Abstract
The aim of this study was to discriminate the children malignant peripheral neuroblastic tumors (PNTs) from those with benign histotype ganglioneuroma (GN) based on clinical and biological characteristics in all PNTs. Four hundred and seventy-six patients were included in this study, containing 345 patients for model development and 131 patients for external validation. Multivariate logistic regression analysis was conducted to select potentially useful characteristics for discrimination of histopathology. External validation was performed for model evaluation. Compared with the main characteristics of GN (85/345, 24.6%), those of malignant PNTs (260/345, 75.4%) showed significant differences. Multivariate analysis was performed to further find the characteristics linked to histopathology. The results indicated that for the patients younger than 49 months, the primary site of adrenal and thoracic, the level of serum neuron-specific enolase (NSE) > 33 ng/mL, and tumor encasing blood vessels were the extremely important discrimination factors of malignant PNTs. The area under the receiver-operating-characteristic of the discrimination model was 0.96. The accuracy rate, sensitivity and specificity were 93.4%, 96.3% and 83.8%, respectively. Meanwhile, the accuracy rate of the external validation from the 131 patients was 97.0%. Overall, histopathologic type of childhood malignant PNTs can be discriminated based on age, primary site, NSE level and the relationship between primary tumor and blood vessels.
Introduction
Peripheral neuroblastic tumors (PNTs) occur mostly in children and arise in cells derived from the neural crest. PNT is a heterogeneous disease, varying in terms of location, histopathologic appearance, biologic characteristics and prognosis. Histopathologic types of PNTs include the malignant histotypes neuroblastoma (NB), ganglioneuroblastoma nodular (GNBn), and the stroma-rich ganglioneuroblastoma intermixed (GNBi), and benign histotype ganglioneuroma (GN)1. Malignant PNTs, especially NB, account for most of PNTs, with nearly 50% of patients have a high risk phenotype characterized by widespread disease dissemination and poor long-term survival, are responsible for 12% of deaths associated with cancer in children younger than 15 years of age2.
Former studies have found that malignant PNTs differ from GN in that they affect younger children3, produce elevated amounts of catecholamines and other tumor markers4, and are often symptomatic5,6. For these patients with malignant PNTs, they need a comprehensive assessment of the disease and a multimodality therapy that usually includes chemotherapy, surgical resection, and even high-dose chemotherapy with hematopoietic stem-cell rescue, radiation therapy and immunologic therapy according to the risk group classification7. On the contrary, surgical resection or observation strategy due to the significant morbidity associated with surgery are the common therapies for GNs8.
For a patient initially diagnosed of PNTs, doctors need to make a quick and accurate judgement of the disease condition through the basic clinical characteristics, imaging and bloodwork, and then determine the suitable diagnosis and treatment strategies. The published reports confirmed the numerous clinical and biological differences between diverse histotypes of PNTs. However, in clinical practice, there were no clear indicators and processes to differentiate malignant PNTs from GNs before histological examination. In addition, we often experience the uncertain pathological findings of biopsies for differential diagnostic between malignant PNTs and GNs especially when core needle biopsies were performed because biopsies could not reflect all histological features of the huge tumors. In previous studies it has been demonstrated that sometimes it has a malignant PNT (GNBi or GNBn) on surgery following initial biopsy of GN9. Therefore, it is necessary to build discriminative models based on the clinical and biological factors of PNTs to guide the clinical differential diagnosis and treatment.
In this study, we compare the characteristics between malignant PNTs and GNs. Meanwhile, we further find the best factors to discriminate malignant PNTs in all patients with PNTs, in order to recognize malignant PNTs as soon as possible and conduct a complete evaluation of tumor and give the personalized systemic therapy for these patients.
