Abstract
N6-methyladenosine (m6A) is the most prevalent internal modification of eukaryotic mRNA. Very little is known of the function of m6A in the immune system or its role in host–pathogen interactions. Here we investigated the topology, dynamics, and bidirectional influences of the viral–host RNA methylomes during HIV-1 infection of human CD4 T cells. We show that viral infection triggers a massive increase in m6A in both host and viral mRNAs. In HIV-1 mRNA, we identified 14 methylation peaks in coding and noncoding regions, splicing junctions, and splicing regulatory sequences. We also identified a set of 56 human gene transcripts that were uniquely methylated in HIV-1-infected T cells and were enriched for functions in viral gene expression. The functional relevance of m6A for viral replication was demonstrated by silencing of the m6A writer or the eraser enzymes, which decreased or increased HIV-1 replication, respectively. Furthermore, methylation of two conserved adenosines in the stem loop II region of HIV-1 Rev Response Element (RRE) RNA enhanced binding of HIV-1 Rev protein to the RRE in vivo and influenced nuclear export of RNA. Our results identify a new mechanism for the control of HIV-1 replication and its interaction with the host immune system.
INTRODUCTION
RNA plays numerous vital roles in cellular processes ranging from the transfer of genetic information from DNA to protein to the epigenetic modulation of gene transcription1. Similarly to proteins and DNA, RNA undergoes chemical modifications that can influence its metabolism, function, and localization. More than 100 diverse chemical groups are known to modify RNA at one or more of its four nucleotides (A, G, C, and U). Ribosomal and tRNAs sequences incorporate most of the diverse chemical modifications. Other than the 5′-cap structure, mRNAs and IncRNAs contain N6-methyladenosine (m6A) and 5-methyl cytosine (m5C)2,3. Methylation of adenosine at the N6 position (m6A), which was first reported ~40 years ago4-7, is the most prevalent internal modification of eukaryotic mRNA and contributes to its generation, localization, and function8-10. Although our understanding of the importance of m6A to physiological and pathological processes is increasing rapidly11-13, little is known of the function of m6A in the mammalian immune system or its role in host–pathogen interactions.
Adenosine methylation is catalyzed by a “writer”, which is a large 1 MDa RNA methyltransferase complex (MTase) composed of two catalytic subunits (METTL3 and METTL14), a splicing factor (WTAP), a novel protein (KIAA1429), and other subunits not yet identified10,14-16. Conversely, methyl groups are removed by two “erasers” RNA demethylases, FTO and AlkBH512,13. The development of techniques to selectively immunoprecipitate m6A and sequence the associated RNA (MeRIP-seq) has been pivotal to our understanding of the topology and prevalence of m6A in various eukaryotic transcriptomes8,9. Also, the mRNA regions most highly enriched in m6A are transcription start sites, stop codons, and 3′UTRs8-10, raising many hypotheses about their roles. Methylation of RNA adenosines is a dynamic process and the exact sites and frequency of modification may vary in cells exposed to non-physiological conditions such as stress, inflammation, and infection.
Although the precise molecular functions of m6A and its dynamic regulation are not yet fully understood, it has been postulated to play several important roles in RNA metabolism, including translation, stability, splicing, transport, and localization17,18. For example, the enrichment of m6A in regions of the 3′UTR plays a role in RNA stability through specific recognition of m6A sites by RNA-binding proteins involved in mRNA decay pathways19-21. m6A modification is involved in triggering RNA structural switches to affect RNA-protein interactions22 as well as directing translational control of heat shock responses23. Additionally, nuclear export of mRNA is promoted by the increased abundance of m6A resulting from AlkBH5 depletion13. m6A is enriched in exonic regions flanking splicing sites24. Increased m6A resulting from FTO knockout favours the binding of splicing factor SRSF2 to mRNA splicing sites and in turn promotes inclusion of target exons24. Circadian gene expression is delayed upon METTL3 depletion25, and pluripotency of embryonic stem cells is affected by modulation of METTL326,27 and METTL1428 expression. Taken together, these findings substantiate RNA methylation as a new epitranscriptomic mechanism for the regulation of gene expression.
The internal m6A modification is also present in viral RNA. This was first identified in Rous sarcoma virus29, influenza virus30, and SV40 virus31 several decades ago, but the function and relevance of this modification to viral replication remain unclear. Similarly, whether and how viral infections alter the dynamics of the host or viral RNA methylomes remain fundamental and unanswered questions. Notably, m6A has not yet been identified in HIV-1 RNA. In this study, we report the presence of this modification in HIV-1 RNA and examined the topology, functions, and molecular features of m6A in both HIV-1 and host RNA during infection of human CD4+ T cells.
RESULTS
RNA methylation in T cells is enhanced by HIV-1 infection and modulates viral replication
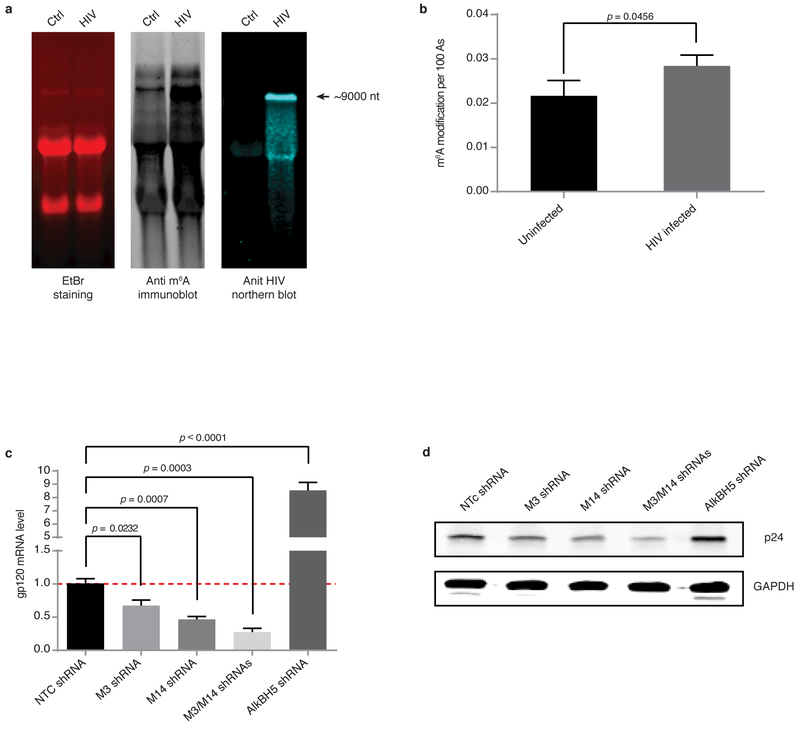
To investigate whether HIV-1 RNA is m6A modified during the course of viral infection, we examined the MT4 human CD4+ T cell line at 3 days post-infection with the HIV-1 LAI clone, the time period when virus is in the active replication phase. The abundance of m6A was analysed by anti-m6A immunoblotting (Fig 1a) and quantified by LC-MS/MS according to published methods 32. Poly(A) RNA from LAI-infected cells showed significantly higher level of m6A (~30% increase) compared with control uninfected cells (Fig. 1b). An increase in methylation was observed in all RNA sizes (Fig. 1a, middle panel), suggesting a widespread effect on cellular mRNA species. These results were also confirmed by m6A immunoblot analysis of poly(A)-enriched RNA where the contribution of ribosomal RNA was removed (Supplementary Information Fig. 1). Total RNA from HIV-1-infected cells showed a high molecular weight band with an intense m6A signal, which was subsequently identified as HIV-1 genomic RNA by northern blot analysis (Fig. 1a, middle and right panels). These results demonstrate that HIV-1 infection of T cells promotes methylation of both viral and host RNA.
