Abstract
A recent report suggested Complement 4 (C4A) gene copy numbers (GCN) as risk factors for schizophrenia. Rodent model showed association of C4 with synaptic pruning suggesting its pathophysiological significance (Sekar, A. et al. (2016)). We, therefore, predicted that C4A GCN would be positively correlated with neuropil contraction in the human brain among schizophrenia patients showing more prominent correlations in ventral regions among young adults and dorsal regions among adolescents since neuromaturation progresses dorsoventrally. Whole-brain, multi-voxel, in vivo phosphorus magnetic resonance spectroscopy (31P MRS) assessed neuropil changes by estimating levels of membrane phospholipid (MPL) precursors and catabolites. Increased MPL catabolites and/or decreased MPL precursors indexed neuropil contraction. Digital droplet PCR-based assay was used to estimate C4A and C4B GCN. We evaluated two independent cohorts (young adult-onset early-course schizophrenia (YASZ = 15) and adolescent-onset schizophrenia (AOSZ = 12) patients), and controls matched for each group, n = 22 and 15, respectively. Separate forward stepwise linear regression models with Akaike information Criterion were built for MPL catabolites and precursors. YASZ cohort: Consistent with the rodent model (Sekar, A. et al. 2016)), C4A GCN positively correlated with neuropil contraction (increased pruning/decreased formation) in the inferior frontal cortex and inferior parietal lobule. AOSZ cohort: C4A GCN positively correlated with neuropil contraction in the dorsolateral prefrontal cortex and thalamus. Exploratory analysis of C4B GCN showed positive correlation with neuropil contraction in the cerebellum and superior temporal gyrus among YASZ while AOSZ showed neuropil contraction in the prefrontal and subcortical structures. Thus, C4A and C4B GCN are associated with neuropil contraction in regions often associated with schizophrenia, and may be neuromaturationally dependent.
Introduction
Schizophrenia is a severe brain disorder which costs over $155 billion a year in the United States1. Available treatments are symptomatic leading to poor long-term social outcome2–5. A better understanding of pathophysiology may help develop new treatments. One of the neurodevelopmental models that propose excessive loss of synapses6 may be one such mechanism. Convergent animal7,8, human developmental9, neuroimaging10,11, postmortem12,13, and computational modeling14,15 data suggest that increased neuropil loss predates16–23 and continues after the onset of psychosis24,25, and may predict short-term outcome26. The genetic underpinnings of excessive synaptic pruning are poorly understood in humans.
Recently, independent lines of evidence suggest that Complement 4 (C4A) gene copy numbers (GCN) are associated with schizophrenia risk and synaptic pruning. This is important because a number of prior studies reported altered peripheral blood complement protein levels in schizophrenia but the results were inconsistent27–36 and the pathophysiological significance of such alterations was unclear since the peripheral complement proteins may not cross blood brain barrier37.
Persuasive results from a study by Sekar et al. (2016)38 demonstrated that a copy number variant (CNV) accounts for a portion of the risk in Human Leukocyte Antigen (HLA) region reported repeatedly in genetic association studies of schizophrenia39–42. This CNV consists of ‘cassettes’, denoted by ‘R-C-C-X’ that comprises STK19 (RP1), C4 (C4A or C4B), CYP21A1 or CYP21A2, and TNXB43. The C4 sequences can encode C4A or C4B, which are isotypes of C4 with > 99% sequence homology; however, the translational products differ in antigen affinities and hemolytic activity44–46. A recombination site at CYP21A2 leads to mono-, bi-, and tri-modular RCCX cassettes (and rarely, 4 modules) that can generate multiple functional copies of C4A/C4B, while retaining just one functional copy of the remaining genes47. Thus, each chromosome commonly has 1–3 functional copies of C4A/C4B, (rarely, 0 or 4 copies), for a typical total of 0–6 copies/individual; further, transcription of C4A/C4B can be impacted by an intronic human endogenous retroviral (HERV) sequence48. Sekar et al.38 found that C4A, but not C4B GCN are associated with higher risk for schizophrenia. In post-mortem brain samples of schizophrenia patients, the expression of C4A and C4B genes was proportional to the number of C4 GCN with higher expression in 5 brain regions (namely the frontal cortex, cingulate cortex, parietal cortex, cerebellum, corpus callosum and orbitofrontal cortex) by approximately 40% among schizophrenia patients compared to controls38. A rodent model showed decreased synaptic pruning in C4-deficient mice. The RCCX CNV is also associated with risk for auto-immune disorders that have altered prevalence among schizophrenia patients44,49,50. Recently, C4 mRNA levels in plasma have been correlated with severity of psychopathology in schizophrenia51. Another study found correlations between predicted C4A transcription and impairment in memory52. Complement proteins were also associated with risk for schizophrenia53, and with thinning of superior frontal cortex54. Thus, in humans, the complement system serves diverse immune and neural functions55,37 and suggest that abnormal C4A function contributes to schizophrenia pathogenesis.
We examined the relationship of C4 GCN with neuropil contraction/expansion in a human context within two independent cohorts of schizophrenia patients and healthy controls (HC). We examined neuropil because direct examination of synapses in live human subjects is challenging as the synapses are embedded in the neuropil. Neuropil is a synaptically dense region composed of dendrites, unmyelinated axons and glial filaments with relatively few cell bodies56,57. Phosphorus magnetic resonance spectroscopy (31P MRS) is used to assess changes in neuronal membrane expansion/contraction within the neuropil by estimating the availability of membrane phospholipid (MPL) precursors (phosphocholine, PC; phosphoethanolamine, PE) and catabolites (glycerophosphocholine, GPC; glycerophosphoethanolamine, GPE). PC, PE, GPC, and GPE can be reliably measured in the brain by 31P MRS58. Specificity of these measurements to neuropil compared to gray matter measurements is supported by convergent data from animal lesion59, cellular model60, human postmortem61 and neurodevelopmental studies9,62. Greater sensitivity for age-related changes of MPL metabolites to neuropil changes compared to gray matter metrics is provided by human developmental studies9,63. Thus, 31P MRS that assesses molecular biochemistry of neuropil is superior to gray matter measures that represent composite physical measurement of total volume of interneurons, synapses, axonal terminals, dendritic arborization, neuronal soma, glia, microvasculature and interneuronal space64,65.
The rationale for the superiority of 31P MRS has been described in prior publications9,11,62. Briefly, synapse/dendritic spine formation and dendritic branching requires expansion of dendritic/axonal membranes. This expansion requires increased MPL synthesis, with resultant increase in MPL precursor levels (PC + PE). This is supported by elevation of PC + PE at the time and site of neuropil growth spurts59,62. Likewise, neuropil contraction or pruning are associated with breakdown of MPLs leading to elevated GPC + GPE, which is, also, noted at the time and site of synaptic pruning59,66. Since expansion and contraction of neuronal membranes largely occurs at the dendrites and axonal endings during neuropil growth/contraction with a considerably smaller contribution from changes in neuronal soma size, glial expansion and myelin content67–69, changes in MPL metabolites are thought to more specifically and sensitively index changes in axonal endings, dendritic branches and synapses.