Patients and Methods
Patient information
A total of 476 patients diagnosed with PNTs, who were histopathologically proven in Beijing Children’s Hospital (BCH) Affiliated to the Capital Medical University between January 2013 and January 2018 were included retrospectively in this study. The basic information of patients were collected by referring to medical records, including age, sex, laboratory, imaging, and histopathological results. The initial diagnosis of malignant PNT was established by histology, as well as typical bone marrow metastasis in combination with abnormal catecholamines according to the International Neuroblastoma Staging System (INSS) criteria10. In special cases of seriously ill patients without bone marrow metastasis, the initial clinical diagnosis was established by typical tumor localisation with typical metastases (such as bone, liver, lymph node, skin and etc) detected by fluorine-18-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (18F-FDG PET/CT), and combined with abnormal tumor marker levels. However, the histology results of delayed surgery confirmed that these patients were malignant PNTs. All GNs were diagnosed by histology. All methods were carried out in accordance with relevant guidelines and regulations and the study has been approval by the Medical Ethics Committee of Beijing Children’s Hospital (2017-k-89). A waiver of consent was awarded to conduct analyses on the study.
Laboratory analysis
Laboratory analysis was done prior to treatment and the interval between laboratory tests and the biopsy was less than 15 days. Urinary vanillylmandelic acid (VMA) of 24 h was analyzed by High Performance Liquid Chromatography (HPLC) technique. Lactate dehydrogenase (LDH), neuron-specific enolase (NSE), and ferritin were determined on serum, using routine clinical chemistry laboratory methods.
Imaging analysis
The radiology reports of ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and/or 18F-FDG PET/CT were collected and analyzed retrospectively. Tumor lesions were identified on images at the primary site as any mass adjacent to the adrenal glands, in a paravertebral location, or in other tumor-typical regions of the head, thorax, abdomen or pelvis. According to the Response Evaluation Criteria in Solid Tumors (RECIST) guidance, published in 2000 and revised in 2009, tumor evaluation relied on measurement of nonnodal target lesions based on the longest single diameter11. Maximum diameters of primary tumors in CT and/or MRI were analyzed for all patients. Furthermore, by reference to the Image-Defined Risk Factors (IDRFs) in neuroblastic tumors12, we analyzed the relationship between primary tumor and important blood vessels (such as carotid, vertebral artery, subclavian vessels and internal jugular vein in neck; aorta, vena cava and/or major branches in thorax; aorta, vena cava, coeliac axis, superior mesenteric artery, and/or major branches, renal vessels in abdomen; iliac vessels in pelvis), which was one of the most important factors in determining whether the upfront tumor resection could be performed, and the relationship was classified as encasement, displacement, none, and unknown.
Histopathology
Tumors were classified in accordance with the International Neuroblastoma Pathology Classification System (INPC)13. Histologically, the balance between neural-type cells and Schwann-type (Schwannian) cells helped to categorize the tumor as NB, GNB, or GN, and morphologic indicators [grade of neuroblastic differentiation: undifferentiated, poorly differentiated and differentiating; and mitosis-karyorrhexis index (MKI): low, intermediate, and high MKI] for tumors were tested.
Statistical analysis
Statistical analysis was performed using SAS 9.4. Four hundred and seventy-six patients were included in this study, including 345 patients (January 2013 to January 2017) for model development and 131 patients (January 2017 to January 2018) for external validation. Clinical and biological factors of PNTs were analyzed in 345 patients with PNTs (January 2013 to January 2017). Tumor markers with abnormal distribution were presented as median and interquartile range. Mann-Whiney U test of tumor markers was used for comparing between malignant PNT subgroup and GN subgroup. Receiver operating characteristic (ROC) curve analysis was performed to determine the most appropriate cut-off values for age, tumor marker and maximum diameter of primary tumors. Univariate and multivariate logistic regression analyses were conducted to select potentially useful characteristics for discrimination of histopathology subtypes. The area under the receiver-operating-characteristic (AUC-ROC) curves of the model were calculated. External validation was conducted to verify the model effect. Back substitution test was conducted in patients with inconsistent pathological results of biopsy and surgery. P < 0.05 was considered to be statistically significant.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Results
Patients and tumor characteristics
The main characteristics of 345 patients diagnosed between January 2013 and January 2017 with PNTs were analyzed, including 260 malignant PNTs (75.4%) and 85 GNs (24.6%). Among the 260 malignant PNTs, 52 cases were initially diagnosed by upfront surgery, 28 cases by biopsy of surgery, 28 cases by needle core biopsy, 117 cases by typical bone marrow metastasis in combination with abnormal catecholamines and 35 cases were diagnosed by delayed surgery after the initial clinical diagnosis. All 85 GNs were diagnosed by histology of biopsy after surgery. Compared with the main characteristics of GN, those of malignant PNTs showed significant differences (Table 1). Patients with malignant PNTs were more frequently male (54.2% vs 40%) and were diagnosed at a younger median age (33 vs 72 months); the tumor was more often in the adrenal (56.5% vs 17.6%) and less frequently in the retroperitoneal (11.9% vs 28.2%). Moreover, the tumor markers of malignant PNTs were significantly differed from GNs, since they almost had elevated values of serum NSE (P < 0.0001), ferritin (P = 0.0024), LDH (P < 0.0001), and urinary VMA (P = 0.0009). By comparing the imaging findings, we found that, maximum diameter of primary tumor in malignant PNTs was larger than in GNs (P = 0.0002), and malignant PNTs were more prone to encase important blood vessels (72.3% vs 24.7%, P < 0.0001). Furthermore, statistically significant variables in Table 1 were included into multivariate analysis, and continuous variables were determined the most appropriate cut-off values by ROC curve analysis.
