Abstract
The vasodilatory effect of angiotensin-(1-7) seems to vary between sexes, and estradiol (E2) can modulate the magnitude of the Ang-(1-7) vasodilatory response in female rats. However, there are few studies addressing the influence of sex on the age-related vasodilatory effect of Ang-(1-7). Here, we evaluated the vasodilatory response to Ang-(1-7) on vascular ageing. Ang-(1-7) dose–response curves were determined in mice aortic rings from males (old and young) and females (E2 treated/non-treated old and young) mounted in an isolated organ chamber. Abdominal aortic rings were used for protein expression analysis and determination of reactive oxygen species (ROS) and nitric oxide (NO) production. Our results showed that the Ang-(1-7) vasodilatory effect was absent in aorta from old females, contrasting with a full response in vessels from young females. The Ang-(1-7) vasodilatory effect was restored by E2 replacement in old females. A robust increase in Mas receptor, SOD2, NRF-2 and NOX2 expression was observed in aorta from old females, which was normalized by E2. This effect of E2 was also associated with lower production of ROS and normal levels of NO. In conclusion, our data demonstrated that pathways involved in the Ang-(1-7) vasodilatory response in female mice is affected by hormonal changes in ageing and rescued by E2.
Keywords: Angiotensin-(1-7), gender, female, estradiol, vascular response, ageing
Introduction
Cardiovascular diseases are the leading cause of morbidity, mortality and public health costs globally. Up to 45 years of age, men are most affected by cardiovascular diseases such as hypertension and heart failure. The incidence of cardiovascular morbidity and mortality is similar in men and women aged 45–54 years, but after that, it progressively increases in women and surpasses that in men.1–3 This evidence points to the influence of hormonal status on the cardiovascular system of women, suggesting that the absence of cardioprotection after menopause is an important factor for the sex-related differences in cardiovascular diseases.
The natural ageing process induces both structural and functional changes in the arterial wall. Compared to healthy young vessels, aged vessels are characterized by endothelial dysfunction and vascular smooth muscle cell (VSMC) migration to and proliferation in the intima.4 These processes are partially mediated by increased reactive oxygen species (ROS) and vascular inflammation.5 In this regard, few studies have evaluated sex-related differences and the effects of oestrogen (E2) in natural vascular ageing. Indeed, the loss of hormonal protection after menopause has been poorly studied in the context of vascular function, and there are only a few studies on the effects of E2 in vascular ageing.6 Hormone replacement therapy is a strategy that is under discussion. E2 replacement has been shown to prevent certain diseases postmenopause. However, many years after the menopausal state has settled, the oestrogenic actions may not be the same as when the menopause began. Intriguingly, few studies have addressed the effects of E2 on natural ageing and in models of senescence.
In addition to hormonal changes induced by ageing, some components of the classic renin-angiotensin system (RAS) are differentially regulated in the circulation of aged animals. Ageing is associated with a decline in RAS components.7 Ang II is associated with age-related increase in blood pressure, and levels of this peptide do not change in normotensive aged rats.8 Few studies have evaluated the effects of ageing on the local RAS, such as in renal, cerebral and abdominal adipose tissues, and little is known about the effects of ageing on the newly discovered RAS components, such as the angiotensin-(1-7)/Mas receptor axis. Angiotensin-(1-7) (Ang-(1-7)) is a bioactive peptide that counter-regulates the deleterious effects of Ang II. Ang-(1-7), through the activation of the G-coupled protein receptor, the Mas receptor, improves endothelial function, inhibits vascular smooth muscle cell proliferation and migration, induces vasodilation and regulates cardiac remodelling.9
In the present work, we investigated sex- and age-related differences in vascular responses to Ang-(1-7) in mice aorta and evaluated the effects of E2 replacement on vascular responses to Ang-(1-7) in aged female mice.
Methods
Animals
All experiments were approved by the Ethics Committee for the use of animals of the Universidade Federal de Minas Gerais (CEUA-UFMG; 365 and 372/2017). The present study used young (10 weeks old) and old (20 months) C57/Bl6 males and females. The animals were kept in an environment with controlled conditions of light (12 hours light/dark cycle) and temperature (±23°C) and were given water and food ad libitum. In elderly females, a vaginal smear was performed for at least 10 consecutive days to confirm the state of constant dioestrus phase (reduction in endogenous E2). Elderly females treated with E2 received a daily subcutaneous injection of 1µg/day of 17ß-estradiol (Sigma-Aldrich, St. Louis, MO, USA) for seven days.