We examined two independent cohorts–young adult-onset schizophrenia (YASZ) cohort (n = 15) and adolescent-onset schizophrenia (AOSZ) patients (n = 12)- and two HC cohorts age-matched for each group (n = 22 and 15, respectively). Examination of two cohorts helps replicate the findings and explore the association of C4A repeats with neuropil contraction in a developmental context. We selected MRS voxels from five of the six brain regions that showed increased C4A and C4B expression in postmortem tissue38. These included the frontal cortex (the dorsolateral prefrontal cortex (DLPFC), inferior frontal cortex (IFC), ventral PFC (VPFC)), cingulate cortex (anterior cingulate cortex (ACC) and posterior cingulate cortex (PCC)), parietal lobe (inferior parietal lobule (IPL), superior parietal lobule (SPL)), orbitofrontal cortex (OFC) and cerebellum but not corpus callosum because of lower signal-to-noise ratio (SNR) of 31P MRS data for this region. Since prior studies showed progression of brain maturation from the dorsal (e.g., temporal-parietal regions) to the ventral (e.g., prefrontal cortices) regions involving elimination of overproduced synapses that is prominent from late childhood to the third decade of life70–75, we selected the superior temporal gyrus (STG) in addition to the above regions. Subcortical structures (thalamus, caudate, and ventral and dorsal hippocampus) were, also, included because these structures show continued maturation from childhood to late adulthood76–78. Thus, fourteen regions were selected on both hemispheres so that we could examine neuropil alterations in regions that showed increased C4 expression by Sekar et al., and neurodevelopmental changes in the dorsal (STG, PCC, IPL, SPL), ventral (DLPFC, ACC, OFC, IFC, VPFC) and subcortical regions.
Our primary hypothesis was that the GPC + GPE levels would be elevated with increasing C4A repeats in the frontal (DLFPC, IFC, VPFC), cingulate (ACC, PCC), parietal (IPL, SPL), the OFC and the cerebellum in both cohorts. We, further, hypothesized that the GPC + GPE levels would be elevated with increasing C4A repeats in YASZ cohort in the ventral brain regions (DLFPC, IFC, OFC, ACC, and VPFC) whereas the AOSZ would show such elevations in the dorsal regions (STG, PCC, IPL, and SPL). We predicted increased GPC + GPE in the hippocampus, caudate and thalamus in both cohorts because of protracted maturation. We investigated whether the C4A GCN would be associated with decreased PC + PE (MPL precursors) levels suggesting decreased neuropil expansion, and explored whether C4B GCN would be similarly correlated with GPC + GPE and PC + PE levels in these regions similar to C4A associations.
Methods
SAMPLE 1: YASZ and age-matched HC
Clinical evaluations
YASZ between 18–44 years of age with DSM-IV schizophrenia/schizoaffective disorder and ≤ 5 years of illness from the onset of psychotic symptoms were eligible to be enrolled at the University of Pittsburgh Medical Center. The diagnosis was confirmed in a consensus meeting of experienced diagnosticians79 after reviewing the Structured Clinical Interview for DSM diagnosis (SCID-IV)80 data and clinical information. Total antipsychotic dose and duration were collected. Substance abuse in the previous month or dependence 6 months prior to enrollment, mental retardation per DSM-IV, serious neurological/medical illnesses were exclusion criteria. After explaining the experimental procedures, informed consents were obtained from the subjects. University of Pittsburgh IRB approved the study.
Imaging procedures
Details of 31P MRS data acquisition and processing are published11. Briefly, whole-brain, multi-voxel, in vivo 31P MRS data in 3-dimensions was collected on a 3 T Siemens Tim Trio system using a dual-tuned ¹H-³¹P volume head coil and a conventional chemical shift imaging (CSI) sequence. Acquisition parameters were: FOV = 310 × 310 × 160 mm, acquired phase-encoding steps = 14 × 14 × 8 and zero-filled to 16 × 16 × 8 (nominal voxel dimension = 1.94 × 1.94 × 2.0 cm3), TR = 0.54 sec, flip-angle = 330 reflecting the Ernst angle where the average T1 value of phosphocreatine (PCr), PE, PC was 3 sec, complex data points = 2048, spectral bandwidth = 4.0 kHz, 24 averages of the CSI matrix in which the averaging was weighted to the central k-space points conforming to a 3D elliptical function and pre-acquisition delay of 1.4 ms. T1-weighted MPRAGE images were collected and used to guide the extraction of ³¹P MRS signal of hypothesized voxels-of-interest using an innovative procedure81. In the k-space domain of the ³¹P MRS data, a 75% Hamming window was applied, and modeled in the time domain with 23 Gaussian-damped sinusoids (PE, PC, GPE, and GPC as triplets, Pi, MPLbroad, PCr and dinucleotides as singlets, and adenosine triphosphate (ATP) (two doublets and a triplet)).
The post-processing and metabolite quantification of extracted 31P MRS signal was 100% automated81. Due to the lack of ¹H decoupling, the quantification of the individual phosphomonoesters (PE and PC), and phosphodiesters (GPE and GPC) were indistinguishable. Therefore, summated measures (PE + PC, GPE + GPC) were obtained. The proportion of gray and white matter, and CSF/extra-cortical space was estimated for each voxel-of-interest using a fully automated procedure82–84. Since the CSF concentration of ³¹P metabolites is below the detection limit and the ³¹P metabolites are expressed as a percentage relative to the total signal, the correction for CSF fractions is not applicable and do not confound the ³¹P MRS results.
SAMPLE 2: AOSZ and age-matched HC
Clinical evaluations
Early onset was defined as the first appearance of psychotic symptoms before 18 years of age85,86. AOSZ were between 14 and 21 years of age and on stable antipsychotic doses for longer than 1 month. SCID-IV80 for adults and K-SADS-PL87 for adolescents were administered and reviewed for “consensus” diagnosis as noted above. The exclusion criteria were (a) significant present/past history of medical/neurological illness, e.g., epilepsy, head injury, (b) Obstetric complications, e.g., neonatal asphyxia, (c) Mental retardation per DSM-IV, (d) Hyperbilirubinemia requiring transfusion/phototherapy > 2 days, (e) The Apgar score < 7 at 1 and 5 min after birth, (f) Gestational age < 37 or > 42 weeks, (g) Significant substance use including cigarettes > ½ pack a day, alcohol > 2 drinks/day during pregnancy, and h) Preeclampsia/eclampsia during subject’s pregnancy. These data were collected through mothers of subjects and their obstetric records. After explaining the experimental procedures, informed consents were obtained from adult subjects; subjects below 18 years of age provided the assent and then the consent was obtained from the parents or legal guardians. University of Pittsburgh IRB approved the study.
Imaging procedures
For this sample, 1H-decoupled 31P MRS data was acquired. Scout images were acquired to prescribe 31P MRS voxels. Initial maximization of the B0 field homogeneity in the 1H mode (shimming) and optimization of the 31P reference radio frequency (RF) pulse amplitude for a 33° flip angle was conducted. The acquisition sequence includes a single slab-selective excitation RF pulse followed by phase-encoding pulses to spatially encode in 3D. The axial slab was placed parallel to the AC-PC line covering the whole brain. The scanning parameters: FOV = 310 × 310 × 160 mm, slab thickness = 140 mm, phase-encoding steps = 14 × 14 × 8, zero-filled to 16 × 16 × 8 (nominal voxel dimension = 1.94 × 1.94 × 2.0 cm3), TR = 0.54 sec, flip-angle = 33° where the average T1 value of PCr, PE, PC, is 3 sec, complex data points = 2048, spectral bandwidth = 4.0 kHz, 24 averages (weighted-average k-space), which are validated88, elliptical k-space sampling. To minimize the signal attenuation due to spin-spin relaxation plus T2* within the pre-acquisition delay time, the rise and fall time parameters and duration of the phase encoding pulses are reduced giving a pre-acquisition delay of 1.4 ms. T1 images were acquired to shift voxels. Post-processing and quantification was similar to the non-decoupled data.
Genetic assays
Subjects were chosen randomly from the larger YASZ and AOSZ cohorts for genetic assays. Characterization of C4 variants is challenging due to complex linkage disequilibrium in the HLA region and the complexity of C4 locus. We used digital droplet PCR (ddPCR)38 assay to estimate copy numbers of C4 structural elements (C4A, C4B, C4L, and C4S). Briefly, AluI-digested genomic DNA was mixed with primer-probe mix for C4 and a reference locus (RPP30), and 2 × ddPCR Supermix for Probes (Bio-Rad). The oligonucleotide primers and probes used for assaying copy number of C4A, C4B, C4L, and C4S were synthesized (IDT tech), and the master mix was emulsified into droplets, using a micro-fluidic droplet generator (Bio-Rad) that was subjected to PCR. After PCR, the fluorescence in each droplet was read using a QX100 droplet reader (Bio-Rad), and the data analyzed using the QuantaSoft software (Bio-Rad) and copy numbers deduced.
