Abstract
This study aimed to enhance production of polyhydroxybutyrate P(3HB) by a newly engineered strain of Cupriavidus necator NSDG-GG by applying response surface methodology (RSM). From initial experiment of one-factor-at-a-time (OFAT), glucose and urea were found to be the most significant substrates as carbon and nitrogen sources, respectively, for the production of P(3HB). OFAT experiment results showed that the maximum biomass, P(3HB) content, and P(3HB) concentration of 8.95 g/L, 76 wt%, and 6.80 g/L were achieved at 25 g/L glucose and 0.54 g/L urea with an agitation rate of 200 rpm at 30 °C after 48 h. In this study, RSM was applied to optimize the three key variables (glucose concentration, urea concentration, and agitation speed) at a time to obtain optimal conditions in a multivariable system. Fermentation experiments were conducted in shaking flask by cultivation of C. necator NSDG-GG using various glucose concentrations (10–50 g/L), urea concentrations (0.27–0.73 g/L), and agitation speeds (150–250 rpm). The interaction between the variables studied was analyzed by ANOVA analysis. The RSM results indicated that the optimum cultivation conditions were 37.70 g/L glucose, 0.73 g/L urea, and 200 rpm agitation speed. The validation experiments under optimum conditions produced the highest biomass of 12.84 g/L, P(3HB) content of 92.16 wt%, and P(3HB) concentration of 11.83 g/L. RSM was found to be an efficient method in enhancing the production of biomass, P(3HB) content, and P(3HB) concentration by 43, 21, and 74%, respectively.
Keywords: Cupriavidus necator, Glucose utilization, Poly(3-hydroxybutyrate), Response surface methodology (RSM)
Introduction
In various industries, petrochemical plastic has become one of the most intensively used materials all over the world such as automobile, agriculture, medicine, pharmaceutical, cosmetics, food industry, etc. Although the plastic derived from petrol is important in various industrial sectors, their disposal still poses a serious problem to the environment because of their non-degradable characteristics. For this reason, researchers developed biodegradable plastic such as polyhydroxyalkanoate (PHA) (Shah et al. 2008).
Generally, PHA is a variety of biodegradable polymer that is synthesized intracellularly by many genera of bacteria (Narayanan and Ramana 2012). PHA granules have a diameter of around 500 nm that can be considered as carbon and energy storage (Shah et al. 2008). PHA has thermo-mechanical properties similar to petrochemical polymers such as polypropylene (PP) and polyethylene (PE) (Lee 1996; Sudesh 2013) with a biodegradable characteristic in the environment. However, PHA production has some limitations as well. For example, one of the substrates which is used in PHA production is sugar but the use of the food grade sugar purified from plant resources of sugar in the foodstuff industry is expensive. For this reason, the utilization of inedible plant materials such as agro by-products appears to be convenient for PHA production.
In this context, microbial strain Cupriavidus necator and some of its genetically modified organisms (GMO), and also many other PHA producing microorganisms, do not produce enzymes to utilize complex sugars from agro-wastes. This has resulted in the utilization of only simple types of sugar for PHA production (Jiang et al. 2016). Moreover, C. necator wild strain H16 could assimilate some limited simple sugars such as fructose and gluconate, but does not utilize glucose. So far, a few glucose-utilizing mutants of C. necator have been isolated by UV and spontaneous mutagenesis (Raberg et al. 2012), and constructed by targeted genetic engineering (Poirier et al. 1992). The strain C. necator NSDG-GG is one of modified strains to accumulate P(3HB) from glucose.
OFAT is a suitable method to identify the considerable factors and their useful operational ranges. However, they rarely evaluate the effect of more than a factor at the time and their interactions, which is a weakness once the interactions of factors are significant. Generally, the feature of the bioprocesses is characterized by the interactions among the level of their input factors. As a result, when the interactions among the levels of input factors are significant, the major disadvantage of this method is that it hardly gives the optimal conditions for the responses investigated. Furthermore, this method is time consuming, laborious and expensive (Wang and Wan 2009; Surwase et al. 2013).
Hence, in the quantitative optimization of bioprocess, a robust design of experiment (DOE) has to be applied, to which the input parameter levels and their interaction can be analyzed. On the other hand, the purpose of statistical optimization experiment is to build a predictive statistical mathematical model to predict the behavior of the process being investigated. They form graphical statistical models which can be obtained from RSM (Montgomery 1991; Dashti et al. 2016). The central composite design (CCD) is the most popular design for RSM used to fit models determined. It is a Box–Wilson central composite design, but usually called a CCD. This design consists of a factorial design (FD) with center points and rotating points (star or axial points) that allow estimation of curvature and generate a second-order model (Hembree et al. 2010; Emeko et al. 2015).
On the other hand, most of the investigations have been focused on reducing the total cost of PHA by optimization of fermentation process using the statistical approach of RSM (Poirier et al. 1992; Mokhtari-Hosseini et al. 2009; Sheu et al. 2009). Furthermore, the use of GMO can be an alternative to decrease the cost of production. Thus, glucose may be an economically feasible feedstock in optimized systems. RSM is a statistical approach that uses quantitative data from a sequence of systematic experiments to seek for optimal condition in multivariable system by establishing the authority of each element in the organization (Bezerra et al. 2008). The related literature shows that CCD has been successfully applied to optimize microbial cell cultures of Bacillus megaterium and Bacillus subtilis SRKP-3 to produce P(3HB) (Pandian et al. 2010; Sathiyanarayanan et al. 2013).
The aim of this study was to improve the production of biomass and P(3HB) by the statistical design of CCD based on pivotal culture medium parameters, namely the initial concentration of carbon and nitrogen sources including glucose and urea, respectively, and agitation speed. This study has a unique feature of applying statistical optimization of P(3HB) production by C. necator NSDG-GG using RSM with the emphasis on only a single carbon source (glucose).
Materials and methods
Microorganism
Cupriavidus necator NSDG-GG is a glucose-utilizable engineered strain of wild strain H16 which has been constructed by modification of nag operon and replacement of phaC with a mutant gene of phaC derived from Aeromonas caviae on the chromosome (Mifune et al. 2008; Orita et al. 2012). The stock culture was maintained in nutrient rich (NR) broth medium with 50% glycerol at − 80 °C.