Table 1.
Characteristics of patients with malignant PNTs and GN.
| Variables | Malignant PNTsa,b | GN | Results | P | |
|---|---|---|---|---|---|
| n | 260 | 85 | |||
| Gender | Male | 141 (54.2%) | 34 (40.0%) | 5.190 | 0.022 |
| Female | 119 (45.8%) | 51 (60.0%) | |||
| Primary site | Adrenal | 147 (56.5%) | 15 (17.6%) | 43.941 | <0.001 |
| Retroperitoneal | 31 (11.9%) | 24 (28.2%) | |||
| Thoracic | 76 (29.2%) | 41 (48.2%) | |||
| Pelvic | 5 (1.9%) | 5 (5.9%) | |||
| The relationship between primary tumor and blood vessels | Encasement | 188 (72.3%) | 21 (24.7%) | 63.916 | <0.001 |
| Displacement | 34 (13.1%) | 31 (36.5%) | |||
| None | 34 (13.1%) | 33 (38.8%) | |||
| Unknown | 4 (1.5%) | 0 (0.0%) | |||
| Age (months) | 33.00 (18.00, 50.00) | 72.00 (53.50, 97.50) | 9.985 | <0.001 | |
| Maximum diameter of primary tumor (cm) | 8.50 (6.00, 12.23) | 6.71(4.80, 8.80) | −3.779 | <0.001 | |
| NSE (ng/mL)c | 269.00 (63.10, 370.00) | 21.25 (18.70, 24.90) | −11.473 | <0.001 | |
| Ferritin (ng/mL) | 208.70 (60.10, 453.40) | 44.80 (21.98, 81.93) | −3.030 | 0.002 | |
| LDH (U/L) | 572.50 (333.00, 1203.75) | 222.00 (199.00, 255.00) | −11.470 | <0.001 | |
| VMA (mg/24 h) | 13.92 (5.45, 37.53) | 4.99 (3.60, 7.58) | −3.320 | <0.001 | |
aContinuous variables were presented as median and interquartile range;
bClassification variables were presented as numbers (percent);
cReference ranges of tumor markers: serum NSE ≤ 25 ng/mL; serum ferritin 6 ng/mL – 159 ng/mL; serum LDH 110 U/L – 295 U/L; urinary VMA ≤ 13.6 mg/24 h
PNTs: peripheral neuroblastic tumors; GN: ganglioneuroma; NSE: neuron-specific enolase; VMA: vanillylmandelic acid; LDH: lactate dehydrogenase.
