Abstract
IN BRIEF This study explored the impact of correcting vitamin D deficiency on blood pressure, metabolic status, and weight loss in patients with fatigue and obesity refractory to conventional interventions such as diet, exercise, behavioral modification, and pharmacotherapy. Correction of vitamin D deficiency in such patients was found to be significantly associated with weight reduction and improved insulin sensitivity.
Vitamin D deficiency is a prevalent but under-recognized health problem. Subclinical deficiency had been noted with high prevalence in adult medical inpatients, postmenopausal women, and healthy young adults (1). Vitamin D is a steroid hormone involved in calcium homeostasis and exists in several forms; the primary circulating form is 25-hydroxyvitamin D (25[OH]D), and the active form is 1,25-dihydroxyvitamin D (1,25[OH]2D) (2). After digestion, vitamin D is processed by 25-hydroxylase in the liver to generate 25(OH)D and is then converted to 1,25(OH)2D by 25-hydroxyvitamin D-1-α-hydroxylase (3). Serum 25(OH)D is used instead of 1,25(OH)2D as a biomarker for assessing vitamin D deficiency because the secondary increase of parathyroid hormone (PTH) induces renal hydroxylation of 25(OH)D with subsequent normalization of 1,25(OH)2D levels despite systemic vitamin D deficiency (4).
Vitamin D deficiency is usually accompanied by normal blood levels of calcium and phosphorus, high-normal or elevated PTH, normal to elevated levels of total alkaline phosphatase (ALP), a low 24-hour urine calcium excretion rate, and a low level of total 25(OH)D (5). Vitamin D insufficiency is defined as a level <30 ng/mL (75 nmol/L), and deficiency is defined as a circulating level <20 ng/mL (50 nmol/L) (6). Vitamin D deficiency is more common than previously thought; the percentage of adults showing vitamin D sufficiency as defined by 25(OH)D ≥30 ng/mL has been declining (7).
It has been postulated that hypovitaminosis D is associated with bone pain, myalgia, generalized weakness, and chronic fatigue (8). Vitamin D deficiency may be a risk factor for depression and other psychological disorders. This may be a result of malfunctions of either the neuroendocrine or central nervous systems (9–11).
Hypocalcemia is a cause of refractory hypotension, especially in patients with chronic renal disease (12). Cardiac performance is reduced by hypocalcemia, with a subsequent decrease in myocardial contractility, ejection fraction, and cardiac index (13).
Vitamin D deficiency may have a role in the prevalence of obesity and the individual differences in its onset and severity. It may be possible to reverse the increasing prevalence of obesity by improving vitamin D status when associated deficiency is confirmed (14). The prevalence of vitamin D deficiency was found to be 35% higher in obese subjects compared to a eutrophic group and 24% higher than in an overweight group (15). This association would be explained by accumulation of vitamin D in the fatty tissue (16). Vitamin D also has anti-adipogenic properties (17), and limited research suggested that vitamin D may potentiate weight loss and improve metabolic markers (18).
Supplementation of vitamin D, together with exercise or mild caloric restriction, had been shown to improve markers of inflammation (19). Vitamin D acts through an intracellular increase in ionized calcium, which stimulates the apoptosis of adipocytes through sympathetic nervous system activation to augment diet-induced thermogenesis and fat oxidation, increasing energy expenditure. It also acts on the gastrointestinal tract to enhance fecal fat excretion and gastrointestinal hormones that control appetite (19).
Vitamin D supplementation will decrease PTH levels, and this will enhance insulin sensitivity and gene modulation (i.e., increase genes controlling lipolysis in favor of genes controlling lipogenesis) (20).
Total daily energy expenditure (TEE) encompasses the resting metabolic rate (RMR), the thermal effect of food, and physical activity. RMR is the component of energy expenditure that explains the largest proportion of total daily energy requirements. Individuals with a low RMR are at higher risk of significant weight gain than those with a high RMR (21,22).
The aim of this study was to evaluate vitamin D status in patients presenting with fatigue and obesity refractory to conventional interventions such as diet, exercise, behavioral modification, and pharmacotherapy and to assess the impact of vitamin D deficiency correction on blood pressure and metabolic status, including weight loss.