To discriminate the compound structural forms of C4 (AL, AS, BL, BS), we amplified a 5.2 kb product that spans the C4A/B by long-range PCR. The diluted 5.2 kb PCR product was further PCR amplified with primers specific for C4AS and C4BS. The ratio of C4AS to C4BS was used to determine C4AS and C4BS copy numbers.
Statistical analysis
We used Student’s t tests to examine differences in age, and χ2 or Fisher’s exact tests to examine distribution of sex and C4 GCN between schizophrenia and controls. Since the number of univariate tests (2 sides × 2 metabolites × 14 regions) would overwhelm hypothesis testing in this relatively small sample, we used multivariate linear modeling within SPSS 25 controlling for age and sex that would require one model for each group. Multivariate models would also account for correlation among the MPL metabolites in the hypothesized regions. Separate regression models were built for GPC + GPE and PC + PE levels for AOSZ and YASZ by including these metabolite levels for all hypothesized regions, diagnosis, age, sex and education as covariates. Since antipsychotic dose did not significantly contribute to MPL metabolite alterations11, we did not covary for medications. To test primary hypothesis on the association of GPC + GPE levels with C4A GCNs, we built two separate forward stepwise regression models with Akaike Information Criterion (AIC) for the YASZ and the AOSZ cohorts. Smallest AIC was used to guide model selection. The AIC was used as an additional parameter because it penalizes model complexity, similar to other criterion such as Bayesian Information Criterion. We followed a similar approach to test the secondary hypothesis on the relationship of C4A repeats with PC + PE, and to explore the association of C4B repeats with GPC + GPE and PC + PE levels.
Results
Demographic and clinical characteristics
Sample 1: YASZ and age-matched HC
Although the eligibility of subjects to enter the study was 18–44 years, mean age of enrolled YASZ (25.74 ± 8.46 years) did not differ from HC (27.07 ± 7.14 years)(t = 0.52, p = 0.61) (range 18.24 to 43.98 years for YASZ, and 18.60 to 42.10 years for HC with 3 subjects above 35 years in both groups). Mean duration of illness was 2.37 ± 1.62 years. C4A (Fisher’s exact test, p = 0.69) and C4B (Fisher’s exact test, p = 0.23) GCN did not show schizophrenia-HC differences (Table 1). We recapitulated our earlier published results on differences in MPL metabolites in a larger YASZ and HC cohort11.
Table 1.
Demographic and clinical characteristics
| Young adult-onset schizophrenia (n = 15) | Healthy controls (n = 22) | Statistics | Adolescent-onset schizophrenia (n = 12) | Healthy controls (n = 15) | Statistics | |
|---|---|---|---|---|---|---|
| Age (in years) | 25.74 ± 8.46* | 27.07 ± 7.14* | t = 0.52, p = 0.61 | 19.59 ± 1.52* | 19.16 ± 1.32* | t = 0.76, p = 0.47 |
| Sex | ||||||
| Male | 11 | 5 | Fisher’s exact test, p = 0.006 | 10 | 7 | Fisher’s exact test, p = 0.11 |
| Female | 4 | 17 | 2 | 8 | ||
| C4AL repeats | ||||||
| 1 copy | 4 | 4 | Fisher’s exact test, p = 0.69 | 1 | 2 | Fisher’s exact test, p = 1.00 |
| 2 or more copies | 11 | 18 | 11 | 13 | ||
| C4BL repeats | ||||||
| 0 copy | 8 | 15 | Fisher’s exact test, p = 0.23 | 7 | 4 | Fisher’s exact test, p = 0.27 |
| 1 copy | 5 | 2 | 4 | 9 | ||
| 2 or more copies | 2 | 5 | 1 | 2 | ||
| Duration of illness (in years) | 2.37 ± 1.62 | – | – | 3.99 ± 0.90 | – | – |
The quality of MRS data was measured as SNR, mean Gaussian linewidths of PCr and Cramer-Rao Lower Bound (CRLB) values. Gaussian linewidth of PCr represents the resolution of spectra, where narrower linewidths indicate higher resolution of spectra, and lower CRLB value is the lower bound on the variance of spectral measurements. Our larger cohort, published previously, did not show case-control differences in the mean SNR and mean Gaussian linewidths of PCr, and CRLB values for the PC + PE and GPC + GPE11. This sample that was derived from the larger cohort11 showed significant differences for PCr Gaussian linewidth for left caudate, hippocampus, DLPFC, thalamus, and the right ACC, IFC and caudate (all p < 0.05). However, the CRLB and the SNR values did not differ between the groups for these regions except for the CRLB of GPC + GPE of the left hippocampus (p = 0.028).
Sample 2: AOSZ and age-matched HC
Age at onset of psychosis in the AOSZ was 15.60 ± 0.8 years. Age at scan was not significantly different between AOSZ (n = 12; 19.59 ± 1.60 years) and HC (n = 15; 19.16 ± 1.29 years) (t = 0.76, p = 0.47). AOSZ were significantly younger than YASZ (t = 2.74, d. f = 25, p = 0.011) (range 15.33 to 20.92 in both groups). We did not observe AOSZ-HC differences in C4AL (Fisher’s exact test, p = 1.00) and C4BL (Fisher’s exact test, p = 0.27) distribution in this sample, as well (Table 1).
Quality of the 1H-decoupled 31P MRS was high providing improved spectral resolution and peak separation to enable clear separation of PE, PC, GPC, and GPE. The mean SNR of PCr, the mean Gaussian linewidths of the PCr and the CRLB for the PC, PE, GPC, and GPE did not show significant case-control differences except for the CRLB of PC and GPC of the left ventral hippocampus (p = 0.025), and the CRLB of PE of the right anterior cingulate (p = 0.037). These regions did not show differences associated with C4 GCN.
C4A variants and MPL metabolites
Sample 1: YASZ and age-matched HC
In the combined sample of YASZ and HC, increasing C4A GCN were associated with elevated GPC + GPE levels in the ACC while IFC showed a concurrently decreased GPC + GPE and PC + PE levels. The smallest AIC for model selection was −46.20 for GPC + GPE and −43.69 for PC + PE.
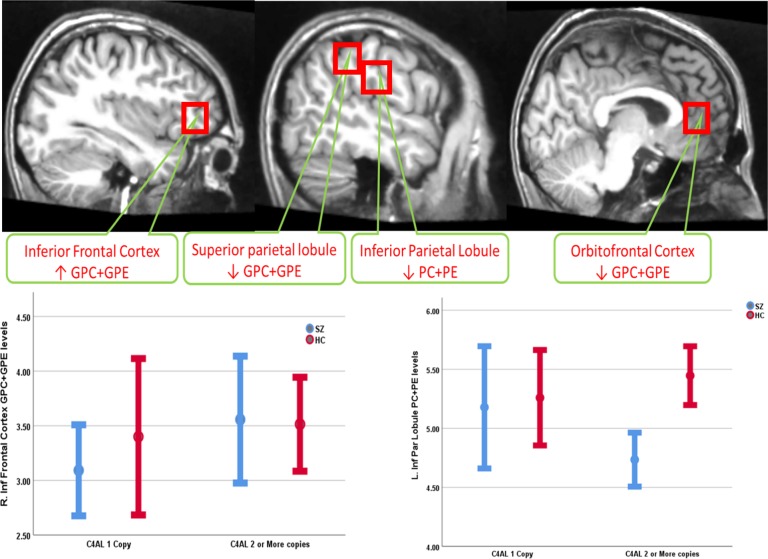
Among YASZ, increasing C4A GCN was associated with elevated GPC + GPE levels in a ventral brain region, namely the right IFC with a large effect size (Cohen’s d = 1.15) but not among HC. We, also, observed decreased GPC + GPE levels in the ventral regions among YASZ (the left OFC; Cohen’s d = 0.85) and HC (left IFC; Cohen’s d = 0.52); however, HC showed a concurrent decrease in the left IFC (Cohen’s d = 0.57). YASZ patients showed decreased GPC + GPE levels (the right SPL; Cohen’s d = 1.8) and decreased PC + PE levels (left IPL; Cohen’s d = 0.84) in relation to increasing C4A repeats. Overall model AIC was −23.87 for GPC + GPE and −19.72 for PC + PE (Table 2; Fig. 1).
