ABSTRACT
Spermatogenesis in mammals is a very complex, highly organized process, regulated in part by testosterone and retinoic acid (RA). Much is known about how RA and testosterone signaling pathways independently regulate this process, but there is almost no information regarding whether these two signaling pathways directly interact and whether RA is crucial for steroidogenic cell function. This study uses a transgenic mouse line that expresses a dominant-negative form of RA receptor α (RAR-DN) and the steroidogenic cell-specific Cre mouse line, Cyp17iCre, to generate male mice with steroidogenic cells unable to perform RA signaling. Testes of mutant mice displayed increased apoptosis of pachytene spermatocytes, an increased number of macrophages in the interstitium and a loss of advanced germ cells. Additionally, blocking RA signaling in Leydig cells resulted in increased permeability of the blood-testis barrier, decreased levels of the steroidogenic enzyme cytochrome P450 17a1 and decreased testosterone levels. Surprisingly, the epididymides of the mutant mice also displayed an abnormal phenotype. This study demonstrates that RA signaling is required in steroidogenic cells for their normal function and, thus, for male fertility.
KEY WORDS: Leydig cells, Spermatogenesis, Testis, Epididymis, Retinoic acid
Summary: Expression of a dominant-negative form of retinoic acid receptor α in Leydig cells reveals a role for this receptor in male infertility.
INTRODUCTION
Testosterone and the active metabolite of vitamin A, retinoic acid (RA), are both known to be important for spermatogenesis, and misregulation of either RA or androgens causes testicular dysfunctions, usually resulting in male infertility. Testosterone, which is produced by the steroidogenic cells of the testis, known as Leydig cells, regulates the end of meiosis, the establishment and maintenance of the blood-testis barrier (BTB), and spermiation (Walker, 2011). Similarly, RA is essential for spermatogonial differentiation, meiotic initiation, the formation of BTB, spermiogenesis and spermiation (Huang and Hembree, 1979; Griswold et al., 1989; Chung and Wolgemuth, 2004; Wolgemuth and Chung, 2007; Clagett-Dame and Knutson, 2011; Hogarth et al., 2011; Raverdeau et al., 2012). After spermiation, sperm are released into the epididymis where they undergo maturation and are stored until ejaculation. Although there is a significant amount known about how RA and testosterone independently regulate spermatogenesis, it is unknown whether there is crosstalk between the RA and testosterone signaling pathways. In addition, it has not been investigated whether Leydig cells require the RA signaling mechanism for their normal function and, thus, for normal production of testosterone.
RA signaling is mediated by two families of nuclear receptors: retinoic acid receptors (RARs) and retinoid X receptors (RXRs). These receptors form RAR/RXR heterodimers that translocate into the nucleus in a ligand-dependent or -independent manner. In the nucleus, the RAR/RXR dimer, without the RA ligand, binds to retinoic acid response elements (RAREs) in the genome and recruits co-repressors, suppressing transcription of target genes. In the presence of the ligand, RA binds to the RAR/RXR dimer, inducing dissociation of co-repressors and recruiting co-activators, resulting in transcription of RA responsive genes (van Pelt et al., 1992; Kastner et al., 1996; Bastien and Rochette-Egly, 2004; Vernet et al., 2006; Mendoza-Parra and Gronemeyer, 2013). Immunohistochemistry and in situ hybridization studies have detected the presence of all six RARs (α, β, γ) and RXRs (α, β, γ) in the Leydig cells (Martin and Tremblay, 2010). However, it is unknown whether RARs and/or RXRs are essential in Leydig cells for their normal function.
Testosterone, which is secreted by the Leydig cells in the interstitium, signals through the androgen receptor (AR) located in the Sertoli cells inside the seminiferous epithelium, where spermatogenesis takes place. Multiple published reports have demonstrated that a lack of testosterone or depletion of Ar within Sertoli cells results in a blockage in spermatogenesis at the end of meiosis, causing decreased production of round spermatids and a lack of elongated spermatids (O'Donnell et al., 1994, 1996; De Gendt et al., 2004, 2005). Additionally, testosterone depletion causes problems during BTB formation and spermiation (Holdcraft and Braun, 2004; Meng et al., 2005). In the rat, all of these processes require high levels of testosterone, at least 70 nM (Zirkin et al., 1989).
Interestingly, testosterone and RA are essential for normal spermatogenesis, but it is not known whether there is an interplay between these two signaling pathways. In vitro studies, performed using immortalized Leydig cell lines and cultured primary rat Leydig cells, demonstrated that RA and retinol can enhance the production of steroidogenic proteins, such as steroidogenic acute regulatory (STAR) protein and cytochrome P450 17a1 (CYP17A1), thus, regulating testosterone synthesis (Chaudhary et al., 1989; Lefevre et al., 1994; Manna et al., 2013). Microarray studies have also revealed that testicular feminized mice, which have lower than normal levels of testosterone, display decreased expression of transcripts encoding vitamin A-metabolizing proteins (O'Shaughnessy et al., 2007). Supporting these data, depletion of vitamin A in rats also resulted in decreased levels of 3β-hydroxysteroid dehydrogenase (HSD3B1) activity (Jayaram et al., 1973). These studies imply that Leydig cell function might be regulated by RA signaling, yet the function of RARs and RXRs in Leydig cells is still unclear.
In this present study, we investigated whether the RA signaling mechanism is needed for steroidogenic cell function. We used a conditional transgenic mouse line that expresses a dominant-negative form of RARα (RAR-DN) but only in the presence of Cre recombinase (Rosselot et al., 2010). The RAR-DN is a truncated form of human RARα mutated in its activation site. Previous studies have demonstrated that the RAR-DN inhibits RA signaling by sequestering the RXRs and preventing the formation of heterodimers with endogenous RARs in a dose-dependent manner (Damm et al., 1993; Rosselot et al., 2010; Chen et al., 2016). Thus, we crossed this RAR-DN mouse line to the steroidogenic cell-specific Cre recombinase mouse line Cyp17iCre (Bridges et al., 2008), to generate male mice with steroidogenic cells unable to perform RAR/RXR signaling. It has previously been published that the Cyp17icre is highly expressed in Leydig cells (Jauregui et al., 2018). Unexpectedly, in this study, we also found expression of the Cyp17icre in some of the principal cells within the epididymis. We discovered that inhibition of RAR/RXR signaling in steroidogenic cells caused alterations in spermatogenesis and in the structure of the epithelial cells in the epididymis, resulting in male infertility. Taken together, these findings demonstrate, for the first time, that RA signaling activity is needed in the steroidogenic cells for normal spermatogenesis and epididymal function.
RESULTS
Loss of RA signaling in Leydig cells results in spermatogenetic errors
To determine whether RA signaling activity is needed in Leydig cells for their normal function, we expressed the RAR-DN only in Leydig cells by using the Cyp17iCre (Bridges et al., 2008; Rosselot et al., 2010). Morphological analyses were performed on RAR-DN-Flox/Flox; Cyp17iCre-negative (control) and RAR-DN-Flox/Flox; Cyp17iCre-positive (mutant) testes collected at 15, 30, 60, 90 and 180 days postpartum (dpp) to examine the progression of spermatogenesis in mutant mice (Fig. 1). At 15 dpp, control (Fig. 1A) and mutant (Fig. 1B) testes displayed normal spermatogenesis and no difference in the testis:body weight ratio. However, by 30 dpp, vacuoles had appeared in 20% of the seminiferous tubule cross-sections in the mutant testes (Fig. 1D), a phenotype not seen in the controls (Fig. 1C). At 60 dpp, mutant testes possessed seminiferous tubules that contained vacuoles, lacking advanced germ cells or with no germ cells at all (Fig. 1F,G). Similarly, the 90 dpp mutant testes displayed seminiferous tubules with vacuoles, degraded seminiferous tubules with missing advanced germ cells or no germ cells, and also seminiferous epithelium with normal spermatogenesis (Fig. 1I,J), whereas almost all of the seminiferous tubules in the 60 (Fig. 1E) and 90 dpp (Fig. 1H) control testes displayed normal spermatogenesis. Quantification of 90 dpp mutant testes revealed a significant decrease in the number of normal tubules (24.5%), a significant increase in the number of degraded tubules (45.25%), and a significant increase of tubules with vacuoles (30.24%) in comparison with 90 dpp control testes (Fig. 1P). No significant difference was observed in the testis:body weight ratios between the 90 dpp mutant and controls testes (Fig. 1K). The 180 dpp mutant testes also contained seminiferous tubules with no advanced germ cells or no germ cells at all (Fig. 1M,N), in comparison with control testes where normal spermatogenesis was observed (Fig. 1L). In the 180 dpp mutant testes, there was a significant decrease in the number of normal tubules (15.3%), and a significant increase in the number of degraded tubules (76%) in comparison with the control testes (Fig. 1Q). Additionally, there was a significant decrease in the testis:body weight ratio of the 180 dpp mutant mice compared with the controls (Fig. 1O). In 60, 90 and 180 dpp mutant animals, the testicular phenotype varied between animals, but it became more severe with age.
Fig. 1.
