Abstract
Purpose of review
Although it has been known for some time that increases in body mass enhance aldosterone secretion, particularly in women, the origin of this elevation in aldosterone production is not well defined. Adipocyte-derived factors have emerged as potential candidates to increase aldosterone production in obesity.
Recent findings
Emerging evidence suggests the presence of a mechanistic link in which the adipocyte-derived hormone leptin stimulates aldosterone production in obesity, thereby creating a positive feedback loop for obesity-associated cardiovascular disease. In addition, recent reports give credence to the concept that this leptin–aldosterone stimulation pathway in obesity is an underlying mechanism for sex-discrepancies in obesity-associated cardiovascular disease.
Summary
Leptin appears as a new direct regulator of adrenal aldosterone production and leptin-mediated aldosterone production is a novel candidate mechanism underlying obesity-associated hypertension, particularly in females.
Keywords: aldosterone, hypertension, leptin, obesity, sex differences
INTRODUCTION
Aldosterone levels are characteristically upregulated in obese patients. Although obesity is characterized by a dysfunction of many hormone systems, obesity-associated increases in circulating aldosterone is not particularly associated with disruption of aldosterone’s classical activators: angiotensin II (ANGII), plasma potassium level and adrenocorticotropin release. Many studies have suggested the existence of an adipocyte-derived aldosterone-stimulating factor, and subsequently, the adipocyte-derived hormone leptin has emerged as a major candidate mechanism underlying increases in aldosterone secretion in the obesity condition. This review will discuss evidence in the literature for the presence of a link between leptin and aldosterone secretion, as well as the contribution of leptin-mediated aldosterone secretion in cardiovascular disease.
THE IDENTIFICATION OF AN ADIPOCYTE-DERIVED ALDOSTERONE-SECRETING FACTOR IN OBESITY: EVIDENCE IMPLICATING LEPTIN IN THIS ROLE
It was observed several decades ago that obesity is associated with an increase in aldosterone, which has since been confirmed by a large number of clinical and basic science studies [1–4]. This is also true in rodents as high-fat diet-induced obese mice present with a higher level of aldosterone compared with lean mice [5,6]. The positive relationship of aldosterone to body mass is evidently specific to the adipose tissue. Studies have shown that adiposity itself is directly correlated to plasma aldosterone levels in an American population [3] as well as additionally tied to adiposity-associated hypertension in African-American and French Canadian patients [7]. Although these studies have evaluated both men and women, others have uncovered sex-dependent relationships of body mass and aldosterone. In an elegant study, Goodfriend et al. [3] analyzed the correlation between aldosterone plasma levels and fat mass in normotensive women and normotensive men and found that plasma aldosterone in women correlates directly with visceral adipose tissue, independent of plasma renin activity, whereas no correlation is evident in men. Following a weight-loss regimen, significant reductions in plasma aldosterone are observed in both sexes; however, the correlation of aldosterone with adipose tissue persists in women, potentially indicating a cause-and-effect relationship that may be sex-specific. [3].
Aldosterone is primarily synthesized in and secreted from the outer layer of the adrenal cortex, the zona glomerulosa, although, secondary sources of aldosterone have been identified including adipose tissue itself [2,4]. Aldosterone secretion is traditionally regulated by three factors: plasma potassium, ANGII and adrenocorticotropin, as has been reviewed elsewhere [8]; however, several studies have indicated that these factors are not directly correlated with aldosterone in obese patients [9–14]. Many studies have confirmed that aldosterone production is correlated with adipose tissue mass, particularly white adipose tissue [15]. It was later confirmed that an adipocyte-derived aldosterone-stimulating factor is present in rodents [16], and in addition, that the presentation of obesity in rodents increases the production of this factor [16]. The establishment of this concept led to the suggestion of an adipose-derived aldosterone secretion factor. Ehrhart-Bornstein et al. [17] then investigated the direct effects of adipocytes on adrenocortical aldosterone release and cocultured NCI-H295R adrenocortical cells with media from adipocyte cultures and observed that aldosterone production was increased. These studies indicate that factor(s) upregulated by and derived from adipose tissue stimulates increased aldosterone production, which prompted investigation into the role of adipocyte-derived hormones (‘adipokines’) and their role in aldosterone regulation.
