Abstract
Background:
Manganese (Mn) is an essential metal that can become neurotoxic at elevated levels with negative consequences on neurodevelopment. We have evaluated the influence of single nucleotide polymorphisms (SNPs) in Mn transporter genes SLC30A10 and SLC39A8 on Mn concentrations in dentine, a validated biomarker that reflects Mn tissue concentrations early in life.
Methods:
The study included 195 children with variable environmental Mn exposure. Mn concentrations in dentine representing fetal, early postnatal and early childhood developmental periods were measured using laser ablation-inductively coupled plasma mass spectrometry. SLC30A10 rs12064812 (T/C) and SLC39A8 rs13107325 (C/T) were genotyped by TaqMan real time PCR and SLC30A10 rs1776029 (G/A) by pyrosequencing; and SNPs were analyzed in association with Mn in dentine.
Results:
SLC39A8 rs13107325 rare allele (T) carriers had significantly higher Mn concentrations in postnatal dentine (110%, p = 0.008). For all SNPs we also observed non-significant associations with Mn concentrations in dentine in opposite directions for fetal and early postnatal periods. Furthermore, there were significant differences in the influence of SLC30A10 rs1776929 genotypes on Mn concentrations in dentine between sexes.
Discussion:
The findings from this study indicate that common SNPs in Mn transporters influence Mn homeostasis in early development and may therefore be important to consider in future studies of early life Mn exposure and health effects. Our results also suggest that the influence of these transporters on Mn regulation may differ by developmental stage, as well as between girls and boys.
Keywords: Manganese homeostasis, Manganese neurotoxicity, Manganese transporter genetics, Teeth biomarkers, SLC30A10, SLC39A8
1. Introduction
Manganese (Mn) is an essential nutrient involved in a number of physiological processes in humans (Jitrapakdee et al., 2008; Reddi et al., 2009; Takeda, 2003). Mn is required for normal brain function but can become neurotoxic at elevated concentrations (Tuschl et al., 2013) and there appears to be a narrow window between deficiency, essential dose and toxicity in early neurological development (Claus Henn et al., 2010). Elevated internal Mn can occur via exposure from a range of sources including naturally elevated levels in drinking water (Rahman et al., 2016) and exposure from soil, air and dust as a consequence of industrial pollution (Boudissa et al., 2006; Pavilonis et al., 2015). Animal and human studies have shown that Mn crosses the placenta (Krachler et al., 1999) and maternal exposure may therefore reach the fetus.
Several studies have suggested that environmental Mn exposure can interfere with children’s neurodevelopment and has been linked to cognitive, motor and behavioral deficits (Zoni and Lucchini, 2013), and may also modify the neurotoxic effects of other metals, including lead (Claus Henn et al., 2012). Several studies have also shown sex differences in the health effects of Mn exposure (Gunier et al., 2015; Menezes-Filho et al., 2011; Riojas-Rodriguez et al., 2010), suggesting that there may be important differences in Mn homeostasis and toxicity between girls and boys. Furthermore, studies in animals and humans have indicated differences in the effects of exposure related to the developmental stage at which the exposure was experienced (Betharia and Maher, 2012; Gunier et al., 2015; Mora et al., 2015).
In addition to the effects of exposure, internal manganese concentrations can be influenced by variations in genes involved in Mn regulation. Recent studies have shown that common single nucleotide polymorphisms (SNPs) in Mn transporter genes SLC30A10 (efflux transporter) and SLC39A8 (influx transporter) modify Mn concentrations in adults and are also associated with neurological symptoms (Ng et al., 2015; Pickrell et al., 2016; Wahlberg et al., 2016). These finding suggests that SNPs in Mn transporters may influence the brain, possibly via interference with Mn homeostasis.
In the presents study we have investigated the influence of common SNPs in transporter genes SLC30A10 and SLC39A8 on Mn concentrations in tooth dentine from early childhood and evaluated potential differences in genetic influence between different developmental stages (prenatal, early postnatal and early childhood) and between sexes. For this purpose we have applied newly-developed and validated tooth-matrix Mn biomarkers that can directly measure prenatal and early childhood Mn uptake and which has been shown to reflect early-life Mn exposure (Arora et al., 2012). We undertook this study in a well-characterized study of Italian children (n=195) with different background exposures to Mn.
