Abstract
Purpose of review
Metabolic syndrome (MS) describes the co-occurrence of conditions that increase one’s risk for heart disease and other disorders such as diabetes and stroke. The worldwide increase in the prevalence of MS cannot be fully explained by lifestyle factors such as sedentary behavior and caloric intake alone. Environmental exposures, such as heavy metals, have been implicated, but results are conflicting and possible mechanisms remain unclear. To assess recent progress in determining a possible role between heavy metal exposure and MS, we reviewed epidemiological and model system data for cadmium (Cd), lead (Pb), and mercury (Hg) from the last decade.
Recent findings
Data from 36 epidemiological studies involving 17 unique countries/regions, and 13 studies leveraging model systems are included in this review. Epidemiological and model system studies support a possible association between heavy metal exposure and MS or comorbid conditions; however, results remain conflicting. Epidemiological studies were predominantly cross-sectional and collectively, they highlight a global interest in this question and reveal evidence of differential susceptibility by sex and age to heavy metal exposures. In vivo studies in rats and mice, and in vitro cell-based assays provide insights into potential mechanisms of action relevant to MS including altered regulation of lipid and glucose homeostasis, adipogenesis, and oxidative stress.
Summary
Heavy metal exposure may contribute to MS or comorbid conditions; however, available data are conflicting. Causal inference remains challenging as epidemiological data are largely cross-sectional; and variation in study design, including samples used for heavy metal measurements, age of subjects at which MS outcomes are measured, the scope and treatment of confounding factors, and the population demographics vary widely. Prospective studies, standardization or increased consistency across study designs and reporting, and consideration of molecular mechanisms informed by model systems studies are needed to better assess potential causal links between heavy metal exposure and MS.
Keywords: Metabolic syndrome, diabetes, heavy metals, cadmium, Pb, mercury
Introduction
Metabolic syndrome (MS) represents a major public health problem due to its increasing incidence worldwide and its association with serious life-threatening conditions such as cardiovascular disease. MS (also known as insulin resistance syndrome or syndrome X) describes the co-occurrence of conditions that increase an individual’s risk for heart disease and other disorders such as diabetes and stroke. Despite attempts by national and international entities such as the World Health Organization (WHO), National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) and the International Diabetes Federation (IDF) to define the diagnostic criteria for MS, there is still a lack of consensus, reflecting the complexity of the condition and population-based differences in baseline physiology(1). The National Heart, Lung and Blood Institute of the National Institutes of Health describes MS as having at least three of the following five metabolic risk factors: abdominal obesity (≥35 inch circumference for women and ≥40 inches for men), triglyceride level ≥150 mg/dL (or taking medication to treat high triglycerides), HDL cholesterol level <50 mg/dL for women and <40 mg/dL for men (or taking medication to treat low HDL cholesterol), blood pressure ≥130/85 mmHg (or taking medication to treat high blood pressure), and fasting blood sugar ≥100 mg/dL (or taking medicine to treat high blood sugar). The number of metabolic risk factors correlates with the severity of the syndrome.
The prevalence of MS among adults in the US increased from 25% during the period 1988-1994 to 35% during the period 2007-2012(2). Whereas non-Hispanic white men were diagnosed with MS more frequently than non-Hispanic black men, the opposite was true for women. In addition, lower education and advanced age were significantly associated with MS. Globally, it is estimated that approximately one quarter of the adult population has MS(1). Not surprisingly, comorbid conditions such as obesity (body mass index [BMI] ≥30 kg/m2) also increased in recent decades. Approximately 36% of adults and 17% of children in the US were diagnosed as obese during the period 2011-2014(3). According to WHO, the worldwide prevalence of obesity in adults tripled between 1975 and 2016. In 2016, approximately 13% of adults were considered obese and 18% of children were considered overweight or obese.
Although energy imbalance due to sedentary lifestyles has been implicated in the etiology of MS, increasing evidence suggests that environmental exposures, particularly during critical periods of development, may also contribute to the onset of MS and comorbid conditions(4, 5). Heavy metals such as cadmium (Cd), lead (Pb), and mercury (Hg) have been studied for potential associations with MS, diabetes, or obesity(6–9) but results are conflicting. To improve understanding of the potential contribution of these compounds to MS and underlying mechanisms, we provide an updated review of the epidemiological literature in combination with findings from model systems from the previous decade (2007-2017). Collectively, these data provide a summary of epidemiological findings, highlight possible molecular mechanisms based on model systems, enable comparisons between model system and human studies, and identify outstanding research gaps.
Criteria for inclusion
We identified publications for review by querying Medline using the PubMed application. Epidemiological literature was identified on August 2, 2017 using the string: epidemiology AND ((heavy AND metals) OR (cadmium OR mercury OR Pb)) AND (obesity OR metabolic syndrome OR diabetes). Model system literature was restricted to mammalian and fish models and identified on August 18, 2017 using the string: (Mus musculus OR mouse OR rat OR Rattus OR zebrafish OR Danio OR fishes) AND ((heavy AND metals) OR (cadmium OR mercury OR Pb)) AND (obesity OR metabolic syndrome OR diabetes). Both queries were further restricted to the previous 10 years (date range 2007 to present). The resulting corpus was reviewed manually and resulted in the elimination and addition of some articles (Figure 1). Articles were eliminated based on several criteria: 1) lack of relevant information; 2) they were review articles; 3) the full text was not accessible through the University of North Carolina electronic library system; 3) the focus was on different compounds or endpoints; 4) the focus was on potentially ameliorative (e.g., chelation, nutrition) or confounding (e.g., previous health disorders) influences on metal exposure-associated MS; 5) the article was published prior to 2007; or 6) the article had been retracted. Based on these criteria, we ultimately included 36 epidemiological and 13 model system studies for this review. In addition to summarizing the results of the studies comprising this review we provide a high-level summary of the significant findings for prospective human studies in Table 1 and cross-sectional studies in Supplemental Table 1. Summaries of model system studies for Cd, Pb and Hg are provided in Tables 2–4, respectively. In addition, the reader is referred to the publicly available Comparative Toxicogenomics Database (CTD; http://ctdbase.org) where each epidemiology reference was expertly curated by CTD staff. In CTD, more extensive information including exposure levels measured for each epidemiology study is available for download.
Figure 1.
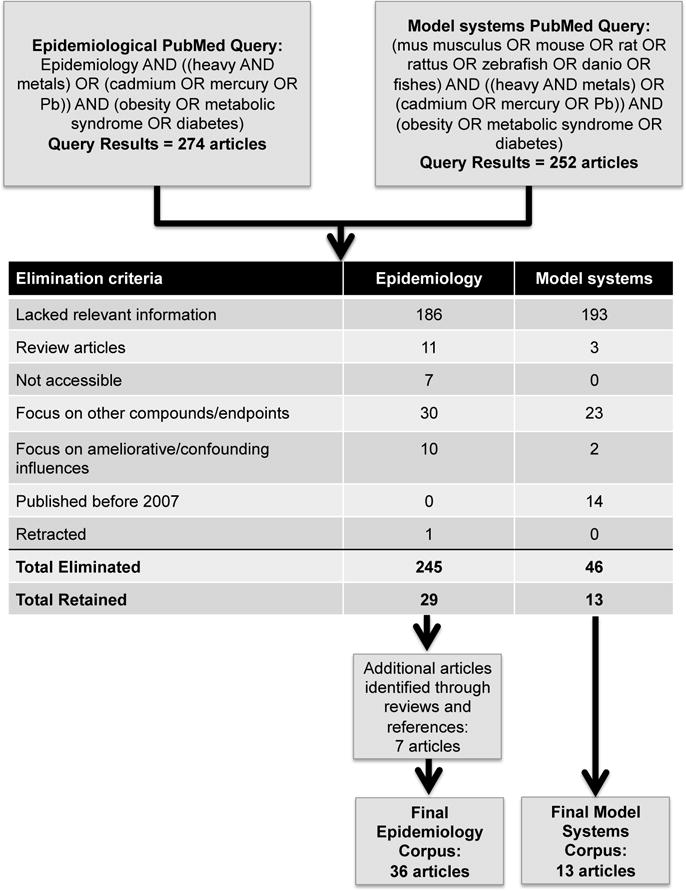
Criteria by which epidemiological and model system studies were identified for review.
Table 1.