Evaluation of vascular reactivity
On the day of the experiment, animals were euthanized by decapitation, and thoracic aortic rings were set up in an isolated organ chamber. Experiments to assay vascular function were performed in an organ bath system, as previously described.10 Briefly, thoracic aortic rings were obtained, mounted in an organ bath system and stabilized for 60 minutes in Krebs–Henseleit solution (110.8 mmol/L NaCl, 5.9 mmol/L KCl, 25 mmol/L NaHCO3, 1.07 mmol/L MgSO4, 2.49 mmol/L CaCl2, 2.33 mmol/L NaH2PO4, 11.51 mmol/L glucose; pH 7.4). The arteries were pre-constricted with an EC50 concentration of phenylephrine at 10 μM (Sigma-Aldrich, St. Louis, MO, USA), and their vascular responses were evaluated 30 minutes after the first concentration–response curve, by adding increasing concentrations of: Ang-(1-7) (10−10 to 10−4 M concentrations) or acetylcholine (10−9 to 10−4 M concentrations) or sodium nitroprusside (10−10 to 10−6 M concentrations). Mechanical activity was recorded isometrically by a force transducer (World Precision Instruments, Inc.) connected to an amplifier-recorder (Model TBM-4; World Precision Instruments, Inc.) and to a personal computer equipped with an analogue-to-digital converter board (DI-720; Dataq Instruments, Inc.), using Windaq data acquisition/recording software (Dataq Instruments, Inc.).
Western blotting analyses
Abdominal aortic segments were removed and stored at −80°C for analyses of protein expression. The proteins from abdominal aorta were isolated with lysis buffer (pH 8.0, 50 mmol/L tris–base, 100 mmol/L NaCl, 5 mmol/L EGTA, Na4P2O7 50 mmol/L, MgCl2 1 M/L, Nonidet p/40 1%, 0.3% triton X-100, sodium deoxycholate 0.5% and 1% protease inhibitor cocktail). For the Western blotting experiments, 50 µg of each protein sample was boiled and denatured in loading buffer containing 5% 2β-mercaptoethanol (Invitrogen, Waltham, MA, USA).Samples were separated by electrophoresis on a 10% sodium dodecyl sulphate-polyacrylamide gel (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were probed with diluted antibodies specific to Mas receptor (1:500; AAR013; Alomone Labs, Jerusalem, Israel), Catalase (1:1000. sc271803, Santa Cruz Biotechnology, Dallas, TX, USA), superoxide dismutase 2 (SOD-2) (1:1000, sc137254, Santa Cruz Biotechnology, Dallas, TX, USA), NRF-2 (1:500, ab31163, Abcam, Cambridge, UK) and NOX2/gp91 (1:500, sc74514, Santa Cruz Biotechnology, Dallas, TX, USA). Pixel density was normalized to the expression of the reference protein glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:1000, Cell Signaling Technology, Danvers, MA, USA; #97166).
Vascular nitric oxide (NO) analyses
NO levels were detected using the DAF-2 fluorescence probe in longitudinal sections from the aortic arch (catalogue no. 023844, Life Technologies, Carlsbad, CA, USA). The sections were washed with phosphate buffered saline (PBS) and incubated with DAF-FM, at a final concentration of 5 μM for 1 hour. The sections were washed twice with PBS, and fluorescence was monitored using a Nikon (Minato, Tokyo, Japan) fluorescent microscope (excitation 488 nm, emission 610 nm). Images were taken from the longitudinal sections of aortic arch (inner and outer segments), consisting of the endothelial, medial and adventitial layers of the vessel. The values of fluorescent intensity were calculated using ImageJ software (Version d1.47, National Institutes of Health, Bethesda, MD, USA).
Measurement of reactive oxygen species (ROS) generation in aortic rings
The oxidative fluorescent dye dihydroethidium (DHE) (catalogue no. D1168, Life Technologies, Carlsbad, CA, USA) was used to measure ROS production in longitudinal sections from the aortic arch. The sections of the aorta were incubated at 37°C for 10 minutes in PBS. Thereafter, the slides were again incubated with DHE (10 μM) in a dark, light-protected chamber at 37°C for 30 minutes. The nucleus was labelled with 4’,6-diamidino-2-phenylindole (DAPI). After this period, the images were captured using the Apotome microscope, equipped with a 545-nm wavelength filter, using the 40× objective (Zeiss, Oberkochen, Baden-Wurttemberg, Germany). Eight images of two randomly selected sections were performed and the integrated density of the fluorescence emitted by the DHE probe per area was quantified through the ImageJ software (Version d1.47, National Institutes of Health, Bethesda, Maryland, USA).
Data and statistical analysis
The data presented herein are expressed as mean ± SEM. Two-way ANOVA followed by a Bonferroni multiple comparisons post hoc test were used to compare concentration–response curves. Student’s t-test or one-way ANOVA followed by a Newman–Keuls Multiple Comparison test were used to compare groups in a graph bar. Analyses were performed by GraphPad Prism software (San Diego, CA, USA). Differences were considered significant at p ⩽ 0.05.