2. Methods
2.1. Study population
The full study cohort consists of 721 adolescents, aged 10–14 years, residing in the three different districts of Valcamonica valley, Bagnolo Mella and Lake Garda within the Province of Brescia, Italy. Valcamonica has a long history of ferromanganese alloy plants which were active in the period 1902–2001, while Bagnolo Mella has ongoing exposure from a plant that has been active since 1973. In contrast, Lake Garda has no history of ferroalloy plants. A first round of recruitment including 311 children from Valcamonica and Lake Garda was completed in 2010 and included sampling of air, water,soil and dust and biomarkers blood, urine, saliva, hair and nails. A second round of recruitment of 410 children from Valcamonica, Lake Garda and Bagnolo Mella took place 2010–2014 and for some of these children shed baby teeth were collected for analysis for metals.
Mn exposure of these children has been carefully monitored by measurements of Mn concentrations in samples from the their home environment and shown increased Mn concentrations in samples from the Valcamonica and Bagnolo Mella compared to Lake Garda (Borgese et al., 2011, 2013; Lucas et al., 2015). The children have also undergone comprehensive neurological and neuropsychological testing at adolescent age, including tests for IQ, motor function and behaviour; however, neurological outcomes were not in included in the present study. Sex of the child was reported in connection with neurological testing. The study design, information about study aims and forms for informed consents were reviewed and approved by the ethics committees of the local Public Health agencies of Valcamonica and Brescia, and carefully explained to participants before recruitment. The present study involves a sub-cohort of 195 children for which Mn concentrations in teeth were measured.
2.2. Tooth metal analysis
Our approach to measuring metals in teeth using laser ablation-inductively coupled plasma mass spectrometry (LA-ICP-MS) and assigning developmental times has been detailed elsewhere (Arora and Austin, 2013; Austin et al., 2013). Briefly, we used the neonatal line (a histological feature formed in enamel and dentine at birth) and daily growth incremental markings to assign temporal information to sampling points. The neonatal line (or birth line) provides a landmark that distinguishes the prenatal and postnatal compartments of teeth. Additional microscopic analysis allows us to identify specific regions of teeth that develop at different ages. These regions of teeth are then sampled using a laser coupled with a mass spectrometer (Arora and Austin, 2013; Austin et al., 2013). Prenatal dentine was sampled from primary dentine (cuspal to the neonatal line) that forms during the second trimester until birth. Postnatal dentine was sampled from primary dentine (cervical to the neonatal line) that forms from birth to approximately 1.5–11 months of age depending on tooth type. Early childhood dentine was sampled from secondary dentine that forms from 1.5–10 years of age depending on tooth type (Berkovitz et al., 2009).
The laser ablation unit used was a New Wave Research NWR-193 system (ESI, USA) equipped with an excimer argon-fluoride laser emitting a nanosecond laser pulse with a wavelength of 193 nm. An approximately 1 m length of Tygon® tubing (i.d. 3 mm) connected the laser ablation unit to an Agilent Technologies 8800 ICP-MS. The instrument was fitted with an ‘s’ lens system for enhanced sensitivity. The system was tuned daily for sensitivity using NIST SRM 612 (trace elements in glass). Polyatomic oxide interference was evaluated and minimized by monitoring the Th+/ThO+ (m/z 232/248) ratio. Typical oxide formation was consistently under 0.3%. The NIST SRM 612 glass standard was run every day to tune the instrument and multiple times during the day to check for signal stability. Ca was also used as an internal standard to account for variation in the mineral content of teeth and any signal drift between runs.
Using the laser we sampled 30 sampling points in primary dentine and 10 sampling points in secondary dentine. Data were analyzed as 55Mn:43Ca ratios to control for any variations in mineral content within a tooth and between samples. The limit of detection (LOD) was 0.001 55Mn:43Ca.
2.3. Genetic analysis
Briefly, DNA was extracted from whole blood using the QIAamp DNA Blood Mini kit (Qiagen, Hilden, Germany). Genotyping of rs12064812 and rs13107325 was performed by TaqMan Real Time PCR using predesigned assays (Thermo Scientific assay IDs C_32155052_10 and C_____1827682_10 respectively) as previously described (Wahlberg et al., 2016). Reactions were performed in 5 μl total volumes containing 10 ng of DNA, and analysed on the ABI 7900HT Fast Real Time PCR System (Applied Biosystems, Thermo Fisher, Waltham, USA), using manufacturer’s recommended standard conditions.