Figure 1. m6A RNA methylation in T cells is promoted by HIV-1 infection and modulates viral replication.
a, Analysis of RNA from HIV-1-infected T cells. Total RNA extracted from uninfected (Ctrl) or HIV-1-infected (HIV) MT4 cells 3 days post-infection was analysed by ethidium bromide (EtBr) staining (left), anti-m6A immunoblotting (middle), or northern blotting for HIV-1 RNA (right). Arrow indicates the predicted size of full-length HIV-1 RNA. The data is a representative of seven independent experiments. b, LC-MS/MS quantification of m6A content in poly(A) enriched RNA fractions. Results are the mean ± s.e.m. of five independent experiments. P values were calculated by unpaired two-tailed Student’s t-test. c, d, HIV-1 replication is reduced by METTL3 and METTL14 silencing and enhanced by AlkBH5 silencing. c, RT-qPCR analysis of gp120 expression in MT4 cells transduced for 3 days with nontargeting control (NTC), METTL3, METTL14, or AlkBH5 shRNA. Cells were then infected with HIV-1 LAI virus, and cells were analysed 3 days later. d, Western blot analysis of HIV-1 p24 intracellular protein expression in cells described in c. Results are the mean ± s.e.m. of four independent experiments. P values were calculated by unpaired two-tailed Student’s t-test.
We next determined whether modulation of m6A abundance might alter the HIV-1 replication rate. MT4 cells were transduced with shRNAs targeting METTL3, METTL14, or AlkBH5, and knockdown efficiency was assessed by western blot and RT-qPCR analysis (Supplementary Information Fig. 2). We chose to deplete AlkBH5 for RNA demethylase function due to its high expression in T cells. Transduced cells were then infected with HIV-1 LAI and 3 days later, viral replication was monitored by RT-qPCR analysis of gp120 mRNA levels and p24 immunoblotting of total cell extracts (Fig. 1c, d). Depletion of METTL3 or METTL14 alone significantly decreased viral replication compared with cells expressing nontargeting control (NTC) shRNA, and an additive effect was observed when both MTases were simultaneously knocked down. Conversely, AlkBH5 silencing caused a striking increase in viral replication (Fig. 1c, d). Taken together, these results demonstrate that modulation of METTL3, METTL14, and AlkBH5 expression directly affects HIV-1 replication.
Topology of RNA methylome during HIV-1 infection
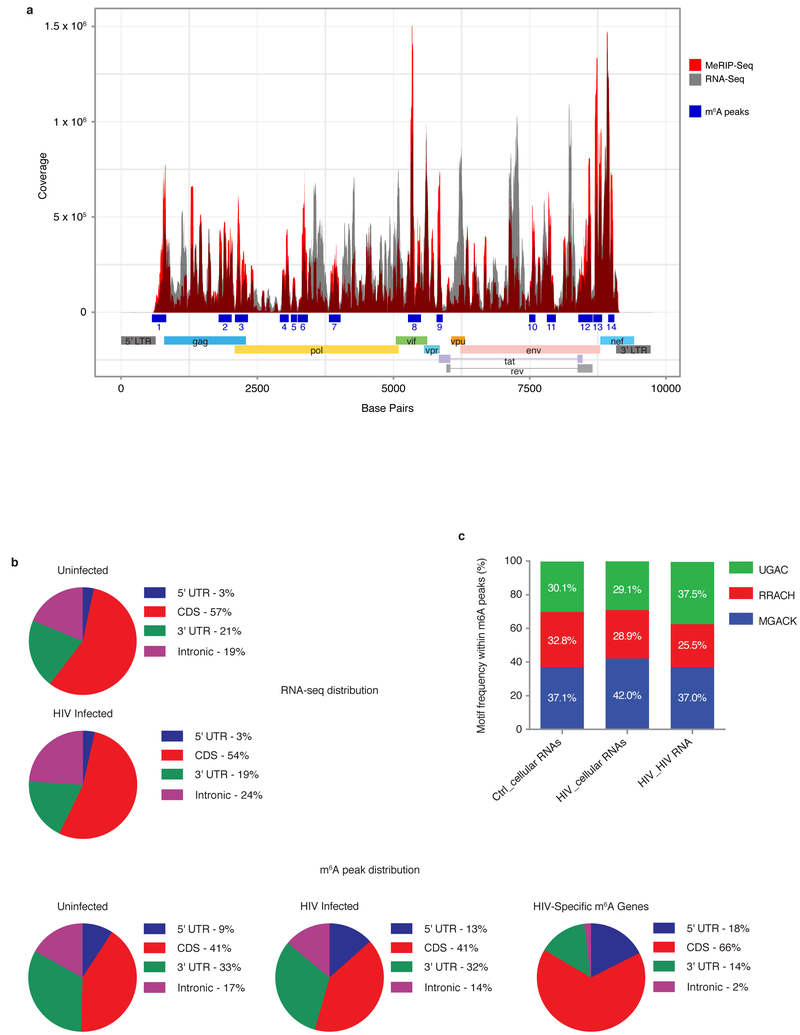
We next examined the topology of the m6A RNA methylome during HIV-1 infection by MeRIP-seq experiments. Poly(A)-enriched RNA from uninfected and HIV-1-infected MT4 cells was fragmented into 60–200 nucleotides and immunoprecipitated using an m6A-specific antibody (see methods), and the associated RNA was then sequenced. Reads were mapped onto the HIV-1 and human genomes to identify regions enriched in m6A. We found ~ 49% of input RNA-seq reads and ~ 42% of MeRIP-seq reads mapped to the HIV genome, respectively (Supplementary Information Table 1). We identified 14 distinct methylation peaks located in coding and noncoding regions, splicing junctions (D1), and splicing regulatory sequences (Fig. 2a; Table 1), with each peaks suggesting potential specific functional roles for each methylation peak. Peak 1 encompasses the primary binding site sequence, dimerization sequence, encapsidation sequence (ψ), D1 splicing site, and gag gene start site. Peak 2 is within the gag coding sequence. Peak 3 overlaps the gag gene stop codon and the pol gene start codon. Peaks 4-7 are within the pol coding sequence. Peak 8 is within the vif coding sequence and also overlaps with the exonic splicing enhancer for vpr (ESEVpr). Peak 9 overlaps with the exonic splicing silencer 2p (ESS2p) the exonic splicing silencer 2 (ESS2) and vpr coding sequence. Peak 10 lies within the env coding sequence. Peak 11 overlaps with the RRE region. Peak 12 is within the env and rev coding sequences, and overlaps the tat gene stop codon and the exonic splicing enhancer 3a (ESS3a). Peak 13 lies upstream of the env gene stop codon and the nef gene start codon. Peak 14 is within the nef coding sequence. These findings identify the topology of the m6A landscape in HIV-1 RNA and indicate widespread methylation throughout the genome.