Summary of Cd, Pb, or Hg and associations with MS-related outcomes in prospective epidemiological studies
| Ref. | Study Name | Study Location | Age group | Sex | N | %♀ | Sample | Endpoints | Cd | Pb | Hg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (17) | Malmo Diet and Cancer study | Sweden | Adults 46-67 y |
♀, ♂ | 4585 | 60 | Blood | T2D | N/S | – | – |
| (29) | EDEN | France | Pregnant adults 18-45y |
♀ | 901 | 100 | Blood | BW, FGR | Neg. for BW OR=1.41 (1.00-1.99) |
N/S | – |
| (32) | Thailand | Adults ≥15y (at baseline) |
♀, ♂ | 484 at baseline | 66 | Urine | HT, T2D, UrS | Pos. HT 36% p=0.03 T2D 11% p=0.02 UrS 16.6% p<0.001 |
– | – | |
| (56) | Kuopio Ischemic Heart Disease Risk Factor study | Finland | Adults 42-60y |
♂ | 2212 | 0 | Hair | T2D | – | – | N/S |
| (57) | HPFS AND NHS | US | Adults Mean ♀ = 54y ♂=61y |
♀, ♂ | 9267 | 73 | Toenail | T2D | – | – | Neg. HR♀=0.86 (0.66 - 1.11) HR♂=0.77 (0.61-0.98) |
| (58) | EDEN | France | Pregnant adults | ♀ | 691 | 100 | Hair | FGR | – | – | N/S |
| (59) | CARDIA Trace Element Study | US | Adults 20-32 y |
♀, ♂ | 3875 | 52 | Toenail | T2D | – | – | Pos. OR=1.65 (1.07-2.56) |
EDEN, Etude des Déterminants pré et post natals du développement et de la santé de l’Enfant; FGR, fetal growth rate; HPFS AND NHS, Health Professionals Follow-up Study and Nurses’ Health Study (NHS); HT, hypertension; Neg., significant negative association; N/S, not significant; OR, odds ratio; Pos., significant positive association; T2D, type II diabetes; UrS, urinary stones; ♀, females; ♂, males.
Table 2.
Exposure to Cd and metabolic parameters in model systems
| Model | N/Sex | Conc. (ppm) | Route (Duration) | Blood glucose | IR/GI | BW/Adiposity | TG/Lipids | Molecular signals | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| In Vivo | |||||||||
| Rats (Albino Wistar) |
25 ♀ |
3.1 | Oral gavage (45 d) |
↑ | ↑ | N/S | ↑ TG, VLDL, HDL ↓ LDL |
↓ Sod and Cat activity, total protein and glycogen ↑ Liver and kidney LPO N/S Gp, G6pc |
(42) |
| Rats (Wistar) |
30-60 ♂ |
32.5 | Water (60, 90, 120 d) |
↑ | ↑ | N/S | ↑ FFA, TG, TC, LDL, VLDL ↓ HDL |
– | (43) |
| Mice (MT-null) |
9-18 ♂ |
0.5, 0.75 | Subcutaneous (7 d) |
– | – | ↓ (0.75 ppm) adipocyte size, BW, WAT | – | ↓ Pparg2, Cebpa, Lep, Retn and Mest mRNA, plasma leptin, BUN ↑ plasma Ast N/S adiponectin |
(44) |
| Mice (Kunming) |
10 ♂ |
613 ppm Cd + HFD (vs STD) |
Diet (12 w) |
↑ (8, 12 w) | – | ↓ (8, 12 w) BW |
↑ TG, TC, LDL-C ↓ HDL-C |
↓ Sod and Cat activity, GSH ↑ Nag, microalbumin, Alt, Ast, LPO, Pco, nitric oxide |
(45) |
| 10 ♂ |
613 ppm Cd + HFD (vs HFD) |
Diet (12 w) |
N/S | – | ↓ (8, 12 w) BW |
N/S | ↑ Nag, microalbumin | (45) | |
| In Vitro | |||||||||
| MT-null (Mouse adipocytes) |
– | 0.011 - 11 | Media (6-48 h) |
– | – | – | ↑ (≥0.56 ppm) FFA, TG |
↓ Pparg, Lipe, Pnpla2, Acaca, Fasn, Lep, Ccl2, Adipoq, Retn, and Plin1 mRNA ↑ Abhd5 and Serpine1 mRNA, Plin1 phosphorylation |
(44) |
| MIN6 (Mouse β-cells) |
– | 0.11 | Media (48 h) |
– | – | – | ↓ GSH ↑ Hmox1 and Gclm mRNA |
(46) | |
| Primary mouse islet cells | – | 0.011 - 0.056 | Media (8, 48 h) |
– | ↓ (0.011 ppm) | – | – | ↓ GSSG/GSH | (46) |
|
RIN-m5F (Rat) |
– | 0.112 - 1.12 |
Media (24 h) |
– | ↓ (0.56 ppm) | – | – | ↑ ROS | (47) |
AA, arachidonic acid; Abhd5, abhydrolase domain containing 5; Acaca, acetyl-Coenzyme A carboxylase alpha; Adipoq, adiponectin, C1Q and collagen domain containing; Alt, alanine aminotransferase; Ast, aspartate aminotransferase; BUN, blood urea nitrogen; BW, body weight; Cat, catalase; Cebpa, CCAAT/enhancer binding protein alpha; Ccl2, chemokine (C-C motif) ligand 2; Conc., concentration; d, days; Fasn, fatty acid synthase; FFA, free fatty acids; G6pc, glucose-6-phosphatase, catalytic subunit; Gclm; glutamate cysteine ligase, modifier subunit; GI, glucose intolerance; Gp, glycogen phosphorylase; GSH, glutathione (reduced); GSSG, glutathione (oxidized); h, hours; HDL, high density lipoproteins (C-particles); HFD, high fat diet; Hmox1, heme oxygenase 1; IR, insulin resistance; LDL, low density lipoproteins (C-particles); Lep, leptin; Lipe, lipase; LPO, lipid peroxides; Mest, mesoderm specific transcript; Nag, N-acetyl-β-glucosaminidase; N/S, not significant; Pparg, peroxisome proliferator activated receptor gamma; ppm, parts-per-million (converted); Pco, protein carbonyl; Plin1, perilipin 1; Pnpla2, patatin-like phospholipase domain containing 2; Retn, resistin; ROS, reactive oxygen species; Serpine1, serpin family E member 1; Sod, superoxide dismutase; TC, total cholesterol; TG, triglycerides; VLDL, very low density lipoproteins; w, weeks; ↑, increased levels; ↓, decreased levels.
Table 4.
Exposure to Hg and metabolic parameters in model systems
| Model | N/Sex | Conc. (ppm) | Route (Duration) | Blood glucose | IR/GI | BW/Adiposity | TG/Lipids | Molecular signals | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| In Vivo | |||||||||
| Mice (Slc:ICR) |
16 ♂ |
0.04, 0.4 | Oral gavage (14, 28 d) |
↑ (0.04 ppm) | ↑ (0.04 ppm) | – | – | ↑ Plasma lipid peroxidation | (65) |
| Mice (Slc:ICR) |
4 ♂3 |
0.7 ppm Hg + HFD (vs STD) |
Subcutaneous (10 d) |
↑ | – | ↑ BW ↓ adipocyte size |
↑ FFA, LDL | ↓ Plasma insulin and leptin, and Lep, CD36, Ampka2, Ppara, and Pparg mRNA ↑ Alt, Ast, BUN, and Aqpap/7 mRNA N/S Liver weight, HDL, adiponectin and Ampka1 mRNA |
(66) |
| 4 | 0.7 ppm Hg + HFD (vs HFD) |
Subcutaneous (10 d) |
↓ | – | ↓ BW, visceral WAT, adipocyte size | ↓ TG, TC, LDL | ↓ Plasma insulin and leptin, and Lep CD36, Ampka2, Ppara, and Pparg mRNA ↑ Alt, Ast, BUN and Aqpap/7 mRNA N/S Liver weight, HDL, adiponectin and Ampka1 mRNA |
||
| In Vitro | |||||||||
| HIT-T15 cells | – | 0.04 | Media (8 h) |
– | ↑ | – | – | ↑ ROS, Pik3 activity | (65) |
| Primary mouse islet cells | – | 0.04 | Media (4h) |
– | ↑ | – | – | ↑ ROS, Pik3 activity | (65) |
Alt, alanine aminotransferase; Ampk1 (Prkaa1), protein kinase AMP-activated catalytic subunit alpha 1; Ampk2 (prkaa2), protein kinase, AMP-activated, alpha 1 catalytic subunit; Aqpap/7 (Aqp7), aquaporin 7; Ast, aspartate aminotransferase; BUN, blood urea nitrogen; BW, body weight; Cd36, Cd36 molecule; Conc., concentration; d, days; FFA, free fatty acids; h, hours; HFD, high fat diet; IR, insulin resistance; Lep, leptin; LDL, low density lipoproteins; N/S, not significant; ppm, parts-per-million (converted); PI3K/Akt (Pik3), phosphoinositide 3-kinase; Ppara, peroxisome proliferator activated receptor alpha; Pparg, peroxisome proliferator activated receptor gamma; STD, standard diet; TC, total cholesterol; TG, triglycerides; WAT, white adipose tissue; ↑, increased levels; ↓, decreased levels.
Overview of heavy metals
Heavy metals are naturally occurring elements exhibiting high atomic weights and high densities. Many metals, including copper, iron, manganese, nickel, and zinc have important biological roles as cofactors in enzymatic reactions, or as constituents of protein secondary and tertiary structure. However, a number of heavy metals, including cadmium (Cd), lead (Pb), and mercury (Hg), have no known biological roles. Instead, these metals exhibit high toxicity when consumed by humans, and are classified as toxic heavy metals. The World Health Organization lists Cd, Pb, and Hg in its list of top ten chemicals of major public health concern, and exposure to these metals has been linked to numerous diseases in humans. Although naturally occurring, these metals are generally found in the subsurface and associated with human activities, particularly through mining and industrial processes, and have led to widespread environmental distribution of toxic metals, thus increasing the likelihood that humans will come into contact with them through air, water, contaminated soil, and food.
