Abstract
In this review, we attempt to integrate the empirical evidence regarding stimulus-specific adaptation (SSA) and mismatch negativity (MMN) under a predictive coding perspective (also known as Bayesian or hierarchical-inference model). We propose a renewed methodology for SSA study, which enables a further decomposition of deviance detection into repetition suppression and prediction error, thanks to the use of two controls previously introduced in MMN research: the many-standards and the cascade sequences. Focusing on data obtained with cellular recordings, we explain how deviance detection and prediction error are generated throughout hierarchical levels of processing, following two vectors of increasing computational complexity and abstraction along the auditory neuraxis: from subcortical toward cortical stations and from lemniscal toward nonlemniscal divisions. Then, we delve into the particular characteristics and contributions of subcortical and cortical structures to this generative mechanism of hierarchical inference, analyzing what is known about the role of neuromodulation and local microcircuitry in the emergence of mismatch signals. Finally, we describe how SSA and MMN are occurring at similar time frame and cortical locations, and both are affected by the manipulation of N-methyl-D-aspartate receptors. We conclude that there is enough empirical evidence to consider SSA and MMN, respectively, as the microscopic and macroscopic manifestations of the same physiological mechanism of deviance detection in the auditory cortex. Hence, the development of a common theoretical framework for SSA and MMN is all the more recommendable for future studies. In this regard, we suggest a shared nomenclature based on the predictive coding interpretation of deviance detection.
Keywords: SSA, MMN, predictive coding, deviance detection, repetition suppression
Introduction: SSA and MMN, Two Faces of Deviance Detection
Throughout their entire life, in each and every moment of it, humans and animals live immersed in an overwhelming flow of acoustic information continuously coming from all kinds of sources within their nearby environment. It is a major task of the auditory system to organize that acoustic jumble into perceptual constructs of biological relevance. Most of the sounds incessantly hitting the eardrum are repetitive and predictable and have meagre functional significance. The capacity of the auditory system to preattentively purge irrelevant foreseeable stimulation and provide perceptual saliency to those sounds that are unique, unpredictable, and therefore highly informative is generally referred to as deviance detection (also referred as change, surprise, or novelty detection, with varying usage across the literature). In other words, deviance detection is the response to a stimulus that diverges from a regularity in the stimulation previously identified by the processing system (Winkler & Schröger, 2015).
Deviance detection in the auditory system finds one of its most well-studied manifestations in the mismatch negativity (MMN), an event-related potential (ERP) recorded from the human scalp. An MMN can be elicited by any discriminable change in the auditory stimulation, peaking at 150 to 250 ms from change onset. That discriminable change has been widely reproduced experimentally using a classic oddball paradigm (Figure 1(a)), in which rare acoustic events (deviant stimuli) are randomly embedded within a series of frequently repeating sounds (standard stimuli; Näätänen, Gaillard, & Mäntysalo, 1978). The differential response to a given tone when presented as deviant or standard first revealed the MMN as an automatic deviance-specific component of the auditory ERP (Figure 2), persistent during sleep (Nashida et al., 2000; Strauss et al., 2015), anesthesia (Koelsch, Heinke, Sammler, & Olthoff, 2006; Quaedflieg, Münte, Kalso, & Sambeth, 2014), or coma (Morlet & Fischer, 2014; Rodríguez, Bussière, Froeschl, & Nathan, 2014), and present in the moment of birth (Cheour et al., 2002; Winkler et al., 2003) and before (Draganova et al., 2005; Draganova, Eswaran, Murphy, Lowery, & Preissl, 2007). The classical notion of MMN has widened in the past decades, proving its capacity to identify deviances inserted in more complex sequences organized by abstract rules (Paavilainen, 2013; Paavilainen, Kaukinen, Koskinen, Kylmälä, & Rehn, 2018; Saarinen, Paavilainen, Schöger, Tervaniemi, & Näätänen, 1992; Schröger, Bendixen, Trujillo-Barreto, & Roeber, 2007; Tervaniemi, Maury, & Näätänen, 1994). The computational feature behind deviance detection is currently thought to be foundation and trigger of higher order cognitive functions (Näätänen, Astikainen, Ruusuvirta, & Huotilainen, 2010) such as attention (Fritz, Elhilali, David, & Shamma, 2007; Sussman, Winkler, & Wang, 2003) and memory (Bartha-Doering, Deuster, Giordano, am Zehnhoff-Dinnesen, & Dobel, 2015; Ranganath & Rainer, 2003). Consequently, it is not surprising that MMN is not only disrupted in patients suffering from neurodevelopmental and psychiatric conditions, with a characteristically prominent reduction in schizophrenia (Baldeweg, Klugman, Gruzelier, & Hirsch, 2004; Damaso, Michie, & Todd, 2015; Ells et al., 2018; Fisher et al., 2018; Haigh et al., 2017; Javitt & Sweet, 2015; Joshi et al., 2018; Kantrowitz, Swerdlow, Dunn, & Vinogradov, 2018; Koshiyama et al., 2018; Näätänen & Kähkönen, 2009; Todd, Harms, Schall, & Michie, 2013), but also altered in other pathologies such as Parkinson’s disease (Brønnick, Nordby, Larsen, & Aarsland, 2010; Heldmann et al., 2017; Minks et al., 2014; Pekkonen, Jousmäki, Reinikainen, & Partanen, 1995; Seer, Lange, Georgiev, Jahanshahi, & Kopp, 2016; Solís-Vivanco et al., 2011), Alzheimer’s disease (Idrizbegovic, Hederstierna, & Rosenhall, 2016; Jiang et al., 2017; Papadaniil et al., 2016; Pekkonen, 2000; Pekkonen, Hirvonen, Jääskeläinen, Kaakkola, & Huttunen, 2001; Tsolaki et al., 2017), autism spectrum disorders (Goris et al., 2018; Hudac et al., 2018; Schwartz, Shinn-Cunningham, & Tager-Flusberg, 2018; Vlaskamp et al., 2017), and language impairments (Davids et al., 2011; Kujala & Leminen, 2017). Because of this, MMN has become a central tool in cognitive and clinical neuroscience (Bartha-Doering et al., 2015; Kujala, Tervaniemi, & Schröger, 2007; Näätänen, Paavilainen, Rinne, & Alho, 2007; Näätänen, Sussman, Salisbury, & Shafer, 2014; Sussman, Chen, Sussman-Fort, & Dinces, 2014), even showing promising potential diagnostic applications (Light & Näätänen, 2013; Näätänen et al., 2012; Näätänen, Petersen, Torppa, Lonka, & Vuust, 2017; Schall, 2016).
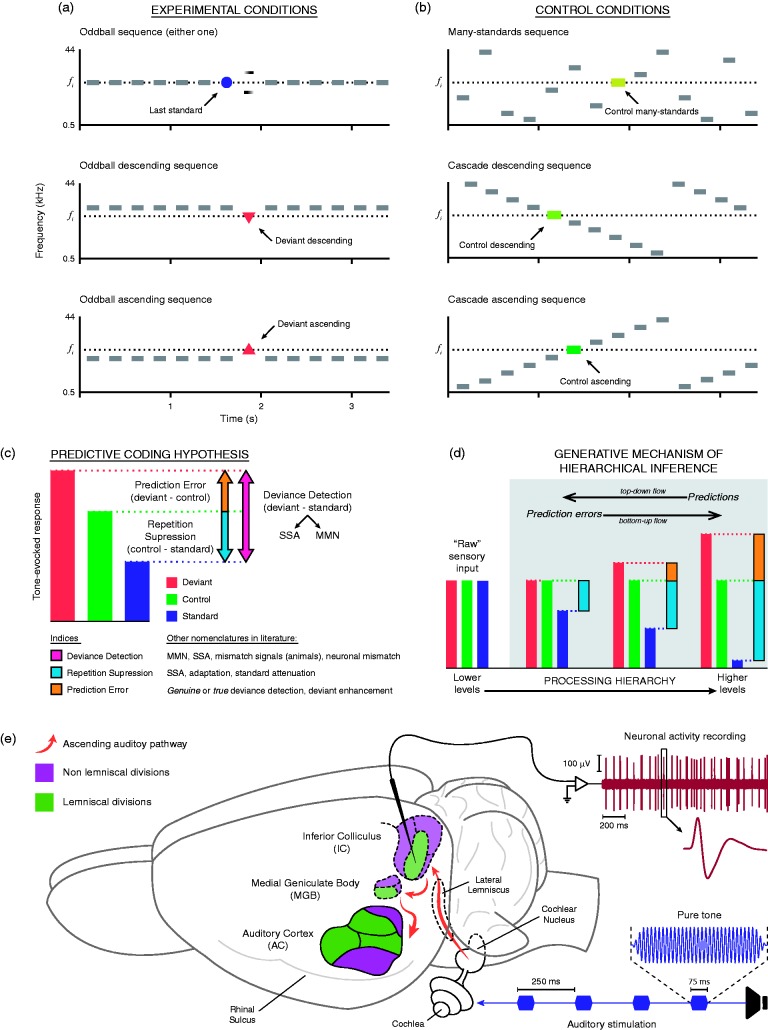
Figure 1.
