Abstract
The aim of this study was to investigate the effects of multiple cold-water immersions (CWIs) on muscle function, markers of muscle damage, systemic inflammation and ECM degradation following exercise-induced muscle damage (EIMD). Thirty physically active males were randomly assigned to either a control (n = 15) or cold-water immersion (CWI) group (n = 15). The CWI group performed one immersion (10 °C for 20 min) at post-exercise and every 24 h for the following 72 h, while the control group remained in a seated position during these corresponding periods. Muscle strength, vertical jump height, muscle thickness, delayed-onset muscle soreness (DOMS), systemic creatine kinase (CK), C-reactive protein (CRP), inflammatory cytokines and matrix metalloproteinase-2 (MMP-2) activity were assessed at Pre, Post, 24, 48, 72, 96 and 168 h following EIMD. No significant time × group interaction was obtained for muscle strength, vertical jump height recovery and MMP-2 activity (p > 0.05). At 24 h, muscle thickness from the CWI group returned to baseline and was lower than the control (p = 0.04). DOMS returned to baseline at 168 h for the CWI group (p = 0.109) but not for the control (p = 0.008). At 168 h, CK showed a time-group difference with a greater peak for the control group (p = 0.016). In conclusion, multiple CWIs attenuated muscle damage, but not altered systemic inflammation and muscle function recovery.
Introduction
The long-lasting impairment in muscle performance subsequent to unaccustomed and/or eccentric exercises has been referred as exercise-induced muscle damage (EIMD)1. EIMD causes an increase in inflammatory markers in the blood, associated with an increase of edema and delayed onset muscle soreness (DOMS), as well as a prolonged impairment in muscle strength, and range of motion2. Therefore, different strategies of muscle recovery have been employed to minimize EIMD. One strategy widely used today in both clinical settings and sports activities is cryotherapy using cold-water immersion (CWI)3 at temperatures lower than 15 °C4.
Although the mechanisms related to the benefits of CWI are not completely understood, it has been suggested that the decrease in muscle temperature causes a reduction in the metabolic rate, reactive oxygen species (ROS) production, and the inflammatory process, which may minimize secondary muscle damage5. In addition, alterations in intramuscular blood and lymphatic flow may occur partly because of cold-induced vasoconstriction and/or hydrostatic pressure4. It has been reported that the magnitude of the potential physiological changes related to CWI depends on the water temperature6–8, immersion duration, and frequency3,9. However, despite the widespread use of CWI, the optimal protocol to elicit the required physiological response remains unknown3,9,10.
The use of cryotherapy has been recommended for the first 72 h after muscle damage11. The theoretical hypothesis that underpins this recommendation is related to its intensive treatment during the destruction phase of muscle regeneration12. The destruction phase, lasting 0–72 h, is characterized by cell membrane rupture, myofiber death, muscle edema and the inflammatory process13. To our knowledge, few studies14–17 have explored the effects of multiple CWIs during the first 72 h after a single bout of EIMD on acute recovery and muscle function. Some of these studies demonstrated that this recovery strategy reduces subjective ratings of DOMS16,17 and perceived exertion17, attenuates indirect markers of muscle damage14,16, and accelerates the recovery of functional muscle performance16,17. However, there is conflicting evidence from previous studies and there is no clear consensus supporting the use of multiple CWIs administration in humans.
We have previously demonstrated in an animal model that multiple administrations of cryotherapy during the destruction phase can reduce the inflammatory process through a decrease in macrophage infiltration18. In the same study, cryotherapy also reduced the release of messenger RNA levels of tumor necrosis factor alpha (TNF-α), and extracellular matrix (ECM) degradation markers (matrix metalloproteinase-9, MMP-9)18. Siqueira and coauthors19 also reported that multiple cryotherapy administrations relieved the production of ROS after muscle injury. Since the balance of pro- versus anti-inflammatory cytokines plays a key role in muscle regeneration by affecting the activation of satellite cells and matrix metalloproteinases (MMPs), a clear understanding of the effects of multiple CWIs during the first 72 h following EIMD on the inflammatory process is important20–23.
Despite these promissory results and the widespread use of cryotherapy after muscle damage/injury in clinical practice, no study has addressed the effects of multiple CWIs on inflammation and ECM debradation markers. In addition, the results related to muscle function recovery remain to be determined in humans. Thus, we aimed to investigate the effects of multiple CWIs during the first 72 h following a single bout of EIMD on muscle function (i.e., muscle strength, and vertical jump height), markers of muscle damage (muscle thickness, DOMS and creatine kinase), systemic inflammation (C-reactive protein, and cytokine kinetics), and ECM degradation. Our hypothesis was that multiple CWIs during the first 72 h post-exercise would attenuate muscle damage and the inflammatory response, which would support a quicker recovery of muscle function.
Results
We assessed the eligibility of thirty-two physically active males between December 2013 and August 2014. We excluded two individuals that had a thigh skinfold greater than 20 mm. Thirty individuals participated voluntarily and were allocated into the (1) control or (2) CWI group. However, one participant from the CWI group was excluded 48 h after exercise due to the manifestation of rhabdomyolysis diagnosed by the medical staff. Physical characteristics of each experimental group are shown in Table 1; there were no significant differences between groups at baseline (p > 0.05). The analysis of primary and secondary outcomes included all twenty-nine participants that finished the study.
Table 1.
Age, body mass, height, body mass index (BMI), thigh skinfold, baseline knee extensors peak torque and countermovement jump (CMJ) height from subjects of each experimental group.
| Physical characteristic | Control group (n = 15) | CWI group (n = 14) | p value |
|---|---|---|---|
| Age (years) | 19.9 ± 1.4 | 20.5 ± 1.4 | 0.284 |
| Body mass (kg) | 71.3 ± 9.4 | 71.3 ± 9.1 | 0.997 |
| Height (cm) | 175.7 ± 7.2 | 176.3 ± 5.0 | 0.791 |
| BMI (kg/m²) | 23.0 ± 2.0 | 22.4 ± 2.8 | 0.496 |
| Thigh skinfold (mm) | 14.3 ± 5.8 | 13.6 ± 4.3 | 0.748 |
| Peak torque (N·m) | 333.5 ± 52.0 | 327.9 ± 47.1 | 0.770 |
| CMJ height (cm) | 46.6 ± 5.1 | 49.6 ± 8.1 | 0.297 |
Data are expressed as mean ± SD. The differences between groups were analyzed using Student’s test. No difference was observed in the physical characteristics between groups (p > 0.05). CWI: cold-water immersion.
Muscle function
Muscle Strength
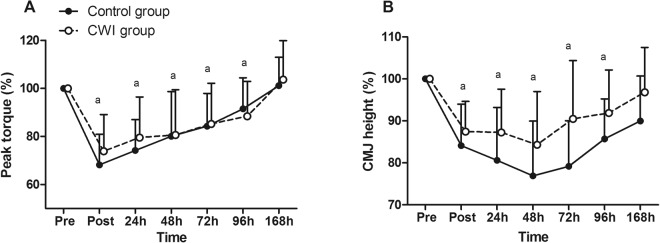
No significant time × group interaction was obtained for muscle strength [F = 0.778, p = 0.588, ηρ2 = 0.027, power = 0.303]. Nevertheless, a significant main time effect was observed [F = 44.305, p < 0.001, ηρ2 = 0.613, power = 0.999]. The lowest values of muscle strength were obtained immediately post-exercise (Fig. 1A). Muscle strength returned to pre-values only at 168 h in both groups (p = 1.0).
Figure 1.
Changes in muscle function following exercise-induced muscle damage. (A) Knee extensor peak torque; and (B) counter-movement jump (CMJ) height. Data are expressed as mean ± SD. The differences within and between groups were analyzed by the two-way mixed-model ANOVA. aDifference when compared to pre-value whatever the group. CWI: cold-water immersion.
