Abstract
Numerous studies investigate morphology in the context of habitat, and lizards have received particular attention. Substrate usage is often reflected in the morphology of characters associated with locomotion, and, as a result, claws have become well‐studied ecomorphological traits linking the two. The Kimberley predator guild of Western Australia consists of 10 sympatric varanid species. The purpose of this study was to quantify claw size and shape in the guild using geometric morphometrics, and determine whether these features correlated with substrate use and habitat. Each species was assigned a Habitat/substrate group based on the substrate their claws interact with in their respective habitat. Claw morphometrics were derived for both wild caught and preserved specimens from museum collections, using a 2D semilandmark analysis. Claw shape significantly separated based on Habitat/substrate group. Varanus gouldii and Varanus panoptes claws were associated with sprinting and extensive digging. Varanus mertensi claws were for shallow excavation. The remaining species’ claws reflected specialization for some form of climbing, and differed based on substrate compliance. Varanus glauerti was best adapted for climbing rough sandstone, whereas Varanus scalaris and Varanus tristis had claws ideal for puncturing wood. Phylogenetic signal also significantly influenced claw shape, with Habitat/substrate group limited to certain clades. Positive size allometry allowed for claws to cope with mass increases, and shape allometry reflected a potential size limit on climbing. Claw morphology may facilitate niche separation within this trophic guild, especially when considered with body size. As these varanids are generalist predators, morphological traits associated with locomotion may be more reliable candidates for detecting niche partitioning than those associated directly with diet.
Keywords: ecomorphology, niche partitioning, semilandmarks, the Kimberley, Varanidae, Western Australia
1. INTRODUCTION
Ecomorphology investigates the functional design of an organism in relationship with its environment, as morphology can limit the ability for said organism to obtain resources (Wainwright, 1991). Numerous systems and morphological traits have been explored to determine how morphology links with performance and habitat (Arnold, 1983; Findley & Black, 1983; James, 1982; Karr & James, 1975; Losos, 1990a; Melville & Swain, 2000; Williams, 1972). Lizards have often been study systems to test such principles, with investigations of body proportions (Herrel, Meyers, & Vanhooydonck, 2001; Thompson & Withers, 1997; Vanhooydonck & Van Damme, 1999), clinging, sprinting, and jumping ability (Irschick et al., 1996,2005; Losos, 1990b; Losos & Sinervo, 1989; Van Damme, Aerts, & Vanhooydonck, 1997; Zamora‐Camacho, Reguera, Rubiño‐Hispán, & Moreno‐Rueda, 2014), retreat choice (Thompson, Clemente, Withers, Fry, & Norman, 2009), limb bone loading and gait (Clemente, Withers, Thompson, & Lloyd, 2011; McElroy & Reilly, 2009), and biting structures (Herrel, Spithoven, Van Damme, & De Vree, 1999; Herrel, Van Damme, Vanhooydonck, & Vree, 2001; Verwaijen, Van Damme, & Herrel, 2002).
Morphological adaptations associated with substrate usage are often reflected in locomotor traits (Grizante, Navas, Garland, & Kohlsdorf, 2010; Losos, 1990b; Vanhooydonck, Andronescu, Herrel, & Irschick, 2005). Claws are therefore well studied, as they are often the first and last structure to interface with substrate during locomotion (Birn‐Jeffery, Miller, Naish, Rayfield, & Hone, 2012). Claw characteristics in lizards have been correlated with performance variables such as clinging and sprinting (Crandell, Herrel, Sasa, Losos, & Autumn, 2014; Tulli, Abdala, & Cruz, 2011, 2012; Zani, 2000) or habitat/microhabitat preference (Ribas et al., 2004; Teixeira‐Filho, Rocha‐Barbosa, Paes, Ribas, & de Almeida, 2001; Tulli, Cruz, Herrel, Vanhooydonck, & Abdala, 2009). Bird claws have also received significant attention (Hahn, Dimitrov, Rehse, Yohannes, & Jenni, 2014), focusing on curvature and its relationship with habitat (Bock & Miller, 1959; Fowler, Freedman, & Scannella, 2009; Glen & Bennett, 2007; Mosto & Tambussi, 2014; Pike & Maitland, 2004). Large comparative studies of mammals have used claws/unguals to determine locomotor, and in particular fossorial, adaptations (MacLeod & Rose, 1993). Modern claws are often correlated with those of nonavian dinosaurs to extrapolate paleo‐behavior (Burnham, Feduccia, Martin, & Falk, 2011; Fowler, Freedman, Scannella, & Kambic, 2011; Lautenschlager, 2014). These studies quantified claw morphology in several ways, including Euclidean distance measures, claw curvature based on triangles, outline‐based morphometrics, and digital modelling (respective examples in Ribas et al., 2004; Feduccia, 1993; MacLeod & Rose, 1993; Manning et al., 2009). Most studies of lizard claw morphology in particular are a variation of the method presented by Zani (2000), which combined Euclidean measures and angular values.
Claws may function as ecomorphologically significant traits within trophic guilds, potentially allowing members to exploit different habitats and reduce interspecific competition. Guilds are defined as multiple species that exploit a similar resource in a similar way (Root, 1967). The monitor lizards (Family: Varanidae) of northern Australia form closely related top‐predator guilds (Wilson & Swan, 2013). The Kimberley (Western Australia) guild consists of 10 sympatric varanid species. Guild members are characterized as generalist, opportunistic predators possessing a degree of dietary overlap (Losos & Greene, 1988; Shine, 1986). The spread of the invasive cane toad (Rhinella marinas) has decreased populations of many varanid species across northern Australia (Doody et al., 2009; Doody, James, et al., 2014; Doody, Mayes, et al., 2014; Doody et al., 2017; Doody, Soanes, et al., 2015; Shine, 2010), potentially changing the nature of these guilds.
The purpose of this study was to quantify the morphological variability in the claws of the Kimberley monitor lizard guild, and determine whether it correlated with function and ecology. Our major hypothesis was if monitor lizard claws interacted with a variety of substrates, then they would have significantly different claw morphologies. We clustered the varanid species into ecological groups based on the substrate their claws typically interact with within their respective habitats. We then measured both forelimb and hindlimb claws, and analyzed them using geometric morphometrics. We also investigated how allometry and phylogeny might also influence claw structure. Lastly we explored how claw morphology could potentially facilitate niche separation in the Kimberley monitor guild.
2. MATERIALS AND METHODS
2.1. Ecological assignments
The Kimberley landscape is diverse, with gorges, boulder fields, riparian zones, and savannahs of flat, open grasslands. As habitat, locomotor mode, and substrate are closely linked, varanid claws may interact with number of substrates to varying degrees. Therefore, varanid species were placed in a priori groups based on these factors. Estimates of species substrate usage and locomotor mode were taken from the relevant literature and personal observations (Clemente, Thompson, & Withers, 2009; Openshaw & Keogh, 2014; Thompson et al., 2009; Wilson & Swan, 2013). This resulted in five Habitat/substrate groups (Table 1):
Table 1.
Kimberley varanid species with Habitat/substrate group, clade according to molecular phylogeny, and number of specimens sampled
| Species | Habitat/substrate group | Clade | N |
|---|---|---|---|
| Varanus acanthurus | Rocky‐field | Acanthurus | 15 |
| Varanus glauerti | Escarpment | Tristis | 7 |
| Varanus glebopalma | Rocky‐field | Tristis | 5 |
| Varanus gouldii | Savannah‐burrower | Gouldii | 12 |
| Varanus kingorum | Rocky‐field | Acanthurus | 3 |
| Varanus mertensi | Riverbed | Gouldii | 14 |
| Varanus mitchelli | Arboreal | Tristis | 10 |
| Varanus panoptes | Savannah‐burrower | Gouldii | 7 |
| Varanus scalaris | Arboreal | Tristis | 18 |
| Varanus tristis | Arboreal | Tristis | 4 |
Arboreal consists of savannah species which are primarily observed climbing trees. Varanus scalaris and Varanus tristis may be found within grassland trees (Pianka, 2004; Smith, Sweet, & King, 2004; Sweet, 2007). Varanus mitchelli frequents mangroves in the riparian zone, and uses branches to launch into rivers (Schultz & Doody, 2004; Shine, 1986).
Escarpment consists of monitors that climb large, vertical faces of sandstone escarpments, typically within gorges. This group is solely composed of the saxicolous Varanus glauerti (Sweet, 2004a).
Riverbed consists solely of Varanus mertensi, which is almost always found in proximity to permanent freshwater (Christian, 2004a; pers. obs.). This species is known to occasionally bask and/or sleep in trees, but is more typically associated with the rocks and soil of the river's edge. It excavates shallow burrows near water, and forages primarily on semi‐ and fully aquatic river prey (Kennett, Christian, & Pritchard, 1993; Losos & Greene, 1988; Mayes, Thompson, & Withers, 2005; Rhind, Jackson, Pezaro, & Doody, 2016).
Rocky‐field consists of monitors found in rocky open fields, composed of spinifex grasses, small trees, boulders, and outcrops. These species cross open terrain, climb up rocks, and refuge within crevices. This group includes Varanus acanthurus, Varanus glebopalma, and Varanus kingorum (Dryden, 2004; King, 2004; Sweet, 2004b).