(a) Classical oddball paradigm, displaying three possible experimental conditions for a given fi target tone. (b) Control sequences highlighting the fi target tone. In the many-standards sequence, the target tone is embedded within a random succession of assorted equiprobable tones, making impossible for the system to establish a predictive rule. The two versions of the cascade sequence (descending and ascending) are compared with the corresponding version of the oddball sequence. In both versions, the target tone is embedded in a predictable succession of equiprobable tones, allowing the system to establish a predictive rule that is not broken by the appearance of the target tone, as opposed to what happens in the oddball sequence. (c). Decomposition of deviance detection signals (deviant–standard) according to the interpretation of the predictive coding hypothesis. The difference between the response to the target tone in the control sequence and its evoked response when presented as a standard in the oddball sequence would constitute the component of repetition suppression. On the other hand, the difference between the deviant-evoked response and the response to that target tone within a control sequence, if positive, would unveil a component of prediction error. (d). Explanation of how the generative mechanism of hierarchical or Bayesian inference would work, showing the modulation of evoked responses normalized to the control condition. “Raw” sensory input (i.e., information about the physical features of the auditory stimuli disregarding its context) would be fed into the mechanism of inference to be modulated along the auditory processing hierarchy according to their contextual features and interstimular relationships. Higher order levels of processing would abstract increasingly complex rules to generate top-down predictions capable of explaining away incoming input and save processing resources. When predictions match the input at lower levels, sensory coding is optimized and perception arises. But when there is a mismatch, lower order levels covey a bottom-up prediction error to higher order levels to update the predictive model. (e) Sketch of a typical experimental setup for cellular recording (in rat brain), in which neuronal activity is recorded from different auditory stations while stimulating with sequences of pure tones.
MMN = mismatch negativity; SSA = stimulus-specific adaptation. Adapted from Parras et al. (2017).
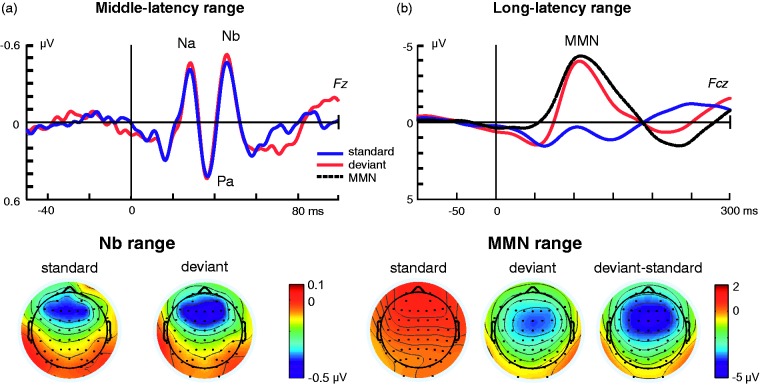
Figure 2.
Auditory-evoked potentials (ERPs) recorded from the human scalp to standard and frequency deviant stimuli presented in an oddball sequence. (a) Middle-latency response (MLR) with its typical morphology (Na, Pa, and Nb) waveforms disclosing larger amplitude for deviant (red) compared with standard (blue) stimuli. The bottom plots correspond to the scalp distribution of the Nb latency range for deviant and standard stimuli. (b) Long-latency auditory-evoked potential for standard (blue) and deviant (red) stimuli, and the corresponding difference waveform (black) disclosing the mismatch negativity (MMN). The bottom plots correspond to the scalp distribution of the MMN latency range for deviant and standard stimuli, as well as the scalp distribution of the MMN (right).
ERP = event-related potential. Adapted from Althen, Grimm, and Escera (2013).
Using the same oddball sequences that elicit the MMN in human ERP studies, an analogue deviance-detection process has been characterized in the response of some neurons distributed along the auditory pathways of several animal species. These neurons show a progressively reduced response to a repetitive standard sound, which is restored when stimulated by an unpredictable deviant sound. This special type of adaptation is considered a form of short-term plasticity, known as stimulus-specific adaptation (SSA). SSA is quantified as the index of change in the firing rate of a neuron in response to a deviant stimulus when compared with its response to that same stimulus played as a standard. Neurons exhibiting SSA are located subcortically within the nonlemniscal divisions of the auditory midbrain (Ayala et al., 2015; Ayala & Malmierca, 2015, 2018; Duque & Malmierca, 2015; Duque, Perez-Gonzalez, Ayala, Palmer, & Malmierca, 2012; Duque, Wang, Nieto-Diego, Krumbholz, & Malmierca, 2016; Malmierca, Cristaudo, Perez-Gonzalez, & Covey, 2009; Parras et al., 2017; Patel, Redhead, Cervi, & Zhang, 2012; Pérez-González, Hernández, Covey, & Malmierca, 2012; Pérez-González & Malmierca, 2012; Pérez-González, Malmierca, & Covey, 2005; Valdés-Baizabal, Parras, Ayala, & Malmierca, 2017; Zhao, Liu, Shen, Feng, & Hong, 2011) and thalamus (Anderson, Christianson, & Linden, 2009; Anderson & Malmierca, 2013; Antunes & Malmierca, 2014; Antunes, Nelken, Covey, & Malmierca, 2010; Bauerle, von der Behrens, Kossl, & Gaese, 2011; Duque, Malmierca, & Caspary, 2014; Parras et al., 2017) and are widely spread over primary (Chen, Helmchen, & Lutcke, 2015; Farley, Quirk, Doherty, & Christian, 2010; Hershenhoren, Taaseh, Antunes, & Nelken, 2014; Klein, von der Behrens, & Gaese, 2014; Natan et al., 2015; Natan, Rao, & Geffen, 2017; Nieto-Diego & Malmierca, 2016; Parras et al., 2017; Szymanski, Garcia-Lazaro, & Schnupp, 2009; Taaseh, Yaron, & Nelken, 2011; Ulanovsky, Las, Farkas, & Nelken, 2004; Ulanovsky, Las, & Nelken, 2003; von der Behrens, Bauerle, Kossl, & Gaese, 2009) and secondary (Nieto-Diego & Malmierca, 2016; Parras et al., 2017) areas of the auditory cortex (AC).
SSA was proposed to be the correlate of the deviance-detection mechanism at the neuronal level (Ulanovsky et al., 2003), which population activity summation would build up until being detectable on the scalp as an MMN (Nelken & Ulanovsky, 2007). Given the clinical potential of MMN, the need of knowing more about its neuronal substrate has encouraged numerous studies to delve into the SSA dynamics, neurochemical mechanisms, and anatomical distribution and connectivity to overcome the initial difficulties of linking the microscopic (SSA) and macroscopic (MMN) manifestations of the allegedly same physiological mechanism of deviance detection (Escera & Malmierca, 2014; Khouri & Nelken, 2015; Malmierca, Sanchez-Vives, Escera, & Bendixen, 2014; Nelken & Ulanovsky, 2007).
Adaptation or Modeling? Different Ways of Understanding Deviance Detection
In the context of an oddball paradigm, both MMN and SSA can be understood as indices of automatic deviance detection, which results from the overall difference between the responses to a given tone when it is presented as a deviant stimulus compared with when it is presented as a standard stimulus. But this contrast between deviant and standard responses could be accounted for in at least two different ways. On one hand, it could be due to an enhancement in the response to the deviant sound, as its appearance represents a violation of a previously established regularity. According to the model-adjustment hypothesis (also called online-comparison hypothesis), that dissonance between the prior expected sound and the actual auditory input would prompt an online update of the established perceptual model, resulting in an increased response to the deviant sound (Garrido, Kilner, Stephan, et al., 2009; Winkler, 2007; Winkler & Czigler, 1998). This interpretation has been traditionally favored by the MMN literature, which usually refers to this enhancement as true or genuine deviance detection. Such epithets are added as a way of emphasizing the active nature of the comparison between the sensory memory trace and the incoming input; a refined processing that has even been proposed as the sign of a “primitive intelligence” present in the auditory system (Näätänen, Tervaniemi, Sussman, Paavilainen, & Winkler, 2001). However, the intricate interplay of sensory processors, memory tracers, and online comparators proposed by this hypothesis has stumbled upon some difficulties in pinning down its neuronal correlates (Fishman, 2014; May & Tiitinen, 2010).
On the other hand, the contrast between deviant and standard could be simply due to attenuation of the response to the repetitive sound, as an effect of mere neuronal adaptation. The appearance of the deviant sound, physically different from the standard stimuli, would elicit the response of other novel afferences. The deviant sound would not produce an enhanced response, but just a nonadapted one (May & Tiitinen, 2010). This much simpler interpretation conforms to the adaptation hypothesis that is favored in most neurophysiological studies about SSA. In spite of its advantageous and versatile simplicity, the adaptation hypothesis turned out to be unsuited to fully explaining deviance detection, as it has many difficulties in accounting for all the aspects of the MMN (Winkler, Denham, & Nelken, 2009). For example, following the adaptation hypothesis rationale, if the deviant stimulus of an oddball sequence was just the standard tone but played softer, that should not release the deviant response from adaptation (Duque et al., 2016). Indeed, the deviant response would be reduced not only because of adaptation but also because of the decrement in the stimulation intensity. By contrast, the empirical fact is that an infrequent random intensity drop within a train of tones of the same frequency does generate an MMN in human participants (Althen, Grimm, & Escera, 2011; Altmann et al., 2013; Escera, Corral, & Yago, 2002; Jacobsen, Horenkamp, & Schröger, 2003; Loewy, Campbell, De Lugt, Elton, & Kok, 2000; Shestopalova, Petropavlovskaia, Semenova, & Nikitin, 2018). Furthermore, if that decrement in intensity is absolute, creating a stimulus omission in the train of standards, that absence of an expected tone is capable of eliciting an MMN (Berlot, Formisano, & DeMartino, 2018; Horváth, Czigler, Winkler, & Teder-Sälejärvi, 2007; Horváth, Müller, Weise, & Schröger, 2010; Oceák, Winkler, Sussman, & Alho, 2006; Raij, McEvoy, Mäkelä, & Hari, 1997; Yabe, Tervaniemi, Reinikainen, & Näätänen, 1997) within a “temporal window of integration” of limited span (Yabe et al., 2001). This seems counterintuitive and difficult to explain in terms of adaptation alone because it means that the auditory system is generating a response to the silence. Some authors have argued that the omission could yield an abrupt release of adaptation that would provoke a rebound of neuronal activity, confounding that activity recorded in the scalp with a genuine response (May & Tiitinen, 2010). However, some MMN studies in humans have challenged the plausibility of this interpretation (Berlot et al., 2018; Dehaene, Meyniel, Wacongne, Wang, & Pallier, 2015; Wacongne et al., 2011) and have put forward alternative explanations that will be discussed in the next section.
