Abstract
Nontarget embolization from transarterial liver-directed therapy for hepatocellular carcinoma is a rare complication. We present a case of flank skin ulceration after embolization of the inferior phrenic artery supplying tumor. This complication of inferior phrenic artery embolization underscores caution when using small particles to embolize extrahepatic supply to hepatocellular carcinoma.
Keywords: Transarterial chemoembolization, TACE, Hepatocellular carcinoma, Embolization
Introduction
Hepatocellular carcinoma (HCC) is the leading primary malignancy of the liver, most often occurring in the setting of chronic liver disease [1]. Transarterial chemoembolization is a proven treatment for HCC, and the primary therapy in the setting of multifocal disease [2]. Additional transarterial treatment for HCC includes bland embolization, embolization using drug-eluting microspheres, and radioembolization using beta-emitting microspheres [3], [4], [5]. Extrahepatic vessels can often supply intrahepatic tumors, particularly those along the capsular surface [6], [7]. The right inferior phrenic artery, for example, can supply tumor at the dome or lateral margins of the liver [8], [9]. Extrahepatic vasculature can be targeted for transarterial treatment of liver tumors. With any transarterial therapy, nontarget embolization is a potential complication either by direct or collateral flow. In rare cases, nontarget embolization has resulted in gastrointestinal ulceration, pulmonary infarction, or skin ischemia [10], [11], [12], [13].
Before treatment with yttrium-90 (Y90) microspheres, a planning angiogram is done to map out the arterial anatomy of the liver and to calculate the hepatopulmonary shunt fraction using microaggregated albumin Tc99m. On occasion, if extrahepatic vessels are seen to supply the liver tumors, embolization of these vessels may be performed to facilitate Y90 delivery via intrahepatic arteries [13], [14]. Additionally, embolization of extrahepatic arterial supply to the tumors can help ensure a complete treatment by treating all pertinent arterial feeders. We present a case of flank skin ischemia and ulceration after transarterial embolization of the right inferior phrenic artery using small particles to address extrahepatic arterial supply during a planning angiogram for radioembolization. The danger of small particle embolization of extrahepatic feeders to HCC leading to nontarget ischemic effects is underscored.
Case presentation
Institutional review board approval is not required for single patient retrospective review at our institution. A 66-year-old male with hepatitis C virus-related cirrhosis and portal hypertension was referred for treatment of an infiltrative 10-cm right hepatic lobe HCC (Fig. 1A). The patient was selected for Y90 radioembolization based on consensus recommendations by our multidisciplinary liver tumor board. The patient's liver function was preserved (Child-Pugh-Turcotte score A5), and he had good functional status with no history of ascites, hepatic encephalopathy, or gastrointestinal bleeding.
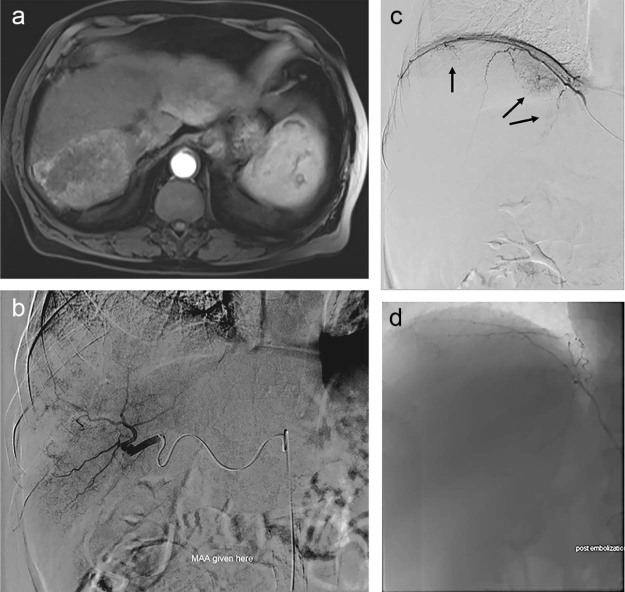
Fig. 1.