Culture conditions and growth media
To prepare the seed inoculum from the C. necator NSDG-GG stock culture, three loopsful of 24-h freshly grown culture on a nutrient rich agar plate at 30 °C were transferred into a 50-mL nutrient rich broth medium containing (g/L): yeast extract, 2; beef extract, 10; and peptone, 10 prepared in a 250-mL Erlenmeyer flask in which no baffles and aeration system were applied. Then, culture was incubated at 30 °C in a rotary shaker running at 200 rpm for 8 h. Subsequently, 3% (v/v) of the seed culture were transferred aseptically into 50 mL of a minimal salts medium (MSM). The MSM comprised (g/L): NaH2PO4, 4.0; Na2HPO4, 4.6; K2SO4, 0.45; Mg2SO4, 0.39; CaCl2, 0.062; and 1 mL trace elements. The trace elements were composed of (g/L): FeSO4·6H2O, 15; MnSO4·H2O, 2.4; ZnSO4·7H2O, 2.4; and CuSO4·5H2O, 0.48 in 0.1 M HCl which was adjusted to pH 6.8 using equimolarity of 1M HCl and 1M NaOH (Budde et al. 2010). In OFAT experiment, glucose as a carbon source was prepared at various concentrations of 5–45 g/L while the urea as a nitrogen source was maintained at 0.54 g/L. The prepared culture was incubated for 48 h at 30 °C and 200 rpm agitation rate.
Determination of cell dry weight (CDW)
The cells were harvested by centrifugation at 8000 rpm at 4 °C for 10 min and they were washed two times with distilled water and frozen overnight into − 20 °C freezer. Then the cells were lyophilized based on weight gravimetrically using a freeze-dryer (LABCONCO FreeZone Freezer-Dryer).
Gas chromatography (GC) analysis of PHA
GC analysis was conducted to determine the PHA content and composition in the cells. Samples were prepared according to Braunegg et al. (1978): 2 mL chloroform and 2 mL methanol acidified with 15% (v/v) H2SO4 were added to approximately 10 mg of lyophilized cells. Methanolysis was conducted at 100 °C for 140 min to convert PHA monomers into hydroxyacyl methyl esters monomers. Having completed the methanolysis, the mixture was cooled to room temperature and 1 mL distilled water was added. Then, it was vortexed for 1 min which led into the formation of a lower chloroform phase and an upper aqueous phase. The samples taken from the lower chloroform phase were used for GC analysis. Caprylic methyl ester (CME) as an internal standard at a ratio of 1:1 was added to the samples. Analysis was performed using a GC-2010 Gas Chromatograph (Shimadzu, Japan) equipped with a Supelco SPB-1 column (Sigma-Aldrich, United States) operated at a temperature of 280 °C. An AOC-20i auto-injector (Shimadzu, Japan) was used with a temperature of 270 °C. The carrier gas was N2 with a flowrate of 14 mL/min. Detection was conducted using a flame ionization detector (FID) with a temperature of 280 °C. Data from the analysis were taken from GC Solution Version 2.30.00 SU3 software (Shimadzu, Japan).
Central composite design (CCD)
According to the screening results of OFAT, the CCD was employed to measure the proportional response amount of P(3HB) content (wt%), P(3HB) concentration (g/L), and biomass (g/L) under different culture conditions. Based on this design the total number of experimental combinations was 2k + 2 k + n0, where k is the number of independent variables and n0 is the number of repetitions of the experiments at the center point (Dashti et al. 2014). Twenty fermentation runs were designed based on the CCD for three independent variables of glucose concentration, urea concentration, and agitation speed. Each variable was coded at three levels of − 1, 0, and + 1, representing low, middle, and high level of the variance, respectively (Sharma et al. 2007). The coded values and the actual levels of the variables are given in Table 1 and the designed matrix of the performed experimental runs is shown in Table 2. As can be seen, experimental runs of 6, 7, 8, 9, 12 and 15 included center point in the experimental design which was repeated six times for the estimation of test error. In CCD experiments, the same incubation time and temperature as that in OFAT experiment was applied.
Table 1.
Test variables and levels of central composite design (CCD)
| Variable | Symbol | Unit | Levels | |||||
|---|---|---|---|---|---|---|---|---|
| Actual range | Coded value | |||||||
| Low | Middle | High | Low | Middle | High | |||
| Glucose | X 1 | g/L | 10 | 30 | 50 | − 1 | 0 | + 1 |
| Urea | X 2 | g/L | 0.27 | 0.54 | 0.73 | − 1 | 0 | + 1 |
| Agitation | X 3 | rpm | 150 | 200 | 250 | − 1 | 0 | + 1 |
Table 2.
Central composite design and experimental results for biomass (g/L), P(3HB) content (wt%), and P(3HB) concentration (g/L) produced by C. necator NSDG-GG in batch fermentation at 30 °C for 48 h
| Run | Glucose | Nitrogen | Agitation (rpm) | Biomass (g/L) | P(3HB) content (wt%) | P(3HB) concentration (g/L) |
|---|---|---|---|---|---|---|
| 1 | − 1 | − 1 | 1 | 5.21 | 74.01 | 3.85 |
| 2 | − 1 | 0 | 0 | 4.91 | 50.35 | 2.47 |
| 3 | 1 | − 1 | − 1 | 5.28 | 55.04 | 2.91 |
| 4 | 0 | − 1 | 0 | 7.51 | 89.81 | 6.74 |
| 5 | 1 | 1 | − 1 | 12.89 | 80.43 | 10.36 |
| 6 | 0 | 0 | 0 | 10.51 | 90.11 | 9.47 |
| 7 | 0 | 0 | 0 | 11.87 | 88.23 | 10.47 |
| 8 | 0 | 0 | 0 | 10.51 | 89.69 | 9.42 |
| 9 | 0 | 0 | 0 | 10.69 | 87.59 | 9.36 |
| 10 | 1 | − 1 | 1 | 4.48 | 74.81 | 3.35 |
| 11 | 0 | 0 | 1 | 10.39 | 88.51 | 9.19 |
| 12 | 0 | 0 | 0 | 11.69 | 87.69 | 10.25 |
| 13 | 1 | 1 | 1 | 13.32 | 59.47 | 7.92 |
| 14 | 1 | 0 | 0 | 9.11 | 70.68 | 6.43 |
| 15 | 0 | 0 | 0 | 9.81 | 89.51 | 8.78 |
| 16 | − 1 | − 1 | − 1 | 5.75 | 30.11 | 1.73 |
| 17 | 0 | 1 | 0 | 12.28 | 91.61 | 11.24 |
| 18 | − 1 | 1 | − 1 | 5.01 | 49.33 | 2.47 |
| 19 | 0 | 0 | − 1 | 10.48 | 78.27 | 8.21 |
| 20 | − 1 | 1 | 1 | 4.78 | 43.45 | 2.07 |
Statistical modeling
The experimental data from CCD design (Table 2) were used to fit a second-order polynomial regression model (1). A full model for independent variables is represented as follows:
| 1 |
where y is the predictive measured response, and are the independent variables, represents the intercept, , , and are the regression coefficients of the model (Kadier et al. 2018). The generated model for three independent variables was expressed as follows:
| 2 |
where y is the predictive measured response as P(3HB) content (wt%), P(3HB) concentration (g/L), and biomass (g/L), , , and are linear coefficients, , , and denote quadratic coefficients, , , and are interaction coefficients, , , and represent coded values of glucose concentration (g/L), urea concentration (g/L), and agitation speed (rpm), respectively. The numerical optimization was applied to synchronize optimization of the multiple responses in which all the independent variables were kept within the levels determined to maximize the responses. In order to validate the optimum conditions determined by the regression model, a set of fermentations was performed under the optimum conditions. The results of responses obtained from verification experiment were compared to the values predicted by the regression model. Statistical analysis of the data was performed by Design-Expert software (version 6.0.6 Stat-Ease, Inc.). The same software was used for optimization of the variables.