Rs1776029 was genotyped using pyrosequencing due to its location in a repeat region. This SNP is highly linked to rs2275707 in this cohort, and due to its location in a genomic region with signatures of regulatory elements (i.e. histone acetylation and DNAse I hypersensitivity), it is the more likely causative variant of the two and was therefore selected in this study. The assay was designed by PyroMark Assay Design 2.0 software (Qiagen, Germany) with primers targeting sequences flanking the repeat, which allowed the generation of a specific PCR product for sequencing. The following primer sequences were used: forward primer 5’TTAGTCATACCATGGGTCATGTCT, reverse primer (biotinylated) 5’ACTCTTGGAAGGCATGATGATT and sequencing primer 5’CTCCTGCCTCAGCCT. PCR and sequencing was performed using the PyroMark reagents and PSQ HS96 Pyrosequencing System (Qiagen) according to manufacturer’s recommended protocol.
For all SNPs, >5% of samples were re-analysed to verify genotypes with a 100% agreement between duplicate. Data quality was also assessed by evaluating Hardy-Weinberg equilibrium using the conventional Chi-Square test.
2.4. Statistical methods
Differences in area of residency and allelic distribution between sexes were evaluated using Chi-square test and differences between Mn concentrations in dentine and sex by Mann-Whitney test.
The dentine Mn biomarker provides data as 55Mn counts per sec, which are then normalized to 43Ca as an internal standard to correct for variations in tooth mineral density. The data are thus, 55Mn:43:Ca × 104. These data were explored using univariate scatter plots to identify potential outliers. Subsequently, area under the curve was calculated for prenatal and postnatal time periods to estimate cumulative Mn exposure during these periods. For the cumulative childhood time measure, an average of ten measurements was used as each sampling point approximated the same time period in secondary dentine.
Given that dentine Mn concentrations were not normally distributed, log transformations were used to achieve a more balanced distribution; however, the data still failed to meet assumptions of normality for postnatal and early childhood dentine. Accordingly, two statistical approaches were used to analyze associations between genotypes and Mn: for prenatal dentine, general linear models with ln-transformed values were used while for postnatal and early childhood dentine, non-parametric tests (Kruskal-Wallis or Mann-Whitney) were used. Separate analyses were performed for each age period and, in some analyses, stratified for each sex. Due to a low representation of rare allele homozygotes (TT) for rs13107325 (only 2 individuals), these were combined with the heterozygotes in associations analyses to generate a group representing all carriers of the rare allele.
In order to test for interactions among sex and genotype, an additional analytical approach was implemented whereby Mn values in dentine were dichotomized relative to the median and tested in generalized (logistic) linear models. Factors included in these models included genotype, sex, developmental stage (prenatal, postnatal, early childhood), and genotype*develop mental stage and genotype*sex interactions, with development status modeled as a random (within-subjects repeated measures) factor.
We used two sided p-values and considered our results to be marginally significant at p < 0.1 and significant at p < 0.05. Where interactions in regression models yielded significant effects, raw p-values and bonferroni-adjusted p-values were reported for post hoc pairwise comparisons.
3. Results
3.1. Cohort characteristics
In Table 1, characteristics are presented for children with measurements for at least one of the three dentine markers. The distribution of the children’s residency was fairly even between the three study sites and 75% of children resided in exposed regions (Bagnolo Mella and Valcamonica). For all three SNPs (SLC30A10 rs1776029, rs12064812; SLC39A8 rs13107325), the distributions of genotypes in the cohort were in Hardy-Weinberg equilibrium and allele frequencies were in agreement with publically available data for European populations from the National Centre for Biotechnology Information (NCBI, 2017). We did not observe any significant differences between girls and boys in respect to area of residency, Mn concentrations in dentine or allele frequencies.
Table 1.
Summary statistics for study cohort.
| Girls | Boys | All children | ||
|---|---|---|---|---|
| Number of participants | 105 | 90 | 195 | |
| Distribution between study sites (%) | Bagnolo Mella | 43 | 41 | 42 |
| Valcamonica | 29 | 39 | 33 | |
| Lake Garda | 29 | 20 | 25 | |
| Median manganese conc. in dentinea (5, 95 percentile) | Prenatal | 0.42 | 0.47 | 0.43 |
| (0.23, 0.74) | (0.20, 1.2) | (0.21, 0.85) | ||
| Postnatal | 0.17 | 0.17 | 0.17 | |
| (0.11, 8.9) | (0.10, 8.7) | (0.10, 8.8) | ||
| Cumulative early childhood | 5.59 | 4.29 | 5.48 | |
| (0.00049, 9.3) | (0.00037, 9.1) | (0.00048, 9.3) | ||
| Minor allele frequencies (%) | rs1776029 | 22 | 20 | 21 |
| rs12064812 | 30 | 29 | 29 | |
| rs13107325 | 9 | 12 | 10 |
a Area under curve 55Mn:43Ca × 104 for prenatal and postnatal periods. Average of ten measurements as 55Mn:43Ca counts/sec, for cumulative eraly childhood.