Savannah‐burrower consists of large, widely foraging, savannah monitors who burrow extensively in soil (Doody, James, et al., 2014). These species are rarely found in trees, and include Varanus gouldii and Varanus panoptes (Christian, 2004b; Thompson, 1995).
2.2. Specimens and data collection
Claw morphometrics were taken from both wild caught and preserved specimens. Wild‐caught specimens were captured at El Questro Wilderness Park, situated in the Kimberley, Western Australia (15°53′42.1″S, 128°7′56.8″E), during the Dry Season. Lizards were caught using a combination of trapline fences equipped with pit‐ and funnel‐traps (similar to Doody, Clulow, et al., 2015), noosing, and hand‐capture. Field researchers would survey the park in teams, and noose specimens when encountered. Specimens were placed in a breathable cloth bag upon capture, taken back to camp, processed, marked, and released the following day in the same place. Dry‐ and ethanol‐preserved specimens were from the Division of Reptiles and Amphibians in the University of Michigan Museum of Zoology (UMMZ). The only specimens in the collection omitted had damage to the distal tissue where the claw erupted, or the claw was broken or visibly worn. All specimens had their snout‐vent length (SVL) taken using measuring tape.
The claw of digit IV of both the forelimb and hindlimb of each side was placed lateral‐side up against a light background with a scale. Photographs were taken with a Canon Rebel T3 EOS and 60 mm Macro lens held perpendicular to the claw. A camera stand ensured proper perspective in the museum. For wild specimens, the lizard was held in position by a researcher while another photographed it (Figure 1).
Figure 1.
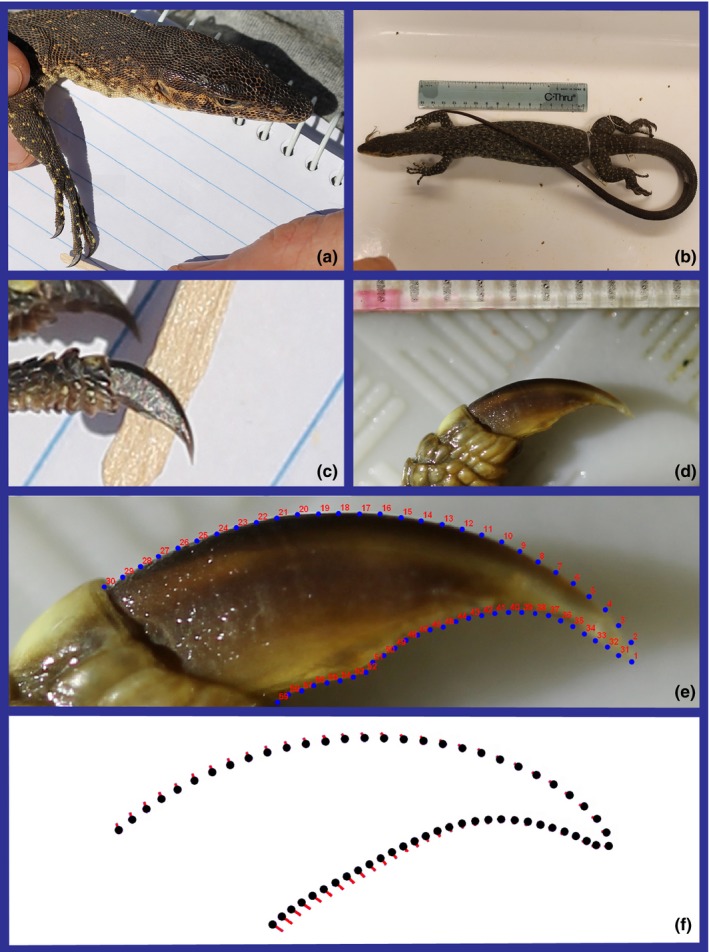
Claws were photographed from both wild caught (a,c; Varanus mitchelli, Vmi12) and preserved museum (b,d; V. mitchelli, UMMZ 210576) specimens. (e) Fifty‐nine equidistant coordinates were plotted, and semilandmarks were slid using minimum bending energy. (f) Shape variance was represented through vector diagrams
Tinius and Russell (2017) proposed the use of “pseudolandmarks” (referred to here as semilandmarks) when measuring claws, and we adopted a similar method. This approach, nested in geometric morphometrics (Bookstein, 1997; Zelditch, Swiderski, Sheets, & Fink, 2004), best assumes the totality of claw shape. The margin of the claw was traced from photographs in TpsDig 2.16 using the curve drawing tool (Rohlf, 2010). Tracing started at the base where the claw erupts to the tip, on both the dorsal and palmer/plantar sides (Figure 1). The base of the claw itself was not traced, because (a) our method as is accurately depicted the height of the claw at the base and (b) differences in distal scale morphology would add shape variance that is not relevant to claw function. The two traced margins were transformed into 30 equidistant coordinates, and the coordinates at the tip were combined into one. This resulted in three landmarks and 56 semilandmarks, the latter of which were slid to minimize the bending energy (Gunz & Mitteroecker, 2013; Perez, Bernal, & Gonzalez, 2006) using TpsRelw 1.53 (Rohlf, 2013). This program also performed a generalized least squares Procrustes superimposition on the data, and calculated centroid size (CS). CS is the square root of the sum‐squared distances from the landmarks to the centroid (Zelditch et al., 2004). It is technically a linear measurement, but measures overall size as opposed to a single Euclidean dimension. Bilateral symmetry was assumed; the superimposed coordinates and CS were averaged between left and right sides.
2.3. Ordination and statistics
All analyses were conducted in MorphoJ (Klingenberg, 2011) and SPSS Version 19.0 (IBM Corp, Armonk, NY). A 10,000 permutations test of the Procrustes distance between forelimb and hindlimb claws determined there was no significant difference between them (p = 0.0514). Forelimb and hindlimb claws were therefore analyzed together from here on.
Numerous genetic phylogenies of Varanidae exist (Ast, 2001; Clemente et al., 2009; Fitch, Goodman, & Donnellan, 2006; Vidal et al., 2012), and the consensus is that four major clades are present in Australia; the pygmy monitors (“Odatria” clade), the sand monitors (“Gouldii” clade), the lace monitors (“Varius” clade), and the mangrove monitors (“Indicus” clade). Odatria may be further divided into the “Tristis” and “Acanthurus” clades (Table 1). Claw morphometrics were mapped onto a molecular phylogeny with branch lengths in MorphoJ (based on Thompson et al., 2009), to evaluate shape and Habitat/substrate group in reference to relatedness. A 10,000 permutations test against the null hypothesis indicated that phylogenetic signal did influence claw shape (p = 0.0034), so this relationship was investigated further. MorphoJ generated phylogenetically independent contrasts (PICs) as a way of measuring relative shape change decoupled from relatedness (Felsenstein, 1985; Klingenberg & Marugán‐Lobón, 2013). The output was in x‐y coordinates for each branching on the phylogeny. These coordinates were converted to Procrustes distances, and a greater value indicated a greater relative shape change decoupled from phylogenetic history. Sample claws and line drawings of species means were also plotted on the phylogeny for qualitative visual comparison.
Allometric reduced major axis regressions (sensu Clarke, 1980) and their residuals were produced using PAST (Hammer, Harper, & Ryan, 2001), plotting CS and shape coefficients against SVL for all individuals. Allometry concerning CS was defined by a statistically significant (p < 0.05) slope deviating from 1. Consistency in shape indicates isometry; therefore, allometric shape change is any significant slope. Residuals were then analyzed to determine significant differences between Habitat/substrate groups once the data were normalized. These residuals were not normally distributed within certain groups according to Shapiro–Wilk tests, but homoscedastic according to Levene's test. Therefore, they were analyzed using a nonparametric analysis of variance (Kruskal–Wallis).
Procrustes distances were calculated between Habitat/substrate groups to determine the pairwise differences in mean shapes. The statistical significance of these distances was assessed with 10,000 permutations tests. A principal components analysis (PCA) was conducted to determine the degree of shape variance within the data set. All PCs representing over 10% of the variance were considered, and were plotted as x–y scatter‐based morphospaces. The aforementioned phylogeny was also plotted on the same morphospace resulting in a phylomorphospace.
3. RESULTS
3.1. Phylogenetic context of Habitat/substrate groups
Habitat/substrate groups were exclusive to either the Odatria or Gouldii clades, a separation reflected by a relatively large PIC at the root (Figure 2). All Riverbed and Savannah‐burrower taxa were exclusive to Gouldii, as another large PIC signified the separation between these two groups. Although Odatria consisted of three Habitat/substrate groups, the clade mostly showed low PICs with one exception. The entirety of the Acanthurus clade was Rocky‐field with a relatively low PIC. The Tristis clade had one Rocky‐field representative branching off early, with the remaining members mostly being Arboreal. The branching off of the Escarpment taxon from the Arboreal resulted in the largest PIC in our data set.
Figure 2.
