated oxidative stress and ALD have been extensively studied. Studies in rodents showed that evident induction of CYP2E1 by chronic alcohol feeding parallels with significant ALD [24–26], and inhibition of CYP2E1 attenuated ALD [27, 28]. CYP2E1 inhibitors such as diallyl sulfide (DAS) [29], phenethyl isothiocyanate (PIC) [30, 31] and chlormethiazole (CMZ) [32], blocked hepatic LPO and ameliorated alcohol-induced hepatic pathological changes in rats. Polyenylphosphatidylcholine (PPC), which was effective in suppressing alcohol-induced oxidative stress [33], also had an inhibitory effect on CYP2E1 [34]. Bardag-Gorce et al [35] found that chronic ethanol-induced liver injury and oxidative stress were blunted in cyp2e1−/− mice. In contrast, ALD was enhanced in transgenic mice overexpressing mouse CYP2E1 gene [36].
We applied cyp2e1−/− mice, and cyp2e1−/− Tg mice to examine the role of CYP2E1 in ALD. While cyp2e1−/− mice are CYP2E1-deficient, cyp2e1−/− Tg mice are cyp2e1−/− mice reconstituted with human CYP2E1. These mice were developed in the laboratory of Dr. Fank Gonzalez, National Cancer Institute [37, 38] and generously provided by Dr. Gonzalez to allow evaluation of the role of CY2E1 in the biochemical and toxicological actions of ethanol. After chronic ethanol feeding, alcoholic fatty liver was observed in WT mice but not in the cyp2e1−/− mice [39, 40]. Ethanol-induced oxidative stress was higher in WT mice than in cyp2e1−/− mice. Peroxisome proliferator-activated receptor alpha (PPARα), a regulator of fatty acid oxidation, was upregulated by ethanol in cyp2e1−/− mice but not in WT mice. A PPARα target gene, acyl CoA oxidase (AOX), was decreased by ethanol in WT mice but not in cyp2e1−/− mice. CMZ, an inhibitor of CYP2E1, blunted alcoholic fatty liver in WT mice; when CYP2E1 was reintroduced into cyp2e1−/− mice by injection with adenovirus expressing CYP2E1, alcoholic fatty liver was restored [39]. When the human CYP2E1 gene was reintroduced and expressed in cyp2e1−/− mice (humanized cyp2e1−/− Tg mice), ethanol-induced steatosis was again observed, and liver necroinflammation and oxidant stress were even more severe than those observed in WT mice [40]. These experiments suggest that CYP2E1 plays an important role in alcoholic liver injury produced by oral alcohol feeding.
A common rodent model for chronic ALD is the Lieber-DeCarli model [41]. The Lieber-DeCarli diet is a high fat diet, including dextrose control diet and ethanol diet. The ethanol diet contains lower calories than the dextrose control diet, so animals will develop less steatosis in the ethanol diet group compared to the dextrose control diet group. However, when a certain amount of ethanol is supplied, the calories in the ethanol diet will become equal to the dextrose control diet, and animals will develop much more steatosis and steatohepatitis in the ethanol diet group compared to the dextrose control diet group. This shows synergism between ethanol and high fat. Usually, steatosis and steatohepatitis are evident in this model, but liver fibrosis is rare and serum ALT and AST were just slightly increased. Dr. Bin Gao’s group in NIAAA developed a Gao-Binge-on-chronic model e.g. 2 weeks of ethanol feeding followed by one single binge alcohol administration (5 g/kg) to enhance alcohol-induced liver injury (elevated serum ALT/AST) [42]. In the Gao-Binge-on-chronic model, both steatosis and serum ALT/AST are increased; however, liver fibrosis is still not evident. Common liver fibrosis models include carbon tetrachloride (CCL4) and thioacetamide (TAA). CCL4 and TAA are substrates of CYP2E1, both of them are bio-activated by CYP2E1 to hepatotoxins. CCL4- and TAA-induced liver injuries are CYP2E1-dependent because acute liver injury induced by CCL4 or TAA was observed in WT mice but not in the cyp2e1−/− mice [43, 44]. We treated mice with CCL4 or TAA twice a week for 1 month to induce liver fibrosis and found that CCL4 or TAA induced chronic liver injury and liver fibrosis in WT mice but not in cyp2e1−/− mice [45]. These results indicate that CYP2E1 metabolism is indispensable for CCL4- and TAA-induced liver injury, either acute or chronic. Moderate alcohol drinking plus lower dose of CCL4 (ethanol/CCL4) have been used for study of alcoholic liver fibrosis [46, 47], but apparently this model is not suitable for studying on the role of CYP2E1 in alcoholic liver fibrosis in cyp2e1−/− mice.
To examine the role of CYP2E1 in alcohol-induced liver fibrosis, we modified the Gao-Binge-on-chronic model to increase the times of alcohol binge administration and duration of the chronic ethanol feeding. The mice were fed for 5 weeks instead of 2 weeks, and binge ethanol was orally administrated at 5 g/kg weekly for 5 times instead of one single time during the chronic ethanol feeding. We found that chronic-binge alcohol-induced liver fibrosis was observed in cyp2e1−/− Tg mice but not in cyp2e1−/− mice (Fig. 1), suggesting a connection of CYP2E1 to alcohol-induced liver fibrosis.
In addition to a chronic alcohol feeding model, in binge alcohol models alcohol-induced liver injury is also CYP2E1-dependent. As mentioned above, CYP2E1 plays a major role in chronic ethanol metabolism and chronic alcohol feeding models were applied to study the role of CYP2E1 in ALD. Does CYP2E1 have an influence on acute alcohol-induced liver injury? Liver injury by different administration doses, times or patterns of acute or binge alcohol were investigated in cyp2e1−/− mice and WT mice. The acute-alcohol-induced liver injuries in these different models were all less severe in cyp2e1−/− mice than in WT mice [48, 49], suggesting that acute alcohol-induced liver injury is also CYP2E1-dependent. We treated cyp2e1−/− mice and cyp2e1−/− Tg mice with binge alcohol at 6 g/kg: 4 h later, serum TG, ALT, and AST were all increased in cyp2e1−/− Tg mice but not in cyp2e1−/− mice (Fig. 2). Interestingly, the difference in serum TG, ALT, and AST was observed in older mice (older than 10 months) but not in younger mice (3-month old), suggesting that aging is a risk factor for CYP2E1-mediated ALD. This requires further study.
Binge alcohol can induce severe steatohepatitis in obese animals [50, 51]. Binge alcohol induced CYP2E1 in obese fa/fa rats to a greater extent than in lean Fa/? rats, and steatohepatitis was also more severe in in obese fa/fa rats than in lean Fa/? rats [51]. Oral administered ethanol induced massive steatohepatitis in Otsuka Long-Evans Tokushima fatty (OLETF) rats but not in control Otsuka Long-Evans Tokushima (OLET) rats. Staining for CYP2E1 demonstrated marked increases in the hepatic tissue of ethanol-treated OLETF rats compared with ethanol-treated OLET rats [52]. These studies suggest that CYP2E1 may play a significant role in alcohol-enhanced steatohepatitis.
4. CYP2E1 AND HYPOXIA
CYP2E1-mediated ethanol metabolism directly consumes O2, and ethanol consumption can induce hypoxia, which is considered as an important risk factor for liver injury. Thus, CYP2E1 might play an important role in ethanol-induced hypoxia. We applied wild type mice (WT), cyp2e1−/− mice, and cyp2e1−/− Tg mice in which the human CYP2E1 gene was reintroduced and expressed in cyp2e1−/− mice to examine the role of CYP2E1 in ethanol metabolism and development of hypoxia. After chronic ethanol feeding, CYP2E1 was induced much more dramatically in the cyp2e1−/− Tg mice than in WT mice, but liver ADH levels was comparable in these mice (Fig. 3A). While blood ethanol levels are comparable in WT mice and cyp2e1−/− mice, blood levels of ethanol in cyp2e1−/− Tg mice were strikingly decreased (Fig. 3B), suggesting a relationship between CYP2E1 induction and alcohol metabolism (clearance). Ethanol metabolism by ADH and CYP2E1 may consume oxygen and induce hypoxia [53]. Hypoxia was higher in the ethanol-fed cyp2e1−/− Tg mice than in the ethanol-fed WT mice and lowest in the ethanol-fed cyp2e1−/− mice (Fig. 3C). These results suggest that highly induced CYP2E1 metabolize more ethanol, which consumes more oxygen and induces more severe hypoxia.
Hypoxia Inducible factors (HIFs) are a family of transcription factors in important transcriptional response to low oxygen tension, which promotes cell homeostasis under hypoxia. Mature HIF is composed of one of three isoforms of an alpha- subunit (HIF-1α, HIF-2α, or HIF-3α) and a β subunit, HIF-1β which is also termed the Aryl-Hydrocarbon Receptor Nuclear Translocator (ARNT). Under normal conditions, the alpha subunits of HIF are rapidly hydroxylated, ubiquitinated, and degraded. Under hypoxia, HIF alpha subunits are stabilized and dimerize with HIF-1β/ARNT, translocate to the nucleus, and activate hypoxia response elements (HRE) [54]. HIF-1α can be induced by alcohol consumption in the liver [48, 55]. In humans, CYP2E1 activity and protein levels were found to be positively correlative with liver HIF-1α levels and blood alcohol concentrations, and binge alcohol promoted apoptosis in liver with significant correlations between CYP2E1 and HIF-1α. Binge alcohol-induced HIF-1α activation and subsequent apoptosis was more severe in WT mice than in cyp2e1−/− mice [48]. In a chronic model, HIF-1α and HIF-1α-regulated downstream targets were elevated in the ethanol-fed cyp2e1−/− Tg mice compared to the WT and cyp2e1−/− mice; HIF prolyl hydroxylase 2, a degrader of HIF-1α, was reduced in the ethanol-fed cyp2e1−/− Tg mice in association with the increases in HIF-1α [56]. These results suggest that CYP2E1 contributes to activation of HIF-1α. In CYP2E1 over-expressing HepG2 cells, 2-Methoxyestradiol, an inhibitor of HIF-1α, blunted the ethanol-related cytotoxic effects in association with inhibition of HIF-1α, suggesting that HIF-1α contributes to CYP2E1-dependent ethanol-induced cytotoxicity [56]. In a binge model, a HIF-1α inhibitor, PX-478, prevented alcohol-induced HIF-1α elevation and apoptosis in mice [48]. The results from an in vitro cell model, in vivo mouse models, and human samples support the roles of CYP2E1 in HIF-1α induction and ALD development.
5. CYP2E1 AND AUTOPHAGY
In cells, long-lived proteins and cellular organelles become damaged. Cells develop a mechanism called autophagy to degrade and recycle those damaged protein or organelles through lysosomes. This pathway helps maintaining cellular energy homeostasis under conditions of limited nutrients or stress by degrading cellular components [57–61]. The process of autophagy is complex, and the regulation of this process is controlled by the coordinated actions of autphagy-related genes (Atgs). Removal of damaged mitochondria by cells is via a process of mitophagy and removal of lipid droplets by cells is via lipophagy, both of these are selective forms of macroautophagy [59–61]. Removal of damaged mitochondria protects the cell against mitochondrial oxidative stress, while removal of lipid droplets limits the accumulation of lipids by hepatocytes in liver and adipocytes in adipose tissue. Defects in lipophagy can contribute to hepatic steatosis, and hepatic lipid accumulation also decreases autophagy; hepatic steatosis might be a mechanism of defective autophagy in liver [62–64]. Autophagy is generally considered as a cell survival pathway but it can also mediate cell death under certain conditions or when over-activated.
Autophagy can either be increased or decreased by ethanol depending on the model used, the dose, the tissue evaluated and the experimental condition [65–72]. Chronic ethanol consumption was recently shown to increase autophagy [68, 71] and in some cases this increase was associated with a decrease in activity of the other major cellular proteolyic system, the proteasome complex [72]. Many studies suggest that autophagy serves a protective function against ALD [60, 65, 66]. For example, in HepG2 E47 cells which express CYP2E1, ethanol-induced cytotoxicity, intracellular fat accumulation and generation of ROS were potentiated by 3-methyladenine (3-MA), an inhibitor of autophagy, but these toxic effects were decreased by rapamycin, which stimulates autophagy [73], suggesting that autophagy protects against ethanol-induced cytotoxicity. The fact that no effect was found with C34 control HepG2 cells which do not express CYP2E1 suggests that autophagy protection is associated with CYP2E1. Similarly, other CYP2E1-related toxicity models such as those induced by CCL4, arachidonic acid (AA), or depletion of GSH by buthionine sulfoximine in the E47 cells, were also enhanced by 3-MA or by SiRNA against Atg 7 [74]. In an acute alcohol model, there was modest liver injury, fat accumulation and increased ROS production in WT and cyp2e1−/− Tg mice but not in cyp2e1−/− mice [75]. These effects were magnified by 3-MA injection but blunted by rapamycin injection along with ethanol [75].
Autophagy is also protective against CYP2E1-dependent liver injury after chronic ethanol treatment [76]. WT mice, cyp2e1−/− mice, and cyp2e1−/− Tg mice were fed an ethanol liquid diet for 4 weeks. In the last week, some mice received 3-MA, an inhibitor of autophagy, or rapamycin, an inducer of autophagy. The autophagy inhibition by 3-MA or induction by rapamycin was indicated by decreased or increased levels of Beclin-1 and Atg 7, respectively, but increased or decreased levels of p62, respectively. While the inhibition of autophagy by 3-MA enhanced the ethanol-induced increases in serum levels of ALT, AST, and TG in the WT and cyp2e1−/− Tg mice but not in cyp2e1−/− mice, rapamycin prevented the ethanol-induced liver injury in WT mice and cyp2e1−/− Tg mice. Treatment with 3-MA potentiated the ethanol-induced hepatic fat accumulation in WT mice and caused hepatocyte necrosis in the cyp2e1−/− Tg mice, but little or no effect was found in the ethanol-fed cyp2e1−/− mice [76]. These results suggest that autophagy is protective against CYP2E1-dependent liver injury in the chronic ethanol-fed mouse model. These experiments, including in vitro and in vivo, acute and chronic models of ethanol intake, support the notion that autophagy is protective against CYP2E1-dependent ALD.
6. CYP2E1 AND LPS/TNFα
Not all studies support the CYP2E1’s contributive effect on ALD. For example, Kono et al did not observe any differences in steatosis and steatohepatitis between WT mice and cyp2e1−/− mice in an intragastric infusion model [77]. Instead, many evidences support the idea that endotoxemia is a major contributor to ALD [78]. Lipopolysaccharide (LPS) is a component of the outer wall of gram-negative bacteria. Ethanol consumption can alter microflora which normally inhabit the gut and increase the permeability of the gut, thus elevating the absorption of LPS from the gut lumen into the portal circulation (endotoxemia). Kupffer cells, the resident macrophages in liver, are activated by LPS and release chemical mediators including tumor necrosis factor (TNFα) and ROS or reactive nitrogen species (RNS), which subsequently causes the development of ALD [79, 80]. Indeed, destruction of Kupffer cells with gadolinium chloride attenuated ALD [81] and anti-TNFα antibodies protect against ALD [82]. The role of TNFα in ALD was further validated by the findings that the ethanol-induced pathology was nearly blocked in TNFα receptor 1 knockout mice [83]. In vitro studies showed that osteopontin (OPN) can bind to LPS and blunt LPS-activated production of TNFα in macrophage cells [84]. In in vivo studies, bovine milk osteopontin (mOPN) powder supplemented into the ethanol diet at 200 mg/L can protect against ALD as indicated by reduced serum ALT/AST levels, decreased hepatic triglyceride levels, less hepatic neutrophil infiltration, and decreased translocation of gram-negative bacteria. Serum levels of LPS and TNF-α were lowered by mOPN, too. These results suggest that a LPS-TNFα axis plays an important role in the development of ALD [85].
