Abstract
The rice arable system is of importance to both society and the environment. The emergence of rice paddies was a crucial step in the transition from pre-domestic cultivation to systematic land use and management. However, many aspects of the formation of rice farming systems remain unclear. An important reason is the lack of reliable methods for identifying early rice paddies. One possible means of remedying this knowledge deficit is through analysis of phytolith assemblages, which are closely related to their parent plant communities. In this study, phytolith assemblages from 27 surface soil samples from wild rice fields, 91 surface soil samples from modern rice paddies, and 50 soil samples from non-rice fields were analysed to establish a discriminant function. This discriminant function enabled classification of 89.3% of the samples into appropriate groups. Further, the results suggested that phytolith assemblages can be used to identify rice fields and differentiate between wild rice fields and domesticated rice fields. The method was demonstrated to be an effective way of utilising the large amounts of unidentifiable phytoliths discovered at archaeological sites to provide a modern analogue that may be a valuable key to unlocking the past.
Introduction
Rice paddies are crucial for global food security because rice is the staple food of nearly half the world population1. Eastern Asia, Southern Asia, and South-Eastern Asia account for almost 90% of rice production and 88% of the rice cultivation area2. The history of Asian societies, human populations and the evolutionary history of rice as a crop are inseparable3; rice production was a prerequisite for the rise of civilization in monsoon Asia. The earliest confirmed rice paddy cultivation began in the coastal wetlands of Eastern China about 7,700 cal yr B.P.4; however, phytolith evidence showed that rice domestication might have begun as early as 9,400 cal yr B.P. in the lower Yangtze Basin5,6. Exploring the transformation from pre-domestic cultivation to current systematic, large-scale land use and management is the key to understanding the formation of rice farming systems; however, when, where, and how rice paddies emerged remains unclear, largely because of the lack of reliable methods to identify early rice paddies.
Currently, the methods for studying ancient and present-day rice paddies mainly focus on the physical and chemical properties of paddy soil7,8, such as micromorphological features9, organic matter10–12 (including organic carbon13–18 and nitrogen19,20, fatty acids and polycyclic aromatic hydrocarbons21–26), forms of iron27,28, mineral characteristics29, bacterial communities30–34, soil fertility35, pollen features36, and phytolith accumulation37. Although these methods are effective for analysing the characteristics of paddy fields, they can only be applied to known paddy fields.
Phytoliths are siliceous bodies present in certain plant tissues; plants absorb silica in a soluble state from groundwater and accumulate solid silica in both intracellular and extracellular locations38. Phytoliths can be used to identify particular genera or species based on shape, size, and other anatomical features38–40. Rice bulliform phytoliths41,42 and double-peak phytoliths43,44, which differ between wild and domesticated rice, have been employed to study the origin and domestication of rice5,6,45. Fujiwara et al.46 established the paddy identification standard as 5000 rice bulliform phytoliths in 1 g of dried soil sample and this standard has been applied to identify Neolithic paddies in the Shandong and Zhejiang Provinces47,48. However, careful consideration is required when applying this standard because if the crop is harvested by uprooting or basal cutting, only a few bulliform phytoliths will remain in the field49,50.
Notably, a rice system does not contain paddy soil alone; it is a relatively independent ecosystem including other plant species that might be of use for identification. Rice arable systems are of importance to both the society3,51–54 and the environment55–57. As rice arable systems are associated with specific grasses, weed assemblages58–61 can be used to determine changes in arable systems. Recently, phytolith assemblages, based on the proportion of fixed and sensitive types have been used to distinguish between wet and dry cultivation systems62–64; however, similar studies using phytolith assemblages to differentiate wild and domesticated rice paddies are rare.
In the present study, a detailed discriminant analysis was conducted on modern soil samples (Fig. 1) to develop a method for identifying rice fields using phytolith assemblages as indicators. The results demonstrated that modern phytolith assemblages can be used to differentiate between wild rice fields, domesticated rice paddies, and non-rice fields.
Figure 1.
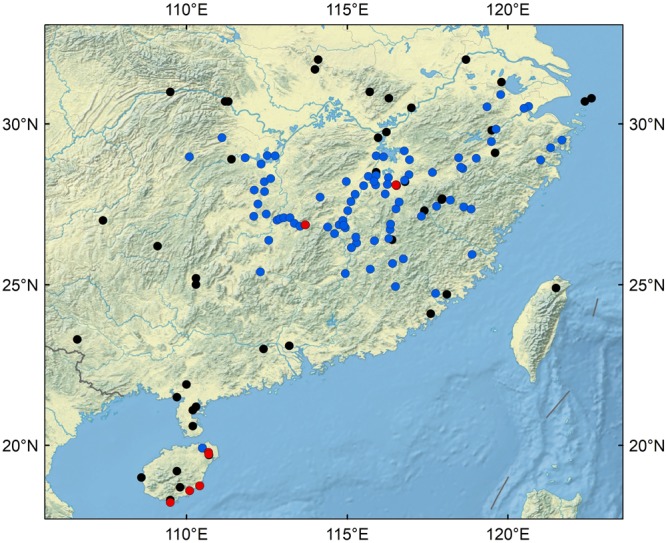
Geographic locations of samples. Red dots represent soil samples from wild rice fields; blue dots represent soil samples from domesticated rice paddies; black dots represent soil samples from non-rice fields. (The figure was generated using GRASS GIS 7.2.1: GRASS Development Team, 2017. Geographic Resources Analysis Support System (GRASS) Software, Version 7.2. Open Source Geospatial Foundation. Electronic document: http://grass.osgeo.org).