Study Design and Methods
Patient Selection
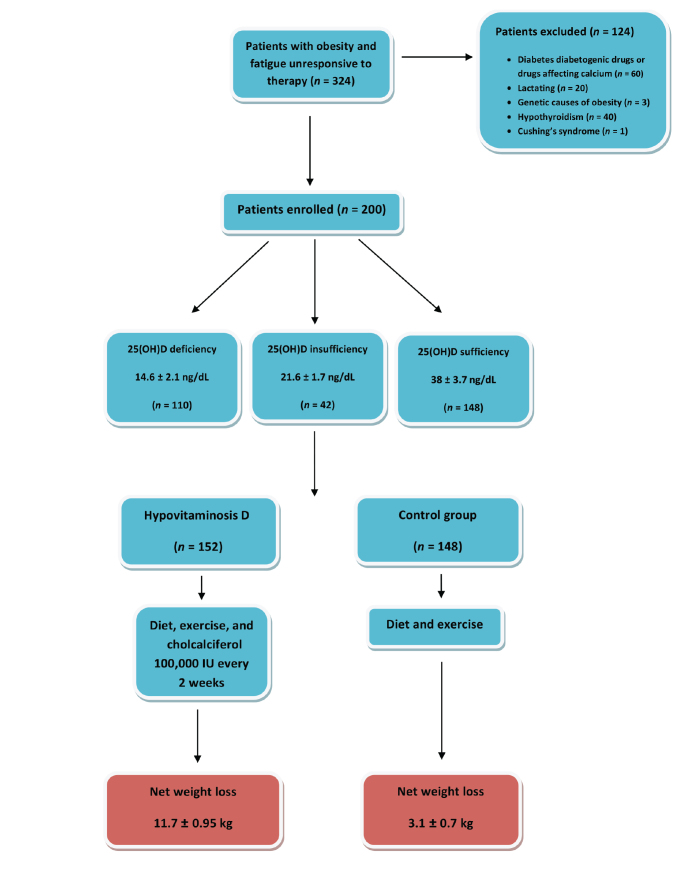
Two hundred patients, aged 20–40 years, were selected after application of the inclusion criteria from 324 patients who were evaluated because of fatigue, hypotension, and refractory obesity, which is defined as failure to achieve weight loss ≥5% of initial body weight after 1 year, despite diet control, regular exercise, behavioral intervention, and pharmacotherapy (23) (Figure 1). Fatigue was defined as the perception of generalized weakness and reduced capacity to maintain activity and difficulties with concentration and memory (24). The study was conducted from February 2014 to October 2016.
FIGURE 1.
Flow diagram of the study patient selection and intervention.
All the patients had tried conventional weight loss interventions, including pharmacotherapies such as maximum tolerated doses of orlistat (120 mg three times daily [n = 144]) or sibutramine (10 mg/day [n = 56]). An age- and sex-matched control group was selected that included patients (n = 148) who were obese and had a deficient level of 25(OH)D. All patients were recruited from the internal medicine outpatient clinic.
The study was conducted at the internal medicine outpatient clinic and Department of Physiology of Zagazig University in Zagazig, Egypt, and was approved by the Zagazig University Ethical Committee. Written informed consent was obtained from patients before their participation in the study.
Potential participants completed a questionnaire regarding their personal and family medical history and drug history. Patients were excluded if their obesity was the result of endocrine causes such as hypothyroidism, genetic causes, pregnancy, lactation, or medications that affect vitamin D status (e.g., calcium, vitamin D, bisphosphonates, or previous treatment with antiviral or immunosuppressive drugs). Patients with hypotension caused by drugs or endocrine causes, diabetes, or medications that affect blood glucose levels (e.g., oral antidiabetic agents, β-blockers, steroids, immunosuppressants, thiazides, or antipsychotics such as clozapine, olanzapine, or risperidone) were also excluded.
Methods
Thorough medical histories and clinical examinations were performed on all patients. Clinical signs of endocrine causes of hypotension and obesity were evaluated. Fatigue was evaluated with regard to characteristics (i.e., onset, course, and duration), as were risk factors for specific diseases such as malignancies, autoimmune diseases, and diabetes. Use of drugs that cause fatigue was ruled out. Fatigue was measured by a visual analog scale that rates patients’ fatigue on a 0–10 scale.
Clinical Evaluation
Clinical evaluation included:
Waist circumference, which was measured in the horizontal plane at the midpoint between the lowest rib and the iliac crest at the end of normal expiration
BMI calculated as weight in kilograms divided by the square of height in meters
Resting energy expenditure (REE), which was calculated by using the Mifflin-St. Jeor equations (Eq. 1 and Eq. 2) before and after vitamin D replacement (25); these equations consider weight, height, and age for both men and women and can efficiently predict measured RMR better than other methods (26).
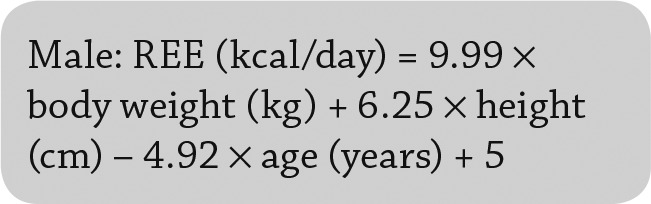 |
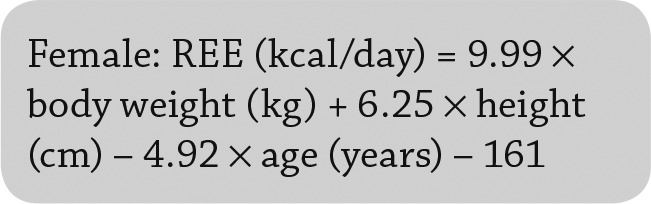 |
TEE, which is calculated according to Eq. 3.
