Abstract
A new study shows that astrocytes are involved in the development of chronic itch in a mouse model. This dependent on upregulation of lipocalin 2 (LCN2) by the transcription factor STAT-3 and astrogliosis.
Acute itch, or pruritus, serves as a biological warning that protects against parasites, disease-carrying insects and poisonous plants. The past decade has provided new insights into the molecular mechanisms that underlie the transmission of itch, identifying new receptors and neural circuits1–3. Although acute itch is protective, chronic itch can be a devastating illness. Pruritus that lasts longer than 6 weeks is deemed chronic and is accompanied by a host of co-morbidities including depression, sleep and anxiety disorders4. Millions of people suffer from chronic itch, and it is associated with a wide variety of diseases, ranging from diabetes and cancer to kidney disorders5. Up until now, our understanding of how acute itch transitions into a state of chronic itch has not been well understood. Here Shiratori-Hayashi and colleagues present exciting work that links astrocytes to the maintenance of chronic itch6.
Previous studies of itch have focused on the peripheral afferent and spinal neural circuits and molecular pathways. Characterizing pruritus has been no easy task, with studies showing multiple receptors responsible for mediating differing itch-inducing substances such as histamine or the malarial drug chloroquine7,8. Moreover, itch neurons seem to be a heterogeneous population with subtypes that share only certain commonalities. However, the consensus at present is that these peripheral itch neurons project signals to the dorsal horn, an area of the spinal cord that receives and processes sensory information. Shiratori-Hayashi and colleagues6 took a different approach to study chronic itch by investigating the role of glia—specifically astrocytes—a type of support cell in the central nervous system.
To explore the role of non-neuronal cells in maintaining itch, the authors used a mouse model of atopic dermatitis. These mice, when removed from a pathogen-free environment, developed the hallmarks of human chronic itch, including repetitive spontaneous scratching and skin lesions. Using this animal model, Shiratori-Hayashi et al.6 then examined how the morphology of astrocytes in the spinal cord had changed. When the spinal cord was fluorescently labeled and examined using a confocal microscope, the astrocytes displayed enlarged cell bodies and overly arborized processes characteristic of astrogliosis, a type of change in glia that occurs only after injury or infection (Fig. 1). Compared to what they observed in healthy mice, the authors also saw increased levels of the astrocyte marker GFAP in segments of the spinal cord that signal to areas of the body that the animal scratched6. This phenotype could also be recapitulated by injecting wild-type mice with diphenylcyclopropenone (DCP), an immunotherapy drug known to produce pruritus as a side effect. However, glia from segments of the spinal cord that did not show itch behavior were normal. Up until now, astrogliosis has primarily been thought of as a feature found in nerve-injury models of chronic pain; however, the findings here also point to it being an important marker for chronic itch.
Figure 1.
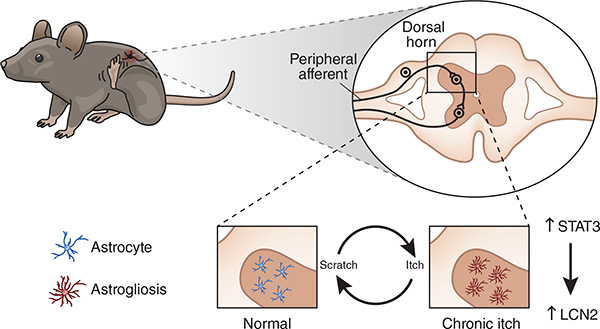
Astrogliosis contributes to chronic itch. In a chronic itch state, scratching results in the astrocytes of the dorsal horn becoming reactive (right). Shiratori-Hayashi et al.6 show that the protein LCN, under the control of the transcription factor STAT3, underlies this reactive astrogliosis.
To study the possible role that scratching has in producing astrogliosis, the investigators ablated a subset of itch-sensing nerve fibers expressing the ion channel TRPV1, using high concentrations of the potent plant toxin resiniferatoxin. Mice whose TRPV1+ fibers were abolished showed both reduced GFAP expression and scratching behavior. These results provide a possible link between peripheral skin lesions caused by chronic itch and reactive astrocytes in the dorsal horn and are consistent with previous studies in which ablation of peptidergic-expressing C-fibers exhibited near-complete loss of both heat-and histamine-evoked itch9.