Figure 2. RNA methylation profiles of HIV-1 and HIV-1-infected T cells.
a, Methylation peaks in the full-length HIV-1 RNA genome. Viral gene annotation is shown below. Poly(A) RNA from uninfected and HIV-1-infected MT4 cells was fragmented, subjected to RNA-seq and MeRIP-seq, and analysed as described previously8. The grey shading corresponds to the total RNA-seq mapping distribution (MeRIP input), and the red signal corresponds to the MeRIP-seq mapping distribution. On the x-axis, the dark blue boxes and numbers indicate the m6A peaks called by MeRIPPeR analysis and referred to in the text. b, Topology of m6A in the cellular and viral transcriptomes. Pie charts showing the distribution of total RNA sequence (upper) and m6A peaks (middle) in four regions (5′UTR, CDS, 3′UTR, and intronic) of transcripts from uninfected and HIV-1-infected MT4 cells. m6A peak distribution in HIV-1-specific RNA is also shown (bottom). c, Frequency of TGAC, RRACH, and MGACK sequence motifs within m6A peaks in cellular RNAs from control and HIV-1-infected MT4 cells (Ctrl_cellular RNAs and HIV_cellular RNAs, respectively) and HIV-1 LAI RNA (HIV_HIV RNA).
Table 1. HIV m6A peaks.
| Peak number | Position (nt) | Features |
|---|---|---|
| 1 | 625-875 | PBS, DIS, ψ |
| 2 | 1950-2075 | gag CDS |
| 3 | 2150-2375 | gag STOP, pol START |
| 4 | 3000-3150 | pol CDS |
| 5 | 3175-3275 | pol CDS |
| 6 | 3325-3475 | pol CDS |
| 7 | 3845-4075 | pol CDS |
| 8 | 5325-5450 | vif CDS, ESEVpr |
| 9 | 5850-5950 | ESS2p, ESS2, vpr CDS |
| 10 | 7550-7650 | env CDS |
| 11 | 7875-8000 | RRE, env CDS |
| 12 | 8450-8700 | env CDS, rev CDS, tat STOP, ESS3a |
| 13 | 8725-8875 | env CDS, nef START |
| 14 | 9000-9100 | nef CDS |
PBS: primary binding site; DIS: dimerization sequence; ψ: encapsidation sequence; CDS: coding sequence; STOP: stop codon; START: start codon; ESE: exonic splicing enhancer; ESS: exonic splicing silencer; RRE: Rev-responsive element.
Next, we performed MeRIP-seq experiments to determine the dynamics of RNA methylation in the host cells after HIV-1 infection, and identified a set of 56 gene transcripts that were specifically methylated upon viral infection (Supplementary information Table 2 and Supplementary information Fig 3). We performed gene ontology (GO) analysis of the 56 genes and found that the most represented category was “viral gene expression”, suggesting that methylation of many of the transcripts is functionally important for viral infection (the top 10 most enriched categories are shown in Supplementary Information Fig. 3a). In fact, of the 56 genes, 19 have a known association with HIV replication, and in some cases, the protein products directly interact with viral components. Remarkably, the majority of these genes encode proteins with proviral functions. Depletion or inhibition of MOGS, PSIP1 (LEDGF), HNRNPK, TRAF2, TUBA1B, CCT2, PABPC3, CBFB and ETS2 have been reported to negatively affect different viral replication steps (see Supplementary information Table 2 and references therein); expression of GTF2I, MYL12A, and UBA52 is induced by the infection process or by viral proteins; HLA-DPA2, EIF3M, DUT, MBD2, and RPS5 proteins have been shown to interact with viral proteins; and HSPA1A and SRSF5 overexpression is known to promote viral replication.
The exact mechanism by which m6A regulates the stability, splicing, and translation of RNA transcripts remains unknown. To gain insight, we compared the distribution of m6A peaks in the 56 HIV-1-specific methylated RNAs versus the total infected cellular transcriptome and found some striking differences (Fig. 2b). Specifically, the percentage of total m6A peaks mapping to the human genome located in the 5′UTR, CDS, 3′UTR, and intronic regions was 17.5%, 66.0%, 14.4%, and 2.1% for the 56 HIV-specific m6A peaks, respectively, compared with 13.4%, 41.0%, 31.6%, and 14.0% for the same regions in the total cellular transcriptome of infected cells (Fig. 2b). Thus, for the HIV-1 specific methylated genes, m6A is more abundant in the 5′UTR and CDS and less abundant in the 3′UTR and intronic regions of HIV-1 RNA compared with total cellular mRNA. These findings suggest that m6A modification may have more prominent functions in RNA splicing and translation than in RNA stabilisation. Additionally, m6A appears to be more prevalent on mature than immature mRNAs, as indicated by the large reduction of m6A peaks in the intronic region of HIV-1 RNA versus total cellular mRNA.
RNA methylation is a dynamic process controlled by the catalytic activities of the m6A writer (MTases) and eraser (demethylases) enzymes. Because the enzymes have preferred target sequences8,9,13,18,19, we performed consensus sequence motif analysis within m6A peaks to determine the preferred motifs in HIV and cellular RNA, and determined whether the preferential motif usage in the host cells was altered by HIV-1 infection. In uninfected T cells, the frequency of MGACK (A/C-GAC-G/U), RRACH (G/A-G/A-AC-A/C/U), and UGAC motifs within m6A peaks was ~37%, 33%, and 30%, respectively (Fig. 2c). After HIV-1 infection, enrichment of m6A in motif MGACK was increased (37% to 42%), indicating a large change in motif preference during viral infection. In HIV-1 RNA, UGAC and MGACK were equally preferred (~37%), but UGAC usage was ~7% higher than in the cellular transcripts. Although the mechanisms by which viral infection drives a change in the preferred host m6A motif are not clear, several plausible possibilities exist. For example, the assembly and catalytic rate of the methylation complex may be changed by viral infection such that MGACK becomes the preferred substrate. Conversely, infection-driven changes in demethylase activities may decrease recognition of methylated MGACK but not of the other sequence motifs. Or, the accessibility of these motifs is altered in this context, either by sequestering or secondary structure. Future characterization of methylation complexes in HIV-1-infected cells will undoubtedly enhance our understanding of the dynamics of mammalian and viral RNA methylation during host–virus interactions.