Beyond the classical oddball paradigm, adaptation seems also insufficient to explain how an MMN can be generated by the violation of regularities established by patterns more complex than sheer one-stimulus repetition (Heilbron & Chait, 2017). For example, in a two-tone pattern or alternation sequence (ABABABAB…), the repetition of one of the tones (ABABABBA or ABABABAA) prompts an MMN in human participants (Alain, Woods, & Ogawa, 1994; Cornella, Leung, Grimm, & Escera, 2012; Ells et al., 2018; Nordby, Roth, & Pfefferbaum, 1988; Saarinen et al., 1992; Sculthorpe, Collin, & Campbell, 2008; Todorovic, van Ede, Maris, & de Lange, 2011). Likewise, in the so-called local/global paradigm, a token consisting of an arrangement of several tones (e.g., AAAB, being A a local standard and B a local deviant) repeats over time. The resulting sequence outlines a regular pattern, in which each token as a whole acts like a global standard (AAAB AAAB AAAB…). In some rare and random occasions, an additional repetition of the local standard is introduced instead of the local deviant (AAAA; Bekinschtein et al., 2009; Chennu et al., 2016; Wacongne et al., 2011) or just before it (AAAAB; Recasens, Grimm, Wollbrink, Pantev, & Escera, 2014; Sussman, Ritter, & Vaughan, 1998). Those global deviants within the sequence pattern can elicit an MMN in human participants. According to the adaptation hypothesis sensu stricto, in both the alternation sequence and the local/global paradigm, the repetition of a tone should lead to an adapted response, but instead it is generating a deviance-detection signal. The same occurs when the patterns are built with low and high intensities of the same frequency (e.g., LHLHLHHL; Macdonald & Campbell, 2011). Furthermore, the unexpected omission of one of the tones conforming the alternating (ABABAB_B) or the local/global (AAAB AAAB AAA_) pattern generates an MMN in human participants (Chennu et al., 2016; Recasens & Uhlhaas, 2017; Shinozaki et al., 2003; Todorovic et al., 2011; Wacongne et al., 2011), which is all the more interesting and difficult to account for relying exclusively on adaptation. The only possible explanation based on a broad interpretation of the adaptation hypothesis would suggest the existence of higher order neurons capable of adapting to tonal relationships, for example, specifically adapting to an AB tone-pair (May & Tiitinen, 2010).
In addition, the theoretical inference of a genuine deviance-detection component being present in SSA (Hershenhoren et al., 2014; Taaseh et al., 2011) has been recently confirmed empirically in the visual (Hamm & Yuste, 2016) and auditory systems (Chen et al., 2015; Parras et al., 2017). All the limitations hindering both the adaptation and the model-adjustment hypothesis are encouraging the adoption of a new perspective, capable of integrating MMN and SSA data in a common theoretical framework, and fully accountable for the neurobiological mechanisms underlying deviance detention at every level of measurement. This framework is referred to as the hierarchical-inference hypothesis, most commonly known as predictive coding.
Predictive Coding: Moving to a Common Framework
Predictive coding is one of the most influential and comprehensive theories of neural function addressing how the brain makes sense of the world (Heilbron & Chait, 2017). It has become very popular in the past decade, although some of the insights comprehended in this theoretical framework have a long tradition in the literature. Early in the history of cognitive psychology, Neisser (1976) already introduced the concept of perceptual cycle, which might be considered an ancestor of predictive coding. As the biological basis for Bayesian theories of perception and cognition, predictive coding offers compelling explanations for multiple phenomena from neuroanatomy (Friston, 2005) and electrophysiology (Rao & Ballard, 1999) to psychology (Knill & Pouget, 2004). Regarding neuroscience of perception, predictive coding was initially adopted in the study of visual processing (Lee & Mumford, 2003; Rao & Ballard, 1999), and the application of its principles to research in the auditory system is gaining momentum as of late (Denham & Winkler, 2018; Heilbron & Chait, 2017; Schröger et al., 2014; Schröger, Marzecová, & SanMiguel, 2015; Winkler & Schröger, 2015).
According to the predictive coding theory, perception emerges from integrating sensory information from the environment and our predictions based on an internal representation of that information (Auksztulewicz & Friston, 2016; Bastos et al., 2012; Friston, 2005). As in the model-adjustment hypothesis, current inputs are predicted from past events through a model, and the aim of the system is to minimize errors in the prediction by continuously updating the model. The reduction of prediction error is achieved through recurrent interactions among levels of a processing hierarchy, organized in distinct anatomical structures and neuronal populations. Each level of processing generates abstractions (models) to fit the sensory information relayed from lower levels of processing, sending top-down predictions (or expectations) to the lower levels to explain away those inputs, instead of investing resources in processing them yet another time. Convergence of inputs and predictions configures a multilevel representation of the sensory information, and thereby perception arises at the minimum expense of processing resources. But when those top-down predictions do not fit the actual input, the first-level neuronal populations convey a prediction error signal to the higher levels to favor the processing of unpredicted features. This prediction error is functionally analogous to the aforementioned genuine deviance detection. Hence, lower and higher processing stages keep communicating iteratively in reciprocal pathways until the suppression of the error signal is accomplished, which indicates that perceptual encoding is optimized.
Optimization of perceptual representation throughout this hierarchical chain of processing levels of increasing abstraction complexity requires managing the relative influence of top-down prior expectations and bottom-up prediction errors. This process would require short-term synaptic plasticity. Mechanisms operating at the input of the neuron, such as synaptic depression and facilitation or inhibition, would differentially affect diverse parts of its dendritic tree to optimize the postsynaptic sensitivity of neurons acting as deviance-detection units, that is, neurons showing SSA (Garrido, Kilner, Stephan, et al., 2009). Thus, when repetitive stimuli can be predicted precisely by top-down afferents, bottom-up influences are reduced by decreasing the postsynaptic responsiveness of the neurons to the redundant sensory inputs, like the adaptation hypothesis predicted. Notwithstanding, the limitation is that this is the only effect within deviance detection that adaptation can effectively account for: repetition suppression (Auksztulewicz & Friston, 2016; Garrido, Kilner, Kiebel, et al., 2009; Grill-Spector, Henson, & Martin, 2006; Summerfield, Monti, Trittschuh, Mesulam, & Egner, 2008; Todorovic et al., 2011), that is, the attenuation of the evoked response to a certain repeated stimulus feature, and namely to a certain repeated frequency in the case of auditory stimulation (Duque et al., 2016).
Predictive coding provides a much more extensive explanation, as it postulates that any regularity, simple or complex, is susceptible to being encoded at some level of processing, thereby subduing suppressive effects at that level. That is, regularity encoding may lead to automatic expectation suppression (Grotheer & Kovács, 2016; Pajani, Kouider, Roux, & de Gardelle, 2017; Todorovic & de Lange, 2012; but see Barascud, Pearce, Griffiths, Friston, & Chait, 2016; Southwell et al., 2017), which can be understood as the functional footprint of those perceptual representations held by the processing network of a certain neuronal population. Any stimulus fitting in the represented regularity does not have to be represented anew, saving processing resources and thus evoking attenuated responses. Throughout progressive levels of hierarchical processing, this mechanism acts like a concatenation of filters of redundant information. From the predictive coding standpoint, adaptation can only account for the simplest form of expectation suppression, which is repetition suppression. Hence, less computationally demanding regularities, such as repetition of a physical feature (e.g., frequency), can be encoded and locally reflect suppression already at lower levels of the processing hierarchy. But with the accumulation of successive processing levels in iterative interaction, higher regions could be capable of extracting increasingly complex stimulus patterns (e.g., an AB tone-pair) and even abstract relationships (e.g., “succession of pairs of tones in which the first tone of the pair can be anything, but second tone always has a higher pitch than the first”), as observed in MMN research. As more incoming inputs fit into the attained representation, the strength and confidence in the perceptual model increases, yielding to stronger suppression on the response evoked by those inputs. But when a stimulus diverges from the encoded regularity, a prediction error will be issued. The amplitude and shortened latency of the resultant deviance-detection signal should be proportional to the magnitude of divergence, as well as the confidence in the perceptual model represented in the processing network, as human MMN (Näätänen et al., 2007) and animal SSA (Ulanovsky et al., 2003) evidence seem to indicate.
Deviance can only be defined in relation to something regular (Winkler & Schröger, 2015). Hence, the term deviance detection must refer to the total signal evoked by a stimulus that violates a regularity encoded in a given part of the processing system, when compared with the response to the same stimulus when it fitted in that represented regularity. Then, when we use the term deviance detection, we include two processes. The adjustment of the incoming input to the model represented in the system would yield expectation suppression. But when a deviant event does not adjust to the expectation, the evoked neuronal response is released from suppression, and the local network responsible of encoding the unfitting feature forwards a prediction error to higher levels of processing. The reciprocal signaling between hierarchical levels of processing makes possible that the same stimulus might generate a prediction error in one part of the system while falling under expectation suppression in other part (e.g., local/global paradigm), depending on the representations held at each neuronal population. Navigating these three core concepts (deviance detection, expectation suppression, and prediction error), predictive coding can account for and connect all the evidence coming from MMN and SSA research.