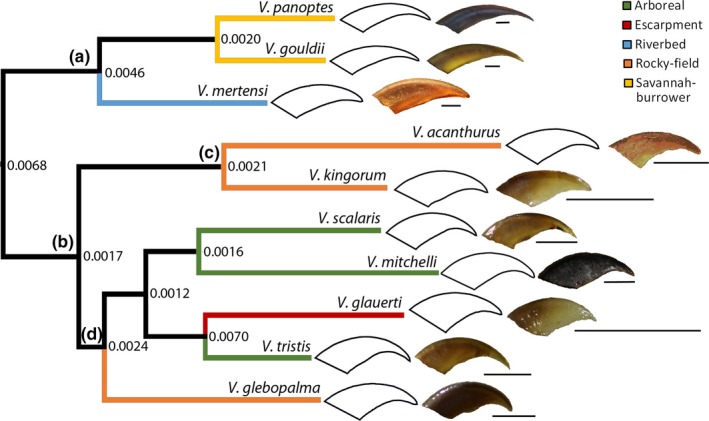
Phylogenetic tree topology of Varanus used in this study, based on Thompson et al. (2009) and generated using Mesquite (Maddison & Maddison, 2017). Major clades are labelled, including (a) Gouldii, (b) Odatria, (c) Acanthurus, and (d) Tristis. Numbers at each node indicate phylogenetically independent contrasts in the form of Procrustes distances for associated branches. Line diagrams indicate the mean shape for each species, and colored branches indicate Habitat/substrate group. Scale = 2 mm
3.2. Allometry
Arboreal, Escarpment, and Rocky‐field taxa typically had smaller SVLs. Claw CS and SVL were highly correlated (r 2 = 0.91), and indicated positive size allometry regardless of species or Habitat/substrate group (Figure 3). Positive shape allometry, although significant, had a much poorer goodness of fit (r 2 = 0.35), as shape appeared to be consistent within species regardless of body size (for example, both a 195.7 mm and 385.6 mm SVL V. gouldii had shape‐coefficents of ~0.12). This resulted in individuals not conforming closely to the regression. For both size and shape residuals, Savannah‐burrower varanids displayed primarily positive values and Riverbed taxa were mostly negative, with other groups plotting on both sides of the regression. This resulted in a significant difference as indicated by Kruskal–Wallis (Table 2).
Figure 3.
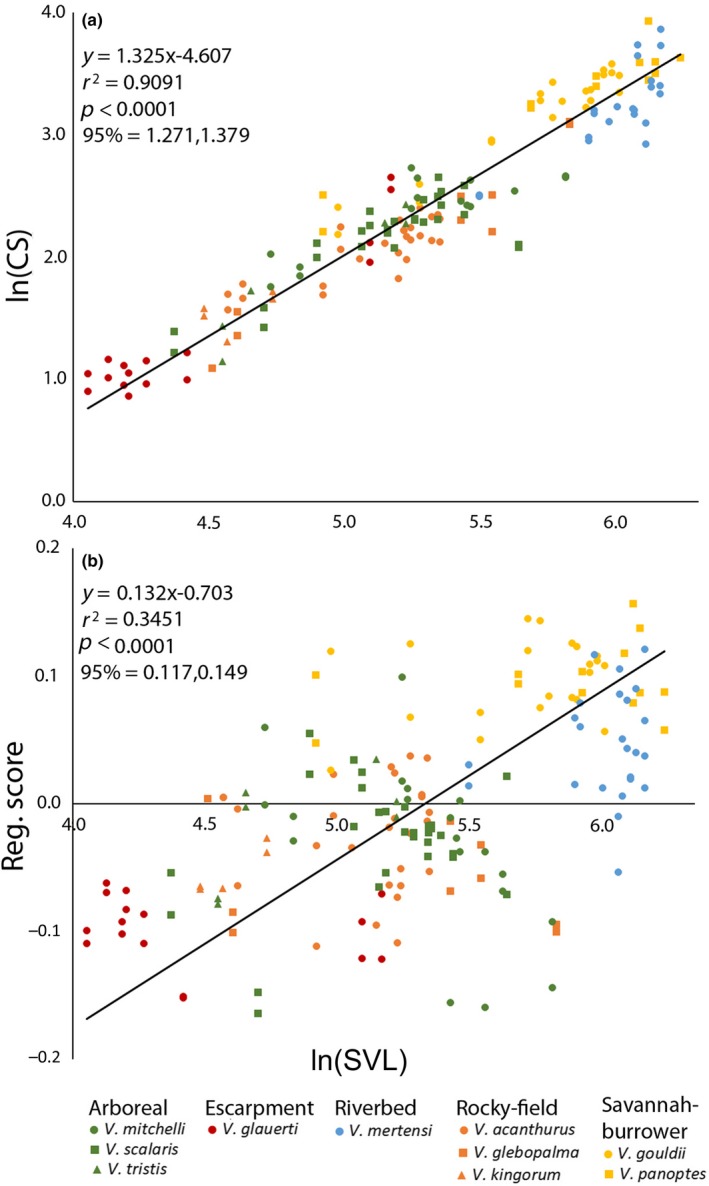
Regression of (a) ln claw centroid size (CS) in millimeters and (b) Shape Coefficients (Reg. Score) versus ln individual Snout‐vent length (SVL) in millimeters for all varanid claws, with regression information and statistics. Habitat/substrate group and species are distinguished by color and shape, respectively
Table 2.
Output of Kruskal–Wallis test of allometric regression residuals between Habitat/substrate groups
| Variable | df | χ 2 | p |
|---|---|---|---|
| Size | 4 | 44.558 | <0.0001 |
| Shape | 4 | 26.667 | <0.0001 |
Regressions plotted in Figure 3.
3.3. Shape variability
All Procrustes distances between Habitat/substrate groups were statistically significant (Figure 4). Savannah‐burrower was closest to Riverbed, and both were farthest from Escarpment. Arboreal and Rocky‐field were the closest to one another out of all groups.
Figure 4.
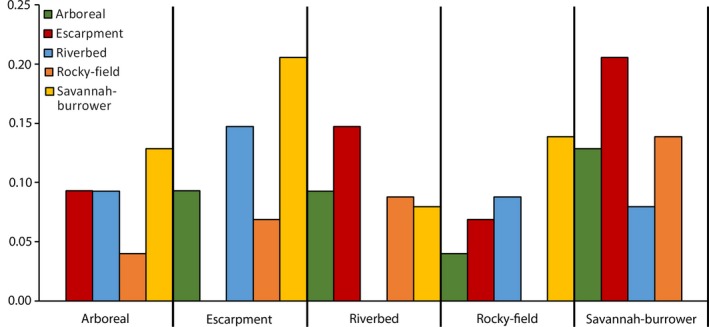
Procrustes distances among Habitat/substrate groups for all claws. All were significant (p < 0.05)
The first two PCs accounted for >80% of the total shape variance. PC1 (61.62%) displayed variation in claw height relative to length. Short, high claws were indicated by positive values, and low, elongate ones as negative (Figure 5a). PC2 (20.87%) could be defined as claw curvature. Positive values defined more curved claws and negative values indicated less curved claws. Curved claws also reflected a narrowing at the tip.
Figure 5.
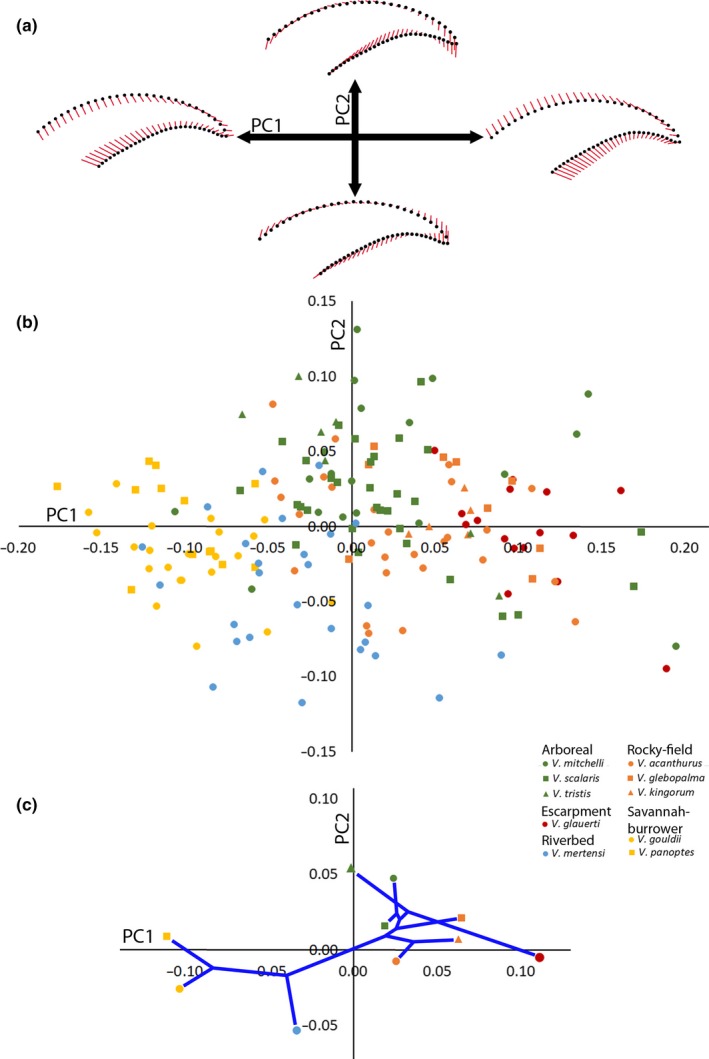
Principal components analysis for all claws. (a) Represents vector diagrams of maximum and minimum shape variance of all varanids for the first and second principal components (PC). Black points indicate the mean claw shape (PC = 0), and red vectors indicate deviation from said mean represented by the most extreme sample along the respective PC axis. (b) Represents principal components scores for all claws in the sample for PC1 and 2. Habitat/locomotor group and species are distinguished by color and shape, respectively. (c) Phylomorphospace of species mean PC scores (based on Thompson et al., 2009)
Savannah‐burrower varanids had some of the most negative PC1 values, and surrounded the mean of PC2 (Figure 5b). Opposite them was the Escarpment species with all positive PC1 scores. Arboreal varanids surrounded the mean of PC1, with majority possessing positive PC2 scores. Riverbed varanids had average to negative PC values, with some of the most negative PC2 values in the sample. The Rocky‐field scores rested between Arboreal and Escarpment, and overlapped both.
