Abstract
Higher intakes of the omega-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) relative to the omega-6 arachidonic acid (AA) have been variably associated with reduced risk of premenopausal breast cancer. The purpose of this pilot trial was to assess feasibility and explore the effects of high-dose EPA and DHA on blood and benign breast tissue risk biomarkers before design of a placebo-controlled phase IIB trial. Premenopausal women with evidence of hyperplasia ± atypia by baseline random periareolar fine needle aspiration were given 1860 mg of EPA + 1500mgofDHAethyl esters daily for 6 months. Blood and benign breast tissue were sampled during the same menstrual cycle phase prestudy and a median of 3 weeks after last dose. Additional blood was obtained within 24 hours of last dose. Feasibility, which was predefined as 50% uptake, 85% retention, and 70% compliance, was demonstrated with 46% uptake, 94% completion, and 85% compliance. Cytologic atypia decreased from 77% to 38% (P = 0.002), and Ki-67 from a median of 2.1% to 1.0% (P = 0.021) with an increase in the ratio of EPA + DHA to AA in erythrocyte phospholipids but no change in blood hormones, adipokines, or cytokines. Exploratory breast proteomics assessment showed decreases in several proteins involved in hormone and cytokine signaling with mixed effects on those in the AKT/mTORpathways. Further investigation of EPA plusDHAfor breast cancer prevention in a placebo-controlled trial in premenopausal women is warranted.
Introduction
In addition to hormonal influences, cytokine production and chronic inflammation are increasingly being recognized as important in breast carcinogenesis (1). A low level of macrophages which function to clear apoptotic cells are present in normal breast tissue; but a progressive increase in activated macrophages in inflammatory infiltrates has been observed in proliferative breast disease, ductal carcinoma in situ (DCIS), and invasive breast cancer (2).
The initial stimulus for the increase in inflammatory cell infiltration might include immunogenic gene alterations in pre-cancerous epithelial cells (3) or abnormal adipocytes in obese women (4); but the ratio of the long chain omega-3 to omega 6 fatty acids plays an important role in the continuation of the inflammatory process. Proinflammatory eicosanoids derived from the omega-6 fatty acid arachidonic acid (AA) released from activated macrophage membranes promote production of cytokines, proteases, proangiogenic factors, and further macrophage infiltration (5). The inflammatory loop can be interrupted with the long chain omega-3 eicosapentaenoic acid (EPA) and doc-osahexaenoic acid (DHA) which compete with AA as substrate for cyclooxygenase and lipoxygenase enzymes and give rise to inflammation resolving metabolites (6). However, continued excess of AA relative to the sum of EPA plus DHA, the norm in western diets, promotes chronic inflammation, and in preclinical models breast carcinogenesis (6–9). The current intake of EPA and DHA in the United States averages only 0.1% to 0.2% of calories with a total omega-3 to omega-6 intake ratio of approximately 1:10 (10). Two case–control studies using dietary recall instruments suggest breast cancer risk reduction in premenopausal women with higher intakes of omega-3 fatty acids (11, 12). It is likely to be easier to increase EPA and DHA from fish oil supplements than reduce omega-6 and AA in the diet frequently found in soybean, corn oil, meat, and eggs (13). Rodent studies showing reduced mammary cancer incidence and multiplicity are generally with EPA plus DHA at 8% to 25% of calories or >1:1 ratio of omega 3:6 ratio in feed (14, 15).
There are multiple potential molecular mechanisms underlying the reduction in breast carcinogenesis in preclinical models with EPA and DHA supplementation (9). The most important are likely to be reductions in NF-κB and protein kinase c (PKC) activation, nuclear translocation, and signaling (16) with downstream reductions in cyclin D-1 and bcl2 (15), and disruption of cell membrane lipid rafts with reduced interaction potential of multiple oncogenic proteins including EGFR, SRC, and HER-2 (9, 17–19). Recent studies suggest that the actual levels of some of these proteins may also be reduced resulting from new juxtaposition to lysosomes and proteasomes (18). Additional mechanisms include reduced adhesion molecule expression and motility and improved insulin sensitivity (20, 21). A sum result is likely reduction in proliferation, increased apoptosis, and reduced mammary carcinogenesis (18, 19, 22).
We conducted a study of blood and tissue fatty acid levels in a high-risk cohort (23), and found that lower levels of omega-3 fatty acids were associated with evidence of cytologic atypia, a known risk predictor for development of breast cancer (24).
Given the lack of information in humans about the level of EPA and DHA supplementation which might be needed to favorably modulate risk biomarkers, we selected a dose of 3.4 g per day of DHA + EPA ethyl esters, providing approximately 2% of calories. This dose is FDA approved for treatment of hypertriglyceridemia and is generally thought to be anti-inflammatory. Although this and higher doses are generally well tolerated, given the gastrointestinal complaints in some women (25), a feasibility study assessing uptake, compliance, and effects on biomarkers was thought to be prudent before a randomized study. Because premenopausal but not postmenopausal women taking EPA and DHA have been reported to have increases in blood, estradiol (26) and omega-3 fatty acids may impact progestin related EGF signaling (27), we conducted separate pilot studies of 6 months of EPA + DHA ethyl esters (4 g/day of Lovaza) in pre- and postmenopausal women. Results of the study in premenopausal women are reported here; those for postmenopausal women are in the subsequent article (28).
Materials and Methods
Eligibility
Premenopausal women between the ages of 30 and 54 years with no prior invasive breast cancer were potentially eligible for tissue screening by random periareolar fine needle aspiration (RPFNA; ref. 24), provided they met risk criteria and had a stable hormone status for at least 6 months. Women < 49 years with intact ovaries but not menstruating had premenopausal status confirmed by follicle-stimulating hormone (FSH), estradiol, and progesterone. Stable hormonal status was defined as not stopping, starting, or significantly changing hormonal birth control within 6 months and no pregnancy or lactation within 12 months. Women over 40 years were required to have a normal mammogram within 6 months before entering study.
Criteria for breast tissue screening for intervention
Risk criteria included any one or more of the following: a first-degree relative or two or more affected second-degree relatives under 60, a prior breast biopsy showing atypical hyperplasia or lobular carcinoma in situ, a prior contra-lateral treated breast cancer, a 5-year Gail model (29) risk ≥1.67% or a 10 year Tyrer-Cuzick (IBIS II) risk at least 2× that for age group, >50% mammographic breast density, RPFNA evidence of hyperplasia with atypia within the prior 3 years, or radiation to the neck or chest before the age of 30 years. Excluded were those who (i) had received tamoxifen or were on a clinical prevention study within the prior 6 months, (ii) had used fish oil or flaxseed supplements within 3 weeks before RPFNA, (iii) regularly used NSAIDs, (iv) had a BMI of 40 kg/m2 or higher, or (v) had breast implants.
Participants signed separate consents for IRB-approved protocols for screening and repeat RPFNAs (HSC 4601) and administration of Lovaza (HSC 12349; NCT01252277). Protocols were approved by the University of Kansas Medical Center Human Subjects Committee (Kansas City, KS) and were conducted in accordance with the Declaration of Helsinki. The first woman was enrolled January 2011 and the last completed the trial in March 2013.
Breast tissue biomarker eligibility for intervention
Women were required to have cytologic evidence of hyperplasia with atypia or borderline atypia (Masood score of 14 or higher) in specimens obtained by RPFNA (30). Initially, women were not required to have evidence of Ki-67 expression but later selection criteria were tightened such that Ki-67 expression was required (the latter based on assessment of 500 epithelial cells). We acquired two and four vials of non-bloody frozen tissue for assessment of fatty acids, gene expression, adipocytokines, and proteomics.