Table 2.
Association of C4AL copy number repeats with MPL metabolite levels among young adult-onset schizophrenia (YASZ) and matched healthy controls
| GPC + GPE ↑ | GPC + GPE ↓ | PC + PE↑ | PC + PE↓ | |
|---|---|---|---|---|
| Sample 1: Young adult-onset schizophrenia and age-matched HC | ||||
| YASZ + HC | L. Anterior Cingulate Cortex (AIC = −45.70; β = 0.45, t = 2.62, p = 0.013) (d = 0.43) | L. Inferior Frontal Cortex (AIC = −43.48; β = −0.27, t = 3.48, p = 0.001) (d = 0.57) | – | L. Inferior Frontal Cortex (AIC = −43.69; β = −0.11, t = 2.26, p = 0.03) (d = 0.37) |
| YASZ | R. Inferior Frontal Cortex (AIC = −20.73; β = 0.37, t = 3.81, p = 0.007) (d = 1.15) |
R. Superior Parietal Lobule (AIC = −15.82; β = −0.58, t = 5.98, p = 0.001) (d = 1.8) L. Orbitofrontal Cortex (AIC = −23.87; β = −0.15, t = 2.83, p = 0.03) (d = 0.85) |
– | L. Inferior Parietal Lobule (AIC = −18.06; β = −0.88, t = 3.26, p = 0.007) (d = 0.84) |
| HC | – | L. Inferior Frontal Cortex (AIC = −25.90; β = −0.17, t = 2.42, p = 0.026) (d = 0.52) | – | L. Inferior Frontal Cortex (AIC = −29.06; β = −0.17, t = 2.72, p = 0.014) (d = 0.57) |
| Sample 2: Adolescent-onset schizophrenia and age-matched HC | ||||
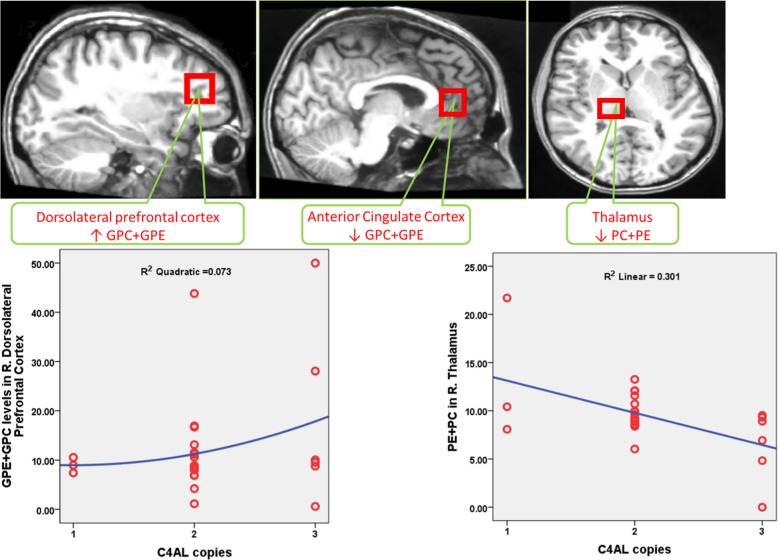
| AOSZ + HC | R. Dorsolateral Prefrontal Cortex (AIC = −44.78; β = 0.019, t = 2.40, p = 0.024) (d = 0.46) | L. Anterior Cingulate Cortex (AIC = −41.49; β = −0.088, t = 5.05, p < 0.001) (d = 0.99) | – | R. Thalamus (AIC = −35.54; β = −0.096, t = 2.96, p = 0.007) (d = 0.57) |
Increased GPC + GPE suggest increased neuropil contraction while decreased PC + PE suggest decreased neuropil expansion
Fig. 1.
MRS voxels that showed significant MPL metabolite changes among adult-onset schizophrenia (YASZ)
Sample 2: AOSZ and age-matched HC
Because there was one AOSZ and two controls with 1 copy of C4A, we examined the association of C4A repeats in the entire sample of AOSZ and HC. We noted elevated GPC + GPE levels in the DLPFC (Cohen’s d = 0.46) but decreasing GPC + GPE levels in the left anterior cingulate with increasing C4A GCN.
In the same combined sample, we noted decreasing PC + PE levels in the right thalamus associated with increasing C4A GCN (Cohen’s d = 0.57). Overall model AIC was −44.78 for GPC + GPE and −36.52 for PC + PE (Table 2; Fig. 1).
Post hoc tests for the association of C4BL repeats with MPL metabolite changes
Sample 1: YASZ and age-matched HC
Combined sample of YASZ + HC showed increased GPC + GPE levels in the left IPL with increasing C4BL repeats. Right ventral hippocampus showed elevated PC + PE and right DLFPC showed decreased PC + PE. The AIC for overall model selection was −77.07 for GPC + GPE and −18.13 for PC + PE. YASZ subjects showed elevated GPC + GPE levels in the cerebellar vermis and the left STG along with decreased PC + PE levels in the left IPL and OFC with increasing C4BL repeats. HC showed increased GPC + GPE levels in multiple regions with decreased PC + PE levels in the DLPFC and caudate. The lowest AIC for model selection was 10.92 for GPC + GPE and −13.89 for PC + PE for within YASZ and HC.
Sample 2: AOSZ and age-matched HC
Because of small n in each cell, AOSZ and HC groups were not examined separately. MPL metabolite changes in relation to C4B GCN were observed in the prefrontal and subcortical regions in the combined sample of AOSZ and HC. PC + PE levels did not differ between with C4BL repeats (Table 3; Fig. 2). AIC for overall model selection was −53.13 for GPC + GPE.
Table 3.