The phylomorphospace (Figure 5c) clustered the Gouldii clade together with average to negative PC1 and 2 values, and Odatria primarily in the opposite quadrant with average to positive PC1 and 2 values. Varanus gouldii and V. panoptes clustered with low PC1 scores, reflecting long claws with shallow curves and low heights. Varanus mertensi separated from them with a low PC2 score, as its claws were uncurved. Within the Odatria, V. glauerti stood out with a high PC1 score that displayed a very short and high claw. Varanus tristis had the highest PC2, indicating its claws both curved and tapered to a distinct point. The remainder of the Odatria clustered together.
4. DISCUSSION
4.1. Summary and hypotheses
There were significant and noticeable differences between the claw morphology of each Habitat/substrate group. Therefore, our primary hypothesis that claw morphology correlated with substrate use was supported. The claws of Riverbed species were long and straight. The Savannah‐burrower species were even longer, slightly curved, and relatively large. Arboreal, Rocky‐field, and Escarpment groups had claws that were all relatively high, with varied curvature. On average, Escarpment had the highest and shortest claws, and Arboreal had the most curved claws with a distinct “pointed” tip. The Rocky‐field group overlapped both these groups, with means rested in‐between. Allometry and phylogeny influenced claws as well, as Odatria were all relatively small climbers and Gouldii were both large and terrestrial.
4.2. Habitat‐related explanations for claw morphology
The claws of the Kimberley varanid guild shared striking similarities with those of previously studied taxa, especially other lizards and birds. Because we did not directly measure performance here, our conclusions about the influence of habitat on claw morphology were based on analogy with previously studied taxa.
Greater claw height and curvature are considered indicative of climbers (Crandell et al., 2014; Ribas et al., 2004; Tulli et al., 2009), and especially true for mostly vertical climbers (Glen & Bennett, 2007). It is not surprising that the Arboreal, Rocky‐field, and Escarpment taxa collectively reflected a similar condition, considering accounts of some sort of climbing existed for all species (see section 2.1). Claws interlock with substrate when climbing, thereby generating nonvertical contact surfaces (Biewener, 2003). These surfaces are typically perpendicular to adductor forces, resulting in a vertical reaction force supporting the animal (Cartmill, 1985). A relatively high claw has a mechanical advantage, and can withstand these forces as the animal clings to substrate (Alexander, 1968). The curved, narrow‐tipped Arboreal claws were specialized for creating these contact surfaces by penetrating the wood and bark they often climb (Biewener, 2003). The Escarpment claws were significantly shorter, and consequently higher, than those of their arboreal relatives. Two possible explanations exist for why this is. First, this particular claw morph may be specialized to deal with rough substrates (Zani, 2000) such as sandstone. These surfaces cannot be penetrated like wood, and the claw must therefore interlock with sand‐size particles when climbing. Having long claws would project the varanid's center of mass away from its own supports, increasing its likelihood of toppling (Cartmill, 1985). An alternative explanation is that the hard sandstone substrate could have worn away part of the claw. Constantly climbing the escarpment may have grinded down the tip, giving the claw a shorter, less curved appearance. There was little evidence of claw wear apparent to the naked eye, so we feel the former is more likely.
Members of the Rocky‐field group may be considered locomotor generalists, as accounts indicate they both free‐roam and rock‐climb. Varanus glebopalma is especially proficient in both behaviors (Sweet, 2004b); it habitually rushes after prey, sprints across boulder fields, and ascends vertical rock faces. Rocky substrate was therefore reflected in claw shape; claws were relatively short and high, similar to (although to a lesser degree than) Escarpment taxa. The Rocky‐field morphotype might allow these varanids to interact with rocky substrate in a more versatile way than V. glauerti, which is considered to be “wholly saxicolous” in the Kimberley (Sweet, 2004a p.369).
The Savannah‐burrower and Riverbed claws reflected terrestrial locomotion primarily. A shallow‐curved claw would be ideal for providing grip as the foot is rotated against substrate when running, as seen in ground birds (Birn‐Jeffery et al., 2012; Feduccia, 1993; Glen & Bennett, 2007; Pike & Maitland, 2004). Both biomechanical models and empirical studies correlate relative limb lengths and speed (Garland, 1985; Losos, 1990b; Sinervo & Losos, 1991), because increasing stride length will transport the animal farther with each step (Biewener, 2003). Replacing claw height with length would increase overall limb length and consequently sprint speed (Teixeira‐Filho et al., 2001; Tulli et al., 2009, 2012), and the Savannah‐burrower varanids are known to have especially high sprint speeds (Clemente, Withers, & Thompson, 2012; Clemente et al., 2009).
The need to loosen and move resistant material is the main obstacle of a digging vertebrate (Hildebrand, 1985). This requires much force, and the digging tool must be modified to resist said forces. Vertebrates that “scratch‐dig” extensively tend to have long, and disproportionately large, claws (Bramble, 1982; Lautenschlager, 2014; Taylor, 1978; Warner, Tucker, Filoramo, & Towey, 2006). The Savannah‐burrower taxa share this trait, as they are arguably the most proficient diggers of the Kimberley. Both species maintain communal warrens consisting of multiple burrows (Christian, 2004b). Varanus gouldii can dig several burrows up to 5 meters long in sequence to excavate invertebrates (Thompson, 2004; Whitford, 1998). Varanus panoptes produces a complex spiraling burrow, the deepest reptile nest sites on record (Doody, James, Colyvas, McHenry, & Clulow, 2015; Doody et al., 2018).
The degree to which other Kimberley taxa dig is either much less extreme, or unknown (Husband, 1980). This is limited to the shallow excavation of tree hollows (Sweet, 2004a; Thompson & Pianka, 1999), termite mounds (Smith et al., 2004), and turtle egg nests (Christian, 2004a; Kennett et al., 1993). Although of similar body size, V. mertensi nests are much shallower and simpler than V. gouldii or V. panoptes (Rhind et al., 2016), so there is less selection pressure to enlarge or elongate claws.
4.3. Nonhabitat‐related explanations for claw morphology
It was not surprising that phylogenetic signaling plays a significant role in varanid claw morphology, considering it also strongly influences claw shape in other lizards (Tulli et al., 2009, 2011, 2012) and birds (Birn‐Jeffery et al., 2012; Fowler et al., 2009). We could not develop rigorous phylogenetic conclusions about Australian varanids as a whole, but tentative inferences may be drawn. High PICs tended to link with the establishment of new Habitat/substrate groups within phylogenetic history. The ancestral state of Odatria was likely a high claw for climbing, which became only slightly more specialized to cope with the Arboreal and Rocky‐field conditions. Varanus glauerti went through a substantial morphological change when it transitioned to vertical sandstone. A relatively long claw may have been the ancestral condition of the Gouldii group. The Savannah‐burrower morphotype appeared after the crown group separated from the V. mertensi line, when they adopted their digging‐heavy lifestyle.
The positive size allometry apparent in claws has also been seen in other locomotor structures such as limbs (Christian & Garland, 1996). This is most likely a consequence of the cubic scaling of mass with size increase (Alexander, 1985; McMahon, 1973), as mass is highly correlated with SVL in varanids (Thompson, 2004). Claws can bear much of the weight of the animal when running and/or climbing, and, as a varanid's body size increases, mass increases at a greater rate than claw cross‐sectional area. Therefore, an allometric increase in relative claw size would compensate for this. The fact that shape allometry was heavily influenced by Habitat/substrate group may be an indicator of how body size limits locomotor mode in Varanidae. Large size could be detrimental to a climbing animal, as this increases the likelihood of structural failure associated with falling (Biewener, 1982; Cartmill, 1985). The climbing varanids were limited to ≤340 mm SVL, which the terrestrial species surpassed. This resulted in a general correlation between climbing/terrestrial claw morphotypes and body size. Other monitor lizards have shown a similar connection, as Varanus komodoensis changes from climbing to terrestrial locomotion as it grows larger (Auffenberg, 1981). This was not the case in the Kimberley taxa, as no ontogenetic changes in claw shape within species were exhibited.