Determination of cut off value
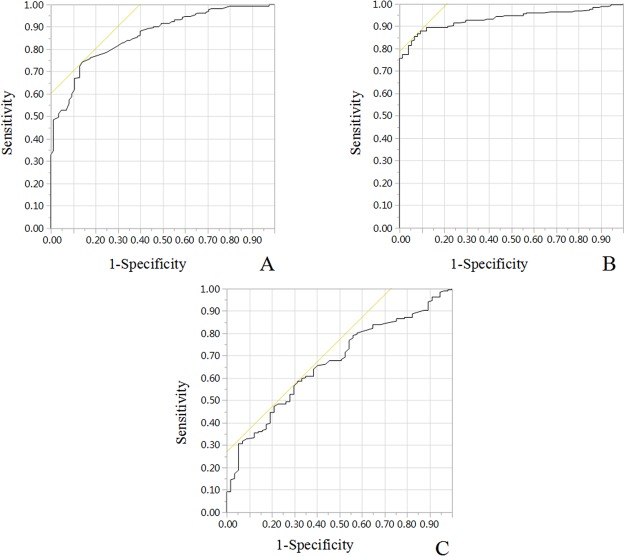
According to the maximum joint sensitivity and specificity values, stratification value of age, NSE, maximum diameter of primary tumor were calculated by ROC curve analyses. The cut off value for the characteristics above were 49 (months), 33 (ng/mL), and 7.97 (cm), respectively (Table 2 and Fig. 1).
Table 2.
Results of the ROC analysis.
| Variables | AUC-ROC | Cut-off value |
|---|---|---|
| Age | 0.86 | 49.00 (months) |
| Serum NSE | 0.93 | 33.00 (ng/mL) |
| Maximum diameter of primary tumor | 0.67 | 7.97 (cm) |
AUC-ROC: area under the receiver-operating-characteristic; NSE: neuron-specific enolase.
Figure 1.
ROC curve analyses. Stratification value of (A) age; (B) serum NSE; (C) maximum diameter of primary tumor were calculated by ROC curve analyses.
Multivariate logistic regression analysis
To further identify the characteristics linked to histopathology, multivariate analysis was conducted. After multivariate analysis, we found that age, primary site, serum NSE and the relationship between primary tumor and blood vessels were significant independent factors to discriminate histopathology between malignant PNTs and GNs. The adjusted OR (odds ratio) of the characteristics above were shown in Table 3. The results showed that there were four vital discrimination factors of malignant PNTs as followed, age ≤ 49 months, primary site of adrenal and thoracic, level of serum NSE > 33 ng/mL, and tumor encasing blood vessels, especially level of serum NSE > 33 ng/mL [OR, 36.154 (10.681, 122.379)] and age ≤ 49 months [OR, 19.049 (6.470, 56.088)]. The AUC of the model was 0.96 (Fig. 2). The accuracy rate, sensitivity and specificity were 93.4%, 96.3% and 83.8%, respectively. In our algorithm, small proportion (21 out of 317, 6.6%) of patients were misclassified, including 9 malignant PNTs missclassified as GNs and 12 GNs missclassified as malignant PNTs (Table 4).
Table 3.
Multivariate logistic regression analysis of prediction of malignant PNTs.
| Characteristics | Estimate | Standard Error | Wald | P | OR |
|---|---|---|---|---|---|
| Intercept | −10.73 | ||||
| Age > 49 (months) | Ref | ||||
| Age ≤ 49 (months) | 2.95 | 0.55 | 28.61 | <0.001 | 19.049 (6.470, 56.088) |
| Primary site of retroperitoneal | Ref | ||||
| Primary site of adrenal | 2.47 | 0.85 | 8.48 | 0.004 | 11.806 (2.242, 62.179) |
| Primary site of thoracic | 1.99 | 0.74 | 7.25 | 0.007 | 7.280 (1.717, 30.869) |
| Primary site of pelvic | 1.55 | 1.35 | 1.31 | 0.253 | 4.695 (0.331, 66.507) |
| Serum NSE ≤ 33 (ng/mL) | Ref | ||||
| Serum NSE > 33 (ng/mL) | 3.59 | 0.62 | 33.26 | <0.001 | 36.154 (10.681, 122.379) |
| None relationship between primary tumor and blood vessels | Ref | ||||
| Tumor encasing blood vessels | 1.82 | 0.60 | 9.49 | 0.002 | 6.163 (1.937, 19.608) |
| Tumor displacing blood vessels | −0.21 | 0.65 | 0.10 | 0.751 | 0.813 (0.227, 2.916) |
PNTs: peripheral neuroblastic tumors; OR, odds ratio; NSE, neuron-specific enolase.