Results
Phytolith assemblages in selected samples
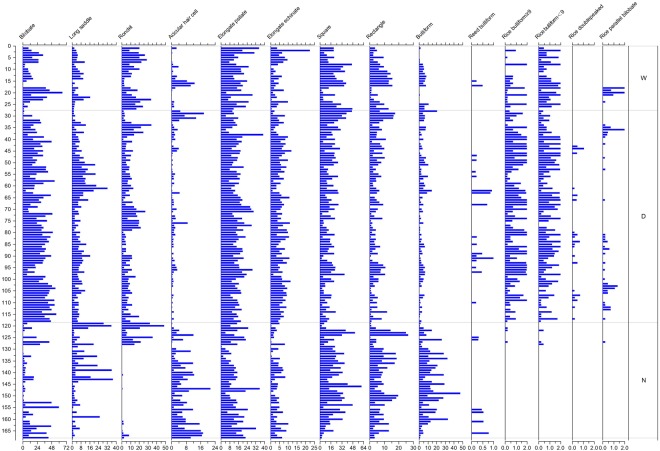
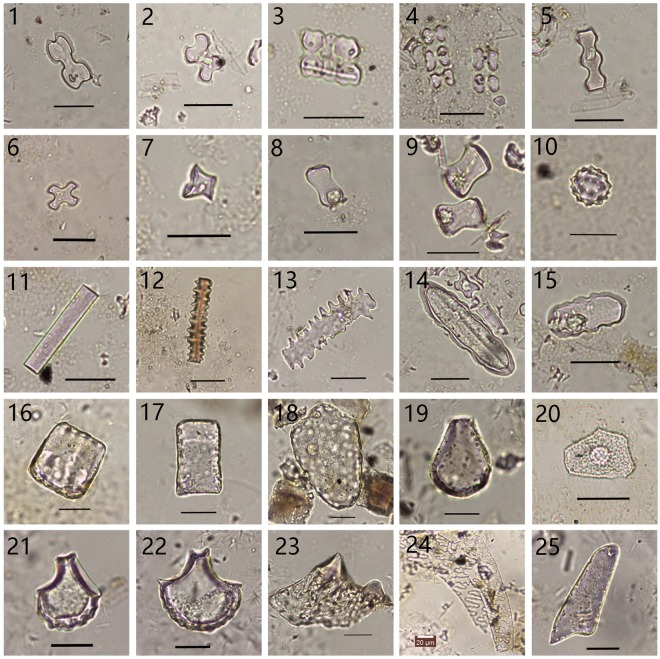
A total of 170 soil samples were collected and 168 of these contained abundant phytoliths. Twenty-one phytolith morphotypes were identified (Fig. 2), including bilobate, cylindrical polylobate, cross, long saddle, short saddle, rondel, trapeziform sinuate, acicular hair cell, elongate psilate, elongate echinate, square, rectangle, bulliform, Cyperaceae-type, globular echinate and barnyard grass husk-type. Soil samples from wild rice and domesticated rice fields were characterised by the presence of bulliform phytoliths with fish-scale decorations. Characteristic phytolith types are shown in Fig. 3.
Figure 2.
Percentage diagram of the major phytolith morpho-types in selected samples. Group code on the right: W represents samples from wild rice fields; D represents samples from domesticated rice paddies; N represent soil samples from non-rice fields. For sample codes, refer Supplementary Table S2.
Figure 3.
Major phytolith morpho-types in rice paddy soil 1–2: Bilobate; 3–4: Parallel-bilobate 5: Cylindrical polylobate; 6: Cross; 7: Rondel; 8–9: Long saddle; 10: Globular echinate; 11: Elongate psilate; 12–13: Elongate echinate; 14–15: Trapeziform sinuate; 16: Square; 17: Rectangle; 18: Reed bulliform; 19: Bulliform; 20: Cyperaceae; 21: Rice bulliform with < 9 fish-scale decorations; 22: Rice bulliform with ≥ 9 fish-scale decorations; 23: Rice double-peaked; 24: Barnyard grass husk; 25: Acicular hair cell (Scale bar 20 μm).