Blood and tissue acquisition and specimen processing
Screening and off-study RPFNAs were performed between the first and tenth day after the onset of menses. For premenopausal women who were not regularly menstruating, follicular phase of cycle was confirmed by FSH, estradiol, and progesterone levels. For the RPFNA procedure, two sites per breast were aspirated under local anesthesia as previously described with needle tip preferentially guided to areas of increased resistance (24, 31). One-fifth of the RPFNA material was immediately placed in 0.25 mL aliquots of PBS in cryovials, placed in liquid nitrogen, and transferred to a −80° C freezer within 12 hours until assessments for fatty acid analyses, adipocytokines (Luminex), gene expression (RT-qPCR), and proteomics. Remaining material was pooled in a single 15 cc tube with 9 mL CytoLyt and 1 mL of 10% formalin (Hologic, Inc.) for cytomorphology and Ki-67. Cells were pelleted, washed in CytoLyt, and transferred to PreservCyt. Aliquots were then transferred to slides via ThinPrep methodology for pap staining for cytomorphology or Ki-67.
Frozen RPFNA samples for each subject were quickly thawed in a cool water bath, transferred to ice, pooled and mixed. Of note, 250 μL of sample was removed and mixed with 750 μL TRIzol LS (Life Technologies) and stored at −80°C until ready to extract RNA. Another 150 to 250 μL aliquot was transferred to a sterile 1.5 mL tube, quickly frozen in pulverized dry ice, and then stored at −80°C before Luminex assays for adipocytokines. The remainder of the RPFNA sample on ice was briefly sonicated (Fisher 100 Sonic Dismembrator) using three 10 second pulses at low settings (≤10–15W), and additional 250 μL aliquots were quick frozen in dry ice and stored at – 80° C for reverse-phase proteomics and fatty acid analyses.
Fasting blood (31) was obtained at the on-study visit and at 6 months within 24 hours of scheduled drug discontinuation. Insulin, glucose, adipocytokines, and fatty acid analyses were performed on fasting blood. A nonfasting sample was obtained with each RPFNA for sex hormone-binding globulin (SHBG) and hormones. Per protocol, the RPFNA was to be delayed 2 weeks after drug discontinuation to reduce chance of bruising. All biomarker assays except cytomorphology and Ki-67 were batch processed with samples stored in aliquots at − 80°C so that pre-and postintervention specimens were run together.
Cytomorphology
Cytomorphology was assessed by a single cytopathologist (C.M. Zalles) and classified by both a categorical method (24) and a semiquantitative index score (30, 31). The cytopathologist was aware that the subject was undergoing eligibility screening or off study aspiration but scoring of slides was performed without knowledge of prior score which was kept in the prevention lab database. Scores of 12–14 generally correspond to hyperplasia and 15–18 to atypia (30). The number of epithelial cells per slide was estimated and categorized in the following ranges: <10, 10–99, 100–499, 500–999, 1,000–4,999, or >5,000.
Ki-67
Ki-67 assessment was performed after citrate buffer antigen retrieval, using a MIB-1 monoclonal antibody (M7240; Dako Cytomation) at a 1:20 dilution with a Dako Autostainer Plus (Dako Cytomation). At baseline, only hyperplastic breast cell clusters were assessed, but at the 6-month follow-up, if no hyperplastic clusters were present, clusters containing the highest proportion of cells staining for Ki-67 were evaluated. Number of nuclear staining cells out of 500 cells was recorded by two independent readers without knowledge of prior Ki-67 until readings were complete. In case of a difference between the two readers, the scores were averaged (31).
Anthropometric and dietary variables
Height and weight were measured and a Dual Energy X-ray Absorptiometry or DEXA scan (Lunar Prodigy, GE Healthcare) was performed at baseline and postintervention for body mass, fat mass, lean body mass, and android fat mass. Subjects completed the online National Cancer Institute Diet History Questionnaire II (32) pre- and postintervention.
Mammographic density
Pre- and postintervention mammograms were all acquired on digital units at the University of Kansas Medical Center. A file was made of the cranial and caudal images of the left breast and images were coded and then de-identified by B.F. Kimler for assessment by two readers (C.J. Fabian and W.L. Hensing) using Cumulus software (33). Images were paired so that the reader was aware that they belonged to the same subject (but did not know which was preintervention and which postintervention) as this is associated with reduced variability (33). Images were assessed in batches of 14 including some sets multiple times such that variance could be assessed. The percent area of breast at increased density estimated by the two readers was averaged and used for analysis.
Adverse events, quality of life, and compliance
Adverse events were assessed monthly by the study coordinator, and in the event of a significant symptom, they were assessed by the protocol chair. Overall quality of life was assessed pre- and postintervention by the Breast Cancer Prevention Trial (BCPT) symptom scale as used previously (34).
High-dose omega-3 intervention
Women initiated the intervention within 6 months of their baseline RPFNA and took two capsules of Lovaza each containing 465 mg EPA and 375 mg DHA as the ethyl ester twice daily (total dose 3360 mg) with meals for a planned 6 months. Per protocol, the duration of the intervention could be modified for scheduling considerations.
Fatty acid analyses
Blood samples were collected in 5 mL sodium–EDTA tubes (BD Vacutainer) and placed on ice immediately. Plasma and erythrocytes were separated by centrifugation (3,000×g, 10 minutes; 4°C), frozen, and stored under nitrogen at −80°C until analysis. Lipids from plasma, erythrocytes, and breast tissue were isolated according to a modified Folch method (35), and fractionated by thin-layer chromatography. All lipid fractions were transmethylated with boron trifluoride-methanol, and the resulting fatty acid methyl esters (FAME) were separated using a Varian 3900 gas chromatograph with an SP-2560 capillary column (100 m, Sigma Aldrich) and a Star 6.41 Chromatography Workstation for peak integration and analysis (35). Injector and detector temperatures were programmed at 260°C The column temperature program for the 41-minute column run was: 5 minutes at 140°C; 4°C increase/minute to 240° C; and 240° C, 11 minutes. Individual peaks were identified by comparison with qualitative standards (PUFA 1 and PUFA 2, Sigma Aldrich) and a weighed standard mixture (Supelco 37 Component FAME mix, Sigma Aldrich) was used to adjust fatty acids for area/weight to calculate a final weight percent of total fatty acids.
Hormones and growth factors
Serum was frozen at −80°C in aliquots to avoid thawing and refreezing until assays were performed. Baseline and off-study samples were run together in batch assays, with control pooled sera included to assess inter and intra-batch variation.
Serum assays performed in our laboratory were all ELISAs using commercially available kits from Diagnostics Biochem Canada Inc. except as noted. Estradiol (CAN-E430), testosterone (CAN-TE-250), and SHBG (CAN-SHBG-410) results were used to calculate bioavailable estradiol and testosterone (30). Progesterone (CAN-P-305) and high-sensitivity C-reactive protein (CAN-CRP-4360) kits were also assayed. High-molecular weight adiponectin (DHWAD0), insulin-like growth factor I (IGFI; DG100), and insulin-like growth factor-binding protein 3 (IGFBP-3; DGB300) kits were purchased from R&D Systems, Inc.
Insulin was assessed by immunoassay and pro-insulin by immunochemo-luminescent assay in the CLIA-approved clinical laboratories at the University of Kansas Hospital and Mayo Medical Laboratories, respectively. Insulin resistance (IR), plus insulin sensitivity (%S) and β cell function (%B) relative to normal young adults, was estimated from fasting glucose and insulin levels using a calculator for the updated Homeostasis Model Assessment (HOMA2; ref. 36), available from ref. 37.