While an MMN evoked by a simple deviant like an infrequent decrement in tone intensity or an omission is difficult to explain in terms of adaptation alone (Duque et al., 2016), predictive coding accounts not only for that but also for how an MMN can be generated by the violations of abstract rules based on complex interstimulus relationships or transitional probabilities (Dehaene et al., 2015; Mittag, Takegata, & Winkler, 2016). The omission of an expected tone implies a violation of the established perceptual representation in the system, so the perceptive model would require an update. In other words, even if no sound has occurred, the auditory system must encode the no-tone event as a prediction error. Thus, that auditory response to the silence is in truth a pure prediction error, signaling the unexpected gap in the sequence. Regarding the alternation and the local/global sequences, the rationale is similar but instead features an unpredictable repetition of a tone. Note that, as that repeated tone is indeed a local standard (e.g., AAAAB), its corresponding evoked response will undergo repetition suppression at lower levels of the processing hierarchy (“tone A is already represented on the system”). But as that same stimulus also creates global deviance in the pattern (AAAB AAAB AAAAB), that repetition will entail a prediction error in higher levels of the processing hierarchy (“tone B was expected after three iterations of tone A, but tone A repeated a fourth time”), as some studies with human ERPs indicate (Recasens et al., 2014). Likewise, the local deviant will provoke a prediction error at lower levels, but as a global standard, it will subdue expectation suppression at higher levels in the processing hierarchy (Recasens et al., 2014). At the higher order stages, expectation suppression and prediction error could emerge, respectively, from encoding and violating abstract features of interstimulus relationships (Paavilainen, 2013; Paavilainen et al., 2018; Saarinen et al., 1992; Schröger et al., 2007; Tervaniemi et al., 1994).
Understanding the brain essentially as a prediction machine has delivered great integrative potential to the scientific literature on perception and cognitive neuroscience (Clark, 2013; Hohwy, 2012). Several authors have been able to explain previous ERP evidence on auditory processes such as deviance detection, stream segregation, auditory scene analysis, and attention to sound, all together under the common theoretical framework of predictive coding (Garrido, Kilner, Kiebel, et al., 2009; Schröger et al., 2014, 2015; Wacongne, Changeux, & Dehaene, 2012; Winkler et al., 2009; Winkler & Schröger, 2015). As discussed earlier, predictive coding is also capable of reconciling the two classic MMN-based and SSA-based interpretations of deviance detection, postulating the adjustment of a generative model of the current stimulus train (as in the model-adjustment hypothesis) founded on plastic changes in synaptic connectivity (as in the adaptation hypothesis; Garrido, Kilner, Stephan, et al., 2009). In this review, we intend to thoroughly expand that integrative endeavor into SSA research. Using a predictive coding perspective, we reinterpret the evidence obtained from neuronal-level recordings, such as extracellular single-unit and multiunit activity (Figure 1(e)) or patch clamp recordings (for reviews more focused on macrocellular recording techniques, see, e.g., Escera & Malmierca, 2014; Fishman, 2014; Garrido, Kilner, Stephan, et al., 2009; Grimm, Escera, & Nelken, 2016; May & Tiitinen, 2010), as an attempt to reconcile SSA data with MMN observations within a common framework of Bayesian hierarchical inference.
Control Sequences for a Renewed Methodology in SSA Research
The oddball paradigm faces a major methodological limitation conforming to the predictive coding perspective: It confounds the effects of adaptation and expectation (Ruhnau, Herrmann, & Schröger, 2012). In other words, it does not allow the distinction between repetition suppression and more complex forms of predictive activity (Fishman & Steinschneider, 2012; Nelken & Ulanovsky, 2007; Taaseh et al., 2011). Repetition suppression is the result of abstracting the less computationally demanding interstimulus relationship (repetition) and establishing the simplest expectation in consonance: “The next input is going to be similar to the previous one encoded.” Since the input information is already represented in the system, there is no need to mobilize processing resources to represent it again. This type of prediction should require few encoding capabilities, so it could be resolved already at the lower levels of the processing hierarchy (Figure 1(d)), as suggested by the presence of SSA as early as the auditory midbrain (Bibikov, 1977; Malone, & Semple, 2001; Pérez-González et al., 2005).
In consequence, during the oddball sequence, the unchanging pattern formed by the repeated presentation of standards quickly generates top-down predictions that efficiently explain away the sensory input and suppress prediction error, which leads to a reduction of standard-evoked response by means of short-term synaptic plasticity (adaptation). But when the deviant stimulus interrupts the train of standards, two distinguishable processes take place, yielding two distinct components in the difference signal we call deviance detection (also neuronal mismatch, when recorded from single units; Parras et al., 2017). On one hand, the repetition rule has been violated, freeing the neuronal response from repetition suppression. Strictly speaking, this activity could be considered as a prediction error emerging, but for the purpose of clarity, we refer to this particular component as repetition suppression, as it accounts for the suppressive effects of representing the repetition rule in the processing network. On the other hand, the random appearance of the deviant sound could not be predicted, generating an additional prediction error signal that is transmitted bottom-up to higher levels in the processing hierarchy. Those higher order processing stations might be capable of fitting the deviant event in some new rule and explain it, generating new predictions that might be more accurate. This component of the deviance-detection signal is the one we refer to as prediction error, as done elsewhere (Parras et al., 2017).
Thus, deviance-detection measurements obtained using the oddball paradigm have the two components mingled: repetition suppression and prediction error. It is interesting how other sound sequences used in human MMN research, like the omission (Berlot et al., 2018; Raij et al., 1997; Yabe et al., 1997) or sequences based on complex patterns or abstract rules (Dehaene et al., 2015; Heilbron & Chait, 2017; Paavilainen et al., 2018; Symonds et al., 2017; Wacongne et al., 2011, 2012), are able to elicit and manipulate these two components separately to a certain extent. But this is impossible to do in SSA studies, by definition. So to disentangle the two effects of extracting the standard-repetition rule, we need the inclusion of a control condition.
From a predictive coding standpoint, a sequence must meet at least two theoretical requirements to be considered an apt control for the oddball paradigm. First, the control sequence cannot feature the recurrent repetition of an individual stimulus, making it possible to assess the effect of repetition suppression yielded by the representation of that particular interstimulus relationship during the oddball sequence. This would also allow us to estimate the amount of prediction error generated from the remaining deviance-detection signal. Note that other more complex interstimulus relationships featuring in the design of the control sequence could exert expectation suppression on incoming stimulation if those patterns are identified at higher levels of processing. However, that attenuation would certainly not be due to the repetition suppression tested, which effect during the oddball paradigm we want to estimate. Second, the control sequence must be able to account for the general state of refractoriness originated in the processing system during the oddball sequence. This is the reason why muting the train of standards and testing the response to a stimulus over a background of silence (the so-called deviant-alone control) do not provide a valid benchmark for the decomposition of deviance detection. To date, two control sequences have been proposed meeting those criteria: the many-standards control and the cascade control (Figure 1(b)).
The many-standards control was pioneered in human MMN studies (Schröger & Wolff, 1996) and has since been introduced in a growing number of SSA studies as well (Chen et al., 2015; Farley et al., 2010; Hershenhoren et al., 2014; Parras et al., 2017; Taaseh et al., 2011). In the many-standards sequence, the target tone (fi, the tone that evoked responses we are interested in measuring) is presented immersed in a random sequence compounded by a handful of tones, every one of each with the same probability of appearance as the deviant of the oddball sequence. Because the train of standards has been replaced with a random succession of assorted tones, this sequence does not generate repetition suppression in response to the target tone while controlling for the state of refractoriness of the auditory system (Schröger & Wolff, 1996). The comparison between the response of the target tone within the control sequence with the standard-evoked response accounts for repetition suppression, while the rest of the deviance detection can be imputed to prediction error (Figures 1(c) and 3 for examples of real neurons).
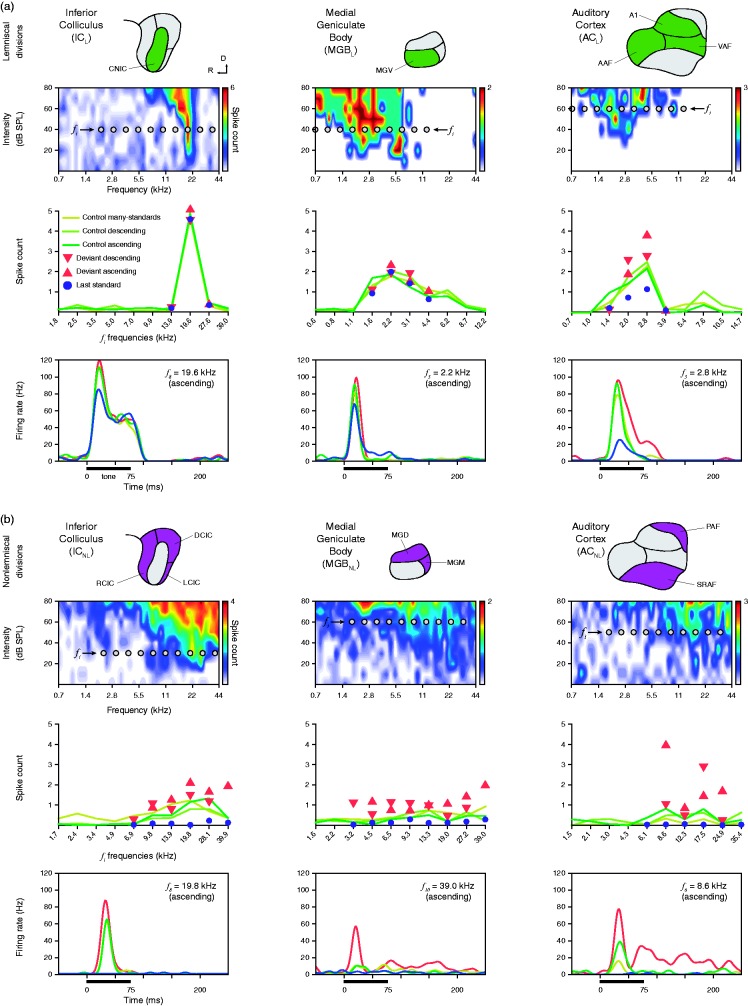
Figure 3.