All surface soil samples from the wild rice fields were characterised by high rates of square (22.17%), bilobate (18.19%) and elongate psilate (17.62%) phytoliths. Furthermore, the proportion of bulliform phytoliths with ≥9 fish-scale decorations was 0.55%, whereas that for bulliform phytoliths with <9 fish-scale decorations was 1.55%. The average phytolith concentration in these samples was ~1.18 million particles/g; the highest phytolith concentration was 6.34 million particles/g (WN-BT8), whereas the lowest concentration was 5,200 particles/g (WN-BT6).
Among 93 soil samples from domesticated rice paddies, no phytoliths were observed in samples DTZJ9 and DTZJ11. Phytolith assemblages from the other 91 domesticated rice paddy samples were characterised by fairly high rates of bilobate (30.54%), square (15.96%), and elongate psilate (15.90%) phytoliths. The proportion of bulliform phytoliths with ≥9 fish-scale decorations was 1.43%, whereas that for bulliform phytoliths with <9 fish-scale decorations was 1.14%. The average phytolith concentration in these samples was ~3.03 million particles/g. The highest concentration was 6.61 million particles/g (HL-OS1), whereas the lowest concentration was 15800 particles/g (DTZJ3).
Phytolith assemblages from the 50 non-rice field samples were different from those of rice field samples in that high rates of square (22.14%), elongate psilate (13.67%), bulliform (12.78%) and bilobate (11.47%) phytoliths were present. In particular, four samples (10CL-FB1, 10CL-FB2, WN-BT5, and ZX-4) in this group contained a low rate of rice bulliform phytolith particles. Although the four soil samples did not come from wild rice or domesticated rice fields, their sampling locations were extremely close to wild or domesticated rice fields. Therefore, it is possible that these samples contained rice bulliform phytoliths because of soil tillage or other soil-disturbing activities.
Numerical analysis of modern phytolith data
We used the data on 21 phytolith types (Supplementary Table S2) in a discriminant analysis. The discriminant scores of 168 samples were plotted along the first two discriminant functions, accounting for 64% and 36% of variance, respectively (Fig. 4). The results showed that the three group centroids were clearly separated, marginally overlapping with each other (Fig. 4; black square).
Figure 4.
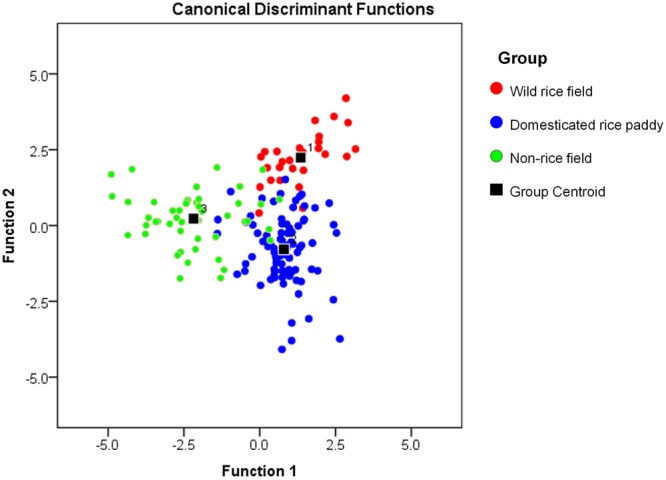
One hundred and sixty-eight samples plotted against the canonical discriminant functions 1 and 2 and their group centroids corresponding to three groups.
The results of the discriminant analysis showed that 89.3% (150 out of 168) of the original grouped cases were classified correctly (Table 1). Only two of the 27 samples from the wild rice field were misclassified. Of the samples from the domesticated rice paddies, 90.1% were classified correctly and nine samples were misclassified. Seven samples in the non-rice field group were classified in the rice-field group. Regarding cross-validation (each case was classified using the functions derived from all the other cases), 82.1% of the cross-validated grouped cases were classified correctly (Table 1). The high degree of correct classification suggested that the classification of the surface soil samples into the three groups was statistically robust.
Table 1.
Classification results of discriminant analysis of 168 samples.
| Groupa | Predicted Group Membership | Total | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| Originalb | Count | 1 | 25 | 2 | 0 | 27 |
| 2 | 6 | 82 | 3 | 91 | ||
| 3 | 2 | 5 | 43 | 50 | ||
| % | 1 | 92.6 | 7.4 | 0.0 | 100.0 | |
| 2 | 6.6 | 90.1 | 3.3 | 100.0 | ||
| 3 | 4.0 | 10.0 | 86.0 | 100.0 | ||
| Cross-validatedc, d | Count | 1 | 24 | 3 | 0 | 27 |
| 2 | 11 | 74 | 6 | 91 | ||
| 3 | 2 | 8 | 40 | 50 | ||
| % | 1 | 88.9 | 11.1 | 0.0 | 100.0 | |
| 2 | 12.1 | 81.3 | 6.6 | 100.0 | ||
| 3 | 4.0 | 16.0 | 80.0 | 100.0 | ||
aGroup 1: Wild rice group; Group 2: Domesticated rice group; Group 3: Non-rice group.
b89.3% of original grouped cases correctly classified.
cCross-validation was performed only for those cases in the analysis. In cross-validation, each case was classified by the function derived from all other cases.
d82.1% of cross-validated grouped cases correctly classified.