Assays for the presence of Cd, Pb and Hg in humans can take place in different ways, depending on whether short- or long-term exposures are under investigation. Half-lives of metals in different tissues may result in measurements reflecting different exposure periods. Measuring blood metal levels typically captures acute exposures over the preceding several months, whereas measurements in hair or toenails can capture exposures over the past year depending on how the sample is collected, and are not considered reliable predictors of toxicity. Cd accumulates in the kidney and has relatively long resident half-lives; therefore urine Cd can capture exposures over many years, whereas Pb accumulates in bone and bone Pb measurements capture exposures over the past years to decades. Studies that rely on assessment of metals in biosamples may underestimate the effect of the metal’s presence on a disease of interest if the exposure period reflected by the biosample measurement does not include the exposure window of relevance for the disease. Cross sectional studies are also more subject to possible reverse causation whereby the disease state affects the measured metal concentration rather than vice-versa. For greater depth of coverage of these metals and their role in diseases other than MS, we refer the reader to several recent reviews(10–12).
Cadmium (Cd)
Epidemiology
Among the 27 epidemiological studies evaluating associations between Cd and MS components, three were prospective and the remaining were cross-sectional or case-control studies; 15 (two prospective) studies reported significant associations. Four cross-sectional studies of adults in South Korea, Norway, China and the US found no significant association between Cd in blood(13, 14), urine(15), or spot urine(16) and Type II Diabetes (T2D) diagnoses. Two studies examined associations with blood Cd in prospective studies of adults. The Swedish Malmo Diet and Cancer Study excluded people with diabetes at baseline and followed participants over a mean of 15.2 years(17). Although blood Cd levels were positively associated with glycated hemoglobin (HbA1c) at baseline, they were not associated with T2D at follow-up. In the international Modeling the Epidemiological Transition Study (METS; including participants from Ghana, Jamaica, Seychelles, South Africa, US) 78% of participants were classified as overweight or obese by BMI at baseline (prevalence of diabetes was not indicated); follow-up assessments were conducted annually over a three-year period(18). Cross-sectional analysis of a random sampling of 150 blood samples at baseline did not find a significant association between Cd and elevated fasting glucose (FG) levels. Cross-sectional analysis of a prospective occupational study of adults working in one of the world’s largest nonferrous manufacturing operations (the Jinchang cohort) found no significant association between urinary Cd levels and FG(19). Similarly, MS was not significantly associated with hair or blood Cd levels in two cross-sectional studies of adults in South Korea(20, 21). A cross-sectional study did not find a significant association for Cd alone; however, when combined with Pb and Hg levels, the adjusted odds ratios for MS were significant for quartiles 2-4 (1.344, 1.638 and 1.556, respectively) relative to the lowest quartile(22). A cross-sectional analysis of a subset of 65-year old women with diabetes, or impaired or normal glucose tolerance did not detect significant differences between baseline blood or urinary Cd concentrations and diagnoses at baseline or with insulin production, blood glucose or glycated hemoglobin(23). In a follow-up study of adolescents with and without MS from the cross-sectional Iranian CASPIAN III study, blood Cd levels were detected that exceeded the WHO guidelines(24). Although Cd levels correlated with high diastolic blood pressure, serum TGs, FG, cholesterol within low density lipoprotein particles (LDL-C), and liver enzymes, the correlations were not statistically significant.
Four studies reported significant negative associations with MS or related conditions. Analysis of the cross-sectional NHANES 99-02 study population, consisting of children, adolescents and adults, reported a negative association between urinary Cd and BMI and waist circumference (WC); however, when the analysis was restricted to adolescents (6–18 years) or to adults (≥19 years), the association in adolescents was not significant(25). In contrast to Cd, negative associations for Pb exposure remained significant in both groups suggesting metal-and age-specific sensitivities to weight gain(25). Analysis of children 6-19 years of age from the cross-sectional NHANES 1999-2011 study identified a negative association between urinary levels of Cd and obesity; this association was strongest among the 6-12 year-old children(26). There are conflicting data about the possible connection between low birth weight and obesity later in life(27, 28). To determine the effects of developmental exposure to metals on birth weight, data from the prospective mother-child EDEN cohort were evaluated(29). Smoking status was positively correlated with Cd levels in mid-pregnancy maternal blood, and cord blood. Analysis of either smoking status or, separately, maternal blood Cd levels found negative associations between both exposures and birth weight and fetal growth. Notably, significant associations were not observed for Pb in this study. Cross-sectional analysis of adult males in Poland with and without MS identified a negative association between blood Cd and insulin levels but no associations were found with T2D, obesity or MS(30).
Eleven studies (one prospective, the remainder cross-sectional) reported positive associations between Cd levels and MS or comorbid conditions. Four studies specifically evaluated associations with cardiovascular outcomes. Urinary Cd levels were positively associated with hypertension (HT) among adults from heavily contaminated rural villages in northwestern Thailand(31). Notably, associations with HT were more significant in women who were ever or never smokers whereas the association was less significant in men that were never smokers. No significant associations were identified between Cd and T2D for either sex. A prospective study by the same authors compared renal function and diagnoses of HT, T2D and urinary tract stones among highly exposed individuals in rural Thailand in 2005 and at a five-year follow-up evaluation(32). Despite efforts to reduce Cd exposure during the interim period, decreases in renal function and increases in the incidence of HT, T2D, and urinary tract stones were observed at follow-up. Myocardial infarction was positively associated with blood Cd levels and the higher tertiles of urinary Cd levels among adults in the general US population(33, 34). T2D was positively associated with blood and particularly hair Cd levels among adults in the general population from Norway(35) and Pakistan(36), respectively. Urinary Cd was also associated with T2D in Chinese coke oven workers exposed occupationally and in male Korean residents living near abandoned metal mines(37, 38). A large cross-sectional study in China revealed positive associations between blood Cd levels and BMI and FG levels(39). In two separate analyses of different KNHANES data subsets, the same group reported positive associations between blood Cd levels and MS in males only(40, 41). Notably, although blood Cd levels in females were not reported in the first study, similar to other populations blood Cd levels in females were found to be higher than those in males in the second study (females: 1.157 ug/L ± 0.026 [SEM] vs males: 0.823 ug/L ± 0.019 [SEM]). Thus, of the 15 studies that found associations between Cd and MS components, two were prospective cohort studies and the remainder were cross-sectional or case-control studies but measured Cd in urine, to estimate long-term exposure.
Overall, there is very little evidence to suggest that exposure to Cd is associated with MS in humans, consistent with an earlier systematic analysis of the effect of Cd exposure on the incidence of T2D(9). The presence of confounders, methodological differences in data collection, including how Cd levels are determined (for example, urine for long-term exposure or blood for short-term exposure) or study design (e.g. cross-sectional vs. prospective), genetics, and other factors could significantly affect data interpretation.