3.2. Associations of SNPs in Mn transporter genes with Mn concentrations in dentine
Mn concentrations in dentine representing prenatal period, early postnatal and early childhood periods were analyzed in association with genotypes and presented in Figs. 1–3. We observed noticeable differences in the associations between dentine Mn concentrations and genotypes between sexes, particularly for rs1776029 in the Mn efflux transporter gene SLC30A10 (see Section below); therefore, in addition to analyses of all children together, we also evaluated associations separately for girls and boys.
Fig. 1.
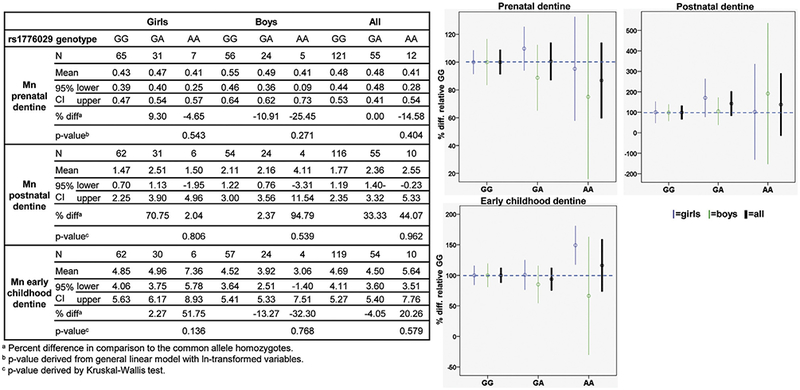
Associations of manganese concentrations in dentine from different developmental stages across SLC30A10 rs1776029 genotypes. Graphs show difference in Mn concentrations in percent between SLC30A10 rs1776029 genotypes with Mn concentrations for the common allele homozygotes (GG) set to 100%.
Fig. 3.
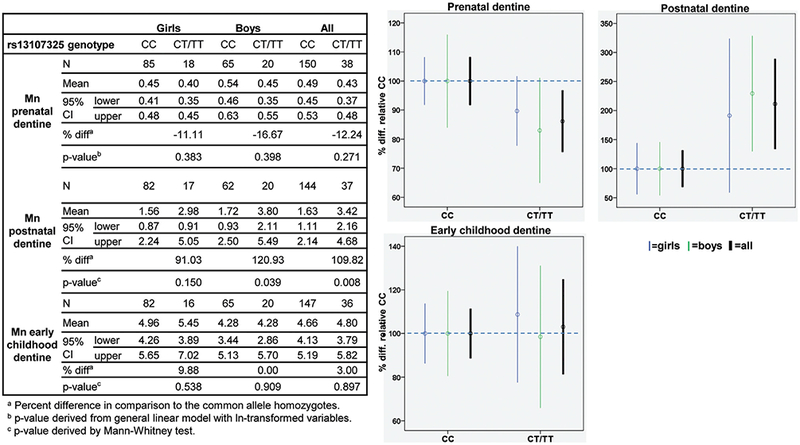
Associations of manganese concentrations in dentine from different developmental stages across SLC39A8 rsl3107325 genotypes. Graphs show difference in Mn concentrations in percent between SLC39A8 rs13107325 genotypes with Mn concentrations for the common allele homozygotes (CC) set to 100%.
3.2.1. SLC30A10 rs1776029
For rs1776029, we observed that the rare allele (A) was weakly associated with decreased Mn concentrations in prenatal dentine for all children and the association was mainly represented by the boys (Fig. 1). The A-allele was also weakly associated with increased Mn in postnatal dentine. However, none of these associations were significant. For early childhood dentine, we observed opposite, but non-significant, associations for girls and boys. The rare allele homozygotes showed a weak association with decrease Mn concentrations for boys and increased Mn concentrations for girls compared to the common allele homozygotes.
3.2.2. SLC30A10 rs12064812
Compared to rs1776029, rs12064812 showed opposite directions of associations with dentine Mn; the rare allele (C) was weakly associated with increased Mn concentrations in prenatal dentine and decreased Mn in postnatal dentine (Fig. 2). The increase in Mn concentrations associated with the C allele in postnatal dentine was represented by a marginally significant increase of 37% (p = 0.067) for the girls, while little association was observed for the boys. For postnatal dentine, the negative association of the rare allele with Mn concentrations was mainly represented by a marginally significant decrease of 69% for the boys (p = 0.077). As for rs1776029, we observed opposite but non-significant associations of early childhood dentine Mn concentrations with genotypes between girls and boys, however, in contrast to rs1776029, the rare allele was associated with increased Mn in boys and decreased Mn in girls.