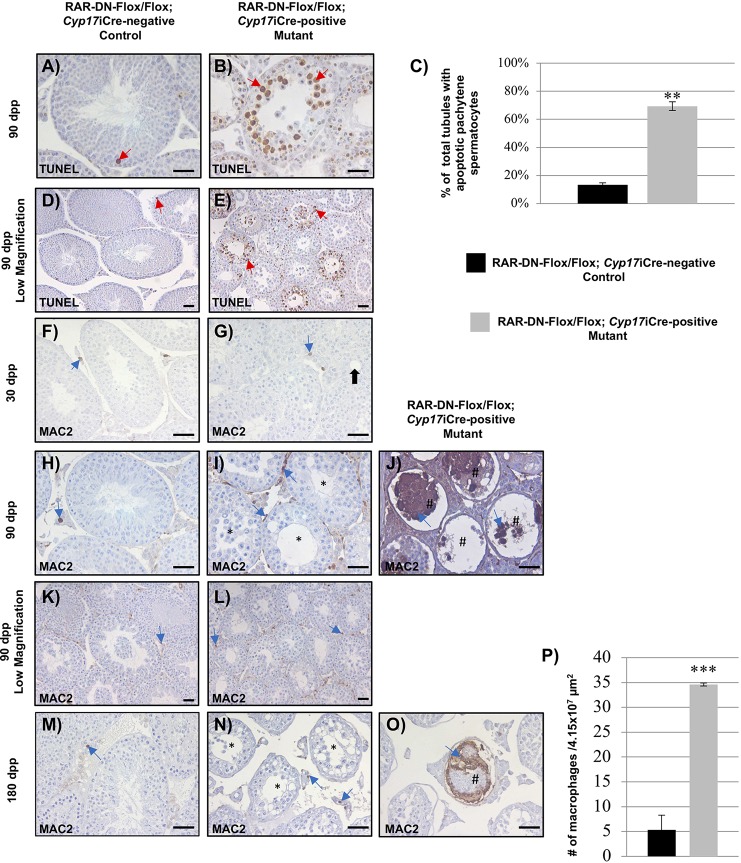
Loss of RA signaling from Leydig cells results in spermatogenic errors. (A,C,E,H,L) Representative cross-sections of testes stained with Harris Hematoxylin from the RAR-DN-Flox/Flox; Cyp17iCre-negative control mice at 15 (A), 30 (C), 60 (E), 90 (H) and 180 dpp (L). (B,D,F,G,I,J,M,N) Representative cross-sections of testes from RAR-DN-Flox/Flox; Cyp17iCre-positive mutant mice are also shown at 15 (B), 30 (D), 60 (F,G), 90 (I,J) and 180 dpp (M,N). The pictures representing the mild (F,I,M) and the severe (G,J,N) phenotypes are from two different RAR-DN-Flox/Flox; Cyp17iCre-positive mutant mice from each time point. The phenotype ratio of mild to severe was 1:2. Black arrows indicate vacuoles, hashes indicate degraded tubules and asterisks indicate missing elongated spermatids. Scale bars: 100 μm. (K,O) The average of the testis/body weight ratios (y-axis) for 90 (K) and 180 dpp (O) control (black bars) and mutant (gray bars) animals (n=10 for each genotype). (P,Q) Quantification of normal tubules, degraded tubules and tubules with vacuoles in the mutant (gray bars) and control (black bars) testes of 90 (P) and 180 dpp (Q) tubules, and calculated based on percentage of total tubules (y-axis) (n=3 for each genotype with mild phenotype). Data are mean±s.e.m. (**P<0.01 and *P=0.04).
Normal STRA8 and PLZF expression in the seminiferous tubules of the mutant testis
To investigate whether the lack of RA signaling in Leydig cells affected the population of spermatogonia, we examined the expression of markers for undifferentiated spermatogonia, zinc-finger and BTB domain containing 16 (ZBTB16) (Buaas et al., 2004; Costoya et al., 2004), and for differentiating spermatogonia, stimulated by retinoic acid gene 8 (STRA8) (Zhou et al., 2008; Snyder et al., 2010), in the 30, 90 and 180 dpp control and mutant testes by immunohistochemistry. As expected, there was normal ZBTB16 (Fig. 2A,E,I,M) and STRA8 (Fig. 2C,G,K,O) expression in the 30, 90 and 180 dpp control testes. In the 30 dpp mutant testes, normal ZBTB16 (Fig. 2B) and STRA8 (Fig. 2D) expression was detected within the seminiferous tubules, including the seminiferous tubules with vacuoles. In the 90 dpp mutant testes, there was expression of ZBTB16 (Fig. 2F,N) and STRA8 (Fig. 2H,P) in normal seminiferous tubules, in the seminiferous tubules with missing advanced germ cells and in the seminiferous tubules with vacuoles. Surprisingly, there was also ZBTB16 (Fig. 2J) and STRA8 (Fig. 2L) expression within the seminiferous tubules in the 180 dpp mutant testes. The detection of ZBTB16 and STRA8 markers in the mutant testes suggests that a lack of RA signaling in Leydig cells does not affect the spermatogonial population and spermatogonial differentiation still occurs, even though there is disruption of meiotic and haploid germ cell development.
Fig. 2.
ZBTB16 and STRA8 expression in male mice lacking RA signaling within Leydig cells. Representative cross-sections of testes stained for ZBTB16 (brown staining) via immunohistochemistry. (A,B,E,F,I,J,M,N) ZBTB16 expression in undifferentiated spermatogonia (yellow arrows) was detected in 30 (A), 90 (E,M) and 180 dpp (I) control RAR-DN-Flox/Flox; Cyp17iCre-negative mice, and in 30 (B), 90 (F,N) and 180 dpp (J) mutant RAR-DN-Flox/Flox; Cyp17iCre-positive mice. Representative cross-sections of testes were also stained for STRA8 (brown staining) using immunohistochemistry. (C,D,G,H,K,L,O,P) STRA8-positive preleptotene spermatocytes (red arrows) and STRA8-positive differentiated spermatogonia (blue arrows) were detected in 30 (C), 90 (G,O) and 180 dpp (K) control RAR-DN-Flox/Flox; Cyp17iCre-negative mice, and in 30 (D), 90 (H,P) and 180 dpp (L) mutant RAR-DN-Flox/Flox; Cyp17iCre-positive mice. Scale bars: 50 μm. Black arrows indicate vacuoles, hashes indicate degraded tubules and asterisks indicate missing elongated spermatids.
Inhibition of RA signaling in Leydig cells results in increased number of apoptotic pachytene spermatocytes and macrophages
The vacuoles and the lack of advanced germ cells, specifically missing round and elongated spermatids, suggested that there may be germ cells undergoing apoptosis in the mutant testes. To investigate this, we used the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay to detect fragmented DNA in the mutant and control 90 dpp testes. In the control testes, there were very few TUNEL-positive cells within the seminiferous epithelium (Fig. 3A,D). In the mutant testes, we observed pachytene spermatocytes undergoing apoptosis, especially in tubules with missing advanced germ cells (Fig. 3B,E). The percentage of total tubules with apoptotic pachytene spermatocytes was significantly higher in the mutant (69.4%) compared with the control (13.35%) testes (Fig. 3C).
Fig. 3.
Lack of RA signaling in Leydig cells causes increased numbers of apoptotic cells and macrophages. (A,B,D,E) The representative images depict immunohistochemistry of apoptotic cells (brown staining, red arrows indicate pachytene spermatocytes) detected by TUNEL assays performed on cross-sections of 90 dpp RAR-DN-Flox/Flox; Cyp17iCre-negative control (A,D) and RAR-DN-Flox/Flox; Cyp17iCre-positive mutant (B,E) testes. (C) Quantification of the percentage of total tubules with pachytene spermatocytes (y-axis) was performed in 90 dpp control (black bar) and mutant (gray bar) testes. (F-O) Immunohistochemistry for MAC2 (brown staining, indicated by blue arrows) was performed in testis cross-sections of 30 (F), 90 (H,K) and 180 (M) dpp control, and 30 (G), 90 (I,J,L) and 180 dpp (N,O) mutant animals. (P) The number of macrophages in the interstitial of 4.15×107 µm2 sections (y-axis) in control (black bar) and mutant (gray bar) testes (n=3 for each genotype). Data are mean±s.e.m. (***P=0.001; **P=0.003). Scale bars: 50 μm. Black arrows indicate vacuoles, hashes indicate degraded tubules and asterisks indicate missing elongated spermatids.
Increased amounts of apoptotic cells in testis usually result in immune response and increased number of phagocytic cells such as macrophages (Hedger, 2002). To determine whether there was an increased number of inflammatory macrophages within the mutant testes, immunohistochemistry for lectin, galactose binding, soluble 3 (MAC2), a marker for M2 inflammatory macrophages, was performed in 30, 90 and 180 dpp control and mutant testes. A similar number of cells expressing MAC2 were observed in the interstitial space of the 30 dpp mutant and control testes (Fig. 3F,G). As predicted, we observed an increased number of macrophages in the interstitial space of the 90 (Fig. 3I,L) and 180 dpp (Fig. 3N) mutant testes compared with the control testes (Fig. 3H,K,M). Interestingly, the 90 and 180 dpp mutant testes displayed tubules with infiltrating macrophages (Fig. 3J,O). Quantification revealed a significantly increased number of macrophages in the interstitial space in the 90 dpp mutant testes compared with the control (Fig. 3P).
Normal numbers of Sertoli and Leydig cells in the mutant testis
Macrophages are closely associated with Leydig cells in the interstitium and regulate Leydig cell development and function (Hales, 2002; Bhushan and Meinhardt, 2017). Additionally, the Leydig cell population is also known to be regulated by Sertoli cells (Rebourcet et al., 2014). To investigate whether the numbers of Sertoli cells and Leydig cells were normal in the mutant testis, we performed immunohistochemistry for the Sertoli cell marker SRY (sex determining region Y) box 9 (SOX9) and for the Leydig cell marker HSD3B1 to determine whether the numbers of these cells were affected in the mutant testis. Immunostaining cross-sections of 30, 90 and 180 dpp control and mutant testes revealed normal expression of SOX9 (Fig. 4A-F) in Sertoli cells and of HSD3B1 in Leydig cells (Fig. 4G-L). Interestingly, in the mutant testes, SOX9 (Fig. 4E,F) and HSD3B1 (Fig. 4K,L) were normally expressed in tubules with missing germ cells. Counts of Sertoli cells (Fig. 4M) and Leydig cells (Fig. 4N) per tubule did not reveal any significant difference between the 90 dpp control and mutant testes. However, tubules that shrunk due to the mass germ cell death in the mutant testes contained few Sertoli cell nuclei sloughing from the basement membrane (Fig. 4E,F). These data imply that even though the seminiferous tubule diameter is smaller in the mutant compared with the control, because of a lack of advanced germ cells, the number of Leydig and Sertoli cells per tubule did not change. As a result, it is unlikely that the observed testis phenotype is caused by altering the numbers of these somatic cells.