Leptin is an adipokine whose levels increase in accordance with increasing adipose mass and is dramatically increased in obesity, which made leptin a candidate for an aldosterone-stimulating factor. Our first finding indicating that leptin may be implicated in aldosterone secretion was observed in male C57Bl/6 mice, in which we found that leptin infusion (10 μg/day) increases aldosterone plasma levels in mice on a control and high-fat diet, data that have been recapitulated in female mice [5,18▪▪]. These data were strengthened by a similar finding in protein tyrosine phosphatase 1b (PTP1b) knockout mice, a model of leptin hypersensitivity, PTP1b being an endogenous molecular restraint of the leptin-signaling pathway. In PTP1b knockout female mice we observed that leptin hypersensitivity per se triggers aldosterone production, as well as adrenal CYP11B2 (aldosterone synthase) expression [18▪▪]. The studies that followed this observation were published in the same report in which we found that aldosterone levels were increased in correlation with increasing leptin levels in both Agouti female hyperleptinemic mice, which are hyper-phagic and obese because of a mutation in the leptin-mediated appetite suppression pathway, as well as high-fat diet-induced obese female mice, which are hyperleptinemic [18▪▪]. To further confirm the role of leptin as a mediator of this obesity-associated elevation in aldosterone in obese female mice, we measured aldosterone in three rodent models of obesity in which leptin activity is deleted: ob/ob mice (deficient in leptin), db/db mice (deficient in leptin receptor) and Zucker rats (expressing a nonfunctional leptin receptor), all of which failed to have increased aldosterone plasma level and adrenal CYP11B2 gene or protein expression despite the presence of obesity [18▪▪]. Furthermore, administration of the leptin receptor antagonist Allo-Aca blunts elevated aldosterone plasma levels in female PTP1b knockout mice and obese Agouti mice [19▪▪] suggesting that leptin receptor activation mediates aldosterone production. These data demonstrate that increases in aldosterone production observed in obese mice are independent of obesity per se, but rather dependent on leptin signaling. Other reports show conflicting data with regard to these models and their respective aldosterone levels [2,4,20–22]. The reason for the discrepancy between our findings and others remains unclear. The strength of our data resides in the diversity of the animals (mouse and rats) and of the genetic backgrounds (Balb/c, C57Bl/6, Agouti) all housed in the same animal facility and fed an identical diet (0.2% Sodium, Teklad Global #2018).
Although leptin receptors are expressed in a variety of cell types and organ systems, recent data indicates that the leptin-aldosterone stimulation pathway is a direct relationship, and unlikely involving a secondary mediator. We [18▪▪] and others [23–25] have shown that leptin receptors are expressed in adrenal tissues of humans and rodents. Our study specifically showed that leptin receptors are colocalized with CYP11B2 in human adrenal cortical cells, implying that the regulation of aldosterone secretion in the adrenal cortex by leptin signaling is a more direct relationship mediated by the leptin–CYP11B2 interaction in adrenal cells [18▪▪] (Fig. 1). This direct relationship of leptin-mediated aldosterone secretion is suggested by evidence of a concentration-dependent increase in aldosterone production and CYP11B2 expression in human adrenal zona glomerulosa cells in vitro [18▪▪]. Leptin-mediated release of aldosterone by adrenal cells also appears to be a calcium-dependent process, in a similar fashion to ANG Il-induced aldosterone production. Similar to ANG II, leptin increases calmodulin and calmodulin-dependent protein kinase expressions in HAC15 cells (human adrenocortical carcinoma cell line) [18▪▪]. Furthermore, chelating the intracellular Ca2+ with BAPTA-AM abolishes leptin-mediated as well as ANG II-mediated increases in CYP11B2 promoter activity suggesting that leptin-mediated aldosterone production is Ca2+ maneuver-dependent [18▪▪]. However, further data are required to determine the exact intracellular pathways that mediate the leptin–CYP11B2 aldosterone-stimulating relationship. The single direction (i.e. not a feedback mechanism) implied by these findings are strengthened by data in human patients with alterations in aldosterone signaling. Neither patients with congenital adrenal cortical hyperplasia (aldosterone deficiency) [26] nor those with primary aldosteronism (elevated aldosterone levels) [27,28] present with correlating alterations in leptin levels, indicating that the stimulation of aldosterone by leptin is not a circular feedback mechanism in a physiological system.