Fig. 2.
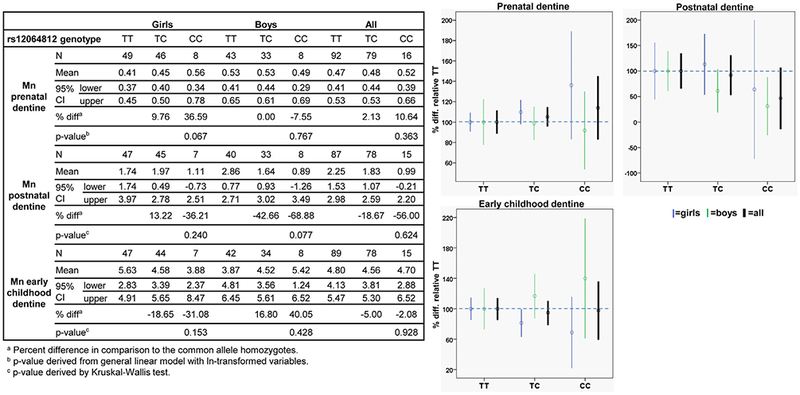
Associations of manganese concentrations in dentine from different developmental stages across SLC30A10 rs12064812 genotypes. Graphs show difference in Mn concentrations in percent between SLC30A10 rs12064812 genotypes with Mn concentrations for the common allele homozygotes (TT) set to 100%.
3.2.3. SLC39A8 rs13107325
For rs13107325 in the Mn in`flux transporter SLC39A8, we observed that the rare allele (T) was weakly negatively associated with Mn concentrations in postnatal dentine, however not significantly (Fig. 3). In contrast, the T-allele was significantly positively associated with Mn concentrations in postnatal dentine for all children (110%, p = 0.008) with the strongest association observed for boys (121%, p = 0.039). We did not observe any noteworthy associations of rs13107325 genotypes with Mn concentrations in dentine representing early childhood.
3.3. Interactions of genotypes with sex and developmental stages
Additional analyses were undertaken to test for interactions between genotype and sex, as well as between genotype and developmental stage in multivariate models. For rs1776029, we found no differences between Mn in dentine from different developmental stages (i.e. prenatal, postnatal and early childhood), but genotype differences were embedded in a sex-dependent interaction (genotype*sex interaction p < 0.0048) with significant differences between girls and boys in dentine Mn within the GG and GA genotypes (for GA only pre-Bonferroni adjustment) (Table 2), which supported the sex-differences observed from associations analyses between genotypes and Mn in dentine.
Table 2.
Pairwise comparison within sex by rs1776029 genotype interaction in multivariate models with dichotomized dentine Mn as the outcome.
| Genotype | Sex | Genotype | Sex | P-valuea | Adjusted p-valueb |
|---|---|---|---|---|---|
| GG | F | GG | M | 0.01 | 0.09 |
| GA | F | GA | M | 0.03 | 0.27 |
| AA | F | AA | M | 0.31 | 1 |
a Values refer to post-hoc comparisons of sex*genotype effects observed in early childhood. The estimate is the associated test statistics (t-value) for a given pairwise comparison.
b Bonferroni-adjusted p-values.
For rs13107325, a marginally significant interaction was observed between genotype and developmental stage (prenatal vs postnatal; p < 0.064), which supported differences in genotype-dentine Mn associations between different developmental stages observed from associations analyses, though we did not confirm this with additional post-hoc tests as the underlying interaction was only marginally significant. No significant interactions were observed for SLC30A10 rs12064812.
4. Discussion
In this study we have evaluated three SNPs in Mn transporter Mn transporter genes SLC30A10.... SLC30A10 and SLC39A8 that have previously been shown to have a significant influence on blood Mn concentrations in adults and have also been associated with neurological symptoms (Ng et al., 2015; Pickrell et al., 2016; Wahlberg et al., 2016). This is however the first study evaluating the influence of these SNPs on Mn regulation in early-life human development and we have observed interesting consistent patterns of associations between these SNPs with Mn concentrations in dentine representative of different early life stages.