m6A methylation modulates the interaction between Rev protein and RRE RNA
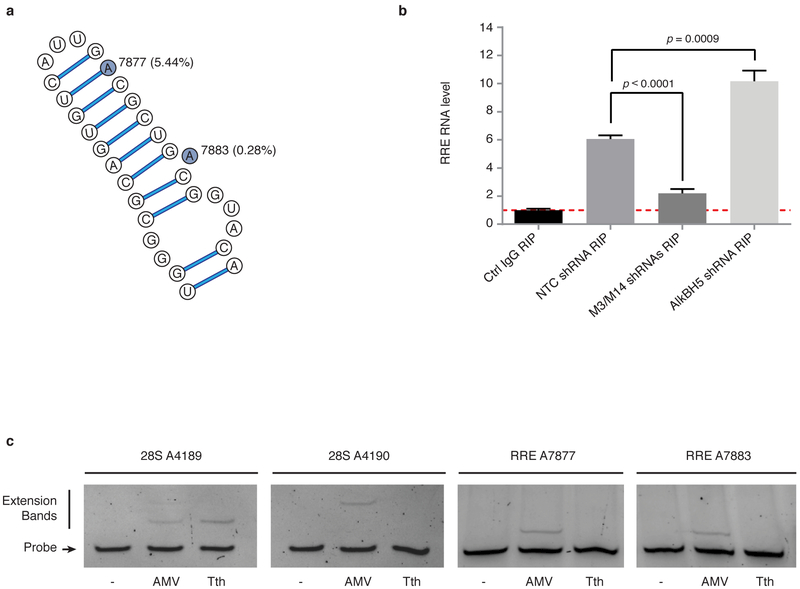
In considering the mechanism by which m6A might alter RNA function to promote HIV-1 replication, we hypothesized that m6A might alter the secondary or tertiary structures of RNA and subsequently its ability to interact with proteins. To test this, we examined binding of viral Rev protein to RRE RNA. RRE is a structurally and functionally well-characterized RNA element within the HIV-1 env gene. After translation, Rev proteins are imported back into the nucleus where they assemble at the RRE to form active nuclear export complexes that facilitate transit of viral transcripts into the cytoplasm33-35. This is an essential step in viral replication. Intriguingly, we found that peak 11 of the HIV-1 methylation sites (Fig. 2a) spanned the stem IIB region of RRE RNA, which contains the high-affinity binding site for Rev (Fig. 3a). The stem loop secondary structure of RRE is critical for Rev protein binding and its function in nuclear export of HIV-1 RNA33-35. The solution structure of a Rev peptide bound to stem loop IIB showed that Rev binds in the major groove of the RRE in a distinctive binding pocket formed by two purine-purine base pairs36. Therefore, we reasoned that methylation of A7877 and A7883 in stem loop IIB (Fig. 3a) might affect the RRE affinity for Rev and thus alter the function of the RNA-protein complex.
Figure 3. m6A methylation modulates the interaction between Rev protein and RRE RNA.
a, Secondary structure of the hairpin loop IIB of HIV-1 RRE. The two highlighted adenosines represent the methylation sites identified in peak 11 on Figure 2a. Nucleotide numbering is based on the pLAI2 proviral vector sequence, and the mutation frequency of the adenosines in 2,501 HIV-1 sequences is shown in parentheses. b, RRE methylation enhances Rev interactions. 293T cells were depleted of METTL3, METTL14, or AlkBH5, and transfected pLAI2 vector. RIP-qPCR experiments were performed by IP with control IgG or anti-Rev antibody followed by qPCR to analyse RRE RNA. Results are the mean ± s.e.m. of 3 independent experiments and are expressed as the fold enrichment of RRE RNA in anti-Rev versus control IgG IPs. P values were calculated by unpaired two-tailed Student’s t-test. c, RRE A7877 and A7883 are methylated in vivo. Primers were designed to probe for m6A modification at these two sites in RRE. AMV and Tth primer extensions for A7877 and A7883 were performed on 1 µg poly(A) enriched RNA from HIV-1 infected MT4 cells. Extension for 28S A4189 and 28S A4190 were performed on 10 µg total RNA and served as negative and positive methylation sites, respectively.
To test this possibility, we first determined whether METTL3/METTL14 or AlkBH5 knockdown in MT4 cells modulated the methylation status of RRE RNA and its interactions with Rev. MeRIP experiments coupled with RT-qPCR specific for the RRE sequence first confirmed that the two MTases and the demethylase are indeed able to methylate and demethylate RRE, respectively (Supplementary Information Fig. 4a). Silencing of METTL3/14 decreased the amount of RRE RNA by Me-RIP while knock down of AlkBH5 enhanced RRE RNA isolated by Me-RIP (Supplementary Information Fig 4a). To determine whether changes in RRE methylation affect its affinity for Rev, we performed experiments in 293T cells transiently expressing the HIV-1 construct pLAI2. Rev–RRE interactions were detected by immunoprecipitation of Rev followed by qPCR to quantify bound RRE RNA. We found that Rev–RRE binding was significantly reduced by silencing of METTL3/METTL14 and, conversely, significantly increased by AlkBH5 knockdown (Fig. 3b). These results demonstrate that methylation of RRE RNA enhanced the formation of Rev–RRE complexes in vivo. Analysis of the REV immunoprecipitates indicated that these effects were not due to differences in the efficiency of Rev immunoprecipitation between cells expressing the various shRNAs (Supplementary Information Fig. 4c).
Because these experiments were performed with 293T cells expressing full-length HIV-1, it is possible that other viral proteins may have influenced Rev–RRE binding. To address this concern, we performed the same experiments with cells expressing FLAG-Rev and the 66-nucleotide minimal RRE stem loop region (Fig. 3a). Consistent with the results in cells expressing the full-length HIV-1 RRE, we found that Rev binding to the minimal RRE sequence was significantly decreased by METTL3/METTL14 knockdown and increased by AlkBH5 knockdown (Supplementary Information Fig. 4b and c). These data strongly suggest that modulation of A7877 and A7883 methylation in the RRE stem loop IIB region affects its binding to Rev protein.
Rev-RRE interactions and their function in nuclear export of viral RNAs are required events in HIV-1 replication cycle. We asked if the changes in the binding ability of Rev to RRE due to the alteration of RNA methylation could affect the function of viral RNA export. We depleted METTL3/METTL14 or AlkBH5 in 293 cells by corresponding shRNAs followed by infections with HIV-1 LAI virus. Next, we prepared cytoplasmic and nuclear fractions from these cells and quantified RRE RNA distribution in the cytoplasm and nucleus. Hypo-methylation of RRE caused by METTL3/METTL14 knockdown significantly decreased viral RNA nuclear export, consistent with the observed reduction in replication rates. On the contrary, hypermethylation of RRE RNAs by AlkBH5 silencing was able to enhance nuclear export and viral replication (Supplementary Information Fig 4d; and Fig 1c).