4.4. Claw morphology and Niche partitioning in the Kimberley
The fact that claw morphology correlated well with function suggests that claws may play a role in niche separation in the Kimberley varanid guild. Competition between sympatric varanid species may be reduced by maintaining disparate foraging locales (Pianka, 1994), and we have shown certain claw morphotypes to be ideal for particular habitats. This may function to isolate guild members from one another and allow for coexistence. Claws may allow the Arboreal taxa to physically separate themselves from the larger monitors of the savannah by living in trees, eliminating them as competition and potential predators. On the ground, the specialized claws of the Savannah‐burrowing taxa allowed them to exploit the terrestrial prey by chasing after and/or excavating them. This claw morphotype limits their climbing of trees though. It also constrains them to the savannah, as rocky terrain would be ill‐suited for burrowing. Varanus glauerti is the only varanid typically found along vertical escarpments in the Kimberley gorges, so its uniquely shaped claws allow it to solely exploit this habitat.
Varanids often use body size differences (Farlow & Planka, 2002) and behavior to faciltate niche separation. These are probably major driving forces in the Kimberley, especially in taxa with similar claw morphologies. Varanus mitchelli is associated with rivers where it eats fish (Losos & Greene, 1988; Schultz & Doody, 2004; Shine, 1986), isolating it from the other, less riparian, arboreal taxa. This diet also separates it from V. mertensi, which specializes in crustaceans and turtle eggs (Losos & Greene, 1988; Shine, 1986). Varanus panoptes may separate itself from V. gouldii in that it is typically larger (Christian, 2004b; Thompson, 2004), and incorporates more riparian‐zone prey (Pianka, 1994; Shine, 1986; Thompson, 1996). Rocky‐field monitors almost certainly partition themselves through size variation, as this correlates with their diet. Varanus kingorum is the smallest and limited to eating small arthropods, whereas V. glebopalma consumes mostly vertebrates (James, Losos, & King, 1992; King, 2004). Varanus glebopalma is also the only monitor known to feed at dusk (Rhind et al., 2013; Swanson, 1979).
5. CONCLUSIONS
Our results showed claw morphology was highly variable in the Kimberley monitor lizards, and correlated well with substrates found in their respective habitats as well as locomotor behaviors. This makes claws likely ecomorphological candidates for niche separation in this predator guild, especially when considered in tandem with body size and habitat selection behaviors. Guilds are often defined by shared trophic resources (Simberloff & Dayan, 1991), and varanids typically have intersecting diets due to their opportunistic feeding strategies (example in Sutherland, 2011). Feeding structures such as skulls and teeth would be potentially poor ecomorphological characters for differentiating niche, as such traits have linked particularly well with diet in monitor lizards (Rieppel & Labhardt, 1979; D'Amore, 2015, unpublished data). We suggest that morphological traits associated with locomotion, such as claws, may be more reliable candidates for niche partitioning in these situations, as they link to the occupation of certain habitats.
A major limitation of our study is the fact we only considered two dimensions, and morphological variation along the medial‐lateral axis almost certainly influences function. For example, the degree of taper along this axis could affect interaction with substrate. Claws built for puncturing wood would benefit from a very narrow tip (Cartmill, 1985), whereas burrowers may benefit from a wide claw as it would help transport soil (Hildebrand, 1985). Excluding the third dimension also prohibits investigating certain biomechanical principles by assessing lateral bending strengths (performed for teeth by Valkenburgh and Ruff (1987)), or conducting finite element modelling (Lautenschlager, 2014; Manning et al., 2009). Future studies should consider in what way medial‐lateral claw characters may influence claw function.
In addition to broader applications, several surveys and testable hypotheses may be developed from our work. Admittedly our assessment of claw function is correlative, as claw performance has yet to be directly tested in these varanids. Performance studies are therefore necessary to confirm our assertions about the functional significance of these claws (sensu Wainwright, 1991; Irschick, 2002). This should then be followed by investigations into how claws specifically influence patterns of resource use in the Kimberley. Although stomach content studies exist for these monitors, more studies investigating diet and prey capture methods would allow for elaboration on habitat use. Habitat/substrate groups may be expanded across most Australian varanids to see if claw shape varies to such a degree in other guilds, as well as determine the extent that phylogenetic signal plays a role in claw morphology.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
DD, SC, and CM conceived the ideas and designed methodology; DD, SC, SD, DR, and CM collected the data; DD analyzed the data and led the writing of the manuscript.
DATA ACCESSIBILITY
Data has been uploaded to Dryad (https://doi.org/10.5061/dryad.cf2hs41).
ACKNOWLEDGMENTS
We thank the Stop the Toad Foundation, Frogwatch Northern Territory, and El Questro Wilderness Park for logistical support. In particular, K. Hands, G. Sawyer, and M. Bass from those respective organizations provided valuable support. Several volunteers assisted in data collection, including L. Blaxland, C. Castellano, S. Cory, J. Lloyd, D. Hamilton, A. Hendy, R. Jaensch, H. James, M. McCurry, M. Parrott, C. and F. McHenry, M. Quayle, R. Soanes, B. Stewart, L. Stroud, D. Tome, C. Walmsley, S. Wilson, and C. Young. The study was funded by the Australian Government (Caring for our Country scheme), the Australian Research Council DP0986471 (to CRM), Monash University, and the Australian Geographic Society. DEC scientific license number was SF009165. The University of Michigan Museum of Zoology Division of Reptiles and Amphibians, and G. Schneider in particular, provided access to specimens. Lodging was provided by Z. Freedman and was funded by the Daemen College Natural Science Department. Statistical help was given by C. Klingenberg. The authors have no conflict of interest to declare.
D'Amore DC, Clulow S, Doody JS, Rhind D, McHenry CR. Claw morphometrics in monitor lizards: Variable substrate and habitat use correlate to shape diversity within a predator guild. Ecol Evol. 2018;8:6766–6778. 10.1002/ece3.4185
REFERENCES
- Alexander, R. M. (1968). Animal mechanics. Seattle, WA: University of Washington Press. [Google Scholar]
- Alexander, R. M. N. (1985). Body support, scaling, and allometry In Hildebrand M., Bramble D. M., Liem K. F., & Bolis L. (Eds.), Functional vertebrate morphology (pp. 26–37). Cambridge, MA: Harvard University Press. [Google Scholar]
- Arnold, S. J. (1983). Morphology, performance and fitness. American Zoologist, 23(2), 347–361. 10.1093/icb/23.2.347 [DOI] [Google Scholar]
- Ast, J. C. (2001). Mitochondrial DNA evidence and evolution in Varanoidea (Squamata). Cladistics, 17(3), 211–226. 10.1006/clad.2001.0169 [DOI] [PubMed] [Google Scholar]
- Auffenberg, W. (1981). The behavioral ecology of the Komodo monitor. Gainesville, FL: University Press of Florida. [Google Scholar]
- Biewener, A. A. (1982). Bone strength in small mammals and bipedal birds: Do safety factors change with body size? Journal of experimental Biology, 98(1), 289–301. [DOI] [PubMed] [Google Scholar]
- Biewener, A. (2003). Animal locomotion. Oxford, UK: Oxford University Press. [Google Scholar]
- Birn‐Jeffery, A. V. , Miller, C. E. , Naish, D. , Rayfield, E. J. , & Hone, D. W. (2012). Pedal claw curvature in birds, lizards and Mesozoic dinosaurs–complicated categories and compensating for mass‐specific and phylogenetic control. PLoS ONE, 7(12), e50555 10.1371/journal.pone.0050555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock, W. J. , & Miller, W. D. (1959). The scansorial foot of the woodpeckers, with comments on the evolution of perching and climbing feet in birds. American Museum Novitates, 1931. Boston, MA: Houghton Mifflin. [Google Scholar]