 |
Laboratory Analysis
All patients underwent a 12-hour overnight fast before blood testing, which included:
Investigations for Secondary Causes of Obesity
Liver function tests, including aspartate aminotransferase (normal range 8–34 IU/L), alanine aminotransferase (19–33 IU/L), serum albumin (3.5–5 g/dL), and total bilirubin (0.2–1.2 mg/dL)
Kidney function tests, including serum creatinine (normal range 0.8–1.3 mg/dL) and urea (8–21 mg/dL)
Complete blood count, including hemoglobin (normal range 13–17 g/dL), white blood cell count (4–10 × 109/L), and platelets (150–400 × 109/L)
Fasting blood glucose (normal range 70–99 mg/dL)
Serum sodium (normal range 136–145 mEq/L)
Potassium (normal range 3.5–5.1 mEq/L)
Morning cortisol level (8:00 a.m.; normal range 5–23 µg/dL)
Thyroid-stimulating hormone (TSH; normal range 0.3–5.0 U/mL)
Specific Investigations
Serum 25(OH)D, estimated by Cobas e 411 immunoassay analyzer by a competitive electrochemiluminescence protein binding assay (Roche Diagnostics, Mannheim, Germany). The intra- and inter-assay coefficients of variation were 3.0–7% and 5–13%, respectively. The normal reference range for serum 25(OH)D was ≥30 ng/mL. The lower limit of detection of 25(OH)D was 3 ng/mL.
PTH, estimated by the Elecsys 2010 PTH immunoassay (Roche Diagnostics) (normal range 10–65 pg/mL)
Serum ionized calcium (normal range 4.4–5.4 mg/dL [1.12–1.32 mmol/L])
Total calcium (normal range 8.6–10.2 mg/dL)
Phosphorus (normal range 3–4.5 mg/dL)
ALP (normal range 36–92 IU/L)
24-hour urinary calcium (normal range 20–275 mg/24 hours)
Serum insulin, measured quantitatively by electrochemiluminescence immunoassay (cut-off value 8.64 µIU/mL)
Quantitative insulin sensitivity check index (QUICKI), which measures insulin sensitivity by calculating the inverse of the sum of the logarithmically expressed values of fasting insulin and glucose according to Eq. 4; a value <0.339 indicates reduced insulin sensitivity (27)
 |
Abdominal Ultrasonography
The patients were examined after a 6-hour fast and evaluated for:
-
Fatty liver disease (FLD), via the ultrasonographic steatosis score, which was defined as follows:
❍ Absent steatosis: normal liver echo texture
❍ Mild steatosis: slight and diffuse increase in fine parenchymal echoes with normal visualization of diaphragm and portal vein borders
❍ Moderate steatosis: slightly impaired visualization of portal vein borders and diaphragm
❍ Severe steatosis: poor or no visualization of portal vein borders, diaphragm, and posterior portion of the right lobe (28)
Abdominal adiposity, by estimation of midline abdominal subcutaneous fat thickness (SFT), which was measured 1 cm above the umbilicus during expiration and corresponds to the distance in centimeters between the skin and the anterior surface of the linea alba (normal value 2.64 ± 1.37 cm [29])
Supra renal masses and the presence of ovarian cysts; patients diagnosed with these were excluded
Statistical Analysis
Data were analyzed using SPSS 20 for Windows (SPSS Inc., Chicago, Ill.). Continuous variables were summarized as mean ± SD or SE, as appropriate. A χ2 test was used for categorical variables such as frequency and percentage. Comparison of the response after vitamin D supplementation was performed using a paired t test.
Patients were classified according to their 25(OH)D level into three subgroups: one with sufficient levels (≥30 ng/mL), one with deficiency (<20 ng/mL), and one with insufficiency (20–29 ng/mL). Between-group differences were tested using one-way analysis of variance for continuous data and χ2 tests for categorical data. Multivariate linear regression analysis was used to detect clinical variables independently associated with 25(OH)D concentrations.
Protocol for Therapy and Follow-Up
The patients were exposed to 12 weeks of a 500-kcal deficit diet and mild physical activity, defined as walking for one-half hour on 5 days/week such that their heart rate was 40–54% of their maximum calculated heart rate (220 – age [years]). The patients monitored their pulse rate (30).
The 500-kcal deficit diet was calculated depending on patients’ body weight (kg), height (cm), age, sex, and activity level. Adherence was assessed by daily records of total energy intake and expenditure kept by the study participants and by regular check of weight loss during attendance at behavioral support counseling sessions.
Patients with proven vitamin D deficiency were given vitamin D supplementation with cholecalciferol (vitamin D3) 100,000 IU every 2 weeks for 12 weeks to achieve a blood level of 25(OH)D >30 ng/mL (31).
Physical activity adherence was defined as successful if participants completed a prescribed exercise schedule for at least two-thirds of the time. Self-reports of minutes of exercise were collected, and we followed up on patients’ commitment to perform regular exercise. To guarantee medication adherence, we supervised vitamin D administration.
Patients were evaluated after 12 weeks for weight loss, improvement in insulin sensitivity, and blood pressure.