In order to define which adenosine in the RRE hairpin structure is methylated in vivo, we performed primer extension experiments using AMV RT and Tth DNA polymerase 37. A7877 and A7883 modifications were analysed by sequence-specific complementary ssDNA probes and enzymatic extensions. In contrast with AMV RT, Tth pol enzyme is sensitive to m6A RNA modification resulting in markedly reduced ability to extend the DNA oligo. As shown in Fig 3c, Tth pol failed to extend the probes for both A7877 and A7883 sites, indicating that these A residues are methylated in vivo. In control experiments using ribosomal RNA, Tth pol extended oliogs for A4189 (unmethylated site) and did not extend to A4190 (methylated site), which are consistent with previously reported results 37. Altogether, these results show that the both A in the RRE hairpin are methylated in vivo. m6A methylation of RRE may influence Rev binding by at least two mechanisms; direct interaction of the methyl group with specific Rev residues and/or by inducing changes in RRE folding and thus the secondary structure required for Rev recognition. Further experiments will be necessary to clarify the mechanisms involved.
HIV-1 replication and RNA nuclear export are perturbed by methylation of A7883 in the RRE bulge region
To determine whether m6A methylation in RRE RNA might confer an evolutionary advantage for HIV-1, we analysed the mutation frequency of A7877 and A7883 in 2,501 HIV-1 sequences isolated from infected humans. For this, we downloaded aligned FASTA sequences from the HIV database and processed the alignments using a custom Python program. At each position in the reference HIV-1 HXB2 genome, we calculated the frequency of mutations (single nucleotide substitutions and insertions/deletions) using the alignment matrix for the 2,501 sequences (Supplementary Information Fig. 5). We found that the mutation frequencies at positions A7877 and A7883 were 5.44% and 0.28%, respectively, which are much lower than the mutation frequencies of other adenosine residues in the HIV-1 sequence or of the adenosines in the stem loop IIB region of RRE (11.16 ± 0.30% s.e.m. and 8.99 ± 6.80% s.e.m, respectively; Supplementary Information Fig. 5). This analysis suggests that A7877 and A7883 in the RRE are conserved and likely to confer survival benefit. Interestingly, A7883 is located immediately above the high-affinity Rev-binding internal bubble structure of RRE stem loop IIB (Fig. 3a)38,39. Collectively, these results demonstrate a significant role for A7877 and A7883 in Rev–RRE binding and function.
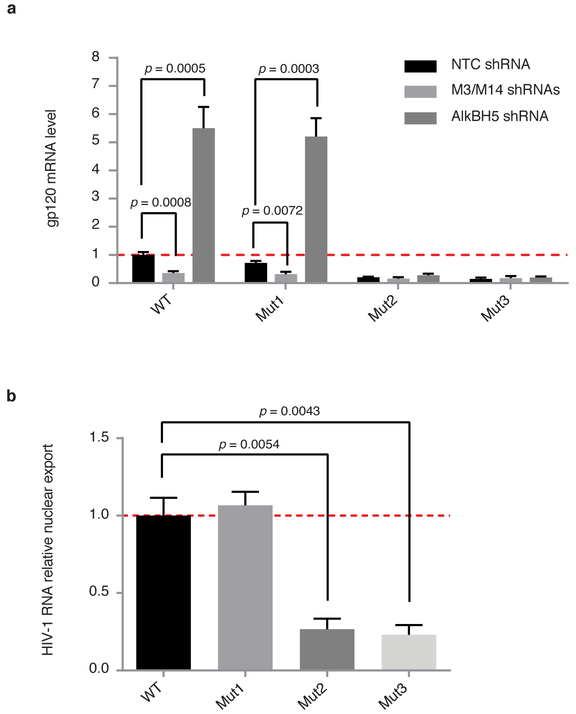
In order to further validate the role of specific methylation at position A7877 and A7883 in HIV-1 replication, we generated LAI mutant constructs in the RRE region (Fig 3a): U7871C + A7877G (Mut1), A7883G (Mut2) and the combination of both (Mut3). The mutant constructs were assessed for viral replication by transfecting 293T cells with WT or mutant pLAI2 plasmids (Fig. 4a). Mut1, which is designed to affect methylation at position A7877, did not show relevant differences in terms of viral expression in NTC, METTL3/METTL14 or AlkBH5 knock-down conditions compared to WT virus. On the other hand, Mut2 and Mut3, which are mutated at the bulge region of RRE A7883, show dramatic decrease in viral replication (~10% compared to WT) and this effect is not modulated by METTL3/METTL14 or AlkBH5 Knock-down, suggesting that mutation at A7883 is not only responsible in severely attenuating viral fitness but also a functionally critical nucleotide modified by the cellular RNA methylation machineries. Furthermore, we analyzed the nuclear export efficiency of the mutant constructs and compared with the WT virus (Fig. 4b). Mutations at the A7877 region (Mut1) did not perturb the nuclear export efficiencies of viral transcripts, while mutations at A7883 in the bulge region (Mut2 and Mut3) drastically affected nuclear export of viral RNA (Fig 4b). Taken together, these results demonstrate that methylation at A7883 is critical for Rev binding, enhanced nuclear export, and viral replication.
Figure 4. HIV-1 replication and RNA nuclear export are perturbed by methylation of A7883 in the RRE bulge region.
a, WT or LAI HIV-1 mutant constructs, U7871C + A7877G (Mut1), A7883G (Mut2), and the combination of both (Mut3), were assessed for replication in 293T cells under METTL3/METTL14 or AlkBH5 knock-down conditions. Results are the mean ± s.e.m. of three independent experimentsand P values were calculated by unpaired two-tailed Student’s t-test. b, Nuclear export of viral RNA was analysed by cytoplasmic and nuclear fractionation in 293T cells transfected with WT or mutant LAI constructs described in a. Viral RNA distribution in the two compartments is relative to the viral RNA content from total cellular RNA. Results are the mean ± s.e.m. of three independent experiments. P values were calculated by unpaired two-tailed Student’s t-test.