Serum and tissue for adipokine and cytokine assay by luminex
Frozen sera or RPFNA aspirates were utilized for adipokines and cytokine assessment using Milliplex MAP Human Adipokine Magnetic Bead Panel 1 and Panel 2 kits from Millipore Corporation. Adipokines and cytokines assessed by Luminex were adiponectin, leptin, macrophage chemoattractant factor 1 (MCP-1), tumor necrosis factor alpha (TNFα), plasminogen activator inhibitor-1 (PAI-1), hepatocyte growth factor (HGF), nerve growth factor (NGF), resistin, and insulin. For tissue assays, results were normalized to total protein content (Bio-Rad Protein Assay, #500-0006, Bio-Rad Laboratories, Inc.). For statistical analysis of changes in tissue, only 25 paired RPFNA samples were included where neither sample was visually bloody.
Tissue for RT-qPCR
Total RNA was extracted from frozen RPFNA samples using TRIzol LS (Life Technologies) according to the manufacturer's instructions. RNA was amplified using MessageAmpII aRNA amplification kit (Life Technologies) and reverse transcribed to cDNA using SMARTScribe Reverse Transcriptase (Clontech Laboratories, Inc.) and random nonamer primers. Real-time PCR (qPCR) was performed using TaqMan chemistry as previously described (38). Levels were expressed as relative to three reference transcripts (PPIA, PPIG, and HRPT1) using the ΔΔCt method. For statistical analysis of changes, 25 paired specimens were included where neither was visually bloody. Genes assessed were ADIPOQ, LEP, ALOX15, ALOX15B, ALOX5, ALOX5AP, CD44, PTSG2, CCNB1, CCND1, ERP44, ESR1, SLC2A4, GREB1, HGF, HPGD, ICAM1, IGFBP2, KISS1, LTA4H, STK11, MCM2, CCL2, PPARG, PGR, TTF1, two splice variants for CXCL12, SDC1, TXNIP, VEGFA, XIAP; plus ACTB, GUSB, and B2M as potential reference transcripts that were eliminated when all data were analyzed by genormPLUS software (Biogazelle).
Tissue for reverse phase protein array
Frozen RPFNA specimens were utilized for RPPA performed at the CCSG Functional Proteomics Core at the University of Texas MD Anderson Cancer Center (Houston, TX). Specimens were spotted onto a glass slide coated with nitrocellulose with each sample represented on the slide as a serial microdilution. Dilution series were replicated on spatially distant portions of the array. Each slide contained multiple positive and negative controls including quantitative peptide and phosphopeptide controls with results normalized to total protein loading (39). Pre- and post-intervention specimens were assessed together.
Assessment of 110 validated peptides and phosphopeptides was performed on 23 pairs of specimens, 16 of which were not visually bloody.
Statistical analysis
The primary endpoint was feasibility defined as 50% or greater protocol acceptance in eligible women, with at least 85% retention and 70% compliance. The target accrual of 40 subjects was designed to provide paired (pre- and postintervention) specimens for biomarker evaluation of at least 30 subjects. Secondary endpoints were (i) change in risk biomarkers including cytomorphology, Ki-67, mammographic breast density, serum fasting insulin, and pro-insulin, HOMA IR, HOMA %S, HOMA %B, IGFI: IGFBP3, adiponectin and leptin and their ratio, BMI, change in body fat, % body fat distribution, and lean body mass; and (ii) change in fatty acid composition primarily in erythrocyte and breast tissue phospholipid compartments. Changes were also examined in molecular mechanism of action biomarkers including proinflammatory cytokines such as TNFα, HGF, PAI-1; chemokines such as MCP-1; and proteins and phosphoproteins of interest in breast carcinogenesis. Adverse events and quality-of-life indices were studied as measures of safety and acceptability.
Data were summarized using frequencies and percentages for categorical variables, and medians, means, and SD for continuous variables.
Given the small sample sizes, nonparametric statistical analysis approaches were used throughout. Change in continuous biomarkers over the course of the intervention was assessed by the Wilcoxon signed rank test. For comparison of paired categorical variables (e.g., atypia), McNemar test was used. All analyses were conducted using SPSS, version 20 (IBM). A two-sided P <0.05 was considered as statistically significant. Because secondary analyses including biomarkers were considered exploratory, they were not corrected for multiple comparisons; rather, uncorrected P values are shown and the reader is advised to interpret results conservatively.
Results
Eligibility testing and trial entry
Of the 145 potentially medically eligible women screened by RPFNA, 66 were not tissue eligible. Of the 79 who were tissue eligible, 42 declined participation of which 28 entered another trial. Thus, 36 entered the trial for an acceptance rate if medically and tissue biomarker eligible of 46% close to the feasibility target rate of 50% (Supplementary Fig. S1). When it became apparent that completion rate and evaluability rate were high, accrual was stopped at 36 entrants as our target was 30 women with available blood and tissue pre and posttreatment.
Baseline demographics and risk factors
Median age was 44.5 (range 32–52) and all were premenopausal. Eleven of the 36 women (31%) were on oral contraceptives at baseline and throughout the trial. Ninety-seven percent of participants self-identified as non-Hispanic white, and 3% as African-American. Eighty-three percent of participants were college graduates, and 47% had postgraduate degrees. Median BMI was 25.0 kg/m2 (range 17.0–34.7 kg/m2) and 25% were obese (BMI 30 kg/m2 or higher). Eighty-three percent had a positive family history of breast cancer, 25% had a prior precancerous biopsy showing atypical hyperplasia, LCIS, or DCIS and 47% had an estimated mammographic density of >50%. (Supplementary Table S1).
Retention, compliance, adverse events, and quality of life
Of the 36 women who enrolled, 34 (94%) completed the intervention and had a repeat RPFNA and at least 85% remaining on study were compliant with study medication. Thirty-three of the 34 women who completed the study took 70% or more of prescribed drug, based on duration on drug (median 190 days, range 121–235 days) and returned pill counts.
A favorable side-effect profile was also documented. Of the 40 Grade 2 or worse adverse events self-reported in 14 participants, only one (easy bruising) was characterized as probably due to study agent. Two subjects went off study early due to grade 1 gastrointestinal symptoms. There were no clinically significant changes in electrolytes or liver or renal function. The median BCPT quality-of-life score was 7.5 at on-study (range 0–29) and 7.0 at off-study (range 0–32). Higher scores denote greater symptoms.
Dietary intake of omega-3 and omega-6 fatty acids
At baseline, the median dietary estimated combined EPA + DHA intake as assessed by the food frequency questionnaire was 90 mg/day with a similar amount for AA. Median intakes of the shorter chain omega 3 ALA and omega-6 LA were 1.0 g and 9.8 g, respectively. The median ratio of total omega-3: omega-6 fatty acids consumed at baseline was approximately 1:10. There were no changes in dietary intake of EPA, DHA, AA, or total omega-3 or omega-6 fatty acids over the course of the study.
Fatty acid profiles in blood and breast aspirates
When assessed within 24 hours after the last dose, there was a decrease in AA, an increase in erythrocyte phospholipid EPA + DHA; resulting in a median 178% increasein (EPA+DHA): AA ratio (Table 1). Participant blood samples for fatty acid analysis were also collected at RPFNA, a median of 21 days after drug discontinuation. Median erythrocyte phospholipid (EPA+DHA):AA ratio at 21 days after drug discontinuation declined to a value of 117% over baseline (see Supplementary Table S2). Change in plasma phospholipid and triacylglyceride was similar to those observed in erythrocyte phospholipids, except for a more rapid decline in (EPA+DHA): AA in plasma phospholipids after study agent discontinuation.