Results
A total of 200 patients (104 male, 96 female) were enrolled in the study. Mean baseline characteristics of the studied population included body weight 98.6 ± 12.3 kg, height 162.2 ± 13.2 cm, BMI 37.57 ± 1.7 kg/m2, and blood pressure 86.6 ± 5.9/47.3 ± 6.5 mmHg. Ultrasonography revealed a mean SFT of 3.3 ± 1.2 cm, and all patients showed variable degrees of FLD (mild: 43 [21.5%]; moderate: 87 [43.5%]; and severe: 70 [35%]). Moderate and severe FLD were significantly predominant in patients with vitamin D deficiency, as shown in Table 1.
TABLE 1.
Demographic, Laboratory, and Ultrasonographic Characteristics of the Studied Population According to 25(OH)D Level
| 25(OH)D Deficiency (n = 110) | 25(OH)D Insufficiency (n = 42) | 25(OH)D Sufficiency (n = 48) | P* | |
|---|---|---|---|---|
| Age (years) | 27.4 ± 4.1 | 29 ± 4.2 | 27.8 ± 4 | 0.6 |
| Sex (female/male) | 56/54 | 20/22 | 20/28 | 0.8 |
| Weight (kg) | 90.6 ± 4.8 | 92 ± 3.6 | 93.8 ± 3.5 | 0.43 |
| Height (cm) | 162.6 ± 4.2 | 166.6 ± 5.3 | 166.9 ± 5 | 0.19 |
| BMI (kg/m2) | 34.3 ± 1.7 | 33.2 ± 1.3 | 33.7 ± 1.7 | 0.04 |
| Unhealthy diet (yes/no) | 82/28 | 32/10 | 32/16 | 0.003 |
| Blood pressure (mmHg) | ||||
| Systolic | 87 ± 6.4 | 86 ± 5 | 90 ± 6 | 0.58 |
| Diastolic | 47 ± 6.6 | 49 ± 6.4 | 60 ± 6.4 | 0.66 |
| Sodium (mEq/L) | 139 ± 2.9 | 140 ± 3.4 | 139.5 ± 3.1 | 0.52 |
| Potassium (mEq/L) | 4.06 ± 0.36 | 4.1 ± 0.42 | 4.1 ± 0.4 | 0.21 |
| Cortisol (µg/dL) | 13.5 ± 3.7 | 13.4 ± 4.8 | 14.7 ± 5.5 | 0.31 |
| QUICKI | 0.328 ± 0.02 | 0.332 ± 0.02 | 0.336 ± 0.03 | 0.01 |
| TSH (U/mL) | 2.2 ± 0.9 | 2.13 ± 0.85 | 1.9 ± 0.2(SE) | 0.7 |
| Calcium (mg/dL) | 8.23 ± 0.6 | 8.4 ± 0.7 | 9 ± 0.4 | 0.03 |
| Ionized calcium (mg/dL) | 3.6 ± 0.63 | 3.6 ± 0.52 | 4.2 ± 0.6 | 0.02 |
| Urine calcium (mg/24 hours) | 38.2 ± 16 | 71 ± 13.8 | 99.5 ± 27.1 | 0.02 |
| Phosphorus (mg/dL) | 3.68 ± 0.54 | 3.6 ± 0.57 | 4 ± 0.5 | 0.23 |
| ALP (IU/L) | 101 ± 14.6 | 131 ± 13.6 | 79.5 ± 12.7 | 0.09 |
| PTH (pg/mL) | 102.5 ± 16.8 | 85.6 ± 16.4 | 48.1 ± 11 | 0.000 |
| 25OHD (ng/mL) | 12.9 ± 1.8 | 22.6 ± 1.3 | 37.2 ± 2.9 | 0.000 |
| FLD | ||||
| Mild (n = 43) | 8 | 12 | 23 | 0.001 |
| Moderate (n = 87) | 60 | 12 | 15 | 0.001 |
| Severe (n = 70) | 42 | 18 | 10 | 0.001 |
| SFT (cm) | 4.2 ± 0.8 | 3.7 ± 0.6 | 3 ± 0.5 | 0.001 |
Values are expressed as mean ± SD except where otherwise noted.
Bold type denotes statistical significance.
The mean value of 25(OH)D for all patients (n = 200) was 21.6 ± 1.1 (SE) ng/mL; PTH was 81.5 ± 27.5 pg/mL; serum calcium was 8.44 ± 0.63 mg/dL; ionized calcium was 3.78 ± 0.63 mg/dL; ALP was 94.6 ± 16.1 IU/L; and 24-hour urinary calcium was 59.8 ± 3.2 mg/24 hours. TSH, cortisol, sodium, and potassium levels were normal; however, subjects showed reduced insulin sensitivity as indicated by a reduced mean QUICKI value of 0.338 ± 0.03 (n = 120 [60%]). Hypovitaminosis D was prevalent in 152 patients (76%), with a mean value of 16.1 ± 5.3 ng/mL.