Exploratory analysis of association of C4BL copy number repeats with MPL metabolite levels among young adult-onset schizophrenia (YASZ) and matched healthy controls, and a combined sample of AOSZ + HC
| GPC + GPE↑ | GPC + GPE↓ | PC + PE↑ | PC + PE↓ | |
|---|---|---|---|---|
| Sample 1: Young adult-onset schizophrenia and age-matched HC | ||||
| YASZ + HC | L. Inferior Parietal Lobule (AIC = −32.32; β = 0.32, t = 3.43, p = 0.002) (d = 0.56) | – | R. Vent Hippocampus (AIC = −17.92; β = 0.25, t = 2.31, p = 0.027) (d = 0.38) | R. Dorsolateral Prefrontal Cortex (AIC = −19.50; β = −0.38, t = 2.65, p = 0.012); (d = 0.44) |
| YASZ | Cerebellar Vermis (AIC = −9.64; β = 0.42, t = 2.69, p = 0.02) (d = 0.69) | – | L. Inferior Parietal Lobule (AIC = −13.44; β = 0.91, t = 3.37, p = 0.006) (d = 0.87) | – |
| L. Superior Temporal Gyrus (AIC = −10.89; β = 0.48, t = 2.60, p = 0.025) (d = 0.67) | – | L. Orbitofrontal Cortex (AIC = −10.25; β = 0.18, t = 2.74, p = 0.019) (d = 0.71) | – | |
| HC | L. Superior Parietal Lobule (AIC = −18.04; β = 1.86, t = 37.20, p < 0.001) (d = 7.93) | L. Superior Temporal Gyrus (AIC = −30.15; β = −1.05, t = 31.78, p < 0.001) (d = 6.77) | Posterior Cingulate Cortex (AIC = −12.43; β = 0.55, t = 3.45, p = 0.003) (d = 0.73) | R. Dorsolateral Prefrontal Cortex (AIC = −10.78; β = −0.38, t = 3.50, p = 0.003) (d = 0.75) |
| Posterior Cingulate Cortex (AIC = −15.18; β = 1.42, t = 31.15, p < 0.001) (d = 6.61) | L. Dorsolateral Prefrontal Cortex (AIC = −15.18; β = −0.74, t = 25.72, p < = 0.001) (d = 5.41) | L. Hippocampus (AIC = −18.13; β = 0.37, t = 2.37, p = 0.031) (d = 0.50) | R. Caudate (AIC = −15.36; β = −0.71, t = 3.40, p = 0.004) (d = 0.73) | |
| L. Caudate (AIC = −41.64; β = 0.45, t = 12.31, p < 0.001) (d = 2.61) | L. Dorsal Hippocampus (AIC = −46.70; β = −0.65, t = 13.34, p < 0.001) (d = 2.87) | |||
| L. Ventral Hippocampus (AIC = −74.51; β = 0.08, t = 4.74, p = 0.001) (d = 1.00) | R. Anterior Cingulate Cortex (AIC = −37.77; β = −0.26, t = 12.57, p < 0.001) (d = 2.63) | |||
| Sample 2: Adolescent-onset schizophrenia and age-matched HC | ||||
| AOSZ + HC | R. Caudate AIC = −34.93; (β = 0.16, t = 10.07, p < 0.001) (d = 1.95) | R. Thalamus (AIC = −37.35; β = −0.14, t = 6.68, p < 0.001) (d = 1.26) | – | – |
| R. Hippocampus (AIC = − 34.92; β = 0.16, t = 8.07, p < 0.001) (d = 1.56) | R. Anterior Cingulate Cortex (AIC = −42.36; β = −0.03, t = 5.27, p < 0.001) (d = 1.09) | – | – | |
| R. Dorsolateral Prefrontal Cortex (AIC = −49.12; β = 0.03, t = 6.21, p < 0.001) (d = 1.19) | L. Dorsolateral Prefrontal Cortex (AIC = −41.64; β = −0.03, t = 2.18, p = 0.046) (d = 0.44) | – | – | |
| Ventral Prefrontal Cortex (AIC = −46.07; β = 0.04, t = 5.50, p < 0.001) (d = 1.03) | – | – | – | |
| L. Orbitofrontal Cortex (AIC = −34.93; β = 0.03, t = 5.34, p < 0.001) (d = 1.01) | – | – | – | |
| L. Hippocampus (AIC = −34.93; β = 0.09, t = 2.95, p = 0.011) (d = 0.56) | – | – | – | |
Increased GPC + GPE suggest increased neuropil contraction while decreased PC + PE suggest decreased synapse expansion
Fig. 2.
MRS voxels that showed significant MPL metabolite changes among adolescent-onset schizophrenia (AOSZ) in relation to C4AL repeats
Discussion
This is the first study, to our knowledge, to demonstrate the association of C4A repeats with increased neuropil contraction as shown by elevated GPC + GPE levels and/or decreased PC + PE levels in two independent cohorts of schizophrenia patients. Neuropil contraction was observed in the prefrontal and parietal regions among adult-onset schizophrenia patients whereas adolescent subjects (AOSZ and controls) showed neuropil contraction in the prefrontal and thalamic regions. Our hypothesis that the GPC + GPE levels would be elevated with increasing C4A repeats in the frontal (DLFPC, IFC, VPFC), cingulate (ACC, PCC), parietal (IPL, SPL), the OFC and the cerebellum in both cohorts was partly supported. Our second hypothesis was, also, partly supported for the YASZ cohort in that the YASZ showed elevated GPC + GPE and/or decreased PC + PE levels in a ventral region (IFC) but not all hypothesized regions. Likewise, AOSZ + HC group showed decreased PC + PE levels in the thalamus. Overall, our results on patients and controls support the association of C4 deficiency with synaptic pruning observed in the rodent model, although the pattern of associations are intriguing. Further, this study extends Sekar et al.38 rodent model findings to human subjects with schizophrenia and for C4B associations with neuropil contraction although C4B was not associated with risk for schizophrenia.
Changes in MPL metabolite levels measured through 31P MRS reflects expansion/contraction of membranes that contain the MPLs (phosphatidylcholine, PtdC; phosphatidylethanolamine, PtdE). Expansion/contraction of cell membranes that primarily occurs at the axonal endings and dendritic branches during neuropil formation/contraction are associated with elevated MPL precursors (PE, PC) and catabolites (GPC, GPE), respectively. Changes in myelination, neuronal soma size and glial cells contribute considerably less to MPL metabolite signals on 31P MRS59. Thus, major source of MPL metabolite signals is likely to be the synaptically dense neuropil68,69 (see our prior publication for details11). Since postmortem studies have not consistently reported atrophy of neuropil89, observed changes in MPL metabolites are unlikely to be due to general neuropil atrophy.
The first in vivo evidence of altered neuropil development in first-episode neuroleptic-naïve schizophrenia was provided by 31P MRS data (decreased PC + PE and increased GPC + GPE), later supported by postmortem neuropil morphology data90–92. Neuropil reduction may be linked to dendritic spine loss91,93–95 and decreased neuronal soma size96,97 as observed in postmortem brain tissue of schizophrenia patients. Since dendritic spines receive majority of cortical excitatory synapses, dendritic spine loss may suggest loss of cortical excitatory synapses. Thus, the association of C4A repeats with neuropil contraction may support synaptic pruning in the rodent model38. However, other factors may also contribute to neuropil reduction, e.g., peripheral blood inflammatory mediators C-reactive protein (CRP) and Interleukin-6 (IL-6) levels11 but unlikely to be due to long-term administration of antipsychotics. Association of changes in MPL metabolites with antipsychotic medications has been examined in several studies. These studies show that MPL metabolites were altered with short-term but not long-term treatment98–101. Given short duration of illness of both cohorts and lack of association of medications with MPL metabolites, antipsychotics may not have contributed a major variance to MPL metabolite differences in this study. Therefore, genetic influence of a variant that showed genomewide significance on neuropil changes exemplify an attempt to explore in vivo biological significance of such variants.
Among six brain regions that showed increased C4A and C4B RNA expression associated with C4 repeats in postmortem brain tissue of schizophrenia patients38, we found neuropil alterations associated with C4A GCN in four of five regions examined. While the frontal (DLPFC and the IFC) and parietal (IPL) regions showed increased neuropil contraction, the ACC and the OFC showed decreased neuropil contraction without altered neuropil formation. HC showed decreased neuropil turnover in the IFC. Further, the thalamus (that was not examined in the previous postmortem study38) showed decreased neuropil formation in the AOSZ + HC cohort. Cerebellum did not show neuropil contraction associated with higher C4A GCN. Taking our in vivo 31P MRS data with the published data on increased RNA expression in these regions suggests that abnormal function of C4 GCN contributes to neuropil changes in schizophrenia. However, YASZ showed variations in widespread cortical regions with greater heterogeneity in neuropil contraction/expansion compared to HC. Precise reasons for such heterogeneous associations are unclear. Data on differential expression of C4A in various regions of the brain within the context of illness course or neurodevelopment are not available. Examination of whole brain 31P MRS data allowed us to identify brain regions beyond those examined in the postmortem study that suggests that C4AL may be associated with decreased neuropil synthesis, as well as neuropil contraction, both of which may be observed as dendritic spine loss–a proxy measure of synaptic pruning in postmortem studies. In the combined AOSZ + HC sample, C4AL showed neuropil changes in the ventral and subcortical regions. Partial support for both hypothesis may possibly be because our YASZ were not in the third decade and AOSZ were not in early adolescence where the neurodevelopment would be more active such as in early to mid-adolescence. Future postmortem and animal studies should examine these issues.