Discussion and Conclusions
Earlier studies on phytolith assemblage focused mainly on palaeoenvironment reconstruction65,66. Only a few specific crop-related phytoliths are highly valued for their application in archaeology41,43,67–71. Therefore, a large proportion of unidentifiable phytoliths discovered at archaeological sites are undervalued. Excluding a few cases62–64, phytoliths could be exploited differently for archaeological applications by combining with statistical analyses, such as correspondence analysis, discriminant analysis, and principal component analysis.
This study demonstrated that rice paddies could be distinguished by their phytolith assemblages. Soil samples from both the wild rice fields and domesticated rice paddies were characterised by high proportions of bilobate, square and elongate psilate phytoliths, whereas the non-rice field samples exhibited diverse phytolith assemblages dominated by the rondel type. Furthermore, according to phytolith grouping for fixed and sensitive types64, a higher proportion of bilobate phytoliths (a fixed type) was observed in the soil samples from the domesticated rice paddies, whereas a higher rate of elongate psilate (a sensitive type) phytoliths was obtained from the wild rice fields. This probably indicates that wild rice habitats are swampier than those of domesticated rice. The proportion of rice bulliform phytoliths is informative, although it is relatively low. Rice bulliform phytoliths are unique to rice plants and the decorations on the edges of rice bulliform phytoliths can be used to differentiate wild rice from domesticated rice41,42. However, because of the limited number of samples from Southern China, the results were not compared with samples from Northern China, South Asia, and Southeast Asia, which are also major rice-producing areas.
This study analysed the phytoliths present in 168 soil samples collected from wild and domesticated rice fields and non-rice fields to establish a discriminant function, and demonstrated that these phytolith assemblages correctly classified 89.3% of the samples. The results statistically support the development of a robust method for identifying rice paddies and distinguishing between wild and domesticated rice fields. The method was demonstrated to be an effective way of utilising the large numbers of unidentifiable phytoliths from archaeological sites and this modern analogue should provide a valuable key to unlocking the past.
Methods
In this study, a total of 170 soil samples were collected for phytolith analysis (Fig. 1). These included (1) 27 surface soil samples from wild rice fields in Jiangxi, Hu’nan and Hainan, (2) 93 surface soil samples from domesticated rice paddy fields in Zhejiang, Jiangxi, Hu’nan, Fujian and Hainan, and (3) 50 soil samples from non-rice fields in Southern China (40 of these were selected from the modern phytolith database for Chinese surface soil65). The geographic distribution of rice fields is 18.23°N–30.91°N and 109.5°E–121.33°E, so we chose non-rice fields samples located at 18.3°N–32°N and 106.6°E–122.6°E to ensure all studied samples came from similar climatic regions. Surface soils with a depth of 0–5 cm (in most cases from the top 5 cm, but sometimes from the top 3 cm) were collected and mixed evenly before analysis. Leaves, roots, and plant litter were removed prior to sampling. Details of all the samples are presented in Supplementary Table S1. All necessary permits for sample collection from Jiangxi were obtained from the Jiangxi Academy of Agricultural Sciences. No permit was required to collect samples from the other locations.
Phytoliths were extracted from the soil samples according to the procedure described by Zhang72, with minor modifications. Initially, 5 g of soil samples was weighed. Subsequently, 30% H2O2 and 15% HCl were added to the samples to remove organic matter and carbonates. The samples were then subjected to heavy liquid flotation using ZnBr2 (density, 2.35 g/cm3) to separate the phytoliths, which were subsequently mounted on a slide with Canada Balsam. The phytoliths were counted and identified under a Leica microscope at 400X magnification. More than 400 phytolith particles from grass were counted in each sample. A few woody phytoliths were identified in this study; however, because these can be highly influenced by local environment and climatic factors, they were excluded to minimize possible bias73. Identification of phytoliths was performed using previous studies as references39,41,67,74.
Discriminant analysis was performed using SPSS version 22.0. It is a method of statistical inference for deriving linear combinations of variables, called discriminant functions, which are mutually independent75,76. These discriminant functions ensure maximum separation among priori sample groups and can also be used to classify new samples with unknown group memberships into one of the priori groups75–77. In this study, 168 samples were initially divided into three priori groups (actual); subsequently percentage data of 21 phytolith types were used to establish the discriminant functions and the samples were classified into predicted groups.