Table 1. Change in fatty acid content for the 34 subjects who completed the trial.
| Median values | |||||
|---|---|---|---|---|---|
|
| |||||
| Tissue/lipid compartment fatty acid component | Percent of total fatty acid content | Relative change baseline to FNA2 | P (Wilcoxon) for baseline to FNA2c | ||
|
| |||||
| Baseline | 6 moa | 6.5 mob | |||
| Erythrocyte PLs | N = 34 | N = 34 | N = 32 | N = 32 | |
| AA | 12.8 | 10.7 | 10.8 | −16% | <0.001 |
| EPA | 0.40 | 2.24 | 1.27 | 235% | <0.001 |
| DHA | 2.95 | 5.46 | 4.71 | 64% | <0.001 |
| EPA+DHA:AA ratio | 0.27 | 0.76 | 0.56 | 117% | <0.001 |
| Plasma PLs | N = 34 | N =; 34 | N = 32 | N = 32 | |
| AA | 9.9 | 8.2 | 8.6 | −11% | <0.001 |
| EPA | 0.54 | 2.80 | 0.64 | 30% | 0.0013 |
| DHA | 2.50 | 4.50 | 3.35 | 37% | <0.001 |
| EPA+DHA:AA ratio | 0.30 | 0.91 | 0.45 | 44% | <0.001 |
| Plasma TAGs | N = 34 | N = 34 | N = 31 | N = 31 | |
| AA | 1.58 | 1.44 | 1.50 | −10% | 0.29 |
| EPA | 0.18 | 1.02 | 0.24 | 28% | 0.017 |
| DHA | 0.47 | 1.94 | 0.67 | 61% | <0.001 |
| EPA+DHA:AA ratio | 0.38 | 1.97 | 0.73 | 49% | <0.001 |
| Breast TAGs | N = 32 | N =32 | N = 32 | ||
| AA | 0.32 | 0.34 | 6.5% | 0.47 | |
| EPA | 0.033 | 0.096 | 165% | <0.001 | |
| DHA | 0.073 | 0.147 | 42% | <0.001 | |
| EPA+DHA:AA ratio | 0.29 | 0.51 | 96% | <0.001 | |
| Breast PLs | N = 33 | N = 33 | N = 33 | ||
| AA | 0.65 | 0.57 | 2.9% | 0.54 | |
| EPA | 0.051 | 0.049 | 21% | 0.30 | |
| DHA | 0.129 | 0.149 | 18% | 0.16 | |
| EPA+DHA:AA ratio | 0.23 | 0.32 | 35% | 0.044 | |
NOTE: Erythrocyte phosholipids (PLs), plasma PLs and triacylglycerides (TAGs), and breast aspirate PLs and TAGs.
Blood collected within a day of scheduled discontinuation of drug, after a nominal 6 months of intervention.
Second blood specimen collected on the day of the second aspiration, a median of 21 days after scheduled drug discontinuation.
P-values <0.05 are indicated in bold.
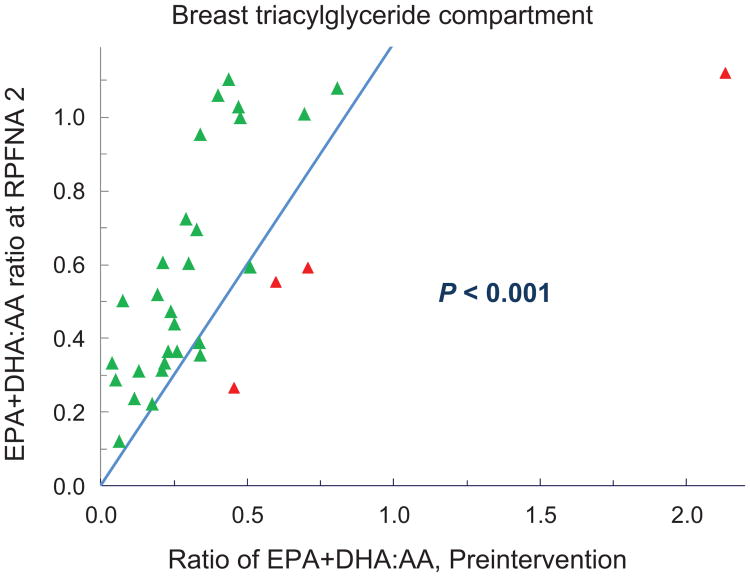
Although proportional amounts of fatty acids in breast aspirates were 8- to 40-fold lower than in erythrocytes or plasma, the baseline (EPA+DHA):AA ratios were in the same range (Table 1). There was a decided effect of supplementation on breast triacylgylcerides with a median increase of 96% for the (EPA+DHA): AA ratio and 88% of participants exhibiting an increase (P < 0.001; Fig. 1).
Figure 1.
Changes in the ratio of (EPA+DHA):AA in the breast triacylglyceride compartment. Baseline aspiration values are shown on the x-axis; repeat aspiration on the y-axis. The line represents no change in value; symbols above the line denote an increase and symbols below the line a decrease. P value via Wilcoxon signed rank test.
The above observations are consistent with omega-3 pharmacokinetics in which the half-life of EPA in serum triglycerides is 5 to 7 days, 1 month for erythrocyte phospholipids, and 6 months to a year for adipose; with incorporation and washout generally longer for membrane phospholipids than for triacylgylcerides, and DHA longer than EPA (40).
Anthropometric variables
At baseline, median BMI was 25.0 kg/m2, body fat 39.7%, android fat 43.3%, waist circumference 85 cm, and waist hip ratio 0.81. There was a statistically significant change for waist circumference (median decrease of 2 cm; P = 0.0091) and the corresponding waist to hip ratio (median decrease of 3%; P = 0.014; Supplementary Table S3).
Serum hormones, serum and tissue adipokines, and cytokines
There were no statistically significant changes in serum levels of bioavailable estradiol, or testosterone, progesterone, high-sensitivity CRP, high-molecular weight adiponectin, leptin, insulin, TNFα, resistin, PAI-I, HGF, NGF, and MCP-1 (Table 2). Glucose, insulin, HOMA IR, HOMA%S, and HOMA%B showed no significant changes although a numerical increase (improvement) was observed for HOMA% B (Table 2). Although 33 paired RPFNA samples were available for adipocytokines, seven were omitted as due to visual blood contamination resulting in 25 paired specimens for tissue adipocytokines. No differences were noted in tissue cytokines except for a small but significant increase (61%; P = 0.015) in TNFα (Supplementary Table S4).