- Bookstein, F. L. (1997). Landmark methods for forms without landmarks: Morphometrics of group differences in outline shape. Medical Image Analysis, 1(3), 225–243. 10.1016/S1361-8415(97)85012-8 [DOI] [PubMed] [Google Scholar]
- Bramble, D. M. (1982). Scaptochelys: Generic revision and evolution of gopher tortoises. Copeia, 1984(4), 852–867. 10.2307/1444097 [DOI] [Google Scholar]
- Burnham, D. A. , Feduccia, A. , Martin, L. D. , & Falk, A. R. (2011). Tree climbing–a fundamental avian adaptation. Journal of Systematic Palaeontology, 9(1), 103–107. 10.1080/14772019.2010.522201 [DOI] [Google Scholar]
- Cartmill, M. (1985). Climbing In Hildebrand M., Bramble D. M., Liem K. F., & Bolis L. (Eds.), Functional vertebrate morphology (pp. 73–88). Cambridge, MA: Cambridge University Press. [Google Scholar]
- Christian, K. (2004a). Varanus mertensi In Pianka E., & King D. (Eds.), Varanoid lizards of the world (pp. 410–415). Bloomington, IN: Indiana University Press. [Google Scholar]
- Christian, K. (2004b). Varanus panoptes In Pianka E., & King D. (Eds.), Varanoid lizards of the world (pp. 423–429). Bloomington, IN: Indiana University Press. [Google Scholar]
- Christian, A. , & Garland, T. Jr (1996). Scaling of limb proportions in monitor lizards (Squamata: Varanidae). Journal of Herpetology, 30, 219–230. 10.2307/1565513 [DOI] [Google Scholar]
- Clarke, M. R. B. (1980). The reduced major axis of a bivariate sample. Biometrika, 67(2), 441–446. 10.1093/biomet/67.2.441 [DOI] [Google Scholar]
- Clemente, C. , Thompson, G. , & Withers, P. (2009). Evolutionary relationships of sprint speed in Australian varanid lizards. Journal of Zoology, 278(4), 270–280. 10.1111/j.1469-7998.2009.00559.x [DOI] [Google Scholar]
- Clemente, C. J. , Withers, P. C. , & Thompson, G. (2012). Optimal body size with respect to maximal speed for the yellow‐spotted monitor lizard (Varanus panoptes; Varanidae). Physiological and Biochemical Zoology, 85(3), 265–273. 10.1086/665275 [DOI] [PubMed] [Google Scholar]
- Clemente, C. J. , Withers, P. C. , Thompson, G. , & Lloyd, D. (2011). Evolution of limb bone loading and body size in varanid lizards. The Journal of Experimental Biology, 214(18), 3013–3020. 10.1242/jeb.059345 [DOI] [PubMed] [Google Scholar]
- Crandell, K. E. , Herrel, A. , Sasa, M. , Losos, J. B. , & Autumn, K. (2014). Stick or grip? Co‐evolution of adhesive toepads and claws in Anolis lizards. Zoology, 117(6), 363–369. 10.1016/j.zool.2014.05.001 [DOI] [PubMed] [Google Scholar]
- D'Amore, D. C. (2015). Illustrating ontogenetic change in the dentition of the Nile monitor lizard, Varanus niloticus: A case study in the application of geometric morphometric methods for the quantification of shape–size heterodonty. Journal of Anatomy, 226(5), 403–419. 10.1111/joa.12293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody, J. , Clulow, S. , Kay, G. , D'Amore, D. , Rhind, D. , Wilson, S. , … Bass, M. (2015). The dry season shuffle: Gorges provide refugia for animal communities in tropical savannah ecosystems. PLoS ONE, 10(7), e0131186 10.1371/journal.pone.0131186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody, J. , Green, B. , Rhind, D. , Castellano, C. , Sims, R. , & Robinson, T. (2009). Population‐level declines in Australian predators caused by an invasive species. Animal Conservation, 12(1), 46–53. 10.1111/j.1469-1795.2008.00219.x [DOI] [Google Scholar]
- Doody, J. S. , James, H. , Colyvas, K. , Mchenry, C. R. , & Clulow, S. (2015). Deep nesting in a lizard, déjà vu devil's corkscrews: First helical reptile burrow and deepest vertebrate nest. Biological Journal of the Linnean Society, 116(1), 13–26. 10.1111/bij.12589 [DOI] [Google Scholar]
- Doody, J. S. , James, H. , Ellis, R. , Gibson, N. , Raven, M. , Mahony, S. , … McHenry, C. R. (2014). Cryptic and complex nesting in the yellow‐spotted monitor, Varanus panoptes . Journal of Herpetology, 48(3), 363–370. 10.1670/13-006 [DOI] [Google Scholar]
- Doody, J. S. , Mayes, P. , Clulow, S. , Rhind, D. , Green, B. , Castellano, C. M. , … Mchenry, C. (2014). Impacts of the invasive cane toad on aquatic reptiles in a highly modified ecosystem: The importance of replicating impact studies. Biological Invasions, 16(11), 2303–2309. 10.1007/s10530-014-0665-6 [DOI] [Google Scholar]
- Doody, J. S. , McHenry, C. , Brown, M. , Canning, G. , Vas, G. , & Clulow, S. (2018). Deep, helical communal nesting in the sand monitor: Ecology informing paleoecology? Journal of Zoology, (In press). 10.1111/jzo.12543 [DOI] [Google Scholar]
- Doody, J. S. , Rhind, D. , Green, B. , Castellano, C. , McHenry, C. , & Clulow, S. (2017). Chronic effects of an invasive species on an animal community. Ecology, 98(8), 2093–2101. 10.1002/ecy.1889 [DOI] [PubMed] [Google Scholar]
- Doody, J. S. , Soanes, R. , Castellano, C. M. , Rhind, D. , Green, B. , McHenry, C. , & Clulow, S. (2015). Invasive toads shift predator‐prey densities in animal communities by removing top predators. Ecology, 96(9), 2544–2554. 10.1890/14-1332.1 [DOI] [PubMed] [Google Scholar]
- Dryden, G. (2004). Varanus acanthurus In Pianka E., & King D. (Eds.), Varanoid lizards of the world (pp. 298–307). Bloomington, IN: Indiana University Press. [Google Scholar]
- Farlow, J. O. , & Planka, E. R. (2002). Body size overlap, habitat partitioning and living space requirements of terrestrial vertebrate predators: Implications for the paleoecology of large theropod dinosaurs. Historical Biology, 16(1), 21–40. 10.1080/0891296031000154687 [DOI] [Google Scholar]
- Feduccia, A. (1993). Evidence from claw geometry indicating arboreal habits of Archaeopteryx . Science, 259(5096), 790–793. 10.1126/science.259.5096.790 [DOI] [PubMed] [Google Scholar]
- Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39(4), 783–791. 10.1111/j.1558-5646.1985.tb00420.x [DOI] [PubMed] [Google Scholar]
- Findley, J. S. , & Black, H. (1983). Morphological and dietary structuring of a Zambian insectivorous bat community. Ecology, 64(4), 625–630. 10.2307/1937180 [DOI] [Google Scholar]
- Fitch, A. J. , Goodman, A. E. , & Donnellan, S. (2006). A molecular phylogeny of the Australian monitor lizards (Squamata: Varanidae) inferred from mitochondrial DNA sequences. Australian Journal of Zoology, 54(4), 253–269. 10.1071/ZO05038 [DOI] [Google Scholar]
- Fowler, D. W. , Freedman, E. A. , & Scannella, J. B. (2009). Predatory functional morphology in raptors: Interdigital variation in talon size is related to prey restraint and immobilisation technique. PLoS ONE, 4(11), e7999 10.1371/journal.pone.0007999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, D. W. , Freedman, E. A. , Scannella, J. B. , & Kambic, R. E. (2011). The predatory ecology of Deinonychus and the origin of flapping in birds. PLoS ONE, 6(12), e28964 10.1371/journal.pone.0028964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland, T. (1985). Ontogenetic and individual variation in size, shape and speed in the Australian agamid lizard Amphibolurus nuchalis . Journal of Zoology, 207(3), 425–439. [Google Scholar]
- Glen, C. L. , & Bennett, M. B. (2007). Foraging modes of Mesozoic birds and non‐avian theropods. Current Biology, 17(21), R911–R912. 10.1016/j.cub.2007.09.026 [DOI] [PubMed] [Google Scholar]
- Grizante, M. B. , Navas, C. A. , Garland, J. , & Kohlsdorf, T. (2010). Morphological evolution in Tropidurinae squamates: An integrated view along a continuum of ecological settings. Journal of evolutionary biology, 23(1), 98–111. 10.1111/j.1420-9101.2009.01868.x [DOI] [PubMed] [Google Scholar]
- Gunz, P. , & Mitteroecker, P. (2013). Semilandmarks: A method for quantifying curves and surfaces. Hystrix, the Italian Journal of Mammalogy, 24(1), 103–109. [Google Scholar]
- Hahn, S. , Dimitrov, D. , Rehse, S. , Yohannes, E. , & Jenni, L. (2014). Avian claw morphometry and growth determine the temporal pattern of archived stable isotopes. Journal of Avian Biology, 45(2), 202–207. 10.1111/j.1600-048X.2013.00324.x [DOI] [Google Scholar]
- Hammer, Ø. , Harper, D. A. T. , & Ryan, P. D. (2001). PAST‐palaeontological statistics, ver. 1.89. Palaeontol Electron, 4(1), 1–9. [Google Scholar]
- Herrel, A. , Meyers, J. J. , & Vanhooydonck, B. (2001). Correlations between habitat use and body shape in a phrynosomatid lizard (Urosaurus ornatus): A population‐level analysis. Biological Journal of the Linnean Society, 74(3), 305–314. 10.1111/j.1095-8312.2001.tb01394.x [DOI] [Google Scholar]