Deviance detection and prediction error in representative neurons of the anesthetized rat. (a) Examples of lemniscal single-unit responses in each recorded auditory station (columns). The first row shows schematics of the lemniscal subdivisions (green) within each nucleus. The second row shows the frequency-response area (representation of neuronal sensitivity to different frequency-intensity combinations) of representative lemniscal neurons from each nucleus. Ten gray dots within each frequency-response area represent the 10 tones (fi) selected to build the experimental sequences (Figure 1(a)). The third row displays the measured responses of the particular neuron to each fi tone (baseline-corrected spike counts) for each tested condition. Note that measured conditions tend to overlap in the subcortical stations (ICL and MGBL) and only start differentiating from each other once auditory information reaches the cortex (ACL). The fourth row shows sample peristimulus time histograms (PSTH) comparing the neuronal responses with each condition tested for an indicated fi tone. A thick horizontal line represents stimulus duration. (b) Examples of nonlemniscal neuronal responses in each recorded auditory nuclei, organized as in (a). The first row highlights nonlemniscal divisions in purple. In the second row, note frequency-response areas tend to be more broadly tuned, when compared with lemniscal neurons. In the third row, responses to deviant conditions tend to relatively increase and distance themselves from their corresponding controls as information ascends in the auditory pathway. Also note that responses to last standards are feeble or even completely missing across all nonlemniscal stations (ICNL, MGBNL, and ACNL). In the last row, the strong influence of the experimental condition over the neuronal response to the same tone can be clearly appreciated in the three nuclei.
AAF = anterior auditory field; CNIC = central nucleus of the inferior colliculus; DCIC = dorsal cortex of the inferior colliculus; LCIC = lateral cortex of the inferior colliculus; MGB = medial geniculate body of the thalamus; MGD = dorsal division of the MGB; MGM = medial division of the MGB; MGV = ventral division of the medial geniculate body of the thalamus; PAF = posterior auditory field; RCIC = rostral cortex of the inferior colliculus; SPL = sound pressure level; SRAF = suprarhinal auditory field; VAF = ventral auditory field. Adapted from Parras et al. (2017).
Nevertheless, the many-standards control might be somewhat conservative in the identification of prediction error. This is because there is a conceptual difference between the oddball and the many-standards sequences. During the oddball sequence, albeit punctually violated by the deviant, an internal rule is actually being established by the repetition of standard tone, giving rise to predictions based on it (Ruhnau et al., 2012). During the many-standards sequence, conversely, the random succession of assorted stimuli never allows for the substantiation of a reliable internal representation. Presumably, no efficient predictions can be made based on randomness, which keeps any effective expectation suppression from happening. Even if we accept that randomness in its different degrees may constitute a category of interstimulus relationship at some level of processing, it would always generate much feebler expectation suppression than a comparable regularity. As the system tries to fit the random sequence into an abstract rule unsuccessfully, the many-standards control could be still eliciting a considerable amount of prediction error itself, thereby overestimating the effect of repetition suppression during the oddball sequence. To overcome this caveat, the cascade sequence (Ruhnau et al., 2012) presents the control stimulation in an organized fashion; for example, in an increasing or decreasing frequency succession (Figure 1(b)). Hence, the target tone is presented embedded in a predictable series of tones, minimizing the emergence of prediction error signals. In addition, the two directions of cascade sequence allow the comparison with the respective versions of the oddball sequence (ascending or descending; Figure 1(a)), thereby controlling for the possible cross-frequency adaptation and pitch gliding effects that the preceding stimulus could exert over the target tone. Comparisons between control and oddball conditions are similar to the many-standards sequence (Figures 1(c) and 3). Despite been regarded as a better control than the many-standards from a theoretical standpoint, the use of the cascade sequence is just starting to pass into SSA research (Parras et al., 2017).
The Two Axes of Predictive Coding Hierarchy in the Auditory System
Using both many-standards and cascade controls, a recent study has provided evidence of a generative system of prediction error distributed hierarchically along the auditory pathway of anesthetized rats and confirmed it in awake mice (Parras et al., 2017), supporting predictive coding as a plausible interpretation of the organization and functioning of auditory neurons. Two vectors of increasing prediction error were identified in the auditory hierarchy: from lemniscal to nonlemniscal subdivisions, and from subcortical toward cortical structures (Parras et al., 2017). In the following, we adopt this view of hierarchical disposition to revisit the evidence of deviance detection at neuronal levels along the auditory pathway, to trace the roots of predictive activity in the auditory system and provide a robust neurophysiological basis for the MMN.
Lemniscal Versus Nonlemniscal Processing: Two Parallel Pathways of Auditory Information
Auditory information is transmitted along a series of nuclei arranged in a hierarchical manner, where different acoustic and contextual features are progressively extracted at each level of processing. Originating at the midbrain level of the auditory neuraxis, two parallel pathways can be distinguished marking each station they cross with structural and functional characteristic features. Almost half a century ago, Graybiel (1973) first coined and defined the so-called lemniscal line system and lemniscal adjunct system as a general categorization of sensory conduction routes referred to the lemniscus. Since then, the distinction between lemniscal (also referred as core or primary) and nonlemniscal (also referred as belt or nonprimary) pathways have been widely used in auditory research (Hu, 2003; Jones, 2003; Lee & Winer, 2008). Making this simple distinction, we can easily classify and understand the role of the multiple subdivisions present in the inferior colliculus (IC), the medial geniculate body of the thalamus (MGB), and the AC (Figures 1(e) and 4).
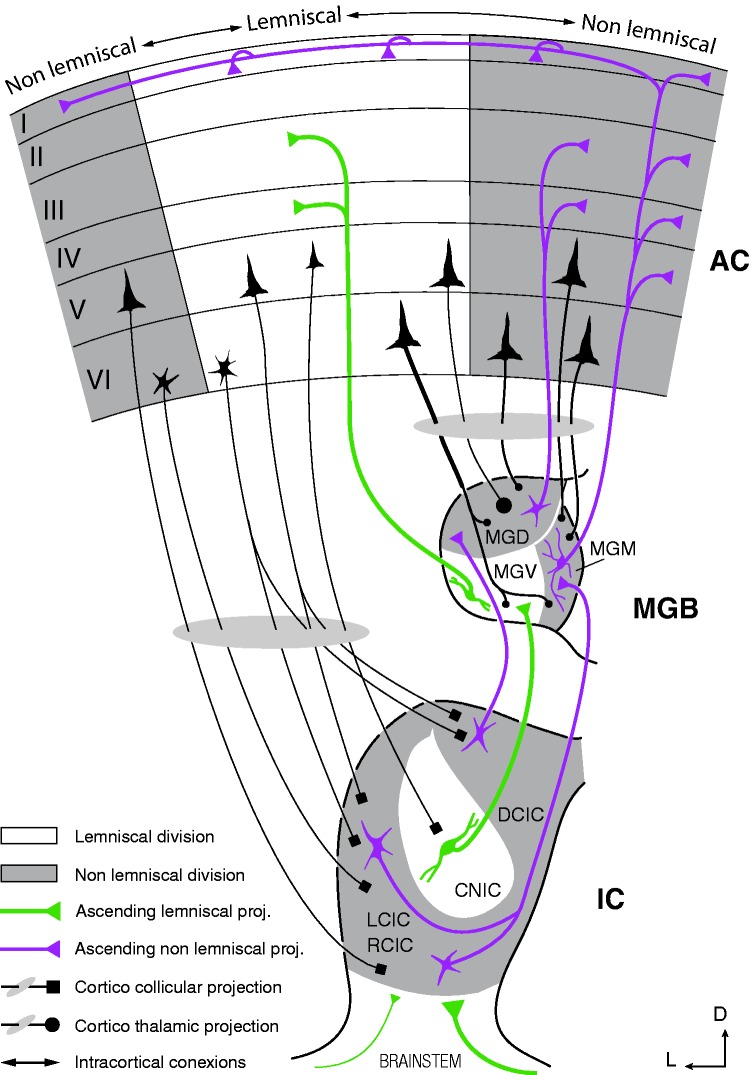
Figure 4.
Schematic diagram of the auditory pathway, showing the major stations and projections that constitute the lemniscal and nonlemniscal pathways. Note that divisions in subcortical nuclei are well preserved across species, while AC fields vary markedly (Malmierca & Hackett, 2010). As a rule of thumb, lemniscal tonotopic laminae tend to project to their analogous lamina in the next lemniscal division and receive few cortical projections, shaping a sort of straightforward pathway to the cortex. Conversely, nonlemniscal divisions tend to project mostly to other nonlemniscal divisions and receive dense cortical projections, shaping a loop-like connectivity network ideal for hosting a generative mechanism of hierarchical inference (Figure 1(d)).
AC = auditory cortex; CNIC = central nucleus of the inferior colliculus; DCIC = dorsal cortex of the inferior colliculus; IC = inferior colliculus; LCIC = lateral cortex of the inferior colliculus; MGB = medial geniculate body of the thalamus; MGD = dorsal division of the MGB; MDM = medial division of the MGB; MGV = ventral division of the MGB; RCIC = rostral cortex of the inferior colliculus. Adapted from Malmierca et al. (2015).