Model systems
Experimental studies using adult animals and cell culture models of Cd exposure have shown Cd to have both diabetogenic and antiobesogenic effects (Table 2). Adult female rats (180 days) exposed to high levels of Cd (3.1 ppm) via daily oral gavage for 45 days exhibited hyperglycemia, reduced serum insulin, progesterone and low density lipoproteins (LDL); increased serum estradiol, TGs, very low density lipoproteins (VLDL), and high density lipoproteins (HDL); and reduced glucose clearance at the end of treatment(42). Liver and muscle tissue was harvested and assayed at the termination of the experiment, revealing a significant association between Cd and decreased superoxide dismutase (Sod) and catalase activity, decreased total protein and glycogen content, and increased liver and kidney lipid peroxides (LPO) and serum markers of hepatic dysfunction. No significant differences were observed in body weight, glycogen phosphorylase (Gp) or glucose-6-phosphatase (G6pc). Juvenile male rats (4 weeks) exposed in drinking water to 32.5 ppm Cd for 60, 90, and 120 days, exhibited increased FG, insulin, free fatty acids (FFAs), TGs, total cholesterol, LDL, and VLDL as well as a decrease in HDL after 60 days(43). Glucose and insulin tolerance testing showed insulin resistance and glucose intolerance, along with increases in all indexes of insulin resistance/sensitivity tested (homeostatic model assessment-insulin resistance [HOMA-IR], HOMA-S%, QUICKI, Matsuda-DeFronzo) at 60, 90, and 120 days. Tissue specific sensitivity and resistance analyses showed decreased hepatic and muscle insulin sensitivity and increased hepatic, adipose, and cardiovascular resistance at 60, 90, and 120 days. No significant difference in body weight, BMI, or fat percentage was observed in any of the exposed rats. Male metallothionein (MT)-null mice (7-8 weeks) exposed to 0.5 or 0.75 ppm Cd by subcutaneous injection for seven days, showed increased liver weight and plasma aspartate aminotransferase (Ast; a sign of liver damage) and decreased plasma blood urea nitrogen (BUN) at 0.5 and 0.75 ppm Cd, and decreased body and white adipose tissue weight when exposed to 0.75 ppm Cd(44). The decrease in white adipose tissue was associated with a significant decrease in adipocyte size. In addition to the decrease in body weight and adiposity, all exposed mice showed decreases in peroxisome proliferator activated receptor gamma (Pparg), CCAAT/enhancer binding protein alpha (Cebpa), leptin (Lep), and resistin (Retn) expression along with decreases in plasma leptin without significant changes in adiponectin (Adipoq). Additionally, MT-null mouse adipocytes exposed to 0.56 ppm or higher Cd for 48 hours showed decreased Pparg, hormone-sensitive lipase (Lipe), patatin-like phospholipase domain containing 2 (Pnpla2), acetyl-Coenzyme A carboxylase alpha (Acaca), fatty acid synthase (Fasn), perilipin 1 (Plin1), adiponectin (Adipoq), Lep, Retn, and chemokine (C-C motif) ligand 2 (Ccl2) expression along with increased AB-hydrolase containing 5 (Abhd5) and Serpine1 expression, Plin1 phosphorylation, and FFA and TG efflux. Adult male mice exposed via a high fat diet (HFD) to 613 ppm Cd for 12 weeks were evaluated in comparison to mice fed either a standard diet (STD) or HFD alone(45). At eight weeks, HFD+Cd mice had significantly lower body weight than either control group and significantly higher blood glucose levels than STD controls. At 12 weeks mice fed HFD+Cd vs STD exhibited significant increases in N-acetyl-b-glucosaminidase (Nag), microalbumin, total cholesterol, TGs, alanine transaminase (Alt), Ast, and LDL-C along with decreases in HDL-C, and changes in markers of oxidative stress (lipid peroxides, Sod, GSH, protein carbonyl [Pco], Cat, and nitric oxide [NO]). At 12 weeks, Nag and microalbumin levels were significantly higher in HFD+Cd mice than in HFD controls(45). Mouse MIN6 pancreatic β-cell line exposed to 110 ppb Cd for 48 hours decreased glucose-stimulated insulin release and GSH levels and increased heme oxygenase 1 (Hmox1) and glutamate-cysteine ligase modifier (Gclm) mRNA expression(46). Exposure of primary mouse β-cells to 11 ppb Cd for 48 hours decreased glucose-stimulated insulin secretion and oxidized glutathione (GSSG)/GSH ratio(46). The rat β-cell line, RIN-m5F, exposed to ≥300 ppb Cd exhibited mitochondrial dysfunction whereas at 560 ppb, sub-G1 hypodiploidy, decreased insulin secretion and cell death were observed(47).
These data suggest that Cd promotes hepatic and adipose tissue release of lipids and identify two potential mechanisms for Cd-induced disruption in glucose homeostasis: increased hepatic function and disruption of pancreatic islet function. These findings are supported by a recent study demonstrating that Cd exposure during the prenatal period was associated with obesity in children as young as five years of age. They also were unlikely to be confounded by unmeasured non-chemical stressors as they were recapitulated in a zebrafish model (Green, Hoyo, Mattingly, and Planchart, unpublished data). While these data offer modest support for the diabetogenic effects of Cd more studies are needed to provide greater weight of evidence to facilitate public health policy changes. Further, making comparisons across studies is challenging considering differences in exposure route, dose, and animal model used. To allow for improved validity and comparison across studies, internal measurements of blood and tissue specific Cd levels would be invaluable. Finally, care must be taken when reporting insulin or glucose levels as they can be influenced by the disease state of the animal. As shown in a study characterizing a rat model of T2D, insulin levels are higher even in the absence of increased blood glucose presumably to overcome rising insulin resistance; once β-cell function declines, blood glucose levels rise (48). Consequently, it may be necessary to monitor glucose and insulin at multiple timepoints during extended studies.
In conclusion, there is modest evidence that Cd exposure in adult animals is associated with MS-related effects in vitro and in vivo, and that the mechanisms linking Cd to MS may be associated with perturbations of gluconeogenesis and pancreatic islet dysfunction. The data show that Cd may have antiobesogenic activity in adult animals by promoting the release of lipids from hepatic and adipose tissue, resulting in dyslipidemia, whereas prenatal exposure may increase the risk of lipid accumulation. Finally, there is a dearth of data evaluating the association between developmental exposure to Cd and MS, for which further studies are warranted.
Lead (Pb)
Epidemiology
Among 18 studies evaluating associations between Pb and MS components, one was prospective and the remaining were cross-sectional or case control studies; nine (none were prospective) studies reported significant associations. Four studies conducted in South Korea(13), the US(16), Norway(35), and China(49) found no significant associations between urine or blood concentrations and diabetes diagnoses in adult populations. Three Korean studies(20, 40, 41) of adult males and females as well as a Polish study(30) of exclusively older men (50-75 years of age) found no significant association between blood Pb levels and MS as determined by standard anthropometric measures. Unlike the findings for Cd, no significant association was identified between Pb levels in maternal and cord blood and low birth weight or fetal growth restriction in the mother-child, prospective EDEN study(29).
Seven studies reported significant positive associations between Pb exposure and MS or associated disorders. A study of coke oven workers (86% men) in China revealed that participants in the highest tertile of urinary Pb levels had a 1.45-fold risk of hyperglycemia(37). Urinary Pb levels were also positively and significantly associated with impaired FG among the general adult Chinese population(15). Two small studies including adults in Pakistan and Norway reported significantly higher Pb levels in scalp hair and blood, respectively, in diabetic than in control participants(14, 36). Blood Pb levels were associated with increased TGs(21, 22) and WC(22) in two different subsets of KNHANES data. A small prospective study of randomly selected blood samples from the multinational METS study reported a significant and positive association specifically between Pb and elevated FG levels(18). Only two studies focused on Pb effects in children or adolescents. The earlier study evaluated 1999-2002 NHANES data for adolescents (defined as ages 6-18 years old) and adults (≥19 years old) and reported a significant negative association between urinary Pb levels and both BMI and WC in adolescents and adults(25). Analysis of data for children (ages 6-12 years) and adolescents (ages 13-19 years) from the 1999-2011 NHANES study also revealed significant negative correlations between urinary Pb levels and obesity, with more significant results found in the younger age group (ages 6-12 years)(26). Notably, the mean Pb levels measured in these children were lower than the CDC reference level for children, which was reduced in 2012 from 10 to five ug/dL based on NHANES data from 2007-2008 and 2009-2010(50).
Together, the majority of studies evaluating associations between Pb exposure and MS and related disorders reported positive associations, regardless of whether blood or urine was used to measure Pb. Studies that did not find associations were largely cross-sectional with Pb measured in peripheral blood or its components.
Model systems
We identified five studies during the past decade that evaluated potential effects of Pb exposure on development of MS or associated conditions (Table 3). Among these studies, mammalian and cell culture models were used to evaluate diabetes-related phenotypes and regulators of glucose homeostasis. Juvenile female rats (6 weeks) exposed to 319 ppm Pb in drinking water for 20 weeks exhibited fasting hyperglycemia after 8 weeks and glucose intolerance after 12 weeks. Livers were harvested and assayed at the termination of the experiment, revealing a significant association between Pb exposure and increased TGs and transcription of phosphoenolpyruvate carboxykinase 1 (Pck1) and glucose-6-phosphatase (G6pc)(51). Both genes play important roles in glucose homeostasis; Pck1 is a key regulator of gluconeogenesis and G6pc is a key enzyme in both gluconeogenesis and glycogenolysis. No significant changes in adiposity or body weight were observed(51). Expression of Pck1 and G6pc were also increased in FAO rat hepatoma cells exposed to 1 ppm Pb(51). Adult male rats exposed to drinking water without or with Pb (319 or 1274 ppm Pb as lead acetate) for 32 days exhibited significant increases in fasting blood insulin (1274 ppm Pb only), area under the curve (AUC) following an oral glucose tolerance test (GTT), Pck1 and G6pc activity, and expression of oxidative stress markers (TBARS, protein carbonyl (Pco) and 8-hydroxyguanosine (8-OHG)), whereas glycogen phosphorylase (Gp) activity was decreased(52). Interestingly effects were seen at the low dose despite the fact that there was no significant difference detected in blood Pb levels between this group and control animals. No significant differences were observed for HOMA-IR or tumor necrosis factor alpha (Tnfa), a marker of inflammation. Cultured islets of Langerhans exposed to Pb had decreased cell viability and glucose-stimulated insulin secretion as well as altered markers of endoplasmic reticulum stress (e.g., glucose regulated protein 78 (Grp78), nuclear factor kappa-light-chain-enhancer of activated B cells (Nfkb), and CCAAT/enhancer-binding protein homologous protein (Chop), and increased baseline insulin levels, glycogen synthase kinase 3 beta (Gsk3b) activity, and ROS production. In another study, adult male rats (8 weeks) were exposed for 21 weeks to a STD without or with Pb (500, 1500 or 4500 ppm) administered via drinking water, or to HFD alone(53). STD+500 ppm Pb animals exhibited significantly greater weight gain, as well as fasting glucose and insulin levels, HOMA-IR, TGs and glucose intolerance compared to all other STD±Pb animals. Notably, changes in weight gain, glucose intolerance and insulin resistance in the STD+500 ppm Pb animals approximated those observed in animals fed a HFD only. No significant changes in hyperglycemia were observed with Pb exposure(53). Whole genome bisulfite sequencing of liver tissue from STD+500 ppm Pb animals revealed hypermethylation at the whole genome level and differentially methylated genes were enriched in pathways involved in lipid and glucose metabolism.