- Herrel, A. , Spithoven, L. , Van Damme, R. , & De Vree, F. (1999). Sexual dimorphism of head size in Gallotia galloti: Testing the niche divergence hypothesis by functional analyses. Functional Ecology, 13(3), 289–297. 10.1046/j.1365-2435.1999.00305.x [DOI] [Google Scholar]
- Herrel, A. , Van Damme, R. , Vanhooydonck, B. , & Vree, F. D. (2001). The implications of bite performance for diet in two species of lacertid lizards. Canadian Journal of Zoology, 79(4), 662–670. 10.1139/z01-031 [DOI] [Google Scholar]
- Hildebrand, M. (1985). Digging in quadrupeds In Hildebrand M., Bramble D. M., Liem K. F., & Bolis L. (Eds.), Functional vertebrate morphology (pp. 89–109). Cambridge, MA: Harvard University Press; 10.4159/harvard.9780674184404 [DOI] [Google Scholar]
- Husband, G. (1980). Notes on a nest and hatchlings of Varanus acanthurus . Herpetofauna, 11, 29–30. [Google Scholar]
- Irschick, D. J. (2002). Evolutionary approaches for studying functional morphology: Examples from studies of performance capacity. Integrative and Comparative Biology, 42(2), 278–290. 10.1093/icb/42.2.278 [DOI] [PubMed] [Google Scholar]
- Irschick, D. J. , Austin, C. C. , Petren, K. , Fisher, R. N. , Losos, J. B. , & Ellers, O. (1996). A comparative analysis of clinging ability among pad‐bearing lizards. Biological Journal of the Linnean Society, 59(1), 21–35. 10.1111/j.1095-8312.1996.tb01451.x [DOI] [Google Scholar]
- Irschick, D. J. , Carlisle, E. , Elstrott, J. , Ramos, M. , Buckley, C. , VanHooydonck, B. , … Herrel, A. (2005). A comparison of habitat use, morphology, clinging performance and escape behaviour among two divergent green anole lizard (Anolis carolinensis) populations. Biological Journal of the Linnean Society, 85(2), 223–234. 10.1111/j.1095-8312.2005.00487.x [DOI] [Google Scholar]
- James, F. C. (1982). The ecological morphology of birds: A review. Annales Zoologici Fennici, 19, 265–275. [Google Scholar]
- James, C. D. , Losos, J. B. , & King, D. R. (1992). Reproductive biology and diets of goannas (Reptilia: Varanidae) from Australia. Journal of Herpetology, 26(2), 128–136. 10.2307/1564852 [DOI] [Google Scholar]
- Karr, J. , & James, F. (1975). Eco‐morphological configurations and convergent evolution in species and communities In Cody M. L., & Diamond J. M. (Eds.), Ecology and evolution of communities (pp. 258–291). Harvard, MA: Harvard University Press. [Google Scholar]
- Kennett, R. , Christian, K. , & Pritchard, D. (1993). Underwater nesting by the tropical fresh‐water turtle, Chelodina rugosa (Testudinata, Chelidae). Australian Journal of Zoology, 41(1), 47–52. 10.1071/ZO9930047 [DOI] [Google Scholar]
- King, M. (2004). Varanus kingorum In Pianka E., & King D. (Eds.), Varanoid lizards of the world (pp. 406–409). Bloomington, IN: Indiana University Press; 10.2307/j.ctt2005wjp [DOI] [Google Scholar]
- Klingenberg, C. P. (2011). MorphoJ: An integrated software package for geometric morphometrics. Molecular Ecology Resources, 11(2), 353–357. 10.1111/j.1755-0998.2010.02924.x [DOI] [PubMed] [Google Scholar]
- Klingenberg, C. P. , & Marugán‐Lobón, J. (2013). Evolutionary covariation in geometric morphometric data: Analyzing integration, modularity, and allometry in a phylogenetic context. Systematic biology, 62(4), 591–610. 10.1093/sysbio/syt025 [DOI] [PubMed] [Google Scholar]
- Lautenschlager, S. (2014). Morphological and functional diversity in therizinosaur claws and the implications for theropod claw evolution. Proceedings of the Royal Society B: Biological Sciences, 281(1785), 20140497 10.1098/rspb.2014.0497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losos, J. B. (1990a). Ecomorphology, performance capability, and scaling of West Indian Anolis lizards: An evolutionary analysis. Ecological Monographs, 60(3), 369–388. 10.2307/1943062 [DOI] [Google Scholar]
- Losos, J. B. (1990b). The evolution of form and function: Morphology and locomotor performance in West Indian Anolis lizards. Evolution, 44(5), 1189–1203. 10.1111/j.1558-5646.1990.tb05225.x [DOI] [PubMed] [Google Scholar]
- Losos, J. B. , & Greene, H. W. (1988). Ecological and evolutionary implications of diet in monitor lizards. Biological Journal of the Linnean Society, 35(4), 379–407. 10.1111/j.1095-8312.1988.tb00477.x [DOI] [Google Scholar]
- Losos, J. B. , & Sinervo, B. (1989). The effects of morphology and perch diameter on sprint performance of Anolis lizards. Journal of Experimental Biology, 145(1), 23–30. [Google Scholar]
- MacLeod, N. , & Rose, K. D. (1993). Inferring locomotor behavior in Paleogene mammals via eigenshape analysis. American Journal of Science, 293(A), 300–355. 10.2475/ajs.293.A.300 [DOI] [Google Scholar]
- Maddison, W. P. , & Maddison, D. R. (2017). Mesquite: A modular system for evolutionary analysis. Version 3.31. Retrieved from http://mesquiteproject.org
- Manning, P. L. , Margetts, L. , Johnson, M. R. , Withers, P. J. , Sellers, W. I. , Falkingham, P. L. , … Raymont, D. R. (2009). Biomechanics of dromaeosaurid dinosaur claws: Application of X‐Ray microtomography, nanoindentation, and finite element analysis. The Anatomical Record, 292(9), 1397–1405. 10.1002/ar.20986 [DOI] [PubMed] [Google Scholar]
- Mayes, P. , Thompson, G. , & Withers, P. (2005). Diet and foraging behaviour of the semi‐aquatic Varanus mertensi (Reptilia: Varanidae). Wildlife Research, 32(1), 67–74. 10.1071/WR04040 [DOI] [Google Scholar]
- McElroy, E. J. , & Reilly, S. M. (2009). The relationship between limb morphology, kinematics, and force during running: The evolution of locomotor dynamics in lizards. Biological journal of the Linnean Society, 97(3), 634–651. 10.1111/j.1095-8312.2009.01230.x [DOI] [Google Scholar]
- McMahon, T. (1973). Size and shape in biology: Elastic criteria impose limits on biological proportions, and consequently on metabolic rates. Science, 179(4079), 1201–1204. 10.1126/science.179.4079.1201 [DOI] [PubMed] [Google Scholar]
- Melville, J. , & Swain, R. O. Y. (2000). Evolutionary relationships between morphology, performance and habitat openness in the lizard genus Niveoscincus (Scincidae: Lygosominae). Biological Journal of the Linnean Society, 70(4), 667–683. [Google Scholar]
- Mosto, M. C. , & Tambussi, C. P. (2014). Qualitative and quantitative analysis of talons of diurnal bird of prey. Anatomia, Histologia, Embryologia, 43(1), 6–15. 10.1111/ahe.12041 [DOI] [PubMed] [Google Scholar]
- Openshaw, G. , & Keogh, J. (2014). Head shape evolution in monitor lizards (Varanus): Interactions between extreme size disparity, phylogeny and ecology. Journal of Evolutionary Biology, 27(2), 363–373. 10.1111/jeb.12299 [DOI] [PubMed] [Google Scholar]
- Perez, S. I. , Bernal, V. , & Gonzalez, P. N. (2006). Differences between sliding semi‐landmark methods in geometric morphometrics, with an application to human craniofacial and dental variation. Journal of Anatomy, 208(6), 769–784. 10.1111/j.1469-7580.2006.00576.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianka, E. R. (1994). Comparative ecology of Varanus in the Great Victoria desert. Australian Journal of Ecology, 19(4), 395–408. 10.1111/j.1442-9993.1994.tb00505.x [DOI] [Google Scholar]
- Pianka, E. , (2004). Varanus tristis In Pianka E., & King D. (Eds.), Varanoid lizards of the world (pp. 477–487). Bloomington, IN: Indiana University Press; 10.2307/j.ctt2005wjp [DOI] [Google Scholar]
- Pike, A. , & Maitland, D. (2004). Scaling of bird claws. Journal of Zoology, 262(1), 73–81. 10.1017/S0952836903004382 [DOI] [Google Scholar]
- Rhind, D. , Doody, J. S. , Ellis, R. , Ricketts, A. , Scott, G. , Clulow, S. , & McHenry, C. R. (2013). Varanus glebopalma (Black‐palmed Monitor) nocturnal activity and foraging. Herpetological Review, 4(4), 687–688. [Google Scholar]
- Rhind, D. , Jackson, C. , Pezaro, N. , & Doody, J. S. (2016). A Nest of Varanus mertensi (Glauert, 1951) in Northern Australia. Biawak, 10(1), 18–21. [Google Scholar]
- Ribas, S. C. , Velloso, A. , Teixeira‐Filho, P. , Rocha‐Barbosa, O. , Evangelista, H. , & Dos Santos, E. (2004). Structure of claws and toes of two tropidurid lizard species of Restinga from Southeastern Brazil: Adaptations to the vertical use of the habitat. Revista Chilena de Historia Natural, 77(4), 599–606. [Google Scholar]
- Rieppel, O. , & Labhardt, L. (1979). Mandibular mechanics in Varanus niloticus (Reptilia: Lacertilia). Herpetologica, 35, 158–163. [Google Scholar]
- Rohlf, F. J. (2010). TpsDig 2.16. Stony Brook, NY: Department of Ecology and Evolution, State University of New York. [Google Scholar]