DISCUSSION
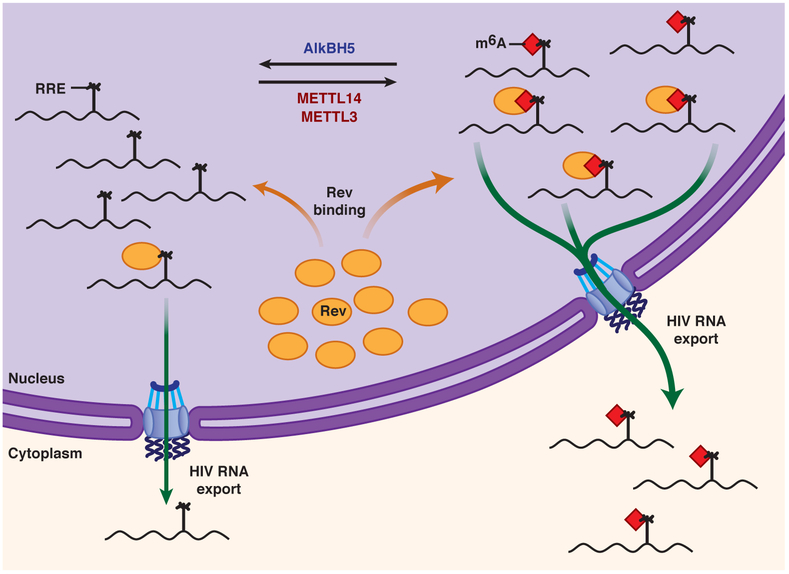
Based on these studies, we propose a model for m6A methylation of RRE RNA and its effects on HIV-1 replication (Fig. 5). Silencing of METTL3/METTL14 or AlkBH5 induces m6A hypomethylation or hypermethylation of HIV-1 RRE sites at positions A7877 and A7883, respectively. Rev protein preferentially interacts with methylated RRE. Therefore, AlkBH5 silencing increases RRE RNA methylation and thus promotes Rev binding, increases nuclear export of viral RNA, and subsequently, viral replication. In contrast, METTL3/14 silencing reduces methylation of RRE RNA and subsequent Rev recruitment onto the RRE, thereby reducing export of viral RNA and suppressing replication. Our results presenting the significance of A7883 in Rev-RRE interactions is further supported by in vitro Rev-RRE binding and NMR studies. In vitro experiments determined that efficient Rev binding required A7883 in the RRE structure 40. NMR structural studies have shown that A7883 is flipped out from the stem region of RRE and directly interacts with W45 of Rev through van der Waals contacts36. Notably, mutation of W45 inactivates Rev41. Recent studies further highlight the significance of m6A RNA modification in regulating the dynamics of RNA structure and RNA-protein interactions involved in biological mechanisms 22,42-44.
Figure 5.
Proposed model for modulation of the m6A modification in RRE RNA and its effect on HIV-1 replication. See text for details. For simplicity, only monomeric Rev–RRE interactions are shown.
In summary, this work highlights the significance of RNA methylation in the context of HIV-1 infection in human T cells. The demonstration that viral infection significantly increases m6A abundance in both viral and host cell RNA provides an impetus to further investigate the role played by RNA methylation in HIV-1 biology and in the mammalian response to viral infection. The topology of m6A methylation in HIV-1 RNAs shows unique features compared to cellular mRNAs. m6A is abundant across the full-length viral genome and is present in regulatory regions, coding sequences, and structural regions, suggesting that multiple aspects of viral RNA biology may be regulated by m6A, possibly through distinct mechanisms. We found that the RNA methylation status positively correlates with viral replication, revealing a previously unknown layer of regulation of HIV-1 replication. Precise loci of methylation can be further investigated by single-molecule technologies, which are just emerging 45 and also enabled fully-phased epitranscriptomic characterization of viruses, thus revealing the co-occurrence of these dynamic m6A sites. Finally, our finding that Rev–RRE binding is regulated by MTases and demethylases suggests that the pathways could be exploited pharmacologically to disrupt Rev function. Overall, these results identify a new mechanism for the control of HIV-1 replication and its interaction with the host immune system. These findings also suggest that other RNA viruses may similarly utilize the m6A RNA modification as a mechanism to regulate viral and host RNA metabolism and gene regulation.
METHODS
Additional Methods are available on Online Content
HIV-1 virus production and MT4 infection.
HIV-1 LAI virus was produced by transfection of 293T cells with the proviral pLAI2 plasmid (NIH AIDS Reagent Program) at 3 µg/106 cells using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s instructions. After 4 h incubation, the medium was replaced with DMEM supplemented with 10% foetal bovine serum (FBS). Two days after transfection, the virus-containing supernatant was collected and filtered. The human T cell line MT4 was then infected with HIV-1 LAI virus (106 cpm of RT activity/106 cells), and 12 h later, the cells were centrifuged to remove the virus, washed with PBS, and resuspended in RPMI/10% FBS for further analysis. MT4 and 293T cells were obtained from ATCC and were tested for the absence of mycoplasma.
m6A immunoblot analysis.
Total RNA was extracted from uninfected or HIV-1-infected MT4 cells (at 3 days post-infection) with TRIzol reagent (Invitrogen). Aliquots of 5 µg total RNA were separated on 1% agarose/0.4% formaldehyde gels in 20 mM HEPES and 1.5 mM EDTA, pH 7.8. RNA was stained with ethidium bromide and transferred overnight to nitrocellulose membranes in 10X SSC buffer (1.5 M NaCl, 150 mM sodium citrate). RNA was UV-crosslinked to membrane and blocked in 5% milk PBS Tween 0.1%. Rabbit polyclonal anti m6A antibody was used at 1:1000 dilution in PBS Tween 0.1% with incubation at room temperature for 1 hour. Subsequent steps for m6A immunoblotting were performed according to previously reported protocols8. For m6A dot blotting, poly(A) RNA was purified with an mRNA Direct Kit (Ambion) and the remaining steps were as described above. Densitometric quantification of the m6A signal intensity was performed using ImageJ software (NIH).
m6A-modified RNA immunoprecipitation sequencing (MeRIP-seq) and analysis.
The quality of the RNA samples was assessed using an Agilent Bioanalyzer RNA 6000 Nano kit to ensure all RNA integrity numbers were >9.0. For poly(A) RNA purification, Dynabeads Oligo(dT)25 (#61005, Life Technologies) were incubated with an aliquot of 75 µg RNA, washed, and subjected to a second incubation with the same RNA sample, according to the manufacturer’s instructions. RNA was obtained by ethanol precipitation overnight, fragmented using Ambion® RNA Fragmentation Reagents (#AM8740, Life Technologies), and then repurified by ethanol precipitation overnight. An aliquot of fragmented RNA was retained as a control for RNA sequencing.
For MeRIP, fragmented RNA was denatured for 5 min at 75°C, cooled on ice for 2–3 min, and incubated with the anti-m6A-coupled beads in 300 µl of 140 mM IP Buffer for 2 h at 4°C. Unbound RNA was removed by centrifugation, and beads were washed three times with 140 mM IP buffer and then incubated in elution buffer (5 mM Tris HCl, pH 7.5, 1 mM EDTA, 0.05% SDS, and proteinase K) for 1.5 h at 50°C. The eluted RNA was extracted with phenol/chloroform and precipitated with ethanol. An aliquot of RNA was retained as a control for round 1 MeRIP RNA, and the remainder was subjected to a second round of MeRIP, as described above. Libraries for sequencing (input RNA-Seq and MeRIP-Seq) were prepared using Illumina TruSeq Stranded mRNA kits, entering the protocol at the Elute-Prime-Fragment step, with the modification that samples were heated to 80 °C for 2 mins to only prime but not further fragment. Samples were sequenced on a HiSeq 2000 across 2 lanes with single read 50. RNA-seq data were aligned to a combined hg19 (chr 1-22, X, Y) and HIV-1 (https://aidsreagent.org/, pLAI2) genome FASTA using the STAR aligner46 keeping only uniquely mapping reads. Peaks were called with MeRIPPeR8, using Fisher’s Exact Test on 25 bp genome-based windows to test for statistically significant enrichment in the IP relative to the control input RNA, with Benjamini-Hochberg p-value adjustment and a p-value cutoff of 0.05. Windows were merged into peaks and peaks smaller than 100 bp were filtered out. Differentially methylated peaks were called by splitting the union of all peaks into 100 bp windows in 25 bp steps. MeRIP-seq and RNA-seq counts were tabulated using bedtools47) and input into edgeR48 to identify windows with significant changes in methylation. Read counts mapping to genes were obtained using HTSeq (HTSeq cite), gene features using R-Make count (http://physiology.med.cornell.edu/faculty/mason/lab/r-make/usage.html), and all other features using BedTools. RNA-Seq data are publicly available at GEO GSE74016.