Vertical jump height
Vertical jump height showed no significant time × group interaction [F = 1.220, p = 0.301, ηρ2 = 0.058, power = 0.465]. However, a significant main time effect was obtained [F = 17.116, p < 0.001, ηρ2 = 0.461, power = 0.999]. Vertical jump height declined immediately post-exercise with the lowest values at 48 h (Fig. 1B). Finally, the vertical jump height returned to pre-values at 168 h in both groups (p = 0.188).
Markers of muscle damage
Muscle thickness
Muscle thickness showed a significant time × group interaction [F = 2.204, p = 0.04, ηρ2 = 0.109, power = 0.758] with a significant difference between groups at 24 h [F = 6.089, p = 0.024, ηρ2 = 0.253, power = 0.646]. A significant main time effect was also observed [F = 12.024, p < 0.001, ηρ2 = 0.400, power = 0.999]. The immediate increase in muscle thickness was similar in both groups, however, each group recovered differently over time (Fig. 2). Muscle thickness returned toward pre-values at 24 h in the CWI group (p = 0.900) and only at 168 h in the control group (p = 0.900).
Figure 2.
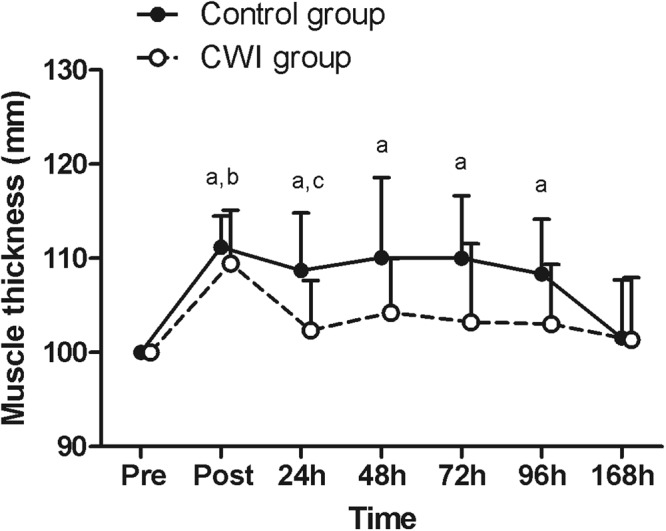
Changes in muscle thickness following exercise-induced muscle damage. Data are expressed as mean ± SD. The differences within and between groups were analyzed by the two-way mixed-model ANOVA and Tukey post-hoc test. aDifference when compared to pre-value for the control group; bdifference when compared to pre-value for the CWI group; cdifference between control and CWI groups. CWI: cold-water immersion.
Delayed-onset muscle soreness
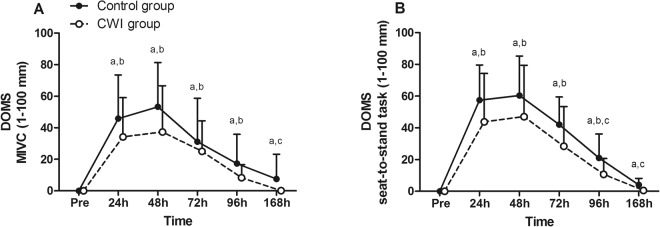
Time-group differences were found in both the maximum isometric voluntary contraction (MIVC) and the seat-to-stand assessment of DOMS: at 168 h (U = 30.5, p = 0.009, d = 0.678, power = 0.190) during MIVC (Fig. 3A); and at 96 h (U = 41, p = 0.046, d = 0.717, power = 0.468) and 168 h (U = 33, p = 0.014, d = 1.131, power = 0.626) during the seat-to-stand task (Fig. 3B). The CWI and control groups rated peak DOMS at 48 h post-exercise during both indices. The CWI group returned to pre-exercise values at 168 h (W = 1, p = 0.317, d = 0.318, power = 0.586 in MIVC; and W = 6, p = 0.109, d = 0.339, power = 0.368 in seat-to-stand task), whereas the control group did not recover over the investigated time period (W = 45, p = 0.008, d = 0.485, power = 0.163 in MIVC; and W = 45, p = 0.008, d = 1.017, power = 0.143 in seat-to-stand task).
Figure 3.
Changes in delayed-onset muscle soreness (DOMS) following exercise-induced muscle damage, during: (A) maximal voluntary isometric contraction (MIVC); (B) seat-to-stand task. Data are expressed as mean ± SD. The differences between groups were analyzed using a Mann-Whitney test and the differences in time for each recovery modality using the Wilcoxon test. aDifference when compared to pre-value for the control group; bdifference when compared to pre-value for the CWI group; cdifference between control and CWI groups. CWI: cold-water immersion.
Biochemical markers of muscle damage, systemic inflammation and ECM degradation
There was no difference in the pre-values of any investigated biochemical markers between both experimental groups (p > 0.05, Table 2).
Table 2.
Changes in biochemical markers following exercise-induced muscle damage.
| Biochemical markers | Group | Time | ||||
|---|---|---|---|---|---|---|
| Pre | 24 h | 48 h | 72 h | 168 h | ||
| CK (U/L) | Control | 84.1 ± 31.3 | 313.5 ± 169.4a | 231.8 ± 128.0a | 840.4 ± 1707.1a | 2932.0 ± 6446.9a,c |
| CWI | 86.4 ± 35.8 | 630.5 ± 1058.6b | 216.3 ± 117.5b | 127.5 ± 65.5 | 113.2 ± 61.1 | |
| CRP (mg/L) | Control | 0.4 ± 0.4 | 0.8 ± 1.0 | 0.5 ± 1.1 | 0.3 ± 0.6 | 0.5 ± 0.8 |
| CWI | 0.4 ± 0.5 | 1.2 ± 1.3b | 0.5 ± 0.5 | 0.2 ± 0.2 | 0.4 ± 0.7 | |
| IL-6 (pg/mL) | Control | 4.0 ± 4.9 | 5.8 ± 11.6 | 4.3 ± 8.9 | 1.0 ± 0.1 | 1.1 ± 0.4 |
| CWI | 4.1 ± 10.4 | 5.9 ± 16.2 | 3.7 ± 8.8 | 10.9 ± 22.0 | 3.5 ± 6.9 | |
| TNF-α (pg/mL) | Control | 20.3 ± 35.0 | 43.4 ± 60.2a | 52.2 ± 106.4 | 13.8 ± 24.4 | 23.9 ± 31.6 |
| CWI | 9.3 ± 19.1 | 30.3 ± 54.4 | 32.9 ± 334.6 | 7.8 ± 16.0 | 7.6 ± 13.9 | |
| IL-1α (pg/mL) | Control | 25.4 ± 84.1 | 64.7 ± 147.5 | 36.6 ± 68.1 | 86.6 ± 142.7 | 100.7 ± 187.8 |
| CWI | 23.4 ± 46.0 | 31.6 ± 68.1 | 31.5 ± 73.6 | 75.8 ± 120.3b | 44.8 ± 91.0 | |
| IL-10 (pg/mL) | Control | 129.4 ± 229.3 | 229.2 ± 555.9 | 505.7 ± 811.7 | 136.9 ± 252.9 | 164.1 ± 238.9 |
| CWI | 39.5 ± 99.7 | 98.9 ± 178.3 | 85.5 ± 249.5 | 436.4 ± 1203.7 | 62.4 ± 145.6 | |
Data are expressed as mean ± SD. The differences between groups were analyzed by a Mann-Whitney test and the differences in time for each recovery modality by Wilcoxon test. aDifference when compared to pre-value of the control group; bdifference when compared to pre-value of the CWI group; cdifference between control and CWI groups. CK: creatine kinase; CRP: C-reactive protein; IL-6: interleukin-6; TNF-α: tumor necrosis factor alpha; IL-1α: interleukin-1 alpha; IL-10: interleukin-10; CWI: cold-water immersion.