Table 2. Change in serum biomarkers for 34 subjects who completed the trial.
| Biomarker | Median prestudy | Median poststudy | Median absolute change | Median relative change | P (Wilcoxon) |
|---|---|---|---|---|---|
| ELISA methodology | |||||
| High-molecular weight adiponectin, μg/mL | 5.8 | 5.7 | −0.09 | −2% | 0.73 |
| hsCRP, μg/mL | 1.4 | 1.2 | −0.2 | −23% | 0.16 |
| IGFI, nmol/L | 18.5 | 16.7 | 0.4 | 3% | 0.98 |
| IGFBP3, nmol/L | 116 | 110 | −4 | −4% | 0.080 |
| IGF-1:IGFBP3 ratio | 0.16 | 0.17 | 0.01 | 5% | 0.20 |
| SHBG, nmol/L (with estradiol) | 114.5 | 112.6 | 2.5 | 5% | 0.33 |
| Estradiol, pg/mL | 73.3 | 77.4 | −10.5 | −11% | 0.13 |
| Estradiol, pmol/L | 0.27 | 0.29 | −0.4 | −11% | |
| Bioavailable estradiol, pmol/L | 2.71 | 3.08 | −0.31 | −15% | 0.13 |
| Progesterone, ng/mL | 0.90 | 0.97 | −0.03 | −4% | 0.98 |
| Progesterone, nmol/L | 2.9 | 3.1 | −0.1 | −4% | |
| SHBG, nmol/L (with testosterone) | 99.4 | 105.5 | 0.6 | 1% | 0.86 |
| Testosterone, ng/mL | 0.55 | 0.46 | −0.03 | −5% | 0.38 |
| Testosterone, nmol/L | 1.88 | 1.60 | −0.11 | −5% | |
| Bioavailable testosterone, pmol/L | 16.6 | 16.5 | −0.12 | −2% | 0.38 |
| Luminex methodology | |||||
| Adiponectin, μg/mL | 17.0 | 18.4 | −1.3 | −8% | 0.44 |
| Leptin, ng/mL | 12.3 | 12.3 | 1.2 | 9.4% | 0.67 |
| Adipo:Leptin ratio | 1,170 | 1,559 | −36 | −6% | 0.37 |
| HGF, pg/mL | 264 | 240 | −17 | −8% | 0.74 |
| Insulin, pg/mL | 168 | 140 | −18 | −9% | 0.22 |
| MCP-1, pg/mL | 179 | 166 | −7 | −5% | 0.21 |
| NGF, pg/mL | 5.9 | 5.5 | −0.3 | −6% | 0.18 |
| PAI-1, ng/mL | 30.3 | 32.1 | −0.8 | −3% | 0.97 |
| Resistin, ng/mL | 23.4 | 24.5 | −0.8 | −4% | 0.54 |
| TNF-α, pg/mL | 2.7 | 2.8 | 0.0 | 0% | 0.88 |
| Assessment of insulin resistance | |||||
| Glucose, mg/dL | 95.0 | 93.0 | −2.5 | −3% | 0.075 |
| Immunoreactive Insulin, μIU/ml | 5.3 | 4.7 | 0.0 | 0% | 0.55 |
| Pro-Insulin, pmol/L | 8.7 | 9.2 | 0.2 | 2% | 0.89 |
| HOMA2 %B | 61.2 | 66.7 | 5.1 | 9% | 0.13 |
| HOMA2 %S | 142.6 | 160.3 | −0.1 | 0% | 0.78 |
| HOMA2 IR | 0.7 | 0.6 | 0.0 | 0% | 0.32 |
Cytomorphology, Ki-67, mammographic density
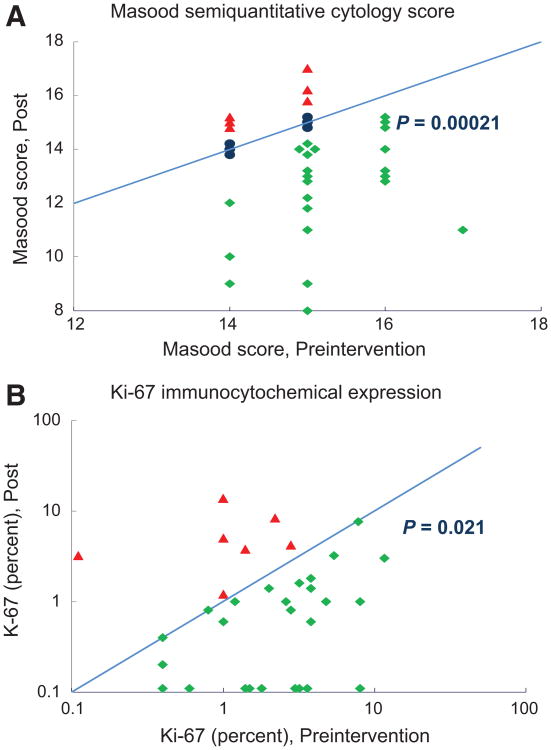
The off-study RPFNA was obtained a median of 21 days (range 5–78 days) after drug discontinuation. Favorable modulation was observed for the Masood cytomorphology index score, decreasing from a median of 15 at baseline to 14 after intervention (P < 0.001; Table 3). A decrease in Masood score was observed in 23 women versus an increase in 6 (P < 0.001; Fig. 2A). There was also a significant change in the designation of cytologic atypia, decreasing from 77% to 38% (P = 0.0024). For all 34 women, Ki-67 decreased from a median of 2.1% at baseline to1.0% off-study, with 23 decreasing versus 6 increasing (P = 0.021; Fig. 2B). Mammographic breast density which was a median of 17% at baseline showed a modest median decrease in absolute density of − 1.9% (relative value of −17%) with an average of 10 months between mammograms.
Table 3. Change in risk and response biomarkers for 34 subjects who completed the trial.
| Biomarker | Median prestudy | Median poststudy | Median absolute change | Median relative change | P Wilcoxon (McNemar) |
|---|---|---|---|---|---|
| RPFNA specimens | |||||
| Masood score | 15 | 14 | −1 | −7% | <0.001 |
| Frequency of atypia, percent | 77 | 38 | 2 “gain” atypia 15 “lose” atypia |
0.0024 | |
| Estimated epithelial cell number per slide | 1–5 × 103 | 1–5 × 103 | 16 decrease 3 increase |
0.0015 | |
| Ki-67, percent | 2.1 | 1.0 | −1.5 | −62% | 0.021a |
| Mammographic breast density Percent area of increased density (average of two readers) |
16.8 | 15.8 | −1.9 | −17% | 0.0036 |
If omit one baseline specimen exhibiting 0% (not possible to decrease), then P = 0.0091. P-values < 0.05 are indicated in bold.
Figure 2.
A, change in Masood semiquantitative cytology index score over the course of the intervention. Baseline aspiration values are shown on the x-axis; repeat aspiration on the y-axis. The line represents no change in value; symbols above the line denote an increase and symbols below the line a decrease. P value via Wilcoxon signed rank test. B, change in Ki-67 expression (percent of cells staining positive) over the course of the intervention. Baseline aspiration values are shown on the x-axis; repeat aspiration on the y-axis. The line represents no change in value; symbols above the line denote an increase and symbols below the line a decrease. P value via Wilcoxon signed rank test.
Benign breast tissue gene expression (mRNA)
No change was observed for 32 mRNA transcripts assessed in 29 pairs of frozen specimens, following normalization to reference transcripts. (Supplementary Table S5).
Benign breast tissue proteomics
Results from 23 paired pre- and postintervention RPFNA samples were available from Reverse Phase Protein Array (RPPA) for a set of 161 proteins and phosphoproteins. Restricting analysis to only 16 women with non-bloody paired samples, changes at the P < 0.05 level were observed for 24 of 110 validated peptides and phosphopeptides (Table 4). A complete listing of all peptides assessed, plus analysis including bloody specimens, is available in Supplementary Table S6. Changes significant at the P < 0.01 level were decreases in Bcl-2, eukaryotic initiation factor 4E (EIF4E), fibronectin, progesterone receptor, phosphorylated proline-rich Akt substrate (PRAS40_pT246), regulatory associated protein of mTOR (Raptor), stearoyl-CoA desaturase, and Smad3; and increases in 4E binding protein 1 (4E-BP1) phosphorylated at threonine 37 and 46, PKCα, and tuberous sclerosis 2 protein (tuberin) phosphorylated at threonine 1462.