6.1 CYP2E1 promotes gut LPS transporting into blood
The Keshavarzian and the BJ Song groups initiated studies on CYP2E1 and intestinal LPS translocation. CYP2E1 protein in colon tissue from chronic alcohol-fed mice was increased [86]. To test whether colon CYP2E1 contributes to gut LPS leaking, Caco-2 cells were applied. Caco-2 cells are human intestinal epithelial cells, which can grow confluent monolayer and are often used as an in vitro model to test intestinal epithelium permeability. When Caco-2 cell monolayers were incubated with alcohol, the monolayers exhibited an increase in permeability. When Caco-2 cells were exposed to alcohol, CYP2E1 protein and activity were elevated. When CYP2E1 expression was knocked down via siRNA, alcohol-induced hyperpermeability was decreased. N-acetylcysteine (NAC), an antioxidant, prevented the alcohol effects [86, 87]. These data suggest a possible novel role for intestinal CYP2E1 in alcohol-induced intestinal hyperpermeability via CYP2E1-dependent induction of oxidative stress.
Results from in vivo studies with WT mice and cyp2e1−/− mice are consistent with the Caco-2 in vitro data [88]. The mice were treated with three doses of binge ethanol at 6g/kg by oral gavage at 12-h intervals. Epithelial alteration and blebbing of lamina propria in intestines, which were accompanied by increased levels of serum endotoxin and TNFα, were only observed in WT mice but not in cyp2e1−/− mice. Likewise, elevated steatosis and scattered inflammatory foci in liver were also observed in WT mice but not in cyp2e1−/− mice. The above changes were significantly reversed by a specific inhibitor of CYP2E1 (CMZ) and an antioxidant (NAC). These data suggest that intestinal CYP2E1 is critical in binge alcohol-induced increased gut leakage, endotoxemia, and resultant steatosis and steatohepatitis [88].
6.2 CYP2E1 potentiates LPS-induced liver injury
In the intragastric infusion model, the ethanol diet is fed through catheters surgically installed on the wall of the stomach, which may produce endotoxemia and have masked differences in ALD between WT and cyp2e1−/− mice [77]. We examined whether CYP2E1 synergizes with LPS to promote ALD. Besides ethanol, pyrazole, a potent inhibitor of alcohol dehydrogenase and of the oxidation of ethanol, is a well characterized inducer of CYP2E1 [4, 5]. Rats were injected intraperitonally with pyrazole at 200 mg/kg body wt, once a day for 2 days, which causes hepatic CYP2E1 induction but without evident liver injury. Following pyrazole induction of CYP2E1, LPS was injected via the tail vein at 10 mg/kg body wt. Neither pyrazole alone nor LPS alone caused liver injury as reflected by serum levels of ALT/AST or liver histopathology. However, the combination of LPS plus pyrazole increased serum ALT/AST about four-fold over the levels in the pyrazole alone or LPS alone groups, suggesting that CYP2E1 inducer pyrazole enhances LPS liver injury in rats [89]. To test whether pyrazole enhancing effects on LPS liver injury is CYP2E1 dependent, WT mice and cyp2e1−/− mice were injected with pyrazole (150 mg/kg), LPS (4 mg/kg), or combination of pyrzole plus LPS. While pyrazole alone or LPS alone caused no liver injury, the combination of pyrazole and LPS induced severe liver injury in WT mice but not in cyp2e1−/− mice, suggesting that CYP2E1 is indispensible for the pyrazole enhancing effect on LPS liver injury. The role of CYP2E1 in the potentiation of LPS toxicity by pyrazole was validated by experiments with CMZ, an inhibitor of CYP2E1, and cyp2e1−/− mice [90].
LPS can induce nitric oxide synthase (NOS) and cause an increase in RNS including nitric oxide (NO) and peroxynitrite (OONO-) production, which results in protein nitration. Level of 3-nitro-tyrosine protein adduct (3-NT), a marker for oxidized nitrated protein formation, was lower in saline control livers. Treatments with either LPS alone or pyrazole alone slightly elevated 3-NT protein adduct levels; however, striking increases in 3-NT were found in the combined LPS plus pyrazole group, which was also observed in WT mice but not in cyp2e1−/− mice. Thus CYP2E1 enhances LPS liver injury might be through LPS-stimulated mediators such as RNS [89, 90]. In addition, LPS can potentiate ALD [91–94]. We found that LPS injection following chronic alcohol feeding enhanced ALD in WT mice to a greater extent than in cyp2e1−/− mice, suggesting that CYP2E1 contributes to LPS-enhanced ALD [39].
6.3 CYP2E1 Potentiates TNFα-induced liver injury
In addition to RNS, TNFα is a major proinflammatory mediator of LPS. TNFα levels are elevated after LPS administration and TNFα plays an important role in the effects of LPS. Does pyrazole induction of CYP2E1 potentiate TNFα toxicity? The same approaches for pyrazoe plus LPS were used, with injection of TNFα (50 µg/kg body wt) replacing the LPS treatment [95, 96]. Treatment with TNFα elevated serum ALT/AST levels by about 2–3-fold, but treatment with pyrazole/TNFα elevated serum ALT/AST levels by more than 3-fold over the TNFα alone-treated mice. Necrotic loci were observed in liver sections from pyrazole/TNFα-treated mice but not in sections from pyrazole alone- or TNFα alone-treated mice. These results suggest that the combined pyrazole plus TNFα treatment produces more severe liver injury compared to TNFα alone or pyrazole alone. After TNFα administration to pyrazole-treated mice, large increases in serum ALT and AST levels were found in WT mice but not in cyp2e1−/− mice. After pyrazole plus TNFα treatment, severe liver necrosis was observed in WT mice, but normal liver pathology was observed in cyp2e1−/− mice. The failure of TNFα to induce liver injury in pyrazole-treated cyp2e1−/− mice supports a critical role for CYP2E1 in the potentiated injury observed in the WT mice.
6.4 MAP Kinases and pyrazole/TNFα-induced liver injury
Mitogen-activated protein kinases (MAPKs), a group of serine-threonine kinases, mediate intracellular signaling associated with a variety of cellular activities including cell proliferation, differentiation, survival, death, and transformation. The mammalian MAPK family consists of extracellular signal-regulated kinase (ERK), p38 MAPK, and c-Jun NH2-terminal kinase (JNK) [97]. The MAPK signaling cascade includes MAP kinase (MAPK), MAPK kinase (MAPKK), and MAPKK kinase (MAPKKK). MAPKKK phosphorylates and thereby activates MAPKK, which in turn phosphorylates and activates MAPK. Activated MAPK translocates to the cell nucleus to regulate activities of transcription factors and thereby control gene expression [98]. In either in vivo or in vitro models of ALD, an increase of gene expression and activation of the MAPK pathway was found [99–101].
We applied CYP2E1 overexpressing HepG2 cells (E47 cells) to examine whether CYP2E1-dependent cytotoxicity is associated with ERK, JNK or p38 MAPK. Cisplatin, a potent anticancer drug, has adversary effects such as kidney damage, liver injury, and ototoxicity. Cisplatin cytotoxicity was increased in E47 cells compared to control HepG2 cells without CYP2E1 expression (C34 cells), suggesting that CYP2E1 contributes to cisplatin cytotoxicity [102]. ERK and JNK phosphorylation was observed in both E47 cells and C34 cells. While JNK phosphorylation was comparable in E47 and C34 cells, ERK phosphorylation was stronger in E47 cells than in C34 cells, suggesting that ERK but not JNK phosphorylation is associated with CYP2E1. Indeed, inhibition of JNK by SP600125 had no effect on cisplatin cytotoxicity. Inhibition of ERK by U0126 protected against cisplatin cytotoxicity, suggesting that ERK activation contributes to cisplatin cytotoxicity but unlike the usually protection by ERK against cell injury [102]. While cisplatin treatment did not activate P38 MAPK, the polyunsaturated fatty acid arachidonic acid (AA) phosphorylated P38 MAPK in E47 cells. SB203580, a p38 MAPK inhibitor, prevented AA cytotoxicity in E47 cells [103]. These results suggest that different MAPK pathways play different roles in different models of toxicity.
We evaluated for possible activation of MAP kinases in ur pyrazole/LPS or pyrazole/TNFα hepatotoxicity models. LPS treatment alone or pyrazole alone did not cause significant phosphorylation of JNK or p38 MAPK. However, both JNK and p38 MAPK were phosphorylated in livers of the pyrazole plus LPS-treated mice. ERK activation was not detectable. A similar hepatic activation of JNK and p38 MAPK was also observed in mice treated with pyrazole plus TNFα but not in mice treated with TNFα alone [95]. Administration of SP600125 or SB203580 prevented liver injury induced by LPS or TNFα plus pyrazole. These results suggest that the CYP2E1 elevation of LPS/TNFα liver injury is MAPK dependent.
JNK is encoded for by three genes, each of which is alternatively spliced to yield α and β forms of both a 54 and 46 kDa protein. In hepatocytes, only two of the genes, JNK1 and JNK2 are expressed [104]. Mice deficient in either JNK1 (jnk1−/− mice) or JNK2 (jnk2−/− mice) are viable but double knockouts are lethal, suggesting some redundant functions [104]. Liver injury produced by either LPS/D-galactosamine or TNFα/D-galacosamine was comparable in WT and jnk1−/− mice but lower injnk2−/− mice [105]. Singh et al. [106] reported that jnk1−/− mice fed a high fat diet did not gain weight or develop steatohepatitis as did the WT and jnk2−/− mice. We evaluated whether JNK1 or JNK2 or both play critical roles in the pyrazole/TNFα-induced hepatotoxicity [107]. Male jnk1 −/−, jnk2−/−, and WT mice were injected either pyrazole alone, TNFα alone, or TNFα following pyrazole pretreatment. Liver injury was significantly higher in the WT and jnk2−/− mice treated with pyrazole plus TNFα than that in the jnk1−/− mice treated with pyrazole plus TNFα, suggesting that JNK1 but not JNK2 plays a major role in pyrazole/ TNFα-induced liver injury. Mechanisms responsible for this JNK 1-dependent toxicity require further study.
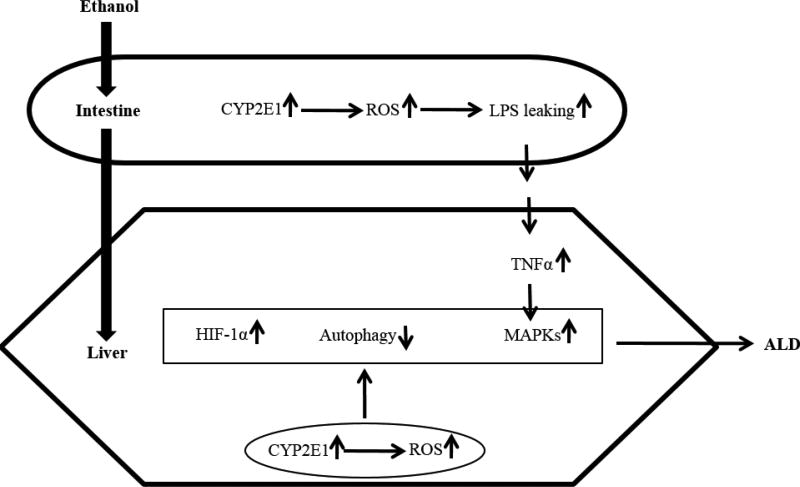
Relationship between CYP2E1 and ALD summarized in this review is schematically presented in Figure 4. Alcohol in intestines induces CYP2E1, which causes lumen LPS translocating to blood and entering liver. In liver, LPS induces production of TNFα and subsequent activation of MAPKs, which causes liver injury. Alcohol induction of CYP2E1 in liver leads to ROS generation and results in HIF-1α induction, autophagy inhibition, and activation of MAPKs. All these events contribute to ALD. While liver CYP2E1 inhibition is being considered in the therapy strategy, intestine CYP2E1 should not be ignored as intestine CYP2E1 promotes alcoholic inflammation by promoting LPS leakiness in guts.
Figure 4.
Schema of CYP2E1-mediated liver injury.
7. CYP2E1-CYP2A5 INTERACTIONS
Pyrazole is also an effective inducer of CYP2A5 in mice [108]. While CYP2E1 was induced by pyrazole in WT mice but not in cyp2e1−/− mice, CYP2A5 induction was also higher in WT mice than in cyp2e1−/− mice [90]. When CYP2E1 was inhibited by CMZ, CYP2A5 was also decreased [90]. These suggest that CYP2E1 has an influence on CYP2A5 induction by pyrazole. Does ethanol induce CYP2A5? If yes, is ethanol induction of CYP2A5 also CYP2E1-dependent? In human, is CYP2A6 induction also associated with CYP2E1? In patients with ALD, both CYP2E1 and CYP2A6 expression were found to be induced in the livers [109]. In vitro studies showed that ethanol treatment up-regulated CYP2A6 expression in a human U937 monocyte cell line, which is also associated with CYP2E1 upregulation [110]. However, when drinking water containing ethanol was fed to mice, activity of COH, a CYP2A5 marker enzyme, was not induced [111]. We examined mice subjected to oral alcohol feeding with a Lieber-DeCarli liquid diet and found that COH activity was increased dramatically, and CYP2A5 protein and mRNA levels were strikingly up-regulated [12, 13]. Interestingly, a time course study showed that elevation of CYP2E1 activity was earlier than CYP2A5 elevation. In rats, CYP2A3, the ortholog to mouse CYP2A5 and human CYP2A6, is not detectable in the liver, even after treatments with pyrazole [112, 113]. Most studies on microsomal ethanol metabolism were performed with rat microsomes, but COH activity (CYP2A3) is absent in rat liver. This might be the major reason why ethanol induction of COH was not detected before.
Considering the well-known increase in CYP2E1 by ethanol, we were especially interested in whether CYP2E1 also affects ethanol induction of CYP2A5. The Lieber-DeCarli diet has been widely used to study the induction of CYP2E1 [114]. Therefore, the Lieber-DeCarli model was applied in both chronic and acute model to study the interaction between CYP2E1 and CYP2A5 [12, 13]. After one week of ethanol feeding, CYP2E1 was induced but CYP2A5 was not yet induced. After two weeks of ethanol feeding, CYP2A5 was induced and CYP2E1 remained elevated. After 3 weeks of ethanol feeding, CYP2A5 was further increased and CYP2E1 still remained the similar levels to the 2 weeks of feeding. These results suggest that CYP2E1 induction by ethanol is earlier than CYP2A5 induction. Interestingly, those zones of the liver from the ethanol-fed mice with the highest levels of CYP2E1 as detected immunohistochemically also contained the highest amounts of CYP2A5, i.e. CYP2E1 and 2A5 co-localized in the liver. With respect to human liver, the levels of both CYPs varied widely from liver to liver. The levels of CYP2A6 paralleled the levels of CYP2E1. Those livers with lower CYP2E1 content also had lower CYP2A6 content. These studies suggest a close association between induction of CYP2E1 and induction of CYP2A5/2A6.