Fig. 4.

Normal numbers of Sertoli cells and Leydig cells detected within the mutant testes. (A-F) Representative cross-sections of 30 (A), 90 (B) and 180 dpp (C) control RAR-DN-Flox/Flox; Cyp17iCre-negative testes, and 30 (D), 90 (E) and 180 dpp (F) mutant RAR-DN-Flox/Flox; Cyp17iCre-positive testes stained for SOX9 (brown staining, red arrows) by immunohistochemistry. (G-L) The cross-sections of 30 (G), 90 (H) and 180 dpp (I) control RAR-DN-Flox/Flox; Cyp17iCre-negative testes, and 30 (J), 90 (K) and 180 dpp (L) mutant RAR-DN-Flox/Flox; Cyp17iCre-positive testes were also stained for HSD3B1 (brown staining, blue arrows). (M,N) Graphs show the quantification of numbers of Sertoli cells (M) and Leydig cells (N) per testis tubule (y-axis) in the 90 dpp control (black bars) and mutant (gray bars) testes (n=3 for each genotype). Scale bars: 50 μm. Black arrows indicate vacuoles, hashes indicate degraded tubules and asterisks indicate missing elongated spermatids. Data are mean±s.e.m.
Lack of RA signaling in Leydig cells affects their function and results in male infertility
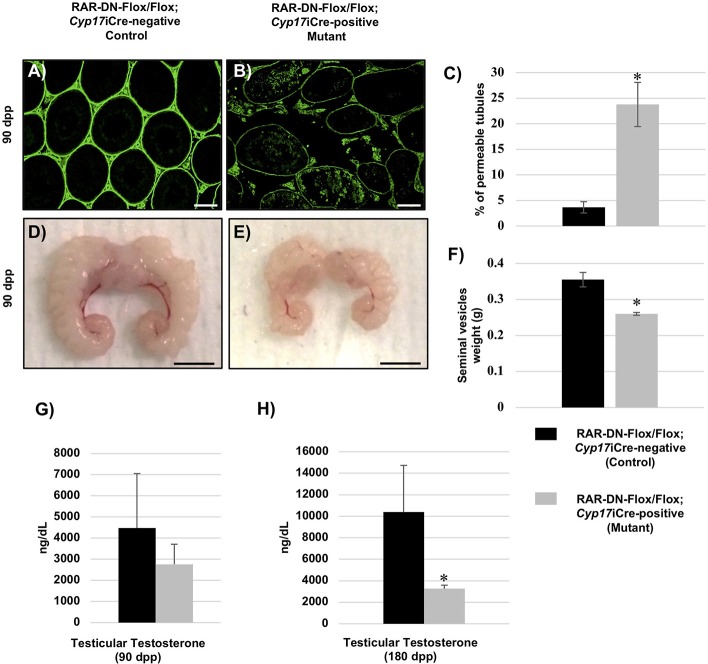
Previous publications have shown that decreased levels of testosterone results in decreased production of round spermatids and missing elongated spermatids (Chang et al., 2004; De Gendt et al., 2004; Tan et al., 2005). In the Leydig cells, testosterone is synthesized as a result of several steroidogenic enzymes (Verhoeven et al., 2010). Therefore, to investigate whether a lack of RA signaling in Leydig cells has a direct effect on their function, we measured transcript levels for different known steroidogenic enzymes. Quantitative RT-PCR measurements of the 90 dpp control and mutant testes showed significantly decreased levels of Cyp17a1 (Fig. 5A), a significant increase in cytochrome P450, family 11, subfamily a, polypeptide 1 (Cyp11a1) expression (Fig. 5D), and no difference in the expression of either Hsd3b1 (Fig. 5B) or Star (Fig. 5C). As decreased levels of Cyp17a1 were detected in the 90 dpp mutant testes, we then investigated whether the BTB, which is known to be regulated by testosterone, was affected in the mutant mice. The BTB, which is formed between Sertoli cells, prevents large molecules from penetrating into the seminiferous epithelium, thereby protecting the cells undergoing meiosis (Meng et al., 2005). Therefore, we performed a biotin-permeability assay on the 90 dpp mutant and control testes. An increase in the permeability of the BTB was observed in the 90 dpp mutant testes (Fig. 6B) when compared with controls (Fig. 6A), and there was a significant increase in the percentage of tubules with a disrupted BTB in the 90 dpp mutant (23.77%) compared with the control testes (3.65%) (Fig. 6C).
Fig. 5.
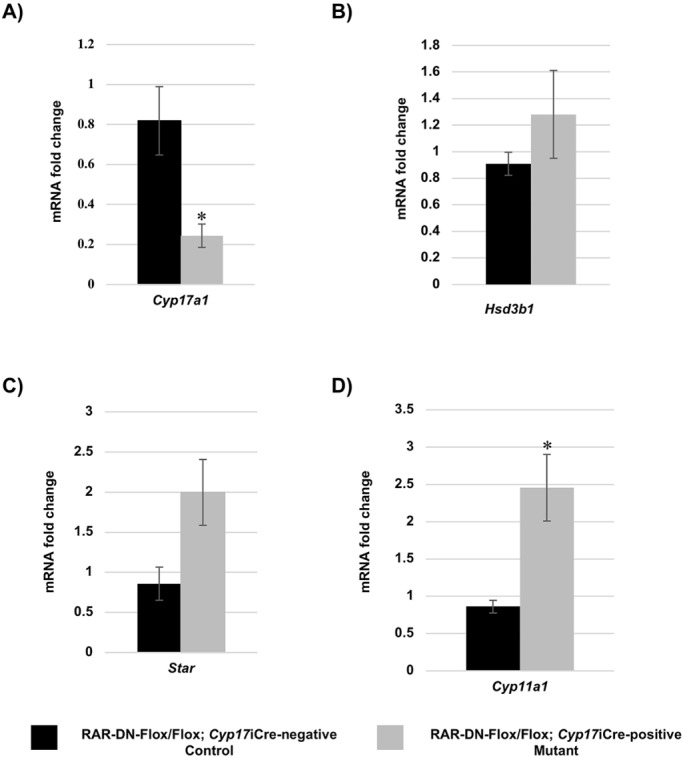
Blocking RA signaling in Leydig cells leads to alterations in the expression of steroidogenic transcripts. (A-D) Fold change in mRNA expression (y-axis) of Cyp17a1 (A), Hsd3b1 (B), Star (C) and Cyp11a1 (D) between 90 dpp RAR-DN-Flox/Flox; Cyp17iCre-negative control (black bars) and RAR-DN-Flox/Flox; Cyp17iCre-positive mutant (gray bars) testes (n=3 for each genotype; mild phenotype). Data are mean±s.e.m. (*P=0.03).
Fig. 6.
Inhibition of RA signaling in steroidogenic cells results in BTB permeability, small seminal vesicles and decreased testosterone levels. (A,B) Representative cross-sections of 90 dpp RAR-DN-Flox/Flox; Cyp17iCre-negative control (A) and RAR-DN-Flox/Flox; Cyp17iCre-positive mutant (B) testes used in the biotin assay to detect BTB permeability (green staining). (C) Quantification of the percentage of permeable tubules (y-axis) in the 90 dpp control (black bars) and mutant (gray bars) testes. (D,E) Representative photos of the seminal vesicles from the 90 dpp control (D) and mutant (E) animals. (F) The average weights of the seminal vesicles from the 90 dpp control and mutant animals are shown in the graph. (G,H) Testicular testosterone measurements of 90 (G) and 180 (H) dpp in ng/dL (y-axis) were performed in control (black bars) and mutant (gray bars) animals (n>6 for each genotype). Data are mean±s.e.m. (*P<0.03). Scale bars: 50 μm in A,B; 0.5 cm in D,E.
Seminal vesicle weight is also directly related to circulating testosterone levels in mice (Higgins and Burchell, 1978; Ortiz and Cavicchia, 1990). We collected and weighed seminal vesicles of the 90 dpp mutant and control animals, and found that the seminal vesicles from the mutant mice were smaller and weighed significantly less than the seminal vesicles from the control mice (Fig. 6D-F). As smaller seminal vesicles are often an indication of decreased testosterone levels, we also measured testicular testosterone concentrations in the 90 and 180 dpp mutant and control animals. There was no significant difference in testosterone levels in the 90 dpp mutant and control testes (Fig. 6G). However, at 180 dpp the testosterone levels were significantly decreased in the mutant compared with controls (Fig. 6H).
To determine whether a lack of RA signaling in Leydig cells altered male fertility, adult male control and mutant mice were paired with wild-type females of known fertility and mating behaviors, and litters sired were recorded over several months. Four out of five adult mutant mice plugged their female companion at least twice, but no confirmed matings ever produced pups. The three control mice plugged their female companion at least twice, and all matings resulted in litters of seven to nine pups (Table 1). These data demonstrate that RA signaling in Leydig cells is needed for their function and for normal spermatogenesis.
Table 1.