Results and discussion
Initial culture and P(3HB) production
Cultivation of C. necator NSDG-GG was run at 30 °C and 200 rpm using the medium introduced in the “Materials and methods”. Figure 1 shows that variations in glucose and urea concentrations in the range 5–25 and 0.34–0.54 g/L, respectively. As presented in Fig. 1, the glucose concentration more than 25 g/L and urea with a concentration more than 0.54 g/L resulted in a negative effect on the P(3HB) production and the production decreased. However, the results indicated that the highest biomass achieved was 8.95 g/L with P(3HB) content of 76 wt% and P(3HB) concentration of 6.80 g/L at 48 h and 200 rpm when glucose and urea concentrations were set at optimal concentrations of 25 and 0.54 g/L, respectively (Fig. 1). This indicates that the optimized combination of carbon and nitrogen sources at pH 6.8 and 200 rpm significantly increased biomass compared to that found by Aramvash et al. (2015). They measured a biomass of 1.37 g/L from C. necator ATCC 17699 with 20 g/L glucose and 2.0 g/L (NH4)2SO4 at pH 7.0 after 48 h. The results revealed that this new mutant strain (C. necator NSDG-GG) in the present study is capable of increasing bio-transforming of glucose into biomass.
Fig. 1.
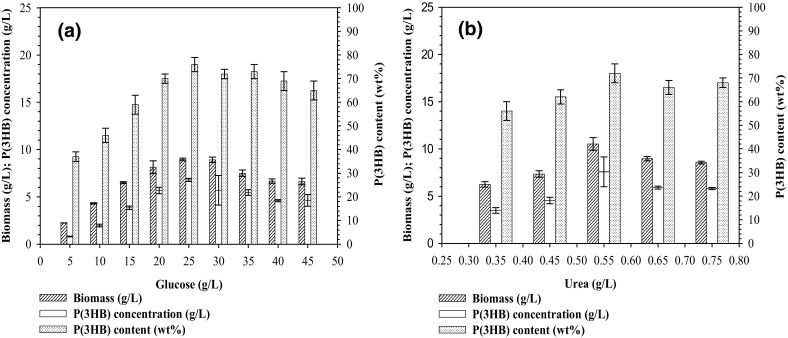
The effect of glucose concentration at 0.54 g/L urea (a); the effect of urea concentration at 25 g/L glucose (b) on biomass, P(3HB) content, and P(3HB) concentration
Biomass production
The second-order model obtained from the regression analysis of experiments of CCD is represented as follows:
| 3 |
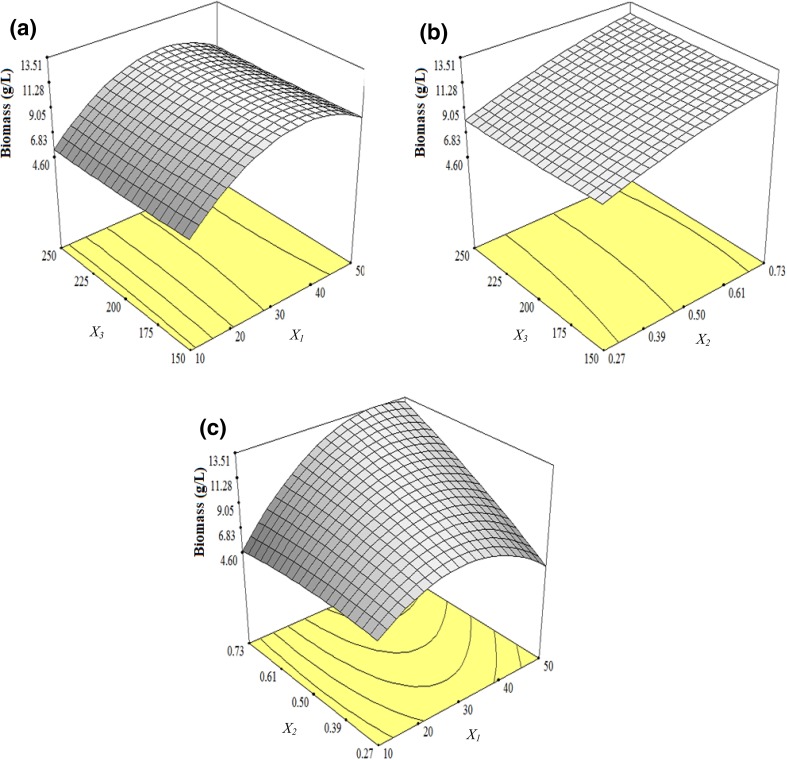
The statistical test for the ANOVA of the model is shown in Table 3. Table 3 demonstrates the result of biomass in response to the combination of input variables (X1, X2, X3) according to the experimental design. The regression coefficient and determination coefficient (R2) for a second-order model of biomass are given in Table 3. This regression model was significant (p < 0.01) with a reasonably large value of the determination coefficient (R2 = 0.9753). The response surface fitted to the model showed the effect of glucose (X1) and urea (X2) on biomass production. Therefore, it demonstrates that this quadratic model has a maximum predicted point and the interaction between its considerable linear terms. Referring to the fitted model (3), the positive coefficient of X1, X2, and X1X2 infers that glucose and urea are important and they synergistically interacted to improve biomass production (Table 3). The maximum actual production of biomass by C. necator NSDG-GG after 48 h cultivation was obtained when the initial concentration of glucose (X1) and urea (X2) was 50 and 0.73 g/L, respectively, at 250 rpm in Run 13 (Table 2).
Table 3.