Creatine kinase
Both experimental groups showed significant increases in CK activity at 24 h. The CWI group returned to pre-values at 72 h (W = 34.5, p = 0.155, d = 0.500, power = 0.547), remaining stable for all subsequent measurements (Table 2). However, the control group did not return to pre-values at any investigated time period (W = 63, p = 0.008, d = 0.442, power = 0.091 at 168 h). There was a time-group difference in CK activity at 168 h (U = 18, p = 0.016, d = 0.618, power = 0.162), as the control group interestingly reached peak CK activity.
Markers of systemic inflammation
Changes in markers of systemic inflammation are presented in Table 2. There was a significant increase in CRP levels at 24 h post-exercise in the CWI group (W = 52, p = 0.013, d = 0.430, power = 0.267). In regards to IL-6 and IL-10 levels, there was no difference between groups at any time point (p > 0.05) and no time effect (p > 0.05) for both control and CWI groups. TNF-α levels significantly increased at 24 h post-exercise in the control group (W = 28, p = 0.018, d = 0.334, power = 0.104) and IL-1α levels at 72 h in the CWI group (W = 15, p = 0.043, d = 0.413, power = 0.304).
Active MMP-2
MMP-2 activity showed no significant time × group interaction [F = 1.139, p = 0.356, ηρ2 = 0.125, power = 0.316] and no main time effect following EIMD [F = 1.415, p = 0.251, ηρ2 = 0.150, power = 0.389] (Table 3).
Table 3.
Changes in MMP-2 activity following exercise-induced muscle damage.
| Peak area | Group | Time | ||||
|---|---|---|---|---|---|---|
| Pre | 24 h | 48 h | 72 h | 168 h | ||
| Active MMP-2 (p.d.u.) | Control | 19,449 ± 4,767 | 21,774 ± 5,021 | 17,614 ± 8,067 | 19,963 ± 3,809 | 22,188 ± 4,733 |
| CWI | 16,753 ± 9,275 | 21,988 ± 8,257 | 19,511 ± 8,431 | 19,420 ± 5,392 | 17,340 ± 5,877 | |
Data are expressed as mean ± SD. The differences within and between groups were analyzed using the two-way mixed-model ANOVA. MMP-2: matrix metalloproteinase-2; p.d.u.: procedure defined unit; CWI: cold-water immersion. No difference was observed in the MMP-2 activity between groups (p > 0.05).
Skin temperature, thermal sensation and thermal comfort
Skin temperature was similar between the control and CWI groups prior to the recovery procedures (control: 32.0 ± 1.7 °C vs CWI: 32.5 ± 1.4 °C, U = 95, p = 0.910, d = 0.316, power = 0.944). Throughout the 20 min recovery period, no significant skin temperature change was observed in the control group (p > 0.05, for all time-points). On the other hand, the CWI group had a significant decrease in skin temperature after the first 5 min of immersion (13.1 ± 2.4 °C, W = 7, p = 0.004, d = 6.070, power = 1.000). Over the remaining period of immersion, the skin temperature gradually reduced (10 min: 15.1 ± 1.3 °C; 15 min: 12.2 ± 1.7 °C; 20 min: 12.0 ± 1.5 °C), keeping relatively constant until the end and showing no difference between the time-points during the cooling procedures (p > 0.05).
Prior to recovery procedures, the median rates of thermal sensation were not different between both experimental groups (U = 95, p = 0.683, d = 0.190, power = 0.733). Participants from both groups rated their thermal sensation as “slightly warm”. However, the rates of thermal sensation were different between groups after 5 min of the recovery procedures (U = 5, p < 0.001, d = 2.752, power = 0.914). After 5 min into the cooling procedures, participants from the CWI group reported a “cool” sensation (W = 0, p = 0.001, d = 1.850, power = 0.321). Then, from 5 min until the end of the cooling procedures, they reported a “slightly cool” sensation (p = 0.001, for all time-points). Those from the control group reported a “neutral” sensation at 10 min and until the end of the recovery period (p < 0.05).
In regard to thermal comfort, there was no difference between groups prior to the recovery procedures, as both groups rated it as “comfortable” (U = 98, p = 1.000, d = 0, power = 1.000). After 5 min into the recovery procedures, a difference in thermal comfort rates between groups was observed (U = 182, p < 0.001, d = 2.264, power = 0.962). Compared to the baseline, the CWI group felt “slightly uncomfortable” during the cooling procedures (p = 0.002, for all time-points) while the control group continued feeling “comfortable” during the entire recovery period (p > 0.05).
Discussion
The current study was conducted to determine the effects of a multiple CWIs recovery strategy on muscle function, markers of muscle damage, systemic inflammation and ECM degradation. The initial hypothesis was confirmed partially. Multiple CWIs during the first 72 h attenuated muscle damage by an early reduction of muscle thickness, and a delayed reduction of DOMS and CK activity. However, the recovery of maximal muscle strength, vertical jump height, systemic cytokine kinetics and ECM degradation did not differ between control and CWI groups. Our findings may help sports medicine professionals to better understand the effects of multiple CWI administrations on recovery following muscle-damaging exercise.
The present study demonstrated that four CWI administrations were not effective to accelerate the recovery of muscle function. Previous studies investigating the effectiveness of multiple CWIs during the first 72 h following a single bout of EIMD have reported conflicting results14–17. Discrepancies between the findings may be attributed to the CWI protocol (i.e. water temperature and duration). In an attempt to investigate the effects of different water temperatures during a multiple-CWI protocol, Machado and coauthors17 found that four immersions with durations of 15 min at 14 °C was more effective when compared to 9 °C. Moreover, Vaile and coauthors16 found that four immersions with durations of 14 min at 15 °C improved muscle function recovery. Therefore, a less harsh CWI temperature might optimize muscle function recovery7,17. Secondly, in the present study, CWI was applied with a longer duration (20 min) in contrast to the above mentioned studies (14 min16 and 15 min17). Actually, Peiffer and coauthors24 demonstrated that the magnitude of change in tissue temperature was associated with a longer CWI duration. Furthermore, it has been reported that an excessive tissue cooling could exacerbate the inflammatory response, which in consequence could even impair the recovery following EIMD6.
As expected, an increase in muscle thickness was observed immediately post-exercise in both groups. This parameter provides a measure of muscle swelling as a consequence of muscular and connective tissue damage, increased vessel permeability, or the combination of these factors2. We observed different muscle thickness kinetics between groups with the control group returning toward pre-values at 168 h, whereas the CWI group returned at 24 h post-exercise. CWI may have potentially reduced lymphatic and capillary cell permeability through peripheral vasoconstriction induced by low temperatures25 and/or the effect of hydrostatic pressure4. A decrease in fluid diffusion might assist in the reduction of the pro-inflammatory cell infiltration and edema formation5. In addition, time-course differences between CWI and control groups could be associated to the capacity of cryotherapy to decrease cellular metabolism and reduce pro-inflammatory cytokines and ROS release, which may minimize secondary muscle damage and preserve myofibers and other local muscle structures19.
Systematic reviews and meta-analyses have suggested that, for management of DOMS, CWI is better than conditions involving rest or no intervention3,8–10. Accordingly, the present study showed lower rates of DOMS in the CWI group compared to the control group at 168 h. This prolonged effect of DOMS is in agreement with a previous study that administered the same damaging procedure, even though a single bout of CWI showed no significant effect on DOMS7. Although, there is an inconsistency between the time of peak serum CK activity and peak DOMS, the longer presence of DOMS can be partially explained by the magnitude of muscle damage26. Among studies that investigated multiple CWIs effects15–17, only one17 has shown beneficial results, particularly, on soreness ratings immediately post CWI and 40 min post-exercise. Short-term analgesia after cryotherapy has been associated with reductions in the neural conductance velocity of sensory and motors neurons, which limits pain and reflexive spasms, respectively27. Beyond the CWI-invoked physiological changes already mentioned, long term analgesia might be ascribed to alterations in intracellular-intravascular fluid shifts which favor nutrition and waste transportation28 as well as attenuation of muscle edema, which prevents nerve compression12. Moreover, lower levels of pro-inflammatory cytokines could reduce rates of DOMS, since nociceptor activity in muscle tissues might be mediated by IL-1β, IL-6, and TNF-α29.