(A) Arterial phase MRI showing an infiltrative arterial enhancing mass in the posterior right lobe of the liver. (B) Angiogram from the right posterior division hepatic artery showing hypertrophy of the artery and dominant supply to the right lobe HCC. (C) Angiogram from the right inferior phrenic artery showing supply to the superior and medial aspects of the tumor (arrows). (D) Postembolization angiogram showing decreased tumor blush supplied by the right inferior phrenic artery. HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging.
Planning angiography demonstrated dominant tumor arterial supply from the posterior division of the right hepatic artery (Fig. 1B). In addition, angiography of the right inferior phrenic artery showed approximately 10% supply to the overall tumor burden (Fig. 1C). Particle embolization of the right inferior phrenic supply was performed at the time of planning angiography with plans for subsequent Y90 treatment to the dominant hepatic arterial supply. One-hundred micron microspheres were used to embolize the right inferior phrenic artery vascular supply to the right lobe HCC. Postembolization angiography showed decreased tumor enhancement and preserved antegrade flow in the right inferior phrenic artery (Fig. 1D).
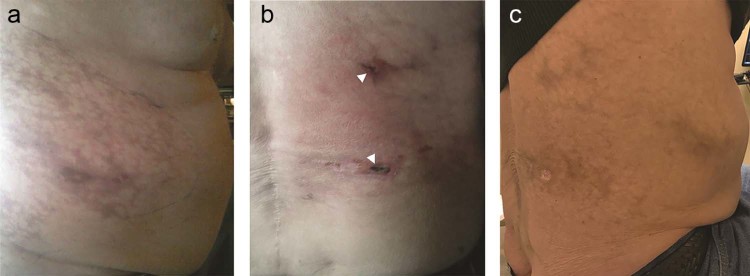
Approximately 8 hours after treatment, the patient reported right flank pain and had slight mottling to the skin in a dermatomal pattern (Fig. 2A). The skin changes worsened over the ensuing several hours, prompting a punch biopsy of the affected area. The biopsy specimen revealed microspheres within the vasculature, suggesting ischemic dermatopathy as the cause of the observed skin changes (Fig. 3). The patient was managed conservatively with emollient application to the affected area for moist healing, and was given oral narcotic pain medication for associated discomfort. The skin was monitored for the next 3 weeks, during which time 2 focal areas of skin ulceration measuring approximately 1 cm each were observed (Fig. 2B). Conservative management continued, and his skin healed over the next 3 months without progressive ulceration or infectious complication, leaving only focal residual pigmentation (Fig. 2C).
Fig. 2.
(A) Right flank mottling immediately after right inferior phrenic artery particle embolization. (B) Photograph of the right flank 3 weeks postembolization. Note two small 1-cm eschars from focal necrosis and ulceration (arrowheads). (C) Photograph of the right flank approximately 3 months later showing healing of the ulcerated areas with residual skin pigmentation.
Fig. 3.
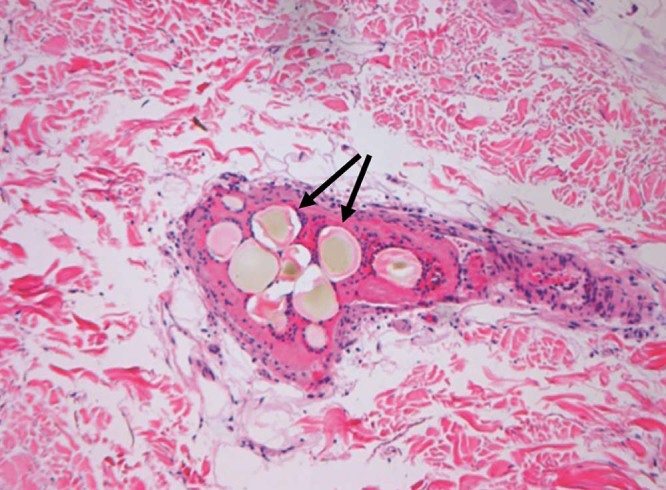
Photomicrograph of biopsy specimen showing microspheres within the epidermal tissue vasculature (arrows). Surrounding blue-stained cells represent reactive perivascular inflammation.