Analysis of variance for the second-order polynomial model of biomass synthesis by C. necator NSDG-GG in batch fermentation at 30 °C for 48 h
| Source | Polynomial coefficient | Sum of squares | df | Mean squares | F value | Prob > F |
|---|---|---|---|---|---|---|
| Model | 178.95 | 9 | 19.88 | 43.82 | < 0.0001** | |
| Intercept | 10.64 | |||||
| X 1 | 1.94 | 37.71 | 1 | 37.71 | 83.11 | < 0.0001** |
| X 2 | 2.01 | 40.20 | 1 | 40.20 | 88.59 | < 0.0001** |
| X 3 | − 0.12 | 0.15 | 1 | 0.15 | 0.33 | 0.5764 |
| − 3.31 | 30.16 | 1 | 30.16 | 66.47 | < 0.0001** | |
| − 0.43 | 0.50 | 1 | 0.50 | 1.10 | 0.3181 | |
| 0.11 | 0.035 | 1 | 0.035 | 0.078 | 0.7862 | |
| X 1 X 2 | 2.2 | 38.81 | 1 | 38.81 | 85.52 | < 0.0001** |
| X 1 X 3 | 0.05 | 0.02 | 1 | 0.02 | 0.044 | 0.8379 |
| X 2 X 3 | 0.19 | 0.30 | 1 | 0.30 | 0.65 | 0.4377 |
| Residual | 4.54 | 10 | 0.45 | |||
| Lack of fit | 1.45 | 5 | 0.29 | 0.47 | 0.7858 | |
| Pure error | 3.08 | 5 | 0.62 |
X 1: glucose (g/L); X2: urea (g/L); X3: agitation (rpm); , , : the quadratic terms; X1X2, X1X3, X2X3: the interaction terms. R2 = 0.9753
**Statistically significant at 99% probability level
As shown in Table 3, the data indicated that the linear terms of X1, X2 and their interaction X1X2 were significant (p < 0.01). It shows that the changes in the concentration of X1 and X2 could extremely reduce or increase the growth. The coefficient of the model term X2 was relatively greater than X1, i.e., the glucose effects on biomass production was less than urea. As depicted in Fig. 2c, it can be explained that the increasing glucose concentration at a maximum concentration of urea contributes to the quadratic biomass production. However, both excessive additions of urea and glucose in medium decreased biomass production. Additionally, the results indicated that the synergistic interactions between glucose and urea concentration (X1 X2) increased the biomass production to a certain limit. The related literature shows that the carbon and nitrogen concentration are critical to balance biomass production; for example, some researchers (Sangkharak and Prasertsan 2007; Wisuthiphaet and Napathorn 2016) also found similar effects using various carbon and nitrogen sources with different concentrations. In this study, only glucose and urea were used as carbon and nitrogen sources and they sufficed to improve biomass production. This is because the organism used was genetically modified to accumulate biomass from glucose. In addition, urea was selected through the optimization of different nitrogen sources by C. necator NSDG-GG prior to this study.
Fig. 2.
3D surface showing the interactive effect of input-independent variables on biomass production after 48 h of cultivation: a the interaction of glucose (X1) and agitation speed (X3); b the interaction of urea (X2) and agitation speed (X3) and c the interaction of glucose (X1) and urea (X2)
Figure 2a, b shows that the increasing or decreasing of agitation rate and its interaction with glucose and urea concentrations lead to an insignificant effect on biomass production. The interactions between the variables can also be inferred from the shapes of the contour plots in the same Fig. 2a, b. The elliptical and saddle surfaces indicate the evidence of the interactions (Yu et al. 2008; Daneshi et al. 2010).
P(3HB) accumulation
Experimental results of P(3HB) content obtained from CCD are shown in Table 2. The results obtained from multiple regression analysis of CCD experiments were fitted to second-order polynomial model. P(3HB) content fitted in the terms of coded variables was obtained as the following model:
| 4 |
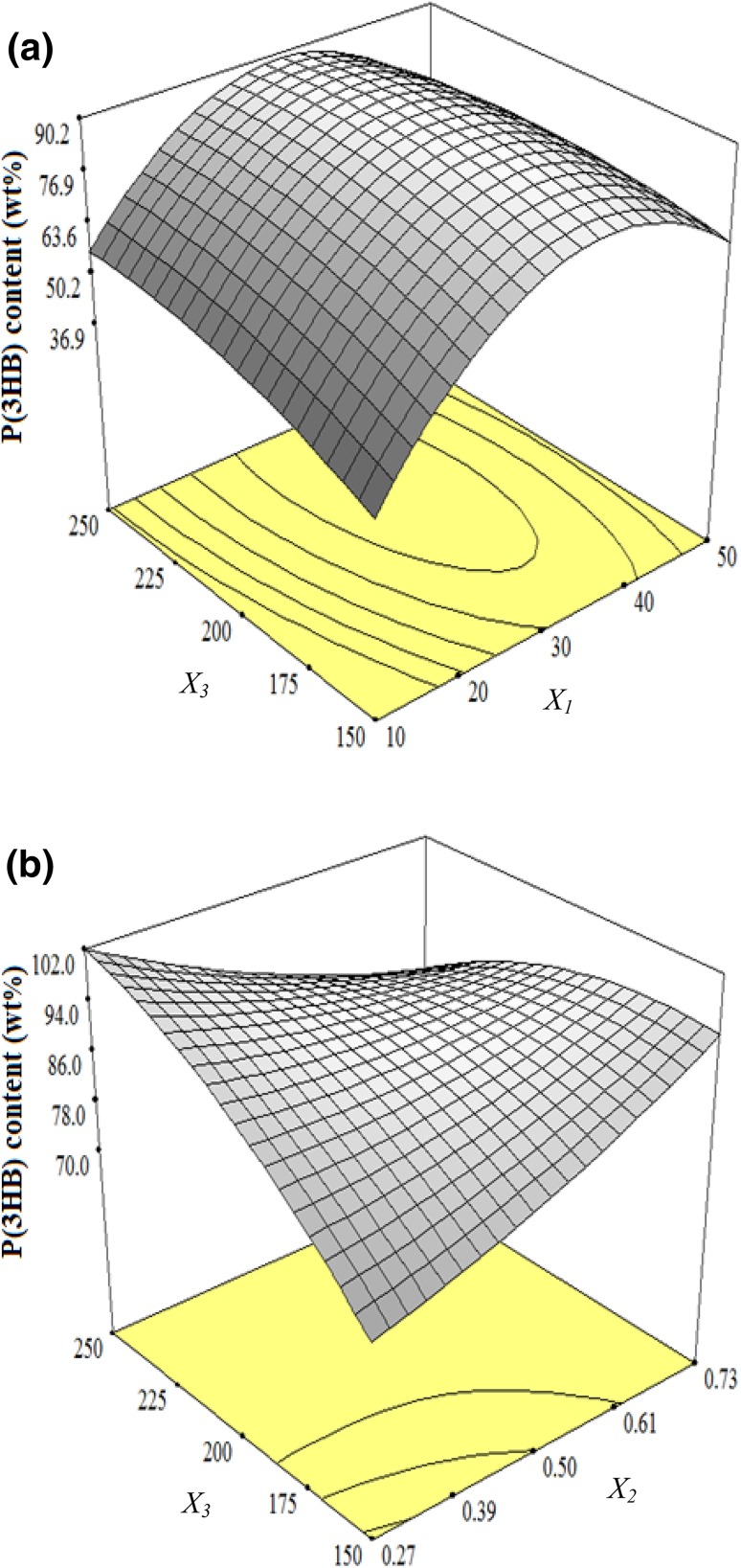
This model implies that the constant linear (X1, X3), quadratic (), and interaction terms (X1X3 and X2X3) are significant (Table 4). The negative polynomial coefficient in interaction terms implies that the interaction is antagonistic. The difference in the values of the coefficient between X1 (9.32) and X3 (4.71) might be a possible justification for their negative interaction and as a result an unbalanced effect on P(3HB) content as reflected in the response surface displayed in Fig. 3a. In other words, the unbalanced effect can be explained as a result of the magnitude effect from glucose concentration which is higher than agitation rate.