Electronic supplementary material
Acknowledgements
We thank Xu E.Q. and Tang X.G. for providing many essential samples. We also thank Xu D.K., Zhang D. and Cui A.N. for their assistance in generating several of the figures. This research was financially supported by the “Strategic Priority Research Program” of the Chinese Academy of Sciences (Grant No. XDPB0503), the National Key R&D Program of China (Grant No. 41430103), 973 Program (Grant No. 2015CB953801), National Natural Science Foundation of China (Grant No. 41472154), Youth Innovation Promotion Association CAS (No. 2017096).
Author Contributions
X.H. and H.L. designed the study. H.X., J.Z., H.L. and C.W. collected the samples and performed the experiments. X.H. analysed the data and prepared all the figures and tables. X.H., H.L. and J.Z. wrote the main manuscript. All authors discussed the results and reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29172-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Rice Research Institute. Bringing Hope, Improving Lives: Strategic Plan 2007–2015. 1–61 (Manila, Philippines, 2006).
- 2.Food and Agriculture Organization of the United Nations. FAO Statistical Databases (2016). (Rome, 2017).
- 3.Fuller DQ. Pathways to Asian Civilizations: Tracing the Origins and Spread of Rice and Rice Cultures. Rice. 2011;4:78–92. doi: 10.1007/s12284-011-9078-7. [DOI] [Google Scholar]
- 4.Zong Y, et al. Fire and flood management of coastal swamp enabled first rice paddy cultivation in east China. Nature. 2007;449:459–462. doi: 10.1038/nature06135. [DOI] [PubMed] [Google Scholar]
- 5.Zuo X, et al. Dating rice remains through phytolith carbon-14 study reveals domestication at the beginning of the Holocene. Proceedings of the National Academy of Sciences. 2017;114:6486–6491. doi: 10.1073/pnas.1704304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huan X, et al. Fan-shaped phytoliths reveal the process of rice domestication at Shangshan site, Zhejiang province. Quaternary Sciences. 2014;34:106–113. doi: 10.2306/scienceasia1513-1874.2014.40.106. [DOI] [Google Scholar]
- 7.Cao, Z. Origin and evolution og irrigated rice fields and related ancient and present paddy soil’s quality in China. (Beijing, 2016)
- 8.Cao Z. Study of prehistoric irrigated paddys and ancient paddy soils in China. Acta predologica Sinica. 2008;05:784–791. [Google Scholar]
- 9.Zhuang Y, Ding P, French C. Water management and agricultural intensification of rice farming at the late-Neolithic site of Maoshan, Lower Yangtze River, China. Holocene. 2014;24:531–545. doi: 10.1177/0959683614522310. [DOI] [Google Scholar]
- 10.Wen Z, Sun G, Xie L, Sun Y. The geochemical characteristics and significance of soil organic matter in the Tianluoshan Site of Hemudu cultural. Geochimica. 2014;02:166–173. [Google Scholar]
- 11.Zou P, Fu J, Cao Z, Ye J, Yu Q. Aggregate dynamics and associated soil organic matter in topsoils of two 2,000-year paddy soil chronosequences. Journal of Soils and Sediments. 2015;15:510–522. doi: 10.1007/s11368-014-0977-2. [DOI] [Google Scholar]
- 12.Nakahara S, et al. Stability of soil organic matter accumulated under long‐term use as a rice paddy. Journal of Geophysical Research Biogeosciences. 2016;121:67–77. doi: 10.1002/2015JG003104. [DOI] [Google Scholar]
- 13.Xiao Y, et al. Characteristics of Organic Carbon Mineralization in Ancient Paddy Soils at Liyang Plain. Hunan Agricultural Sciences. 2015;03:53–55. [Google Scholar]
- 14.Xiao Y, et al. Distribution characteristics of different organic carbon forms in ancient paddy soils at Liyang plain. Chinese Journal of Ecology. 2015;06:1644–1649. [Google Scholar]
- 15.Zhang, J. et al. Effects of land use change on soil organic carbon sources and molecular distributions: 6280 years of paddy rice cropping revealed by lipid biomarkers. Journal of Soils & Sediments, 1–12 (2017).