Discussion
Nontarget embolization is a rare complication of transarterial therapies for hepatic neoplasms [10], [13]. Particularly when treating intrahepatic tumors via extrahepatic collateral circulation, caution must be diligently applied to avoid embolization of anatomic perfusion pathways. Structures at risk for such nontarget embolization include the lungs, diaphragm, skin, bowel, and gallbladder [9], [10], [11], [12], [13].
In the case presented herein, embolization of the right inferior phrenic artery (IPA) resulted in right flank skin ischemia and focal ulceration secondary to nontarget embolization. This was presumably through collateral channels with intercostal arteries resulting in embolization of dermal microvasculature. Reports of embolic complications involving the IPA most often involve the diaphragm or lungs, and at times the stomach or esophagus [8]. Skin ulceration from inferior phrenic artery embolization has not been reported. Although pain involving the C3-5 dermatomal area has been described, this was thought to be referred pain from the diaphragm [8]. Collaterals exist between the inferior phrenic and intercostal arteries (see Fig. 4), and intercostal arteries also supply the skin [8], [9]. Miyayama et al. reported a case of skin necrosis from nontarget embolization of the intercostal arteries from transcatheter arterial chemoembolization via the right internal mammary artery [15]. Embolization of the right inferior phrenic artery, therefore, can cause distal embolization to the skin, particularly if small embolic particles are used. When specific branches of extrahepatic parasitized arteries exclusively supplying tumor cannot be catheterized, small particle embolization should be avoided to minimize risk of distal end organ ischemia. In our case, larger diameter particles may have had less risk of distal ischemia via skin collaterals. Recognizing the risk of skin ischemia from embolization of the inferior phrenic artery using small particles will be important for the safe treatment of this often involved extrahepatic vessel in liver directed therapies.
Fig. 4.
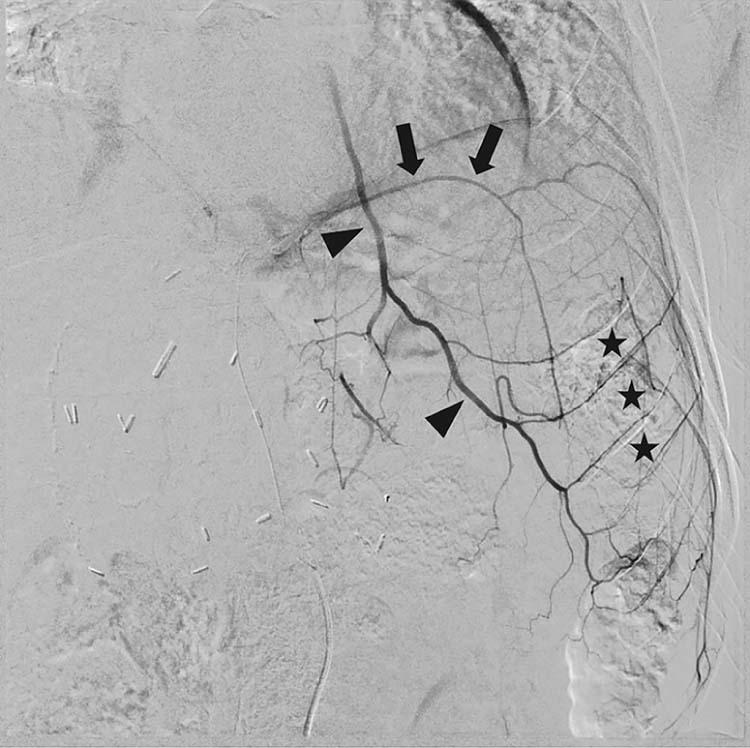
Angiogram of the left inferior phrenic artery (arrows) in a different patient showing collateralization to the left internal mammary artery (arrowheads) and multiple intercostal arteries (asterisks).
Conclusion
Extrahepatic arterial supply to hepatic tumors is occasionally encountered, the most common being the inferior phrenic artery. Embolization of hepatic tumors through extrahepatic vessels can be performed, although with risk of nontargetischemia. Small particles should be avoided when possible during embolization of extrahepatic vessels to minimize this risk.