In accordance with previous studies, we observed that multiple CWIs were efficient in reducing CK activity following EIMD14,16. However, the studies showed differences on the time-points of reduction in CK activity, which might be due to the fatiguing exercise, the training level of the participants, as well as the CWI protocol. For instance, Eston and Peters14 observed a reduction in CK activity at 48 h and 72 h following a bout of eccentric exercise on the elbow flexors of the dominant arm and seven immersions at 15 °C for 15 min. Vaile and coauthors16 found an earlier reduction in CK activity, at 24 h and 72 h following an eccentric bi-lateral leg press protocol and four immersions at 15 °C with for 14 min. On the other hand, the present study demonstrated a later effectiveness on reduction in CK activity following a drop jump protocol and four immersions at 10 °C for 20 min, once the CK activity reduced only at 168 h. Indeed, the upper limbs muscles are more susceptible to muscle damage then lower limbs30. Moreover, the untrained participants of the present study were potentially more susceptible to muscle damage than the trained participants2 from the Vaile and coauthors16 study. Interestingly, the control group of the present study demonstrated a biphasic pattern of CK activity with a peak at 24 h post-exercise and a greater second peak at 168 h. Previous studies7,31 that used a similar protocol to induce muscle damage also found this biphasic pattern. This may be related to CK being leaked from the interstitial fluid and conducted to the lymphatic system before reaching the blood stream2. In addition, the time-course of CK may be related to the large inter-subject variability of CK release and clearance which can be influenced by many factors such as ethnicity, genetic factors, and ability to generate energy32.
An adequate balance between pro-inflammatory (such as TNF-α, IL-1α) and anti-inflammatory (such as IL-10) cytokines is important for muscle and surrounding connective tissue regeneration20. Furthermore, the magnitude of the inflammatory response affects muscle function23. In general, the ambiguous findings of inflammatory cytokines in the present study do not suggest that our multiple CWIs protocol would be more effective in reducing inflammatory responses than passive recovery. The results presented here showed that the CWI group had a significant increase in CRP levels at 24 h. In general, CRP is associated with the inflammatory response, because it would attract macrophages to the damaged tissue, among other roles33. Our results disagree with a recent meta-analysis that found differences in CRP levels favoring cooling at 48 h after EIMD9. Nevertheless, we must stress the heterogeneity of cooling modalities among the studies included in this meta-analysis9. Furthermore, the authors highlighted that this result may not represent the true effect of cryotherapy, since the few studies which measured CRP levels had only small sample sizes, and in the presence of one more inflammatory markers, no statistical difference between groups were found9.
IL-6 has been termed an “inflammation-responsive” myokine, since IL-6 is responsive to the mechanical loading associated with exercise, suggesting that it exerts a pro-inflammatory effect20; and on the other hand, IL-6 inhibits TNF-α production and enhances IL-10 production, suggesting that it plays a part in the anti-inflammatory effect20. White and coauthors6 noticed a relationship between long time periods (30 min) of a single bout of CWI protocols and significant increases in IL-6 levels immediately post and 2 h post immersion. The authors hypothesized that a longer duration of a single bout of CWI could lead multiple cell types, not only muscle cells, to produce IL-6, resulting in a sustained highly inflammatory process within 2 h post-exercise6. Previous studies showed that systemic concentrations of IL-6 usually return to baseline within 24 h post-exercise6,34,35. According to our data and others16, it remains unclear whether multiple CWIs could exacerbate IL-6 concentrations, since blood samples were not collected within 24 h post-exercise and immediately after each cooling procedure either. Moreover, a study into multiple whole body cryotherapy versus passive recovery, analyzing inflammatory markers within 24 h post-exercise, indicated no differences in IL-6 and IL-10 levels but revealed a significant increase in CRP and IL-1 receptor alpha and suppression of IL-1β in favor of the cryotherapy group36.
At 24 h post-exercise, the control group showed an increase in TNF-α levels. In fact, TNF-α mediates macrophage activation (to M1 phenotype), which in turn produces potent pro-inflammatory mediators, which together could start a breakdown of the damaged muscle and induce local edema37. Interestingly, it is possible to observe that changes in some biochemical markers (TNF-α and CK levels) during the early inflammatory phase coupled with long lasting edema and muscle performance impairment in the control group. The IL-1α increase observed in the CWI group at 72 h corroborates the hypothesis that reductions in muscle temperature by cryotherapy could delay the inflammatory cascade38. Muscle damage can cause the cell membrane to rupture and allow calcium ions to invade damaged tissue and activate calpain. Calpain performs the catalysis of the IL-1α precursor, making this molecule active39. Therefore, multiple cryotherapy administrations might have delayed IL-1α activation, until it was observed to peak in the blood stream at 72 h in the CWI group. Takagi and coauthors38 described that this delay in the inflammatory cascade after cryotherapy might be associated with an excessive deposition of collagen in damaged tissue. However, as the MMP-2 activity assessed in the present study was not changed even by exercise, it was not possible to suggest that collagen deposition was altered by CWI during muscle regeneration. Likewise, Tayebjee and coauthors40 found no alteration in MMP-2 following treadmill exercise testing. However, it was observed, even without any intervention, that there were participants showing high levels of MMP-2, in contrast to others being undetectable40. The authors highlighted the importance to elucidate the categorical distribution of MMP-2 concentration which remains unclear to date40. Interestingly, a study using an animal model showed that only high-intensity exercise promoted an increase in MMP-2 expression and mainly in muscles composed predominantly of fast fibers41. Most likely, these incongruent findings of MMP-2 following exercise also indicate the differences between intramuscular versus systemic analyses, as ECM degradation and remodeling have been explored using both plasma and muscle sample analyses40,41.
All recovery strategies, including CWI, have been challenged by the placebo effect42. Due to the popularity of CWI for recovery, it can be assumed that most participant had heard about CWI’s purported advantages, leading to a confounding influence on the assessments10. It has been demonstrated that the placebo effect not only influences subjective measures, but also muscle performance43. Broatch and coauthors34 performed a study that supported, at least in part, the placebo effect on the beneficial effects attributed to CWI. The experimental procedure made participants to believe that a liquid added to the thermoneutral water immersion was advantageous to muscle recovery34. Surprisingly, the placebo strategy was as effective as CWI on muscle strength recovery and ratings of readiness for exercise, pain, and vigor34. Whether the placebo effect affected the data from studies on CWI is an important issue that remains to be elucidated. Moreover, as previously observed44, subjective feeling during and after recovery modalities is of paramount importance. Even if no effect on performance was obtained, CWI could be perceived as effective. This feeling could bolster individuals’ positive attitudes toward subsequent exercises.
It is important to highlight the limitations in the present study. Firstly, we only focused on the use of multiple CWIs in young and healthy individuals. Although, the majority of participants in studies on CWI for preventing and treating DOMS were untrained, trained athletes are more likely to use CWI regularly3. Therefore, additional comparative studies are required investigating this population. Secondly, in order to determine regeneration processes, additional measurements would have been interesting. For example, muscle biopsies45 and intramuscular temperature46 measurements would have provided greater insight of local changes. Nonetheless, multiple immersions could increase the risk of contamination of the wound due to the invasive procedures. Also, exploring neuromuscular function using evoked contractile properties, voluntary activation measurements, and motoneuronal excitability would have helped to clarify the real physiological effects of CWI rather than any placebo effect. Thirdly, the addition of a single CWI group would have been interesting to determine whether a beneficial or a harmful magnitude effect exists between single and multiple immersions, especially regarding systemic inflammation and ECM degradation. Finally, although MMP-9 activity was detected in the zymography gels, MMP-9 was not measured because of the interference by coagulation/fibrinolytic pathways that may have increased the MMP-9 content in serum47.