Patients were divided into three subgroups according to 25(OH)D level:
25(OH)D deficiency group: 110 patients (56 male, 54 female); vitamin level 12.9 ± 1.8 ng/mL
25(OH)D insufficiency group: 42 patients (24 male, 18 female); vitamin level 22.6 ± 1.3 ng/mL
25(OH)D sufficiency group: 48 patients (20 male, 28 female); vitamin level 37.2 ± 2.9 ng/mL (normal)
Statistically significant differences were noted with regard to BMI (P = 0.04); consumption of an unhealthy diet (defined as a diet that provides high calories derived from fat and sugars with little fiber, protein, minerals, and vitamins) (P = 0.003); QUICKI (P = 0.01); total, ionized, and 24-hour urine calcium (P = 0.03, 0.02, and 0.02, respectively); PTH; 25(OH)D level (P = 0.000); degree of FLD; and SFT (P = 0.001) (Table 1).
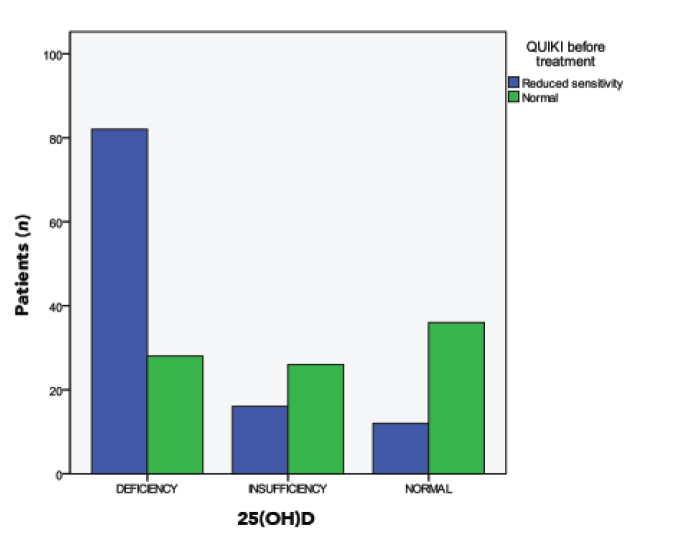
Reduced insulin sensitivity (defined as a QUICKI value <0.339) was evident in 120 patients (60%); it was significantly prevalent in the 25(OH)D deficiency group (82 patients [74.5%]) and insufficiency group (26 patients [61.9%]) (Table 2 and Figure 2). Patients with decreased 25(OH)D formed a hypovitaminosis D subgroup that included 152 patients with 25(OH)D deficiency (n = 110) or 25(OH)D insufficiency (n = 42). Their baseline demographic and laboratory characteristics before weight loss intervention, as well as the characteristics of the control group that included 148 obese patients with hypovitaminosis D, are shown in Table 3.
TABLE 2.
Effect of Correction of Vitamin D Status on Insulin Sensitivity
| Patients With Reduced QUICKI | 25(OH)D Deficiency (n = 110) | 25(OH)D Insufficiency (n = 42) | 25(OH)D Sufficiency (n =48) | P* |
|---|---|---|---|---|
| Before treatment (n [%]) | 82 (74.5) | 26 (61.9) | 12 (25) | 0.0001 (χ2 = 16.118) |
| After vitamin D supplementation (n [%]) | 18(16.4) | 9 (21.4) | 12 (25) | 0.45 (χ2 = 1.62) |
Bold type denotes statistical significance.
FIGURE 2.
Prevalence of reduced insulin sensitivity among the study patients according to 25(OH)D status.
TABLE 3.
Improvement in Metabolic Parameters After Correction of 25OHD Deficiency
| Variable | Hypovitaminosis D (80 Male/72 Female) |
P (Paired t Test) | Control Group (76 Male/72 Female) |
P (Paired t Test) | ||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| Hypovitaminosis D (ng/mL) | 18.4 ± 2.4 | 35.2 ± 4.3 | 0.000 | 19.6 ± 3.9 | 17.8 ± 2.6 | 0.08 |
| Weight (kg) | 93 ± 4.9 | 81.3 ± 3.95 | <0.05 | 92.3 ± 4.5 | 89.2 ± 3.8 | <0.05 |
| BMI (kg/m2) | 34.2 ± 1.4 | 30.6 ± 2.05 | <0.05 | 33.4 ± 0.85 | 32.4 ± 1.1 | NS |
| Waist circumference (cm) | 97.5 ± 6.7 | 91.2 ± 5.3 | <0.05 | 96.8 ± 8.8 | 94.5 ± 5.3 | NS |
| SFT (cm) | 4 ± 0.8 | 2.8 ± 0.6 | <0.05 | 3.2 ± 0.7 | 2.9 ± 0.4 | NS |
| REE (kcal/day) | 1,500 ± 103 | 1,350 ± 56 | NS | 1,760 ± 127 | 1,602 ± 76 | NS |
| TEE (kcal/day) | 2,240 ± 257 | 2,050 ± 109 | NS | 2,570 ± 190 | 2,450 ± 82 | NS |
NS, not significant.