- Rohlf, F. J. (2013). TpsRelw 1.53, relative warps analysis. Stony Brook, NY: Department of Ecology and Evolution, State University of New York. [Google Scholar]
- Root, R. B. (1967). The niche exploitation pattern of the blue‐gray gnatcatcher. Ecological Monographs, 37(4), 317–350. 10.2307/1942327 [DOI] [Google Scholar]
- Schultz, T. , & Doody, S. (2004). Varanus mitchelli In Pianka E., & King D. (Eds.), Varanoid lizards of the world (pp. 416–422). Bloomington, IN: Indiana University Press. [Google Scholar]
- Shine, R. (1986). Food habits, habitats and reproductive biology of four sympatric species of varanid lizards in tropical Australia. Herpetologica, 42(3), 346–360. [Google Scholar]
- Shine, R. (2010). The ecological impact of invasive cane toads (Bufo marinus) in Australia. The Quarterly Review of Biology, 85(3), 253–291. 10.1086/655116 [DOI] [PubMed] [Google Scholar]
- Simberloff, D. , & Dayan, T. (1991). The guild concept and the structure of ecological communities. Annual Review of Ecology and Systematics, 22(1), 115–143. 10.1146/annurev.es.22.110191.000555 [DOI] [Google Scholar]
- Sinervo, B. , & Losos, J. B. (1991). Walking the tight rope: Arboreal sprint performance among Sceloporus occidentalis lizard populations. Ecology, 72(4), 1225–1233. 10.2307/1941096 [DOI] [Google Scholar]
- Smith, L. , Sweet, S. , & King, D. (2004). Varanus scalaris In Pianka E., & King D. (Eds.), Varanoid lizards of the world (pp. 453–461). Bloomington, IN: Indiana University Press. [Google Scholar]
- Sutherland, D. R. (2011). Dietary niche overlap and size partitioning in sympatric varanid lizards. Herpetologica, 67(2), 146–153. 10.1655/HERPETOLOGICA-D-10-00053.1 [DOI] [Google Scholar]
- Swanson, S. (1979). Some rock‐dwelling reptiles of the Arnhem Land escarpment. Northern Territory Naturalist, 1(2), 14–18. [Google Scholar]
- Sweet, S. (2004a). Varanus glauerti In Pianka E., & King D. (Eds.), Varanoid lizards of the world (pp. 366–372). Bloomington, IN: Indiana University Press. [Google Scholar]
- Sweet, S. (2004b). Varanus glebopalma In Pianka E., & King D. (Eds.), Varanoid lizards of the world (pp. 373–379). Bloomington, IN: Indiana University Press. [Google Scholar]
- Sweet, S. (2007). Comparative spatial ecology of two small arboreal monitors in northern Australia. In Boehme W., Horn H. G., & Krebs U. (Eds.), Advances in monitor research III. Mertensiella, 16 (pp. 378–402) Rheinbach. [Google Scholar]
- Taylor, B. K. (1978). The anatomy of the forelimb in the anteater (Tamandua) and its functional implications. Journal of Morphology, 157(3), 347–367. 10.1002/(ISSN)1097-4687 [DOI] [PubMed] [Google Scholar]
- Teixeira‐Filho, P. , Rocha‐Barbosa, O. , Paes, V. , Ribas, S. C. , & de Almeida, J. R. (2001). Ecomorphological relationships in six lizard species of Restinga de Barra de Maricá, Rio de Janeiro, Brazil. Revista Chilena de Anatomía, 19(1), 45–50. [Google Scholar]
- Thompson, G. (1995). Foraging patterns and behaviours, body postures and movement speed for goannas, Varanus gouldii (Reptilia: Varanidae), in a semi‐urban environment. Journal of the Royal Society of Western Australia, 78, 107–114. [Google Scholar]
- Thompson, G. (1996). Notes on the diet of Varanus gouldii in a semi‐urban environment. Western Australian Naturalist, 21, 49–54. [Google Scholar]
- Thompson, G. (2004). Varanus gouldii In Pianka E., & King D. (Eds.), Varanoid lizards of the world (pp. 380–400). Bloomington, IN: Indiana University Press. [Google Scholar]
- Thompson, G. G. , Clemente, C. J. , Withers, P. C. , Fry, B. G. , & Norman, J. A. (2009). Is body shape of varanid lizards linked with retreat choice? Australian Journal of Zoology, 56(5), 351–362. [Google Scholar]
- Thompson, G. , & Pianka, E. (1999). Reproductive ecology of the black‐headed goanna Varanus tristis (Squamata: Varanidae). Journal of the Royal Society of Western Australia, 82, 27–31. [Google Scholar]
- Thompson, G. G. , & Withers, P. C. (1997). Comparative morphology of Western Australian varanid lizards (squamata: Varanidae). Journal of Morphology, 233(2), 127–152. 10.1002/(ISSN)1097-4687 [DOI] [PubMed] [Google Scholar]
- Tinius, A. , & Russell, A. P. (2017). Points on the curve: An analysis of methods for assessing the shape of vertebrate claws. Journal of Morphology, 278(2), 150–169. 10.1002/jmor.20625 [DOI] [PubMed] [Google Scholar]
- Tulli, M. , Abdala, V. , & Cruz, F. (2011). Relationships among morphology, clinging performance and habitat use in Liolaemini lizards. Journal of Evolutionary Biology, 24(4), 843–855. 10.1111/j.1420-9101.2010.02218.x [DOI] [PubMed] [Google Scholar]
- Tulli, M. J. , Abdala, V. , & Cruz, F. B. (2012). Effects of different substrates on the sprint performance of lizards. The Journal of Experimental Biology, 215(5), 774–784. 10.1242/jeb.065490 [DOI] [PubMed] [Google Scholar]
- Tulli, M. , Cruz, F. , Herrel, A. , Vanhooydonck, B. , & Abdala, V. (2009). The interplay between claw morphology and microhabitat use in neotropical iguanian lizards. Zoology, 112(5), 379–392. 10.1016/j.zool.2009.02.001 [DOI] [PubMed] [Google Scholar]
- Valkenburgh, B. V. , & Ruff, C. B. (1987). Canine tooth strength and killing behaviour in large carnivores. Journal of Zoology, 212(3), 379–397. 10.1111/j.1469-7998.1987.tb02910.x [DOI] [Google Scholar]
- Van Damme, R. , Aerts, P. , & Vanhooydonck, B. (1997). No trade‐off between sprinting and climbing in two populations of the Lizard Podarcis hispanica (Reptilia: Lacertidae). Biological Journal of the Linnean Society, 60(4), 493–503. 10.1006/bijl.1996.0115 [DOI] [Google Scholar]
- Vanhooydonck, B. , Andronescu, A. , Herrel, A. , & Irschick, D. J. (2005). Effects of substrate structure on speed and acceleration capacity in climbing geckos. Biological Journal of the Linnean Society, 85(3), 385–393. 10.1111/j.1095-8312.2005.00495.x [DOI] [Google Scholar]
- Vanhooydonck, B. , & Van Damme, R. (1999). Evolutionary relationships between body shape and habitat use in lacertid lizards. Evolutionary Ecology Research, 1(7), 785–805. [Google Scholar]
- Verwaijen, D. , Van Damme, R. , & Herrel, A. (2002). Relationships between head size, bite force, prey handling efficiency and diet in two sympatric lacertid lizards. Functional Ecology, 16(6), 842–850. 10.1046/j.1365-2435.2002.00696.x [DOI] [Google Scholar]
- Vidal, N. , Marin, J. , Sassi, J. , Battistuzzi, F. U. , Donnellan, S. , Fitch, A. J. , … Couloux, A. (2012). Molecular evidence for an Asian origin of monitor lizards followed by Tertiary dispersals to Africa and Australasia. Biology Letters, 8, 853–855. 10.1098/rsbl.2012.0460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright, P. C. (1991). Ecomorphology: Experimental functional anatomy for ecological problems. American Zoologist, 31(4), 680–693. 10.1093/icb/31.4.680 [DOI] [Google Scholar]
- Warner, D. A. , Tucker, J. K. , Filoramo, N. I. , & Towey, J. B. (2006). Claw function of hatchling and adult red‐eared slider turtles (Trachemys scripta elegans). Chelonian Conservation and Biology, 5(2), 317–320. 10.2744/1071-8443(2006)5[317:CFOHAA]2.0.CO;2 [DOI] [Google Scholar]
- Whitford, W. G. (1998). Contribution of pits dug by goannas (Varanus gouldii) to the dynamics of banded mulga landscapes in eastern Australia. Journal of Arid Environments, 40(4), 453–457. 10.1006/jare.1998.0464 [DOI] [Google Scholar]
- Williams, E. E. (1972). The origin of faunas. Evolution of lizard congeners in a complex island fauna: A trial analysis In Dobzhansky T., Hecht M. K. & Steere W. C. (Eds.), Evolutionary biology (pp. 47–89). New York, NY: Springer; 10.1007/978-1-4684-9063-3 [DOI] [Google Scholar]
- Wilson, S. , & Swan, G. (2013). Complete guide to reptiles of Australia. Chatswood, NSW: New Holland Press. [Google Scholar]
- Zamora‐Camacho, F. J. , Reguera, S. , Rubiño‐Hispán, M. V. , & Moreno‐Rueda, G. (2014). Effects of limb length, body mass, gender, gravidity, and elevation on escape speed in the lizard Psammodromus algirus . Evolutionary Biology, 41(4), 509–517. 10.1007/s11692-014-9285-4 [DOI] [Google Scholar]
- Zani, P. (2000). The comparative evolution of lizard claw and toe morphology and clinging performance. Journal of Evolutionary Biology, 13(2), 316–325. 10.1046/j.1420-9101.2000.00166.x [DOI] [Google Scholar]
- Zelditch, M. L. , Swiderski, D. L. , Sheets, H. D. , & Fink, W. L. (2004). Geometric morphometrics for biologists: A primer. Cambridge, MA: Academic Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data has been uploaded to Dryad (https://doi.org/10.5061/dryad.cf2hs41).