Firstly, for all dentine markers, we observe consistently opposite directions of associations for the rs1776029 and rs12064812 rare alleles with Mn concentrations. This pattern is in line with previous studies which have shown that rs1776029 rare allele (A) (representing rs2275707 allele C by linkage) is associated with decreased SLC30A10 gene-expression levels and increased Mn concentrations in blood, while, in contrast, rs12064812 rare allele (C) is weakly associated with increased SLC30A10 gene-expression and decreased Mn concentrations in blood (Wahlberg et al., 2016). These findings indicate that these two non-coding SNPs may alter different gene-regulatory elements and exert opposite effects on SLC30A10 gene-expression, thereby influencing Mn transport capacity differently.
We also observed consistently different trends of genotypic influence on Mn concentrations between prenatal and postnatal dentine. Postnatal dentine showed associations of Mn concentrations with rs1776029 genotypes that are consistent with patterns previously observed in blood of adults where rs1776029 rare allele (A) was associated with higher Mn and rs12064812 rare allele C with lower Mn (Ng et al., 2015; Wahlberg et al., 2016), while for prenatal dentine, the associations were in opposite directions. We hypothesize that this pattern could reflect the new conditions experienced by the child after birth in respect to nutritional uptake and homeostasis. We propose that the influence that we observe in the prenatal dentine may in fact be a weak reflection of the maternal genotype which could be the determinant influencing factor on fetal nutritional status, by affecting the transfer of Mn from mother to child. For instance, the SLC30A10 rs1776029 rare allele (A) has previously been associated with increased Mn concentrations in whole blood as an indication of reduced Mn efflux capacity (Wahlberg et al., 2016). Mothers carrying this allele may have the majority of Mn trapped in the cells and less Mn available for transfer over the placenta with lower fetal Mn concentrations as a consequence. Due to low expression of SLC30A10 in placenta (Genevestigator, 2017; IST online2017), it is less likely that the associations reflect the involvement of SLC30A10 in placental transfer of Mn. A stronger influence of the mother’s SLC30A10 genotype over the child’s own genotype in fetal life is also supported by low gene-expression of SLC30A10 in fetal mice with an upregulation around birth (Genevestigator, 2017). In postnatal life, the observed associations may instead reflect the child’s own capacity of Mn efflux. For rs12064812, the opposite mechanism to rs1776029 could then possibly explain the observed associations, with less Mn bound to the cells and available for transfer in the mother during the fetal period and increased efflux ability by the child in the postnatal period.
The reduced Mn concentrations observed in association with the SLC39A8 influx transporter rare allele (T), which has previously been associated with reduced blood Mn, may reflect lower Mn status in the mother due to her reduced Mn uptake ability and/or a consequence of the fetal genotype causing reduced efficiency of Mn uptake in the fetus. In contrast to SLC30A10, SLC39A8 is highly expressed in human placenta and during the fetal period in mice (Genevestigator, 2017), and may thus be involved in placental transfer of Mn. The strong positive association of this allele with Mn concentrations in the postnatal period is however more difficult to explain; it may be a compensatory reaction to a deficiency in fetal life.
Furthermore, we observe differences in the associations of SNPs in Mn transporters between girls and boys. Generally women in menstruating ages are known to absorb more Mn concentrations than men due to lower iron stores (Finley et al., 1994), which allows increased uptake of Mn via shared transporters such as DMT1 (Au et al., 2008). However, several other studies have shown differences between sexes in Mn related neurotoxic also in early life (Gunier et al., 2015; Mora et al., 2015; Riojas-Rodriguez et al., 2010). Using the same tooth matrix Mn biomarker as the current study, Mora et al. and Gunier et al. showed that girls were generally more sensitive to early life Mn exposure than boys. The mechanisms behind potential sex differences in Mn toxicity in early life are not established- it may be differences in Mn uptake and regulation as suggested here, possibly due to different nutritional demands between girls and boys at certain developmental periods. It has also been suggested that there may be differences between sexes in how metals influences the central nervous system such as interactions with hormones and neurotransmitters (Llop et al., 2013).