FIGURE 1.
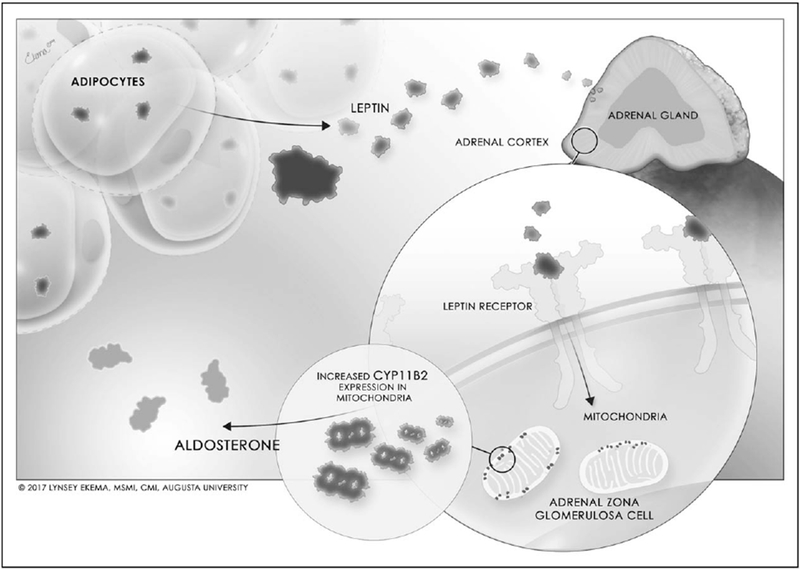
Regulation of aldosterone secretion by leptin.
Although it is tempting to simplify that hyperleptinemia alone leads to hyperaldosteronism in obesity, it is unlikely that leptin is the only adipokine contributing to aldosterone secretion in obese patients. Other adipocyte-derived products, such as the innate immune cytokine interleukin-6, may play a role in aldosterone secretion [29,30], and is also notably increased in obesity, particularly visceral adiposity [31]. In addition, Bollag and colleagues have demonstrated that very low-density lipids (VLDL) are capable of stimulating CYP11B2 expression and aldosterone secretion in different adrenal cell model systems such as: primary cultures of human and bovine adrenal cells and the adrenocortical cell line H295R [32]. Therefore, further investigation is needed into the individual contributions of various obesity-associated factors, including leptin, that stimulate hyperaldosteronism in obesity.
FEMALE PREDOMINANCE OF THE LEPTIN–ALDOSTERONE AXIS
Women characteristically have an increased leptin level per unit of body mass [33,34] compared with men. In accordance, aldosterone rises in obese women are more correlated to body mass in women compared with men [3]. Additionally, in rodents, only female PTP1B knockout leptin-sensitive mice have elevated aldosterone levels and adrenal CYP11B2 expression [18▪▪], indicating a heightened leptin–aldosterone stimulation in female mice that begins with an increased production of leptin. As the leptin–aldosterone stimulation pathway has only recently been proposed, the evolutionary basis for the sex discrepancy in this relationship is speculative at this point, but may be tied to fertility needs that are unique to women.
As has been reviewed elsewhere [35], leptin likely serves as a ‘nutrition sensing’ hormone in women, indicating adequate adipose stores for fertility. Animal models as well as human studies have demonstrated that adequate leptin signaling is required for reproductive puberty to progress in females [36,37]. In addition, leptin follows a cyclical pattern throughout the menstrual cycle in women, peaking at the luteal phase [38], in correlation with the cyclical pattern of aldosterone [39], which is the phase during which embryonic implantation occurs. Pregnancy is a state of heightened physiological need for salt and fluid retention to sustain blood volume expansion [40]. A steady rise in leptin levels is observed throughout all three trimesters, in correlation with trends of rising aldosterone levels throughout pregnancy, indicating that leptin may contribute to rising aldosterone levels [40,41]. The potential function of this role for leptin-induced aldosterone in pregnancy is demonstrated in mice lacking CYP11B2, which are unable to maintain adequate blood pressure to sustain fetal growth [42]. Although ANG II levels are also increased in pregnancy, plasma renin activity peaks in early trimesters of a healthy pregnancy whereas aldosterone levels peak in the third trimester [40]. Furthermore, angiotensin II type I receptor (AT1R) sensitivity is reduced in a normal pregnancy [43], and it may be postulated that leptin predominates as the primary aldosterone-stimulating factor in late pregnancy, however, if adrenal AT1R sensitivity is also reduced in normal pregnancies is in need of investigation.