Results
Vascular responses to Ang-(1-7) in young and old males
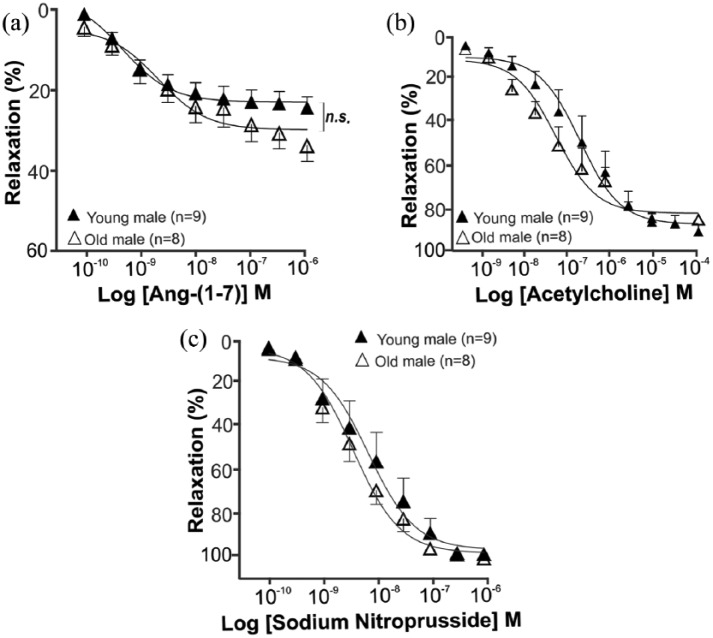
The effect of Ang-(1-7) on isolated thoracic aorta from young (10 weeks’ age) and elderly (20 months’ age) males was evaluated by the concentration–response curve to Ang-(1-7) (10−10 to 10−4 M). Ang-(1-7) induced a dose-dependent vasodilator effect in aorta of both young and elderly males (Figure 1(a)). In addition, there were no differences in acetylcholine endothelium-dependent and sodium nitroprusside endothelium-independent vasorelaxation curves between young and old males (Figure 1(b) to (c)).
Figure 1.
Ang-(1-7) induces a dose-dependent vasodilatory effect in aorta of both young and elderly male mice. (a) Concentration–response curves to Ang-(1-7) (10−10 to 10−6M) in thoracic aortic rings from male mice. Ang-(1-7) induces a dose-dependent vasodilation in aortic rings from both young (n=9) and old (n=8) male mice. (b) Concentration–response curves to acetylcholine (10−9 to 10−4 M) in thoracic aortic rings from young (n=9) and old (n=8) males. (c) Concentration–response curves to sodium nitroprusside (10−10 to 10−6 M) in thoracic aortic rings from young (n=9) and old (n=8) males. The endothelial function of thoracic aorta was preserved in response to both vasodilatory drugs. ▲: young males; △: old males. ANOVA followed by Newman–Keuls Multiple Comparison post hoc test was performed as statistical analysis. Each point represents the mean ± SEM. *p < 0.05.
The influence of ageing on female vascular responses to Ang-(1-7)
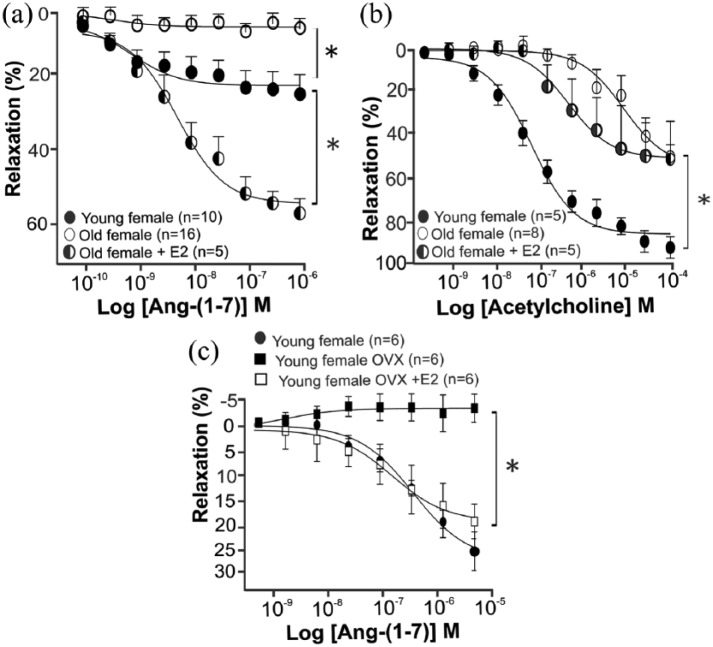
Considering the importance of sex- and ageing-related differences for cardiovascular morbidity and mortality, we investigated the response to Ang-(1-7) in young (10 weeks of age) and elderly females (20 months of age). The effect of Ang-(1-7) on isolated thoracic aorta from young (10 weeks of age) and elderly (20 months of age) females was evaluated by the concentration–response curve to Ang-(1-7) (10−10 to 10−4 M). Similar to males, Ang-(1-7) also induced a dose-dependent vasodilator effect in the aorta of young females (Figure 2(a)). Surprisingly, the aortic rings of elderly females did not respond to any dose of Ang-(1-7) (Figure 2(a)). This effect of age may be related to the loss of cardioprotective effects observed after menopause.2 Thus, to analyze whether the loss of the Ang-(1-7) vasorelaxation response was related to the absence of E2, old female mice were treated with E2 for seven days (1 µg/day, subcutaneously), and after that, the concentration–response curve to Ang-(1-7) was performed. The vasodilatory effects of Ang-(1-7) in isolated aorta were restored after E2 replacement therapy in old female mice. This effect of Ang-(1-7) was dose-dependent and at a greater magnitude than in the aorta of young females (Figure 2(a)). Altogether, the vascular response data of males and females indicate that the vasodilator effect of Ang-(1-7) in isolated mice aorta may be sex- and age-dependent. Aorta from old female mice had a decrease in vasorelaxation in response to acetylcholine as compared to those from young females, and E2 replacement had no effect on this result (Figure 2(b)). Interestingly, while the cessation of E2 production induced by ovariectomy in young mice abrogates the vasodilatory effects of Ang-(1-7), E2 treatment in ovariectomized mice restores Ang-(1-7) vascular responses (Figure 2(c)).