In summary, multiple CWIs were effective to attenuate indirect markers of muscle damage, such as DOMS, muscle thickness, and CK activity. However, this recovery strategy appears to be ineffective on systemic inflammation and ECM degradation markers and muscle function recovery. Thus, the use of multiple CWIs could be recommended as a strategy that may reduce muscle damage following exercises, but without the expectative to enhance recovery between training sessions or competitive events.
Methods
Study design
Thirty physically active males voluntarily participated in this parallel randomized controlled trial study and were allocated into one of the two parallel groups: (1) control group or (2) CWI group. The cross-over design was not applied to avoid the influence of the repeated bout effect (faster recovery of muscle function after a second bout of similar eccentric-type exercise) on the magnitude of muscle damage between conditions31,48. This study was conducted according to the Declaration of Helsinki and approval for the project was obtained from the local ethics committee (University of Brasília Research Ethics Committee, Brasília, Brazil, protocol number 243/13). The trial was retrospectively registered at the US National Institutes of Health (ClinicalTrials.gov, on 16/12/2014, protocol number NCT02341612).
Study population
Study inclusion required participants to be physically active males, practicing mild to moderate intensity aerobic activities (e.g. running and cycling) and/or recreational sports (such as soccer), 2–3 times per week. Participants were excluded if they: (1) had participated in regular strength training or intensive plyometric exercise during the last 3 months; (2) answered “yes” to any Physical-Activity Readiness Questionnaire (PAR-Q) questions49; (3) had any inflammatory disease or had taken any anti-inflammatory medications during the last 4 weeks; (4) had history of adverse reactions to cold temperatures; (5) had a thigh skinfold greater than 20 mm, as the amount of adipose tissue affects intramuscular cooling50; (6) had knee extensor torque less than 185 Nm, in order to achieve paired values between participants. Participants were instructed to maintain their usual hydration regimen; not to consume stimulants (e.g. alcohol, caffeine, chocolate) and anti-inflammatory medications; and not to exercise during their participation in the experiment. To avoid circadian influences, participants were asked to visit the Strength Laboratory of Physical Education Faculty at the same time of day each day, between 1 and 4 pm. Once informed of the purpose, procedures, discomforts, risks, and benefits, each participant signed an informed consent form.
Sample size was calculated based on the knee extensor peak torque data (considered the study’s primary outcome) that was reported in a similar study51, using G*Power (version 3.1.9.2; Heinrich Heine University Düsseldorf, Germany). The following design specifications were taken into account: α = 0.05; (1-ß) = 0.8; effect size ƒ = 0.2; test family = F test, and statistical test = analysis of variance (ANOVA) repeated measures, within-between interaction, groups = 2 and measurements = 7. Sample size estimation indicated 20 participants (10 per group). However, we decided to include more participants in order to increase statistical power.
Randomization
Following the participants’ eligibility screening, the randomization scheme was generated using the website Randomization.com.
Study interventions
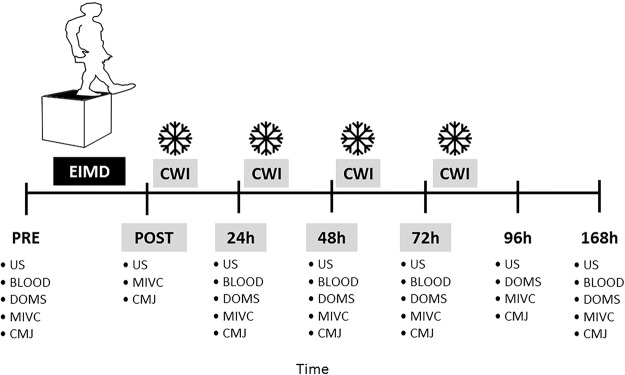
The participants visited the laboratory on seven occasions (Fig. 4). The first visit consisted of: (1) anthropometric measurements; and (2) familiarization with the experimental procedures. At three to seven days after familiarization, participants performed a muscle damaging protocol. In order to investigate the effects of multiple CWIs during the first 72 h following EIMD on muscle function, markers of muscle damage, systemic inflammation, and ECM degradation, were assessed in this sequence: (1) ultrasound assessment; (2) blood collection; (3) DOMS during seat-to-stand task; (4) peak torque and DOMS during MIVC; and (5) CMJ. These assessments were repeated: before exercise (Pre), and immediately (Post), 24, 48, 72, 96 and 168 h post-exercise. DOMS was not measured at Post, as it increases several hours’ post-exercise26. Blood samples were not collected at Post and 96 h, to limit the number of invasive measurements.
Figure 4.
Experimental design. Represented are the times of each assessment procedure and multiple cold-water immersion (CWI) administrations. Maximum isometric voluntary contraction (MIVC), counter-movement jump (CMJ) and ultrasound (US) were measured at pre-exercise (Pre), immediately post (Post), 24 h, 48 h, 72 h, 96 h and 168 h post exercise-induced muscle damage (EIMD). Delayed onset muscle soreness (DOMS) was not measured at Post and blood samples (BLOOD) were not collected at Post and 96 h. Cold-water immersion (CWI) was performed 10 min post-exercise and every 24 h thereafter for the following 3 days after EIMD.
Protocol to induce muscle damage
The exercise protocol consisted of five sets of 20 drop jumps from a 60-cm box with two minutes of rest between sets31. After dropping down from the box and landing on the floor, participants were instructed to perform a maximal explosive vertical jump and then land on the floor. They were instructed to flex their knees to at least 90° (0° = full extension) during all landings and to keep their hands on their hips during the jumps. They were verbally encouraged to exert maximal effort during each jump repetition.
Cold-water immersion and passive recovery
CWI was applied 10 min post-exercise and every 24 h thereafter for the following three days. In total participants completed four bouts of immersion at 10 ± 1 °C for a duration of 20 min each. The duration was chosen based on studies that investigated a similar population7,52. Water temperature was selected based upon the most popular CWI range of 10 to 15 °C3. During the cooling procedure, participants remained seated with knees extended, while immersed up to the iliac crest, ensuring the lower limbs were fully submerged in the water bath. Water temperature was checked every 5 min and was maintained at the target temperature by adding crushed ice when necessary. Participants were instructed to make movements with their legs every 2 min to prevent the formation of the warmer boundary layer of water that forms immediately surrounding the skin. Participants in the control group remained comfortably seated at room temperature (21 ± 1 °C) during the corresponding periods of CWI sessions. After completing their respective interventions, subjects could continue with their regular daily activities.
Study outcomes
Muscle function
Muscle strength: Maximal isometric voluntary knee extensor torque was measured at 60° (0° = full extension), using a commercial dynamometer (Biodex System 3, Biodex Medical, Inc., Shirley, New York, USA). Participants were comfortably positioned on the dynamometer seat with belts fastened across the trunk and pelvis to minimize body movements that could affect torque output53. The lateral epicondyle of the femur was aligned to the dynamometer’s axis and the chair and dynamometer settings for each subject were recorded during the familiarization session and were used throughout the study. Subjects were asked to cross their arms across the chest54 and to maximally contract their right knee extensors for 4 s55. They had two attempts to achieve their maximal isometric torque with 1 min of rest between attempts and received verbal encouragements throughout the tests56. Testing procedures were conducted by the same blinded examiner each time. The greatest torque was retained for further analysis.