Both the hypovitaminosis D subgroup and the control group were exposed to 12 weeks of a 500-kcal deficit diet and mild activity. The hypovitaminosis D subgroup was also given cholecalciferol 100,000 IU every 2 weeks for 12 weeks. Subjects were evaluated after 12 weeks for weight loss, insulin sensitivity, and blood pressure.
After 12 weeks of vitamin D supplementation with a weight loss intervention, the hypovitaminosis D subgroup showed significant weight loss in both males and females compared to the control group. However, RMR and TEE were not significantly changed in either group (Table 3). Thus, adequate vitamin D status may cause weight loss through enhanced insulin sensitivity without change in TEE (Table 2 and Table 3).
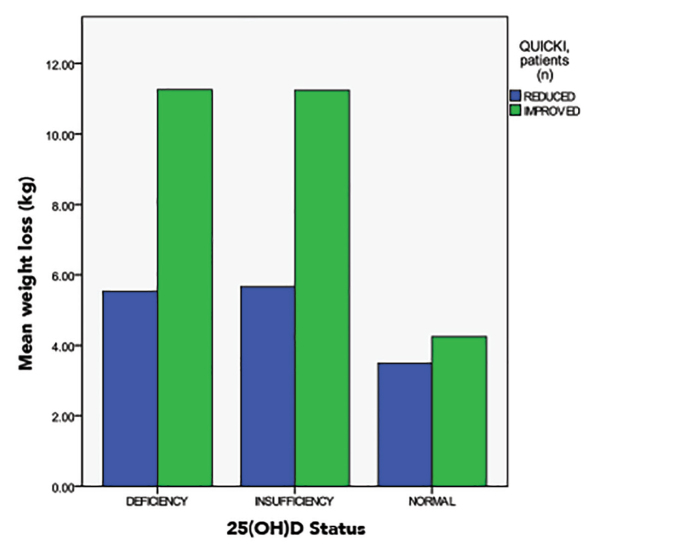
The hypovitaminosis D subgroup showed a highly significant increase in 25(OH)D, significant improvement in insulin sensitivity with a decrease in the number of patients with reduced insulin sensitivity, and significant weight loss (11.7 ± 0.95 kg) as shown in Table 3, Table 4, and Figure 3.
TABLE 4.
Comparison of Clinical and Laboratory Parameters of Hypovitaminosis D Subgroup After Correction of 25(OH)D Deficiency
| Hypovitaminosis D Subgroup |
Paired t Test (95% CI) | ||
|---|---|---|---|
| Before Treatment | After Treatment | ||
| 25(OH)D (ng/mL) | 18.4 ± 2.4 | 35.2 ± 4.3 | 0.001 (–18.12 to –15.9) |
| Blood pressure (mmHg) | |||
| Systolic | 86.5 ± 5.7 | 98.7 ± 6.3 | 0.001 (9.94–14.1) |
| Diastolic | 48 ± 6.5 | 63.2 ± 4.7 | 0.001 (13.8–17.5) |
| QUICKI | 0.330 ± 0.02 | 0.34 ± 0.01 | 0.001 (–0.02 to –0.01) |
| Weight (kg) | 93 ± 4.9 | 81.3 ± 3.95 | 0.006 (1.51–8.9) |
FIGURE 3.
Significant weight loss and improvement in insulin sensitivity in patients with 25(OH)D deficiency after vitamin D supplementation.
The hypovitaminosis D subgroup had a significant reduction in SFT from 4 ± 0.8 to 2.8 ± 0.6 cm after vitamin D supplementation versus a nonsignificant reduction in SFT from 3.2 ± 0.7 to 2.9 ± 0.4 cm in the control group.
Variables most highly correlated with 25(OH)D were QUICKI (r = 0.504, P = 0.000), degree of FLD (r = –0.644, P = 0.000), SFT (r = –0.534, P = 0.000), BMI (r = –0.487, P = 0.000), and PTH (r = –0.842, P = 0.000). Stepwise multivariate linear regression analysis revealed that 25(OH)D is independently associated with PTH level (β= –0.520, P = 0.000), BMI (β= –0.134, P = 0.002), degree of FLD (β = –0.324, P = 0.000), and SFT (β = –0.432, P = 0.000).
Discussion
The vitamin D–parathyroid axis is altered in obesity such that circulating 25(OH)D is reduced in obese individuals (32). Improvement in vitamin D status in obesity is associated with many benefits for general health.
There is a link between vitamin D status, glucose metabolism, and type 2 diabetes; an inverse association between A1C and 25(OH)D has been found, and sufficient vitamin D status provides a protection against type 2 diabetes (33). 25(OH)D deficiency is associated with reduced insulin sensitivity and impaired glucose tolerance; repletion of vitamin D is associated with improvement of this metabolic derangement (34).