Gene ontology (GO) analysis of the uniquely methylated transcripts identified during HIV-1 infection was performed using AmiGO software. The top 10 categories are shown.
Rev–RRE interaction experiments.
For the Rev-RRE interaction experiments, we transfected 3 µg pLAI2 plasmid per 106 293T cells. After 4 h, the medium was replaced with fresh DMEM/10% FBS and incubated for a further 44 h time period. Cells were then lysed in IP lysis buffer (Pierce) and Rev protein was immunoprecipitated by incubation overnight at 4 °C with anti-Rev antibody 49 (NIH AIDS Reagent Program) (5 µg/10 × 106 cell equivalents) and 50 µl protein A/G magnetic beads (Pierce). For overexpression of Rev protein, the Rev coding sequence was amplified from cDNA isolated from HIV-1-infected MT4 cells and cloned into pFLAG-CMV-2 (Sigma) using EcoRI and SalI restriction sites. A minimal RRE corresponding to a 66 nucleotide region (GCA CTA TGG GCG CAC GGT CAA TGA CGC TGA CGG TAC AGG CCA GAC AAT TAT TGT CTG GTA TAG TGC) was PCR amplified and cloned into pcDNA3 (pcDNA3_minRRE) using HindIII and EcoRI restriction sites. The FLAG-Rev construct (1.5 µg) was co-transfected with pLAI2 (1.5 µg) or pcDNA3_minRRE (1.5 µg) into 106 293T cells with Lipofectamine 2000. After 4 h, the medium was replaced with fresh DMEM/10% FBS and incubated for a further 44 h. Cells were then lysed in IP lysis buffer (Pierce) and Rev protein was immunoprecipitated by incubation for 2 h at room temperature with anti-FLAG antibody (2 µg/3 × 106 cell equivalents) and 10 µl protein A/G magnetic beads (Pierce). IPs with mouse IgG (Santa Cruz Biotechnology) were performed in parallel as negative controls. In all the experiments, the beads were washed three times with IP lysis buffer and divided into equal volumes. One aliquot was extracted with TRIzol for RRE RNA quantification by qPCR, with normalization to β-actin RNA. The second aliquot was subjected to western blotting to assess anti-FLAG IP efficiency.
MeRIP-qPCR analysis of RRE methylation.
MT4 cells (5 × 106) were transduced with lentiviruses encoding METTL3, METTL14, or AlkBH5 shRNA as described above. After 3 days, cells were infected with LAI virus and RNA was extracted 48 h later. Aliquots of 40 µg total RNA were poly(A) enriched using mRNA direct isolation kit (Ambion) and enriched fractions were fragmented into 60–200 nucleotides with RNA fragmentation reagents (Life Technologies #AM8740). Fragmented RNA was incubated with 3 µg anti-m6A and 15 µl protein A/G magnetic beads for 2 h at room temperature in MeRIP buffer (10 mM Tris HCl, 150 mM NaCl, 0.1% NP-40, pH 7.4). IPs with rabbit normal IgG (10500C, Invitrogen) were performed in parallel and served as input controls. Beads were washed three times with MeRIP buffer, and m6A-containing RNAs were eluted by incubation with 20 mM m6A for 20 min at room temperature. Eluted RNA fractions were precipitated using the sodium acetate/ethanol precipitation method, and cDNA synthesis and qPCR analysis were performed as described above.
AMV Reverse Transcriptase and Tth DNA polymerase primer extension analyses for probing site-specific m6A modification.
These experiments were performed as previously described 37 with the following modifications. ssDNA oligos were 5′ radio-labelled with 32P. For 28S 4189 and 28S 4190 (negative and positive controls) probing 10 µg total RNA from HIV-1 infected MT4 cells was used. For A7877 and A7883 1 µg poly(A) RNA from HIV-1 infected MT4 cells was used. RNA and radio-labeled DNA oligos (1 µl) were annealed in 2 × annealing solution in a total volume of 15 µl with 1 × Tth pol buffer with 1 mM MnCl2 (Promega) or AMV buffer (Promega), The mixture was denatured at 95 °C for 10 min and slowly annealed cooling to room temperature. 3 µl of annealed solution were mixed with 2 µl of enzyme and heated at 37 °C (AMV Reverse Transcriptase) or 55 °C (Tth DNA Polymerase) for 2 min. dTTP solution (final dTTP concentration: 100 µM) was added and the reactions were incubated for 30 min at 37 °C (AMV) or at 55 °C (Tth). Reactions were resolved on a 20% denaturing polyacrylamide gel and exposed overnight on phosphoimaging screen. SsDNA probe sequences are listed in Supplementary Information Table 3.
Accession numbers.
RNA-Seq data are publicly available at GEO GSE74016.
Supplementary Material
Acknowledgments
We gratefully acknowledge Steve Head and the staff of the Next Generation Sequencing core facility at the Scripps Research Institute for help with early phase of the project and HT-seq. We thank Jennifer Klabis for her help with preparation of figures, and members of the Rana lab for helpful discussions and advice, especially Drs. Ti-Chun Chao and Kungyen Chang.
Footnotes
Author Contributions
G.L. designed and performed experiments, analyzed data, wrote the paper; S.G. performed experiments and analyzed data; Y.S. performed experiments and analyzed data; G. M. G. performed experiments and analyzed data; V.B. analyzed data; Y.W. analyzed data; C.M. analyzed data; T.M.R. concept, design, data analysis, manuscript writing, and financial support.
Additional information
Supplementary information is available online. Reprints and permissions information is available online at www.nature.com/reprints. Correspondence and requests for materials should be addressed to T.M.R.
Competing interests
The authors declare no competing financial interests.