To examine possible association between CYP2E1 and 2A5 more directly, we determined if chronic ethanol feeding induced CYP2A5 in cyp2e1−/− mice and cyp2e1−/− Tg mice, in which the human CYP2E1 is added back into the cyp2e1−/− mice to reestablish expression of human CYP2E1 [38, 115]. While CYP2A5 was induced in WT mice, there was no increase in CYP2A5 in the cyp2e1−/− mice; when CYP2E1 is restored in cyp2e1−/− Tg mice, ethanol feeding resulted in an induction of CYP2A5 in the cyp2e1−/− Tg mice. These results indicate that induction of CYP2A5 by chronic ethanol requires CYP2E1.
To further validate the role of CYP2E1 in the induction of CYP2A5 by chronic ethanol feeding, the effect of CMZ, an effective chemical inhibitor of CYP2E1, was determined. CMZ has been previously used to block ethanol-induced liver pathology in the intragastric infusion model of chronic ethanol feeding [32] and to block ethanol-induced steatosis and oxidant stress in the Lieber-DeCarli liquid ethanol diet model [39]. Treatment with CMZ decreased the chronic ethanol–induction of CYP2E1 and 2A5 comparably. Thus, either genetic knockout of CYP2E1 or chemical inhibition of CYP2E1 blunts ethanol induction of CYP2A5
As mentioned above, one week of chronic ethanol feeding did not elevate CYP2A5 levels in WT mice although CYP2E1 was elevated two-fold. We evaluated whether earlier ethanol induction of CYP2A5 could be developed using the cyp2e1−/− Tg mice with higher levels of CYP2E1 than the WT mice. The cyp2e1−/− mice and cyp2e1−/− Tg mice were fed the Lieber-DeCarli liquid diet for 1, 2 and 3 days instead of 1, 2,and 3 weeks. CYP2E1 increased after 1 to 3 days of the ethanol feeding respectively in the cyp2e1−/− Tg mice. With respect to CYP2A5, in the cyp2e1−/− Tg mice there was no induction after 1 day of the ethanol feeding but CYP2A5 was increased after 2 and 3 days of ethanol feeding, respectively. No such increases were found with the cyp2e1−/− mice. Thus, acute ethanol feeding can induce CYP2A5 when levels of CYP2E1 are high and this induction occurs after CYP2E1 is initially and sufficiently elevated.
Is induction of CYP2E1 by ethanol modulated by CYP2A5? The above experiments indicate that acute and chronic ethanol induction of CYP2A5 is strongly potentiated by CYP2E1. Is ethanol induction of CYP2E1 influenced by CYP2A5? The cyp2a5−/− mice were provided as a generous gift from Dr Xinxin Ding (Wadsworth center, Albany NY) and fed ethanol chronically along with their genetic control WT mice. The ethanol feeding produced a comparable 2.5–3 fold increase in CYP2E1 protein and catalytic activity in the WT and the cyp2a5−/− mice as compared to dextrose-fed controls. The ethanol feeding elevated CYP2A5 protein and activity in the WT mice and not in the cyp2a5−/− mice. Thus, the increase in CYP2E1 by ethanol is independent of the presence of CYP2A5, in contrast to the increase in CYP2A5 by ethanol which requires CYP2E1.
In other experiments we found that cadmium induction of CYP2A5 occurred to similar extents in cyp2e1−/− Tg mice and cyp2e1−/− mice, indicating that CYP2A5 is capable of being induced in the cyp2e1−/− mice, depending on the inducer i.e. the cadmium induction of CYP2A5 is independent of CYP2E1. Thus, CYP2E1 and CYP2A5/6 seem to be interactive during their co-induction by ethanol and pyrazole but not by cadmium.
8. MECHANISM OF ETHANOL INDUCTION OF CYP2A5
Induction of CYP2E1 by chronic ethanol is largely through a posttranscriptional mechanism involving stabilization of the CYP2E1 protein by ethanol against proteasome-mediated degradation, but CYP2E1 mRNA levels are not altered [116, 117]. However, 3 weeks of ethanol feeding did increase CYP2A5 mRNA levels. Thus, while chronic ethanol feeding increases two important CYPs in the liver, the mechanism of induction for each CYP appears to differ. Hepatic CYP2A5 mRNAs can be induced by a PPARα ligand, Wy14,643 [118]; suppression of CYP2A5 expression by LPS was abolished in PPARα KO (pparα−/−) mice (119). PPARα is a well-known ALD regulator [120]. We therefore examine whether PPARα regulates the ethanol induction of CYP2A5. The pparα−/− mice and WT mice were fed ethanol diets for 3 weeks. CYP2E1 and CYP2A5 expression and activities were identically induced in WT mice and pparα −/− mice, suggesting that PPARα doesn’t regulate the ethanol induction of CYP2A5 [14, 121].
The redox-sensitive transcription factor nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2) is a leucine zipper transcription factor which binds to the electrophilic- or antioxidant-response element of many proteins to upregulate their expression [122–131]. Under basal cellular conditions, Nrf2 activation and translocation to the nucleus is suppressed by its binding to Keap1 (Kelch-like erythroid cell derived protein with CNC homology-associated protein 1). Modification of thiol groups of Nrf2 and/or Keap 1 by oxidants and electrophiles dissociates Nrf2 from Keap1, followed by degradation of Keap1 by the proteasome complex and translocation of Nrf2 into the nucleus where it can dimerize with small Maf proteins to function as a transcription factor [122, 123, 126]. CYP2A5 and 2A6 are regulated by Nrf2 [132–135]. Is the ethanol induction of CYP2A5 regulated by Nrf2? Alcohol-induced liver injury was enhanced in Nrf2 KO (Nrf2−/−) mice [136]. The alcohol feeding comparably induced CYP2E1 in the WT and Nrf2−/− mice, suggesting that Nrf2 did not significantly modulate levels of CYP2E1 or its induction by alcohol [136]. Samples of liver from these mice were kindly provided to us by Dr. Vogel of the study in [136]. Our results show that induction of CYP2A5 protein by alcohol was lower in the Nrf2−/− mice (1.6 fold) than the WT mice (2.5 fold) and CYP2A5 catalytic activity was increased 9-fold by alcohol in the WT mice as compared to a 4-fold increase in the Nrf2−/− mice. Thus, maximal induction of CYP2A5 by alcohol requires Nrf2.
In view of the induction of CYP2E1 by ethanol, the increase in ROS by CYP2E1, the activation of Nrf2 by CYP2E1-derived ROS, and the critical role of Nrf2 in regulating the expression of the cyp2a5 gene, a hypothesis was formulated in which ethanol induction of CYP2E1 leads to an increase in ROS, which then leads to activation of Nrf2, which then activates expression of CYP2A5. The next studies evaluated whether the acute and chronic ethanol treatments activated a CYP2E1-ROS-Nrf2-CYP2A5 pathway in mouse livers.
One day alcohol feeding resulted in an increase in hepatic Nrf2, nuclear Nrf2, and Nrf2-DNA binding activity in cyp2e1−/− Tg mice but not in the cyp2e1−/− mice. Thus, CYP2E1 was required for acute ethanol activation of Nrf2. The acute ethanol activation of Nrf2 was blocked when the cyp2e1−/− Tg mice were treated with antioxidants NAC or vitamin C (Vc), indicating that CYP2E1-derived ROS is important in the acute ethanol activation of Nrf2. The acute ethanol feeding elevated both CYP2E1 and CYP2A5. Treatment with the antioxidants blunted the increase in CYP2A5 but had no effect on the induction of CYP2E1 by the acute ethanol treatment. Thus, the induction of CYP2A5 but not CYP2E1 by acute ethanol involves, at least in part, a role for ROS. Similar results were found for the chronic ethanol feeding model as Nrf2 was activated in WT mice fed ethanol for 3 weeks. Feeding ethanol chronically along with either NAC or Vc blunted activation of Nrf2 and this was associated with prevention of the induction of CYP2A5, but not CYP2E1.
Does alcohol-induced human CYP2A6 share the mechanism similar to the alcohol induction of CYP2A5? CYP2A6, like CYP2A5, has been shown to be regulated by Nrf2 [135]. As mentioned above, there appears to be an association between levels of CYP2A6 and CYP2E1 in human livers from patients with alcoholic liver disease and with cirrhosis. Perhaps such livers can be assayed for levels of Nrf2 or extent of oxidative stress. Incubation of human hepatocytes with ethanol and determining whether an in vitro system for alcohol induction of CYP2A6 can be developed might prove useful for this. The human monocyte cell line U937 when incubated with ethanol exhibited higher levels of CYP2A6 [110]. Blood monocytes isolated from alcoholic patients may be a simple and feasible way to examine ethanol induction of CYP2A6 in humans.
9. CYP2A5 AND ALCOHOLIC LIVER DISEASE
Besides generating oxidants including ROS, CYP2E1 can also upregulate antioxidants, which may reflect an adaptive mechanism to remove CYP2E1-derived oxidants. Due to the increase in ROS produced by the elevated levels of CYP2E1, Nrf2 is upregulated in CYP2E1 over-expressing HepG2 (E47) cells and livers from ethanol-fed rodents [137, 138]. Nrf2 up-regulates the expression of those antioxidant enzymes (such as HO-1, GCSR, and GCSC) that were found to be elevated in E47 cells and liver cells with high expression of CYP2E1. Nrf2 mRNA levels and Nrf2-DNA binding activity were two-fold higher in the E47 cells. Transfecting the E47 cells with SiRNA Nrf2 lowered levels of Nrf2 mRNA and protein as compared to SiRNA scramble and this resulted in a decline in levels of the upregulated antioxidant enzymes and GSH. Formation of ROS and LPO were enhanced in the E47 cells treated with SiRNA Nrf2, and cell viability was lowered. Thus, the upregulation of Nrf2 in response to CYP2E1-derived ROS in the E47 cells results in elevated antioxidant defense in these cells. Ethanol-induced liver injury was more severe in Nrf2−/− mice compared with WT mice, suggesting that upregulation of Nrf2 may protect against ALD [136]. The CYP2E1-dependent induction of CYP2A5 by ethanol is regulated by Nrf2, thus the ethanol-induced CYP2A5 may be among the panel of Nrf2-regulated antioxidant genes to protect against ALD.
We treated WT and cyp2a5−/− mice with alcohol and measured biochemical assays of liver function to assess the possible effects of CYP2A5 on the alcohol actions. Our results suggest that in contrast to CYP2E1, CYP2A5 protects against and does not promote alcohol-induced oxidative stress. After ethanol feeding, thiobarbituric acid-reactive substances (TBARS), a marker of LPO, was increased by ethanol feeding more than 2-fold in cyp2a5−/− mice, but it was increased only 50% in WT mice [48]. Formations of 4-hydroxy-nonenal adduct (HNE) and 3-NT, markers of oxidative stress, were detected in cyp2a5−/− mice to a greater extent than in WT mice [14]. These results suggest that CYP2A5 induction inhibits alcohol-induced oxidative stress, which is not surprising considering the facts that ethanol induction of CYP2A5 is regulated by the Nrf2 pathway. The cyp2a5−/− mice developed more severe experimental ALD than the WT mice did, so alcohol-induced oxidative liver injury is also protected by CYP2A5 [14].
Chronic ethanol feeding does not induce HO-1 in rodent liver [139]. Thus, other Nrf2-regulated antioxidant mechanisms may be involved in ALD protection. Alcohol can cause an increase in levels of heme, and free heme can catalyze the production of ROS through Fenton chemistry [140, 141]. HO-1 exhibits antioxidant effects because HO-1 can decrease heme levels through degrading heme with the production of iron, carbon monoxide, and the bile pigments biliverdin and bilirubin [140–143]. Biliverdin can be reduced by ubiquitous biliverdin reductase to bilirubin [144–146]. Bilirubin is an effective antioxidant at concentrations ranging from 0.01 to 10 µM, but it is cytotoxic at concentrations >20 µM [144, 145]. Thus, HO-1/bilirubin might have a dual role in antioxidant responses: elevated levels of HO-1 or its substrate heme can produce bilirubin to high concentrations that may be detrimental and not protective. Recently, bilirubin was identified as an endogenous substrate for CYP2A5, and CYP2A5 can oxidize bilirubin to biliverdin [144–146]. Both HO-1 and CYP2A5/2A6 are regulated by Nrf2, it is possible that HO-1 could cause induction of bilirubin, and CYP2A5 could control the intracellular concentration of bilirubin to a level with antioxidant instead of cytotoxic properties [145]. HO-1 and CYP2A5/6 may coordinate to maintain intracellular bilirubin at an antioxidant concentration. CYP2A5 is highly induced in liver [12, 13], but chronic ethanol feeding does not induce HO-1 (139). It will be interesting to address whether no or weak induction of HO-1and highly induced CYP2A5 coordinate to protect against alcohol-induced oxidative liver injury.
Kirby et al first raised the notion that CYP2A5 may play a role in energy metabolism [8]. Pyrazole, an inducer of CYP2A5, causes hepatic glycogen depletion and decreases serum glucose levels [8]. We found that chronic ethanol feeding induced hyperglycemia in cyp2a5−/− mice but not in WT mice [14]. The cyp2a5−/− mice exhibited more obvious glucose intolerance than WT mice did [147]. These results suggest that CYP2A5 may participate in regulation of glucose homeostasis. Glucose homoestasis regulating factors such as glucagon, cyclic adenosine monophosphate (cAMP), and protein kinase A (PKA) can induce CYP2A5 [148, 149]. Glucagon mediates cAMP production, and cAMP activates PKA, which causes cAMP response element-binding protein (CREB) phosphorylation at Ser133, followed by subsequent translocation to the nucleus and binding to the cAMP response element (CRE) [150–152]. CREB cannot directly regulate CYP2A5 transcription because the cyp2a5 promoter lacks CRE. However, CREB can act through the coactivator peroxisome proliferator-activated receptor γ coactivator-1 α (PGC-1α) [151] and PGC-1α mediates CYP2A5 induction via hepatocyte nuclear factor 4 (HNF-4) [153]. Interestingly, chronic ethanol increases cAMP levels in the liver [154, 155]. It is possible that ethanol induction of CYP2A5 is regulated, at least in part, by a cAMP-PKA-CREB pathway via PGC-1α /HNF-4. It will be interesting to address whether CYP2A5/2A6 regulate glucose homoestasis via the cAMP-PKA-CREB pathway.