Table 4.
Analysis of variance for the second-order polynomial model of P(3HB) content produced by C. necator NSDG-GG in batch fermentation at 30 °C for 48 h
| Source | Polynomial coefficient | Sum of squares | df | Mean squares | F value | Prob > F |
|---|---|---|---|---|---|---|
| Model | 6733.46 | 9 | 748.16 | 355.31 | < 0.0001** | |
| Intercept | 88.61 | |||||
| X 1 | 9.32 | 868.25 | 1 | 868.25 | 412.34 | < 0.0001** |
| X 2 | 0.051 | 0.026 | 1 | 0.026 | 0.012 | 0.9137 |
| X 3 | 4.71 | 221.56 | 1 | 221.56 | 105.22 | < 0.0001** |
| − 27.81 | 2126.42 | 1 | 2126.42 | 1009.85 | < 0.0001** | |
| 2.39 | 15.68 | 1 | 15.68 | 7.45 | 0.0212 | |
| − 4.93 | 66.90 | 1 | 66.90 | 31.77 | 0.0002 | |
| X 1 X 2 | 2.67 | 57.19 | 1 | 57.19 | 27.16 | 0.0004 |
| X 1 X 3 | − 4.90 | 192.18 | 1 | 192.18 | 91.27 | < 0.0001** |
| X 2 X 3 | − 11.31 | 1024.01 | 1 | 1024.01 | 486.31 | < 0.0001** |
| Residual | 21.06 | 10 | 2.11 | |||
| Lack of fit | 15.02 | 5 | 3.00 | 2.49 | 0.1697 | |
| Pure error | 6.03 | 5 | 1.21 |
X 1: glucose (g/L); X2: urea (g/L); X3: agitation (rpm); , , : the quadratic terms; X1X2, X1X3, X2X3: the interaction terms. R2 = 0.9969
**Statistically significant at 99% probability level
Fig. 3.
3D surface showing the interactive effect of input independent variables on P(3HB) content after 48 h of cultivation: a the interaction of glucose (X1) and agitation speed (X3) and b the interaction of urea (X2) and agitation speed (X3)
The analysis of quadratic model shows that input independent variables of glucose concentration (X1) and agitation rate (X3) were important. The analysis indicated that the linear terms coded as X1, X3, and their interaction (X1X3) are significant with the probability value of p < 0.01. This obviously implies that the effect of coded variable X1, X3, and their interaction is meaningful to P(3HB) accumulation in the design. Glucose concentration is as important as agitation rate. However, the linear terms of them positively correlated with the P(3HB) accumulation rise to a certain limit while the interactive term was negatively correlated. In other words, this indicates that the glucose and agitation rate cause P(3HB) content to be accumulated, but their interactive terms are antagonistic.
Given the interaction of nitrogen concentration with agitation (X2X3), the antagonistic effect seemed to be more obvious on P(3HB) accumulation. Therefore, increasing agitation rate and decreasing urea concentration gained more P(3HB) accumulation as demonstrated in Fig. 3b. This implies that the antagonistic interaction results from the terms X2X3 is stronger than that of X1X3.
The agitation rate effect seems to be relative in the production of P(3HB). However, it is considered critical in the concentration of glucose and urea since the availability of dissolved oxygen to the cells is necessary in the formation of P(3HB) (Almeida et al. 2010) and the metabolism of nutrients. On the other hand, it should be noted that the increased agitation rates impose shear stress on microorganism (Tripathi et al. 2013; Aramvash et al. 2015; Wisuthiphaet and Napathorn 2016).
As displayed in Fig. 3a, increasing glucose concentration either with low or high agitation rate increased P(3HB) content. On the contrary, maintaining glucose at a low level and increasing agitation produced an insignificant response.
Regarding the determination coefficient (R2 = 0.9969), 99.69% variability predicted by the model; only 0.31% can be accounted for the model inadequacy. The highest P(3HB) content occurred in Run 17 of CCD (Table 2).
In general, P(3HB) is accumulated under stress conditions that lead to a reduction in cell growth. Stress can be considered in the form of limited aeration or limited nitrogen among other things. High nitrogen and high aeration will promote the growth of bacterial cells and therefore P(3HB) accumulation will be hindered because the acetyl-CoA pool will be channelled to TCA cycle to be oxidized. On the other hand, under limited nitrogen, microbial cells cannot multiply. So, when aeration is increased, microbial cells will accumulate P(3HB) as a form of electron sink (Dawes and Senior 1973).