CARDIOVASCULAR CONSEQUENCES OF THE ACTIVATION OF THE LEPTIN–ALDOSTERONE AXIS: IMPLICATIONS PREDOMINANTLY FOUND IN FEMALE ANIMALS
Increasing BMI is associated with a heightened risk for cardiovascular disease in both sexes, however, the mechanisms underlying the development of obesity-associated cardiovascular diseases are likely sex-specific. It is well established that leptin induces increases in sympathetic tone and hypertension in obese men, however, a comparable increase in sympathetic activation in obese female animals who are not postmenopausal has not been similarly observed [44–46]. What is common among both sexes is the contribution of leptin to obesity-associated hypertension, as animal models as well as human studies have indicated that hyperleptinemia contributes to increased blood pressure [5,47–49]. Therefore, an alternative mechanism to leptin-induced sympathetic activation is likely implicated in obesity-associated hypertension in female animals and emerging evidence suggests the leptin–aldosterone stimulation pathway may be this key mechanism.
Clinical studies have shown that obese women are more responsive to the blood pressure-lowering effects of mineralocorticoid receptor antagonists than obese men [50], indicating that this pathway plays a significant role in vascular tone in women in obesity. At this time, no clinical report has investigated sex differences in leptin inhibition on blood pressure in the clinical population, however, emerging data indicates a need for such studies.
Our recent works have shown that leptin mediates aldosterone production in association with induction of endothelial dysfunction, cardiac fibrosis and hypertension in female mouse models. Female Balb/C wild-type mice infused with leptin (0.9 mg/kg/day) present with endothelial dysfunction and increased cardiac pro-fibrotic markers after 7 days of treatment [18▪▪]. Importantly, mineralocorticoid receptor blockade with spironolactone blunts leptin-mediated endothelial dysfunction and increased cardiac fibrosis in female mice [18▪▪]. Affirming that elevations in leptin are critical for obesity-induced vascular dysfunction in obese female mice, female ob/ob mice do not spontaneously present with vascular and cardiac abnormalities, however, leptin replacement in ob/ob female mice induces endothelial dysfunction and elevates pro-fibrotic cardiac markers, which is prevented by concurrent spironolactone treatment [18▪▪]. We also have shown that leptin sensitization with PTP1b deletion or high leptin levels in Agouti female mice promotes impaired vascular dilation to acetylcholine (a measure of endothelial dysfunction) and elevates mean arterial pressure. In addition, pharmacological leptin receptor antagonism (Allo-Aca) reverts impaired endothelial dilation and reduces high blood pressure in both PTP1b knockout and Agouti mouse models [19▪▪]. No development of endothelial dysfunction presents spontaneously in male PTP1b knockout or Agouti mice, indicating that female leptin-sensitive and hyperleptinemic mice are more responsive to the vascular effects of the leptin–aldosterone stimulation pathway. Taken together, these data indicate that leptin induces cardiovascular dysfunction by increasing aldosterone production in the adrenal glands exclusively in female mice (Fig. 2). Although many have attributed increased leptin production in women to their predisposition of subcutaneous adipose tissue, further studies are needed to establish mechanistic pathways via which the leptin–aldosterone stimulation pathway is not only more efficient in women to produce aldosterone, but also mechanisms describing the increased sensitivity female mice have for the pro-hypertensive effects of aldosterone.
FIGURE 2.