Pyrazole also causes hepatic lipid accumulation, which might be associated with AMP-activated protein kinase (AMPK), a regulator of lipid metabolism [8]. Inhibition of AMPK plays a key role in ethanol-induced fatty liver by enhancing lipogenesis and depressing the rate of fatty acid β-oxidation (156). The previously published results obtained from WT and cyp2a5−/− mice chronically fed ethanol [14] or from primary hepatocytes isolated from WT and cyp2a5−/− mice incubated with ethanol, or from WT and cyp2a5−/− mice gavaged with binge ethanol all show that ethanol-induced steatosis was higher in cyp2a5−/− mice or hepatocytes compared with WT mice or hepatocytes [12]. After chronic ethanol feeding, hypertriglyceridemia was induced in cyp2a5−/− mice but not in WT mice and liver TG contents are increased more strikingly in cyp2a5−/− mice than in WT mice [14]. These results suggest that in contrast to CYP2E1, CYP2A5 protects against but does not promote alcohol-induced steatosis. In patients with alcoholic and nonalcoholic fatty liver, CYP2A6 was found to co-localize with lipid droplets [109], which thus might be extrapolated to be a compensatory or adaptive response to antagonize fat accumulation in the liver. Perhaps inducers of CYP2A5 may have therapeutic potential in ameliorating alcoholic fatty liver.
10. INTERACTION BETWEEN CYP2A5 AND PPARα-FGF21 AXIS
PPARα regulates mitochondrial and peroxisomal fatty acid oxidation. AOX, a key enzyme in peroxisomal fatty oxidation, is regulated by PPARα. Carnitine palmitoyltransferase I (CPT I), which promotes fatty acid uptake into the mitochondria for mitochondrial fatty acid oxidation, is also regulated by PPARα (157, 158). Interestingly, basal level of PPARα expression is up-regulated in cyp2a5−/− mice. To test whether the upregulation of PPARα in cyp2a5−/− mice is attributed to the enhanced alcoholic fatty liver in cyp2a5−/− mice, we designed a model to abrogate the upregulation of PPARα in cyp2a5−/− mice by creating PPARα and CYP2A5 double knockout (pparα −/−/cyp2a5−/−) mice. The comparison between cyp2a5−/− mice not expressing PPARα (pparα−/−/cyp2a5−/−) and cyp2a5−/− mice still expressing PPARα (pparα+/+/cyp2a5−/−) could help define the role of PPARα in the enhanced alcoholic fatty liver in cyp2a5−/− mice. The results showed that alcoholic fatty liver is more severe in pparα −/−/cyp2a5−/− mice than in pparα+/+/cyp2a5−/− mice, suggesting that PPARα interacts with CYP2A5 to protect against the development of alcoholic fatty liver [121]. Both AOX and CPT I are regulated by PPARα, but only basal AOX is upregulated in cyp2a5−/− mice, and basal CPT I is identical in cyp2a5−/− mice and WT mice [121]. Thus, it is possible that PPARα interacts with CYP2A5 via regulating AOX (the peroxisomal fatty acid oxidation pathway) but not CPT I (the mitochondrial fatty acid oxidation pathway).
Fibroblast growth factor 21 (FGF21) is a novel metabolic regulator [159, 160] and FGF21 knockdown promoted both hepatic steatosis and hypertriglyceridemia in HFD-fed mice [161]. Liver expresses the highest levels of FGF21 [162]. Liver produced FGF21 can be released into blood and act on extra-hepatic tissues in an endocrine manner [163]. FGF21 exerts its effect via binding to FGF receptor 1 (FR1) [163]. A major source of serum FGF21 is liver [163]. In liver, FGF21 is regulated by PPARα [161, 164, 165]. Therefore, we examined FGF21 expression in cyp2a5−/− mice.
We found that basal levels of serum FGF21 in Cyp2a5−/− mice were higher than those in WT mice; but ethanol feeding induced FGF21 in WT mice but not in Cyp2a5−/− mice. Serum FGF21 was almost undetectable in pparα−/− mice, and injection of recombinant mouse FGF21 (rFGF21. restored serum FGF21 to a level similar to WT mice. Injection of rFGF21 also blunted the increase in serum TG in pparα−/− mice. Likewise, serum FGF21 was much lower (almost undetectable) in pparα−/−/cyp2a5−/− mice compared to pparα+/+/cyp2a5−/− mice, suggesting that PPARα interacts with CYP2A5 probably through its downstream signaling molecule FGF21 [121].
To further test the role of FGF21 in the development of alcoholic fatty liver, we created liver specific FGF21 knockout (Fgf21alb-cre) mice and liver specific FR1 knockout (Fr1alb-cre) mice. Serum FGF21 was almost undetectable in the Fgf21alb-cre mice while it was unchanged in the Fr1alb-cre mice, suggesting that deletion of FR1 in liver does not have an influence on liver FGF21 expression. Serum FGF21 was induced by ethanol in the Fr1alb-cre mice but it was not induced in the Fgf21alb-cre mice. Correspondingly, Fgf21alb-cre mice developed more severe alcoholic fatty liver compared with the control mice that express FGF21 in liver, suggesting that FGF21 plays an indispensable role in protecting against the development of alcoholic fatty liver. However, alcoholic fatty liver was comparable in the Fr1alb-cre mice and the control mice that express FR1 in liver, suggesting that FGF21 does not exert its effects through liver FR1 [121].
Adipose tissues have comparatively high expression of FR1; FR1 expression in liver is only about 10% the level of expression detected in adipose tissue [166], which leads to a suggestion that FGF21 acts on adipose tissue to a greater extent than on liver. About 60% of hepatic fat is derived from adipose tissues [167], and adipose tissues play a very important role in the development of ALD [168–170]. It will be interesting to address whether FGF21 exerts its effects through adipose FR1 by using adipose tissue specific FR1 knockout mice.
11. CYP2A5 AND LIVER FIBROSIS
As discussed above, the Lieber-decarli ALD animal model does not induce evident liver fibrosis. We applied CCL4 and TAA models to test whether CCL4- and TAA-induced liver fibrosis may be enhanced in cyp2a5−/− mice compared with WT mice [45]. Acute liver injury induced by one single injection of CCL4, either higher dose or lower dose, was comparable in cyp2a5−/− mice and WT mice; chronic liver injury and liver fibrosis induced by multiple injection for one month were comparable, too. However, acute liver injury induced by one single injection with TAA at lower doses (75 and 100 mg/kg) was enhanced in cyp2a5−/− mice compared with WT mice, while high dose of TAA (200mg/kg) induced liver injury in cyp2a5−/− mice to the same extent as in WT mice. Therefore, we selected the dose of 200 mg/kg to induce chronic liver injury and liver fibrosis. As a result, we found that TAA-induced liver fibrosis was more severe in cyp2a5−/− mice than in WT mice, while TAA-induced chronic liver injury as indicated by serum ALT/AST and pathological necrosis evaluation was comparable in cyp2a5−/− mice and WT mice, suggesting that CYP2A5 protects against TAA-induced liver fibrosis, and this protection against liver fibrosis is independent of the protection against liver injury [45]. We found that CYP2A5 but not CYP2E1 was expressed in hepatic stellate cells (HSC), a major fibrogenic cell type in liver (Fig. 5). Thus it will be interesting to examine the role of CYP2A5 in activation of HSC.
12. CYP2A5 AND NICOTINE
Tobacco cigarette smoking is another worldwide major cause of preventable morbidity and mortality. Nicotine is a major stimulant and dependence-forming alkaloid in tobacco smoke [171]. Pre- and post-natal nicotine exposure might be a contributing factor for the occurrence of metabolic disorders later in life [172]. In humans, CYP2A6 is a major enzyme that metabolizes nicotine [173]; in mice, 80% of nicotine is metabolized by CYP2A5 [174, 175]. Alcohol consumption induces CYP2A6, and the induced enzyme metabolizes nicotine very quickly and decrease blood levels of nicotine dramatically. Thus, one has to smoke more tobacco to maintain blood nicotine levels. As a matter of fact, alcohol and tobacco are frequently co-abused [176, 177]. Cigarette smoke exposure promotes alcoholic cirrhosis, increases certain cancers, and possibly increases mortality in heavy alcohol drinkers [178]. Likewise, tobacco smoke exposure increases experimental ALD in apoE-deficient mice [179].
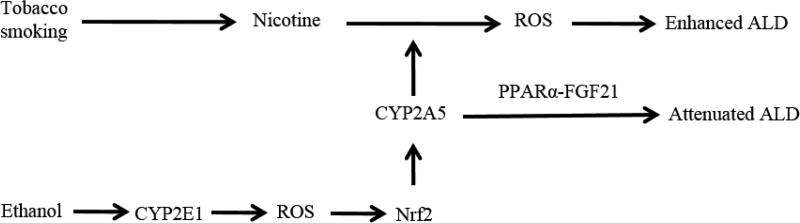
We recently reported that administration of nicotine increased chronic ethanol-induced steatosis [180]. While nicotine treatment or ethanol feeding alone failed to induce hypertriglyceridemia and hyperglycemia, the combination of ethanol feeding and nicotine administration caused increases in serum levels of TG and glucose [180]. To further study the relationship of CYP2A5 and nicotine/alcohol-induced fatty liver, we fed nicotine/alcohol to WT mice and cyp2a5−/− mice and found that nicotine enhanced alcoholic fatty liver in WT mice but not in cyp2a5−/− mice. Cotinine is a major metabolite of nicotine produced by CYP2A5 [175], but we found that cotinine still enhanced alcoholic fatty liver in WT mice but not in cyp2a5−/− mice (unpublished results). It is still being under investigation in our lab whether CYP2A5-dependent nicotine-enhanced alcoholic fatty liver is attributed to nicotine metabolites. Figure 6 shows relationship between CYP2A5, nicotine, and ALD. If tobacco and alcohol are co-abused, CYP2A5 induction may enhance ALD due to CYP2A5-mediated nicotine metabolism.
Figure 6.
Schema for CYP2A5, nicotine, and ALD.
13. CONCLUSIONS AND STRATEGIES FOR ALD THERAPY
Since CYP2E1 has been found to be a major component in MEOS which is induced by alcohol consumption, the studies on CYP2E1 mainly focus on its metabolic action on alcohol and its pathogenesis in ALD. Due to being loosely coupled with NADPH-cytochrome P450 reductase (18), CYP2E1 is a more active generator of ROS compared to other CYPs. Therefore, a lot of studies focus on CYP2E1-mediated oxidative stress and liver injury induced by alcohol consumption.
Now it is generally accepted that CYP2E1 induction is one of the major risk factors for ALD. CYP2A5/2A6 induction by alcohol consumption is a novel new finding. Based on the requirement for CYP2E1 for effective induction of CYP2A5 and Nrf2 by acute and chronic alcohol treatment, the requirement for Nrf2 for maximal induction of CYP2A5, and the prevention by antioxidants of this induction of Nrf2 and CYP2A5, we suggest that increased generation of ROS produced from the induction of CYP2E1 by acute or chronic alcohol treatment activates Nrf2 and activated Nrf2 transcriptionally activates CYP2A5. While the increase in CYP2E1 by alcohol has generally been studied from either a drug metabolism point of view e.g. oxidation of substrates such as nitrosamines, anesthetics, alcohols, etc or from a toxicological point of view e.g. metabolism of hepatotoxins such as acetaminophen, CCL4, TAA, benzene or generation of ROS such as superoxide anion radical and hydrogen peroxide, CYP2E1-generated ROS can also modulate transcription factor activity and thereby regulation of expression of various genes e.g. the activation of Nrf2 by CYP2E1-generated ROS was previously shown to increase the expression of GCS (and subsequently GSH levels), HO-1 and GST activity. Perhaps this reflects initial efforts of liver cells to protect against the alcohol/CYP2E1 toxicity. As summarized in this review, it appears novel that another consequence of the increase in CYP2E1-generated ROS and activation of Nrf2 is the induction of another member of the cytochrome P450 family, CYP2A5. CYP2A5 is found to be protective but not detrimental against ALD. As monooxygenases, in the presence of substrates and O2, CYPs can produce a small amount of ROS. CYP2E1 is special because CYP2E1 can generate ROS even in the absence of substrates. Therefore, CYP2E1 is pro-oxidant and detrimental. However, how CYP2A5 protects against ALD still needs further studies.
For ALD therapy or prevention, the strategies should be blocking CYP2E1 activity without inhibiting CYP2A5 activity, or inhibiting CYP2E1 and simultaneously inducing CYP2A6/2A5. CYP2A3, a rat ortholog of CYP2A5 and CYP2A6, is not expressed in liver of rat, so CYP2E1 inhibitors will not affect CYP2A3 in rat liver. Therefore, there are no concerns about CYP2E1-dependent induction of CYP2A3. As mentioned above, CYP2E1 inhibitors, such as DAS, PIC and CMZ, have been shown to partially block hepatic LPO and ameliorate alcohol-induced hepatic pathological changes in rats [29–32]. Now more studies are performed in mice. For example, CMZ can inhibit ethanol-induced liver injury in mice through suppression of Akt phosphorylation [181]. Apigenin, a plant flavonoid, can also protect against ALD in mice through regulating hepatic CYP2E1-mediated oxidative stress and PPARα-mediated lipogenic gene expression [182]. MicroRNAs (miRNAs) can regulate ALD in mice [183–185]. MiRNAs can bind to 3′-untranslated region (3′-UTR) and inhibit CYP2E1 expression [186–189]. Ethanol induction of CYP2A5/2A6 is CYP2E1-dependent. Thus, inhibition of CYP2E1 may inhibit CYP2A5 induction by ethanol in mice. Due to the protective effects of CYP2A5, it is possible that CYP2A5 inhibition resulted from CYP2E1 inhibition may compromise the therapeutic effects of CYP2E1 suppression
Induction of CYP2A5/2A6 may prevent ALD. Although many toxic chemicals can induce CYP2A5, until now, there are no convincing pharmacological inducer of CYP2A5/2A6 that can be used for studies on ALD. Fatty acids can induce CYP2A5 in HFD-fed mice [190, 191]. Since fatty acids contribute to non-alcoholic fatty liver disease (NAFLD), it is possible that CYP2A5 induction by fatty acids is a compensatory response in NAFLD. In contrast, inhibition of CYP2A5/2A6 will enhance ALD. Celery extract can inhibit both CYP2A5 and CYP2A6 [192]. While celery extract exhibits antioxidant, hypoglycemic, hypolipidemic and anti-platelet aggregation effects, it is unclear whether these pleiotropic effects will be compromised when it is applied for ALD.
Recently, liver cancer was also included in the spectrum of ALD. Upregulation of CYP2E1 and subsequent production of ROS play a significant role in the pathogenesis of liver cancer during chronic ingestion of ethanol [193]. Alcohol consumption can cause formation of carcinogenic etheno-adduct and CYP2E1 plays an important role in the generation of etheno-adduct [194, 195]. CYP2E1 can also metabolize and bioactivate procarcinogens such as nitrosamines to direct carcinogenic metabolites [195–196]. Thus, inhibition of CYP2E1 can suppress ALD as well as carcinogenesis in liver.
For CYP2A5/2A6, it is more complicated. Like CYP2E1, CYP2A5 can also bioactivate carcinogens. For example, nicotine-derived nitrosamine ketone [NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone], a potent lung carcinogen, is bio-activated by CYP2A5 (COH) [197, 198]. CYP2A5-mediated bioactivation of NNK in mouse lung is inhibited by 8-methoxypsoralen (MOP), a potent inhibitor of CYP2A, leading to the prevention of NNK-induced lung adenoma [199, 200]. Thus, for the people co-abusing alcohol and tobacco, inhibition of CYP2A5 suppresses carcinogenesis, but it may enhance ALD.