Lack of RA signaling within Leydig cells results in male infertility

Expression of RAR-DN in the steroidogenic cells results in an abnormal epididymal phenotype
Spermatogenic errors and blockage in sperm development usually result in decreased sperm production and, thus, male infertility. To determine whether decreased sperm production was the cause of infertility in the mutant mice, we dissected the epididymides from the mutant and control mice for sperm count. Interestingly, epididymides of the 90 dpp mutant mice contained a white mass in the cauda (Fig. 7D,E), not visible in the control epididymides (Fig. 7A,B), making it impossible to perform sperm counts. The severity of the epididymides in the mutant mice varied from animal to animal, but 80% of the mutant animals have an epididymal phenotype. As expected, the epididymal phenotype of 180 dpp mutant mice (Fig. 7F) was more severe in comparison with the epididymides of the 90 dpp mutant mice, whereas the epididymides of the 90 dpp and 180 dpp control mice were normal (Fig. 7C). Morphological analyses of the epididymides from 90 dpp (Fig. 8D-F) and 180 dpp (Fig. 8J-L) mutant animals revealed an abnormal epididymal phenotype. In the caput of the 90 dpp mutant mice, there were vacuoles in the epithelium lining of the duct and there was a decreased amount of sperm visible in comparison with controls (Fig. 8D). In the corpus, there was an increased number of cells, extra tissue within the duct and no sperm (Fig. 8E). In the cauda, however, the integrity of the epithelium lining the duct was lost, there were increased numbers of cells and extra tissue within the duct, and increased numbers of interstitial cells (Fig. 8F). In the 180 dpp mutant mice, the epididymal phenotype was more severe in comparison with the 90 dpp mutant mouse. In the caput, the interstitial space between the ducts increased and an increased number of cells appeared in the interstitium. In addition, the epithelial cells lining the duct had an abnormal appearance (Fig. 8J). Additionally, the corpus had abnormal epithelial cells lining the duct and increased numbers of cells in the interstitium (Fig. 8K), whereas in the cauda of the mutant mouse, the duct degenerated and there were increased numbers of abnormal epithelial cells in the interstitial space and a thicker layer of epithelium lining the duct. Interestingly, there were also increased numbers of cells in the interstitium (Fig. 8L). The histology of the epididymides from controls 90 (Fig. 8A-C) and 180 (Fig. 8G-I) dpp mice was normal.
Fig. 7.
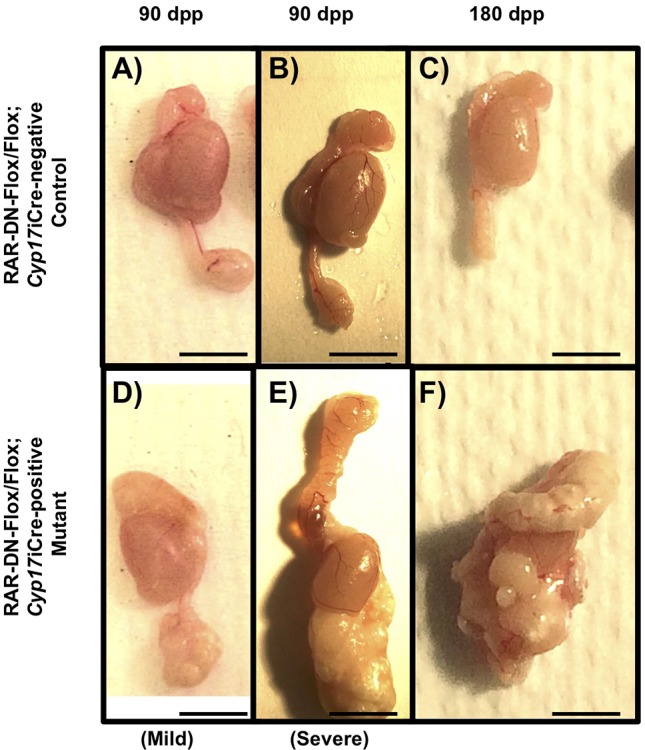
Expression of RAR-DN in steroidogenic cells results in an abnormal epididymal phenotype. (A,B,D,E) The epididymal phenotype observed in 90 dpp control (A,B) and mutant (D,E) mice after dissection. (C,F) The phenotype of the epididymis observed in 180 dpp control (C) and mutant (F) mice (n>10 for each genotype). Scale bars: 0.5 cm.
Fig. 8.
Blocking RA signaling in steroidogenic cells results in an epididymal phenotype. (A-F) Histology of the caput (A), corpus (B), and cauda (C) from 90 dpp control mice, and cross-section histology of the caput (D), corpus (E) and cauda (F) from 90 dpp mutant mice. (G-L) Histology of the caput (G), corpus (H) and cauda (I) from 180 dpp control mice, and the caput (J), corpus (K) and cauda (L) of 180 dpp mutant mice (n>10 for each genotype). Black arrows indicate increase interstitial cells, red arrows indicate abnormal epithelial cells lining duct, purple arrows indicate missing epithelial cells lining the duct, blue arrows indicate vacuoles and asterisks indicate degenerated duct. Scale bars: 50 μm.
Increased expression of cytokeratin 14 and macrophages in the epididymides of mutant mice
Many of the epithelial cells in the mutant epididymis have the characteristic of squamous metaplasia: a change in the morphology of the epithelial cells into a more squamous-like cells. Published data have demonstrated that overexpression of a dominant-negative RAR in the epididymis caused cells to undergo squamous metaplasia (Costa et al., 1997). Therefore, in order to investigate whether the epithelial cells were undergoing squamous metaplasia in the mutant epididymides, we performed immunohistochemistry for cytokeratin 14 (CK14), a marker used to indicate early squamous metaplasia (Vaidyanathan et al., 2003). In the control 90 dpp epididymis, expression of CK14 was detected in the epithelial cells (Fig. 9A-C). However, in the epididymides of the 90 dpp mutant mice, the CK14 expression increased throughout the epithelial cells (Fig. 9D-F). Expression of CK14 was also detected in the abnormal-looking epithelial cells lining the duct and within the duct. These data suggest that the epithelial cells in the mutant epididymis, specifically in the cauda, are undergoing squamous metaplasia. The increased CK14 expression and the expansion of the connective tissue space surrounding the ducts in the mutant epididymides indicated an immune response. Therefore, we performed immunohistochemistry for MAC2 to detect macrophages. In the 90 dpp control epididymis, MAC2 was detected in the epithelial cells (Fig. 9G). However, there was an increased expression of MAC2 in the interstitium between the ducts of the 90 dpp mutant epididymis (Fig. 9H).
Fig. 9.
Increased cytokeratin 14 expression and macrophages in the epididymis of the mutant mice. (A-F) Immunohistochemistry for CK14 in the representative cross-sections of the caput (A), corpus (B) and cauda (C) from 90 dpp control mice and cross-sections of the caput (D), corpus (E) and cauda (F) from 90 dpp mutant mice. Brown staining depicts CK14 expression. (G,H) Immunohistochemistry for MAC2 in representative cross-sections of the cauda in 90 dpp control (G) and mutant (H) mice. Brown staining depicts MAC2 expression (n=5). Scale bars: 50 μm.
Testosterone implant prevented the epididymal phenotype in mice with principal cells lacking RA signaling
Previous studies have shown that all three RAR isomers are expressed in the epididymis and lack of RA results in epithelial cells of the epididymis undergoing squamous metaplasia (Wan et al., 1992; Akmal et al., 1996; Bartlett et al., 1990; Costa et al., 1997). Therefore, we wanted to investigate whether the RAR-DN was expressed in the mutant mice. To investigate this, we crossed the Cyp17icre to a reporter mouse line, RiboTag, and performed immunohistochemistry to detect the human influenza hemagglutinin (HA) tag expression, which is indicative of Cre excision, in the epididymis (Sanz et al., 2009). Interestingly, the principal cells within the epididymis expressed the HA-tag, but the uniformity of it varied between the three regions: the caput, the corpus and the cauda (Fig. 10A-C). The principal cells located in the corpus and the cauda had the highest expression of the HA-tag, which is indicative of Cyp17iCre expression (Fig. 10B,C). While in the caput, only a few principal cells expressed the HA-tag (Fig. 10A). Therefore, it is unknown whether decreased levels of testosterone or lack of RA receptor signaling is the main cause of the epididymal phenotype in the mutant mice.
Fig. 10.
Testosterone implant prevents severe epididymal phenotype in the mutant mouse. (A-C) RiboTag activation in the epididymis of adult RiboTag-positive/Cyp17iCre-positive mice were verified via immunohistochemistry using an anti-HA antibody (brown staining) in the caput (A), corpus (B) and cauda (C) sections. Black arrows indicate immunopositive principal cells. (D,E) Representative images of epididymides and testes from 90 dpp mutant mice with the control implant (D) (n=5) or the testosterone implant (E) (n=8). Scale bars: 50 μm in A-C; 0.5 cm in D,E.
To investigate whether the epididymal phenotype in the mutant mouse was caused by decreased testosterone levels or the expression of RAR-DN in the epididymis, we implanted a testosterone implant (Fig. 10E) or an empty implant (Fig. 10D) into 30 dpp mutant mice and euthanized the mice at 90 dpp. The epididymal phenotype in the 90 dpp mutant mouse with the control implant contained the expected epididymal phenotype with a big white mass in the cauda (Fig. 10D). The severe epididymal phenotype observed in the mutant mouse was partially prevented by the testosterone implant in the 90 dpp mutant mouse and the size of the testis was decreased (Fig. 10E). These data suggests that the low testosterone levels in the testis might be contributing to the epididymal phenotype in the mutant mice. The epididymal phenotype of the mutant mice might be caused by both, the expression of the RAR-DN in the epididymis and the low levels of testosterone. However, we could not determine whether infertility in the mutant animals was caused by the testicular phenotype or by the epididymal phenotype. In conclusion, this study demonstrates that lack of RA signaling in steroidogenic cells results in spermatogenic errors and in an epididymal phenotype that causes male infertility.
DISCUSSION
The main function of Leydig cells is to produce testosterone that is essential for the normal progression of spermatogenesis (O'Donnell et al., 1994, 1996; De Gendt et al., 2004, 2005). Until this study, it was unclear whether the RA and testosterone essential for normal spermatogenesis interact with each other. This study provides the first evidence to detail the importance of RA signaling in Leydig cells for their normal function and proper spermatogenesis. Inhibition of RA signaling activity within Leydig cells, via the expression of the RAR-DN, resulted in alterations of Leydig cell function causing spermatogenic errors, cell death, decreased levels of testosterone and increased numbers of macrophages. In addition to the testicular phenotype, we also found that the expression of the RAR-DN in the steroidogenic cells caused an epididymal phenotype. This study demonstrated, for the first time, that RA signaling is important in steroidogenic cells for normal testosterone production and epididymal development.