Figure 2.
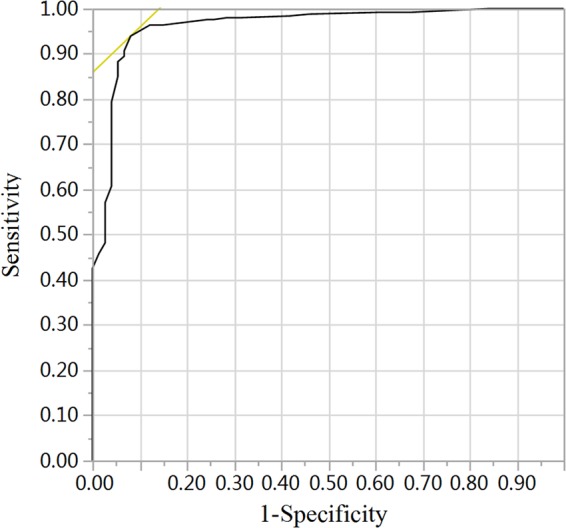
ROC for discrimination of malignant PNTs. Combined age ≤ 49 months, primary site of adrenal and thoracic, level of serum NSE > 33 ng/mL, and tumor encasing blood vessels to discriminate of malignant PNTs and the AUC of the discrimination model was 0.96.
Table 4.
Comparison between results using the model produced and the actual diagnosis produced by pathology.
| Practicea | Discriminationb | |
|---|---|---|
| Malignant PNTs | GN | |
| Malignant PNTs | 234 | 9 |
| GN | 12 | 62 |
aPractice represents the truth observed by pathology.
bDiscrimination represents the predicted value from the model.
PNTs: peripheral neuroblastic tumors; GN: ganglioneuroma.
External validation
One hundred and thirty-one patients with PNTs diagnosed between January 2017 and January 2018 were collected to conduct the external validation of the model. The accuracy rate was 97.0% (127/131). Four patients were misdiscriminated, including 2 malignant PNTs and 2 GNs.
Patients with inconsistent pathological results of biopsy and surgery
Four hundred and seventy-six patients were included in this study, containing 345 patients for model development and 131 patients for external validation. Among the 476 patients of this study, there were 12 patients had inconsistent pathological results of biopsy and surgery. The biopsy results of these patients showed benign tumor of GN, but the final surgery pathological results were malignant PNTs (Table 5). Thus, in order to verify the model effects of these patients, back substitution was conducted to discriminate malignant PNTs. The predicted results of 11 patients were consistent with the practice pathological results (all predicted values were > 0.90), which proved that the discrimination model was of high accuracy, even applied to cases that were difficult to identify of pathological examination.
Table 5.
Discrimination results of model in patients with inconsistent pathological results of biopsy and surgery.
| Patient | Age (months) | Serum NSE (ng/mL) | Primary site | Relationship between primary tumor and blood vessels | Biopsy method | Surgical pathology result | Predicted |
|---|---|---|---|---|---|---|---|
| 1 | 37 | 159.0 | Adrenal | Encasing | Surgery (skin metastasis) | NB | 0.99868 |
| 2 | 45 | 21.4 | Thoracic | Encasing | Core needlea | GNBi | 0.92828 |
| 3 | 40 | 70.4 | Adrenal | Encasing | Core needle | GNBi | 0.99868 |
| 4 | 15 | 43.2 | Adrenal | None | Surgery (cervical lymph node metastasis) | NB | 0.99194 |
| 5 | 41 | 146.4 | Adrenal | None | Core needle | NB | 0.99194 |
| 6 | 75 | 28.0 | Adrenal | None | Core needle | GNBi | 0.15166 |
| 7 | 11 | 98.0 | Thoracic | Encasing | Core needle | GNBn | 0.99787 |
| 8 | 18 | 63.2 | Retroperitoneal | Encasing | Core needle | NB | 0.98468 |
| 9 | 78 | 320.1 | Adrenal | Encasing | Core needle | NB | 0.97551 |
| 10 | 39 | 52.5 | Retroperitoneal | Encasing | Core needle | GNBi | 0.98468 |
| 11 | 28 | 370.0 | Adrenal | Encasing | Core needle | NB | 0.99868 |
| 12 | 77 | 63.3 | Adrenal | Encasing | Core needle | GNBn | 0.97551 |
aCore needle represented the core needle biopsy to primary tumor.