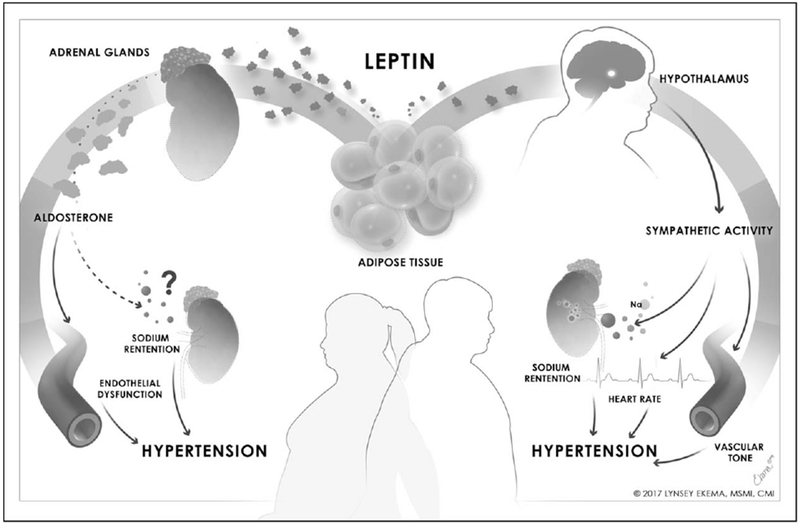
Proposal for sex differences in obesity-associated leptin-mediated hypertension.
CONCLUSION
Dysregulation of the renin–angiotensin–aldosterone system has been well characterized in obesity and it has long been known that aldosterone levels are increased, however, these emerging data tie together for the first time an apparent role for leptin as a novel regulator of aldosterone production. The implications of this relationship appear to be more heavily pronounced in women as they have increased production of both hormones in obesity and evidence in animal models indicates that the cardiovascular effects of this relationship are especially impactful in obese female mice. The implications of this relationship have a significant potential impact as pharmaceutical agents suppressing aldosterone activation (i.e. mineralocorticoid receptor antagonists) are currently commercially available and may be on the advent of becoming an aspect of personalized medicine treatment for obese women at risk for cardiovascular disease.
KEY POINTS.
Emerging evidence suggests that obesity-induced increases in aldosterone production are mediated by increases in leptin production in obesity.
Leptin-induced aldosterone secretion is a novel pathway that may have significant effects on the development of cardiovascular disease in obesity.
Leptin-induced aldosterone production contributes to obesity-associated hypertension and endothelial dysfunction in a sex-specific manner, predominately in female.
Acknowledgements
We would like to thank Lynsey Ekema, MSMI, CMI, Department of Technology Enhanced Learning and Innovation at Augusta University, for assistance in preparing the medical illustration.
Financial support and sponsorship
This work was supported by NIH 1R01HL130301–01, AHA 16IRG27770047, 1 F32 HL136191–01A1 and AHA 17POST33410363.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Andronico G, Cottone S, Mangano MT, et al. Insulin, renin-aldosteronesystem and blood pressure in obese people. Int J Obes Relat Metab Disord 2001; 25:239–242. [DOI] [PubMed] [Google Scholar]
- 2.Briones AM, Nguyen Dinh Cat A, Callera GE, et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 2012; 59:1069–1078. [DOI] [PubMed] [Google Scholar]
- 3.Goodfriend TL, Egan BM, Kelley DE. Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins Leukot Essent Fatty Acids 1999; 60:401–405. [DOI] [PubMed] [Google Scholar]
- 4.Silva MA, Cau SB, Lopes RA, et al. Mineralocorticoid receptor blockade prevents vascular remodelling in a rodent model of type 2 diabetes mellitus. Clin Sci (Lond) 2015; 129:533–545. [DOI] [PubMed] [Google Scholar]
- 5.Belin de Chantemele EJ, Mintz JD, Rainey WE, Stepp DW. Impact of leptin-mediated sympathoactivation on cardiovascular function in obese mice. Hypertension 2011; 58:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schafer N, Lohmann C, Winnik S, et al. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J 2013; 34:3515–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Gharbawy AH, Nadig VS, Kotchen JM, et al. Arterial pressure, left ventricular mass, and aldosterone in essential hypertension. Hypertension 2001; 37:845–850. [DOI] [PubMed] [Google Scholar]
- 8.Bollag WB. Regulation of aldosterone synthesis and secretion. Compr Physiol 2014; 4:1017–1055. [DOI] [PubMed] [Google Scholar]
- 9.Bochud M, Nussberger J, Bovet P, et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension 2006; 48:239–245. [DOI] [PubMed] [Google Scholar]
- 10.Grim CE, Cowley AW Jr, Hamet P, et al. Hyperaldosteronism and hypertension: ethnic differences. Hypertension 2005; 45:766–772. [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu K, Yamada T, Ichikawa K, et al. Changes in endocrine activities relative to obesity in patients with essential hypertension. J Am Geriatr Soc 1981; 29:25–30. [DOI] [PubMed] [Google Scholar]