Previous reports have shown that a lack of testosterone leads to a block in meiosis, resulting in decreased numbers of round spermatids and missing elongated spermatids (O'Donnell et al., 1994, 1996; De Gendt et al., 2004, 2005). In the current study, morphological analysis revealed that the testes of animals with Leydig cells lacking RA signaling contained seminiferous tubules with missing round spermatids, missing elongated spermatids and no spermatozoa. The phenotype of the mutant testes worsened with age, an indication that RA is needed in adult Leydig cells for their function and normal spermatogenesis. In the murine testis, the adult Leydig cells are not completely formed until 49-56 dpp (Hardy et al., 1989). Before the appearance of the adult Leydig cells, the fetal Leydig cells, in combination with the Sertoli cells, are the main producers of testosterone in the murine testis (Shima et al., 2013). Fetal Leydig cells are known to express only very low levels of Cyp17a1 (Ge and Hardy, 1998; Almeida et al., 2011). However, Cyp17a1 expression increases as the adult Leydig cell progenitor population differentiates, first into immature Leydig cells by ∼28 dpp and then into adult Leydig cells (Ge and Hardy, 1998). Therefore, it is most likely that the expression of Cre by the adult Leydig cells of the mutant animals also increases with age (Bridges et al., 2008). This indicates that the RAR-DN transgene is first expressed by the immature Leydig cells at ∼28 dpp (Hardy et al., 1989), which correlates with the initial appearance of vacuoles in the 30 dpp mutant testes, with expression reaching maximum levels at ∼56 dpp when the adult Leydig cells are completely formed. As the RAR-DN inhibits RAR/RXR activity in a dose-dependent manner (Damm et al., 1993), it is highly likely that the increasing phenotype severity seen with age is directly associated with the increased amount of RAR-DN in the cell following the differentiation of the adult Leydig cells.
The observed phenotype of the mutant testis is similar to mice either lacking or containing only low levels of testosterone (Walker, 2011). Multiple studies have demonstrated that either a loss of androgen receptor (AR) in Sertoli cells or low testicular testosterone concentrations results in a blockage at the end of meiosis, inducing germ cell death (O'Donnell et al., 1994, 1996; De Gendt et al., 2004, 2005). Using a TUNEL assay, we were able to detect apoptotic germ cells in the adult mutant testis in seminiferous tubules missing advanced germ cells. The majority of the germ cells undergoing apoptosis were pachytene spermatocytes and their loss is likely the reason for the observed spermatogenic arrest. Increased cell death usually leads to an increased number of macrophages and greater phagocyte function within the testis (Perez et al., 2013). Therefore, the increased macrophages in the interstitial space of the 90 dpp mutant testes suggest that there is likely an immune response taking place. Macrophages are known to interact with Leydig cells and to regulate their function by suppressing testosterone production (Hales, 2002; Bhushan and Meinhardt, 2017). Additionally, Sertoli cells are also known to correlate with the number of Leydig cells and to regulate their development (Rebourcet et al., 2014). The numbers of Sertoli or Leydig cells did not change between the mutant and control testes, which means that the testicular phenotype observed in the mutant mice is not caused by alterations in the numbers of these somatic cells. However, the increased number of macrophages in the mutant testes could possibly be regulating Leydig cell function and decreasing their testosterone production.
The production of testosterone by Leydig cells is regulated by several different steroidogenic enzymes (Verhoeven et al., 2010). The rate-limiting step in the production of androgens is the translocation of cholesterol into the mitochondria and is controlled by the steroidogenic acute regulatory (StAR) protein (Hasegawa et al., 2000). The levels of StAR and other steroidogenic enzymes, such as CYP11A1, HSD3B1 and CYP17A1, determine the capacity of the Leydig cells to produce androgens (Verhoeven et al., 2010). Previously published reports have shown that retinoids or RA increase the levels of cytochrome P450 enzymes and testosterone production (Chaudhary et al., 1989; Chaudhary and Stocco, 1990; Lefevre et al., 1994; Udhane et al., 2015). In addition, an earlier study demonstrated that vitamin A-deficient animals have decreased levels of testosterone, a consequence of decreased CYP11A1 (Appling and Chytil, 1981). These results were also supported by an in vitro study using MA-10 mouse Leydig cells showing that RA signaling enhanced StAR (Manna et al., 2013). Therefore, it is possible that inhibiting RA signaling in Leydig cells results in decreased levels of steroidogenic enzymes and, thus, low levels of testosterone. Interestingly, Cyp17a1 was significantly decreased, but Cyp11a1 was increased in the 90 dpp mutant testes. This observation implies that RA is either directly or indirectly regulating the expression of the steroidogenic enzymes. Further studies, such as ChIP-on-ChIP assays, need to be performed to identify whether there are steroidogenic genes directly regulated by RA signaling.
In addition to decreased levels of Cyp17a1, the mutant mice displayed smaller seminal vesicles than controls and contained tubules with disrupted BTBs. The weight of the seminal vesicles is directly correlated with testosterone levels (Higgins and Burchell, 1978; Ortiz and Cavicchia, 1990) and BTB formation is known to be regulated by testosterone (Walker, 2011). However, testosterone measurements within 90 dpp mutant testes were not significantly lower than controls, even though there was a decreasing trend. This could be due to animal-to-animal testosterone level variation in the controls, as well in the mutants. Interestingly, at 180 dpp the testosterone levels were significantly decreased in the mutant testis compared with controls. At both 90 and 180 days, there is still enough testosterone for normal spermatogenesis to occur, albeit in a very small percentage of the seminiferous tubules. Histological analyses of mutant epididymides show a decreased amount of sperm visible in the caput. These data suggests that there might be decreased production of sperm. However, sperm counts were not possible because of the unexpected epididymal phenotype in the mutant mice.
Published studies have shown that lack of RA signaling in the epididymis resulted in epithelial cells of the epididymis undergoing squamous metaplasia and destruction of the duct in the cauda (Bartlett et al., 1990). This similar epididymal phenotype was observed in the mutant mice, suggesting that lack of RA in the principal cells might be the main cause of the abnormal epididymis in the mutant mouse. Thus, it is possible that RA signaling is playing a role in the structure and function of the epididymis. The increased expression of CK14 in the basal cells could indicate that cells are trying to differentiate in order to replace the abnormal epithelial cells (Vaidyanathan et al., 2003). In addition, published studies have demonstrated that RA signaling negatively regulates the inflammation response in various tissues (Pino-Lagos et al., 2010). The expansion of the interstitial space in the cauda and the increased numbers of macrophages in the epididymides of the mutant mice indicate increased inflammation in the epididymis. This study suggests that RA signaling might be playing a role in suppressing the inflammation response during the development of the epididymis. Together, these findings support published data hypothesizing that RA signaling is crucial for the function and morphology of the cauda in comparison with the other regions of the epididymis mainly because of the increased expression of components involved in RA signaling and detection of high levels of RA (Porter et al., 1985; Pappas et al., 1993; Deltour et al., 1997).
The epididymal phenotype observed in the mutant mice could also be caused by altered Leydig cell function or the low levels of testosterone in the testis. Dihydrotestosterone produced in the testis is necessary for epididymal structure and function (Robaire and Hamzeh, 2011). Low levels of androgens in the epididymis result in decreased epididymal weight, epithelial cell shrinkage, increased cell death, vacuolization in the epithelial cells and increased endocytosis (Moore and Bedford, 1979; Orgebin-Crist and Davies, 1974). The partially recovered epididymal phenotype in the mutant mice by the testosterone implants suggests that the levels of testosterone are also contributing to the abnormal development of the epididymis. However, the lack of published data regarding the function of testosterone and RA in the epididymis makes it difficult to find out whether the epididymal phenotype observed in the mutant mice is the result of lack of RA signaling in the principal cells or decreased testosterone levels, or a combination of both. Therefore, further studies have to be carried out to investigate the role of RA and testosterone signaling in the development of the epididymis.
This is the first study to report an in vivo investigation of the role of RA signaling in the Leydig cells of the mouse testis. It was clearly demonstrated that a lack of RA signaling in Leydig cells resulted in alterations of events regulated by testosterone, such as meiosis, the establishment and maintenance of the BTB, and spermiation. However, there is a possibility that RA is not directly altering testosterone production, but other functions of the Leydig cells that are essential for sperm production. Additionally, it was also found that the Cyp17icre is expressed in the principal cells of the epididymis and that expression of the RAR-DN in the steroidogenic cells also resulted in an epididymal phenotype. Collectively, these data suggest that altering levels of RA in the body can affect the steroidogenic cell function and cause spermatogenic errors, epididymal dysfunctions and, thus, male infertility. Further studies need to be performed to investigate in more depth the molecular mechanisms underlying the effects of RA in the steroidogenic cells of the testis and epididymis. Additionally, the interaction between testosterone and RA need to be investigated further because it could have major repercussions on the development of drugs for fertility treatments and contraceptives.
MATERIALS AND METHODS
Animal care and handling
All mouse use and experiments were performed following the guidelines stated in the United States Public Health Service's Guide for the Care and Use of Laboratory Animals and were approved by the Washington State University Institutional Animal Care and Use Committee. Mouse colonies were housed in a temperature- and humidity-controlled environment, and food and water were provided ad libitum. The adult mice were euthanized by CO2 asphyxiation followed by cervical dislocation, whereas mice younger than 10 dpp were decapitated. Tissues from the control and experimental animals were dissected and stored for further analyses as described below.