A potential mechanism that supports the benefit of vitamin D supplementation is improved insulin secretion, as vitamin D receptors and 1-α-hydroxylase enzymes are expressed in pancreatic β-cells (35). 1,25(OH)2D activates transcription of the human insulin gene, enhancing insulin responsiveness for glucose transport in the muscles, upregulation of glucose transporter 4, and glucose utilization in adipocytes (36). Vitamin D supplementation improves adipose tissue inflammation through enhancing adiponectin, reducing interleukin 6 in adipocytes, and inhibiting the nuclear factor κB pathway and macrophage recruitment (37).
In this study, which enrolled 200 patients with a triad of obesity resistant to conventional measures, hypotension, and fatigue, we measured vitamin D status by detecting 25(OH)D levels and insulin sensitivity by QUICKI. Reduced insulin sensitivity was seen in 60% of patients with hypovitaminosis D (χ2 = 16.118, P = 0.0001).
Because overweight and obese patients with hypovitaminosis D might require higher doses of vitamin D to achieve vitamin D repletion compared to individuals with normal body weight (38), the hypovitaminosis D subgroup was given oral vitamin D in a dose of 100,000 IU every 2 weeks for 12 weeks. In response, this group showed significant weight reduction (11.7 ± 0.95 vs. 3.1 ± 0.7 kg in the control group), and that finding was associated with improved insulin sensitivity and hypotension. Adequate vitamin D status may cause weight loss through enhancement of insulin sensitivity without change in TEE.
Interestingly, our results showed inverse correlations between SFT and severity of FLD and 25(OH)D level.
Mild caloric restriction is an accessible and tolerable intervention and is associated with substantial reduction in insulin needs in patients with type 2 diabetes and insulin resistance (39). Correction of vitamin D level was associated with improved QUICKI from 0.33 to 0.34, weight loss from 93 to 81.3 kg, and SFT reduction from 4 ± 0.8 to 2.8 ± 0.6 cm. In the control group, mild caloric restriction with mild activity led to a nonsignificant weight reduction of 3.1 ± 0.7 kg and a nonsignificant reduction in SFT from 3.2 ± 0.7 to 2.9 ± 0.4 cm.
In a 4-week inpatient study on a metabolic rehabilitation consisting of individualized caloric restriction and aerobic physical exercise in obese subjects (40), researchers assessed the acute effects of 600,000 IU cholecalciferol given orally. Cholecalciferol administration increased 25(OH)D levels (P <0.001) and promoted a significant increase of high molecular weight adiponectin (HMW-A) with a significant decrease of the leptin/HMW-A ratio (P <0.05).
Our study, which was conducted on an adequate number of obese subjects, showed that hypovitaminosis D is prevalent in obesity; 152/200 patients (76%) had an abnormally low vitamin D level. It is not known whether hypovitaminosis D contributes to, or is a complication of, obesity or whether there are parallel interactions between excess fatty tissue and vitamin D activity or bioavailability.
In cases in which 25(OH)D detection is unavailable or cannot be performed because of costs, we can depend accurately on PTH and serum and 24-hour urinary calcium measurements to provide indirect evidence of hypovitaminosis D in obese patients.
In conclusion, recognition and correction of vitamin D deficiency in patients with refractory obesity, fatigue, and hypotension is associated with significant weight reduction and improvements in metabolic derangement and general health.
Acknowledgments
Acknowledgment
The authors thank the doctors and staff of the Internal Medicine and Physiology Department of Zagazig University for their help.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
A.S.H. and H.A.E. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. A.S.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med 2002;112:659–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginde AA, Liu MC, Camargo CA. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med 2009;169:626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006;81:353–373 [DOI] [PubMed] [Google Scholar]
- 4.Binkley N, Ramaurthy R, Krueger D. Low vitamin D status: definition, prevalence, consequences, and correction. Endocrinol Metab Clin North Am 2010;39:287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurt AK, Matthew TD, Daniel LH. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc 2010;85:752–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA, Giovannucci AE, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28 [DOI] [PubMed] [Google Scholar]
- 7.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr 2007;85:6–18 [DOI] [PubMed] [Google Scholar]
- 8.Guo YD, Strugnell S, Black DW, Jones G. Transfected human liver cytochrome P-450 hydroxylates vitamin D analogs at different side-chain positions. Proc Natl Acad Sci U S A 1993;90:8668–8671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DM, Tajar A, O’Neill TW, et al. ; EMAS Study Group. Lower vitamin D levels are associated with depression among community-dwelling men. J Psychopharmacol 2011;25:1320–1329 [DOI] [PubMed] [Google Scholar]