Many of the associations described in this study are weak and do not reach significance which may be due to lack of statistical power in the relative small study cohort, the major weakness of the study. The limited power available in this sample is particularly relevant to the analysis of developmental stage- and sex-based interactions, which we accordingly consider exploratory. Nonetheless our detection of significant effects and statistical trends (i.e., where 0.05 < p < 0.1) suggests effects that should be explored in greater detail in future studies. Further, the validity of the associations is supported by the observation of consistent trends which are also in agreement with previous studies of genotypes vs Mn concentrations in adults (i.e. opposite directions for rs1776029 and rs12064812 rare alleles). Another weakness of the study is that exposure was only measured for the study participants in later childhood and we therefore lack exposure data for fetal and early childhood periods when the analyzed dentine was generated. We can therefore not evaluate whether the SNPs modify the relationship between exposure and internal Mn concentrations in early life and thereby potentially contribute to differences in the sensitivity to exposure between individuals which would have provided interesting information on the importance of these SNPs for gene-environment interactions. The strength of the study is the advanced methodology of using teeth Mn biomarkers allowing us to study early human development in a unique and non-invasive manner. Tooth Mn measures have been validated in human and animal studies and are a direct fetal biomarker of exposure (Andra et al., 2016). Having multiple time points of fetal exposure, as opposed to a single measure, allowed us to uncover developmental stage specific differences in Mn uptake across genotypes, something that has not been reported before.
5. Conclusions
In conclusion, the results from this study suggest that common SNPs in Mn transporter genes may be important contributors to Mn concentrations in early human development and that their influence on Mn concentrations may differ between developmental stages as well as between sexes. These SNPs are therefore be important to consider in studies of early-life Mn exposure as they could contribute to differences in the sensitivity to exposure between children.
Acknowledgements
We gratefully acknowledge the participation of all children, parents and teachers who took part in the study and the staff from the University of Brescia for their assistance with data collection. We also acknowledge Daniela Pineda for performing genotyping analyses.
Funding
The project was supported by funding from the European Union through its Sixth Framework Program for RTD [contract no FOODKCTK2006K016253]. It reflects only the authors’ views, and the European Commission is not liable for any use that may be made of the information contained therein. The project was also supported by the National Institute of Environmental Health Sciences (NIEHS) [Award Number R01ES019222, R01ES019222–06A1]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health. Other supporting funding agencies are the Swedish Research Council for Health, Working Life and Welfare (FORTE) and the Karolinska Institutet. The study sponsors had no role in the design, collection, analysis, or interpretation of data, in the writing of this article, or in the decision to submit the article for publication.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- Andra SS, Austin C, Arora M, 2016. The tooth exposome in children’s health research. Curr. Opin. Pediatr 28, 221–227. doi: 10.1097/mop.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M, Austin C, 2013. Teeth as a biomarker of past chemical exposure. Curr. Opin. Pediatr 25, 261–267. doi: 10.1097/MOP.0b013e32835e9084. [DOI] [PubMed] [Google Scholar]
- Arora M, Bradman A, Austin C, Vedar M, Holland N, Eskenazi B, et al. , 2012. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environ. Sci. Technol 46, 5118–5125. doi: 10.1021/es203569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au C, Benedetto A, Aschner M, 2008. Manganese transport in eukaryotes: the role of dmt1. Neurotoxicology 29, 569–576. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin C, Smith TM, Bradman A, Hinde K, Joannes-Boyau R, Bishop D, et al. , 2013. Barium distributions in teeth reveal early-life dietary transitions in primates. Nature 498, 216–219. doi: 10.1038/nature12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betharia S, Maher TJ, 2012. Neurobehavioral effects of lead and manganese individually and in combination in developmentally exposed rats. Neurotoxicology 33, 1117–1127. doi: 10.1016/j.neuro.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Borgese L, Zacco A, Pal S, Bontempi E, Lucchini R, Zimmerman N, et al. , 2011. A new non-destructive method for chemical analysis of particulate matter filters: the case of manganese air pollution in vallecamonica (Italy). Talanta 84, 192–198. doi: 10.1016/j.talanta.2010.