Furthermore, since the AOSZ cohort was significantly younger than the YASZ, our prediction that the AOSZ and YASZ would show regionally distinct patterns of MPL metabolite changes in relation to C4 GCNs broadly reflecting neurodevelopmental trajectory was not supported. This may be because the age at scan was about 19 years and the neurodevelopment might have progressed beyond dorsal regions into the ventral regions. Another reason may be inadequate power of our sample size to detect such differences. Further studies are needed to investigate developmental effects of C4A and C4B repeats.
We did not observe increased neuropil contraction among young adult HC although a concurrent reduction in PC + PE and GPC + GPE was observed in relation to C4AL. Exploratory analysis on the association of C4BL with MPL metabolites showed significant associations with increased neuropil contraction among young-adult HC compared to YASZ patients. Precise reasons are unclear; however, it is possible that the pattern of expression of C4A and C4B proteins may be different in patients compared to healthy subjects.
This study supported our secondary hypothesis of decreased neuropil expansion. It can be postulated that C4 GCN may affect each brain region through increased neuropil contraction or decreased formation. Although postmortem examination allows direct examination of spines, differentiation of decreased spine formation from increased pruning by cross-sectional examination is challenging. Unique advantage of in vivo MRS data is its ability to distinguish between increased contraction and diminished formation to the neuropil density. Thus, the findings of this study, if replicated, raises the possibility of the contribution of C4 GCN to decreased neuropil formation for future cellular and animal studies.
C4BL GCN were associated with MPL metabolite changes in the dorsal and subcortical regions among adult-onset schizophrenia patients. However, controls showed a similar pattern but in a distinct set of regions and were more extensive compared to patients. The associations were noted in the ventral and subcortical regions among adolescent cohort contrary to our predictions. One likely reason is that nearly half of subjects in both groups did not have any copies of C4BL, effectively making it a comparison between subjects with and without C4BL GCN. Further studies with larger samples are required.
The quality of the spectral data acquired with (AOSZ) and without (YASZ) 1H-decoupling was reasonably good, although the 1H-decoupled MRS data was expectedly of higher quality. The SNR and CRLB values of 31P MRS data without decoupling did not show significant differences but the PCr Gaussian linewidth was longer for selected voxels suggests that the variability among the groups was within reasonable limits. The higher quality of 1H-decoupled data suggests that higher strength magnets may be able to offer better peak separation and quantification of spectral components.
The strengths of our study include examination of two independent schizophrenia cohorts and HC groups matched for each group that shows consistency of association of C4A repeats with neuropil contraction, and extends the findings to decreased neuropil formation. Examining 31P MRS data acquired with and without 1H-decoupling show predicted associations with neuropil contraction supporting robustness of the findings. Innovative ddPCR assays with excellent quality control adds to these strengths. Demonstrating the associations of C4 GCN within the human context is important because C4A is human-specific and supplements the rodent model observations. Examining early course patients minimizes the confounds of prolonged medication exposure, illness chronicity, and comorbid illnesses and their treatment that may affect postmortem data. Limitations of our study include relatively small sample sizes. Temporal changes could not be inferred in this cross-sectional study. 31P MRS data suggests changes in the neuropil that is a synaptically dense region with few cell bodies but does not directly measure changes in synapses, dendritic arborization or spine density.
In summary, our study reports association of increased contraction and decreased formation of neuropil with the GCN of C4 variants. Although data from gene expression studies on postmortem brain tissues and animal models are persuasive, corroboration with live human patients is critical to advance the knowledge of biological significance of gene variants observed in repeatedly replicated GWA studies. Although C4B was not associated with schizophrenia risk in Sekar et al. study, it is likely that C4B may contribute to neuropil alterations that requires further studies. Larger sample sizes need to be examined to replicate our findings.
Acknowledgements
This study was funded through the National Institute of Mental Health grants MH72995, MH93540, and MH101566 (KMP), and MH63480 and the Stanley Medical Research Institute (SMRI) (V.L.N.). We thank Ms. Diana Mermon, Ms. Alicia Thomas, Ms. Karol Rosengarth, Mr. Kevin Eklund, Mr. Dhruman Goradia, Ms. Jean Miewald and Dr. Debra Montrose in characterizing subjects and help with data management.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cloutier M, et al. The Economic Burden of Schizophrenia in the United States in 2013. J. Clin. Psychiatry. 2016;77:764–771. doi: 10.4088/JCP.15m10278. [DOI] [PubMed] [Google Scholar]
- 2.Robinson DG, Woerner MG, McMeniman M, Mendelowitz A, Bilder RM. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am. J. Psychiatry. 2004;161:473–479. doi: 10.1176/appi.ajp.161.3.473. [DOI] [PubMed] [Google Scholar]
- 3.Henry LP, et al. The EPPIC follow-up study of first-episode psychosis: longer-term clinical and functional outcome 7 years after index admission. J. Clin. Psychiatry. 2010;71:716–728. doi: 10.4088/JCP.08m04846yel. [DOI] [PubMed] [Google Scholar]
- 4.Jaaskelainen E, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr. Bull. 2013;39:1296–1306. doi: 10.1093/schbul/sbs130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modestin J, Huber A, Satirli E, Malti T, Hell D. Long-term course of schizophrenic illness: Bleuler’s study reconsidered. Am. J. Psychiatry. 2003;160:2202–2208. doi: 10.1176/appi.ajp.160.12.2202. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J. Psychiatr. Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 7.Mayilyan KR, Weinberger DR, Sim RB. The complement system in schizophrenia. Drug. News Perspect. 2008;21:200–210. doi: 10.1358/dnp.2008.21.4.1213349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer U. Developmental neuroinflammation and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;42:20–34. doi: 10.1016/j.pnpbp.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Stanley JA, et al. Evidence of developmental alterations in cortical and subcortical regions of children with attention-deficit/hyperactivity disorder: a multivoxel in vivo phosphorus 31 spectroscopy study. Arch. Gen. Psychiatry. 2008;65:1419–1428. doi: 10.1001/archgenpsychiatry.2008.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettegrew JW, et al. Alterations in brain high-energy phosphate and membrane phospholipid metabolism in first-episode, drug-naive schizophrenics. A pilot study of the dorsal prefrontal cortex by in vivo phosphorus 31 nuclear magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 1991;48:563–568. doi: 10.1001/archpsyc.1991.01810300075011. [DOI] [PubMed] [Google Scholar]
- 11.Prasad KM, Burgess A, Nimgaonkar VL, Keshavan MS, Stanley JA. Neuropil pruning in early-course schizophrenia: immunological, clinical and neurocognitive correlates. Biol. Psychiatry: Cognitive Neuroscience & Neuroimaging. 2016;1:528–538. doi: 10.1016/j.bpsc.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirnics K, Middleton FA, Lewis DA, Levitt P. Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci. 2001;24:479–486. doi: 10.1016/S0166-2236(00)01862-2. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman RE, Dobscha SK. Cortical pruning and the development of schizophrenia: a computer model. Schizophr. Bull. 1989;15:477–490. doi: 10.1093/schbul/15.3.477. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman RE, McGlashan TH. Neural network models of schizophrenia. Neuroscientist. 2001;7:441–454. doi: 10.1177/107385840100700513. [DOI] [PubMed] [Google Scholar]