P(3HB) concentration
The results obtained from multiple regression analysis of CCD experiments were fitted to a second-order polynomial model. P(3HB) concentration in the terms of coded variables is expressed as the following equation:
| 5 |
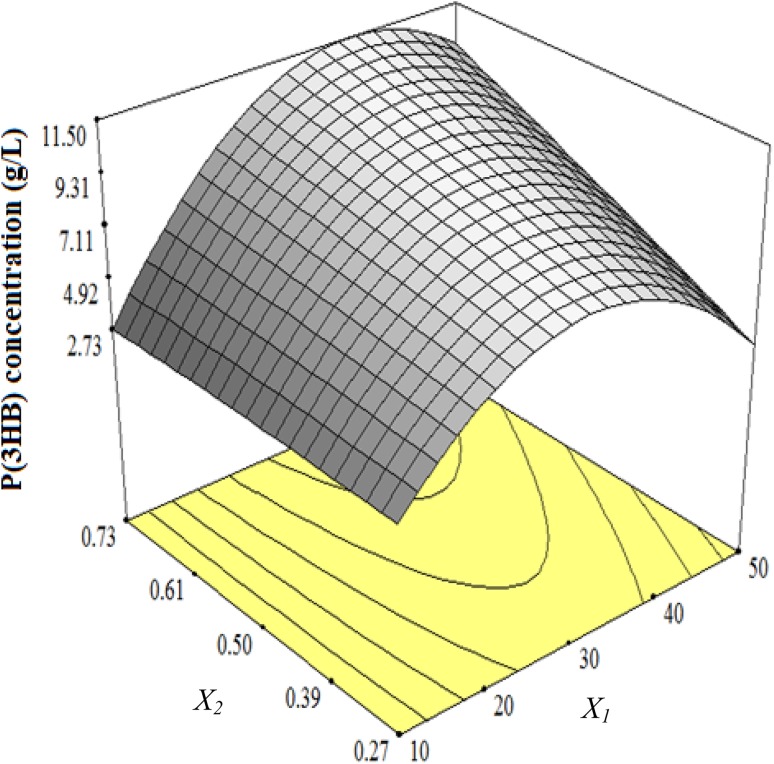
The ANOVA for P(3HB) concentration is presented in Table 5. The model was significant (p < 0.01) with a fairly large coefficient of determination, R2 = 0.9784. This indicates that the obtained experimental data were in a good fit with the model. The resulting surface response generated based on this model shows the effect of glucose (X1) and urea (X2) concentration on P(3HB) concentration.
Table 5.
Analysis of variance for the second-order polynomial model of P(3HB) concentration produced by C. necator NSDG-GG in batch fermentation at 30 °C for 48 h
| Source | Polynomial coefficient | Sum of squares | df | Mean squares | F value | Prob > F |
|---|---|---|---|---|---|---|
| Model | 208.01 | 9 | 23.11 | 50.22 | < 0.0001** | |
| Intercept | 9.42 | |||||
| X 1 | 1.84 | 33.78 | 1 | 33.78 | 73.40 | < 0.0001** |
| X 2 | 1.55 | 23.96 | 1 | 23.96 | 52.06 | < 0.0001** |
| X 3 | 0.070 | 0.049 | 1 | 0.049 | 0.11 | 0.7509 |
| − 4.65 | 59.47 | 1 | 59.47 | 129.22 | < 0.0001** | |
| − 0.11 | 0.034 | 1 | 0.034 | 0.073 | 0.7927 | |
| − 0.40 | 0.44 | 1 | 0.44 | 0.96 | 0.3507 | |
| X 1 X 2 | 1.63 | 21.32 | 1 | 21.32 | 46.32 | < 0.0001** |
| X 1 X 3 | − 0.47 | 1.73 | 1 | 1.73 | 3.76 | 0.0813 |
| X 2 X 3 | − 0.68 | 3.65 | 1 | 3.65 | 7.92 | 0.0183 |
| Residual | 4.60 | 10 | 0.46 | |||
| Lack of fit | 2.65 | 5 | 0.53 | 1.35 | 0.3737 | |
| Pure error | 1.95 | 5 | 0.39 |
X 1: glucose (g/L); X2: urea (g/L); X3: agitation (rpm); , , : the quadratic terms; X1X2, X1X3, X2X3: the interaction terms. R2 = 0.9784
**Statistically significant at 99% probability level
As presented in Tables 3 and 5, the results of ANOVA show that P(3HB) bio-transformation is dependent on biomass production. Moreover, as shown in Fig. 4, increasing glucose and urea concentrations resulted in the increase of P(3HB) concentration. However, higher increment in glucose concentration had a deleterious effect on P(3HB) concentration. In this regard, biomass production and P(3HB) accumulation have been found to be dependent on glucose and urea concentration to a certain limit. This dependency could be attributed to the increased biomass production. This indicates that P(3HB) bio-transformation is related to bacterial growth and biomass production. As biomass rises in the culture broth, the bacterial strains begins to accumulate P(3HB) to a highest concentration so that accumulated P(3HB) is dwindling after maximum biomass production. This could be because of exhaustion of nutrients which makes bacterial cell to utilize P(3HB) as an energy source (Sangkharak and Prasertsan 2007; Getachew and Woldesenbet 2016; Wisuthiphaet and Napathorn 2016). On the other hand, PHA forms mainly under certain growth conditions such as a limiting concentration for at least one nutrient essential for growth (Anderson and Dawes 1990). In this regard, a quite low nitrogen content of culture medium leads to an increment in C/N ratio which in turn enhances P(3HB) accumulation (Getachew and Woldesenbet 2016).
Fig. 4.
3D surface showing the interactive effect of glucose (X1) and urea (X2) on P(3HB) concentration
The interaction term (X1X2) was also found to be highly significant and synergistic to the P(3HB) formation (Table 5). Table 2 shows that the highest amount of P(3HB) concentration (11.24 g/L) was obtained in Run 17 when the initial concentration of glucose and urea was 30 and 0.73 g/L, respectively.
Validation of the regression model and assay reproducibility studies
An analysis of the quadratic models using Design-Expert software was applied to obtain the optimum conditions for achieving the highest production of biomass, P(3HB) content and P(3HB) concentration. The optimum conditions determined by the model analysis were 37.70 g/L glucose, 0.73 g/L urea, and 200 rpm agitation speed as shown in Table 6.
Table 6.
Optimized cultivation conditions, predicted and experimental values for biomass, P(3HB) content, and P(3HB) concentration production using C. necator NSDG-GG
| Method | Response | Variables | ||||
|---|---|---|---|---|---|---|
| Biomass (g/L) | P(3HB) content (wt%) | P(3HB) concentration (g/L) | X 1 a (g/L) | X 2 b (g/L) | X 3 c (RPM) | |
| OFAT | 8.95 | 76 | 6.80 | 25 | 0.54 | 200 |
| Predicted optimal RSM | 13.38 | 91.06 | 11.48 | 37.70 | 0.73 | 200 |
| Validated optimal RSM | 12.84 | 92.16 | 11.83 | 37.70 | 0.73 | 200 |
aGlucose
bUrea
cAgitation rate
Validation experiments were carried out to verify the predicted optimum and to confirm the model. The average value of maximum responses obtained from three replications of the experimental runs in optimum conditions are shown in Table 6. Compared with the medium in OFAT experiment, the concentration of biomass, concentration of P(3HB), and P(3HB) content improved around 1.43, 1.7, and 1.2 times after 48 h, respectively.