Vertical jump height: CMJ height was measured using an AMTI force plate (model BP400600-HF-2000; Advanced Mechanical Technology, Inc., Watertown, MA, USA) with a sampling rate of 1,000 Hz. Subjects were asked to keep their hands on their hips and jump as high as possible. They had three attempts to achieve their best jump performance with 1 min of rest between attempts7. The greatest vertical displacement was considered as the maximal jump height, which was used for further analysis. A self-determined range of motion for the knee was permitted and they received verbal encouragement by the same blinded examiner. Data obtained during vertical jumps were captured from the manufacturer’s software (AMTI Acquisition Software, v 4.2; Advanced Mechanical Technology, Inc.) and processed using a custom MATLAB code (v R2008a7, The MathWorks Inc., Natick, MA, USA). From the ground reaction force, the force-displacement curve was calculated and then integrated to obtain the displacement of the center of mass at each instant of movement57.
Indirect markers of muscle damage
Muscle thickness: All sonograms were acquired by a portable ultrasound device (Philips-VMI, Ultra Vision Flip, Model BF, Minas Gerais, Brazil) equipped with a 7.5 MHz linear array transducer. A standardized protocol (including transducer placement, anatomic landmarks, and subject position) was set by the same examiner. It also included ultrasound settings (e.g. frequency, gain, tissue compression) that were kept constant between subjects and across all time periods, except the depth that was adjusted for each subject to display the entire muscle. Subjects were assessed in the supine position with their knee in maximal extension and neutral rotation. A water-soluble transmission gel was applied to the ultrasound probe to allow acoustic contact without depressing the dermal surface. The anterior images of the anterior thigh were obtained with the transducer placed perpendicular to the long axis of the thigh on its anterior surface, at 60% of the distance from the greater trochanter to the lateral epicondyle and 3 cm lateral to the midline of the anterior thigh58. Once the technician was satisfied with image quality, the image was kept for further analysis59. In order to assure replication of image location on repeated ultrasound assessments, a mark was drawn on the subject’s leg using indelible ink. All image measurements were performed by a blinded examiner in triplicate using Image J Software (NIH, Bethesda, MD, USA). Mean values were used as representative of the thigh muscles (rectus femoris and vastus intermedius) thickness. Muscle thickness, expressed in mm, was defined as the distance from the subcutaneous adipose tissue interface to the muscle-bone interface60.
Delayed-onset muscle soreness: Perceived muscle soreness of quadriceps muscles was assessed using a 100-mm visual analog scale. The scale ranged from “no soreness” (0) to “severe soreness” (100)31. Subjects rated their quadriceps soreness during two situations: (1) sit-to-stand task: three consecutive sit-to-stand movements from a 43-cm chair, which were performed with constant cadence (2 s to sit and 2 s to stand) and (2) MIVC7.
Biochemical markers of muscle damage, systemic inflammation and ECM degradation
Blood samples and biochemical analyses: Approximately 12 mL of blood was collected from the antecubital vein by the standard venipuncture technique using a commercially produced vacuum sealed kit. Tubes were centrifuged (Centrifugal machine, 3250 RPM, Model Centurion, São Paulo, Brazil) at room temperature for 20 min at 2500 rotations per minute (≈1000 × g). Serum was aliquoted (250 µL) and directly stored at −20 °C until analyzed by a blinded examiner. Blood analyses included biochemical markers of: muscle damage by measurements of creatine kinase (CK) activity; systemic inflammation by measurements of C-reactive protein (CRP) levels and cytokine levels, among them interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), interleukin-1 alpha (IL-1α) and interleukin-10 (IL-10); and ECM degradation by measurements of matrix metalloproteinase-2 (MMP-2) activity.
The biochemical analyses were done in duplicate, according to the manufacturers’ protocols by a blinded examiner. All calibration curves displayed linear coefficients (R2) ≥ 0.95 and inter-assay coefficients of variation <5%. CK activity was determined by enzymatic assay using a test kit for total CK (Siemens Medical System, Erlangen, Germany) with a limit of detection of zero (U/L) and linearity of the measurement of 1300 U/L. CRP levels were analyzed by latex particle enhanced immunoturbidimetric assay using a test kit for the width-range C-reactive protein (Siemens Medical Systems, Erlanger, Germany) with a limit of detection of 0.003 mg/L and linearity of the measurement of 156–164 mg/L. Serum levels of IL-6, TNF-α, IL-1α, IL-10 were obtained by commercial test kits from Quantikine® ELISA Human Immunoassay (R&D Systems, Inc., Minneapolis, USA) using an absorbance plate reader (ELx800, BioTek instruments, Inc., Winooski, EUA). Thresholds of detection were experimentally determined at 1.0 pg/mL for IL-6 and IL-10, 1.5 pg/mL for TNF-α and 0.4 pg/mL for IL-1α.
MMP-2 gelatinolytic activity was measured by zymography. Samples containing 0.5 µL of serum were added to 0.5 µL of SDS (8%) (v:v) and subsequently added 10 µL of buffer without β-mercaptoethanol-containing SDS (20%). Samples were resolved by electrophoresis in polyacrylamide gel containing SDS 10% (SDS_PAGE) and gelatin at a final concentration of 1 mg/mL. After electrophoresis, the gels were washed twice for 20 min in 2.5% of Triton X-100 to remove SDS. Gels were incubated in buffer substrate (50 mM Tris-HCl pH 8.0; 5 mM of CaCl2 and 0.02% NaN3) at 37 °C for 20 h. Gels were stained with Coomassie brilliant-blue for 1.5 h and distained with acetic acid: methanol: water (1:4:5) for activity bands visualization. The gelatinolytic activity was visualized as clear bands in the stained gel61. Densitometric semi-quantitative analysis of the MMPs protein bands was performed as previously described by Hu and Beeton62. The analyses were done in triplicate by a single blinded examiner using Image J Software (NIH, Bethesda, MD, USA) and the mean value of peak area were used in the final analysis.
Thermal comfort, thermal sensation, and skin temperature: Subjects were asked to rate their thermal sensation and thermal comfort before and every 5 min throughout the 20 min of each recovery procedure. Thermal sensation was rated on a nine-point Likert-type scale where “−4” = very cold, “−3” = cold, “−2” = cool, “−1” = slightly cool, “0” = neutral, “1” = slightly warm, “2” = warm, “3” = hot, and “4” = very hot. Thermal comfort was rated on a five-point Likert-type scale where “0” = comfortable, “1” = slightly uncomfortable, “2” = uncomfortable, “3” = very uncomfortable, and “4” = extremely uncomfortable63. Every 5 min, subjects were also asked to stand up and gently towel-dry their right thigh to have their skin temperature measured. The anterior thigh temperature was measured in the drawn landmark for ultrasound measurements (above the rectus femoris muscle). An infrared thermometer was used (Fluke, 566, China), which was kept perpendicularly positioned 8 cm from the skin. Over the 4 days, the mean value of skin temperature was calculated at each time-point in each group, as well as the median value of Likert-type scales.
Statistical analysis
Data are described as means and standard deviation, except Likert-type measurement scales used for rates of thermal comfort and thermal sensation that are described as medians. The Shapiro-Wilk test was used to verify data distribution. Peak torque, CMJ height, muscle thickness and MMP-2 activity presented normal distributions. Thereby, they were analyzed using a two-way (group × time) mixed-model ANOVA. In case of significant main effect or interaction (time × group), a Tukey post-hoc test was applied. DOMS, CK, markers of systemic inflammation, skin temperature, and rates of thermal comfort and thermal sensation did not show a normal distribution and were analyzed using nonparametric tests. The Mann-Whitney test was performed to assess differences between groups and the Wilcoxon test was undertaken to evaluate differences in time for each recovery modality. The subjects’ physical characteristics, baseline peak torque and CMJ height were evaluated using an independent t-test. SPSS (Statistical Package for Social Sciences) version 20.0 (IBM, USA) was used for statistical analyses with an alpha level set at 5%. Additionally, effect sizes and statistical power were calculated. Effect sizes from data analyzed by ANOVA were determined using partial eta squared (ηρ2). Cohen64 has provided benchmarks to define small (ηρ2 = 0.01), medium (ηρ2 = 0.06) and large (ηρ2 = 0.14) effects64. To calculate the effect sizes and power from the data analyzed by non-parametric test, it was used the G*Power (version 3.1.9.2; Heinrich Heine University Düsseldorf, Germany). Effect sizes (d) from the data analyzed by non-parametric test were calculated with values of 0.2, 0.5 and above 0.8 considered to represent small, medium and large differences, respectively64. Power were calculated by the asymptotic relative efficiency (A.R.E.) method65.