Animals and tissues
The Cyp17iCre mice (C57Bl/6 background) were generously donated by the CheMyong laboratory (University of Kentucky) (Bridges et al., 2008), the RAR-DN-Flox/Flox mice were a generous gift from the Mendelsohn laboratory (Columbia University) (Rosselot et al., 2010) and the RiboTag mouse line was donated by Dr Paul Ameiux (University of Washington) (Sanz et al., 2009). The experiments were performed using RAR-DN-Flox/Flox; Cyp17iCre-negative mice as controls and RAR-DN-Flox/Flox; Cyp17iCre-positive mice as mutants. Additionally, RiboTag-positive; Cyp17iCre-positive male mice were used to detect expression of the Cyp17iCre. Animals were euthanized at different time points in the morning, and their testes and seminal vesicles were dissected. For each adult animal, one testis was fixed in Bouin's (M7831-88; EMD Millipore) for 4-6 h, whereas for 15 dpp animal, the testis was fixed for 1-3 h. The other testis and the seminal vesicles were snap frozen and stored at −80°C until use. The fixed testes were embedded in paraffin wax, sectioned (4 μm width, 20 μm apart) and placed on slides (Superfrost Plus, Fisher Scientific) for analysis. Blood from each animal was collected and the serum separated from the cells by allowing the samples to clot at 4°C overnight prior to centrifugation for 10 min at 2000 g. The serum was stored at −80°C until used for testosterone measurements.
Histology and immunohistochemistry
Histology was performed on cross-sections of testes and epididymides collected from control and mutant mice. Slides were washed in xylene (twice for 5 min) and a graded ethanol series (100%, 95% and 70%) followed by staining with a 1:3 dilution of Harris Hematoxylin (HHS32-IL, Sigma-Aldrich). Immunohistochemistry was also carried out on testes and epididymides cross-sections of the control mice, mutant mice and RiboTag-positive; Cyp17iCre-positive male mice using rabbit anti-ZBTB16 (0.2 μg/ml, sc-22839, Santa Cruz Biotechnology), rabbit anti-STRA8 (1:1000, made in-house) (Hogarth and Griswold, 2013), rabbit anti-SOX9 (0.4 μg/ml, AB5535, EMD Millipore), goat anti-HSD3B1 (0.4 μg/ ml, sc-30820, Santa Cruz Biotechnology), rat anti-MAC2 (0.4 μg/ml, CL8942AP, Cedarlane), mouse anti-HA (1:1000, MMS-101R, BioLegend) or mouse anti-CK14 (1:500, ab7800, Abcam). The protocol described by Hogarth and Griswold (2013) was followed. Briefly, slides were washed in xylene (2×5 min) and a graded ethanol series (100%, 95% and 70%), followed by an antigen-retrieval step in 10 mM citrate buffer [2.94 mg/ml sodium citrate dihydrate in double distilled H20 (pH 6)] at 100°C for 7.5 min. The slides were then left to cool down for ∼20 min. The cross-sections were then treated with 3% hydrogen peroxide for 5 min at room temperature, washed with 1×PBS and incubated in blocking solution (5% goat or rabbit serum in 0.1% bovine serum albumin/PBS) for 30 min at room temperature. Following block, primary antibody, diluted in the blocking solution, was added to the sections and incubated overnight at room temperature. The next day, slides were washed three times with 1×PBS and then the secondary antibodies, biotinylated goat anti-rat (2 μg/ml, AB97178, Abcam), biotinylated rabbit anti-goat (4 μg/ml, A16140, Invitrogen) and biotinylated goat anti-rabbit solution (Histostain SP Bulk Kit, 956143B, Invitrogen) diluted in blocking solution were added to the sections and incubated at room temperature for 1 h. Horseradish peroxidase streptavidin (5A-5704, Vector Laboratories) was then added to slides and incubated for 30 min before antibody binding was visualized using the metal-enhanced 3,3′-diaminobenzidine tetrahydrochloride substrate kit (34065, Thermo Fisher Scientific) according to the manufacturer's instructions and counterstained with Harris Hematoxylin solution (1:3 dilution, HHS32-1L, Sigma-Aldrich) for 10 s. The sections were washed for 5 min with tap water followed by a graded series of ethanol (70%, 95% and 100%) and xylene. Finally, the slides were mounted with DPX (360294H; VWR International) and used for analyses. A minimum of three technical duplicates and biological replicates were performed.
TUNEL assay
TUNEL assays were performed using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (S7100, Millipore) following the manufacturer's instructions. Briefly, slides of 90 dpp control and mutant testis cross-sections were washed with xylene followed by a graded series of ethanol (100%, 95% and 70%). The slides were then washed with 1×PBS for 5 min and then proteinase K (20 mg/ml) was added to the sections and incubated at room temperature for 15 min. Following protein digestion, the sections were washed in double distilled H2O and incubated at room temperature for 5 min with 3% hydrogen peroxide. A second 1×PBS wash was then performed and the equilibration buffer was added to the sections for 10 s. Immediately after this, working strength TdT enzyme was pipetted on top of the slide and covered with a coverslip. The slide was then incubated at 37°C for 1 h. After the 1 h incubation, wash buffer was added for 10 min followed by three washes of 1×PBS. Anti-digoxigenin peroxidase was pipetted on each cross-section and incubated for 30 min at room temperature. The slides were then washed with 1×PBS and developed using a metal-enhanced 3,3′-diaminobenzidine tetrahydrochloride substrate kit (34065, ThermoScientific) according to the manufacturer's instructions and counterstained with Harris Hematoxylin solution (1:3 dilution, Sigma-Aldrich) for 10 s. Washes of 100%, 95% and 70% ethanol were performed followed by two washes of 2 min each of xylene. The slides were then mounted with DPX (VWR International) and observed under the microscope. Positively stained cells were counted in at least 200 tubules (n=3 for each genotype) and the percentage of tubules with positive pachytene spermatocytes cells was calculated. Statistical analysis was performed using Student's t-test.
Biotin permeability assay
Freshly dissected testes from 90 dpp mutant and control animals (n=4) were used in the biotin permeability assay to determine whether the BTB was disrupted. Similar procedures were followed as previously described (Perez et al., 2012). One testis from each animal was injected with PBS containing 1 mM of CaCl2 (10% of testis weight) (control) and the other testis with 10 mg/ml of EZ-Link Sulfo-NHS-LC-Biotin (#21335; Pierce) dissolved in 1×PBS containing 1 mM of CaCl2 (10% testis weight). The testes were incubated at room temperature for 30 min, then washed with 1×PBS and fixed with Bouin's as described above. After embedding, the testes were sectioned and washed with xylene and a graded ethanol series (100%, 95% and 70%). Slides were then blocked with blocking solution (5% normal goat serum/0.1% bovine serum albumin in 1× PBS) and incubated for 30 min at room temperature. Slides were washed again with 1×PBS and incubated for 1 h with Alexa Fluor 488 streptavidin conjugate (4 μg/ml; S11223; Invitrogen) diluted in blocking solution. Finally, slides were washed in 1×PBS and mounted with fluoroshield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (ab104139, Abcam). The number of tubules displaying infiltration of the biotin tracer into the lumen was counted (a minimum of 200 tubules). Student's t-test was used to determine statistical significance between the control and mutant testes.
Testosterone measurements
Testosterone measurements for testes of control (n=6) and mutant (n=6) mice were performed using a mouse and rat IBL ELISA (Kit Lot 29K105, Rodent QC Lot 012615). Tissue was homogenized in 250 μl of 1×PBS with a mechanical homogenizer and then sonicated for 60 s. Measurements were performed by The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) grant P50-HD28934. The intra-assay CV for testicular testosterone measurements was from 0.3 to 4.96% and for serum measurements from 1.5 to 17.8%. Significance between the control and mutant were determined using Student's t-test.
Quantitative RT-PCR
RNA from 90 dpp mutant and control (at least n=3 for each) testes was extracted using TRI-ZOL (Invitrogen). The extracted mRNAs were used to generate cDNA following the iScript kit (170-8891, Bio-Rad Laboratories) manufacturer's instructions. With the cDNA, quantitative RT-PCR (two-step) was performed as previously described (Zhou et al., 2005) using SYBR Green and primers for Cyp17a1 (5′-AGGTCCTTCAATGAGATCCCTT-3′ and 5′-GTACCCAGGCGAAGAGAATAGA-3′), Cyp11a1 (5′-AGGTCCTTCAATGAGATCCCTT-3′ and 5′-TCCCTGTAAATGGGGCCATAC-3′), Star (primers: 5′-CGGGTGGATGGGTCAAGTTCG-3′ and 5′-GCTCCGGCATCACCCCAAAAT -3′), Hsd3b1 (primers: 5′ CAGGAGCAGGAGGGTTTGT-3′ and 5′- GTGGCCATTCAGGACGAT-3′) and internal control Rps2 (primers: 5′-CTGACTCCCGACCTCTGGAAA-3′ and 5′- GAGCCTGGGTCCTCTGAACA-3′), which was used to normalized the mRNA expressions. Student's t-test was performed for statistical analysis.
Fertility studies
Adult mutant (n=5) and control (n=3) male animals (60 dpp) were caged with fertile females. Females were checked for plugs every morning for at least 4 consecutive weeks. Then, the number of offspring resulted from each plug was recorded. The fertility of the male mutant animals was assessed by comparing them with the control male animals.
Testosterone implants
Following an injectable anesthesia carried out using a combination of ketamine (87 mg/kg; 501072; MWI Animal Health) and xylazine (13 mg/kg; 003437; MWI Animal Health), a bland sterile ophthalmic ointment was applied on the eyes of the mouse. The mouse was then shaved (3-4 cm) at the dorsal midline where the surgery took place. The skin was cleaned up with iodine followed by an alcohol rinse, repeated three times. The mouse was moved into the surgical area and sterile drape was used to isolate the incision site. A 1-1.5 cm incision was made and a 1 cm long sterile silastic implant (Laboratory tubing, 508-006, Dow Corning) with testosterone (5 mg, A6950-000, Steraloids) for the experimental mouse (n=8) or without testosterone for control mouse (n=5) was inserted under the skin. The incision was then closed using wound clip and injected with buprenorphine (0.2 mg/kg, 060969, MWI Animal Health). The mouse was left to recover for 8 weeks. At 90 dpp, experimental and control mice were euthanized and their epididymides collected for further analyses.