14. FUTURE DIRECTIONS
Several directions for future research which may develop from these studies are the following:
A: Is alcohol a ligand or a substrate for oxidation by CYP2A5? Many substrates for CYPs induce their own metabolism via increasing the levels of the corresponding CYP that metabolizes them. Alcohol is a substrate for CYP2E1 to be oxidized to acetaldehyde and alcohol binds to CYP2E1 producing a modified type II binding spectrum [114]. Could the alcohol induction of CYP2A5 reflect alcohol serving as a metabolic substrate for CYP2A5? This can be readily addressed using isolated microsomes expressing only CYP2A5 or microsomes from cyp2a5−/− mice or studying the oxidation of ethanol to acetaldehyde in the absence and presence of inhibitors of CYP2A5 or inhibitory CYP2A5 antibodies. When microsomes from WT and cyp2a5−/− mice were incubated with ethanol, acetaldehyde production was 15% higher in WT microsomes than CYP2A5 microsomes, suggesting CYP2A5 plays a minor role in ethanol metabolism [12]. Alternatively, ligands for CYP2E1 like alcohol can increase the half-life of CYP2E1 protein by stabilizing the protein against proteosome –mediated degradation [116, 117]. Although, unlike CYP2E1, CYP2A5 is induced by alcohol probably via transcriptional upregulation (mRNA levels are increased), it still deserves to investigate whether alcohol can work as a possible ligand to increase the stability and half-life of CYP2A5.
B: As discussed above, bilirubin was recently identified as an endogenous substrate for CYP2A5, and CYP2A5 can function as a hepatic bilirubin oxidase to decrease intracellular bilirubin [144–146]. Common bile duct ligation (BDL), a model for portal fibrosis, can induce cholestasis in which bilirubin accumulates [201]. BDL-induced hepatic fibrosis was more severe in cyp2a5−/− mice than in WT mice [14]. Consistently, liver injury as indicated by serum ALT and ALP (alkaline phosphatase) were more severe in cyp2a5−/− mice than in WT mice, but serum bilirubin levels including unconjugated free bilirubin and conjugated bilirubin and total bilirubin were not significantly higher in cyp2a5−/− mice than in WT mice due to a huge individual variation among the mice (Gordon M. Kirby, personal communication). Future studies should evaluate whether CYP2A5 might be related to cholestasis or sickle cell disease, which displays a higher levels of serum bilirubin [202].
C: What other transcription factors besides Nrf2 upregulate expression of CYP2A5? Normally, Nrf2 heterodimerizes with small MAF proteins to function as a transcription factor. As discussed above, although lower, there was still significant induction of CYP2A5 by alcohol in Nrf2−/− mice. Besides Nrf2, other transcription factors or receptors e. g. pregnane X receptor (PXR), retinoid x receptor α (RXRα) [118], co-activator PGC-1α [153], HIF-1β/ARNT [203] and AhR [204] are known to be capable of upregulating the basal and the inducible expression of the CYP2A5. In addition, tumor suppression gene P53 was also found to be a new regulator of CYP2A6 [205]. The effects of alcohol on these factors deserve to be assessed.
As discussed above, chronic ethanol increases cAMP levels in the liver [154, 155] and cAMP mediates upregulation of CYP2A5 in hepatocytes [148, 149]. cAMP activates PKA, which phosphorylates CREB and causes phosphorylated CREB (p-CREB) translocation to the nucleus followed by binding to the cAMP response element (CRE) [150–152]. P-CREB results in recruitment of the coactivators CREB-binding protein (CBP) and transducer of regulated CREB activity 2 (TORC2) to form the CREB-CBP-TORC complex. With CBP and TORC2 as coactivators, CREB binds to the cAMP response element (CRE). However, the cyp2a5 promoter lacks CRE, which means CREB cannot directly regulate CYP2A5 transcription. It was reported that CREB can act through PGC-1α [151] and PGC-1α mediates CYP2A5 induction via HNF-4α [91]. It remains to be determined whether ethanol induction of CYP2A5 is regulated, in part, by the cAMP-PKA-CREB pathway via PGC-1α/HNF-4α.
CBP possesses intrinsic histone acetyltransferase (HAT) activity. CBP and p300 share a high degree of homology. P300/CBP serves as transcription coactivators to acetylate core histones, which facilitate chromatin decondensation and recruit basic RNA polymerase machinery [152]. Many nonhistone proteins, particularly transcription factors, are also substrates for p300/CBP [206]. CBP and P300 were shown to interact with Nrf2 and to enhance Nrf2-dependent reporter gene activities [207]. Further study indicates that acetylation of Nrf2 augments promoter-specific DNA binding and activity of Nrf2 during the antioxidant response [208]. In contrast, the deacetylase sirtuin 1 (SIRT1) decreased acetylation of Nrf2 and Nrf2-dependent gene transcription [209]. CBP may act as a histone transacetylase to cause acetylation of Nrf2 and subsequently enhance activity of Nrf2 [208]. It will be interesting to address whether the cAMP-PKA-CREB-CBP pathway regulates CYP2A5 through enhancing Nrf2 transcriptional activity especially when nuclear Nrf2 decreases.
D: CYP2A5 and tobacco: Alcohol induction of CYP2A5 will accelerate blood nicotine clearance and increase the amount of tobacco smoking necessary for maintaining blood nicotine levels, thus alcohol induction of CYP2A5 perhaps alter smoking behavior. Such issues are being studied by Tyndale and associates [210, 211]. Nicotine-derived nitrosamine ketone [NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone] was found to be associated with enhanced ALD in rats [212]. NNK is a potent lung carcinogen and is bio-activated by CYP2A5 (COH) [197, 198]. CYP2A5 inhibition can inhibit pulmonary carcinogenesis [199, 200]. We found that alcohol feeding also induced CYP2A5 expression and COH activity in lung (Fig. 7). Thus, it deserves to investigate whether alcohol synergizes with tobacco to induce lung cancer.
We recently reported that injection of nicotine increased chronic ethanol –induced steatosis and collagen deposition [180]. Nicotine can induce fibrogenic changes in human liver via nicotinic acetylcholine receptors expressed on HSC [213]. As mentioned above, the Lieber-DeCarli model does not induce evident liver fibrosis. Whether nicotine plus alcohol diet will induce evident alcoholic liver fibrosis needs to be addressed.
E: Like CYP2E1, CYP2A5 can also be detected in mitochondria [214, 215]. It is still unclear whether alcohol can cause an elevation of CYP2A5 in mitochondria and if so, what are the toxicological significance of mitochondrial CYP2A5.
F: An interesting speculation is the possibility that other CYPs which can be regulated by Nrf2 may be up-regulated by alcohol induction of CYP2E1 analogous to CYP2A5. Recently, Cyp2b10 expression was shown to be increased by activators of Nrf2 such as phorone and phenobarbital in wild type but not in Nrf2−/− mice [216]. Alcohol has been shown to increase hepatic CYP2B [176], but we are not aware of any studies specifically evaluating Cyp2b10.
Acknowledgments
Studies from the author’s laboratories were supported by USPHS Grants AA-018790 and AA-021362 (To AIC) and AA-020877 and AA-024723 (to YL) from the National Institute on Alcohol Abuse and Alcoholism, NIH and ABMRF/the Foundation for Alcohol Research.
The authors appreciate Dr. Frank Gonzalez (NCI) for cyp2e1−/− mice and cyp2e1−/− Tg mice, Dr. Xinxin Ding (SUNY College of Nanoscale Science and Engineering, Albany, NY) for cyp2a5−/− mice, Dr. Philippe Soriano (Department of Development and Regenerative Biology, Icahn School of Medicine at Mount Sinai, New York, NY) for FR1 floxed mice, Dr. Arndt Vogel and Jutta Lamlé (Department of Hepatology, Gastroenterology and Endocrinology, Hannover, Germany) for liver samples from Nrf2−/− mice and WT mice, Dr. Risto Juvonen (Department of Pharmacology and Toxicology, University of Kuopio, Kuopio, Finland) for CYP2A5 antibody, Dr. Jerome Lasker (Hackensack Biomedical Research Institute, Hackensack, NJ) for CYP2E1 antibody, Professor Paul Van Veldhoven (K.U. Leuven, Belgium) for AOX antibody.
Abbreviation
- 3-MA
3-methyladenine
- 3-NT
3-nitro-tyrosine protein adduct
- 4-HNE
4-hydroxy-nonenal adduct
- AA
arachidonic acid
- ADH
alcohol dehydrogenase
- ALD
alcoholic liver disease
- ALDH
aldehyde dehydrogenase
- ALP
alkaline phosphatase
- ALT
alanine transaminase
- AMPK
AMP-activated protein kinase
- AOX
Acyl-CoA oxidase
- AST
aspartate transaminase
- Atgs
autophagy-related genes
- BDL
Common bile duct ligation
- C34 cells
HepG2 cells transfected with empty plasmid that do not express CYP2E1
- cAMP
Cyclic adenosine monophosphate
- CB
conjugated bilirubin
- CBP
CREB-binding protein
- CCL4
carbon tetrachloride
- CMZ
chlormethiazole
- COH
coumarin 7-hydroxylase
- CoA
Coenzyme A
- CPT I
Carnitine palmitoyltransferase I
- CRE
cAMP response element
- CREB
cAMP response element-binding protein
- CYPs
Cytochromes P450s
- cyp2a5−/− mice
CYP2A5 knockout mice
- cyp2e1−/− mice
CYP2E1 knockout mice
- cyp2e1−/− Tg mice
humanized CYP2E1 transgenic mice
- DAS
diallyl sulfide
- E47 cells
HepG2 cells transfected with CYP2E1-plasmid that overexpress CYP2E1
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinases
- FGF21
fibroblast growth factor 21
- Fgf21alb-cre
liver-specific FGF21 knockout mice
- FR1
FGF receptor 1
- Fr1alb-cre
liver-specific FR1 knockout mice
- GCSC
γ-glutamyl cysteine synthase catalytic subunit
- GCSR
γ-glutamyl cysteine synthase regulatory subunit
- GSH
reduced glutathione
- GST
glutathione transferase
- HAT
histone acetyltransferase
- HFD
high fat diet
- HIF-1α
hypoxia inducible factor 1α
- HIF-1β/ARNT
hypoxia inducible factor-1β/aryl hydrocarbon receptor nuclear translocator
- HNF-4α
hepatocyte nuclear factor 4 α
- HO-1
heme oxygenase 1
- HRE
hypoxia response elements
- HSC
hepatic stellate cells
- IgG
immunoglobulin G
- i.p
intraperitoneal
- Keap1
Kelch-like erythroid cell derived protein with CNC homology-associated protein 1
- KO
knockout
- JNK
c-Jun NH2-terminal kinase
- Jnk1−/− mice
JNK1 knockout mice
- Jnk2−/− mice
JNK2 knockout mice
- LPO
lipid peroxidation
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinases
- MAPKK
MAPK kinase
- MAPKKK
MAPKK kinase
- MEOS
microsomal ethanol oxidizing system
- MOP
8-methoxypsoralen
- mOPN
milk osteopontin
- NAC
N-acetyl-L-cysteine
- NAD+
oxidized nicotinamide adenine dinucleotide
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NNK
nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NO
nitric oxide
- NOS
nitric oxide synthase
- Nrf2
nuclear factor erythroid 2 (NFE2) -related factor 2
- Nrf2−/− mice
Nrf2 knockout mice
- O2
molecular oxygen
- OPN
osteopontin
- OONO−
peroxynitrite
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator-1α
- PIC
phenethyl isothiocyanate
- PKA
protein kinase A
- PPARα
peroxisome proliferator-activated receptor α
- pparα−/− mice
PPARα knockout mice
- pparα−/−/cyp2a5−/− mice
PPARα and CYP2A5 double knockout mice
- PPC
polyenylphosphatidylcholine
- PXR
pregnane X receptor
- rFGF21
recombinant mouse FGF21
- RNS
reactive nitrosative species
- ROS
reactive oxygen species
- RXR
retinoid X receptor
- SER
smooth endoplasmic reticulum
- SiRNA
small interference RNA
- SIRT1
deacetylase sirtuin 1
- TAA
thoiacetamide
- TB
total bilirubin
- TBARS
thiobarbituric acid-reactive substances
- TG
triglyceride
- TNFα
tumor necrosis factor alpha
- TORC2
transducer of regulated CREB activity 2
- UB
unconjugated bilirubin
- Vc
vitamin C
- WT
wild type
Footnotes
CONFLICT OF INTEREST:
None
References
- 1.Guengerich FP. Oxidative cleavage of carboxylic esters by cytochrome P-450. J Biol Chem. 1987;262:8459–8462. [PubMed] [Google Scholar]
- 2.Nebert DW, Nelson DR, Coon MJ, Estabrook RW, Feyereisen R, Fujii-Kuriyama Y, et al. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991;10:1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- 3.Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, et al. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44(5):723–38. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su T, Ding X. Regulation of the cytochrome P450 2A genes. Toxicol Appl Pharmacol. 2004;199:285–94. doi: 10.1016/j.taap.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Raunio H, Rahnasto-Rilla M. CYP2A6: genetics structure regulation function. Drug Metabol. Drug Interact. 2012;27:73–88. doi: 10.1515/dmdi-2012-0001. [DOI] [PubMed] [Google Scholar]
- 8.Kirby GM, Nichols KD, Antenos M. CYP2A5 induction and hepatocellular stress: an adaptive response to perturbations of heme homeostasis. Curr Drug Metab. 2011;12:186–97. doi: 10.2174/138920011795016845. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Bakar A, Hakkola J, Juvonen R, Rahnasto-Rilla M, Raunio H, Lang MA. Function and regulation of the Cyp2a5/CYP2A6 genes in response to toxic insults in the liver. Curr Drug Metab. 2013;14:137–50. [PubMed] [Google Scholar]
- 10.Benowitz NL, Swan GE, Jacob P, Lessov-Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin Pharmacol Ther. 2006;80:457–467. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Zhuo X, Xie F, Kluetzman K, Shu YZ, Humphreys WG, et al. Role of CYP2A5 in the clearance of nicotine and cotinine: insights from studies on a CYP2a5-null mouse model. J Pharmacol Exp Ther. 2010;332:578–587. doi: 10.1124/jpet.109.162610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y, Zhang XH, Cederbaum AI. Ethanol induction of CYP2A5: role of CYP2E1-ROS-Nrf2 pathway. Toxicol Sci. 2012;128(2):427–38. doi: 10.1093/toxsci/kfs164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y, Zhuge J, Wu D, Cederbaum AI. Ethanol induction of CYP2A5: permissive role for CYP2E1. Drug Metab Dispos. 2011;39(2):330–6. doi: 10.1124/dmd.110.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong F, Liu X, Ward SS, Xiong H, Cederbaum AI, Lu Y. Absence of cytochrome P450 2A5 enhances alcohol-induced liver injury in mice. Dig Liver Dis. 2015;47(6):470–7. doi: 10.1016/j.dld.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieber CS. Metabolism of alcohol. Clin Liver Dis. 2005;9:1–35. doi: 10.1016/j.cld.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Gorsky LD, Koop DR, Coon MJ. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450: Products of oxygen reduction. J Biol Chem. 1984;259(11):6812–7. [PubMed] [Google Scholar]