Table 4.
Significant (P < 0.05) changes in levels of 26 peptides and phosphopeptides in RPFNA specimens, assessed by RPPA
| Number of paired specimens where levels | ||||
|---|---|---|---|---|
|
| ||||
| Protein (See Supplementary Table for gene name) | Antibody name and specific phosphorylation site | Decrease | Increase | P (Wilcoxona two-sided) |
| 4E-BP1 | 4E-BP1_pS65 | 5 | 11 | 0.049 |
| 4E-BP1 | 4E-BP1_pT37_T46 | 4 | 12 | 0.0045 |
| Akt | Akt_pS473 | 3 | 13 | 0.039 |
| Bcl-2 | BCL2 | 13 | 3 | 0.0072 |
| C-Raf | C-Raf | 13 | 3 | 0.039 |
| Cyclin D1 | Cyclin D1 | 11 | 5 | 0.044 |
| Eukaryotic elongation factor 2 kinase | eEF2K | 4 | 12 | 0.011 |
| Eukaryotic initiation factor 4E | eIF4E | 14 | 2 | 0.0016 |
| Fibronectin | Fibronectin | 13 | 3 | 0.0052 |
| GATA binding protein 3 | GATA3 | 13 | 3 | 0.023 |
| Her-3 | HER3 | 13 | 3 | 0.011 |
| Myosin isoform IIa | Myosin IIa_pS1943 | 12 | 4 | 0.011 |
| Phosphoinositide-dependent protein kinase 1 | PDK1_pS241 | 4 | 12 | 0.049 |
| Phosphoprotein enriched in diabetes/phosphoprotein enriched in astrocytes—15 kDa | PED/PEA15_pS116 | 12 | 4 | 0.023 |
| Protein kinase c α | PKC α | 2 | 14 | 0.0038 |
| Progesterone receptor | Progesterone receptor | 12 | 4 | 0.0027 |
| Proline-rich Akt substrate | PRAS40_pT246 | 13 | 3 | 0.0027 |
| Regulatory associated protein of mTOR | Raptor | 13 | 3 | 0.0052 |
| Rb | Rb_pS807_S811 | 5 | 11 | 0.044 |
| Stearoyl-CoA desaturase | SCD | 13 | 3 | 0.0045 |
| Smad3 | Smad3 | 14 | 2 | 0.0023 |
| Src | Src_pY627 | 3 | 13 | 0.017 |
| Stat5a | Stat5a | 11 | 5 | 0.044 |
| Spleen tyrosine kinase | Syk | 14 | 2 | 0.013 |
| Transglutaminase II | TGM2 | 11 | 5 | 0.044 |
| Tuberin | TSC2 Tuberin_pT1462 | 4 | 12 | 0.0061 |
P-values <0.01 are indicated in bold.
Discussion
Our single arm pilot study suggests the feasibility of assessing high-dose omega-3 ethyl esters effects on risk biomarkers in larger placebo-controlled phase IIB trials. Uptake by potentially eligible premenopausal women was reasonable (46%) especially considering a competing trial. Side effects were minimal with excellent completion (94%) and compliance (85%) rates. Importantly, it appeared that the 3-fold increase in the ratio of erythrocyte combined EPA + DHA to AA is associated with change in some breast tissue risk and mechanism of action biomarkers, although confirmation is needed in a placebo-controlled trial. The primary endpoint for the IIB trial would likely be Ki-67 given the observed reduction in median Ki-67 from 2% at baseline to 1% at conclusion, despite lack of change in bioavailable hormones. Cross-sectional and prospective studies in women undergoing diagnostic breast biopsies suggest that women with hyperplasia ± atypia with a Ki-67 of 2% or higher have an increased short-term risk of breast cancer (41, 42). The observed change in Ki-67 while not definitive due to lack of a placebo, permits us to both select a primary endpoint biomarker and determine a likely sample size of approximately 50 women per arm if care is taken to make the cohort as homogenous as possible presuming a minimum baseline of 1.5% to 2%.
Baseline EPA and DHA intakes were only 90 mg per day similar to average U.S. intake and that in our cross-sectional study of high-risk women (10, 23). Despite the marked increase in blood EPA plus DHA to AA ratio, we found no change in blood proinflammatory adipocytokines, a similar result to that reported by Yee and colleagues in a dose-escalation trial (25). Those supplementation studies of EPA + DHA in which change was observed in blood inflammatory markers used either cohorts with baseline evidence of systemic inflammation or assayed cytokines following monocyte activation with lipopolysaccharide (43). We found only a median 1.7% absolute reduction in the risk biomarker mammographic density over the short study period. Whether this was due to the omega-3 supplementation, aging (44), or chance cannot be ascertained since there was no placebo group. A recent cross-sectional study did not find an association between erythrocyte fatty acid composition and mammographic density (45).
We found no effect of EPA and DHA on 32 mRNA transcripts in benign breast tissue of a number of genes of interest including those involved in estrogen signaling, proliferation, and inflammation. This is not surprising as the activity of EPA and DHA is likely to be translational or posttranslational in nature, with alteration of protein spatial location in lipid rafts and inhibition of compartmental translocation (17).
The breast tissue reverse-phase proteomics assay was exploratory with the primary objective of identifying likely mechanisms of action. Given the large number of assays increasing the chance of a false positive, changes we observed are hypothesis generating only. However, similar to findings in preclinical models, EPA and DHA ethyl ester supplementation was observed to decrease cyclin D1 and bcl2, which are downstream from NF-κB and AKT. This suggests a decrease in NF-κB nuclear translocation impairing nuclear signaling (16, 45). Whether this might be due in turn to a reduction in eicosanoid intermediates (we did not measure) or changes in lipid raft structure is uncertain. Reduction in cyclin D1 and phosphorylated PED/PEA15 along with decreases in intermediates active in cytokine and growth factor signaling (STAT 5 and SMAD3), are consistent with reduction in proliferation (46–48). Decreases were also observed in progesterone receptor associated with estrogen-related signaling and matrix-associated proteins implicated in epithelial-to-mesenchymal transition (EMT) such as the adhesion-related protein fibronectin and a phosphorylated myosin-related protein myosin IIa (49).
However, changes in proteins associated with AKT/mTOR were mixed, possibly the result of omega-3 fatty acid induced increases in insulin signaling/sensitivity after an amino acid load (as RPFNAs were performed after a meal) combined with inhibition of some aspects of AKT/mTOR pro-oncogenic signaling. We observed increases in pPDK1, pAKT, pTSC2, eEF2K, and pE-BP1, suggesting a permissive effect on mTORC1 and an increase in protein synthesis. Absence of an increase in p70S6K, 4E-BP1 phosphorylated at serine 65, or phosphorylated S6 suggests lack of strong oncogenic activity (50–52). In fact, the observed decreases in eIF4E and several other proteins necessary for mTOR signaling such as Raptor, PRAS40, cyclin D1, and stearoyl CoA desaturase (SCD), a key enzyme in fatty acid synthesis, suggest reduction in mTOR-associated cell proliferation and growth (53, 54). A mixed effect pattern with failure of DHA to block PDK1 phosphorylation of AKT despite reduction in mammary carcino-genesis has previously been observed in preclinical models (15).