The lemniscal pathway represents a core of neurons in every auditory nucleus that tend to be sharply tuned and organized in rather clear tonotopic fashion made of anatomical laminae or bands. The majority of the neurons in each frequency lamina project to their corresponding homologous lamina in the next station of the lemniscal pathway (Malmierca, 2015), shaping a sort of straightforward pathway that relays sensory information mainly in bottom-up fashion (Figure 4). In addition to the sharp tuning of their frequency-response areas (Figure 3(a)), lemniscal neurons also show in general a better consistency in their response to the sound, including shorter latencies, higher firing rates, more overall spikes fired per stimulus, and higher spontaneous activity than their nonlemniscal counterparts (Malmierca, 2015). In other words, the response of these very tonotopically organized neurons is fundamentally driven by the physical features of the sound, receiving mostly (albeit not exclusively) ascending inputs from lower lemniscal stations in the auditory neuraxis. Hence, lemniscal divisions are thought to be in charge of accurately relaying sensory input about the stimulus characteristics, fundamentally disregarding its context or other abstract relations between sounds. As expected, recent experiments have demonstrated that subcortical neurons within lemniscal divisions do not generate prediction errors (Parras et al., 2017), so they are likely to be the prime provider of sensory input for their nonlemniscal analogues, distributing and feeding “raw” auditory information to the generative mechanism of hierarchical inference without being an active part of it. It is not until auditory information reaches the cortex that reliable deviance-detection activity can be found in the lemniscal pathway. As a matter of fact, it was lemniscal AC where SSA was discovered and characterized for the first time in the auditory system (Ulanovsky et al., 2003, 2004), and some authors have proposed it as the neural structure where the mechanism of hierarchical inference most probably initiates (Chen et al., 2015; Taaseh et al., 2011). The rat lemniscal pathway consists of the central nucleus of the IC, the ventral division of the MGB, and the primary AC that includes the A1 field, the anterior auditory field, and the ventral auditory field of the AC (Figure 3).
Parallel to the lemniscal pathway, another system referred to as the nonlemniscal pathway lies, in which any trace of tonotopical distribution is at its best diffuse. The nonlemniscal pathway consists of a belt of broadly tuned neurons that get inputs from the lemniscal core they are wrapping, and from other nonlemniscal stations: Subcortical nonlemniscal neurons send ascending projections to the next nonlemniscal station, while cortical neurons send descending projections mostly to the nonlemniscal divisions of the MGB and the IC (Figure 4; Malmierca & Ryugo, 2011; Saldaña, Feliciano, & Mugnaini, 1996). The fact that nonlemniscal neurons shape this loop-like connectivity with heavy cortical modulation, combined with their comparatively longer response latencies, the broadness of their frequency-response areas (Figure 3(b)), and their adjunct anatomical position relative to the lemniscal stream, strongly implies that they must exert an integrative function in the auditory system. In fact, this system of backward and forward connections between stations looks like the perfect network to host the top-down flow of predictions and the bottom-up transmission of prediction errors. Consequently, nonlemniscal divisions seem to form a higher order pathway of processing, constituting a secondary system capable of encoding more complex aspects of the auditory scene and tracking the history of stimulation, as required to account for the emergence of deviance-detection activity in the form of SSA or MMN, and for generating prediction error signals. The rat nonlemniscal pathway includes the rostral, lateral, and dorsal cortices of the IC; the dorsal (MGD) and medial (MGM) divisions of the MGB; and the suprarhinal auditory field and the posterior auditory field of the AC (Figure 3(b)).
Multilevel Hierarchical Auditory Processing: From the Midbrain to the Cortex Through the Thalamus
Anatomically speaking, the earliest generative units of prediction error are found in the cortices of the IC (Parras et al., 2017), at the beginning of the nonlemniscal pathway. Participation of subcortical nuclei in auditory deviance detection has been hinted at by several studies in humans (Althen et al., 2011; Cacciaglia et al., 2015; Cornella et al., 2012; Grimm, Recasens, Althen, & Escera, 2012; Shiga et al., 2015; Skoe, Chandrasekaran, Spitzer, Wong, & Kraus, 2014; Skoe & Kraus, 2010; Skoe, Krizman, Spitzer, & Kraus, 2013; Sonnadara, Alain, & Trainor, 2006; Tervaniemi et al., 2006), despite the technical challenge of recording noninvasively and correctly locating the source of a signal originating from such profound regions of the human brain (Bidelman, 2018; Coffey, Herholz, Chepesiuk, Baillet, & Zatorre, 2016; Coffey, Musacchia, & Zatorre, 2017). This evidence of early deviance detection is most interesting, considering hierarchical-inference hypothesis of predictive coding was in its inception formulated in terms of backward and forward connections between layers and areas of the cortex (Bastos et al., 2012; Friston, 2005). Subcortical involvement is thus somewhat unexpected, even if participation of subcortical structures was never explicitly discarded, or was even foreseen by some authors (Auksztulewicz & Friston, 2016), to the extent that the complex computational machinery of the subcortical auditory system has even led to the speculation of a comparable role of the IC and the primary visual cortex (King & Nelken, 2009). The IC is the auditory center in the midbrain where nearly all ascending pathways converge before sending information to the AC via the thalamus. Excitatory, inhibitory, and neuromodulatory projections originating in the auditory brainstem and cortical regions, as well as nonauditory centers, converge in the IC (Malmierca, 2015). This could provide IC neurons with the necessary inputs to be able to integrate information over time through changes in the efficiency of their synaptic connections based on their history of activation. Finding prediction error signaling as early as the auditory midbrain implies that this generative mechanism of hierarchical inference could be evolutionarily old and deeply rooted in the architecture of the auditory system.
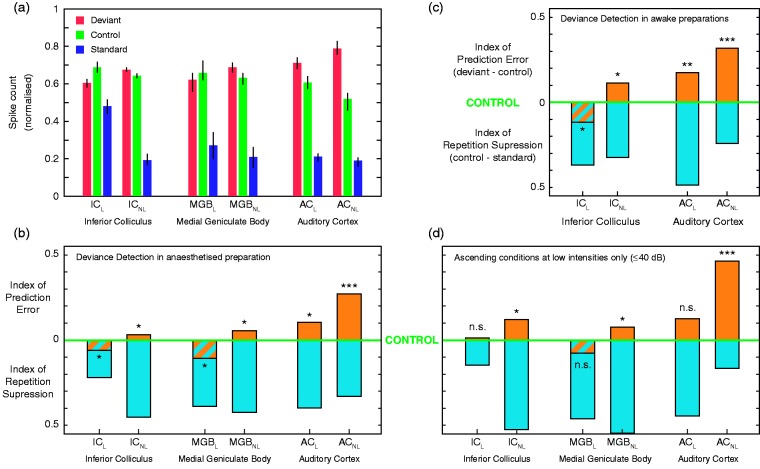
We have seen that most of the deviance-detection activity elicited by the oddball paradigm in the IC cortices can be accounted for by repetition suppression. As auditory information flows up the auditory pathway, the prediction error component keeps growing in proportion (Parras et al., 2017). Starting off in the auditory midbrain with the smallest index of prediction error at the IC cortices, it increases at the level of the nonlemniscal auditory thalamus. Another enlargement ensues when the signal reaches the lemniscal fields of the AC, or primary areas. Finally, it grows again until it is able to explain 50% or more of the overall deviance-detection activity recorded in the nonlemniscal fields of the AC, or belt areas (Figure 5; Parras et al., 2017).
Figure 5.
Emergence of prediction error along the auditory hierarchy. (a) Median normalized tone-evoked responses (lines indicate SEM) to the deviant, standard, and control within each recorded auditory station. (b) Median indices of prediction error (orange) and repetition suppression (cyan) in anesthetized rats, represented with respect to the baseline set by the control. Thereby, the index of prediction error is upward-positive, while the index of repetition suppression is downward-positive. Each median index corresponds to differences between normalized responses in (a). Asterisks denote statistical significance of prediction error against zero median (*p = .05, **p = .01, ***p = .001). (c) Indices of prediction error and repetition suppression in awake mice. (d) Same as in (b), but only representing ascending conditions at low intensities.
AC = auditory cortex; MGB = medial geniculate body of the thalamus; IC = inferior colliculus; L = lemniscal divisions; NL = nonlemniscal divisions. Adapted from Parras et al. (2017).
The proportion of prediction error grows even larger when the animals are awake (Figure 5(c); Cai, Richardson, & Caspary, 2016; Parras et al., 2017) and aged (Cai et al., 2016), as well as when the stimulation has low intensities (Figure 5(d); Parras et al., 2017). When awake mice were presented tone sequences at a low intensity, prediction error reached up to 80% of the overall deviance-detection activity recorded in the nonlemniscal AC (Parras et al., 2017). In accordance with the predictive coding principles, insofar as anesthesia did not efface the trace of prediction error from the neuronal activity of any station, it can be assumed that the generative mechanism of hierarchical inference is automatic and preattentive. Nevertheless, the larger prediction error proportions detected in awake rodents suggest that the state of consciousness, alertness, and attention may play an important role in its modulation. Interestingly, there was an unexpected enhancement of prediction error when the intensity of the stimulation was low. This suggests that the generative mechanism of hierarchical inference may play a crucial role in facilitating perceptual saliency. When perception must be accomplished under challenging sensory conditions, the increased gain of prediction error in the whole auditory system plausibly aids stimulus discrimination (Parras et al., 2017). This saliency facilitation may attain increasing importance with aging, as top-down influences could compensate for degradation and impairments of ascending acoustic information in older individuals (Cai et al., 2016).