PNTs: peripheral neuroblastic tumors; GN: ganglioneuroma; NSE: neuron-specific enolase; NB: neuroblastoma; GNBn: ganglioneuroblastoma nodular; GNBi: ganglioneuroblastoma intermixed.
Discussion
Malignant PNTs and GNs are differed in numerous clinical and biological characteristics, and thus the treatment strategies are not exactly the same. The modern comprehensive treatment of malignant PNTs depends on risk group which is determined on patient age, tumor stage, histopathological appearance, and molecular biological factors. On the contrary, GN is considered to be a benign tumor of neural crest origin13 and tumor resection or observation is recommended3,14. However, in clinical practice, there were no clear indicators and processes to differentiate malignant PNTs from GNs before histological examination. In addition, sometimes we experience the uncertain findings of biopsies for diagnosis of different histopathology subgroups especially when core needle biopsies were performed. Therefore, it is necessary to build discriminative models to guide the clinical diagnosis and treatment. Thus, the result of this study may help us to discriminate PNTs by combining clinical and biological characteristics, and then give different evaluation and treatment strategies to malignant PNTs and GNs respectively.
Former studies found that compared to GNs, malignant PNTs were characterized by a significant increase in tumor marker levels [Serum NSE, ferritin, LDH, urinary VMA and homovanillic acid (HVA)], disseminated disease, younger age, more common primary site of abdomen and abnormal cytogenetics and molecular genetics8,9. As a relative specific tumor marker of PNTs, a large number of studies have analyzed the association between NSE level and initial diagnosis10, response assessment, INPC group15, tumor relapse or progression16, and prognosis17,18, and we found the importance of NSE level in the discriminative of different histopathology subgroups of PNTs. In terms of the primary tumor site, Vo et al.19 found that malignant PNTs were more often in adrenal, but Decarolis et al.8 found that GNs were less frequently in adrenal. In addition, in CT and MRI images, malignant PNTs mostly located in adrenal or in a paravertebral location with vascular embedding, local tissues metastasis and abundant blood supplying, which were reflections of infiltration and invasion features of malignant tumors. Whereas GN had relatively regular morphology and clear boundary, with vascular displacement as a primary manifestation20,21. As for the prognosis, malignant PNTs and GNs were strikingly distinct. Even after aggressive treatment, the prognosis of malignant PNTs especially late stage and high risk patients was still unsatisfactory. On the country, GN could get a good therapeutic effect only by surgical resection without any cytotoxic treatment3,14, and few patients had tumor recurrence or residual lesions of malignant transformation after incomplete resection9,22.
Some researches hypothesized that a good part of GN originated from NB. This theory has first been suggested by Cushing in 192623 and has since been suspected repeatedly for stage 4S24–26 as well as for localized NB27. An indirect support for this hypothesis can be seen by our observation that patients with GNs were older at diagnosis than those with malignant PNTs (median age of 72 vs 33 months). And former reports showed that the grade of neuroblastic differentiation increased with the median age at diagnosis and this was in line with the theory that mature PNTs might just had enough time to mature in situ before they were discovered8. However, whether this is true for all GNs, as proposed by Shimada1, still remains unproven.
NB, GNB, and GN represent tumors of neural crest origin with a continuous spectrum of neuronal maturation. In fact, maturation within a single tumor may not be homogeneous. This may complicate histopathological analysis, and biopsies especially needle core biopsies may not be representative. Although core needle biopsies are usually adequate to identify a tumor as neuroblastic in origin, they do not provide sufficient information about higher-order architecture and regional histological variation that could affect INPC classification and assignment of risk grouping and treatment protocol28. In this study, we can find that the biopsy of 12 patients suggested GN, whereas histological examination after total resection showed GNB or NB. We further used the model to discriminate malignant PNTs of these patients and found that only one patient’s predicted result was incorrect. The core needle biopsy showed GN whereas the eventually surgical histological examination revealed a localized GNBi tumor.