Figure 2.
Absence of Ang-(1-7) vasodilatory response in aortic rings from old females. (a) Concentration–response curves to Ang-(1-7) (10−10 to 10−6 M) in thoracic aortic rings from young (n=10), old (n=16) and old E2-treated (n=5) female mice. Ang-(1-7) induced a concentration-dependent vasodilator effect in young females, but this effect was absent in old females. E2 treatment for seven days (1µg/day) in old females restored Ang-(1-7) response. (b) Concentration–response curves to acetylcholine (10−9 to 10−4 M) in thoracic aortic rings from young (n=5), old (n=8) and old female E2-treated (n=5) mice. (c) Concentration–response curves to Ang-(1-7) (10−10 to 10−6 M) in thoracic aortic rings from young (n=6), young OVX (n=6) and young OVX+E2 (n=6) female mice. Similar to old females, ovariectomized animals have no vasodilatory responses to Ang-(1-7). This response was recovered after E2 replacement. •: young females; ■: young females OVX; □: young females OVX+E2; ○: old females; ◑ : old females E2-treated. ANOVA followed by Newman–Keuls Multiple Comparison post hoc test was performed as statistical analysis. Each point represents the mean ± SEM. *p < 005.
The effects of E2 in NO production stimulated by Ang(1-7)
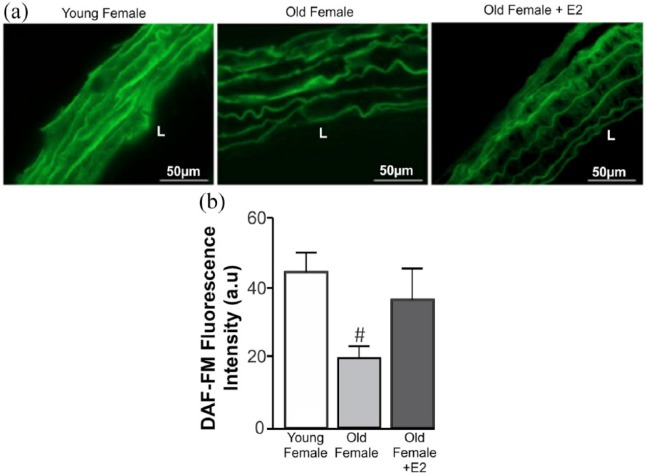
The vasodilation induced by Ang-(1-7) is mediated by NO production.11 Thus, we investigated the effects of ageing on NO production in aortic rings from female mice. As shown in Figure 3, elderly females present a significant decrease in NO as shown indirectly by DAF labelling. However, treatment with E2 for seven days (1 µg/day) did not change NO production (Figure 3(a) and (b)). This shows that the vasodilatory effect induced by E2 treatment in elderly females may not be only NO-dependent.
Figure 3.
Decreased NO production in aortic rings from ageing females. To evaluate the effect of E2 on NO production, aortic arch longitudinal sections from young, elderly and elderly+E2 were analyzed. (a) Representative microphotographs using DAF-FM (green) in aortic arch from young (n=4), old (n=4) and old E2-treated (seven days, 1 µg/day, n=3) female mice. (b) Graph represents NO production by endothelial cells from aortic rings. Elderly females presented a decreased NO production compared with young females. Statistical analyses: ANOVA followed by Newman–Keuls Multiple Comparison post hoc test was performed as statistical analysis. #p < 0.05 compared to the control group. DAF-FM: 4,5-diaminofluorescein-diacetate probe; L: lumen.