Table 3.
Exposure to Pb and metabolic parameters in model systems
| Model | N/Sex | Conc. (ppm) | Route (Duration) | Blood glucose | IR/GI | BW/Adiposity | TG/Lipids | Molecular signals | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| In Vivo | |||||||||
| Rats (Zucker Diabetic Fatty) |
4 ♀ |
319 | Water (20 w) |
↑ (319 ppm) | ↑ | N/S | ↑ (319 ppm) TG |
↑ Pck1 and G6pc mRNA | (51) |
| Rats (Wistar) |
6 ♂ |
319, 1274 | Water (32 d) |
N/S | ↑ (319 ppm) | – | – | ↑ Pck1 and G6pc activity; TBARS, Pco, and 8-ohg levels ↓ Gp activity N/S Tnfa, HOMA-IR |
(52) |
| Rats (Wistar) |
5 ♂ |
500, 1500, 4500 | Water (21 w) |
N/S | ↑ (500 ppm) | ↑ (500 ppm) | ↑(500 ppm) TG |
Differential methylation of lipid and glucose metabolism genes | (53) |
| Mice (C57BL/6J) |
5-6 ♂3 |
50 (+ LFD or HFD) | Lactation and water (56, 77, 119 d) |
↑ (see text) | N/S | N/S | – | ↓ Bone stiffness, trabecular bone volume ↑ Leptin, Trap5b, Sost and Dkk1 protein |
(54) |
| Mice (Agouti) |
11-14 ♂ ♀ |
1, 10, 20 | Water (56 d) |
N/S | ↑ (10 ppm) ♂ |
↑ (10 ppm) ♂ BW, fat |
– | – | (55) |
| In Vitro | |||||||||
| FAO rat hepatoma cells | – | 1 | Media (8 h) |
– | ↑ | – | – | ↑ Pck1 and G6pc mRNA | (51) |
| Islets of Langerhans | – | 207 | Media (24 h) |
– | ↑ | – | – | ↑ Gsk3b activity, ROS, Chop mRNA ↓ Cell viability, Nfkb and Grp78 mRNA |
(52) |
| Primary Bone MSCs | – | 50 | Media (varied due to differentiation factors) |
– | – | ↑ Adiposity | – | ↓ Osteoblastogenic capacity (alizarin red staining) ↑ Adipogenic capacity (Oil Red O staining) and osteoclastogenic capacity (Trap expression, although N/S) |
(54) |
| MC3T3 cells | – | 0.4 |
Media (5 d) |
– | – | – | – | ↑ Fabp4 and Sost mRNA ↑ Pparg- and Sost-mediated DNA binding (luciferase assay) ↓ Runx2 and β-catenin mRNA |
(54) |
8-ohg, 8-hydroxyguanosine; BW, body weight; Chop, CCAAT/enhancer-binding protein (C/EBP) homologous protein; Conc., concentration; d, days; Dkk1, dickkopf WNT signaling pathway inhibitor 1; Fabp4, fatty acid binding protein 4; G6pc, glucose-6-phosphatase, catalytic subunit; Gp, glycogen phosphorylase; Grp78, glucose regulated protein 78; Gsk3b, glycogen synthase kinase 3 beta; h, hours; HFD, high fat diet; IR, insulin resistance; LFD, low fat diet; Nfkb, nuclear factor kappa-light-chain-enhancer of activated B cells; N/S, not significant; ppm, parts-per-million (converted); Pck1, phosphoenolpyruvate carboxykinase; Pco, protein carbonyl; Pparg, peroxisome proliferator activated receptor gamma; ROS, reactive oxygen species; Runx1, runt related transcription factor 2; Sost, sclerostin; TBARS, thiobarbituric acid reactive substances; TG, triglycerides; Tnfa, tumor necrosis factor alpha; Trap, tartrate-resistant acid phosphatase; w, weeks; ↑, increased levels; ↓, decreased levels.
Two studies evaluated the metabolic effects of early-life exposure to Pb. In one study, male mice were chronically exposed to control conditions or Pb via lactation from dams exposed to 0 or 50 ppm Pb-treated water and then via the same drinking water treatment post-weaning. In addition, the mice were fed either a low fat diet (LFD) or HFD from 5-12 weeks(54). After six weeks on the diets, HFD mice exhibited increased weight gain and fat accumulation compared to LFD controls; neither weight nor fat content were affected by Pb. After 11 weeks on the diets, Pb, HFD, and Pb+HFD mice exhibited increased FG, plasma leptin, and tartrate-resistant acid phosphatase isoform 5b (Trap5b) levels compared to LFD controls. Pb alone increased sclerostin (Sost) and dickkopf WNT signaling pathway inhibitor 1 (Dkk1) protein levels but did not alter glucose tolerance. In addition to metabolic effects, Pb treatment significantly decreased bone stiffness, suggesting a difference in bone mineral properties. Analysis of serum proteins as well as in vitro studies using mesenchymal stem cells derived from femur bone marrow and an osteoblast precursor cell line, MC3T3, indicated that Pb exposure promoted adipogenesis while decreasing osteoblastogenesis based on cell type-specific staining and molecular markers (see Table 3 for details). In another study, mice were exposed to Pb (1, 10 or 20 ppm) throughout gestation until postnatal day 21 and phenotyped at three, six, nine and 10 months(55). Exposure resulted in several sexually dimorphic phenotypes: Whereas increased food intake was observed in both males and females, only males exhibited significant increases in body weight and fat; and males exhibited increased insulin levels and HOMA-IR, whereas no differences were observed in females.
Collectively, these data suggest that Pb exposure in juveniles and adults can induce MS-related phenotypes. Although not observed or measured in every study, significant phenotypes included glucose intolerance, insulin resistance, hyperglycemia, increased body weight and adiposity, changes in energy expenditure, and altered pancreatic function. Importantly, several studies also implicated genes, which may be direct or indirect targets of Pb, that are involved in lipid and glucose metabolism, possibly through epigenetic mechanisms, as well as regulators of stem cell differentiation to adipocytes. Only two studies examined the effects of developmental Pb exposure; both showed evidence of insulin resistance, glucose intolerance, or hyperglycemia. Most studies used either male or female animals, but in one study that used both there was a significant effect on body weight only in males, suggesting the possibility of sexually dimorphic sensitivity. There are several caveats to these studies including the small number of animals used and doses that are orders of magnitude greater than human exposure levels.
Mercury (Hg)
Epidemiology
Among 18 epidemiological studies evaluating associations between Hg and MS components, four were prospective and the remaining were cross-sectional or case-control studies; nine (two prospective) studies reported significant associations. Three cross-sectional studies of adult males and females from Norway and South Korea failed to identify a significant association between blood Hg levels and T2D diagnoses(13, 14, 35). Similarly, three studies evaluating different data subsets of the KNHANES cross-sectional study did not find significant associations between blood Hg levels and MS(21, 40, 41). A cross-sectional analysis of blood Hg levels and elevated FG in a multinational cohort of adult males and females(18) found no significant association at baseline. Excluding occupational exposure, Hg is most often encountered in the diet, particularly via consumption of fish. This exposure route also provides significant dietary benefits, including access to high-quality protein, essential fatty acids, and numerous beneficial micronutrients. A prospective study of adult males from Finland(56) evaluated the relationship between T2D and fish consumption, omega-3 polyunsaturated fatty acids, or Hg. After an average follow-up of 19 years, there was no significant association between Hg and T2D. However, omega-3 polyunsaturated fatty acids appeared to be protective, raising the possibility that the benefits of fish consumption could potentially mask the interplay between Hg and T2D or MS-related conditions(56). A large prospective cohort of adult males and females in the US(57) found a negative association between toenail Hg levels and T2D. An earlier evaluation of the EDEN cohort than the one referenced above identified an association between hair Hg levels in pregnant mothers and fetal growth as determined by birth weight but the association was not significant(58). The prospective CARDIA Trace Element Study enrolled 3,875 young adults (ages 20-32 years) free of T2D at baseline(59). Toenail Hg levels at baseline were positively associated with T2D diagnoses at an average follow-up of 18 years, and levels in the highest quintile were significantly correlated with decreased homeostasis model assessment of beta cell function (HOMA-β) suggesting that Hg levels may increase the risk of T2D in young adults(59). A cross-sectional study of adult males in Poland evaluated associations between blood levels of several metals and MS by a range of standard parameters. A significant negative association was identified between Hg and waist-to-hip ratio (WHR) only(30). The following six cross-sectional studies reported positive associations between Hg levels and MS or associated conditions. A study of highly exposed adult Inuits in Greenland found weak associations between blood Hg levels and fasting plasma glucose and T2D(60). Other studies of adults from the US and South Korea found positive associations between blood(22, 61), toenail(62), or hair(20) Hg levels and MS. An additional study found a positive association between blood Hg level and visceral adiposity(63). Based on these studies, associations between Hg levels and MS or associated conditions are inconsistent.