- 16.Zhang J, et al. Land use affects soil organic carbon of paddy soils: empirical evidence from 6280 years BP to present. Journal of Soils & Sediments. 2016;16:767–776. doi: 10.1007/s11368-015-1297-x. [DOI] [Google Scholar]
- 17.Wissing L, et al. Organic carbon accumulation on soil mineral surfaces in paddy soils derived from tidal wetlands. Geoderma. 2014;228–229:90–103. doi: 10.1016/j.geoderma.2013.12.012. [DOI] [Google Scholar]
- 18.Wissing L, et al. Management-induced organic carbon accumulation in paddy soils: The role of organo-mineral associations. Soil & Tillage Research. 2013;126:60–71. doi: 10.1016/j.still.2012.08.004. [DOI] [Google Scholar]
- 19.Zhong M, Zhuang X. Advances of Organic Matter and Nitrogen Study in Ancient Paddy Soils. Chinese Agricultural Science Bulletin. 2011;15:12–15. [Google Scholar]
- 20.Hu L, et al. Different nitrogen supply capacities and nitro genous fertilizer effiencies in ancient and present paddy soils. Acta predologica Sinica. 2007;03:556–560. [Google Scholar]
- 21.Li J, et al. Distribution Characteristics and Sources Identification of PAHs in Ancient Paddy Soil. Environmental Sciences. 2006;06:1235–1239. [PubMed] [Google Scholar]
- 22.Li X, Liu B, Dai J. Composition Features of n-Alkanes and Fatty Acids in Paddy Soil in Chuodun Archaeology Site and Hengjing Site, China. Chinese Journal of Soil Science. 2009;05:977–980. [Google Scholar]
- 23.Li, X. Composition features of biological markers in paddy soil in Chuodun archaeology site and research of the change in the paleocimate and paleoenvironment. Nanjing Agricultural University, Master thesis. (2008)
- 24.Li J, et al. Distribution and origins of polycyclic hydro carbons in a soil profile containing 6000-year old paddy soil. Acta predologica Sinica. 2007;01:41–46. [Google Scholar]
- 25.Li J, et al. Vertical Distribution of Polycyclic Aromatic Hydrocarbons (PAHs) in Two Prehistoric Paddy Soil Profiles. Journal of Agro-Environment Science. 2007;01:224–229. [Google Scholar]
- 26.Jin Z, et al. Change of PAHs with evolution of paddy soils from prehistoric to present over the last six millennia in the Yangtze River Delta region, China. Science of the Total Environment. 2013;449:328–335. doi: 10.1016/j.scitotenv.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 27.Liu P, et al. Evolution of Iron Forms in Ancient Paddy Soils in Liyang Plain. Soils. 2016;06:1151–1156. [Google Scholar]
- 28.Li. J, et al. Distribution Characteristics of Iron Forms in Ancient Paddy Soils of Liyang Plain. Chinese Agricultural Science Bulletin. 2015;15:215–219. [Google Scholar]
- 29.Li, X. Mineralogical and Geochemical Evidence of the Presence of Ancient Paddy Soil Layer in Chuodun Ancient Agricultural Site, Kunshan. Nanjing Agricultural University, Master thesis. (2010)
- 30.Shen W, et al. PCR-DGGE analyses of bacterial and archaeal community diversities in ancient paddy soils discovered in Chuodunshan Site, Suzhou, China. Acta Ecologica Sinica. 2008;06:2916–2924. [Google Scholar]
- 31.Shen W, et al. Microbiological properties of an ancient paddy soil discovered in Chuodunshan relics of Kunshan, China. Acta predologica Sinica. 2006;05:814–820. [Google Scholar]
- 32.Hu L, et al. Effects of rice cultivation on microbial funtional diversity in ancient and present paddy soil. Acta predologica Sinica. 2007;02:280–287. [Google Scholar]
- 33.Ding LJ, Su JQ, Li H, Zhu YG, Cao ZH. Bacterial succession along a long-term chronosequence of paddy soil in the Yangtze River Delta, China. Soil Biology & Biochemistry. 2017;104:59–67. doi: 10.1016/j.soilbio.2016.10.013. [DOI] [Google Scholar]
- 34.Zhu YG, et al. A buried Neolithic paddy soil reveals loss of microbial functional diversity after modern rice cultivation. Science Bulletin. 2016;61:1052–1060. doi: 10.1007/s11434-016-1112-0. [DOI] [Google Scholar]
- 35.Lu J, et al. Characteristics of Soil Fertility of Buried Ancient Paddy at Chuodun Site in Yangtze River Delta. Scientia Agricultura Sinica. 2006;01:109–117. [Google Scholar]
- 36.Li C, et al. Pollen evidence for ancient paddy fields at Chuodun site. Acta predologica Sinica. 2006;03:452–460. [Google Scholar]
- 37.Li Z, Song Z, Jiang P. The production and accumulation of phytoliths in rice ecosystems: a case study to Jiaxing Paddy Field. Acta Ecologica Sinica. 2013;33:7197–7203. doi: 10.5846/stxb201207261061. [DOI] [Google Scholar]
- 38.Piperno, D. R. Phytoliths: a comprehensive guide for archaeologists and paleoecologists. (AltaMira, 2006).
- 39.Wang, Y. & Lu, H. The study of phytolith and its application. (Beijing, 1993).
- 40.Wu, Y., You, H. L. & Li, X. Q. Dinosaur-associated Poaceae epidermis and phytoliths from the Early Cretaceous of China. National Science Review, nwx145, 10.1093/nsr/nwx145 (2017).