- 16.Cannon TD, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol. Psychiatry. 2015;77:147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pantelis C, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 18.Sun D, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr. Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi T, et al. Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr. Res. 2009;111:94–102. doi: 10.1016/j.schres.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch. Gen. Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 21.Ziermans TB, et al. Progressive structural brain changes during development of psychosis. Schizophr. Bull. 2012;38:519–530. doi: 10.1093/schbul/sbq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borgwardt SJ, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr. Res. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Walter A, et al. Hippocampal volume in subjects at high risk of psychosis: a longitudinal MRI study. Schizophr. Res. 2012;142:217–222. doi: 10.1016/j.schres.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Bangalore SS, et al. Untreated illness duration correlates with gray matter loss in first-episode psychoses. Neuroreport. 2009;20:729–734. doi: 10.1097/WNR.0b013e32832ae501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberman J, et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol. Psychiatry. 2001;49:487–499. doi: 10.1016/S0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 26.Prasad KM, Sahni SD, Rohm BR, Keshavan MS. Dorsolateral prefrontal cortex morphology and short-term outcome in first-episode schizophrenia. Psychiatry Res. 2005;140:147–155. doi: 10.1016/j.pscychresns.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Rudduck C, Beckman L, Franzen G, Jacobsson L, Lindstrom L. Complement factor C4 in schizophrenia. Hum. Hered. 1985;35:223–226. doi: 10.1159/000153549. [DOI] [PubMed] [Google Scholar]
- 28.Mayilyan KR, Dodds AW, Boyajyan AS, Soghoyan AF, Sim RB. Complement C4B protein in schizophrenia. World J. Biol. Psychiatry. 2008;9:225–230. doi: 10.1080/15622970701227803. [DOI] [PubMed] [Google Scholar]
- 29.Hakobyan S, Boyajyan A, Sim RB. Classical pathway complement activity in schizophrenia. Neurosci. Lett. 2005;374:35–37. doi: 10.1016/j.neulet.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Shcherbakova I, et al. The possible role of plasma kallikrein-kinin system and leukocyte elastase in pathogenesis of schizophrenia. Immunopharmacology. 1999;43:273–279. doi: 10.1016/S0162-3109(99)00099-5. [DOI] [PubMed] [Google Scholar]
- 31.Maes M, et al. Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res. 1997;66:1–11. doi: 10.1016/S0165-1781(96)02915-0. [DOI] [PubMed] [Google Scholar]
- 32.Mayilyan KR, Arnold JN, Presanis JS, Soghoyan AF, Sim RB. Increased complement classical and mannan-binding lectin pathway activities in schizophrenia. Neurosci. Lett. 2006;404:336–341. doi: 10.1016/j.neulet.2006.06.051. [DOI] [PubMed] [Google Scholar]
- 33.Fananas L, Moral P, Panadero MA, Bertranpetit J. Complement genetic markers in schizophrenia: C3, BF and C6 polymorphisms. Hum. Hered. 1992;42:162–167. doi: 10.1159/000154060. [DOI] [PubMed] [Google Scholar]
- 34.Spivak B, et al. Reduced total complement haemolytic activity in schizophrenic patients. Psychol. Med. 1993;23:315–318. doi: 10.1017/S0033291700028397. [DOI] [PubMed] [Google Scholar]
- 35.Idonije OB, Akinlade KS, Ihenyen O, Arinola OG. Complement factors in newly diagnosed Nigerian schizoprenic patients and those on antipsychotic therapy. Niger J. Physiol. Sci. 2012;27:19–21. [PubMed] [Google Scholar]
- 36.Schroers R, et al. Investigation of complement C4B deficiency in schizophrenia. Hum. Hered. 1997;47:279–282. doi: 10.1159/000154424. [DOI] [PubMed] [Google Scholar]
- 37.Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol. Immunol. 2011;48:1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekar A, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purcell SM, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ripke S, et al. Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi J, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefansson H, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mason MJ, et al. Low HERV-K(C4) copy number is associated with type 1 diabetes. Diabetes. 2014;63:1789–1795. doi: 10.2337/db13-1382. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, et al. Gene copy-number variation and associated polymorphisms of complement component C4 in human systemic lupus erythematosus (SLE): low copy number is a risk factor for and high copy number is a protective factor against SLE susceptibility in European Americans. Am. J. Hum. Genet. 2007;80:1037–1054. doi: 10.1086/518257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dodds AW, Ren XD, Willis AC, Law SK. The reaction mechanism of the internal thioester in the human complement component C4. Nature. 1996;379:177–179. doi: 10.1038/379177a0. [DOI] [PubMed] [Google Scholar]
- 46.Isenman DE, Young JR. The molecular basis for the difference in immune hemolysis activity of the Chido and Rodgers isotypes of human complement component C4. J. Immunol. 1984;132:3019–3027. [PubMed] [Google Scholar]
- 47.Blanchong CA, et al. Genetic, structural and functional diversities of human complement components C4A and C4B and their mouse homologues, Slp and C4. Int. Immunopharmacol. 2001;1:365–392. doi: 10.1016/S1567-5769(01)00019-4. [DOI] [PubMed] [Google Scholar]
- 48.Mack M, Bender K, Schneider PM. Detection of retroviral antisense transcripts and promoter activity of the HERV-K(C4) insertion in the MHC class III region. Immunogenetics. 2004;56:321–332. doi: 10.1007/s00251-004-0705-y. [DOI] [PubMed] [Google Scholar]
- 49.Blanchong CA, et al. Deficiencies of human complement component C4A and C4B and heterozygosity in length variants of RP-C4-CYP21-TNX (RCCX) modules in caucasians. The load of RCCX genetic diversity on major histocompatibility complex-associated disease. J. Exp. Med. 2000;191:2183–2196. doi: 10.1084/jem.191.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nimgaonkar VL, Prasad KM, Chowdari KV, Severance EG, Yolken RH. The complement system: a gateway to gene-environment interactions in schizophrenia pathogenesis. Mol. Psychiatry. 2017;22:1554–1561. doi: 10.1038/mp.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melbourne J. K., Rosen C., Feiner B., Sharma R. P. C4A mRNA expression in PBMCs predicts the presence and severity of delusions in schizophrenia and bipolar disorder with psychosis. Schizophr. Res. Epub ahead of print 2018/02/17 (2018). [DOI] [PMC free article] [PubMed]
- 52.Donohoe G., et al. Genetically predicted complement component 4A expression: effects on memory function and middle temporal lobe activation. Psychol. Med. 48:10:1608–1615 (2018). [DOI] [PubMed]
- 53.English JA, et al. Blood-based protein changes in childhood are associated with increased risk for later psychotic disorder: evidence from a nested case-control study of the ALSPAC longitudinal birth cohort. Schizophr. Bull. 2018;44:297–306. doi: 10.1093/schbul/sbx075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allswede DM, et al. Complement gene expression correlates with superior frontal cortical thickness in humans. Neuropsychopharmacology. 2018;43:525–533. doi: 10.1038/npp.2017.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricklin D, Reis ES, Mastellos DC, Gros P, Lambris JD. Complement component C3 - ‘Swiss Army Knife’ of innate immunity and host defense. Immunol. Rev. 2016;274:33–58. doi: 10.1111/imr.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kandel, E. R. in Principles of Neural Science 5th edn, Vol. I (McGraw-Hill, New York, 2013) 1709 pp.
- 57.Gazzaniga, M. S. in The Cognitive Neurosciences 4th edn, Vol. 17 (MIT Press, Cambridge, 2009) 1294 p.