In summary, EPA plus DHA ethyl esters in a total dose of 3.4 g per day for 6 months resulted in a significant increase in the EPA+DHA: AA ratio in blood and benign breast tissue. We observed a reduction in benign breast epithelial proliferation (Ki-67) and the frequency of cytologic evidence of atypia, concomitant with change in a number of breast proteins of which several have previously been identified as important in breast carcinogenesis. Although these single-arm pilot results must be viewed with caution and modulation of risk and mechanism of action biomarkers confirmed, the 46% uptake and excellent completion (94%) and compliance (85%) rates suggest that a phase II B placebo-controlled trial is feasible.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by grants from the Breast Cancer Research Foundation and the Kansas Bioscience Authority (to C.J. Fabian; KUMC) and CCSG grant (P30 CA16672) from NIH (to G.B. Mills; MD Anderson Cancer Center). GlaxoSmithKline provided the Lovaza for the study.
S.E. Carlson reports receiving commercial research grant from Mead Johnson Nutrition, has honoraria from speakers bureau from DSM and Mead Johnson Nutrition, and is a consultant/advisory board member for DSM.
Footnotes
Note: Supplementary data for this article are available at Cancer Prevention Research Online (http://cancerprevres.aacrjournals.org/).
Authors' Contributions: Conception and design: C.J. Fabian, B.F. Kimler, B.K. Petroff, S.D. Hursting
Development of methodology: C.J. Fabian, B.F. Kimler, G.B. Mills D.K. Sullivan, B.K. Petroff
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): C.J. Fabian, T.A. Phillips, J.A. Box, A.L. Kreutzjans, S.E. Carlson, B.H. Hidaka, T. Metheny, K.R. Powers, B.K. Petroff, W.L. Hensing, S.D. Hursting
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): B.F. Kimler, T.A. Phillips, S.E. Carlson, G.B. Mills, B.L. Fridley, S.D. Hursting
Writing, review, and/or revision of the manuscript: C.J. Fabian, B.F. Kimler, T.A. Phillips, J.A. Box, S.E. Carlson, B.H. Hidaka, G.B. Mills, D.K. Sullivan, B.K. Petroff, W.L. Hensing, S.D. Hursting
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): C.J. Fabian, B.F. Kimler, J.A. Box, C.M. Zalles, G.B. Mills, B.K. Petroff, W.L. Hensing
Study supervision: C.J. Fabian, B.F. Kimler, B.K. Petroff, S.D. Hursting
Other (cytopathology consultant): C.M. Zalles
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed by the other authors.
References
- 1.Baumgarten SC, Frasor J. Minireview Inflammation: an instigator of more aggressive estrogen receptor (ER) positive breast cancers. Mol Endocrinol. 2012;26:360–71. doi: 10.1210/me.2011-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussein MR, Hassan HI. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations. J Clin Pathol. 2006;59:972–7. doi: 10.1136/jcp.2005.031252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott RS, Beuvon F, Pauly M, Pallud C, Vincent-Salomon A, Mosseri V, et al. Tumor antigens and antigen-presenting capacity in breast cancer. Pathobiology. 2002–2003;70:324–9. doi: 10.1159/000071272. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–5S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 5.Wen ZH, Su YC, Lai PL, Zhang Y, Xu YF, Zhao A, et al. Critical role of arachidonic acid-activated mTOR signaling in breast carcinogenesis and angiogenesis. Oncogene. 2013;32:160–70. doi: 10.1038/onc.2012.47. [DOI] [PubMed] [Google Scholar]
- 6.Weylandt KH, Chiu CY, Gomolka B, Waechter SF, Wiedenmann B. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012;97:73–82. doi: 10.1016/j.prostaglandins.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Pollard J. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–30. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (May-wood) 2008;233:674–88. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W, Zhu Z, McGinley JN, El Bayoumy K, Manni A, Thompson HJ. Identification of a molecular signature underlying inhibition of mammary carcinoma growth by dietary N-3 fatty acids. Cancer Res. 2012;72:3795–806. doi: 10.1158/0008-5472.CAN-12-1047. [DOI] [PubMed] [Google Scholar]
- 10.National Research Council. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (Macronutrients) Washington DC: The National Academies Press; 2005. [Google Scholar]
- 11.Chajès V, Torres-Mejía G, Biessy C, Ortega-Olvera C, Angeles-Llerenas A, Ferrari P, et al. ω-3 and ω-6 polyunsaturated fatty acid intakes and the risk of breast cancer in Mexican women: impact of obesity status. Cancer Epidemiol Biomarkers Prev. 2012;21:319–26. doi: 10.1158/1055-9965.EPI-11-0896. [DOI] [PubMed] [Google Scholar]
- 12.Goodstine SL, Zheng T, Holford TR, Ward BA, Carter D, Owens PH, et al. Dietary (n-3)/(n-6) fatty acid ratio: possible relationship to premenopausal but not postmenopausal breast cancer risk in U.S. women. J Nutr. 2003;133:1409–14. doi: 10.1093/jn/133.5.1409. [DOI] [PubMed] [Google Scholar]
- 13.Brenna JT, Salem N, Jr, Sinclair AJ Cunnane SCInternational Society for the Study of Fatty Acids and Lipids, ISSFAL. alpha-linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Yee LD, Agarwal D, Rosol TJ, Lehman A, Tian M, Hatton J, et al. The inhibition of early stages of HER-2/neu-mediated mammary carcinogenesis by dietary n-3 PUFAs. Mol Nutr Food Res. 2013;57:320–7. doi: 10.1002/mnfr.201200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Zhang Y, Jia C, Wang Y, Lai P, Zhou X, et al. mTORC1/2 targeted by n-3 polyunsaturated fatty acids in the prevention of mammary tumorigenesis and tumor progression. Oncogene. 2014;33:4548–57. doi: 10.1038/onc.2013.402. [DOI] [PubMed] [Google Scholar]
- 16.Denys A, Hichami A, Khan NA. n-3 PUFAs modulate T-cell activation via protein kinase C-alpha and -epsilon and the NF-kappa B signaling pathway. J Lipid Res. 2005;46:752–8. doi: 10.1194/jlr.M400444-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Turk HF, Chapkin RS. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2013;88:43–7. doi: 10.1016/j.plefa.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee EJ, Yun UJ, Koo KH, Sung JY, Shim J, Ye SK, et al. Down-regulation of lipid raft-associated onco-proteins via cholesterol-dependent lipid raft internalization in docosahexaenoic acid-induced apoptosis. Biochim Bio-phys Acta. 2014;1841:190–203. doi: 10.1016/j.bbalip.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Ravacci GR, Brentani MM, Tortelli T, Jr, Torrinhas RS, Saldanha T, Torres EA, et al. Lipid raft disruption by docosahexaenoic acid induces apoptosis in transformed human mammary luminal epithelial cells harboring HER-2 overexpression. J Nutr Biochem. 2013;24:505–15. doi: 10.1016/j.jnutbio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Wahle KW, Rotondo D. Fatty acids and endothelial cell function: regulation of adhesion molecule and redox enzyme expression. Curr Opin Clin Nutr Metab Care. 1999;2:109–15. doi: 10.1097/00075197-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Bhaswant M, Poudyal H, Brown L. Mechanisms of enhanced insulin secretion and sensitivity with n-3 unsaturated fatty acids. J Nutr Biochem. 2015;26:571–84. doi: 10.1016/j.jnutbio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Zou Z, Bellenger S, Massey KA, Nicolaou A, Geissler A, Bidu C, et al. Inhibition of the HER2 pathway by n-3 polyunsaturated fatty acids prevents breast cancer in fat-1 transgenic mice. J Lipid Res. 2013;54:3453–63. doi: 10.1194/jlr.M042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hidaka BH, Li S, Harvey KE, Carlson SE, Sullivan DK, Kimler BF, et al. Omega-3 and omega-6 fatty acids in blood and breast tissue of high-risk women and association with atypical cytomorphology. Cancer Prev Res. 2015;8:359–64. doi: 10.1158/1940-6207.CAPR-14-0351. [DOI] [PubMed] [Google Scholar]