- 41.Huan, X. J. et al. Bulliform Phytolith Research in Wild and Domesticated Rice Paddy Soil in South China. Plos One10, 10.1371/journal.pone.0141255 (2015). [DOI] [PMC free article] [PubMed]
- 42.Lu H, et al. Rice domestication and climatic change: phytolith evidence from East China. Boreas. 2002;31:378–385. doi: 10.1080/030094802320942581. [DOI] [Google Scholar]
- 43.Zhao Z, Pearsall D, Benfer R, Piperno D. Distinguishing rice (Oryza sativa poaceae) from wildOryza species through phytolith analysis, II Finalized method. Economic Botany. 1998;52:134–145. doi: 10.1007/BF02861201. [DOI] [Google Scholar]
- 44.Gu Y, Zhao Z, Pearsall DM. Phytolith morphology research on wild and domesticated rice species in East Asia. Quaternary International. 2013;287:141–148. doi: 10.1016/j.quaint.2012.02.013. [DOI] [Google Scholar]
- 45.Ma Y, et al. Rice bulliform phytoliths reveal the process of rice domestication in the Neolithic Lower Yangtze River region. Quaternary International. 2016;426:126–132. doi: 10.1016/j.quaint.2016.02.030. [DOI] [Google Scholar]
- 46.Fujiwara H, Sugiyama S. Fundamental studies of plant opal analysis 5: Investigation of ancient paddy fields before archaeological excavation by plant opal analysis. Kokogaku to Shizen Kagaku. 1985;17:73–85. [Google Scholar]
- 47.Jin G, et al. Neolithic rice paddy from the Zhaojiazhuang site, Shandong, China. Chinese Science Bulletin. 2007;52:3376–3384. doi: 10.1007/s11434-007-0449-9. [DOI] [Google Scholar]
- 48.Zheng Y, Chen X, Ding P. Studies on the archaeological paddy fields at Maoshan site in Zhejiang. Quaternary Sciences. 2014;1:85–96. [Google Scholar]
- 49.Fuller DQ, Allaby RG, Stevens C. Domestication as innovation: the entanglement of techniques, technology and chance in the domestication of cereal crops. World Archaeology. 2010;42:13–28. doi: 10.1080/00438240903429680. [DOI] [Google Scholar]
- 50.Harvey EL, Fuller DQ. Investigating crop processing using phytolith analysis: the example of rice and millets. Journal of Archaeological Science. 2005;32:739–752. doi: 10.1016/j.jas.2004.12.010. [DOI] [Google Scholar]
- 51.Fuller DQ, et al. The contribution of rice agriculture and livestock pastoralism to prehistoric methane levels:An archaeological assessment. The Holocene. 2011;21:743–759. doi: 10.1177/0959683611398052. [DOI] [Google Scholar]
- 52.Fuller DQ, Ling Q, Bogaard A, Whitehouse N. Declining oaks, increasing artistry, and cultivating rice: the environmental and social context of the emergence of farming in the Lower Yangtze Region. Environmental Archaeology. 2010;15:139–159. doi: 10.1179/146141010X12640787648531. [DOI] [Google Scholar]
- 53.Zhuang YJ, Ding P, French C. Water management and agricultural intensification of rice farming at the late-Neolithic site of Maoshan, Lower Yangtze River, China. The Holocene. 2014;24:531–545. doi: 10.1177/0959683614522310. [DOI] [Google Scholar]
- 54.Guo L, Guo J. Types of Early Rice fieldremains and Their Social Correlation. Agricultural History of China. 2016;6:13–28. [Google Scholar]
- 55.Redeker KR, et al. Emissions of Methyl Halides and Methane from Rice Paddies. Science. 2000;290:966–969. doi: 10.1126/science.290.5493.966. [DOI] [PubMed] [Google Scholar]
- 56.Ruddiman WF. The Anthropocene. Annual Review of Earth and Planetary Sciences. 2013;41:45–68. doi: 10.1146/annurev-earth-050212-123944. [DOI] [Google Scholar]
- 57.Ruddiman WF, Guo Z, Zhou X, Wu H, Yu Y. Early rice farming and anomalous methane trends. Quaternary Science Reviews. 2008;27:1291–1295. doi: 10.1016/j.quascirev.2008.03.007. [DOI] [Google Scholar]
- 58.Charles M, Hoppé C, Jones G, Bogaard A, Hodgson JG. Using weed functional attributes for the identification of irrigation regimes in Jordan. Journal of Archaeological Science. 2003;30:1429–1441. doi: 10.1016/S0305-4403(03)00038-4. [DOI] [Google Scholar]
- 59.Jones G, Charles M, Bogaard A, Hodgson J. Crops and weeds: the role of weed functional ecology in the identification of crop husbandry methods. Journal of Archaeological Science. 2010;37:70–77. doi: 10.1016/j.jas.2009.08.017. [DOI] [Google Scholar]
- 60.Kreuz A, Schäfer E. Weed finds as indicators for the cultivation regime of the early Neolithic Bandkeramik culture? Vegetation History and Archaeobotany. 2011;20:333. doi: 10.1007/s00334-011-0294-2. [DOI] [Google Scholar]
- 61.Fuller DQ, Qin L. Water management and labour in the origins and dispersal of Asian rice. World Archaeology. 2009;41:88–111. doi: 10.1080/00438240802668321. [DOI] [Google Scholar]
- 62.Weisskopf, A. A wet and dry story: distinguishing rice and millet arable systems using phytoliths. Vegetation History and Archaeobotany, 1–11 (2016).