Ageing-related changes on vascular oxidative stress balance in female aorta
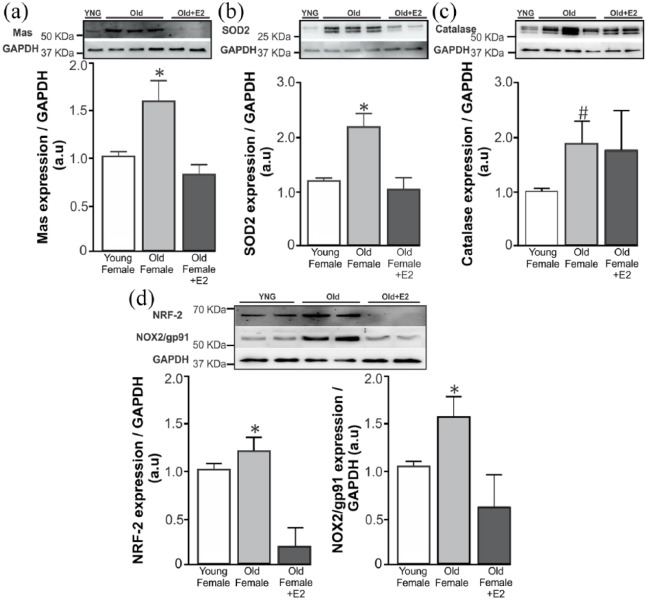
Considering the restoration of the vasoprotective responses to Ang-(1-7) in females after E2 therapy, we decided to investigate pathways related to Ang-(1-7) in abdominal aorta of these animals. As revealed by Western blotting analysis, elderly females exhibited a significantly higher expression of Mas receptor and SOD2 when compared with young females (Figure 4(a) and (b)). In addition, in elderly females treated with E2, the expression of these proteins was again similar to its expression in young females (Figure 4(a) and (b)). Finally, old females demonstrated a significant increase in catalase expression, while no difference was observed between old and old E2-treated females (Figure 4(c)). Although catalase does not appear to be involved in E2-mediated actions in elderly females, the expression of other oxidative stress related factors, namely NRF-2 and NOX2/gp91, both increased in old females and rescued by E2-treatment (Figure 4(d)).
Figure 4.
Ageing-related changes in vascular oxidative stress balance in female aorta. (a–d) Representative Western blotting (top) and the average densitometry values (bottom). Western blotting analysis of abdominal aortic protein homogenates from young (n=7), old (n=7) and old E2-treated (seven days, 1 µg/day, n=5) female mice. (a–c) Elderly females have significantly increased Mas receptor, SOD2 and catalase expression in abdominal aorta. This high expression was rescued in E2-treated old females, except for catalase. (d) NRF-2 and NOX2/gp91 upregulation was also rescued by E2 treatment. ANOVA followed by Newman–Keuls Multiple Comparison post hoc test was performed as statistical analysis. *p < 0.05 compared with the other groups. #p < 0.05 compared with the control group.
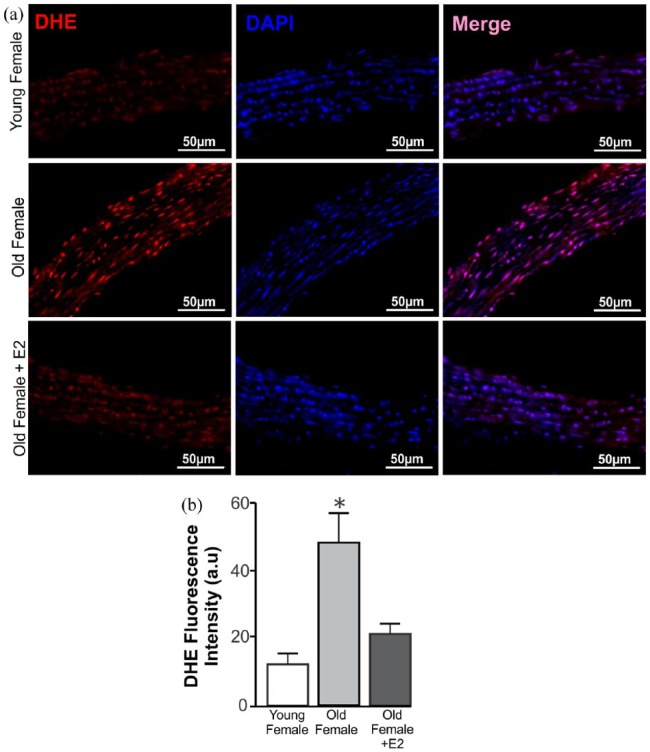
The upregulated antioxidative pathways in our study may be mediating ROS reduction. Thus, the analysis of the indirect measurement of the reactive oxygen species, via DHE, showed that in elderly females, there is a higher concentration of these substances (Figure 5(a) and (b)). Also, treatment with E2 reversed this increase in aorta of elderly females. Thus, correlating the expression of the Mas receptor, SOD2, catalase, NRF-2 and NOX2/gp91, the route of opposition to oxidative stress seems to be upregulated in elderly females and this effect is normalized with E2 replacement therapy in these animals.
Figure 5.
Estradiol treatment rescues increased superoxide concentration in aorta from elderly female mice. (a) Upper panel: representative microphotographs using DHE-derived fluorescence (red) of aortic rings from young (n=4), old (n=4) and old E2-treated (seven days, 1 µg/day, n=3) female mice. Nuclei were stained with DAPI (blue). Scale bar = 50 µm. (b) Elderly females have significantly increased DHE labelling in abdominal aorta. This high expression was rescued in E2-treated old females. Statistical analyses: ANOVA followed by Newman–Keuls Multiple Comparison post hoc test was performed as statistical analysis. *p < 0.05 compared with the other groups. DHE: dihydroethidium.