REFERENCES
- 1.Sharp PA The centrality of RNA. Cell 136, 577–580, doi: 10.1016/j.cell.2009.02.007 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Squires JE et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res 40, 5023–5033, doi: 10.1093/nar/gks144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi C & Pan T Cellular dynamics of RNA modification. Acc Chem Res 44, 1380–1388, doi: 10.1021/ar200057m (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desrosiers RC, Friderici KH & Rottman FM Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5' terminus. Biochemistry 14, 4367–4374 (1975). [DOI] [PubMed] [Google Scholar]
- 5.Perry RP, Kelley DE, Friderici K & Rottman F The methylated constituents of L cell messenger RNA: evidence for an unusual cluster at the 5' terminus. Cell 4, 387–394 (1975). [DOI] [PubMed] [Google Scholar]
- 6.Schibler U, Kelley DE & Perry RP Comparison of methylated sequences in messenger RNA and heterogeneous nuclear RNA from mouse L cells. J Mol Biol 115, 695–714 (1977). [DOI] [PubMed] [Google Scholar]
- 7.Wei CM, Gershowitz A & Moss B Methylated nucleotides block 5' terminus of HeLa cell messenger RNA. Cell 4, 379–386 (1975). [DOI] [PubMed] [Google Scholar]
- 8.Meyer KD et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell 149, 1635–1646, doi: 10.1016/j.cell.2012.05.003 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominissini D et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206, doi: 10.1038/nature11112 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Schwartz S et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5' sites. Cell Rep 8, 284–296, doi: 10.1016/j.celrep.2014.05.048 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frayling TM et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316, 889–894, doi: 10.1126/science.1141634 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia G et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 7, 885–887, doi: 10.1038/nchembio.687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng G et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 49, 18–29, doi: 10.1016/j.molcel.2012.10.015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bokar JA, Shambaugh ME, Polayes D, Matera AG & Rottman FM Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 3, 1233–1247 (1997). [PMC free article] [PubMed] [Google Scholar]
- 15.Ping XL et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res 24, 177–189, doi: 10.1038/cr.2014.3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10, 93–95, doi: 10.1038/nchembio.1432 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia G, Fu Y & He C Reversible RNA adenosine methylation in biological regulation. Trends Genet 29, 108–115, doi: 10.1016/j.tig.2012.11.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee M, Kim B & Kim VN Emerging roles of RNA modification: m(6)A and U-tail. Cell 158, 980–987, doi: 10.1016/j.cell.2014.08.005 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Wang X et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120, doi: 10.1038/nature12730 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu C et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol 10, 927–929, doi: 10.1038/nchembio.1654 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Geula S et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science 347, 1002–1006, doi: 10.1126/science.1261417 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Liu N et al. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518, 560–564, doi: 10.1038/nature14234 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J et al. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594, doi: 10.1038/nature15377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res 24, 1403–1419, doi: 10.1038/cr.2014.151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fustin JM et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell 155, 793–806, doi: 10.1016/j.cell.2013.10.026 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Geula S et al. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science, doi: 10.1126/science.1261417 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Batista PJ et al. m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 15, 707–719, doi: 10.1016/j.stem.2014.09.019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol 16, 191–198, doi: 10.1038/ncb2902 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kane SE & Beemon K Precise localization of m6A in Rous sarcoma virus RNA reveals clustering of methylation sites: implications for RNA processing. Mol Cell Biol 5, 2298–2306 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krug RM, Morgan MA & Shatkin AJ Influenza viral mRNA contains internal N6-methyladenosine and 5'-terminal 7-methylguanosine in cap structures. J Virol 20, 45–53 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finkel D & Groner Y Methylations of adenosine residues (m6A) in pre-mRNA are important for formation of late simian virus 40 mRNAs. Virology 131, 409–425 (1983). [DOI] [PubMed] [Google Scholar]
- 32.Fu L et al. Simultaneous Quantification of Methylated Cytidine and Adenosine in Cellular and Tissue RNA by Nano-Flow Liquid Chromatography-Tandem Mass Spectrometry Coupled with the Stable Isotope-Dilution Method. Anal Chem 87, 7653–7659, doi: 10.1021/acs.analchem.5b00951 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heaphy S et al. HIV-1 regulator of virion expression (Rev) protein binds to an RNA stem-loop structure located within the Rev response element region. Cell 60, 685–693 (1990). [DOI] [PubMed] [Google Scholar]
- 34.Kjems J, Brown M, Chang DD & Sharp PA Structural analysis of the interaction between the human immunodeficiency virus Rev protein and the Rev response element. Proc Natl Acad Sci U S A 88, 683–687 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malim MH et al. HIV-1 structural gene expression requires binding of the Rev trans-activator to its RNA target sequence. Cell 60, 675–683 (1990). [DOI] [PubMed] [Google Scholar]
- 36.Battiste JL et al. Alpha helix-RNA major groove recognition in an HIV-1 rev peptide-RRE RNA complex. Science 273, 1547–1551 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Harcourt EM, Ehrenschwender T, Batista PJ, Chang HY & Kool ET Identification of a selective polymerase enables detection of N(6)-methyladenosine in RNA. J Am Chem Soc 135, 19079–19082, doi: 10.1021/ja4105792 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heaphy S, Finch JT, Gait MJ, Karn J & Singh M Human immunodeficiency virus type 1 regulator of virion expression, rev, forms nucleoprotein filaments after binding to a purine-rich “bubble” located within the rev-responsive region of viral mRNAs. Proc Natl Acad Sci U S A 88, 7366–7370 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiley LS, Malim MH, Tewary HK, Stockley PG & Cullen BR Identification of a high-affinity RNA-binding site for the human immunodeficiency virus type 1 Rev protein. Proc Natl Acad Sci U S A 89, 758–762 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kjems J, Calnan BJ, Frankel AD & Sharp PA Specific binding of a basic peptide from HIV-1 Rev. EMBO J 11, 1119–1129 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammerschmid M et al. Scanning mutagenesis of the arginine-rich region of the human immunodeficiency virus type 1 Rev trans activator. J Virol 68, 7329–7335 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alarcon CR et al. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell 162, 1299–1308, doi: 10.1016/j.cell.2015.08.011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roost C et al. Structure and Thermodynamics of N-Methyladenosine in RNA: A Spring-Loaded Base Modification. J Am Chem Soc, doi: 10.1021/ja513080v (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spitale RC et al. Structural imprints in vivo decode RNA regulatory mechanisms. Nature 519, 486–490, doi: 10.1038/nature14263 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saletore Y et al. The birth of the Epitranscriptome: deciphering the function of RNA modifications. Genome Biol 13, 175, doi: 10.1186/gb-2012-13-10-175 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobin A et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21, doi: 10.1093/bioinformatics/bts635 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quinlan AR & Hall IM BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842, doi: 10.1093/bioinformatics/btq033 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson MD, McCarthy DJ & Smyth GK edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140, doi: 10.1093/bioinformatics/btp616 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalland KH, Szilvay AM, Langhoff E & Haukenes G Subcellular distribution of human immunodeficiency virus type 1 Rev and colocalization of Rev with RNA splicing factors in a speckled pattern in the nucleoplasm. J Virol 68, 1475–1485 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.