Acknowledgements
The authors acknowledge the “Conselho Nacional de Desenvolvimento Científico e Tecnológico” that supported the study under Grant number 401453/2013-8, Brazil; and “Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior” for providing PhD scholarship for Amilton Vieira under Process number BEX 5191/14-9, Brazil. We also wish to thank Sabin Laboratory of Clinical Analyses (Brasília, Brazil) that measured CK activity and CRP levels; Andrei Alkmim Teixeira & José Humberto de Souza Borges for the medical assistance during the study; and Derrick Jason Mulder for the English review. Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil, grant number 401453/2013-8 and 310359/2014-7.
Author Contributions
A.F.S., A.V., J.B.F.J., M.B. and J.L.Q.D. were responsible for the study design. A.F.S., A.V. and J.B.F.J. were involved in the experimental procedure and performed data collection. A.F.S., A.V., O.T.N., V.C.S., R.C.M. were involved in data analyses. A.F.S., A.V. and N.B. performed the statistical analysis. And all authors were involved in manuscript preparation and agree with its submission. We declare that this manuscript is not under consideration for publication by any other journal or scientific event.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ebbeling CB, Clarkson PM. Exercise-induced muscle damage and adaptation. Sport. Med. 1989;7:207–234. doi: 10.2165/00007256-198907040-00001. [DOI] [PubMed] [Google Scholar]
- 2.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002;81:S52–S69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 3.Bleakley, C. et al. Cold-water immersion (cryotherapy) for preventing and treating muscle soreness after exercise (Review). Cochrane Database Syst. Rev. CD0088262 (2012). [DOI] [PMC free article] [PubMed]
- 4.Wilcock IM, Cronin JB, Hing WA. Physiological response to water immersion: a method for sport recovery? Sport. Med. 2006;36:747–765. doi: 10.2165/00007256-200636090-00003. [DOI] [PubMed] [Google Scholar]
- 5.White GE, Wells GD. Cold-water immersion and other forms of cryotherapy: physiological changes potentially affecting recovery from high-intensity exercise. Extrem. Physiol. Med. 2013;2:26. doi: 10.1186/2046-7648-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White GE, Rhind SG, Wells GD. The effect of various cold-water immersion protocols on exercise-induced inflammatory response and functional recovery from high-intensity sprint exercise. Eur. J. Appl. Physiol. 2014;114:2353–2367. doi: 10.1007/s00421-014-2954-2. [DOI] [PubMed] [Google Scholar]
- 7.Vieira A, et al. The effect of water temperature during cold-water immersion on recovery from exercise-induced muscle damage. Int. J. Sports Med. 2016;37:937–943. doi: 10.1055/s-0042-111438. [DOI] [PubMed] [Google Scholar]
- 8.Machado AF, et al. Can water temperature and immersion time influence the effect of cold water immersion on muscle soreness? A systematic review and meta-analysis. Sport. Med. 2016;46:503–514. doi: 10.1007/s40279-015-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohenauer E, Taeymans J, Baeyens JP, Clarys P, Clijsen R. The effect of post-exercise cryotherapy on recovery characteristics: A systematic review and meta-analysis. PLoS One. 2015;10:1–22. doi: 10.1371/journal.pone.0139028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leeder J, Gissane C, van Someren K, Gregson W, Howatson G. Cold water immersion and recovery from strenuous exercise: a meta-analysis. Br. J. Sports Med. 2012;46:233–240. doi: 10.1136/bjsports-2011-090061. [DOI] [PubMed] [Google Scholar]
- 11.Kellett J. Acute soft tissue injuries - a review of the literature. Med. Sci. Sport. Exerc. 1986;18:489–500. doi: 10.1249/00005768-198610000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Swenson, C., Swärd, L. & Karlsson, J. Cryotherapy in sports medicine. Scand. J. Med. Sci. Sport. 193–200 (1996). [DOI] [PubMed]
- 13.Järvinen TAH, et al. Muscle injuries: optimising recovery. Best Pract. Res. Clin. Rheumatol. 2007;21:317–331. doi: 10.1016/j.berh.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Eston R, Peters D. Effects of cold water immersion on the symptoms of exercise-induced muscle damage. J. Sports Sci. 1999;17:231–238. doi: 10.1080/026404199366136. [DOI] [PubMed] [Google Scholar]
- 15.Goodall S, Howatson G. The effects of multiple cold water immersions on indices of muscle damage. J. Sport. Sci. Med. 2008;7:235–241. [PMC free article] [PubMed] [Google Scholar]
- 16.Vaile J, Halson S, Gill N, Dawson B. Effect of hydrotherapy on the signs and symptoms of delayed onset muscle soreness. Eur. J. Appl. Physiol. 2008;102:447–455. doi: 10.1007/s00421-007-0605-6. [DOI] [PubMed] [Google Scholar]
- 17.Machado AF, et al. Dosages of cold-water immersion post exercise on functional and clinical responses: a randomized controlled trial. Scand. J. Med. Sci. Sport. 2017;27:1356–1363. doi: 10.1111/sms.12734. [DOI] [PubMed] [Google Scholar]
- 18.Ramos GV, et al. Cryotherapy reduces inflammatory response without altering muscle regeneration process and extracellular matrix remodeling of rat muscle. Sci. Rep. 2016;6:18525. doi: 10.1038/srep18525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siqueira AF, et al. Multiple cryotherapy applications attenuate oxidative stress following skeletal muscle injury. Redox Rep. 2016;22:323–329. doi: 10.1080/13510002.2016.1239880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peake J, Nosaka K, Suzuki K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc. Immunol Rev. 2005;11:64–85. [PubMed] [Google Scholar]
- 21.Tidball JG. Inflammatory processes in muscle injury and repair. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 22.Urso ML, Pierce JR, Alemany JA, Harman EA, Nindl BC. Effects of exercise training on the matrix metalloprotease response to acute exercise. Eur. J. Appl. Physiol. 2009;106:655–663. doi: 10.1007/s00421-009-1063-0. [DOI] [PubMed] [Google Scholar]
- 23.Paulsen G, Mikkelsen UR, Raastad T, Peake JM. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012;18:42–97. [PubMed] [Google Scholar]
- 24.Peiffer JJ, Abbiss CR, Watson G, Nosaka K, Laursen PB. Effect of cold-water immersion duration on body temperature and muscle function. J. Sports Sci. 2009;27:987–993. doi: 10.1080/02640410903207424. [DOI] [PubMed] [Google Scholar]
- 25.Yanagisawa O, Kudo H, Takahashi N, Yoshioka H. Magnetic resonance imaging evaluation of cooling on blood flow and oedema in skeletal muscles after exercise. Eur. J. Appl. Physiol. 2004;91:737–740. doi: 10.1007/s00421-004-1060-2. [DOI] [PubMed] [Google Scholar]
- 26.Cheung K, Hume PA, Maxwelf L. Delayed onset muscle soreness: treatment strategies and performance factors. Sport. Med. 2003;33:145–164. doi: 10.2165/00007256-200333020-00005. [DOI] [PubMed] [Google Scholar]
- 27.Herrera E, Sandoval MC, Camargo DM, Salvini TF. Motor and sensory nerve conduction are affected differently by ice pack, ice massage, and cold water immersion. Phys. Ther. 2010;90:581–591. doi: 10.2522/ptj.20090131. [DOI] [PubMed] [Google Scholar]
- 28.Yeung SS, et al. Effects of cold water immersion on muscle oxygenation during repeated bouts of fatiguing exercise: a randomized controlled study. Medicine (Baltimore). 2016;95:e2455. doi: 10.1097/MD.0000000000002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang JM, An J. Cytokines, inflammation and pain. Int Anesth. Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nosaka K, Aoki MS. Repeated bout effect: research update and future perspective. Brazilian J. Biomotricity. 2011;5:5–15. [Google Scholar]