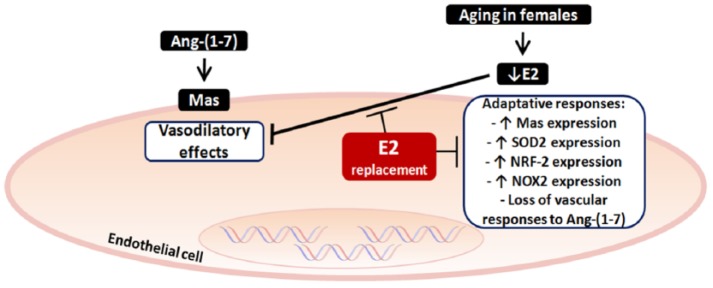
Figure 6.
Proposed model of E2 effects involved in the regulation of vascular responses in ageing. Elderly females have less Ang-(1-7)-induced vasoprotective effects related to low levels of E2. To develop a mechanism to prevent increase in pressure levels, there is an increase in Mas receptor expression and its pathways in old females, for example, increased expression of SOD2/NRF-2/NOX2, a pathway involved in the control of the reactive oxygen species formation, which would be deleterious to the vessel. Despite these adaptive changes, vascular responsiveness remained inadequate in these females and upregulation of the Mas receptor axis was not able to overcome the deleterious effects induced by E2 absence. By replacing E2, there is a reversal of vascular nonresponsiveness to Ang-(1-7) and reestablishment of changes in Mas receptor and SOD2/NRF-2/NOX2 expression present in aorta from these old females. Ang-(1-7): angiotensin-(1-7); E2: estradiol; SOD: superoxide dismutase 2; NRF-2: nuclear factor erythroid 2-related factor 2; NOX2/gp91: isoform 2 from membrane-bound enzyme complex (NADPH).
Discussion
In this study, we showed that age is an essential factor contributing to the sex-related differences in vascular responses to Ang-(1-7). In isolated male aorta, Ang-(1-7) induced a vasodilatory response regardless of whether the artery was from young or old mice. In contrast, isolated aorta from elderly females did not respond to Ang-(1-7). This unfavourable condition was associated with increased expression of Mas receptor, SOD2, NRF-2, NOX2/gp91 and ROS in aortic arteries of elderly females, which in turn were normalized with E2 replacement therapy. These changes may have contributed to the improvement seen in the vasodilator response to Ang-(1-7) in these animals.
In menopause, the reduction in E2 levels is considered to be the major reason for most cardiac and vascular diseases in women. E2 is able to induce vasorelaxation12,13 and sympathoinhibition14 and prevent vascular remodeling.15 In the present work, we showed a sex-related difference in the vasodilator effect of Ang-(1-7), which in turn was associated with the age of the animals. The natural ageing process in women and female mice leads to a drastic reduction in E2 levels, which is associated with loss of cardiovascular protection14,15 and increased morbidity and mortality.16 This hormonal hypothesis has been the most studied to clarify the increased incidence of these pathologies in elderly females.
Our study showed that E2 therapy was able to improve the vasodilatory effect of Ang-(1-7) in the aorta of elderly females, which was even greater than that observed in young females. Recent work by Mompeón et al. showed that E2, acting through ERα, increases intracellular levels of Ang-(1-7) in HUVEC culture after 24 hours of incubation.16 Moreover, in female mice on a high fat diet, ovariectomy reduced Ang-(1-7) plasma levels.17 We hypothesized that the higher levels of E2 in treated groups in relation to elderly females were determinant for the increased aortic responsiveness to Ang-(1-7). Further studies are needed to measure Ang-(1-7) levels in plasma and in aorta from these aged female mice.
E2 has been shown to enhance endothelial-dependent relaxation in arterial rings from different animals and from different vascular beds.18,19 In postmenopausal woman, E2 replacement therapy increases coronary flow and decreases both coronary resistance and peripheral vascular tone.20–22 E2 can increase endothelial NO production, by both stimulation of eNOS gene expression and modulation of NO degrading systems such as ROS generation and antioxidant agents. E2 can also induce eNOS activity and NO release very rapidly.13 However, few studies have investigated the direct effects of E2 treatment in restoring the vascular responses to vasodilators. Here, we show that E2 treatment during natural ageing may potentiate the vasoprotective actions of Ang-(1-7) in females, at least in part, by improving NO production.
Ang-(1-7) exerts its effect by stimulation of the G-protein-coupled Mas receptor. Activation of Mas receptor on the endothelial cell induces the phosphatidylinositol 3-kinases and Akt pathway,23 leading to activation of endothelial NOS and subsequent release of NO inducing VSMC relaxation. Our results suggest that E2 may contribute to the amplification of Ang-(1-7)’s vasodilatory effect in a way that is not only mediated by NO since E2 treatment did not abrogate decreased NO production in aortic rings. Indeed, aortic sections of elderly females showed a decrease in NO production as compared to young females, and E2 replacement showed a strong tendency to reach the control NO levels, but without reaching statistical significance. In this vein, Novella et al. investigated the time-course for ageing-associated effects on contractile and relaxing vascular responses and NO production in aorta from female senescence-accelerated resistant (SAMR1) and prone (SAMP8) mice.24 They demonstrated that treatment with the NOS inhibitor L-NAME markedly increased contractile responses to phenylephrine and attenuated NO release age dependently. However, their study used females aged 3, 6 and 10 months, while our study compared young animals with much older females (20 months of age), which in turn can provide information on the more advanced stages of natural senescence.