The closeness of the results of verification experiments and predicted values of the model established the accuracy of the selected model and reproducibility of the responses. This suggests that the model was adequate with the inadequacy of 0.3–2.5%. Generally, aerobic bacteria consume carbon source for cell growth and biomass synthesis. For this particular case, agitation speed probably improved nutrient and oxygen transferring rate (Kamble et al. 2010) and this condition most likely contributed to the utilization of more glucose to increase biomass as compared to OFAT in which it contained less glucose. Evidently, both glucose and urea were significant as shown in Table 3. In this regard, the study fulfilled by Narayanan and Ramana (2012) for PHB production by Bacillus mycoides DFC1 strain isolated from garden soil showed that PHB yield of 3.32 g/L and PHB content of 76.32% (w/w) were produced in optimum conditions which 17.34 g/L glucose and 7.03 g/L peptone were utilized. In this context, Zafar et al. (2012) utilized sucrose and urea as carbon and nitrogen sources, respectively, in the optimization of culture conditions via RSM for PHB production in shaking flask by the strain Azohydromonas lata MTCC 2311. Experimental results showed that the optimum conditions were 35.20 g/L sucrose and 1.58 g/L urea with a highest predictive PHB concentration of 5.95 g/L.
The measurement of residual glucose of culture medium in validation experiments showed that a quantity of 27.4 g/L of glucose remained at the end of fermentation time, so that 13 g/L of glucose was consumed by the strain C. necator NSDG-GG for production of 12.84 g/L of biomass and 11.83 g/L of P(3HB).
Conclusion
This study investigated the enhancement of P(3HB) production by C. necator NSDG-GG, which is the first glucose-utilizing model strain of C. necator obtained by genetic engineering. For this purpose RSM was employed to optimize the three key variables at a time (glucose concentration, urea concentration, and agitation speed) to obtain optimal conditions in the multivariable system. The optimal conditions obtained in this study included the cultivation conditions of 37.70 g/L glucose, 0.73 g/L urea, 200 rpm at 30 °C, and the incubation period of 48 h. Under optimum conditions the highest biomass of 12.84 g/L, P(3HB) content of 92.16 wt%, and P(3HB) concentration of 11.83 g/L were produced. Furthermore, RSM was able to improve the production of biomass, P(3HB) content, and P(3HB) concentration by 43, 21, and 74%, respectively. The findings of this study suggest that the newly engineered strain of C. necator NSDG-GG has a good potential for production of P(3HB) from glucose. The optimized medium composition will be used to design fed-batch and continuous cultivation modes to scale-up the P(3HB) production in bioreactors.
Acknowledgements
This study was supported by Research University Grant (RUI) from Universiti Sains Malaysia (1001/PBIOLOGI/811328). Also, this study contributed to the international research project PHABIO APP – Polyhydroxyalkanoate Biopolymers from Animal Waste Fats for the Production of Value Added Biobased and Biodegradable Bioplastic Materials, founded by the Federal Ministry of Education and Research of Germany and supervised by the PTJ Jülich. Nazila Biglari expresses her in-depth gratitude towards Universiti Sains Malaysia for supporting her doctoral studies through USM Fellowship.
Author contributions
NB certifies that she has participated full-time to make extensive contributions to conception and design, and acquisition of data, and analysis and interpretation of data for the entire content of the manuscript and writing the manuscript. MGD certifies that she has participated sufficiently in the work to take public responsibility for data analysis and writing the manuscript. PA certifies that she has participated sufficiently in the work to take public responsibility for discussion section and writing the manuscript. IO, TF give final approval of the version to be submitted and any revised version. KS certifies that he has participated in drafting the article or revising it critically for important intellectual content.
References
- Almeida A, Giordano AM, Nikel PI, Pettinari MJ. Effects of aeration on the synthesis of poly(3-hydroxybutyrate) from glycerol and glucose in recombinant Escherichia coli. Appl Environ Microbiol. 2010;76:2036–2040. doi: 10.1128/AEM.02706-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AJ, Dawes WA. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramvash A, Shahabi ZA, Aghjeh SD, Ghafari MD. Statistical physical and nutrient optimization of bioplastic polyhydroxybutyrate production by Cupriavidus necator. Int J Environ Sci Technol. 2015;12:2307–2316. doi: 10.1007/s13762-015-0768-3. [DOI] [Google Scholar]
- Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76:965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Braunegg G, Sonnleitner BY, Lafferty RM. A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Eur J Appl Microbiol Biotechnol. 1978;6:29–37. doi: 10.1007/BF00500854. [DOI] [Google Scholar]
- Budde CF, Mahan AE, Lu J, Rha C, Sinskey AJ. Roles of multiple acetoacetyl coenzyme a reductases in polyhydroxybutyrate biosynthesis in Ralstonia eutropha H16. J Bacteriol. 2010;192:5319–5328. doi: 10.1128/JB.00207-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshi A, Younesi H, Ghasempouri SM, Sharifzadeh M. Production of poly-3-hydroxybutyrate by Cupriavidus necator from corn syrup: statistical modeling and optimization of biomass yield and volumetric productivity. J Chem Technol Biotechnol. 2010;85:1528–1539. [Google Scholar]
- Dashti MG, Abdeshahian P, Yusoff WMW, Kalil MS, Hamid AA. Repeated batch fermentation biotechnology for the biosynthesis of lipid and gamma-linolenic acid by Cunninghamella bainieri 2A1. Biomed Res Int. 2014 doi: 10.1155/2014/831783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashti MG, Abdeshahian P, Sudesh K, Phua KK. Optimization of Salmonella Typhi biofilm assay on polypropylene microtitre plates using response surface methodology. Biofouling. 2016;32:477–487. doi: 10.1080/08927014.2015.1135328. [DOI] [PubMed] [Google Scholar]
- Dawes EA, Senior PJ. The role and regulation of energy reserve polymers in micro-organisms. In: Rose AH, Tempest DW, editors. Advances in microbial physiology. 11. Cambridge: Academic Press; 1973. pp. 135–266. [DOI] [PubMed] [Google Scholar]