To sum up, the results allow the conclusion that combined age ≤ 49 months, primary site of adrenal and thoracic, level of serum NSE > 33 ng/mL, and tumor encasing blood vessels can help us to discriminate malignant PNTs in all PNTs with high accuracy. The result of external validation of the model showed a highly accuracy for the identification of cases, not only in external validity, but also in patients with inconsistent pathological results of biopsy and surgery. However, the generalizability of the model in other ethnicity is unknown. This result may lay the foundation for the development of differential diagnosis and identification strategies to malignant PNTs and GN in the future, and it is helpful to offer different evaluation and treatment strategies to those patients respectively.
Acknowledgements
The authors would like to thank Dr. Lejian He and Dr. Libing Fu (Department of Pathology, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health) for their help to provide detailed pathological diagnosis results of patients with PNTs in this study.
Author Contributions
Xiaoxia Peng and Huanmin Wang were responsible for the conception and design of the study. Xiaoli Ma, Qi Zeng, Hong Qin and Wei Han were responsible for acquisition of data. Siyu Cai performed the data analysis. Shen Yang and Siyu Cai drafted the manuscript. All authors participated in interpretation of the findings and all authors read and approved the final version of the manuscript. All authors confirm that the content has not been published elsewhere and does not overlap with or duplicate their published work.
Competing Interests
The authors declare no competing interests.
Footnotes
Shen Yang, Siyu Cai, Xiaoxia Peng and Huanmin Wang contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaoxia Peng, Email: niuniu@ccmu.edu.cn.
Huanmin Wang, Email: wanghuanmin@bch.com.cn.
References
- 1.Shimada H, et al. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86:349–363. doi: 10.1002/(SICI)1097-0142(19990715)86:2<349::AID-CNCR20>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 2.Irwin MS, Park JR. Neuroblastoma: paradigm for precision medicine. Pediatr Clin North Am. 2015;62:225–256. doi: 10.1016/j.pcl.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Geoerger B, et al. Metabolic activity and clinical features of primary ganglioneuromas. Cancer. 2001;91:1905–1913. doi: 10.1002/1097-0142(20010515)91:10<1905::AID-CNCR1213>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Lucas K, et al. Catecholamine metabolites in ganglioneuroma. Med Pediatr Oncol. 1994;22:240–243. doi: 10.1002/mpo.2950220405. [DOI] [PubMed] [Google Scholar]
- 5.Hayes FA, Green AA, Rao BN. Clinical manifestations of ganglioneuroma. Cancer. 1989;63:1211–1214. doi: 10.1002/1097-0142(19890315)63:6<1211::AID-CNCR2820630628>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Schulman H, et al. Ganglioneuroma: an ‘incidentaloma’ of childhood. Eur Radiol. 1998;8:582–584. doi: 10.1007/s003300050438. [DOI] [PubMed] [Google Scholar]
- 7.Laprie A, et al. High-dose chemotherapy followed by locoregional irradiation improves the outcome of patients with international neuroblastoma staging system Stage II and III neuroblastoma with MYCN amplification. Cancer. 2004;101:1081–1089. doi: 10.1002/cncr.20453. [DOI] [PubMed] [Google Scholar]
- 8.Decarolis B, et al. Treatment and outcome of Ganglioneuroma and Ganglioneuroblastoma intermixed. BMC Cancer. 2016;16:542. doi: 10.1186/s12885-016-2513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bernardi B, et al. Retrospective study of childhood ganglioneuroma. J Clin Oncol. 2008;26:1710–1716. doi: 10.1200/JCO.2006.08.8799. [DOI] [PubMed] [Google Scholar]