Model systems
Studies investigating the relationship between Hg and MS in model systems are sparse. Nearly 15 years ago one study linked inorganic Hg to reduced expression of a glucose transporter (Glut4) and the adipogenic regulator Pparg in 3T3-L1 adipocytes(64). Since then two other studies were conducted in mouse and cell culture models (Table 4). Adult male mice exposed to 0.04 ppm Hg by oral gavage for 14 or 28 days exhibited decreased plasma insulin and increased blood glucose, glucose intolerance and plasma lipid peroxidation(65). These findings were supported by cell culture assays performed in a hamster beta cell line (Hg-exposed HIT-T15) and islet cells isolated from Hg-exposed mice. In these systems, Hg increased Pik3 activity and ROS production, and decreased insulin secretion. Notably, Hg-induced effects in vivo were reversed after terminating mercury exposure, and N-acetylcysteine rescued Hg-induced effects in vivo and in vitro, suggesting that exposure-related effects were not permanent and may be related to oxidative stress(65). In another study, adult male mice were fed a STD or HFD for 34 days with subcutaneous administration of 0.7 mg/kg Hg beginning at day 25(66). Compared to STD or HFD control mice, HFD+Hg mice exhibited significant decreases in adipocyte size, plasma insulin, plasma leptin, Lep mRNA, and mRNA levels of genes known to regulate adipocyte differentiation, fat accumulation, and energy balance (Cd36, AMP activated protein kinase a2 (Ampka2), peroxisome proliferator-activated receptors alpha (Ppara) and Pparg), whereas markers of liver (Alt, Ast) and kidney (BUN) stress and, paradoxically, a marker of fat accumulation (Aqpap/7) were increased. Compared to HFD controls, HFD+Hg mice also exhibited significant decreases in body weight, visceral white adipose tissue weight, blood glucose levels, and plasma lipid parameters (triglycerides [TG], total cholesterol [TC], LDL). In contrast, compared to STD controls, HFD+Hg mice exhibited increases in body weight, blood glucose levels, and lipid parameters (free fatty acids and LDL). With the exception of decreased white adipose tissue weight, STD+Hg vs STD mice did not exhibit significant changes in body weight, markers of liver and kidney stress, blood glucose, insulin or lipid parameters. Interestingly, Hg exposure appears to antagonize some effects of the HFD (blood glucose, body weight, white adipose tissue weight, TGs, total cholesterol, LDL, and plasma insulin and leptin levels) whereas it appears to compound others (BUN, and mRNA levels of Ampka2, ppara and pparg).
Conclusion
Overall, the data from model organisms suggest a possible association between Cd, Pb, and Hg with MS or associated conditions, whereas human data are conflicting. Reasons for conflicting findings are unclear but many factors contribute to this inconsistency and complicate across-study analysis, which we summarize. MS is a complex condition for which there is significant variability among its associated symptoms across cultures, developmental stages, and sexes making it difficult to control for in epidemiological studies or to identify patterns in exposure-related outcomes. Timing of exposure relative to outcome is further complicated by the differences in experimental design (i.e., in vivo, in vitro) and study design (i.e., cross-sectional vs. prospective). Typically, metals are measured in blood, urine or hair because these samples are easily procured from otherwise healthy individuals; however, interpretations about the timing of exposure vary by specimen. While blood is generally thought to assess recent exposure and urine a subacute or chronic exposure, these assumptions can vary based on the properties of the metal and other confounding factors such as age and health of the individual(67). For example, studies have shown that the kidney cortex is a high-affinity storage site for Cd (halftime varies between 18 and 44 years(68)) and that kidney Cd is slowly released into the urine at a rate of approximately 1.7% of total kidney Cd approximately every eight hours. Therefore, assessing the cause and effect relationship between chronic exposure to Cd and MS or MS-related components by sampling urine at one time point partially circumvents the temporal ambiguity inherent in cross-sectional or case-control studies where Cd is measured in blood, wherein the latter sampling reflects exposure for the preceding 30 days(69). However, this assumption is difficult if not impossible to validate because knowledge about the timing and/or duration of exposure is unknown. The issue of metal speciation, specifically oxidation state (Hg) and complexation, which can affect toxicity and environmental partitioning/availability, is largely unaddressed. Increasing evidence indicates that populations in low socioeconomic status (SES) and urban areas carry a greater exposure burden for heavy metals; however, few studies have adequately controlled for residual confounding by SES in relation to MS(39, 70). Although there are shared features, there remains a lack of consensus on the definition of MS, and the outcomes measured and measurement techniques vary across studies(1).
Similar to the epidemiological studies, model systems also suggest a potential association between heavy metal exposure and MS-related outcomes. These systems are uniquely positioned to control for the many complexities associated with exposure studies in human populations while evaluating many of the same outcomes such as adiposity, glucose intolerance, insulin resistance, body weight, and lipid profiles. Of particular importance, model systems offer opportunities to conduct discovery-driven mechanistic analyses in association with physiological endpoints, thereby providing insight into possible mechanisms of action and identifying markers that could be evaluated in human samples such as epigenetic or gene markers. However, similar to epidemiological studies, there are limitations to animal and in vitro studies when extrapolating to human outcomes. These include concentrations, mixtures, differential susceptibilities, and sex differences. Exposure concentrations were typically significantly higher than levels found in human and environmental studies. None of the studies we reviewed addressed exposure to mixtures of heavy metals despite the frequent co-occurrence of these compounds. Few studies addressed differential susceptibility to exposure during development or differentiated between males and females. Common to model system studies, it is unclear whether additional outcomes were evaluated beyond those reported as negative outcomes are often omitted.
As Cd, Pb and Hg are ubiquitous and often co-occur in the environment, the increasing incidence of MS and comorbid conditions worldwide coupled with epidemiological and experimental support for an association between them indicates that longitudinal studies with adequate statistical power to examine the effects of these metals, including the occurrence of sexually dimorphic responses, are warranted. Such prospective studies could be informed by the inclusion of experimental studies in model systems, which together should help to address exposure levels below which human disease is unlikely, mechanisms that could be targeted for screening algorithms, and the design of environmental or therapeutic interventions.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the outstanding team at the Comparative Toxicogenomics Database for helpful discussions and for curating and providing access to additional information about the articles reviewed in this manuscript as well as the Center for Human Health and the Environment, which helped to facilitate collaborations such as this review.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Antonio Planchart, Adrian Green, Cathrine Hoyo, and Carolyn J. Mattingly declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Antonio Planchart, NC State University, Department of Biological Sciences, Center for Human Health and the Environment, Toxicology building, 850 Main Campus Dr., Raleigh, NC 27606.
Adrian Green, NC State University, Department of Biological Sciences, Toxicology building, 850 Main Campus Dr., Raleigh, NC 27606.
Cathrine Hoyo, NC State University, Department of Biological Sciences, Center for Human Health and the Environment, Toxicology building, 850 Main Campus Dr., Raleigh, NC 27606.