- 58.Stanley JA, Pettegrew JW, Keshavan MS. Magnetic resonance spectroscopy in schizophrenia: methodological issues and findings–part I. Biol. Psychiatry. 2000;48:357–368. doi: 10.1016/S0006-3223(00)00949-5. [DOI] [PubMed] [Google Scholar]
- 59.Geddes JW, Panchalingam K, Keller JN, Pettegrew JW. Elevated phosphocholine and phosphatidylcholine following rat entorhinal cortex lesions. Neurobiol. Aging. 1997;18:305–308. doi: 10.1016/S0197-4580(97)80312-0. [DOI] [PubMed] [Google Scholar]
- 60.Marcucci H, Paoletti L, Jackowski S, Banchio C. Phosphatidylcholine biosynthesis during neuronal differentiation and its role in cell fate determination. J. Biol. Chem. 2010;285:25382–25393. doi: 10.1074/jbc.M110.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Brien JS, Sampson EL. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J. Lipid Res. 1965;6:537–544. [PubMed] [Google Scholar]
- 62.Pettegrew JW, Klunk WE, Panchalingam K, McClure RJ, Stanley JA. Molecular insights into neurodevelopmental and neurodegenerative diseases. Brain Res. Bull. 2000;53:455–469. doi: 10.1016/S0361-9230(00)00376-2. [DOI] [PubMed] [Google Scholar]
- 63.Goldstein G, et al. Molecular neurodevelopment: an in vivo 31P-1H MRSI study. J. Int. Neuropsychol. Soc. 2009;15:671–683. doi: 10.1017/S1355617709990233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eriksson SH, et al. Correlation of quantitative MRI and neuropathology in epilepsy surgical resection specimens–T2 correlates with neuronal tissue in gray matter. Neuroimage. 2007;37:48–55. doi: 10.1016/j.neuroimage.2007.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lockwood-Estrin G, et al. Correlating 3T MRI and histopathology in patients undergoing epilepsy surgery. J. Neurosci. Methods. 2012;205:182–189. doi: 10.1016/j.jneumeth.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 66.Pettegrew JW, et al. 31P nuclear magnetic resonance studies of phosphoglyceride metabolism in developing and degenerating brain: preliminary observations. J. Neuropathol. Exp. Neurol. 1987;46:419–430. doi: 10.1097/00005072-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Palay, S. L. & Chan-Palay, V. in Cellular Biology of Neurons. (ed. Kandel, E. R.) 5–37 (American Physiological Society, Bethesda, 1977).
- 68.Pfenninger KH. Plasma membrane expansion: a neuron’s Herculean task. Nat. Rev. Neurosci. 2009;10:251–261. doi: 10.1038/nrn2593. [DOI] [PubMed] [Google Scholar]
- 69.Pfenninger KH, Johnson MP. Membrane biogenesis in the sprouting neuron. I. Selective transfer of newly synthesized phospholipid into the growing neurite. J. Cell. Biol. 1983;97:1038–1042. doi: 10.1083/jcb.97.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson PM, et al. From the Cover: Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. PNAS. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl Acad. Sci. USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 73.Huttenlocher PR. Synaptic density in human frontal cortex-developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 74.Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am. J. Ment. Defic. 1984;88:488–496. [PubMed] [Google Scholar]
- 75.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387:167–178. doi: 10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 76.Goddings AL, et al. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jabes A, Lavenex PB, Amaral DG, Lavenex P. Postnatal development of the hippocampal formation: a stereological study in macaque monkeys. J. Comp. Neurol. 2011;519:1051–1070. doi: 10.1002/cne.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lavenex P, Banta Lavenex P, Amaral DG. Postnatal development of the primate hippocampal formation. Dev. Neurosci. 2007;29:179–192. doi: 10.1159/000096222. [DOI] [PubMed] [Google Scholar]
- 79.Stolz E, et al. Brain activation patterns during visual episodic memory processing among first-degree relatives of schizophrenia subjects. Neuroimage. 2012;63:1154–1161. doi: 10.1016/j.neuroimage.2012.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.First MB. The Structured Clinical Interview for DSM-IV for Axis I disorders: Clinical Version, Administration Booklet. Washington: American Psychiatric Press; 1997. [Google Scholar]
- 81.Wu H., Goradia D. D. & Stanley J. A. A fully automated and robust method of extracting CSI voxels from precise anatomical locations: An application ot a longitudinal 31P MRS study. A fully automated and robust method of extracting CSI voxels from precise anatomical locations: An application ot a longitudinal 31P MRS study. Proceedings of the The International Society of Magnetic Resonance in Medicine (Milan, Italy, 2014).
- 82.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Dale A, Fischl B, Sereno M. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 84.Fischl B, Dale A. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl Acad. Sci. USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maziade M, et al. Long-term stability of diagnosis and symptom dimensions in a systematic sample of patients with onset of schizophrenia in childhood and early adolescence. I: nosology, sex and age of onset. Br. J. Psychiatry. 1996;169:361–370. doi: 10.1192/bjp.169.3.361. [DOI] [PubMed] [Google Scholar]
- 86.Frangou S, Hadjulis M, Vourdas A. The Maudsley early onset schizophrenia study: cognitive function over a 4-year follow-up period. Schizophr. Bull. 2008;34:52–59. doi: 10.1093/schbul/sbm124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-Sads-Pl. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 88.Pohmann R, von Kienlin M. Accurate phosphorus metabolite images of the human heart by 3D acquisition-weighted CSI. Magn. Reson. Med. 2001;45:817–826. doi: 10.1002/mrm.1110. [DOI] [PubMed] [Google Scholar]
- 89.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 90.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol. Psychiatry. 1999;45:17–25. doi: 10.1016/S0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 91.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 92.Glantz LA, Lewis DA. Dendritic spine density in schizophrenia and depression. Arch. Gen. Psychiatry. 2001;58:203. doi: 10.1001/archpsyc.58.2.203. [DOI] [PubMed] [Google Scholar]
- 93.Garey LJ, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J. Neurol. Neurosurg. Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.MacDonald ML, et al. Selective loss of smaller spines in schizophrenia. Am. J. Psychiatry. 2017;174:586–594. doi: 10.1176/appi.ajp.2017.16070814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sweet RA, et al. Pyramidal cell size reduction in schizophrenia: evidence for involvement of auditory feedforward circuits. Biol. Psychiatry. 2004;55:1128–1137. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 97.Sweet RA, Pierri JN, Auh S, Sampson AR, Lewis DA. Reduced pyramidal cell somal volume in auditory association cortex of subjects with schizophrenia. Neuropsychopharmacology. 2003;28:599–609. doi: 10.1038/sj.npp.1300120. [DOI] [PubMed] [Google Scholar]
- 98.Smesny S, et al. Antipsychotic drug effects on left prefrontal phospholipid metabolism: a follow-up 31P-2D-CSI study of haloperidol and risperidone in acutely ill chronic schizophrenia patients. Schizophr. Res. 2012;138:164–170. doi: 10.1016/j.schres.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 99.Volz HP, et al. Increase of phosphodiesters during neuroleptic treatment of schizophrenics: a longitudinal 31P-magnetic resonance spectroscopic study. Biol. Psychiatry. 1999;45:1221–1225. doi: 10.1016/S0006-3223(98)00366-7. [DOI] [PubMed] [Google Scholar]
- 100.Volz HP, et al. 31Phosphorus magnetic resonance spectroscopy of the dorsolateral prefrontal region in schizophrenics–a study including 50 patients and 36 controls. Biol. Psychiatry. 1998;44:399–404. doi: 10.1016/S0006-3223(98)00061-4. [DOI] [PubMed] [Google Scholar]
- 101.Jayakumar PN, et al. High energy phosphate abnormalities normalize after antipsychotic treatment in schizophrenia: a longitudinal 31P MRS study of basal ganglia. Psychiatry Res. 2010;181:237–240. doi: 10.1016/j.pscychresns.2009.10.010. [DOI] [PubMed] [Google Scholar]