The Subcortical Contribution to Deviance Detection
As mentioned previously, auditory SSA was first discovered in A1, and initially it was thought to be absent in subcortical auditory nuclei (Ulanovsky et al., 2003). Added to the cortical origin of MMN and the cortical formulations of predictive coding, authors originally interpreted deviance detection as a purely cortical activity. This cortical nature had to be revisited and reconceptualized after the discovery of SSA in the IC (Ayala et al., 2015; Ayala & Malmierca, 2015, 2018; Duque et al., 2012, 2016; Duque & Malmierca, 2015; Malmierca et al., 2009; Parras et al., 2017; Patel et al., 2012; Pérez-González et al., 2005, 2012; Pérez-González & Malmierca, 2012; Valdés-Baizabal et al., 2017; Zhao et al., 2011) and MGB (Anderson et al., 2009; Anderson & Malmierca, 2013; Antunes et al., 2010; Antunes & Malmierca, 2014; Bauerle et al., 2011; Duque et al., 2014; Parras et al., 2017). Significant and strong deviance detection activity in the form of SSA appeared prominently in the IC cortices (Malmierca et al., 2009), the MGD, and even more intensely in the MGM (Antunes et al., 2010). The nonlemniscal divisions of subcortical nuclei encompassed most of the neurons showing complete SSA, while lemniscal neurons tended to display only partial and rather poor levels of SSA (Antunes et al., 2010; Malmierca et al., 2009). As a consequence, population levels of subcortical deviance detection were substantially higher in the nonlemniscal divisions. Furthermore, positive prediction error in the subcortical auditory system was found only in the nonlemniscal divisions (Parras et al., 2017).
The alleged cortical generation of deviance detection was not completely dismissed after the existence of SSA was demonstrated in subcortical stations. It was suggested that subcortical traces of deviance detection could be imposed by the cortex (Nelken & Ulanovsky, 2007) given the massive corticocollicular projections that the IC cortices receive and the impressively dense corticothalamic projections (Figure 3) that outmatch the thalamocortical output by a factor of 10 (Malmierca, Anderson, & Antunes, 2015). Descending projections must necessarily exert at least a considerable modulatory function (Ayala et al., 2015), but the prime source of deviance detection cannot be pinned down just by investigating connectivity. To address this question, studies of reversible deactivation of the AC using a cooling technique were conducted while recording the MGB (Antunes & Malmierca, 2011) and the IC (Anderson & Malmierca, 2013). The general results demonstrated that the AC clearly modulated the firing rate of the nonlemniscal neurons in a gain-control manner (Malmierca et al., 2015; Pérez-González et al., 2012), helping to increase the contrast between standard and deviant stimuli by affecting the discharge rate to both proportionally (Ayala, Pérez-González, & Malmierca, 2016; Duque, Ayala, & Malmierca, 2015; Pérez-González & Malmierca, 2012). However, the overall subcortical SSA levels and dynamics remained mostly unaffected by cortical deactivation, with only about half of the adapting IC neurons (Anderson & Malmierca, 2013) and almost none in the MGB (Antunes & Malmierca, 2011) showing a significant change in their SSA sensitivity. It would be very interesting to test how cortical deactivation specifically affects the prediction error component of subcortical SSA in future studies.
In light of these results, it is more plausible that deviance detection could be generated de novo at the intrinsic microcircuitry of each auditory station (Ayala & Malmierca, 2013). The great functional diversity of individual neurons suggests they perform as differentiated processing units. Some responses show partial or even absolute repetition suppression but no prediction error at all. Other responses contain partial proportions of repetition suppression and prediction error. Some neurons do not exhibit significant deviance detection despite being in a nonlemniscal division (Parras et al., 2017). Functionally distinct neurons undergo dissimilar effects when subjected to pharmacological manipulation, for example, by cholinergic modulation (Ayala & Malmierca, 2015). These data dovetail with an intrinsic de novo generation of deviance detection. The interaction of neurons with specialized computing roles, arranged in a local hierarchical network, could suffice to extract features and hold representations. Deviance detection in response to unpredictable events would build up as information flows throughout successive neuronal networks and processing stations along the auditory pathway.
Hence, predictive activity emerges from the interaction of neuronal networks hosted in different brain regions, something that must require a delicate balance of neurotransmitters and neuromodulatory influences to coordinate. Human studies on deviance detection have identified the influence of several neurotransmitter systems in MMN (Garrido, Kilner, Stephan, et al., 2009). In animal models, the microiontophoresis technique allows to permeate the vicinity of a recorded neuron with neurotransmitters and modulators that activate (agonists) or block (antagonists) certain membrane receptors, yielding measurable synaptic effects. By means of this precise neuropharmacological manipulation, many SSA studies have characterized the contribution of some of those membrane receptors to the generation of subcortical deviance detection. In the following subsections, we discuss some insights regarding this matter that might be of hypothetical interest to the predictive coding interpretation, despite the limitations imposed by the fact that none of these studies has used the many-standards or cascade controls thus far. Future research may use the proposed methodology to address the influence of neuromodulation over prediction error specifically.
GABAergic Neuromodulation: A Gain Control of Deviance Detection
Synaptic inhibition must be essential for auditory deviance detection, inasmuch as it is responsible for “sculpting” the excitatory activity of the brain (Capano, Herrmann, & De Arcangelis, 2015). γ-Aminobutyric acid (GABA) is the chief inhibitory neurotransmitter in the mammalian central nervous system, binding to two main classes of receptors. GABAA receptors are ionotropic, part of a ligand-gated ion channel. GABAB receptors, on the other hand, are metabotropic receptors, regulating the opening or closing of ion channels via intermediate G proteins. In addition to GABA, glycine is another inhibitory neurotransmitter that can be found in the mammalian brainstem.
Several studies have thoroughly unraveled the role of GABAergic inhibition in subcortical deviance detection using the microiontophoresis technique. The application of the antagonist gabazine in the rat IC (Pérez-González et al., 2012) and MGB (Duque et al., 2014) has demonstrated the strong effect of inhibition exerted through GABAA receptors, which regulates the postsynaptic membrane potential (Sivaramakrishnan et al., 2004). When GABAA receptors were blocked, the general responsiveness of the neuron increased, reducing the proportional difference between deviant and standard responses, thus dampening deviance detection (Figure 6(b)). Conversely and coherently, the injection of the endogenous agonist GABA or the selective GABAA-receptor superagonist gaboxadol increased SSA levels (Duque et al., 2014). This suggests that subcortical deviance detection is modulated by a gain-control mechanism mediated by GABAA receptors that facilitates the relative saliency of unpredicted auditory events over the redundant ones, as in the so-called iceberg effect (Figure 6(a); Pérez-González et al., 2012). The iceberg effect describes the observation whereby the spike output of a neuron under inhibition is more sharply tuned than the underlying membrane potential because only the strongest excitatory input sufficiently depolarizes the membrane to reach threshold for spike generation (Isaacson & Scanziani, 2011).
Figure 6.
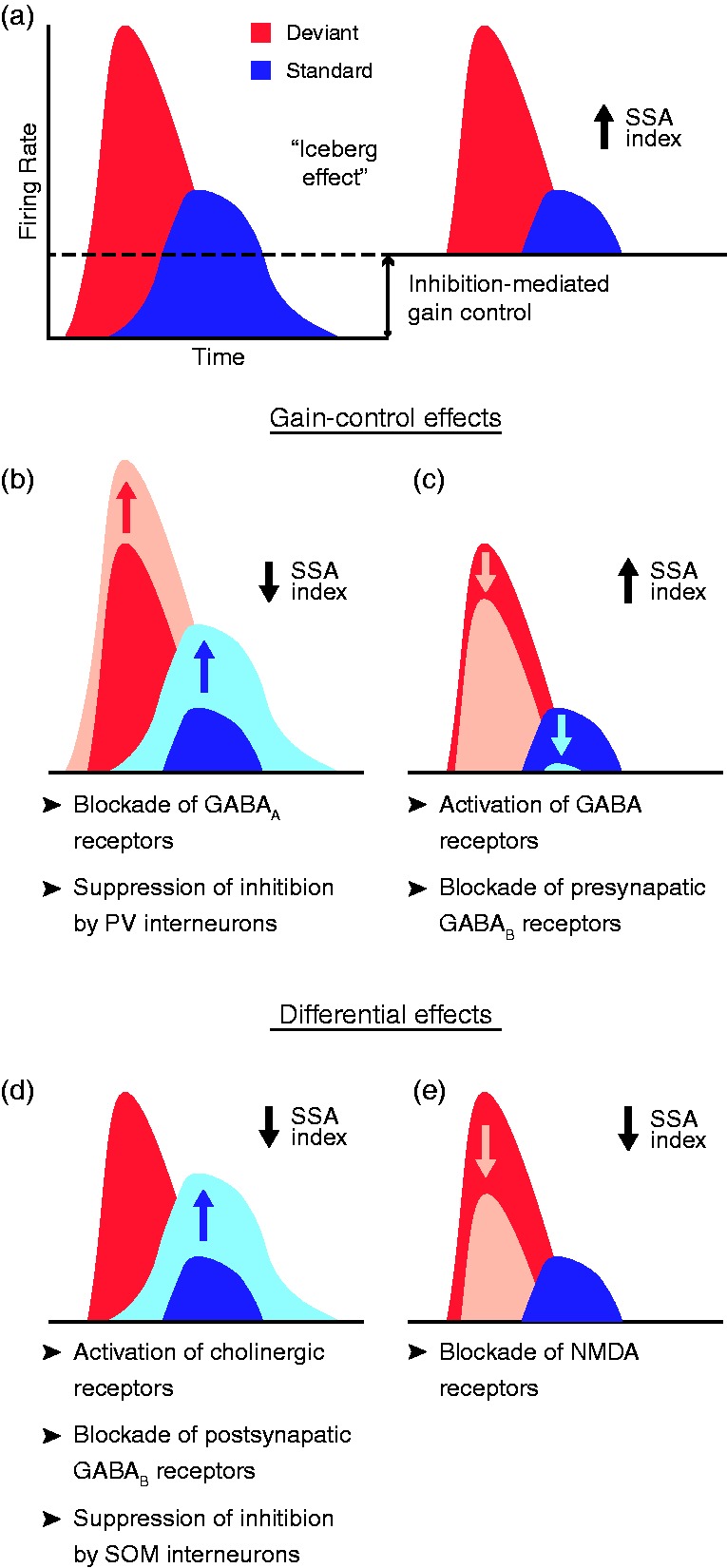
Effect of pharmacological and inhibitory manipulation on SSA index. (a) Schematic representation of the iceberg effect, with a dashed line representing the amount of activity reduced by inhibition. In the absence of inhibition (left), the higher excitability of the neuron yields larger tone-evoked firing rates, thus reducing the relative difference between the response to deviant and standard stimuli. Inhibition reduces both tone-evoked responses (right), increasing the deviant-to-standard ratio and thus enhancing SSA. (b, c) Gain-control effects, that is, effects affecting overall excitability of neurons that produce a change in SSA index, identified as of date via indicated manipulations. (d, e) Manipulations that yield a decrease in SSA index because of the differential effect exerted at the standard- or deviant-evoked responses. Albeit simplified for clarity in figure, this does not mean that the manipulation exerts exclusive effects on deviant or standard responses. Rather, a significantly much larger effect is observed at one that does not generalize to the other, even if the latter does not remain completely unaffected.