Carolyn J. Mattingly, NC State University, Department of Biological Sciences, Center for Human Health and the Environment, Toxicology building, 850 Main Campus Dr., Raleigh, NC 27606.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Kaur J. A comprehensive review on metabolic syndrome. Cardiology research and practice. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015 May 19;313(19):1973–4. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS data brief. 2015 Nov;(219):1–8. [PubMed] [Google Scholar]
- 4.De Long NE, Holloway AC. Early-life chemical exposures and risk of metabolic syndrome. Diabetes, metabolic syndrome and obesity: targets and therapy. 2017;10:101–9. doi: 10.2147/DMSO.S95296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012 Jun;120(6):779–89. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy C, Tremblay PY, Ayotte P. Is mercury exposure causing diabetes, metabolic syndrome and insulin resistance? A systematic review of the literature. Environmental research. 2017 Jul;156:747–60. doi: 10.1016/j.envres.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 7.Tinkov AA, Filippini T, Ajsuvakova OP, Aaseth J, Gluhcheva YG, Ivanova JM, et al. The role of cadmium in obesity and diabetes. The Science of the total environment. 2017 Dec 01;601–602:741–55. doi: 10.1016/j.scitotenv.2017.05.224. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Zhang Y, Wang W, Wu Y. Association of urinary cadmium with risk of diabetes: a meta-analysis. Environmental science and pollution research international. 2017 Apr;24(11):10083–90. doi: 10.1007/s11356-017-8610-8. [DOI] [PubMed] [Google Scholar]
- 9.Kuo CC, Moon K, Thayer KA, Navas-Acien A. Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence. Current diabetes reports. 2013 Dec;13(6):831–49. doi: 10.1007/s11892-013-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thevenod F, Lee WK. Toxicology of cadmium and its damage to mammalian organs. Metal ions in life sciences. 2013;11:415–90. doi: 10.1007/978-94-007-5179-8_14. [DOI] [PubMed] [Google Scholar]
- 11.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011 May 10;283(2–3):65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Gundacker C, Gencik M, Hengstschlager M. The relevance of the individual genetic background for the toxicokinetics of two significant neurodevelopmental toxicants: mercury and lead. Mutation research. 2010 Oct;705(2):130–40. doi: 10.1016/j.mrrev.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Moon SS. Association of lead, mercury and cadmium with diabetes in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Diabetic medicine: a journal of the British Diabetic Association. 2013 Apr;30(4):e143–8. doi: 10.1111/dme.12103. [DOI] [PubMed] [Google Scholar]
- 14.Simic A, Hansen AF, Asvold BO, Romundstad PR, Midthjell K, Syversen T, et al. Trace element status in patients with type 2 diabetes in Norway: The HUNT3 Survey. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements. 2017 May;41:91–8. doi: 10.1016/j.jtemb.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Feng W, Cui X, Liu B, Liu C, Xiao Y, Lu W, et al. Association of urinary metal profiles with altered glucose levels and diabetes risk: a population-based study in China. PLoS One. 2015;10(4):e0123742. doi: 10.1371/journal.pone.0123742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menke A, Guallar E, Cowie CC. Metals in Urine and Diabetes in U.S. Adults. Diabetes. 2016 Jan;65(1):164–71. doi: 10.2337/db15-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borne Y, Fagerberg B, Persson M, Sallsten G, Forsgard N, Hedblad B, et al. Cadmium exposure and incidence of diabetes mellitus–results from the Malmo Diet and Cancer study. PLoS One. 2014;9(11):e112277. doi: 10.1371/journal.pone.0112277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ettinger AS, Bovet P, Plange-Rhule J, Forrester TE, Lambert EV, Lupoli N, et al. Distribution of metals exposure and associations with cardiometabolic risk factors in the “Modeling the Epidemiologic Transition Study”. Environmental health: a global access science source. 2014 Nov 05;13:90. doi: 10.1186/1476-069X-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang A, Liu S, Cheng N, Pu H, Dai M, Ding J, et al. Multiple metals exposure, elevated blood glucose and dysglycemia among Chinese occupational workers. Journal of diabetes and its complications. 2017 Jan;31(1):101–7. doi: 10.1016/j.jdiacomp.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Park SB, Choi SW, Nam AY. Hair tissue mineral analysis and metabolic syndrome. Biological trace element research. 2009 Sep;130(3):218–28. doi: 10.1007/s12011-009-8336-7. [DOI] [PubMed] [Google Scholar]
- 21.Rhee SY, Hwang YC, Woo JT, Sinn DH, Chin SO, Chon S, et al. Blood lead is significantly associated with metabolic syndrome in Korean adults: an analysis based on the Korea National Health and Nutrition Examination Survey (KNHANES), 2008. Cardiovascular diabetology. 2013 Jan 09;12:9. doi: 10.1186/1475-2840-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon SS. Additive effect of heavy metals on metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocrine. 2014 Jun;46(2):263–71. doi: 10.1007/s12020-013-0061-5. [DOI] [PubMed] [Google Scholar]
- 23.Barregard L, Bergstrom G, Fagerberg B. Cadmium exposure in relation to insulin production, insulin sensitivity and type 2 diabetes: a cross-sectional and prospective study in women. Environmental research. 2013 Feb;121:104–9. doi: 10.1016/j.envres.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Kelishadi R, Askarieh A, Motlagh ME, Tajadini M, Heshmat R, Ardalan G, et al. Association of blood cadmium level with cardiometabolic risk factors and liver enzymes in a nationally representative sample of adolescents: the CASPIAN-III study. Journal of environmental and public health. 2013;2013:142856. doi: 10.1155/2013/142856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padilla MA, Elobeid M, Ruden DM, Allison DB. An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99–02. International journal of environmental research and public health. 2010 Sep;7(9):3332–47. doi: 10.3390/ijerph7093332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao W, Liu Q, He X, Liu H, Gu A, Jiang Z. Association between level of urinary trace heavy metals and obesity among children aged 6–19 years: NHANES 1999–2011. Environmental science and pollution research international. 2017 Apr;24(12):11573–81. doi: 10.1007/s11356-017-8803-1. [DOI] [PubMed] [Google Scholar]
- 27.Jornayvaz FR, Vollenweider P, Bochud M, Mooser V, Waeber G, Marques-Vidal P. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: the CoLaus study. Cardiovascular diabetology. 2016 May 03;15:73. doi: 10.1186/s12933-016-0389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schellong K, Schulz S, Harder T, Plagemann A. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS One. 2012;7(10):e47776. doi: 10.1371/journal.pone.0047776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menai M, Heude B, Slama R, Forhan A, Sahuquillo J, Charles MA, et al. Association between maternal blood cadmium during pregnancy and birth weight and the risk of fetal growth restriction: the EDEN mother-child cohort study. Reprod Toxicol. 2012 Dec;34(4):622–7. doi: 10.1016/j.reprotox.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Rotter I, Kosik-Bogacka D, Dolegowska B, Safranow K, Lubkowska A, Laszczynska M. Relationship between the concentrations of heavy metals and bioelements in aging men with metabolic syndrome. International journal of environmental research and public health. 2015 Apr 10;12(4):3944–61. doi: 10.3390/ijerph120403944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swaddiwudhipong W, Mahasakpan P, Limpatanachote P, Krintratun S. Correlations of urinary cadmium with hypertension and diabetes in persons living in cadmium-contaminated villages in northwestern Thailand: A population study. Environmental research. 2010 Aug;110(6):612–6. doi: 10.1016/j.envres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 32•.Swaddiwudhipong W, Limpatanachote P, Mahasakpan P, Krintratun S, Punta B, Funkhiew T. Progress in cadmium-related health effects in persons with high environmental exposure in northwestern Thailand: a five-year follow-up. Environmental research. 2012 Jan;112:194–8. doi: 10.1016/j.envres.2011.10.004. Prospective study with significant associations between Cd exposure and T2D and HT. [DOI] [PubMed] [Google Scholar]
- 33.Everett CJ, Frithsen IL. Association of urinary cadmium and myocardial infarction. Environmental research. 2008 Feb;106(2):284–6. doi: 10.1016/j.envres.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Hecht EM, Arheart KL, Lee DJ, Hennekens CH, Hlaing WM. Interrelation of Cadmium, Smoking, and Cardiovascular Disease (from the National Health and Nutrition Examination Survey) The American journal of cardiology. 2016 Jul 15;118(2):204–9. doi: 10.1016/j.amjcard.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 35.Hansen AF, Simic A, Asvold BO, Romundstad PR, Midthjell K, Syversen T, et al. Trace elements in early phase type 2 diabetes mellitus-A population-based study. The HUNT study in Norway. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements. 2017 Mar;40:46–53. doi: 10.1016/j.jtemb.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Afridi HI, Kazi TG, Kazi N, Jamali MK, Arain MB, Jalbani N, et al. Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes research and clinical practice. 2008 May;80(2):280–8. doi: 10.1016/j.diabres.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Liu B, Feng W, Wang J, Li Y, Han X, Hu H, et al. Association of urinary metals levels with type 2 diabetes risk in coke oven workers. Environmental pollution. 2016 Mar;210:1–8. doi: 10.1016/j.envpol.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 38.Son HS, Kim SG, Suh BS, Park DU, Kim DS, Yu SD, et al. Association of cadmium with diabetes in middle-aged residents of abandoned metal mines: the first health effect surveillance for residents in abandoned metal mines. Annals of occupational and environmental medicine. 2015;27:20. doi: 10.1186/s40557-015-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nie X, Wang N, Chen Y, Chen C, Han B, Zhu C, et al. Blood cadmium in Chinese adults and its relationships with diabetes and obesity. Environmental science and pollution research international. 2016 Sep;23(18):18714–23. doi: 10.1007/s11356-016-7078-2. [DOI] [PubMed] [Google Scholar]