The Ang-(1-7)/Mas axis is essential for cardiovascular protection.25 The increase in Mas receptor expression has been demonstrated in experimental models of muscle atrophy,26 inflammation,23 fibrinogenesis24 and vascular remodelling.27 All these models represent conditions in which the body needs to adapt to prevent the damage caused by the absence or over-stimulation of the receptor. In our model of natural ageing in females, we observed a robust increase in expression of Mas receptor in the aorta, which was associated with an increase in SOD2, catalase, NRF-2 and NOX2/gp91 expression. The increased expression of these targets in aorta of elderly females was rescued by E2 treatment. It is possible that such high levels of oxidative stress in female aged artery may negatively influence the NO-mediated vasorelaxant action of Ang-(1-7), even if Mas receptor is upregulated. Further in vivo experiments with pharmacological inhibitors of NOS or oxygen scavengers would be necessary to obtain direct evidence of this.
There are few studies addressing the role of ovariectomy in aortic vascular reactivity to Ang-(1-7). Grobe and Katovich showed that ovariectomy did not affect the concentration–response curve to Ang-(1-7) in aorta from SD rats as compared to a sham group. In addition, while low doses of E2 did alter the vasorelaxation response to Ang-(1-7), animals treated with high doses of E2 were unresponsive to Ang-(1-7).28 In contrast to their findings, our data showed that ovariectomy in young female mice abolished the vasodilatory response of aortic rings to Ang-(1-7). In agreement, Endlich et al. recently showed that ovariectomy decreased the vasorelaxant responses to Ang-(1-7) in spontaneously hypertensive rats and that E2 therapy slightly improves this response.29 Altogether, our results from young ovariectomized models reinforce the association between E2 and Ang-(1-7). Nevertheless, it is important to note that E2 therapy in elderly female mice leads to an exacerbated vasodilatory response to Ang-(1-7), which was even greater than that in intact young females. Thus, our work shows that both ageing and estradiol deprivation are involved in Ang-(1-7) vascular responses. Further studies are needed to elucidate the mechanistic differences and/or similarities between the two models of E2 deficiency, ovariectomy and natural ageing.
The main findings of our investigation point to a mechanism that considers ageing and sex as determinants for the vasodilatory responses to Ang-(1-7). We propose a mechanism present in elderly females to explain the absence of vasoprotective effects observed (Figure 6). Older females present a loss of cardioprotective oestrogenic actions, leading to an increase in the expression of antioxidative pathways triggered by Mas receptor (Figures 4 and 5). This effect is a way to compensate for the absence of E2 vasoprotection in old females. Despite these adaptive changes, vascular responsiveness remained inadequate in these females, and upregulation of the Mas receptor axis was not able to overcome the deleterious effects induced by the absence of E2. This hormone, in turn, reversed vascular nonresponsiveness to Ang-(1-7) and changes in the expression of pathways present in aorta from these old females.
Conclusions
Our study showed that vasodilatory response to Ang-(1-7) is modified by ageing in females by a mechanism involving increased Mas, SOD2, NRF-2, NOX2/gp91 expression and ROS production. In addition, hormonal replacement therapy with E2 was able to prevent these vascular changes. Our findings provide insight into the mechanisms underlying ageing-dependent actions of E2 and provide a rationale for hormone replacement therapy after menopause. The findings presented here increase our knowledge about the mechanisms elicited by Ang-(1-7) in vessels from old females and suggest new therapeutic strategies for vascular disease in elderly women.
Acknowledgments
We thank Centro de Aquisição e Processamento de Imagens (CAPI) at Federal University of Minas Gerais for technical microscopy assistance.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq) and INCT NanoBiofar. GKG and FPSN were students at the Physiology and Pharmacology Graduate Program of the Universidade Federal de Minas Gerais and recipients of Coodenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and CNPq fellowships.