Quantifications and statistical analysis
The body weight, testis weight and seminal vesicle weight of control and mutant animals were recorded (at least n=10) for comparison. The testis/body weight ratio was then calculated and compared between the mutant and control animals. The number of macrophages, Sertoli cells, Leydig cells, macrophages and tubules was counted in a 4.15×107 µm2 sections (at least 10). The number of total positive cells was then normalized to the number of tubules in each section. Additionally, the number of degraded tubules, tubules with vacuoles and normal tubules were counted in at least 200 tubules in testes of control and mutant animals (at least n=3). Statistical analyses were performed for all experiments using Student's t-test (two-tailed) and significance was determined based on the P value (P<0.05 was significant). Each n represents one animal.
Acknowledgements
The authors thank Dr C. Mendelsohn for providing the RAR-DN mice.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: E.J.J., C.A.H., M.D.G.; Methodology: E.J.J., C.A.H., M.D.G.; Validation: E.J.J.; Formal analysis: E.J.J.; Investigation: E.J.J., D.M., T.T.; Resources: M.D.G.; Writing - original draft: E.J.J.; Writing - review & editing: E.J.J., C.A.H., M.D.G.; Supervision: M.D.G.; Funding acquisition: M.D.G.
Funding
This research was supported by the National Institutes of Health (RO1HD10808 to M.D.G.). Deposited in PMC for release after 12 months.
References
- Akmal K. M., Dufour J. M. and Kim K. H. (1996). Region-specific localization of retinoic acid receptor-alpha expression in the rat epididymis. Biol. Reprod. 54, 1111-1119. 10.1095/biolreprod54.5.1111 [DOI] [PubMed] [Google Scholar]
- Almeida J., Conley A. J., Mathewson L. and Ball B. A. (2011). Expression of steroidogenic enzymes during equine testicular development. Reproduction 141, 841-848. 10.1530/REP-10-0499 [DOI] [PubMed] [Google Scholar]
- Appling D. R. and Chytil F. (1981). Evidence of a role for retinoic acid (vitamin A-acid) in the maintenance of testosterone production in male rats. Endocrinology 108, 2120-2123. 10.1210/endo-108-6-2120 [DOI] [PubMed] [Google Scholar]
- Bartlett J. M. S., Weinbauer G. F. and Nieschlag E. (1990). Stability of spermatogenic synchronization achieved by depletion and restoration of vitamin A in rats. Biol. Reprod. 42, 603-612. 10.1095/biolreprod42.4.603 [DOI] [PubMed] [Google Scholar]
- Bastien J. and Rochette-Egly C. (2004). Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene 328, 1-16. 10.1016/j.gene.2003.12.005 [DOI] [PubMed] [Google Scholar]
- Bhushan S. and Meinhardt A. (2017). The macrophages in testis function. J. Reprod. Immunol. 119, 107-112. 10.1016/j.jri.2016.06.008 [DOI] [PubMed] [Google Scholar]
- Bridges P. J., Koo Y., Kang D.-W., Hudgins-Spivey S., Lan Z.-J., Xu X., DeMayo F., Cooney A. and Ko C. (2008). Generation of Cyp17iCre transgenic mice and their application to conditionally delete estrogen receptor alpha (Esr1) from the ovary and testis. Genesis 46, 499-505. 10.1002/dvg.20428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas F. W., Kirsh A. L., Sharma M., McLean D. J., Morris J. L., Griswold M. D., de Rooij D. G. and Braun R. E. (2004). Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 36, 647-652. 10.1038/ng1366 [DOI] [PubMed] [Google Scholar]
- Chang C., Chen Y.-T., Yeh S. D., Xu Q., Wang R.-S., Guillou F., Lardy H. and Yeh S. (2004). Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl. Acad. Sci. USA 101, 6876-6881. 10.1073/pnas.0307306101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary L. R. and Stocco D. M. (1990). An in vitro cell model system to study the action of retinoids on Leydig cell steroidogenesis. Biochem. Int. 21, 1033-1042. [PubMed] [Google Scholar]
- Chaudhary L. R., Hutson J. C. and Stocco D. M. (1989). Effect of retinol and retinoic acid on testosterone production by rat Leydig cells in primary culture. Biochem. Biophys. Res. Commun. 158, 400-406. 10.1016/S0006-291X(89)80061-0 [DOI] [PubMed] [Google Scholar]
- Chen Y., Ma L., Hogarth C., Wei G., Griswold M. D. and Tong M. H. (2016). Retinoid signaling controls spermatogonial differentiation by regulating expression of replication-dependent core histone genes. Development 143, 1502-1511. 10.1242/dev.135939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. S. and Wolgemuth D. J. (2004). Role of retinoid signaling in the regulation of spermatogenesis. Cytogenet Genome Res. 105, 189-202. 10.1159/000078189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clagett-Dame M. and Knutson D. (2011). Vitamin A in reproduction and development. Nutrients 3, 385-428. 10.3390/nu3040385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa S. L., Boekelheide K., Vanderhyden B. C., Seth R. and McBurney M. W. (1997). Male infertility caused by epididymal dysfunction in transgenic mice expressing a dominant negative mutation of retinoic acid receptor alpha 1. Biol. Reprod. 56, 985-990. 10.1095/biolreprod56.4.985 [DOI] [PubMed] [Google Scholar]
- Costoya J. A., Hobbs R. M., Barna M., Cattoretti G., Manova K., Sukhwani M., Orwig K. E., Wolgemuth D. J. and Pandolfi P. P. (2004). Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 36, 653-659. 10.1038/ng1367 [DOI] [PubMed] [Google Scholar]
- Damm K., Heyman R. A., Umesono K. and Evans R. M. (1993). Functional inhibition of retinoic acid response by dominant negative retinoic acid receptor mutants. Proc. Natl. Acad. Sci. USA 90, 2989-2993. 10.1073/pnas.90.7.2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gendt K., Swinnen J. V., Saunders P. T., Schoonjans L., Dewerchin M., Devos A., Tan K., Atanassova N., Claessens F., Lecureuil C. et al. (2004). A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc. Natl. Acad. Sci. USA 101, 1327-1332. 10.1073/pnas.0308114100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gendt K., Atanassova N., Tan K. A., de Franca L. R., Parreira G. G., McKinnell C., Sharpe R. M., Saunders P. T., Mason J. I., Hartung S. et al. (2005). Development and function of the adult generation of Leydig cells in mice with Sertoli cell-selective or total ablation of the androgen receptor. Endocrinology 146, 4117-4126. 10.1210/en.2005-0300 [DOI] [PubMed] [Google Scholar]
- Deltour L., Haselbeck R. J., Ang H. L. and Duester G. (1997). Localization of class I and class IV alcohol dehydrogenases in mouse testis and epididymis: potential retinol dehydrogenases for endogenous retinoic acid synthesis. Biol. Reprod. 56, 102-109. 10.1095/biolreprod56.1.102 [DOI] [PubMed] [Google Scholar]
- Ge R. S. and Hardy M. P. (1998). Variation in the end products of androgen biosynthesis and metabolism during postnatal differentiation of rat Leydig cells. Endocrinology 139, 3787-3795. 10.1210/endo.139.9.6183 [DOI] [PubMed] [Google Scholar]
- Griswold M. D., Bishop P. D., Kim K. H., Ping R., Siiteri J. E. and Morales C. (1989). Function of vitamin A in normal and synchronized seminiferous tubules. Ann. N. Y. Acad. Sci. 564, 154-172. 10.1111/j.1749-6632.1989.tb25895.x [DOI] [PubMed] [Google Scholar]
- Hales D. B. (2002). Testicular macrophage modulation of Leydig cell steroidogenesis. J. Reprod. Immunol. 57, 3-18. 10.1016/S0165-0378(02)00020-7 [DOI] [PubMed] [Google Scholar]
- Hardy M. P., Zirkin B. R. and Ewing L. L. (1989). Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology 124, 762-770. 10.1210/endo-124-2-762 [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Zhao L., Caron K. M., Majdic G., Suzuki T., Shizawa S., Sasano H. and Parker K. L. (2000). Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol. Endocrinol. 14, 1462-1471. 10.1210/mend.14.9.0515 [DOI] [PubMed] [Google Scholar]
- Hedger M. P. (2002). Macrophages and the immune responsiveness of the testis. J. Reprod. Immunol. 57, 19-34. 10.1016/S0165-0378(02)00016-5 [DOI] [PubMed] [Google Scholar]
- Higgins S. J. and Burchell J. M. (1978). Effects of testosterone on messenger ribonucleic acid and protein synthesis in rat seminal vesicle. Biochem. J. 174, 543-551. 10.1042/bj1740543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth C. A. and Griswold M. D. (2013). Immunohistochemical approaches for the study of spermatogenesis. Methods Mol. Biol. 927, 309-320. 10.1007/978-1-62703-038-0_28 [DOI] [PubMed] [Google Scholar]
- Hogarth C. A., Mitchell D., Evanoff R., Small C. and Griswold M. (2011). Identification and expression of potential regulators of the mammalian mitotic-to-meiotic transition. Biol. Reprod. 84, 34-42. 10.1095/biolreprod.110.086215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdcraft R. W. and Braun R. E. (2004). Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 131, 459-467. 10.1242/dev.00957 [DOI] [PubMed] [Google Scholar]
- Huang H. F. and Hembree W. C. (1979). Spermatogenic response to vitamin A in vitamin A deficient rats. Biol. Reprod. 21, 891-904. 10.1095/biolreprod21.4.891 [DOI] [PubMed] [Google Scholar]
- Jauregui E. J., Mitchell D., Garza S., Topping T., Hogarth C. A. and Griswold M. D. (2018). Leydig cell genes change their expression and association with polysomes in a stage-specific manner in the adult mouse testis. Biol. Reprod. 98, 722-738. 10.1093/biolre/ioy031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram M., Murthy S. K. and Ganguly J. (1973). Effect of vitamin A deprivation on the cholesterol side-chain cleavage enzyme activity of testes and ovaries of rats. Biochem. J. 136, 221-223. 10.1042/bj1360221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P., Mark M., Leid M., Gansmuller A., Chin W., Grondona J. M., Decimo D., Krezel W., Dierich A. and Chambon P. (1996). Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev. 10, 80-92. 10.1101/gad.10.1.80 [DOI] [PubMed] [Google Scholar]