- 31.Miyama M, Nosaka K. Influence of surface on muscle damage and soreness induced by consecutive drop jumps. J. Strength Cond. Res. 2004;18:206–211. doi: 10.1519/R-13353.1. [DOI] [PubMed] [Google Scholar]
- 32.Baird MF, Graham SM, Baker JS, Bickerstaff GF. Creatine-kinase-and exercise-related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012;2012:960363. doi: 10.1155/2012/960363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J. Am. Coll. Cardiol. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 34.Broatch JR, Petersen A, Bishop DJ. Postexercise cold water immersion benefits are not greater than the placebo effect. Med. Sci. Sports Exerc. 2014;46:2139–2147. doi: 10.1249/MSS.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 35.Peake JM, et al. The effects of cold water immersion and active recovery on inflammation and cell stress responses in human skeletal muscle after resistance exercise. J. Physiol. 2017;595:695–711. doi: 10.1113/JP272881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pournot H, et al. Time-course of changes in inflammatory response after whole-body cryotherapy multi exposures following severe exercise. PLoS ONE. 2011;6:e22748. doi: 10.1371/journal.pone.0022748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat. Med. 2014;20:857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 38.Takagi R, et al. Influence of icing on muscle regeneration after crush injury to skeletal muscles in rats. J. Appl. Physiol. 2011;110:382–388. doi: 10.1152/japplphysiol.01187.2010. [DOI] [PubMed] [Google Scholar]
- 39.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 40.Tayebjee MH, Lip GYH, Blann AD, MacFadyen RJ. Effects of age, gender, ethnicity, diurnal variation and exercise on circulating levels of matrix metalloproteinases (MMP)-2 and -9, and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and -2. Thromb. Res. 2005;115:205–210. doi: 10.1016/j.thromres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 41.Carmeli E, Moas M, Lennon S, Powers SK. High intensity exercise increases expression of matrix metalloproteinases in fast skeletal muscle fibres. Exp. Physiol. 2005;90:613–619. doi: 10.1113/expphysiol.2004.029462. [DOI] [PubMed] [Google Scholar]
- 42.Rattray B, Argus C, Martin K, Northey J. & Driller, M. Is it time to turn our attention toward central mechanisms for post-exertional recovery strategies and performance? Front Physiol. 2015;6:79. doi: 10.3389/fphys.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beedie CJ, Foad AJ. The placebo effect in sports performance: a brief review. Sports Med. 2009;39:313–329. doi: 10.2165/00007256-200939040-00004. [DOI] [PubMed] [Google Scholar]
- 44.Peake, J. M. et al. The effects of cold water immersion and active recovery on inflammation and cell stress responses in human skeletal muscle after resistance exercise. 3, 695–711 (2017). [DOI] [PMC free article] [PubMed]
- 45.Sarver, D. C. Cryotherapy minimally impacts the metabolome and transcriptome of human skeletal muscle (2017). [DOI] [PMC free article] [PubMed]
- 46.Mawhinney C, et al. Cold-water mediates greater reductions in limb blood flow than whole body cryotherapy. Med. Sci. Sports Exerc. 2017;49:1252–1260. doi: 10.1249/MSS.0000000000001223. [DOI] [PubMed] [Google Scholar]
- 47.Mannello F. The ‘cinderella’ role of blood collection procedures preanalytical methodological issues influence the release and activity of circulating matrix metalloproteinases and their tissue inhibitors, hampering diagnostic trueness and leading to misinterpretation. Arterioscler. Thromb. Vasc. Biol. 2008;28:611–614. doi: 10.1161/ATVBAHA.107.159608. [DOI] [PubMed] [Google Scholar]
- 48.Miyama M, Nosaka K. Protection against muscle damage following fifty drop jumps conferred by ten drop jumps. J. Strength Cond. Res. 2007;21:1087–1092. doi: 10.1519/R-21056.1. [DOI] [PubMed] [Google Scholar]
- 49.Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q) Can. J. Sport Sci. 1992;17:338–345. [PubMed] [Google Scholar]
- 50.Myrer JW, Myrer KA, Measom GJ, Fellingham GW, Evers SL. Muscle temperature is affected by overlying adipose when cryotherapy is administered. J. Athl. Train. 2001;36:32–36. [PMC free article] [PubMed] [Google Scholar]
- 51.Ferreira-Junior JB, et al. One session of partial-body cryotherapy (−110 °C) improves muscle damage recovery. Scand. J. Med. Sci. Sport. 2015;25:e524–e530. doi: 10.1111/sms.12353. [DOI] [PubMed] [Google Scholar]
- 52.Crystal NJ, Townson DH, Cook SB, Laroche DP. Effect of cryotherapy on muscle recovery and inflammation following a bout of damaging exercise. Eur. J. Appl. Physiol. 2013;113:2577–2586. doi: 10.1007/s00421-013-2693-9. [DOI] [PubMed] [Google Scholar]
- 53.Weir JP, Evans SA, Housh ML. The effect of extraneous movements on peak torque and constant joint angle torque-velocity curves. J. Orthop. Sport. Phys. Ther. 1996;23:302–308. doi: 10.2519/jospt.1996.23.5.302. [DOI] [PubMed] [Google Scholar]
- 54.Stumbo TA, et al. The effect of hand-grip stabilization on isokinetic torque at the knee. J. Stength Cond. Res. 2001;15:372–377. [PubMed] [Google Scholar]
- 55.Brown, L. E. & Weir, J. P. ASEP procedures recommendation I: accurate assessment of muscular strength and power. J. Exerc. Physiol. 4, (2001).
- 56.Mcnair PJ, Depledge J, Brettkelly M, Stanley SN. Verbal encouragement: effects on maximum effort voluntary muscle action. Br. J. Sports Med. 1996;30:243–245. doi: 10.1136/bjsm.30.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linthorne NP. Analysis of standing vertical jumps using a force platform. Am. J. Phys. 2001;69:1198–1204. doi: 10.1119/1.1397460. [DOI] [Google Scholar]
- 58.Chilibeck PD, Stride D, Farthing JP, Burke DG. Effect of creatine ingestion after exercise on muscle thickness in males and females. Med. Sci. Sports Exerc. 2004;36:1781–1788. doi: 10.1249/01.MSS.0000142301.70419.C6. [DOI] [PubMed] [Google Scholar]
- 59.Bemben MG. Use of diagnostic ultrasound for assessing muscle size. J. Strength Cond. Res. 2002;16:103–108. [PubMed] [Google Scholar]
- 60.Abe T, DeHoyos DV, Pollock ML, Garzarella L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur. J. Appl. Physiol. 2000;81:174–80. doi: 10.1007/s004210050027. [DOI] [PubMed] [Google Scholar]
- 61.de Souza Neto IV, et al. Effects of resistance training volume on MMPs in circulation, muscle and adipose tissue. Int. J. Sports Med. 2017;38:307–313. doi: 10.1055/s-0042-123192. [DOI] [PubMed] [Google Scholar]
- 62.Hu X, Beeton C. Detection of functional matrix metalloproteinases by zymography. JoVE. 2010;45:2445. doi: 10.3791/2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Costello JT, Culligan K, Selfe J, Donnelly AE. Muscle, skin and core temperature after −110 °C cold air and 8 °C water treatment. PLoS One. 2012;7:e48190. doi: 10.1371/journal.pone.0048190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen, J. Statistical Power Analysis for the Behavioral Sciences. (Lawrence Erlbaum Associates, 1988).
- 65.Lehmann, E. L. Nonparametrics: statistical methods based on ranks. (McGraw-Hill, 1975).