- 10.Cherniack EP, Troen B, Florez HJ, Roos BA, Levis S. Some new food for thought: the role of vitamin D in the mental health of older adults. Curr Psychiatry Rep 2009;11:12–19 [DOI] [PubMed] [Google Scholar]
- 11.Murphy PK, Wagner CL. Vitamin D and mood disorders among women: an integrative review. J Midwifery Womens Health 2008;10:440–446 [DOI] [PubMed] [Google Scholar]
- 12.Ghent S, Judson MA, Rosansky SJ. Refractory hypotension associated with hypocalcemia and renal disease. Am J Kidney Dis 1994;23:430–432 [DOI] [PubMed] [Google Scholar]
- 13.Hurley K, Baggs D. Hypocalcemic cardiac failure in the emergency department. J Emerg Med 2005;28:155–159 [DOI] [PubMed] [Google Scholar]
- 14.Foss YJ. Vitamin D deficiency is the cause of common obesity. Med Hypotheses 2009;72:314–321 [DOI] [PubMed] [Google Scholar]
- 15.Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev 2015;16:341–349 [DOI] [PubMed] [Google Scholar]
- 16.Worstman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690–693 [DOI] [PubMed] [Google Scholar]
- 17.Rayalam S, Della-Fera MA, Ambati S, et al. . Enhanced effects of 1,25 (OH)(2)D(3) plus genistein on adipogenesis and apoptosis in 3T3-L1 adipocytes. Obesity (Silver Spring) 2008;16:539–546 [DOI] [PubMed] [Google Scholar]
- 18.Zittermann A, Frisch S, Berthold HK, et al. . Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr 2009;89:1321–1327 [DOI] [PubMed] [Google Scholar]
- 19.Slusher AL, McAllister MJ, Huang CJ. A therapeutic role for vitamin D on obesity associated inflammation and weight loss intervention. Inflamm Res 2015;64:565–575 [DOI] [PubMed] [Google Scholar]
- 20.Soares MJ, Pathak K, Calton EK. Calcium and vitamin D in the regulation of energy balance: where do we stand? Int J Mol Sci 2014;15:4938–4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravussin E. Low resting metabolic rate as a risk factor for weight gain: role of the sympathetic nervous system. Int J Obes Relat Metab Disord 1995;19(Suppl. 7):S8–S9 [PubMed] [Google Scholar]
- 22.Buscemi S, Verga S, Caimi G, Cerasola G. Low relative resting metabolic rate and body weight gain in adult Caucasian Italians. Int J Obes 2005;29:287–291 [DOI] [PubMed] [Google Scholar]
- 23.Levy LD, Fleming JP, Klar D. treatment of refractory obesity in severely obese adults following management of newly diagnosed attention deficit hyperactivity disorder. Int J Obes 2009;33:326–334 [DOI] [PubMed] [Google Scholar]
- 24.Markowitz AJ, Rabow MW. Palliative management of fatigue at the close of life: “it feels like my body is just worn out.” JAMA 2007;298:217. [DOI] [PubMed] [Google Scholar]
- 25.Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc 2005;105:775–789 [DOI] [PubMed] [Google Scholar]
- 26.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–247 [DOI] [PubMed] [Google Scholar]
- 27.Katz A, Nambi SS, Mather K, et al. . Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–2410 [DOI] [PubMed] [Google Scholar]
- 28.Shannon A, Alkhouri N, Carter-Kent C, et al. . Ultrasonographic quantitative estimation of hepatic steatosis in children with NAFLD. J Pediatr Gastroenterol Nutr 2011;53:190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diniz ALD, Tomé RAF, Debs CL, et al. . Reproducibility of ultrasonography as a method to measure abdominal and visceral fat. Radiol Bras 2009;42:353–357 (in Portuguese) [Google Scholar]
- 30.Hiilloskorpi HK, Pasanen ME, Fogelholm MG, Laukkanen RM, Manttari AT. Use of heart rate to predict energy expenditure from low to high activity levels. Int J Sports Med 2003;24:332–336 [DOI] [PubMed] [Google Scholar]
- 31.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. . Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 32.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25 hydroxy vitamin D levels in healthy women. J Clin Endocrinol Metab 2003;88:157–161 [DOI] [PubMed] [Google Scholar]
- 33.Dalgård C, Petersen MS, Weihe P, Grandjean P. Vitamin D status in relation to glucose metabolism and type 2 diabetes in septuagenarians. Diabetes Care 2011;34:1284–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu KC, Chu A, Go V, Saad MF. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am J Clin Nutr 2004;79:820–825 [DOI] [PubMed] [Google Scholar]
- 35.Bland R, Markovic D, Hills CE, et al. . Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol 2004;89–90:121–125 [DOI] [PubMed] [Google Scholar]
- 36.Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev 2013;34:33–83 [DOI] [PubMed] [Google Scholar]
- 37.Wöbke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Front Physiol 2014;5:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee P, Greenfield JR, Seibel MJ, Eisman JA, Center JR. Adequacy of vitamin D replacement in severe deficiency is dependent on body mass index. Am J Med 2009;122:1056–1060 [DOI] [PubMed] [Google Scholar]
- 39.Meehan CA, Cochran E, Mattingly M, Gorden P, Brown RJ. Mild caloric restriction decreases insulin requirements in patients with type 2 diabetes and severe insulin resistance. Medicine 2015;94:e1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mai S, Walker GE, Vietti R, et al. . Acute vitamin D3 supplementation in severe obesity: evaluation of multimeric adiponectin. Nutrients 2017;9:459. [DOI] [PMC free article] [PubMed] [Google Scholar]