- Lefevre A., Rogier E., Astraudo C., Duquenne C. and Finaz C. (1994). Regulation by retinoids of luteinizing hormone/chorionic gonadotropin receptor, cholesterol side-chain cleavage cytochrome P-450, 3 beta-hydroxysteroid dehydrogenase/delta (5-4)-isomerase and 17 alpha-hydroxylase/C17-20 lyase cytochrome P-450 messenger ribonucleic acid levels in the K9 mouse Leydig cell line. Mol. Cell. Endocrinol. 106, 31-39. 10.1016/0303-7207(94)90183-X [DOI] [PubMed] [Google Scholar]
- Manna P. R., Cohen-Tannoudji J., Counis R., Garner C. W., Huhtaniemi I., Kraemer F. B. and Stocco D. M. (2013). Mechanisms of action of hormone-sensitive lipase in mouse Leydig cells: its role in the regulation of the steroidogenic acute regulatory protein. J. Biol. Chem. 288, 8505-8518. 10.1074/jbc.M112.417873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. J. and Tremblay J. J. (2010). Nuclear receptors in Leydig cell gene expression and function. Biol. Reprod. 83, 3-14. 10.1095/biolreprod.110.083824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Parra M. A. and Gronemeyer H. (2013). Genome-wide studies of nuclear receptors in cell fate decisions. Semin. Cell Dev. Biol. 24, 706-715. 10.1016/j.semcdb.2013.07.001 [DOI] [PubMed] [Google Scholar]
- Meng J., Holdcraft R. W., Shima J. E., Griswold M. D. and Braun R. E. (2005). Androgens regulate the permeability of the blood-testis barrier. Proc. Natl. Acad. Sci. USA 102, 16696-16700. 10.1073/pnas.0506084102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. D. and Bedford J. M. (1979). Short-term effects of androgen withdrawal on the structure of different epithelial cells in the rat epididymis. Anat. Rec. 193, 293-311. 10.1002/ar.1091930209 [DOI] [PubMed] [Google Scholar]
- O'Donnell L., McLachlan R. I., Wreford N. G. and Robertson D. M. (1994). Testosterone promotes the conversion of round spermatids between stages VII and VIII of the rat spermatogenic cycle. Endocrinology 135, 2608-2614. 10.1210/endo.135.6.7988449 [DOI] [PubMed] [Google Scholar]
- O'Donnell L., McLachlan R. I., Wreford N. G., de Kretser D. M. and Robertson D. M. (1996). Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol. Reprod. 55, 895-901. 10.1095/biolreprod55.4.895 [DOI] [PubMed] [Google Scholar]
- Orgebin-Crist M. C. and Davies J. (1974). Functional and morphological effects of hypophysectomy and androgen replacement in the rabbit epididymis. Cell Tissue Res. 148, 183-201. 10.1007/BF00224581 [DOI] [PubMed] [Google Scholar]
- Ortiz H. E. and Cavicchia J. C. (1990). Androgen-induced changes in nuclear pore number and in tight junctions in rat seminal vesicle epithelium. Anat. Rec. 226, 129-134. 10.1002/ar.1092260202 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy P. J., Abel M., Charlton H. M., Hu B., Johnston H. and Baker P. J. (2007). Altered expression of genes involved in regulation of vitamin A metabolism, solute transportation, and cytoskeletal function in the androgen-insensitive tfm mouse testis. Endocrinology 148, 2914-2924. 10.1210/en.2006-1412 [DOI] [PubMed] [Google Scholar]
- Perez C. V., Sobarzo C. M., Jacobo P. V., Pellizzari E. H., Cigorraga S. B., Denduchis B. and Lustig L. (2012). Loss of occludin expression and impairment of blood-testis barrier permeability in rats with autoimmune orchitis: effect of interleukin 6 on Sertoli cell tight junctions. Biol. Reprod. 87, 122 10.1095/biolreprod.112.101709 [DOI] [PubMed] [Google Scholar]
- Perez C. V., Theas M. S., Jacobo P. V., Jarazo-Dietrich S., Guazzone V. A. and Lustig L. (2013). Dual role of immune cells in the testis: protective or pathogenic for germ cells? Spermatogenesis 3, e23870 10.4161/spmg.23870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas R. S., Newcomer M. E. and Ong D. E. (1993). Endogenous retinoids in rat epididymal tissue and rat and human spermatozoa. Biol. Reprod. 48, 235-247. 10.1095/biolreprod48.2.235 [DOI] [PubMed] [Google Scholar]
- Pino-Lagos K., Guo Y. and Noelle R. J. (2010). Retinoic acid: a key player in immunity. Biofactors 36, 430-436. 10.1002/biof.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S. B., Ong D. E., Chytil F. and Orgebin-Crist M. C. (1985). Localization of cellular retinol-binding protein and cellular retinoic acid-binding protein in the rat testis and epididymis. J. Androl. 6, 197-212. 10.1002/j.1939-4640.1985.tb00836.x [DOI] [PubMed] [Google Scholar]
- Raverdeau M., Gely-Pernot A., Feret B., Dennefeld C., Benoit G., Davidson I., Chambon P., Mark M. and Ghyselinck N. B. (2012). Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc. Natl. Acad. Sci. USA 109, 16582-16587. 10.1073/pnas.1214936109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebourcet D., O'Shaughnessy P. J., Monteiro A., Milne L., Cruickshanks L., Jeffrey N., Guillou F., Freeman T. C., Mitchell R. T. and Smith L. B. (2014). Sertoli cells maintain Leydig cell number and peritubular myoid cell activity in the adult mouse testis. PLoS ONE 9, e105687 10.1371/journal.pone.0105687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaire B. and Hamzeh M. (2011). Androgen action in the epididymis. J. Androl. 32, 592-599. 10.2164/jandrol.111.014266 [DOI] [PubMed] [Google Scholar]
- Rosselot C., Spraggon L., Chia I., Batourina E., Riccio P., Lu B., Niederreither K., Dolle P., Duester G., Chambon P. et al. (2010). Non-cell-autonomous retinoid signaling is crucial for renal development. Development 137, 283-292. 10.1242/dev.040287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz E., Yang L., Su T., Morris D. R., McKnight G. S. and Amieux P. S. (2009). Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl. Acad. Sci. USA 106, 13939-13944. 10.1073/pnas.0907143106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima Y., Miyabayashi K., Haraguchi S., Arakawa T., Otake H., Baba T., Matsuzaki S., Shishido Y., Akiyama H., Tachibana T. et al. (2013). Contribution of Leydig and Sertoli cells to testosterone production in mouse fetal testes. Mol. Endocrinol. 27, 63-73. 10.1210/me.2012-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder E. M., Small C. and Griswold M. D. (2010). Retinoic acid availability drives the asynchronous initiation of spermatogonial differentiation in the mouse. Biol. Reprod. 83, 783-790. 10.1095/biolreprod.110.085811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. A., De Gendt K., Atanassova N., Walker M., Sharpe R. M., Saunders P. T., Denolet E. and Verhoeven G. (2005). The role of androgens in sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology 146, 2674-2683. 10.1210/en.2004-1630 [DOI] [PubMed] [Google Scholar]
- Udhane S. S., Pandey A. V., Hofer G., Mullis P. E. and Flück C. E. (2015). Retinoic acid receptor beta and angiopoietin-like protein 1 are involved in the regulation of human androgen biosynthesis. Sci. Rep. 5, 10132 10.1038/srep10132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan S., McDicken I. W., Mansour P., Soni B. M., Ikin A. J., Singh G. and Sett P. (2003). Detection of early squamous metaplasia in bladder biopsies of spinal cord injury patients by immunostaining for cytokeratin 14. Spinal Cord 41, 432-434. 10.1038/sj.sc.3101464 [DOI] [PubMed] [Google Scholar]
- van Pelt A. M., van den Brink C. E., de Rooij D. G. and van der Saag P. T. (1992). Changes in retinoic acid receptor messenger ribonucleic acid levels in the vitamin A-deficient rat testis after administration of retinoids. Endocrinology 131, 344-350. 10.1210/endo.131.1.1319320 [DOI] [PubMed] [Google Scholar]
- Verhoeven G., Willems A., Denolet E., Swinnen J. V. and De Gendt K. (2010). Androgens and spermatogenesis: lessons from transgenic mouse models. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 1537-1556. 10.1098/rstb.2009.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet N., Dennefeld C., Guillou F., Chambon P., Ghyselinck N. B. and Mark M. (2006). Prepubertal testis development relies on retinoic acid but not rexinoid receptors in Sertoli cells. EMBO J. 25, 5816-5825. 10.1038/sj.emboj.7601447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. H. (2011). Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 1, 116-120. 10.4161/spmg.1.2.16956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y.-J. Y., Wang L. and Wu T.-C. J. (1992). Detection of retinoic acid receptor mRNA in rat tissues by reverse transcriptase-polymerase chain reaction. J. Mol. Endocrinol. 9, 291-294. 10.1677/jme.0.0090291 [DOI] [PubMed] [Google Scholar]
- Wolgemuth D. J. and Chung S. S. (2007). Retinoid signaling during spermatogenesis as revealed by genetic and metabolic manipulations of retinoic acid receptor alpha. Soc. Reprod. Fertil. Suppl. 63, 11-23. [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Shima J. E., Nie R., Friel P. J. and Griswold M. D. (2005). Androgen-regulated transcripts in the neonatal mouse testis as determined through microarray analysis. Biol. Reprod. 72, 1010-1019. 10.1095/biolreprod.104.035915 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Nie R., Li Y., Friel P., Mitchell D., Hess R. A., Small C. and Griswold M. D. (2008). Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol. Reprod. 79, 35-42. 10.1095/biolreprod.107.066795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirkin B. R., Santulli R., Awoniyi C. A. and Ewing L. L. (1989). Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology 124, 3043-3049. 10.1210/endo-124-6-3043 [DOI] [PubMed] [Google Scholar]