- 18.Ekstrom G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1) Biochem Pharmacol. 1989;38:1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- 19.Mari M, Cederbaum AI. CYP2E1 overexpression in HepG2 cells induces glutathione synthesis by transcriptional activation of γ-glutamylcysteine synthetase. J Biol Chem. 2000;275:15563–15571. doi: 10.1074/jbc.M907022199. [DOI] [PubMed] [Google Scholar]
- 20.Mari M, Cederbaum AI. Induction of catalase, alpha and microsomal glutathione S-transferase in CYP2E1-overexpressing HepG2 cells and protection against short-term oxidative stress. Hepatology. 2001;33:652–661. doi: 10.1053/jhep.2001.22521. [DOI] [PubMed] [Google Scholar]
- 21.Nieto N, Mari M, Cederbaum AI. Cytochrome P4502E1-responsiveness in the promoter of glutamate-cysteine ligase catalytic subunit. Hepatology. 2003;37:96–106. doi: 10.1053/jhep.2003.50003. [DOI] [PubMed] [Google Scholar]
- 22.Gong P, Cederbaum AI, Nieto N. Increased expression of cytochrome P4502E1 induces heme oxygenase-1 through ERK MAPK pathway. J Biol Chem. 2003;278:29693–29700. doi: 10.1074/jbc.M304728200. [DOI] [PubMed] [Google Scholar]
- 23.Gong P, Cederbaum AI, Nieto N. Heme oxygenase −1 protects HepG2 cells against cytochrome P4502E1 dependent toxicity. Free Rad Biol Med. 2004;36:307–318. doi: 10.1016/j.freeradbiomed.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Morimoto M, Zern MA, Hagbjork AL, Ingelman-Sundberg M, French SW. Fish oil, alcohol, and liver pathology: role of cytochrome P450 2E1. Proc Soc Exp Biol Med. 1994;207:197–205. doi: 10.3181/00379727-207-43807. [DOI] [PubMed] [Google Scholar]
- 25.Nanji AA, Zhao S, Sadrzadeh SM, Dannenberg AJ, Tahan SR, Waxman DJ. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol Clin Exp Res. 1994;18:1280–1285. doi: 10.1111/j.1530-0277.1994.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 26.Tsukamoto H, Horne W, Kamimura S, Niemelä O, Parkkila S, Ylä-Herttuala S, et al. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995;96:620–630. doi: 10.1172/JCI118077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.French SW, Morimoto M, Reitz RC, Koop D, Klopfenstein B, Estes K, et al. Lipid peroxidation, CYP2E1 and arachidonic acid metabolism in alcoholic liver disease in rats. J Nutr. 1997;127:907S–911S. doi: 10.1093/jn/127.5.907S. [DOI] [PubMed] [Google Scholar]
- 28.Kim ND, Kwak MK, Kim SG. Inhibition of cytochrome P450 2E1 expression by 2-(allylthio)pyrazine, a potential chemoprotective agent: hepatoprotective effects. Biochem Pharmacol. 1997;53:261–269. doi: 10.1016/s0006-2952(96)00647-8. [DOI] [PubMed] [Google Scholar]
- 29.Morimoto M, Hagbjork AL, Nanji AA, Ingelman-Sundberg M, Lindros KO, Fu PC, et al. Role of cytochrome P4502E1 in alcoholic liver disease pathogenesis. Alcohol. 1993;10:459–464. doi: 10.1016/0741-8329(93)90065-v. [DOI] [PubMed] [Google Scholar]
- 30.Morimoto M, Reitz RC, Morin RJ, Nguyen K, Ingelman-Sundberg M, French SW. CYP-2E1 inhibitors partially ameliorate the changes in hepatic fatty acid composition induced in rats by chronic administration of ethanol and a high fat diet. J Nutr. 1995;125:2953–2964. doi: 10.1093/jn/125.12.2953. [DOI] [PubMed] [Google Scholar]
- 31.Albano E, Clot P, Morimoto M, Tomasi A, Ingelman-Sundberg M, French SW. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology. 1996;23:155–163. doi: 10.1002/hep.510230121. [DOI] [PubMed] [Google Scholar]
- 32.Gouillon Z, Lucas D, Li J, Hagbjork AL, French BA, Fu P, et al. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med. 2000;224:302–308. doi: 10.1046/j.1525-1373.2000.22435.x. [DOI] [PubMed] [Google Scholar]
- 33.Lieber CS. The discovery of the microsomal ethanol oxidizing system and its physiologic and pathologic role. Drug Metab Rev. 2004;36:511–29. doi: 10.1081/dmr-200033441. [DOI] [PubMed] [Google Scholar]
- 34.Aleynik MK, Leo MA, Aleynik SI, Lieber CS. Polyenylphosphatidylcholine opposes the increase of cytochrome P-4502E1 by ethanol and corrects its iron-induced decrease. Alcohol Clin Exp Res. 1999;23:96–100. [PubMed] [Google Scholar]
- 35.Bardag-Gorce F, Yuan QX, Li J, French BA, Fang C, Ingelman-Sundberg M, et al. The effect of ethanol-induced cytochrome p4502E1 on the inhibition of proteasome activity by alcohol. Biochem Biophys Res Commun. 2000;279:23–9. doi: 10.1006/bbrc.2000.3889. [DOI] [PubMed] [Google Scholar]
- 36.Butura A, Nilsson K, Morgan K, Morgan TR, French SW, Johansson I, et al. The impact of CYP2E1 on the development of alcoholic liver disease as studied in a transgenicmouse model. J Hepatol. 2009;50(3):572–83. doi: 10.1016/j.jhep.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Lee SS, Buter JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 38.Cheung C, Yu AM, Ward JM, Krausz KW, Akiyama TE, Feigenbaum L, et al. The CYP2E1 -humanized transgenic mouse: role of CYP2E1 in acetaminophen hepatotoxicity. Drug Metab Disp. 2005;33:449–457. doi: 10.1124/dmd.104.002402. [DOI] [PubMed] [Google Scholar]
- 39.Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–94. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y, Wu D, Wang X, Ward SC, Cederbaum AI. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radic Biol Med. 2010;49:1406–16. doi: 10.1016/j.freeradbiomed.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieber CS, DeCarli LM. Animal models of chronic ethanol toxicity. Methods Enzymol. 1994;233:585–594. doi: 10.1016/s0076-6879(94)33061-1. [DOI] [PubMed] [Google Scholar]
- 42.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8(3):627–37. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong FW, Chan WY, Lee SS. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998;153(1):109–18. doi: 10.1006/taap.1998.8547. [DOI] [PubMed] [Google Scholar]
- 44.Kang JS, Wanibuchi H, Morimura K, Wongpoomchai R, Chusiri Y, Gonzalez FJ, et al. Role of CYP2E1 in thioacetamide-induced mouse hepatotoxicity. Toxicol Appl Pharmacol. 2008;228(3):295–300. doi: 10.1016/j.taap.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Hong F, Si C, Gao P, Cederbaum AI, Xiong H, Lu Y. The role of CYP2A5 in liver injury and fibrosis: chemical-specific difference. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(1):33–43. doi: 10.1007/s00210-015-1172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roychowdhury S, Chiang DJ, McMullen MR, Nagy LE. Moderate, chronic ethanol feeding exacerbates carbon-tetrachloride-induced hepatic fibrosis via hepatocyte-specific hypoxia inducible factor 1α. Pharmacol Res Perspect. 2014;2(5):e00061. doi: 10.1002/prp2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roychowdhury S, Chiang DJ, Mandal P, McMullen MR, Liu X, Cohen JI, et al. Inhibition of apoptosis protects mice from ethanol-mediated acceleration of early markers of CCl4 -induced fibrosis but not steatosis or inflammation. Alcohol Clin Exp Res. 2012;36(7):1139–47. doi: 10.1111/j.1530-0277.2011.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yun JW, Son MJ, Abdelmegeed MA, Banerjee A, Morgan TR, Yoo SH, et al. Binge alcohol promotes hypoxic liver injury through a CYP2E1-HIF-1α-dependent apoptosis pathway in mice and humans. Free Radic Biol Med. 2014;77:183–94. doi: 10.1016/j.freeradbiomed.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang L, Wu D, Wang X, Cederbaum AI. Cytochrome P4502E1, oxidative stress, JNK, and autophagy in acute alcohol-induced fatty liver. Free Radic Biol Med. 2012;53(5):1170–80. doi: 10.1016/j.freeradbiomed.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang B, Xu MJ, Zhou Z, Cai Y, Li M, Wang W, et al. Short- or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: an important role for CXCL1. Hepatology. 2015;62(4):1070–85. doi: 10.1002/hep.27921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carmiel-Haggai M, Cederbaum AI, Nieto N. Binge ethanol exposure increases liver injury in obese rats. Gastroenterology. 2003;125(6):1818–33. doi: 10.1053/j.gastro.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 52.Minato T, Tsutsumi M, Tsuchishima M, Hayashi N, Saito T, Matsue Y, et al. Binge alcohol consumption aggravates oxidative stress and promotes pathogenesis of NASH from obesity-induced simple steatosis. Mol Med. 2014 Dec 10;20:490–502. doi: 10.2119/molmed.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arteel GE, Iimuro Y, Yin M, Raleigh JA, Thurman RG. Chronic enteral ethanol treatment causes hypoxia in rat liver tissue in vivo. Hepatology. 1997;25(4):920–6. doi: 10.1002/hep.510250422. [DOI] [PubMed] [Google Scholar]
- 54.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 55.Nath B, Levin I, Csak T, Petrasek J, Mueller C, Kodys K, et al. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53(5):1526–37. doi: 10.1002/hep.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Wu D, Yang L, Gan L, Cederbaum AI. Cytochrome P450 2E1 potentiates ethanol induction of hypoxia and HIF-1α in vivo. Free Radic Biol Med. 2013;63:175–86. doi: 10.1016/j.freeradbiomed.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shintanl T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014;1:92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding WX, Manley S, Ni HN. The emerging role of autophagy in alcoholic liver disease. Exp Biol Med. 2011;236:546–556. doi: 10.1258/ebm.2011.010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin XM, Ding WX, Gao W. Autophagy in the liver. Hepatology. 2008;47:1773–1785. doi: 10.1002/hep.22146. [DOI] [PubMed] [Google Scholar]
- 62.Dong H, Czaja MJ. Regulation of lipid droplets by autophagy. Trends Endocrin. Metab. 2011;22:234–240. doi: 10.1016/j.tem.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu JJ, Quijano C, Wang J, Finkel T. Metabolism meets autophagy. Cell Cycle. 2009;9:4780–4781. doi: 10.4161/cc.9.24.14273. [DOI] [PubMed] [Google Scholar]
- 64.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Osna NA, Thomes PG, Donohue TM., Jr Involvement of autophagy in alcoholic liver injury and hepatitis c pathogenesis. World J Gastroent. 2011;17:2507–2514. doi: 10.3748/wjg.v17.i20.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Von Haefen C, Sifringer M, Menk M, Spies CD. Ethanol enhances susceptibility to apoptotic cell death via down regulation of autophagy-related proteins. Alcoholism: Clin Exp Res. 2011;35:1381–1391. doi: 10.1111/j.1530-0277.2011.01473.x. [DOI] [PubMed] [Google Scholar]
- 68.Lin CW, Zhang H, Li M, Xiong X, Chen X, Chen X, et al. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and nonalcoholic fatty liver conditions in mice. J Hepatology. 2013;58:993–999. doi: 10.1016/j.jhep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noh B, Lee Jl, Jun H, Lee JH, Jia Y, Hoang MH, et al. Restoration of autophagy by puerarin in ethanol--treated hepatocytes via the activation of AMP-activated protein kinase. Biochem Biophys Res Commun. 2011;414:361–366. doi: 10.1016/j.bbrc.2011.09.077. [DOI] [PubMed] [Google Scholar]
- 70.Dolganiuc A, Thomes PG, Ding WX, Lemasters JJ, Donohue TM., Jr Autophagy in alcohol-induced liver diseases. Alcoholism Clin Exp Res. 2012;36:1301–1308. doi: 10.1111/j.1530-0277.2012.01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eid N, Ito Y, Maemura K, Otsuki Y. Elevated autophagic sequestration of mitochondria and lipid droplets in steatotic hepatocytes of chronic ethanol-treated rats: an immunohistochemical and electron microscopic study. J Mol Hist. 2013;44:311–326. doi: 10.1007/s10735-013-9483-x. [DOI] [PubMed] [Google Scholar]
- 72.Thomes PC, Trambly CS, Thiele GM, Duryee MJ, Fox HS, Haorah J, et al. Proteosome activity and autophagosome content in liver are reciprocally regulated by ethanol treatment. Biochem Biophys Res Commun. 2012;417:262–267. doi: 10.1016/j.bbrc.2011.11.097. [DOI] [PubMed] [Google Scholar]
- 73.Wu D, Wang X, Zhou R, Cederbaum AI. CYP2E1 enhances ethanol-induced lipid accumulation but impairs autophagy in HepG2 E47 cells. Biochem Biophys Res Commun. 2010;402:116–122. doi: 10.1016/j.bbrc.2010.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu D, Cederbaum AI. Inhibition of autophagy promotes CYP2E1-dependent toxicity via elevated oxidative stress, mitochondrial dysfunction and activation of p38 and JNK MAPK. Redox Biology. 2013;1:552–565. doi: 10.1016/j.redox.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu D, Wang X, Zhou R, Yang L, Cederbaum AI. Alcohol steatosis and cytotoxicity: the role of cytochrome P4502E1 and autophagy. Free Radical Biol Med. 2012;53:346–1357. doi: 10.1016/j.freeradbiomed.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu Y, Cederbaum AI. Autophagy Protects against CYP2E1/Chronic Ethanol-Induced Hepatotoxicity. Biomolecules. 2015;5(4):2659–74. doi: 10.3390/biom5042659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kono H, Bradford BU, Yin M, Sulik KK, Koop DR, Peters JM, et al. CYP2E1 is not involved in early alcohol-induced liver injury. Am J Physiol. 1999;277:G1259–G1267. doi: 10.1152/ajpgi.1999.277.6.G1259. [DOI] [PubMed] [Google Scholar]
- 78.Rao RK, Seth A, Sheth P. Recent advances in alcoholic liver disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- 79.Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256–G265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 80.Hansen J, Cherwitz DL, Allen JI. The role of tumor necrosis factor-alpha in acute endotoxin-induced hepatotoxicity in ethanol-fed rats. Hepatology. 1994;20:461–474. [PubMed] [Google Scholar]
- 81.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–460. [PubMed] [Google Scholar]