- 12.Kidambi S, Kotchen JM, Grim CE, et al. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension 2007; 49:704–711. [DOI] [PubMed] [Google Scholar]
- 13.Muller-Fielitz H, Lau M, Johren O, et al. Blood pressure response to angiotensin II is enhanced in obese Zucker rats and is attributed to an aldosterone-dependent mechanism. Br J Pharmacol 2012; 166:2417–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocchini AP, Katch VL, Grekin R, et al. Roleforaldosterone in blood pressure regulation of obese adolescents. Am J Cardiol 1986; 57:613–618. [DOI] [PubMed] [Google Scholar]
- 15.Harada E, Mizuno Y, Katoh D, et al. Increased urinary aldosterone excretion is associated with subcutaneous not visceral, adipose tissue area in obese individuals: a possible manifestation of dysfunctional subcutaneous adipose tissue. Clin Endocrinol (Oxf) 2013; 79:510–516. [DOI] [PubMed] [Google Scholar]
- 16.Nagase M, Yoshida S, Shibata S, et al. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol 2006; 17:3438–3446. [DOI] [PubMed] [Google Scholar]
- 17.Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, et al. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci USA 2003; 100:14211–14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.▪▪.Huby AC,Antonova G, Groenendyk J, et al. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 2015; 132:2134–2145. Study identifying leptin as a new direct regulator of aldosterone and demonstrating that activation of the leptin–aldosterone axis leads to endothelial dysfunction and cardiac fibrosis in female preferentially. [DOI] [PubMed] [Google Scholar]
- 19.▪▪.Huby AC, Otvos L Jr, Belin de Chantemele EJ. Leptin induces hypertension and endothelial dysfunction via aldosterone-dependent mechanisms in obese female mice. Hypertension 2016; 67:1020–1028. Study identifying the leptin–aldosterone axis as a major contributor to the development of hypertension in obese female mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredersdorf S, Endemann DH, Luchner A, et al. Increased aldosterone levels in a model of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2009; 117:15–20. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann A, Peitzsch M, Brunssen C, et al. Elevated steroid hormone production in the db/db mouse model of obesity and type 2 diabetes. Horm Metab Res 2017; 49:43–49. [DOI] [PubMed] [Google Scholar]
- 22.Silva MA, Bruder-Nascimento T, Cau SB, et al. Spironolactone treatment attenuates vascular dysfunction in type 2 diabetic mice by decreasing oxidative stress and restoring NO/GC signaling. Front Physiol 2015; 6:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glasow A, Haidan A, Hilbers U, et al. Expression of Ob receptor in normal human adrenals: differential regulation of adrenocortical and adrenomedullary function by leptin. J Clin Endocrinol Metab 1998; 83:4459–4466. [DOI] [PubMed] [Google Scholar]
- 24.Hoggard N, Mercer JG, Rayner DV, et al. Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridization. Biochem Biophys Res Commun 1997; 232:383–387. [DOI] [PubMed] [Google Scholar]
- 25.Malendowicz LK, Neri G, Markowska A, et al. Effects of leptin and leptin fragments on steroid secretion of freshly dispersed rat adrenocortical cells. J Steroid Biochem Mol Biol 2003; 87:265–268. [DOI] [PubMed] [Google Scholar]
- 26.Ariyawatkul K, Tepmongkol S, Aroonparkmongkol S, Sahakitrungruang T. Cardio-metabolic risk factors in youth with classical 21-hydroxylase deficiency. Eur J Pediatr 2017; 176:537–545. [DOI] [PubMed] [Google Scholar]
- 27.Haluzik M, Sindelka G, Widimsky J Jr, et al. Serum leptin levels in patients with primary hyperaldosteronism before and after treatment: relationships to insulin sensitivity. J Hum Hypertens 2002; 16:41–45. [DOI] [PubMed] [Google Scholar]
- 28.Urbanet R, Pilon C, Calcagno A, et al. Analysis of insulin sensitivity in adipose tissue of patients with primary aldosteronism. J Clin Endocrinol Metab 2010; 95:4037–4042. [DOI] [PubMed] [Google Scholar]