- 40.Lee BK, Kim Y. Association of Blood Cadmium Level with Metabolic Syndrome After Adjustment for Confounding by Serum Ferritin and Other Factors: 2008–2012 Korean National Health and Nutrition Examination Survey. Biological trace element research. 2016 May;171(1):6–16. doi: 10.1007/s12011-015-0499-9. [DOI] [PubMed] [Google Scholar]
- 41.Lee BK, Kim Y. Blood cadmium, mercury, and lead and metabolic syndrome in South Korea: 2005–2010 Korean National Health and Nutrition Examination Survey. American journal of industrial medicine. 2013 Jun;56(6):682–92. doi: 10.1002/ajim.22107. [DOI] [PubMed] [Google Scholar]
- 42.Singh PK, Baxi D, Diwedi R, Ramachandran AV. Prior cadmium exposure improves glucoregulation in diabetic rats but exacerbates effects on metabolic dysregulation, oxidative stress, and hepatic and renal toxicity. Drug Chem Toxicol. 2012;35(2):167–77. doi: 10.3109/01480545.2011.589450. 2012/04. [DOI] [PubMed] [Google Scholar]
- 43.Treviño S, Waalkes MP, Flores Hernández JA, León-Chavez BA, Aguilar-Alonso P, Brambila E. Chronic cadmium exposure in rats produces pancreatic impairment and insulin resistance in multiple peripheral tissues. Arch Biochem Biophys. 2015;583:27–35. doi: 10.1016/j.abb.2015.07.010. 2015/10/01/ [DOI] [PubMed] [Google Scholar]
- 44•.Kawakami T, Nishiyama K, Kadota Y, Sato M, Inoue M, Suzuki S. Cadmium modulates adipocyte functions in metallothionein-null mice. Toxicol Appl Pharmacol. 2013;272(3):625–36. doi: 10.1016/j.taap.2013.07.015. 2013/11/01/ In vivo and in vitro study identifying Cd-mediated alterations in molecular markers of metabolism and adiposity. [DOI] [PubMed] [Google Scholar]
- 45.Gong P, Chang X, Chen X, Bai X, Wen H, Pi S, et al. Metabolomics study of cadmium-induced diabetic nephropathy and protective effect of caffeic acid phenethyl ester using UPLC-Q-TOF-MS combined with pattern recognition. Environmental toxicology and pharmacology. 2017;54:80–92. doi: 10.1016/j.etap.2017.06.021. 2017/09// [DOI] [PubMed] [Google Scholar]
- 46.El Muayed M, Raja MR, Zhang X, MacRenaris KW, Bhatt S, Chen X, et al. Accumulation of cadmium in insulin-producing β cells. Islets. 2012;4(6):405–16. doi: 10.4161/isl.23101. 2012/12//Nov- undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang K-C, Hsu C-C, Liu S-H, Su C-C, Yen C-C, Lee M-J, et al. Cadmium induces apoptosis in pancreatic β-cells through a mitochondria-dependent pathway: the role of oxidative stress-mediated c-Jun N-terminal kinase activation. PloS One. 2013;8(2):e54374. doi: 10.1371/journal.pone.0054374. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, et al. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. AJP: Regulatory, Integrative and Comparative Physiology. 2008;295(6):R1782–R93. doi: 10.1152/ajpregu.90635.2008. 2008/10/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Yan C, Yang Z, Zhang W, Niu Y, Li X, et al. Alterations of serum trace elements in patients with type 2 diabetes. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements. 2017 Mar;40:91–6. doi: 10.1016/j.jtemb.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 50.CDC. CDC’s Childhood Lead Poisoning Prevention Program/What Do Parents Need to Know to Protect Their Children? 2017 [cited 2017 November 2017]. Available from: https://http://www.cdc.gov/nceh/lead/acclpp/blood_lead_levels.htm.
- 51.Tyrrell JB, Hafida S, Stemmer P, Adhami A, Leff T. Lead (Pb) exposure promotes diabetes in obese rodents. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements (GMS) 2017;39:221–6. doi: 10.1016/j.jtemb.2016.10.007. 2017/01// [DOI] [PubMed] [Google Scholar]
- 52.Mostafalou S, Baeeri M, Bahadar H, Soltany-Rezaee-Rad M, Gholami M, Abdollahi M. Molecular mechanisms involved in lead induced disruption of hepatic and pancreatic glucose metabolism. Environmental toxicology and pharmacology. 2015 Jan;39(1):16–26. doi: 10.1016/j.etap.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Sun H, Wang N, Nie X, Zhao L, Li Q, Cang Z, et al. Lead Exposure Induces Weight Gain in Adult Rats, Accompanied by DNA Hypermethylation. PLoS One. 2017;12(1):e0169958. doi: 10.1371/journal.pone.0169958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beier EE, Inzana JA, Sheu T-J, Shu L, Puzas JE, Mooney RA. Effects of Combined Exposure to Lead and High-Fat Diet on Bone Quality in Juvenile Male Mice. Environmental Health Perspectives. 2015;123(10):935–43. doi: 10.1289/ehp.1408581. 2015/10// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faulk C, Barks A, Sanchez BN, Zhang Z, Anderson OS, Peterson KE, et al. Perinatal lead (Pb) exposure results in sex-specific effects on food intake, fat, weight, and insulin response across the murine life-course. PloS One. 2014;9(8):e104273. doi: 10.1371/journal.pone.0104273. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Virtanen JK, Mursu J, Voutilainen S, Uusitupa M, Tuomainen TP. Serum omega-3 polyunsaturated fatty acids and risk of incident type 2 diabetes in men: the Kuopio Ischemic Heart Disease Risk Factor study. Diabetes care. 2014;37(1):189–96. doi: 10.2337/dc13-1504. [DOI] [PubMed] [Google Scholar]
- 57.Mozaffarian D, Shi P, Morris JS, Grandjean P, Siscovick DS, Spiegelman D, et al. Methylmercury exposure and incident diabetes in U.S. men and women in two prospective cohorts. Diabetes care. 2013 Nov;36(11):3578–84. doi: 10.2337/dc13-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drouillet-Pinard P, Huel G, Slama R, Forhan A, Sahuquillo J, Goua V, et al. Prenatal mercury contamination: relationship with maternal seafood consumption during pregnancy and fetal growth in the ‘EDEN mother-child’ cohort. The British journal of nutrition. 2010 Oct;104(8):1096–100. doi: 10.1017/S0007114510001947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xun He K, Liu P, Morris K, Reis S, Guallar JE. Mercury exposure in young adulthood and incidence of diabetes later in life: the CARDIA Trace Element Study. Diabetes care. 2013 Jun;36(6):1584–9. doi: 10.2337/dc12-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeppesen C, Valera B, Nielsen NO, Bjerregaard P, Jorgensen ME. Association between whole blood mercury and glucose intolerance among adult Inuit in Greenland. Environmental research. 2015 Nov;143(Pt A):192–7. doi: 10.1016/j.envres.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 61.Eom SY, Choi SH, Ahn SJ, Kim DK, Kim DW, Lim JA, et al. Reference levels of blood mercury and association with metabolic syndrome in Korean adults. International archives of occupational and environmental health. 2014 Jul;87(5):501–13. doi: 10.1007/s00420-013-0891-8. [DOI] [PubMed] [Google Scholar]
- 62.Park K, Seo E. Association between Toenail Mercury and Metabolic Syndrome Is Modified by Selenium. Nutrients. 2016 Jul 12;8(7) doi: 10.3390/nu8070424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park JS, Ha KH, He K, Kim DJ. Association between Blood Mercury Level and Visceral Adiposity in Adults. Diabetes & metabolism journal. 2017 Apr;41(2):113–20. doi: 10.4093/dmj.2017.41.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnes DM, Hanlon PR, Kircher EA. Effects of inorganic HgCl2 on adipogenesis. Toxicol Sci. 2003;75(2):368–77. doi: 10.1093/toxsci/kfg195. 2003/10// [DOI] [PubMed] [Google Scholar]
- 65.Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang CY, et al. The role of phosphoinositide 3-kinase/Akt signaling in low-dose mercury-induced mouse pancreatic beta-cell dysfunction in vitro and in vivo. Diabetes. 2006;55(6):1614–24. doi: 10.2337/db06-0029. 2006/06// [DOI] [PubMed] [Google Scholar]
- 66.Kawakami T, Hanao N, Nishiyama K, Kadota Y, Inoue M, Sato M, et al. Differential effects of cobalt and mercury on lipid metabolism in the white adipose tissue of high-fat diet-induced obesity mice. Toxicol Appl Pharmacol. 2012;258(1):32–42. doi: 10.1016/j.taap.2011.10.004. 2012/01/01/ [DOI] [PubMed] [Google Scholar]
- 67.Wang RY, Caldwell KL, Jones RL. Analytical considerations in the clinical laboratory assessment of metals. Journal of medical toxicology: official journal of the American College of Medical Toxicology. 2014 Jun;10(2):232–9. doi: 10.1007/s13181-014-0381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akerstrom M, Barregard L, Lundh T, Sallsten G. The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol Appl Pharmacol. 2013 May 1;268(3):286–93. doi: 10.1016/j.taap.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 69.Satarug S, Moore MR. Emerging roles of cadmium and heme oxygenase in type-2 diabetes and cancer susceptibility. The Tohoku journal of experimental medicine. 2012 Dec;228(4):267–88. doi: 10.1620/tjem.228.267. [DOI] [PubMed] [Google Scholar]
- 70.King KE, Darrah TH, Money E, Meentemeyer R, Maguire RL, Nye MD, et al. Geographic clustering of elevated blood heavy metal levels in pregnant women. BMC public health. 2015 Oct 09;15:1035. doi: 10.1186/s12889-015-2379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