- 10.Brodeur GM, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Monclair T, et al. TheInternational Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009;27:298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimada H, et al. The International Neuroblastoma Pathology Classification (the Shimada system) Cancer. 1999;86:364–372. doi: 10.1002/(SICI)1097-0142(19990715)86:2<364::AID-CNCR21>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Okamatsu C, et al. Clinicopathological characteristics of ganglioneuroma and ganglioneuroblastoma: a report from the CCG and COG. Pediatr Blood Cancer. 2009;53:563–569. doi: 10.1002/pbc.22106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fendler WP, et al. Combined Scintigraphy and Tumor Marker Analysis Predicts Unfavorable Histopathology of Neuroblastic Tumors with High Accuracy. PLoS One. 2015;10:e0132809. doi: 10.1371/journal.pone.0132809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon T, Hero B, Hunneman DH, Berthold F. Tumour markers are poor predictors for relapse or progression in neuroblastoma. Eur J Cancer. 2003;39:1899–1903. doi: 10.1016/S0959-8049(03)00376-9. [DOI] [PubMed] [Google Scholar]
- 17.Cangemi G, et al. Prognostic value of ferritin, neuron-specific enolase, lactate dehydrogenase, and urinary and plasmatic catecholamine metabolites in children with neuroblastoma. Onco Targets Ther. 2012;5:417–423. doi: 10.2147/OTT.S36366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau L. Neuroblastoma: a single institution’s experience with 128 children and an evaluation of clinical and biological prognostic factors. Pediatr Hematol Oncol. 2002;19:79–89. doi: 10.1080/08880010252825669. [DOI] [PubMed] [Google Scholar]
- 19.Vo KT, et al. Clinical, biologic, and prognostic differences on the basis of primary tumor site in neuroblastoma: a report from the international neuroblastoma risk group project. J Clin Oncol. 2014;32:3169–3176. doi: 10.1200/JCO.2014.56.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang B, Lv DK, Gao SJ, Meng JJ. Differential diagnosis of CT images in children with neuroblastomas and ganglioneuroblastomas. Asian Pac J Cancer Prev. 2014;15:10509–10512. doi: 10.7314/APJCP.2014.15.23.10509. [DOI] [PubMed] [Google Scholar]
- 21.Guan YB, Zhang WD, Zeng QS, Chen GQ, He JX. CT and MRI findings of thoracic ganglioneuroma. Br J Radiol. 2012;85:e365–372. doi: 10.1259/bjr/53395088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Retrosi G, et al. Morbidity after ganglioneuroma excision: is surgery necessary? Eur J Pediatr Surg. 2011;21:33–37. doi: 10.1055/s-0030-1263195. [DOI] [PubMed] [Google Scholar]
- 23.Cushing H, Wolbach SB. The Transformation of a Malignant Paravertebral Sympathicoblastoma into a Benign Ganglioneuroma. Am J Pathol. 1927;3(203-216):207. [PMC free article] [PubMed] [Google Scholar]
- 24.Przkora R, Perez-Canto A, Ertel W, Heyde CE. Ganglioneuroma: primary tumor or maturation of a suspected neuroblastoma? Eur Spine J. 2006;15:363–365. doi: 10.1007/s00586-005-0964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rozmus J, Langer M, Murphy JJ, Dix D. Multiple persistent ganglioneuromas likely arising from the spontaneous maturation of metastatic neuroblastoma. J Pediatr Hematol Oncol. 2012;34:151–153. doi: 10.1097/MPH.0b013e318221ca82. [DOI] [PubMed] [Google Scholar]
- 26.Haas D, Ablin AR, Miller C, Zoger S, Matthay KK. Complete pathologic maturation and regression of stage IVS neuroblastoma without treatment. Cancer. 1988;62:818–825. doi: 10.1002/1097-0142(19880815)62:4<818::AID-CNCR2820620430>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 27.Hero B, et al. Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95-S and NB97. J Clin Oncol. 2008;26:1504–1510. doi: 10.1200/JCO.2007.12.3349. [DOI] [PubMed] [Google Scholar]
- 28.Peuchmaur M, et al. Revision of the International Neuroblastoma Pathology Classification: confirmation of favorable and unfavorable prognostic subsets in ganglioneuroblastoma, nodular. Cancer. 2003;98:2274–2281. doi: 10.1002/cncr.11773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.