- Emeko HA, Olugbogi AO, Betiku E. Appraisal of artificial neural network and response surface methodology in modeling and process variable optimization of oxalic acid production from cashew apple juice: a case of surface fermentation. Bioresources. 2015;10:2067–2082. doi: 10.15376/biores.10.2.2067-2082. [DOI] [Google Scholar]
- Getachew A, Woldesenbet F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res Notes. 2016;9:509. doi: 10.1186/s13104-016-2321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembree B, Prins J, Spagon P, Tobias P, Zey C (2010) NIST/SEMATECH engineering statistics handbook. https://www.itl.nist.gov/div898/handbook. Accessed 17 Sept 2010
- Jiang G, Hill DJ, Kowalczuk M, Johnston B, Adamus G, Irorere V, Radecka I. Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int J Mol Sci. 2016;17:1157. doi: 10.3390/ijms17071157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadier A, Abdeshahian P, Kalil MS, Hamid AA. Optimization of the key medium components and culture conditions for efficient cultivation of G. sulfurreducens strain PCA ATCC 51573 using response surface methodology. Iran J Sci Technol Trans A Sci. 2018;42:237–244. doi: 10.1007/s40995-018-0501-4. [DOI] [Google Scholar]
- Kamble AL, Meena VS, Banerjee UC. Effect of agitation and aeration on the production of nitrile hydratase by Rhodococcus erythropolis MTCC 1526 in a stirred tank reactor. Lett Appl Microbiol. 2010;51:413–420. doi: 10.1111/j.1472-765X.2010.02909.x. [DOI] [PubMed] [Google Scholar]
- Lee SY. Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol. 1996;14:431–438. doi: 10.1016/0167-7799(96)10061-5. [DOI] [Google Scholar]
- Mifune J, Nakamura S, Fukui T. Targeted engineering of Cupriavidus necator chromosome for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from vegetable oil. Can J Chem. 2008;86:621–627. doi: 10.1139/v08-047. [DOI] [Google Scholar]
- Mokhtari-Hosseini ZB, Vasheghani-Farahani E, Heidarzadeh-Vazifekhoran A, Shojaosadati SA, Karimzadeh R, Darani KK. Statistical media optimization for growth and PHB production from methanol by a methylotrophic bacterium. Bioresour Technol. 2009;100:2436–2443. doi: 10.1016/j.biortech.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Montgomery CD. Design and analysis of experiments. New York: Wiley; 1991. [Google Scholar]
- Narayanan A, Ramana KV. Polyhydroxybutyrate production in Bacillus mycoides DFC1 using response surface optimization for physico-chemical process parameters. 3 Biotech. 2012;2:287–296. doi: 10.1007/s13205-012-0054-8. [DOI] [Google Scholar]
- Orita I, Iwazawa R, Nakamura S, Fukui T. Identification of mutation points in Cupriavidus necator NCIMB 11599 and genetic reconstitution of glucose-utilization ability in wild strain H16 for polyhydroxyalkanoate production. J Biosci Bioeng. 2012;113:63–69. doi: 10.1016/j.jbiosc.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Pandian SR, Deepak V, Kalishwaralal K, Rameshkumar N, Jeyaraj M, Gurunathan S. Optimization and fed-batch production of PHB utilizing dairy waste and sea water as nutrient sources by Bacillus megaterium SRKP-3. Bioresour Technol. 2010;101:705–711. doi: 10.1016/j.biortech.2009.08.040. [DOI] [PubMed] [Google Scholar]
- Poirier Y, Dennis DE, Klomparens K, Somerville C. Polyhydroxybutyrate, a biodegradable thermoplastic, produced in transgenic plants. Science. 1992;256:520–523. doi: 10.1126/science.256.5056.520. [DOI] [PubMed] [Google Scholar]
- Raberg M, Kaddor C, Kusian B, Stahlhut G, Budinova R, Kolev N, Bowien B, Steinbüchel A. Impact of each individual component of the mutated PTSNag on glucose uptake and phosphorylation in Ralstonia eutropha G+1. Appl Microbiol Biotechnol. 2012;95:735–744. doi: 10.1007/s00253-012-3911-9. [DOI] [PubMed] [Google Scholar]
- Sangkharak K, Prasertsan P. Optimization of polyhydroxybutyrate production from a wild type and two mutant strains of Rhodobacter sphaeroides using statistical method. J Biotechnol. 2007;132:331–340. doi: 10.1016/j.jbiotec.2007.07.721. [DOI] [PubMed] [Google Scholar]
- Sathiyanarayanan G, Saibaba G, Kiran GS, Selvin J. A statistical approach for optimization of polyhydroxybutyrate production by marine Bacillus subtilis MSBN17. Int J Biol Macromol. 2013;59:170–177. doi: 10.1016/j.ijbiomac.2013.04.040. [DOI] [PubMed] [Google Scholar]
- Shah AA, Hasan F, Hameed A, Ahmed S. Biological degradation of plastics: a comprehensive review. Biotechnol Adv. 2008;26:246–265. doi: 10.1016/j.biotechadv.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Sharma L, Singh AK, Panda B, Mallick N. Process optimization for poly-β-hydroxybutyrate production in a nitrogen fixing cyanobacterium, Nostoc muscorum using response surface methodology. Bioresour Technol. 2007;98:987–993. doi: 10.1016/j.biortech.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Sheu DS, Chen WM, Yang JY, Chang RC. Thermophilic bacterium Caldimonas taiwanensis produces poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from starch and valerate as carbon sources. Enzyme Microb Technol. 2009;44:289–294. doi: 10.1016/j.enzmictec.2009.01.004. [DOI] [Google Scholar]
- Sudesh K. Polyhydroxyalkanoates from palm oil: biodegradable plastics. Heidelberg: Springer Science and Business Media; 2013. [Google Scholar]
- Surwase SN, Jadhav SB, Phugare SS, Jadhav JP. Optimization of melanin production by Brevundimonas sp. SGJ using response surface methodology. 3 Biotech. 2013;3:187–194. doi: 10.1007/s13205-012-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi AD, Srivastava SK, Singh RP. Statistical optimization of physical process variables for bio-plastic (PHB) production by Alcaligenes sp. Biomass Bioenergy. 2013;55:243–250. doi: 10.1016/j.biombioe.2013.02.017. [DOI] [Google Scholar]
- Wang J, Wan W. Experimental design methods for fermentative hydrogen production: a review. Int J Hydrog Energy. 2009;34:235–244. doi: 10.1016/j.ijhydene.2008.10.008. [DOI] [Google Scholar]
- Wisuthiphaet N, Napathorn SC. Optimisation of the use of products from the cane sugar industry for poly(3-hydroxybutyrate) production by Azohydromonas lata DSM 1123 in fed-batch cultivation. Process Biochem. 2016;51:352–361. doi: 10.1016/j.procbio.2015.12.009. [DOI] [Google Scholar]
- Yu L, Lei T, Ren X, Pei X, Feng Y. Response surface optimization of l-(+)-lactic acid production using corn steep liquor as an alternative nitrogen source by Lactobacillus rhamnosus CGMCC 1466. Biochem Eng J. 2008;39:496–502. doi: 10.1016/j.bej.2007.11.008. [DOI] [Google Scholar]
- Zafar M, Kumar S, Kumar S, Dhiman AK. Optimization of polyhydroxybutyrate (PHB) production by Azohydromonas lata MTCC 2311 by using genetic algorithm based on artificial neural network and response surface methodology. Biocatal Agric Biotechnol. 2012;1:70–79. [Google Scholar]