Footnotes
Competing Interests: Authors have no conflicts of interest to disclose.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Llovet J.M., Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 3.Kallini J.R., Gabr A., Salem R., Lewandowski R.J. Transarterial radioembolization with yttrium-90 for the treatment of hepatocellular carcinoma. Adv Ther. 2016;33(5):699–714. doi: 10.1007/s12325-016-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogl T.J., Lammer J., Lencioni R., Malagari K., Watkinson A., Pilleul F. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;197(4):W562–W570. doi: 10.2214/AJR.10.4379. [DOI] [PubMed] [Google Scholar]
- 5.Hodavance M.S., Vikingstad E.M., Griffin A.S., Pabon-Ramos W.M., Berg C.L., Suhocki P.V. Effectiveness of transarterial embolization of hepatocellular carcinoma as a bridge to transplantation. J Vasc Interv Radiol. 2016;27(1):39–45. doi: 10.1016/j.jvir.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Takada K., Ito T., Kumada T., Toyoda H., Tada T., Sone Y. Extra-hepatic feeding arteries of hepatocellular carcinoma: an investigation based on intra-arterial CT aortography images using an angio-MDCT system. Eur J Radiol. 2016;85(8):1400–1406. doi: 10.1016/j.ejrad.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Chung J.W., Kim H.C., Yoon J.H., Lee H.S., Jae H.J., Lee W. Transcatheter arterial chemoembolization of hepatocellular carcinoma: prevalence and causative factors of extrahepatic collateral arteries in 479 patients. Korean J Radiol. 2006;7(4):257–266. doi: 10.3348/kjr.2006.7.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gwon D.I., Ko G.Y., Yoon H.K., Sung K.B., Lee J.M., Ryu S.J. Inferior phrenic artery: anatomy, variations, pathologic conditions, and interventional management. Radiographics. 2007;27(3):687–705. doi: 10.1148/rg.273065036. [DOI] [PubMed] [Google Scholar]
- 9.Kim H.C., Chung J.W., Lee W., Jae H.J., Park J.H. Recognizing extrahepatic collateral vessels that supply hepatocellular carcinoma to avoid complications of transcatheter arterial chemoembolization. Radiographics. 2005;25(Suppl. 1):S25–S39. doi: 10.1148/rg.25si055508. [DOI] [PubMed] [Google Scholar]
- 10.Gates J., Hartnell G.G., Stuart K.E., Clouse M.E. Chemoembolization of hepatic neoplasms: safety, complications, and when to worry. Radiographics. 1999;19(2):399–414. doi: 10.1148/radiographics.19.2.g99mr08399. [DOI] [PubMed] [Google Scholar]
- 11.Ingraham C.R., Johnson G.E., Nair A.V., Padia S.A. Nontarget embolization complicating transarterial chemoembolization in a patient with hepatocellular carcinoma. Semin Intervent Radiol. 2011;28(2):202–206. doi: 10.1055/s-0031-1280665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhalani S.M., Lewandowski R.J. Radioembolization complicated by nontarget embolization to the falciform artery. Semin Intervent Radiol. 2011;28(2):234–239. doi: 10.1055/s-0031-1280672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed O., Patel M.V., Masrani A., Chong B., Osman M., Tasse J. Assessing intra-arterial complications of planning and treatment angiograms for Y-90 radioembolization. Cardiovasc Intervent Radiol. 2017;40(5):704–711. doi: 10.1007/s00270-016-1555-3. [DOI] [PubMed] [Google Scholar]
- 14.Abdelmaksoud M.H., Louie J.D., Kothary N., Hwang G.L., Kuo W.T., Hofmann L.V. Embolization of parasitized extrahepatic arteries to reestablish intrahepatic arterial supply to tumors before yttrium-90 radioembolization. J Vasc Interv Radiol. 2011;22(10):1355–1362. doi: 10.1016/j.jvir.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Miyayama S., Matsui O., Mitsui T., Minami T., Ryu Y., Ito C. Extrahepatic blood supply to hepatocellular carcinoma: angiographic demonstration and transcatheter arterial chemoembolization. Cardiovasc Intervent Radiol. 2006;29(1):39–48. doi: 10.1007/s00270-004-0287-y. [DOI] [PubMed] [Google Scholar]