ORCID iD: Rafaela F. da Silva  https://orcid.org/0000-0002-3335-2542
https://orcid.org/0000-0002-3335-2542
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017; 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics–2013 Update: A Report from the American Heart Association. Circulation 2013; 127: e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maas AHEM, Appelman YEA. Gender differences in coronary heart disease. Neth Heart J 2010; 18: 598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu X, Wang B, Ren C, et al. Age-related impairment of vascular structure and functions. Aging Dis 2017; 8: 590–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El Assar M, Angulo J, Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 2013; 65: 380–401. [DOI] [PubMed] [Google Scholar]
- 6. Novella S, Dantas AP, Segarra G, et al. Vascular aging in women: is estrogen the fountain of youth? Front Physiol 2012; 3: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thompson MM, Oyama TT, Kelly FJ, et al. Activity and responsiveness of the renin-angiotensin system in the aging rat. Am J Physiol Regul Integr Comp Physiol 2000; 279: R1787–1794. [DOI] [PubMed] [Google Scholar]
- 8. Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007; 59: 251–287. [DOI] [PubMed] [Google Scholar]
- 9. Santos RAS, Sampaio WO, Alzamora AC, et al. The ACE2/angiotensin-(1–7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1–7). Physiol Rev 2018; 98: 505–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Capettini LSA, Cortes SF, Lemos VS. Relative contribution of eNOS and nNOS to endothelium-dependent vasodilation in the mouse aorta. Eur J Pharmacol 2010; 643: 260–266. [DOI] [PubMed] [Google Scholar]
- 11. Raffai G, Lombard JH. Angiotensin-(1–7) selectively induces relaxation and modulates endothelium-dependent dilation in mesenteric arteries of salt-fed rats. J Vasc Res 2016; 53: 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu Y, Bian Z, Lu P, et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 2002; 295: 505–508. [DOI] [PubMed] [Google Scholar]
- 13. Caulin-Glaser T, García-Cardeña G, Sarrel P, et al. 17 beta-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res 1997; 81: 885–892. [DOI] [PubMed] [Google Scholar]
- 14. Pinkham MI, Barrett CJ. Estradiol alters the chemosensitive cardiac afferent reflex in female rats by augmenting sympathoinhibition and attenuating sympathoexcitation. Clin Exp Pharmacol Physiol 2015; 42: 622–631. [DOI] [PubMed] [Google Scholar]
- 15. Bonacasa B, Sanchez ML, Rodriguez F, et al. 2-Methoxyestradiol attenuates hypertension and coronary vascular remodeling in spontaneously hypertensive rats. Maturitas 2008; 61: 310–316. [DOI] [PubMed] [Google Scholar]
- 16. Mompeón A, Lázaro-Franco M, Bueno-Betí C, et al. Estradiol, acting through ERα, induces endothelial non-classic renin-angiotensin system increasing angiotensin 1–7 production. Mol Cell Endocrinol 2016; 422: 1–8. [DOI] [PubMed] [Google Scholar]
- 17. Gupte M, Thatcher SE, Boustany-Kari CM, et al. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol 2012; 32: 1392–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borgo MV, Claudio ERG, Silva FB, et al. Hormonal therapy with estradiol and drospirenone improves endothelium-dependent vasodilation in the coronary bed of ovariectomized spontaneously hypertensive rats. Braz J Med Biol Res. Epub ahead of print 17 November 2016. DOI: 10.1590/1414-431X20154655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vacca G, Battaglia A, Grossini E, et al. The effect of 17?-oestradiol on regional blood flow in anaesthetized pigs. J Physiol 1999; 514: 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manhem K, Ahlm H, Milsom I, et al. Transdermal oestrogen reduces daytime blood pressure in hypertensive women [see comment]. J Hum Hypertens 1998; 12: 323–327. [DOI] [PubMed] [Google Scholar]
- 21. White WB, Hanes V, Chauhan V, et al. Effects of a new hormone therapy, drospirenone and 17-beta-estradiol, in postmenopausal women with hypertension. Hypertension 2006; 48: 246–253. [DOI] [PubMed] [Google Scholar]
- 22. Scuteri A, Lakatta EG, Anderson DE, et al. Transdermal 17 beta-oestradiol reduces salt sensitivity of blood pressure in postmenopausal women. J Hypertens 2003; 21: 2419–2420. [DOI] [PubMed] [Google Scholar]
- 23. Capettini LSA, Montecucco F, Mach F, et al. Role of renin-angiotensin system in inflammation, immunity and aging. Curr Pharm Des 2012; 18: 963–970. [DOI] [PubMed] [Google Scholar]
- 24. Pereira RM, Dos Santos RAS, Teixeira MM, et al. The renin-angiotensin system in a rat model of hepatic fibrosis: evidence for a protective role of Angiotensin-(1–7). J Hepatol 2007; 46: 674–681. [DOI] [PubMed] [Google Scholar]
- 25. Santos RA. Angiotensin-(1–7). Hypertension 2014; 63: 1138–1147. [DOI] [PubMed] [Google Scholar]
- 26. Morales MG, Abrigo J, Meneses C, et al. Expression of the Mas receptor is upregulated in skeletal muscle wasting. Histochem Cell Biol 2015; 143: 131–141. [DOI] [PubMed] [Google Scholar]
- 27. Tallant EA, Clark MA. Molecular mechanisms of inhibition of vascular growth by angiotensin-(1–7). Hypertension 2003; 42: 574–579. [DOI] [PubMed] [Google Scholar]
- 28. Grobe JL, Katovich MJ. Alterations in aortic vascular reactivity to angiotensin 1–7 in 17-β-estradiol-treated female SD rats. Regul Pept 2006; 133: 62–67. [DOI] [PubMed] [Google Scholar]
- 29. Endlich PW, Claudio ERG, Lima LCF, et al. Exercise modulates the aortic renin-angiotensin system independently of estrogen therapy in ovariectomized hypertensive rats. Peptides 2017; 87: 41–49. [DOI] [PubMed] [Google Scholar]