Having established the link between chronic itch and abnormal astrocyte growth, the authors then asked what specific cellular mechanism might be involved6. Conditional knockout of the transcription factor STAT3 in astrocytes was previously shown to attenuate some of the features of astrogliosis in mice. Similarly, the authors saw substantial increases in STAT3 immunofluorescence in mice with chronic itch6. Furthermore, Shiratori-Hayashi et al.6 found that injection with AG490, an upstream inhibitor of STAT3, was capable of reducing both STAT3 expression and scratching behavior. Interestingly, the authors found that when STAT3 is genetically ablated in mouse astrocytes, animals still respond normally to injections of histamine and chloroquine, representative assays that induce itch, which implicates STAT3 changes in chronic, but not acute, itch behavior.
Next, Shiratori-Hayashi et al.6 began to look for a possible protein candidate associated with astrogliosis. A screen of mRNAs taken from spinal cord tissue of atopic dermatitis mice showed the Lcn2 gene to be highly upregu lated. Lcn2 encodes the protein LCN, which is involved in the transport of intracellular molecules and has been shown to be released into areas experiencing inflammation, where it can alter glial growth or lead to cell death. This makes it a possible candidate for being involved in astrogliosis. LCN was found to be increased in astrocytes from both chronic-itch mice and mice treated with DCP; likewise, Lcn2- knockout mice had reductions in DCP-induced scratching. When compared to normal mice, DCP-induced upregulation of the Lcn2 gene was reduced in mice whose astrocytes had had STAT3 deleted, a result that connects STAT3 to LCN production (Fig. 1). Collectively, these data point to the inhibition of LCN as a possible option for the treatment of chronic itch.
Over the past decade, studies for understanding chronic itch have been focusing on mapping out the afferent and spinal wiring that generate itching. However, the data presented by Shiratori-Hayashi and colleagues6 provides evidence for astrocytes, via their dysregulation in the spinal cord, as a component in the maintenance of chronic itch. Although it seems that pain and itch have distinct afferent fibers10, this study shows that chronic pain and chronic itch share similar molecular pathways, including the upregulation of STAT3. Nevertheless, in many chronic pain modalities, peripheral nerve injury is the primary cause, whereas in the chronic-itch model, peripheral neurons seem intact. Moreover, LCN protein upregulation appears to be unique to chronic itch.
Questions still remain as to how peripheral input into the dorsal horn can lead to the formation of STAT3-depen dent reactive astrocytes. There is evidence of altered gene regulation during the itch-scratch cycle11. Perhaps maladaptive changes in gene transcription during acute itch lead to the resultant dysregulation of astrocytes in the spinal dorsal horn and thus the transition to a chronic problem. Regardless, chronic itch is still resistant to treatment, but the work presented by Shiratori-Hayashi et al.6 provides new potential therapeutic targets for this debilitating illness.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Dustin Green, Solomon H. Snyder Department of Neuroscience, Department of Neurosurgery, Department of Dermatology and Center for Sensory Biology, Johns Hopkins University, School of Medicine, Baltimore, Maryland, USA.
Xinzhong Dong, Xinzhong Dong is in the Solomon H. Snyder Department of Neuroscience, Department of Neurosurgery, Department of Dermatology, Center for Sensory Biology and the Howard Hughes Medical Institute, Johns Hopkins University, School of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA..
References
- 1.Han L et al. Nat. Neurosci 16, 174–182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross SE et al. Neuron 65, 886–898 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Y-G & Chen Z-F Nature 448, 700–703 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Ständer S et al. Acta Derm. Venereol 87, 291–294 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Weisshaar E & Dalgard F Acta Derm. Venereol 89, 339–350 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Miho Shiratori-Hayashi KK et al. Nat. Med 21, 927–931 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Tani E & Ishikawa T Auris Nasus Larynx 17, 267–274 (1990). [DOI] [PubMed] [Google Scholar]
- 8.Liu Q et al. Cell 139, 1353–1365 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCoy ES et al. Neuron 78, 138–151 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberson DP et al. Nat. Neurosci 16, 910–918 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson SR et al. Nat. Neurosci 14, 595–602 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]