- 82.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alpha attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 83.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, et al. Essential role of TNFα in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 84.Ge X, Leung TM, Arriazu E, Lu Y, Urtasun R, Christensen B, et al. Osteopontin binding to lipopoly saccharide lowers tumor necrosis factor-α and prevents early alcohol-induced liver injury in mice. Hepatology. 2014;59(4):1600–16. doi: 10.1002/hep.26931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ge X, Lu Y, Leung TM, Sørensen ES, Nieto N. Milk osteopontin, a nutritional approach to prevent alcohol-induced liver injury. Am J Physiol Gastrointest Liver Physiol. 2013;304(10):G929–39. doi: 10.1152/ajpgi.00014.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Forsyth CB, Voigt RM, Shaikh M, Tang Y, Cederbaum AI, Turek FW, et al. Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability. Am J Physiol Gastrointest Liver Physiol. 2013;305(2):G185–95. doi: 10.1152/ajpgi.00354.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Forsyth CB, Voigt RM, Keshavarzian A. Intestinal CYP2E1: A mediator of alcohol-induced gut leakiness. Redox Biol. 2014;3:40–6. doi: 10.1016/j.redox.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abdelmegeed MA, Banerjee A, Jang S, Yoo SH, Yun JW, Gonzalez FJ, et al. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic Biol Med. 2013;65:1238–45. doi: 10.1016/j.freeradbiomed.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu Y, Wang X, Cederbaum AI. Lipopolysaccharide-induced liver injury in rats treated with the CYP2E1 inducer pyrazole. Am J Physiol Gastrointest Liver Physiol. 2005;289(2):G308–19. doi: 10.1152/ajpgi.00054.2005. [DOI] [PubMed] [Google Scholar]
- 90.Lu Y, Cederbaum AI. Enhancement by pyrazole of lipopolysaccharide-induced liver injury in mice: role of cytochrome P450 2E1 and 2A5. Hepatology. 2006;44(1):263–74. doi: 10.1002/hep.21241. [DOI] [PubMed] [Google Scholar]
- 91.Deaciuc IV, Nikolova-Karakashian M, Fortunato F, Lee EY, Hill DB, McClain CJ. Apoptosis and dysregulated ceramide metabolism in a murine model of alcohol-enhanced lipopolysaccharide hepatotoxicity. Alcohol Clin Exp Res. 2000;24:1557–1565. [PubMed] [Google Scholar]
- 92.Hansen J, Cherwitz DL, Allen JI. The role of tumor necrosis factor-alpha in acute endotoxin-induced hepatotoxicity in ethanol-fed rats. Hepatology. 1994;20:461–474. [PubMed] [Google Scholar]
- 93.Koteish A, Yang S, Lin H, Huang X, Diehl AM. Chronic ethanol exposure potentiates lipopolysaccharide liver injury despite inhibiting Jun N-terminal kinase and caspase 3 activation. J Biol Chem. 2002;277:13037–13044. doi: 10.1074/jbc.M101632200. [DOI] [PubMed] [Google Scholar]
- 94.Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 95.Wu D, Cederbaum AI. Cytochrome P4502E1 sensitizes to tumor necrosis factor alpha-induced liver injury through activation of mitogen-activated protein kinases in mice. Hepatology. 2008;47:1005–1017. doi: 10.1002/hep.22087. [DOI] [PubMed] [Google Scholar]
- 96.Wu D, Xu C, Cederbaum A. Role of nitric oxide and nuclear factor-kappaB in the CYP2E1 potentiation of tumor necrosis factor alpha hepatotoxicity in mice. Free Radic Biol Med. 2009;46:480–491. doi: 10.1016/j.freeradbiomed.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 97.McCubrey JA, Franklin RA. Reactive oxygen intermediates and signaling through kinase pathways. Antioxid Redox Signal. 2006;8:1745–1748. doi: 10.1089/ars.2006.8.1745. [DOI] [PubMed] [Google Scholar]
- 98.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 99.Bardag-Gorce F, French BA, Dedes J, Li J, French SW. Gene expression patterns of the liver in response to alcohol: in vivo and in vitro models compared. Exp Mol Pathol. 2006;80:241–251. doi: 10.1016/j.yexmp.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 100.Li J, Bardag-Gorce FJ, Oliva J, Dedes J, French BA, French SW. Gene expression modifications in the liver caused by binge drinking, S-adenosylmethionine feeding. The role of epigenetic changes. Genes Nutr. 2009;5:169–179. doi: 10.1007/s12263-009-0158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aroor AR, James TT, Jackson DE, Shukla SD. Differential changes in MAP kinases, histone modifications, and liver injury in rats acutely treated with ethanol. Alcohol Clin Exp Res. 2010;34:1543–1551. doi: 10.1111/j.1530-0277.2010.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu Y, Cederbaum AI. Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol Sci. 2006;89(2):515–23. doi: 10.1093/toxsci/kfj031. [DOI] [PubMed] [Google Scholar]
- 103.Wu D, Cederbaum AI. Role of p38 MAPK in CYP2E1-dependent arachidonic acid toxicity. J Biol Chem. 2003;278(2):1115–24. doi: 10.1074/jbc.M207856200. [DOI] [PubMed] [Google Scholar]
- 104.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 105.Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. Tumor necrosis factor induced liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang X, Wu D, Yang L, Cederbaum AI. Hepatotoxicity mediated by cytochrome P4502E1 plus TNFα occurs in cJun-N terminal kinase 2−/− but not in cJun-N terminal 1−/− mice. Hepatology. 2011;54:1753–1766. doi: 10.1002/hep.24540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kojo A, Viitala P, Pasanen M, Pelkonen O, Raunio H, Juvonen R. Induction of CYP2A5 by pyrazole and its derivatives in mouse primary hepatocytes. Arch Toxicol. 1998;72:336–41. doi: 10.1007/s002040050511. [DOI] [PubMed] [Google Scholar]
- 109.Niemela O, Parkkila S, Juvonen RO, Viitala K, Gelboin HV, Pasanen M. Cytochromes P450 2A6, 2E1, and 3A and production of protein-aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J Hepatol. 2000;33:893–901. doi: 10.1016/s0168-8278(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 110.Jin M, Kumar A, Kumar S. Ethanol-mediated regulation of cytochrome P450 2A6 expression in monocytes: role of oxidative stress-mediated PKC/MEK/Nrf2 pathway. PLoS One. 2012;7:e35505. doi: 10.1371/journal.pone.0035505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Honkakoski P, Autio S, Juvonen R, Raunio H, Gelboin HV, Park SS, et al. Pyrazole is different from acetone and ethanol as an inducer of the polysubstrate monooxygenase system in mice: evidence that pyrazole-inducible P450Coh is distinct from acetone-inducible P450ac. Arch Biochem Biophys. 1988;267:589–98. doi: 10.1016/0003-9861(88)90066-5. [DOI] [PubMed] [Google Scholar]
- 112.Su T, Sheng JJ, Lipinskas TW, Ding X. Expression of CYP2A genes in rodent and human nasal mucosa. Drug Metab Dispos. 1996;24:884–890. [PubMed] [Google Scholar]
- 113.Kimura S, Kozak CA, Gonzalez FJ. Identification of a novel P450 expressed in rat lung: cDNA cloning and sequence, chromosome mapping, and induction by 3-methylcholanthrene. Biochemistry. 1989;28:3798–3803. doi: 10.1021/bi00435a026. [DOI] [PubMed] [Google Scholar]
- 114.Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev. 1997;77:517–44. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 115.Cheung C, Gonzalez FJ. Humanized mouse lines and their application for prediction of human drug metabolism and toxicological risk assessment. J Pharmacol Exp Ther. 2008;327:288–299. doi: 10.1124/jpet.108.141242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Song BJ, Gelboin HV, Park SS, Yang CS, Gonzalez FJ. Complementary DNA protein sequences of ethanol-inducible rat, human cytochrome P-450s. Transcriptional and post-transcriptional regulation of the rat enzyme. J Biol Chem. 1986;261:16689–16697. [PubMed] [Google Scholar]
- 117.Song B, Veech RL, Park SS, Gelboin HV, Gonzalez FJ. Induction of rat hepatic N-nitrosodimethylamine demethylase by acetone is due to protein stabilization. J Biol Chem. 1989;264:3568–3572. [PubMed] [Google Scholar]
- 118.Cai Y, Konishi T, Han G, Campwala KH, French SW, Wan YJ. The role of hepatocyte RXR alpha in xenobiotic-sensing nuclear receptor-mediated pathways. Eur J Pharmacol. 2002;15:89–96. doi: 10.1016/s0928-0987(01)00211-1. [DOI] [PubMed] [Google Scholar]
- 119.Barclay TB, Peters JM, Sewer MB, Ferrari L, Gonzalez FJ, Morgan ET. Modulation of cytochrome P-450 gene expression in endotoxemic mice is tissue specific and peroxisome proliferator-activated receptor-alpha dependent. J Pharmacol Exp Ther. 1999;290:1250–1257. [PubMed] [Google Scholar]
- 120.Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K, et al. Peroxisome proliferator-activated receptor alpha protects against alcohol-induced liver damage. Hepatology. 2004;40(4):972–80. doi: 10.1002/hep.20399. [DOI] [PubMed] [Google Scholar]
- 121.Chen X, Ward SC, Cederbaum AI, Xiong H, Lu Y. Alcoholic fatty liver is enhanced in CYP2A5 knockout mice: The role of the PPARα-FGF21 axis. Toxicology. 2017;379:12–21. doi: 10.1016/j.tox.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hayes SD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox, intermediary metabolism. Trends Biochem. Sciences. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 123.Ma Q. Role of Nrf2 in oxidative stress and toxicity. Ann Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Slocum SL, Kensler TW. Nrf2: control of sensitivity to carcinogens. Arch Toxicol. 2011;85:273–284. doi: 10.1007/s00204-011-0675-4. [DOI] [PubMed] [Google Scholar]
- 125.Baird L, Dinkova-Kostova AT. The cytoprotective role of the keap1-Nrf2 pathway. Arch Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 126.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 127.Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating NRF2q activation in response to chemical stress. Free Radic Biol Med. 2004;37:433–441. doi: 10.1016/j.freeradbiomed.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 128.Ma Q, He X. Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2. Pharmacol Reviews. 2012;64:1055–1081. doi: 10.1124/pr.110.004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the keap 1-Nrf2-ARE pathway. Ann Review Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 130.Niture SK, Kasper JW, Shen J, Jaiswal AK. Nrf2 signaling and cell survival. Toxciol Appl Pharmacol. 2010;244:37–42. doi: 10.1016/j.taap.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cho HY, Reddy SP, Dibiase A, Yamamolto M, Kleeberger SR. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic Biol Med. 2005;38:325–343. doi: 10.1016/j.freeradbiomed.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 132.Abu-Bakar A, Lämsä V, Arpiainen S, Moore MR, Lang MA, Hakkola J. Regulation of CYP2A5 gene by the transcription factor nuclear factor (erythroid-derived 2)-like 2. Drug Metab Dispos. 2007;35:787–94. doi: 10.1124/dmd.106.014423. [DOI] [PubMed] [Google Scholar]
- 133.Lämsä V, Levonen AL, Leinonen H, Ylä-Herttuala S, Yamamoto M, Hakkola J. Cytochrome P450 2A5 constitutive expression and induction by heavy metals is dependent on redox-sensitive transcription factor Nrf2 in liver. Chem Res Toxicol. 2010;23:977–85. doi: 10.1021/tx100084c. [DOI] [PubMed] [Google Scholar]
- 134.Abu-Bakar A, Satarug S, Marks GC, Lang MA, Moore MR. Acute cadmium chloride administration induces hepatic and renal CYP2A5 mRNA, protein and activity in the mouse: involvement of transcription factor NRF2. Toxicol Lett. 2004;148:199–210. doi: 10.1016/j.toxlet.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 135.Yokota S, Higashi E, Fukami T, Yokoi T, Nakajima M. Human CYP2A6 is regulated by nuclear factor-erythroid 2 related factor 2. Biochem Pharmacol. 2011;81:289–294. doi: 10.1016/j.bcp.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 136.Lamle J, Marhenke S, Borlak J, von Wasielewski R, Eriksson CJ, Geffers R, et al. Nuclear factor-erthyoid 2-related factor 2 prevents alcohol-induced fulminant liver injury. Gastroenterology. 2008;134:1159–1168. doi: 10.1053/j.gastro.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 137.Gong P, Cederbaum AI. Nrf2 is increased by CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology. 2006;43:144–153. doi: 10.1002/hep.21004. [DOI] [PubMed] [Google Scholar]
- 138.Gong P, Cederbaum AI. Transcription factor Nrf2 protects HepG2 cells against CYP2E1 plus arachidonic acid-dependent toxicity. J Biol Chem. 2006;281:14573–14579. doi: 10.1074/jbc.M600613200. [DOI] [PubMed] [Google Scholar]
- 139.Mandal P, Park PH, McMullen MR, Pratt BT, Nagy LE. The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology. 2010;51(4):1420–9. doi: 10.1002/hep.23427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–54. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 141.Bauer M, Bauer I. Heme oxygenase-1: redox regulation and role in the hepatic response to oxidative stress. Antioxid Redox Signal. 2002;4:749–58. doi: 10.1089/152308602760598891. [DOI] [PubMed] [Google Scholar]
- 142.Lämsä V, Levonen AL, Sormunen R, Yamamoto M, Hakkola J. Heme and heme biosynthesis intermediates induce heme oxygenase-1 and cytochrome P450 2A5, enzymes with putative sequential roles in heme and bilirubin metabolism: different requirement for transcription factor nuclear factor erythroid- derived 2-like 2. Toxicol Sci. 2012;130:132–44. doi: 10.1093/toxsci/kfs237. [DOI] [PubMed] [Google Scholar]
- 143.Maines MD, Costa LG, Reed DJ, Sassa S, Sipes IG. Current protocols in toxicology. John Wiley & Sons; New York: 1999. Overview of heme degradation pathway. [DOI] [PubMed] [Google Scholar]
- 144.Abu-Bakar A, Moore MR, Lang MA. Evidence for induced microsomal bilirubin degradation by cytochrome P450 2A5. Biochem Pharmacol. 2005;70:1527–35. doi: 10.1016/j.bcp.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 145.Abu-Bakar A, Arthur DM, Aganovic S, Ng JC, Lang MA. Inducible bilirubin oxidase: a novel function for the mouse cytochrome P450 2A5. Toxicol Appl Pharmacol. 2011;257:14–22. doi: 10.1016/j.taap.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 146.Kim SD, Antenos M, Squires EJ, Kirby GM. Cytochrome P450 2A5 and bilirubin: mechanisms of gene regulation and cytoprotection. Toxicol Appl Pharmacol. 2013;270:129–38. doi: 10.1016/j.taap.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 147.Leung TM, Lu Y. Alcoholic liver disease: From CYP2E1 to CYP2A5. Curr Mol Pharmacol. 2017;10(3):172–178. doi: 10.2174/1874467208666150817111846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salonpää P, Pelkonen O, Kojo A, Pasanen M, Negishi M, Raunio H. Cytochrome P4502A5 expression and inducibility by phenobarbital is modulated by cAMP in mouse primary hepatocytes. Biochem Biophys Res Commun. 1994;205:631–7. doi: 10.1006/bbrc.1994.2712. [DOI] [PubMed] [Google Scholar]
- 149.Viitala P, Posti K, Lindfors A, Pelkonen O, Raunio H. cAMP mediated upregulation of CYP2A5 in mouse hepatocytes. Biochem Biophys Res Commun. 2001;280:761–7. doi: 10.1006/bbrc.2000.4195. [DOI] [PubMed] [Google Scholar]