- 29.Judd AM, Call GB, Barney M, et al. Possible function of IL-6 and TNF as intraadrenal factors in the regulation of adrenal steroid secretion. Ann N Y Acad Sci 2000; 917:628–637. [DOI] [PubMed] [Google Scholar]
- 30.Path G, Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA. Interleukin-6 and the interleukin-6 receptor in the human adrenal gland: expression and effects on steroidogenesis. J Clin Endocrinol Metab 1997; 82:2343–2349. [DOI] [PubMed] [Google Scholar]
- 31.Bastard JP, Jardel C, Delattre J, et al. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation 1999; 99:2221–2222. [PubMed] [Google Scholar]
- 32.Xing Y, Rainey WE, Apolzan JW, et al. Adrenal cell aldosterone production is stimulated by very-low-density lipoprotein (VLDL). Endocrinology 2012; 153:721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isidori AM, Strollo F, More M, et al. Leptin and aging: correlation with endocrine changes in male and female healthy adult populations of different body weights. J Clin Endocrinol Metab 2000; 85:1954–1962. [DOI] [PubMed] [Google Scholar]
- 34.Montague CT, Prins JB, Sanders L, et al. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes 1997; 46:342–347. [DOI] [PubMed] [Google Scholar]
- 35.Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr 2008; 99:931–940. [DOI] [PubMed] [Google Scholar]
- 36.Ahima RS, Dushay J, Flier SN, et al. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest 1997; 99:391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 1999; 341:879–884. [DOI] [PubMed] [Google Scholar]
- 38.Riad-Gabriel MG, Jinagouda SD, Sharma A, et al. Changes in plasma leptin during the menstrual cycle. Eur J Endocrinol 1998; 139:528–531. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed AH, Gordon RD, Ward G, et al. Should aldosterone suppression tests be conducted during a particular phase of the menstrual cycle, and, if so, which phase? Results of a preliminary study. Clin Endocrinol (Oxf) 2015; 83:303–307. [DOI] [PubMed] [Google Scholar]
- 40.Lumbers ER, Pringle KG. Roles of the circulating renin-angiotensin-aldosterone system in human pregnancy. Am J Physiol Regul Integr Comp Physiol 2014; 306:R91–R101. [DOI] [PubMed] [Google Scholar]
- 41.Sagawa N,Yura S, Itoh H, et al. Possible role of placental leptin in pregnancy: a review. Endocrine 2002; 19:65–71. [DOI] [PubMed] [Google Scholar]
- 42.Todkar A, Di Chiara M, Loffing-Cueni D, et al. Aldosterone deficiency adversely affects pregnancy outcome in mice. Pflugers Archiv 2012; 464:331–343. [DOI] [PubMed] [Google Scholar]
- 43.Gant NF, Daley GL, Chand S, et al. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 1973; 52:2682–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briant LJ, Charkoudian N, Hart EC. Sympathetic regulation of blood pressure in normotension and hypertension: when sex matters. Exp Physiol 2016; 101:219–229. [DOI] [PubMed] [Google Scholar]
- 45.Sex Hay M., the brain and hypertension: brain oestrogen receptors and high blood pressure risk factors. Clin Sci (Lond) 2016; 130:9–18. [DOI] [PubMed] [Google Scholar]
- 46.Lambert EA, Straznicky NE, Dixon JB, Lambert GW. Should the sympathetic nervous system be a target to improve cardiometabolic risk in obesity? Am J Physiol Heart Circ Physiol 2015; 309:H244–H258. [DOI] [PubMed] [Google Scholar]
- 47.Greenfield JR, Miller JW, Keogh JM, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med 2009; 360: 44–52. [DOI] [PubMed] [Google Scholar]
- 48.Ozata M, Ozdemir IC, Licinio J Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab 1999; 84:3686–3695. [DOI] [PubMed] [Google Scholar]
- 49.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev 2014; 10:131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khosla N, Kalaitzidis R, Bakris GL. Predictors of hyperkalemia risk following hypertension control with aldosterone blockade. Am J Nephrol 2009; 30:418–424. [DOI] [PubMed] [Google Scholar]