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese L, Federici S, Zacco A, Gianoncelli A, Rizzo L, Smith DR, et al. , 2013. Metal fractionation in soils and assessment of environmental contamination in vallecamonica, italy. Environ. Sci. Pollut. Res. Int 20, 5067–5075. doi: 10.1007/s11356-013-1473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudissa SM, Lambert J, Muller C, Kennedy G, Gareau L, Zayed J, 2006. Manganese concentrations in the soil and air in the vicinity of a closed manganese alloy production plant. Sci. Total Environ 361,67–72. doi: 10.1016/j.scitotenv.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Claus Henn B, Ettinger AS, Schwartz J, Tellez-Rojo MM, Lamadrid-Figueroa H, Hernandez-Avila M, et al. , 2010. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology 21, 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Schnaas L, Ettinger AS, Schwartz J, Lamadrid-Figueroa H, Hernandez-Avila M, et al. , 2012. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ. Health Perspect 120, 126–131. doi: 10.1289/ehp.1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JW, Johnson PE, Johnson LK, 1994. Sex affects manganese absorption and retention by humans from a diet adequate in manganese. Am. J. Clin. Nutr 60, 949–955. [DOI] [PubMed] [Google Scholar]
- Gunier RB, Arora M, Jerrett M, Bradman A, Harley KG, Mora AM, et al. , 2015. Manganese in teeth and neurodevelopment in young mexican-american children. Environ. Res 142, 688–695. doi: 10.1016/j.envres.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitrapakdee S, Maurice M, Rayment I, Cleland WW, Wallace JC, Attwood PV, 2008. Structure, mechanism and regulation of pyruvate carboxylase. Biochem. J 413, 369–387. doi: 10.1042/bj20080709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krachler M, Rossipal E, Micetic-Turk D, 1999. Trace element transfer from the mother to the newborn-investigations on triplets of colostrum, maternal and umbilical cord sera. Eur. J. Clin. Nutr 53, 486–494. [DOI] [PubMed] [Google Scholar]
- Llop S, Lopez-Espinosa MJ, Rebagliato M, Ballester F, 2013. Gender differences in the neurotoxicity of metals in children. Toxicology 311, 3–12. doi: 10.1016/j.tox.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Lucas EL, Bertrand P, Guazzetti S, Donna F, Peli M,Jursa TP, et al. , 2015. Impact of ferromanganese alloy plants on household dust manganese levels: implications for childhood exposure. Environ. Res 138, 279–290. doi: 10.1016/j.envres.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Filho JA, Novaes Cde O, Moreira JC, Sarcinelli PN, Mergler D, 2011. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ. Res 111, 156–163. doi: 10.1016/j.envres.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Arora M, Harley KG, Kogut K, Parra K, Hernandez-Bonilla D, et al. , 2015. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the chamacos cohort. Environ. Int 84, 39–54. doi: 10.1016/j.envint.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng E, Lind PM, Lindgren C, Ingelsson E, Mahajan A, Morris A, et al. , 2015. Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum. Mol. Genet 24, 4739–4745. doi: 10.1093/hmg/ddv190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavilonis BT, Lioy PJ., Guazzetti S, Bostick BC, Donna F, Peli M, et al. , 2015. Manganese concentrations in soil and settled dust in an area with historic ferroalloy production.J. Expo. Sci. Environ. Epidemiol 25, 443–450. doi: 10.1038/jes.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell JK, Berisa T, Liu JZ, Segurel L, Tung JY, Hinds DA, 2016. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet 48, 709–717. doi: 10.1038/ng.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman SM, Kippler M, Tofail F, Bolte S, Hamadani JD, Vahter M, 2016. Manganese in drinking water and cognitive abilities and behavior at 10 years of age: a prospective cohort study. Environ. Health Perspect 125, 057003. doi: 10.1289/ehp631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi AR, Jensen LT, Naranuntarat A, Rosenfeld L, Leung E, Shah R, et al. , 2009. The overlapping roles of manganese and cu/zn sod in oxidative stress protection. Free Radic. Biol. Med 46, 154–162. doi: 10.1016/j.freeradbiomed.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riojas-Rodriguez H, Solis-Vivanco R, Schilmann A, Montes S, Rodriguez S, Rios C, et al. , 2010. Intellectual function in mexican children living in a mining area and environmentally exposed to manganese. Environ. Health Perspect 118, 1465–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- `Takeda A, 2003. Manganese action in brain function. Brain Res. Brain Res. Rev 41, 79–87. [DOI] [PubMed] [Google Scholar]
- Tuschl K, Mills PB, Clayton PT, 2013. Manganese and the brain. Int. Rev. Neurobiol 110, 277–312. doi: 10.1016/b978-0-12-410502-7.00013-2. [DOI] [PubMed] [Google Scholar]
- Wahlberg K, Kippler M, Alhamdow A, Rahman SM, Smith DR, Vahter M, et al. , 2016. Common polymorphisms in the solute carrier slc30a10 are associated with blood manganese and neurological function. Toxicol. Sci 149, 473–483. doi: 10.1093/toxsci/kfv252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoni S, Lucchini RG, 2013. Manganese exposure: cognitive, motor and behavioral effects on children: a review of recent findings. Curr. Opin. Pediatr 25, 255–260. doi: 10.1097/MOP.0b013e32835e906b. [DOI] [PMC free article] [PubMed] [Google Scholar]