- 63.Weisskopf A, et al. Archaeobotanical implications of phytolith assemblages from cultivated rice systems, wild rice stands and macro-regional patterns. Journal of Archaeological Science. 2014;51:43–53. doi: 10.1016/j.jas.2013.04.026. [DOI] [Google Scholar]
- 64.Weisskopf A, et al. Phytoliths and rice: from wet to dry and back again in the Neolithic Lower Yangtze. Antiquity. 2015;89:1051–1063. doi: 10.15184/aqy.2015.94. [DOI] [Google Scholar]
- 65.Lu H-Y, et al. Phytoliths as quantitative indicators for the reconstruction of past environmental conditions in China I: phytolith-based transfer functions. Quaternary Science Reviews. 2006;25:945–959. doi: 10.1016/j.quascirev.2005.07.014. [DOI] [Google Scholar]
- 66.Lu HY, Wu NQ, Liu KB, Jiang H, Liu TS. Phytoliths as quantitative indicators for the reconstruction of past environmental conditions in China II: palaeoenvironmental reconstruction in the Loess Plateau. Quaternary Science Reviews. 2007;26:759–772. doi: 10.1016/j.quascirev.2006.10.006. [DOI] [Google Scholar]
- 67.Ge, Y. et al. Phytolith analysis for the identification of barnyard millet (Echinochloa sp.) and its implications. Archaeological and Anthropological Sciences, 1–13(2016).
- 68.Zhang, J. P., Lu, H. Y., Wu, N. Q., Yang, X. Y. & Diao, X. M. Phytolith Analysis for Differentiating between Foxtail Millet (Setaria italica) and Green Foxtail (Setaria viridis). Plos One6, 10.1371/journal.pone.0019726 (2011). [DOI] [PMC free article] [PubMed]
- 69.Ball TB, Ehlers R, Standing MD. Review of typologic and morphometric analysis of phytoliths produced by wheat and barley. Breeding Science. 2009;59:505–512. doi: 10.1270/jsbbs.59.505. [DOI] [Google Scholar]
- 70.Lu, H. Y. et al. Phytoliths Analysis for the Discrimination of Foxtail Millet (Setaria italica) and Common Millet (Panicum miliaceum). Plos One4, 10.1371/journal.pone.0004448 (2009). [DOI] [PMC free article] [PubMed]
- 71.Piperno DR. A Comparison and Differentiation of Phytoliths from Maize and Wild Grasses: Use of Morphological Criteria. American Antiquity. 1984;49:361–383. doi: 10.2307/280024. [DOI] [Google Scholar]
- 72.Zhang J, et al. Phytolith evidence for rice cultivation and spread in Mid-Late Neolithic archaeological sites in central North China. Boreas. 2010;39:592–602. [Google Scholar]
- 73.Tsartsidou G, et al. The phytolith archaeological record: strengths and weaknesses evaluated based on a quantitative modern reference collection from Greece. Journal of Archaeological Science. 2007;34:1262–1275. doi: 10.1016/j.jas.2006.10.017. [DOI] [Google Scholar]
- 74.Lu H, et al. On the meaning of phytolith and its classification in gramineae. Acta Micropalaeontologica Sinica. 2002;04:389–396. [Google Scholar]
- 75.Liu K-B, Lam NS-N. Paleovegetational Reconstruction Based on Modern and Fossil Pollen Data: An Application of Discriminant Analysis. Annals of the Association of American Geographers. 1985;75:115–130. doi: 10.1111/j.1467-8306.1985.tb00062.x. [DOI] [Google Scholar]
- 76.Li Q, Ge Q, Tong G. Modern pollen-vegetation relationship based on discriminant analysis across an altitudinal transect on Gongga Mountain, eastern Tibetan Plateau. Chinese Science Bulletin. 2012;57:4600–4608. doi: 10.1007/s11434-012-5236-6. [DOI] [Google Scholar]
- 77.Lu HY, Liu KB. Phytolith assemblages as indicators of coastal environmental changes and hurricane overwash deposition. Holocene. 2005;15:965–972. doi: 10.1191/0959683605hl870ra. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.