- 24.Fabian CJ, Kimler BF, Zalles CM, Klemp JR, Kamel S, Zeiger S, et al. Short-term breast cancer prediction by random periareolar fine-needle aspiration cytology and the Gail risk model. J Natl Cancer Inst. 2000;92:1217–27. doi: 10.1093/jnci/92.15.1217. [DOI] [PubMed] [Google Scholar]
- 25.Yee LD, Lester JL, Cole RM, Richardson JR, Hsu JC, Li Y, et al. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am J Clin Nutr. 2010;91:1185–94. doi: 10.3945/ajcn.2009.29036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witt PM, Christensen JH, Ewertz M, Aardestrup IV, Schmidt EB. The incorporation of marine n-3 PUFA into platelets and adipose tissue in pre- and postmenopausal women: a randomised, double-blind, placebocontrolled trial. Br J Nutr. 2010;104:318–25. doi: 10.1017/S0007114510000371. [DOI] [PubMed] [Google Scholar]
- 27.Moore MR, King RA. Effects of omega-3 fatty acids on progestin stimulation of invasive properties in breast cancer. Horm Cancer. 2012;3:205–17. doi: 10.1007/s12672-012-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabian CJ, Kimler BF, Phillips TA, Nydegger JL, Kreutzjans AL, Carlson SE, et al. Modulation of breast cancer risk biomarkers by high dose omega-3 fatty acids: Phase II pilot study in post-menopausal women. Cancer Prev Res. 2015;8:922–31. doi: 10.1158/1940-6207.CAPR-14-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Available from: http://bcra.nci.nih.gov/brc/
- 30.Masood S, Frykberg ER, McLellan GL, Scalapino MC, Mitchum DG, Bullard JB. Prospective evaluation of radiologically directed fine needle aspiration biopsy of nonpalpable breast lesions. Cancer. 1990;66:1480–7. doi: 10.1002/1097-0142(19901001)66:7<1480::aid-cncr2820660708>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 31.Fabian CJ, Kimler BF, Phillips TA, Donnelly JE, Sullivan DK, Petroff BK, et al. Favorable modulation of benign breast tissue and serum risk bio-markers is associated with >10% weight loss in postmenopausal women. Breast Cancer Res Treat. 2013;142:119–32. doi: 10.1007/s10549-013-2730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Available from: http://appliedresearch.cancer.gov/DHQ2/forms
- 33.Stone J, Gunasekara A, Martin LJ, Yaffe M, Minkin S, Boyd NF. The detection of change in mammographic density. Cancer Epidemiol Biomarkers Prev. 2003;12:625–30. [PubMed] [Google Scholar]
- 34.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97:448–56. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 35.Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, et al. DHA supplementation and pregnancy outcomes. Am J Clin Nutr. 2013;97:808–15. doi: 10.3945/ajcn.112.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy JC, Matthews DR, Hermans MP. Correct Homeostasis Model Assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 37.Available from: https://www.dtu.ox.ac.uk/homacalculator/.
- 38.Phillips TA, Fabian CJ, Kimler BF, Petroff BK. Assessment of RNA in human breast tissue sampled by random periareolar fine needle aspiration and ductal lavage and processed as fixed or frozen specimens. Reprod Biol. 2013;13:75–81. doi: 10.1016/j.repbio.2013.01.179. [DOI] [PubMed] [Google Scholar]
- 39.Murph MM, Smith DL, Hennessy B, Lu Y, Joy C, Coombes KR, et al. Individualized molecular medicine:linking functional proteomics to select therapeutics targeting the PI3K pathway for specific patients. Adv Exp Med Biol. 2008;622:183–95. doi: 10.1007/978-0-387-68969-2_15. [DOI] [PubMed] [Google Scholar]
- 40.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue:an 18-month controlled study. J Lipid Res. 1997;38:2012–22. [PubMed] [Google Scholar]
- 41.Shaaban AM, Sloane JP, West CR, Foster CS. Breast cancer risk in usual ductal hyperplasia is defined by estrogen receptor-alpha and Ki-67 expression. Am J Pathol. 2002;160:597–604. doi: 10.1016/s0002-9440(10)64879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santisteban M, Reynolds C, Barr Fritcher EG, Frost MH, Vierkant RA, Anderson SS, et al. Ki67: a time-varying biomarker of risk of breast cancer in atypical hyperplasia. Breast Cancer Res Treat. 2010;121:431–7. doi: 10.1007/s10549-009-0534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Rev Biochim Biophys Acta. 2015;1851:469–84. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 44.McCormack VA, Perry NM, Vinnicombe SJ, Dos Santos Silva I. Changes and tracking of mammographic density in relation to Pike's model of breast tissue aging: a UK longitudinal study. Int J Cancer. 2010;127:452–61. doi: 10.1002/ijc.25053. [DOI] [PubMed] [Google Scholar]
- 45.Hudson AG, Reeves KW, Modugno F, Wilson JW, Evans RW, Vogel VG, et al. Erythrocyte omega-6 and omega-3 fatty acids and mammographic breast density. Nutr Cancer. 2013;65:410–6. doi: 10.1080/01635581.2013.760744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghosh-Choudhury T, Mandal CC, Woodruff K, St Clair P, Fernandes G, Choudhury GG, et al. Fish oil targets PTEN to regulate NF kappa B for downregulation of anti-apoptotic genes in breast tumor growth. Breast Cancer Res Treat. 2009;118:213–28. doi: 10.1007/s10549-008-0227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarasewicz E, Jeruss JS. Phospho-specific Smad3 signaling: impact on breast oncogenesis. Cell Cycle. 2012;11:2443–51. doi: 10.4161/cc.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiory F, Formisano P, Perruolo G, Beguinot F. Frontiers: PED/PEA-15, a multifunctional protein controlling cell survival and glucose metabolism. Am J Physiol Endocrinol Metab. 2009;297:E592–601. doi: 10.1152/ajpendo.00228.2009. [DOI] [PubMed] [Google Scholar]
- 49.Derycke L, Stove C, Vercoutter-Edouart AS, De Wever O, Dollé L, Colpaert N, et al. The role of non-muscle myosin IIA in aggregation and invasion of human MCF-7 breast cancer cells. Int J Dev Biol. 2011;55:835–40. doi: 10.1387/ijdb.113336ld. [DOI] [PubMed] [Google Scholar]
- 50.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–90. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaul G, Pattan G, Rafeequi T. Eukaryotic elongation factor-2 (eEF2): its regulation and peptide chain elongation. Cell Biochem Funct. 2011;29:227–34. doi: 10.1002/cbf.1740. [DOI] [PubMed] [Google Scholar]
- 52.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, et al. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–64. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li BD, McDonald JC, Nassar R, De Benedetti A. Clinical outcome in stage I to III breast carcinoma and eIF4E overexpression. Ann Surg. 1998;227:756–63. doi: 10.1097/00000658-199805000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Havel JJ, Li Z, Cheng D, Peng J, Fu H. Nuclear PRAS40 couples the Akt/mTORC1 signaling axis to the RPL11-HDM2-p53 nucleolar stress response pathway. Oncogene. 2015;34:1487–98. doi: 10.1038/onc.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.