- 150.He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, et al. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137:635–46. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–83. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 152.Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 153.Arppianen S, Jarvenpaa SM, Manninen A, Viitala P, Lang MA, Pelkonen O, et al. Coactivator PGC-1 alpha regulated the fasting inducible xenobiotic metabolizing enzyme CYP2A5 in mouse primary hepatocytes. Toxicol Appl Pharmacol. 2008;232:135–141. doi: 10.1016/j.taap.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 154.Nagy LE. Role of adenosine A1 receptors in inhibition of receptor-stimulated cyclic AMP production by ethanol in hepatocytes. Biochem Pharmacol. 1994;48:2091–6. doi: 10.1016/0006-2952(94)90509-6. [DOI] [PubMed] [Google Scholar]
- 155.Nagy LE, DeSilva SE. Ethanol increases receptor-dependent cyclic AMP production in cultured hepatocytes by decreasing G(i)-mediated inhibition. Biochem J. 1992;286:681–6. doi: 10.1042/bj2860681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127(6):1798–808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 157.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc. Natl Acad. Sci. USA. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kliewer SA, Mangelsdorf DJ. Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr. 2010;91(1):254S–257S. doi: 10.3945/ajcn.2009.28449B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 162.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492:203–6. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 163.Luo Y, McKeehan WL. Stressed Liver and Muscle Call on Adipocytes with FGF21. Front Endocrinol (Lausanne) 2013;4:194. doi: 10.3389/fendo.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–25. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 165.Lundåsen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, et al. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun. 2007;360:437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 166.Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, Flier JS, et al. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology. 2011;152:2996–3004. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Poggi M, Di Luzio NR. The role of liver and adipose tissue in the pathogenesis of the ethanol-induced fatty liver. J Lipid Res. 1964;5(3):437–41. [PubMed] [Google Scholar]
- 169.Horning MG, Williams EA, Maling HM, Brodie BB. Depot fat as source of increased liver triglycerides after ethanol. Biochem Biophys Res Commun. 1960;3:635–40. doi: 10.1016/0006-291x(60)90077-2. [DOI] [PubMed] [Google Scholar]
- 170.Zhong W, Zhao Y, Tang Y, Wei X, Shi X, Sun W, et al. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol. 2012;180(3):998–1007. doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Murphy SE. Nicotine Metabolism and Smoking: Ethnic Differences in the Role of P450 2A6. Chem Res Toxicol. 2017;30(1):410–419. doi: 10.1021/acs.chemrestox.6b00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Somm E, Schwitzgebel VM, Vauthay DM, Aubert ML, Hüppi PS. Prenatal nicotine exposure and the programming of metabolic and cardiovascular disorders. Mol Cell Endocrinol. 2009;304(1–2):69–77. doi: 10.1016/j.mce.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 173.Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J Pharmacol Exp Ther. 1997;282:1608–14. [PubMed] [Google Scholar]
- 174.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, et al. Role of human cytochrome P4502A6 in C-oxidation of nicotine. Drug Metab Dispos. 1996;24:1212–7. [PubMed] [Google Scholar]
- 175.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 176.Schoedel KA, Tyndale RF. Induction of nicotine-metabolizing CYP2B1 by ethanol and ethanol-metabolizing CYP2E1 by nicotine: summary and implications. Biochim Biophys Acta. 2003;1619(3):283–90. doi: 10.1016/s0304-4165(02)00487-7. [DOI] [PubMed] [Google Scholar]
- 177.Sellers EM, Tyndale RF, Fernandes LC. Decreasing smoking behaviour and risk through CYP2A6 inhibition. Drug Discov Today. 2003;8(11):487–93. doi: 10.1016/s1359-6446(03)02704-1. [DOI] [PubMed] [Google Scholar]
- 178.Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. Am J Epidemiol. 1992;136(10):1248–57. doi: 10.1093/oxfordjournals.aje.a116433. [DOI] [PubMed] [Google Scholar]
- 179.Bailey SM, Mantena SK, Millender-Swain T, Cakir Y, Jhala NC, Chhieng D, et al. Ethanol and tobacco smoke increase hepatic steatosis and hypoxia in the hypercholesterolemic apoE(−/−) mouse: implications for a “multihit” hypothesis of fatty liver disease. Free Radic Biol Med. 2009;46:928–38. doi: 10.1016/j.freeradbiomed.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu Y, Ward SC, Cederbaum AI. Nicotine enhances ethanol-induced fat accumulation and collagen deposition but not inflammation in mouse liver. Alcohol. 2013;47:353–357. doi: 10.1016/j.alcohol.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zeng T, Zhang CL, Zhao N, Guan MJ, Xiao M, Yang R, et al. Impairment of Akt activity by CYP2E1 mediated oxidative stress is involved in chronic ethanol-induced fatty liver. Redox Biol. 2017;14:295–304. doi: 10.1016/j.redox.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang F, Liu JC, Zhou RJ, Zhao X, Liu M, Ye H, et al. Apigenin protects against alcohol-induced liver injury in mice by regulating hepatic CYP2E1-mediated oxidative stress and PPARα-mediated lipogenic gene expression. Chem Biol Interact. 2017;275:171–177. doi: 10.1016/j.cbi.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 183.Saikia P, Bellos D, McMullen MR, Pollard KA, de la Motte C, Nagy LE. MicroRNA 181b-3p and its target importin α5 regulate toll-like receptor 4 signaling in Kupffer cells and liver injury in mice in response to ethanol. Hepatology. 2017 Aug;66(2):602–615. doi: 10.1002/hep.29144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li M, He Y, Zhou Z, Ramirez T, Gao Y, Gao Y, et al. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47<sup>phox</sup>-oxidative stress pathway in neutrophils. Gut. 2017 Apr;66(4):705–715. doi: 10.1136/gutjnl-2016-311861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Blaya D, Coll M, Rodrigo-Torres D, Vila-Casadesús M, Altamirano J, Llopis M, et al. Integrative microRNA profiling in alcoholic hepatitis reveals a role for microRNA-182 in liver injury and inflammation. Gut. 2016 Sep;65(9):1535–45. doi: 10.1136/gutjnl-2015-311314. [DOI] [PubMed] [Google Scholar]
- 186.Nakano M, Mohri T, Fukami T, Takamiya M, Aoki Y, McLeod HL, et al. Single-Nucleotide Polymorphisms in Cytochrome P450 2E1 (CYP2E1) 3′-Untranslated Region Affect the Regulation of CYP2E1 by miR-570. Drug Metab Dispos. 2015 Oct;43(10):1450–7. doi: 10.1124/dmd.115.065664. [DOI] [PubMed] [Google Scholar]
- 187.Miao L, Yao H, Li C, Pu M, Yao X, Yang H, et al. A dual inhibition: microRNA-552 suppresses both transcription and translation of cytochrome P450 2E1. Biochim Biophys Acta. 2016 Apr;1859(4):650–62. doi: 10.1016/j.bbagrm.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 188.Mohri T, Nakajima M, Fukami T, Takamiya M, Aoki Y, Yokoi T. Human CYP2E1 is regulated by miR-378. Biochem Pharmacol. 2010 Apr 1;79(7):1045–52. doi: 10.1016/j.bcp.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 189.Wang Y, Yu D, Tolleson WH, Yu LR, Green B, Zeng L, et al. A systematic evaluation of microRNAs in regulating human hepatic CYP2E1. Biochem Pharmacol. 2017 Aug 15;138:174–184. doi: 10.1016/j.bcp.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang XH, Cui XX, Sun XQ, Wang XH, Li XC, Qi Y, et al. High Fat Diet-Induced Hepatic 18-Carbon Fatty Acids Accumulation Up-Regulates CYP2A5/CYP2A6 via NF-E2-Related Factor 2. Front Pharmacol. 2017 May 15;8:233. doi: 10.3389/fphar.2017.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cui Y, Wang Q, Yi X, Zhang X. Effects of Fatty Acids on CYP2A5 and Nrf2 Expression in Mouse Primary Hepatocytes. Biochem Genet. 2016 Feb;54(1):29–40. doi: 10.1007/s10528-015-9697-6. [DOI] [PubMed] [Google Scholar]
- 192.Deng X, Pu Q, Wang E, Yu C. Celery extract inhibits mouse CYP2A5 and human CYP2A6 activities via different mechanisms. Oncol Lett. 2016 Dec;12(6):5309–5314. doi: 10.3892/ol.2016.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tsuchishima M, George J, Shiroeda H, Arisawa T, Takegami T, Tsutsumi M. Chronic ingestion of ethanol induces hepatocellular carcinoma in mice without additional hepatic insult. Dig Dis Sci. 2013 Jul;58(7):1923–33. doi: 10.1007/s10620-013-2574-4. [DOI] [PubMed] [Google Scholar]
- 194.Mueller S, Peccerella T, Qin H, Glassen K, Waldherr R, Flechtenmacher C, et al. Carcinogenic Etheno-DNA Adducts in Alcoholic Liver Disease: Correlation with Cytochrome P-4502E1 and Fibrosis. Alcohol Clin Exp Res. 2017 Nov 9; doi: 10.1111/acer.13546. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 195.Linhart K, Bartsch H, Seitz HK. The role of reactive oxygen species (ROS) and cytochrome P-450 2E1 in the generation of carcinogenic etheno-DNA adducts. Redox Biol. 2014;3:56–62. doi: 10.1016/j.redox.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Seitz HK, Wang XD. The role of cytochrome P450 2E1 in ethanol-mediated carcinogenesis. Subcell Biochem. 2013;67:131–43. doi: 10.1007/978-94-007-5881-0_3. [DOI] [PubMed] [Google Scholar]
- 197.Zhou X, D’Agostino J, Xie F, Ding X. Role of CYP2A5 in the bioactivation of the lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in mice. J Pharmacol Exp Ther. 2012;341(1):233–41. doi: 10.1124/jpet.111.190173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Megaraj V, Zhou X, Xie F, Liu Z, Yang W, Ding X. Role of CYP2A13 in the bioactivation and lung tumorigenicity of the tobacco-specific lung procarcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone: in vivo studies using a CYP2A13-humanized mouse model. Carcinogenesis. 2014;35:131–7. doi: 10.1093/carcin/bgt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Takeuchi H, Saoo K, Matsuda Y, Yokohira M, Yamakawa K, Zeng Y, et al. Dose dependent inhibitory effects of dietary 8-methoxypsoralen on NNK-induced lung tumorigenesis in female A/J mice. Cancer Lett. 2006;234:232–8. doi: 10.1016/j.canlet.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 200.Miyazaki M, Yamazaki H, Takeuchi H, Saoo K, Yokohira M, Masumura K, et al. Mechanisms of chemopreventive effects of 8-methoxypsoralen against 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced mouse lung adenomas. Carcinogenesis. 2005;26:1947–55. doi: 10.1093/carcin/bgi156. [DOI] [PubMed] [Google Scholar]
- 201.Marzioni M, Glaser SS, Francis H, Phinizy JL, LeSage G, Alpini G. Functional heterogeneity of cholangiocytes. Semin Liver Dis. 2002;22:227–40. doi: 10.1055/s-2002-34501. [DOI] [PubMed] [Google Scholar]
- 202.Ebert EC, Nagar M, Hagspiel KD. Gastrointestinal and hepatic complications of sickle cell disease. Clin Gastroenterol Hepatol. 2010;8(6):483–9. doi: 10.1016/j.cgh.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 203.Arpiainen S, Lamsa V, Pelkonen O, Yim SH, Gonzalez FJ, Hakkola J. Aryl hydrocarbon receptor nuclear translocator and upstream stimulatory factor regulate cytochrome P450 2a5 transcription through a common E-box site. J Mol Biol. 2007;369:640–652. doi: 10.1016/j.jmb.2007.03.075. [DOI] [PubMed] [Google Scholar]
- 204.Arpiainen S, Raffalli-Mathieu F, Lang MA, Pelkonen O, Hakkola J. Regulation of Cyp2a5 gene involves an aryl hydrocarbon receptor-dependent pathway. Mol Pharmacol. 2005;67:1325–1333. doi: 10.1124/mol.104.008078. [DOI] [PubMed] [Google Scholar]
- 205.Hu H, Yu T, Arpiainen S, Lang MA, Hakkola J, Abu-Bakar A. Tumour suppressor protein p53 regulates the stress activated bilirubin oxidase cytochrome P450 2A6. Toxicol Appl Pharmacol. 2015;289(1):30–9. doi: 10.1016/j.taap.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 206.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 207.Katoh Y, Itoh K, Yoshida E, Miyagishi M, Fukamizu A, Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–868. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 208.Sun Z, Chin YE, Zhang DD. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol. 2009;29:2658–72. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kawai Y, Garduño L, Theodore M, Yang J, Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem. 2011;286:7629–40. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wall TL, Schoedel K, Ring HZ, Luczak SE, Katsuyoshi DM, Tyndale RF. Differences in pharmacogenetics of nicotine and alcohol metabolism: review and recommendations for future research. Nicotine Tobacco Res. 2007;9:S459–S474. doi: 10.1080/14622200701587045. [DOI] [PubMed] [Google Scholar]
- 211.Siu EC, Wildenauer DB, Tyndale RF. Nicotine self-administration in mice is associated with rates of nicotine inactivation by CYP2A5. Psychopharmacology (Berl) 2006;184:401–408. doi: 10.1007/s00213-006-0306-6. [DOI] [PubMed] [Google Scholar]
- 212.Zabala V, Tong M, Yu R, Ramirez T, Yalcin EB, Balbo S, et al. Potential contributions of the tobacco nicotine-derived nitrosamine ketone (NNK) in the pathogenesis of steatohepatitis in a chronic plus binge rat model of alcoholic liver disease. Alcohol Alcohol. 2015;50(2):118–31. doi: 10.1093/alcalc/agu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Soeda J, Morgan M, McKee C, Mouralidarane A, Lin C, Roskams T, et al. Nicotine induces fibrogenic changes in human liver via nicotinic acetylcholine receptors expressed on hepatic stellate cells. Biochem Biophys Res Commun. 2012;417(1):17–22. doi: 10.1016/j.bbrc.2011.10.151. [DOI] [PubMed] [Google Scholar]
- 214.Muhsain SN, Lang MA, Abu-Bakar A. Mitochondrial targeting of bilirubin regulatory enzymes: An adaptive response to oxidative stress. Toxicol Appl Pharmacol. 2015;282(1):77–89. doi: 10.1016/j.taap.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 215.Genter MB, Clay CD, Dalton TP, Dong H, Nebert DW, Shertzer HG. Comparison of mouse hepatic mitochondrial versus microsomal cytochromes P450 following TCDD treatment. Biochem Biophys Res Commun. 2006;342:1375–81. doi: 10.1016/j.bbrc.2006.02.121. [DOI] [PubMed] [Google Scholar]
- 216.Ashino T, Ohkubo-Morita H, Yamammoto M, Yoshida T, Numazawa S. Possible involvement of Nrf2 in the gene expression of Cyb2b10 and Cyp2a5. Redox Biology. 2014;2:284–288. doi: 10.1016/j.redox.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]