AAF = anterior auditory field; GABA = gamma-aminobutyric acid; NMDA = N-methyl-D-aspartate; PV = parvalbumin; SOM = somatostatin; SSA = stimulus-specific adaptation.
Some significant modulatory effects on deviance detection have been observed through pharmacological manipulation of GABAB receptors, although they are not as evident and profound as those mediated by the GABAA type. In the rat IC, the blockade of presynaptic GABAB receptors prompted a decrease of the overall neuronal excitability, yielding a subtle increase of SSA (Figure 6(c)). Conversely, the blockade of postsynaptic GABAB receptors reduced repetition suppression (Figure 6(d)), thereby reducing overall SSA (Ayala & Malmierca, 2018). Because GABAA receptors are ionotropic, blocking them rapidly affected deviance detection, while the effect of metabotropic GABAB receptors was slower (Ayala & Malmierca, 2018) because they are coupled to ion channels through second messengers (Mott, 2015). In the rat MGB, no significant differential effects were yielded by GABA or gaboxadol applications, even though gaboxadol does not bind to GABAB receptors (Bowery, Hill, & Hudson, 1983) while GABA activates both types (Duque et al., 2014). Taken together, in addition to the fact that the region of the MGB in which greater levels of SSA are observed, the MGM (Antunes et al., 2010), lacks GABAB receptors (Smith, Bartlett, & Kowalkowski, 2007) suggests that GABAA receptors may be the ones playing a pivotal role on inhibition-mediated modulation of deviance detection, while GABAB receptors might carry out auxiliary fine adjustments (Duque et al., 2014).
Glycinergic inhibition, on the other hand, is unlikely to play an eminent role in deviance-detection modulation. Pharmacological manipulation of glycine-mediated inhibition in the rat IC produced paradoxical effects on SSA (Ayala & Malmierca, 2018), which is not surprising considering that glycinergic receptors are mainly expressed in the lemniscal IC (Choy, Bishop, & Oliver, 2015; Merchán, Aguilar, López-Poveda, & Malmierca, 2005). Furthermore, rat MGB does not even express glycinergic receptors (Aoki, Semba, Keino, Kato, & Kashiwamata, 1988; Friauf, Hammerschmidt, & Kirsch, 1997), so its implication in deviance detection might be secondary, if any.
Finally, several combinations of GABAA, GABAB, and glycinergic antagonists were applied to test the effects of blocking multiple inhibitory receptors synchronously. Simultaneous coapplications of inhibition antagonists yielded evident augmented effects, generating a gradual increase of neuronal responsiveness that only affected significantly the standard-evoked response. The exacerbated decline of repetition suppression particularly, as a result of a combined application of inhibition antagonists, revealed the importance of a finely balanced and coordinated interplay of inhibitory receptors in deviance detection. Local inhibition could account for about half of the relative difference between the responses to standard and deviant stimuli, although it could not fully account for the generation of deviance detection (Ayala & Malmierca, 2018). This disposition seems to be unfolding a progression of consecutive inhibitory filters of redundant auditory information before reaching AC, emphasizing once again the fundamental contribution of subcortical processing in auditory predictive coding.
Cholinergic Modulation: Tuning Repetition-Sensitivity in Neurons
Cholinergic projections are known to play an important role in arousal, attention, and memory. Human MMN literature has suggested that cholinergic modulation favors the encoding of ongoing stimulation (Hasselmo & McGaughy, 2004; Jääskeläinen, Ahveninen, Belliveau, Raij, & Sams, 2007; Moran et al., 2013; Sarter, Hasselmo, Bruno, & Givens, 2005) and that it enhances responses to afferent sensory input in the AC (Hsieh, Cruikshank, & Metherate, 2000; Metherate & Ashe, 1993), implying that acetylcholine (ACh) could play an important role in deviance detection (Ranganath & Rainer, 2003).
This hypothesis has been tested in a microiontophoresis study in the rat IC (Ayala & Malmierca, 2015) using ACh chloride to activate the two main kinds of cholinergic receptors: nicotinic (ionotropic) and muscarinic (metabotropic) receptors. The infusion of the cholinergic agonist dampened repetition suppression (Figure 6(d)), but only in neurons exhibiting intermediate SSA levels (Ayala & Malmierca, 2015). In other words, ACh application prompted an increase in standard-evoked activity that did not generalize to the deviant-evoked responses. A decrease in deviance detection induced by cholinergic input would cohere with the lower levels of SSA observed in awake animals (Duque & Malmierca, 2015; von der Behrens et al., 2009) in which ACh levels are higher (Kametani & Kawamura, 1990; Marrosu et al., 1995). But concurrently, that reduced deviance detection may come with a relatively larger prediction error component (Parras et al., 2017). The precise relationship of components could be measured by including the aforementioned control sequences during the pharmacological manipulation in future studies. Whatever the case, it is apparent that cholinergic modulation in the IC contributes to persistence of the encoding of regular acoustical stimulation by decreasing repetition suppression (Ayala & Malmierca, 2015).
Interestingly, the excitability of neurons lacking significant SSA or displaying extreme SSA was mostly unaffected by cholinergic modulation, implying the existence of at least two types of deviance-detection units in the cortices of the IC. Neurons exhibiting complete SSA could act as hard static filters of redundant information, insensitive to ACh modulation. On the other hand, neurons showing partial SSA could intervene as a finer dynamic filter of auditory information, influenced by contextual and global brain states, such as deep sleep, wakefulness, attention, or arousal (Ayala & Malmierca, 2015). The presence of such diversity in the neuronal context-driven behavior further speaks in favor of a rich microcircuitry hosting populations of functionally heterogeneous neurons, which hierarchically interconnected should be capable of carrying out predictive coding already at the level of the nonlemniscal IC.
In addition, preparations with antagonists for the two types of cholinergic receptors were also examined. Scopolamine was used for blocking muscarinic receptors, and mecamylamine for the nicotinic receptors. As expected, affected neurons tended to augment their SSA levels for both cholinergic antagonists, but only scopolamine exhibited a significant increase at population level. Thus, muscarinic receptors play a prominent role in the delicate cholinergic modulation, most likely via M1-receptor subtype (Ayala & Malmierca, 2015). The activation of the M1-type receptor induces changes in potassium conductance that could act as an activity-dependent adaptation mechanism (Abolafia, Vergara, Arnold, Reig, & Sanchez-Vives, 2011; Sánchez-Vives, Nowak, & McCormick, 2000a, 2000b), making K+-mediated adaptation a potential mechanism underlying repetition suppression (Abolafia et al., 2011; Ayala & Malmierca, 2015; Malmierca et al., 2014). However, these results cannot be extrapolated to other structures of the auditory pathway, due to the different sources of cholinergic projections. The cholinergic input to the IC comes from the pontomesencephalic tegmentum (Motts & Schofield, 2009; Schofield, Motts, & Mellott, 2011), while the main source of ACh in AC is the basal forebrain (Bajo, Leach, Cordery, Nodal, & King, 2014; Edeline, Hars, Maho, & Hennevin, 1994; Zaborszky, van den Pol, & Gyengesi, 2012), so cortical testing is required.
Endocannabinoids: Modulating the Modulators
Endocannabinoids have been shown to play a role in short-term neural plasticity (Castillo, Younts, Chávez, & Hashimotodani, 2012), so their retrograde signaling could be involved in the modulation of deviance detection at the neuronal level. This was demonstrated by the application of two different agonists of CB1 cannabinoid receptors, anandamide (intravenously) and O-2545 (microiontophoretically). Both systemic and local injections prompted a decrease of repetition suppression in a subset of neurons in the rat IC (Figure 6(d); Valdés-Baizabal et al., 2017). The blockade of CB1 receptors, via microiontophoretic application of the antagonist AM251, leads to nonsignificant population effects. Nevertheless, there was a coherent tendency of some neurons to strengthen their repetition suppression (Valdés-Baizabal et al., 2017).
Those effects could be due to the retrograde modulation of inhibitory and excitatory inputs by cannabinoids, well described along the auditory pathway for both glutamatergic and GABAergic synapses (Zhao, Rubio, & Tzounopoulos, 2008). It is likely that IC neurons showing cannabinoid-mediated modulation received inhibitory input from GABAergic neurons expressing CB1 receptors in their presynaptic terminals (Merchán et al., 2005). The application of CB1 agonists would decrease GABA release of presynaptic inhibitory neurons, thereby increasing the activity and reducing SSA of the recorded postsynaptic neurons (Valdés-Baizabal et al., 2017). A synergistic activity of the endocannabinoid system with other neuromodulators is also a possibility. In any case, the degree of cannabinoid-mediated modulation would depend on the strength and nature of the inputs each neuron receives (Valdés-Baizabal et al., 2017).
The Cortical Contribution to Deviance Detection
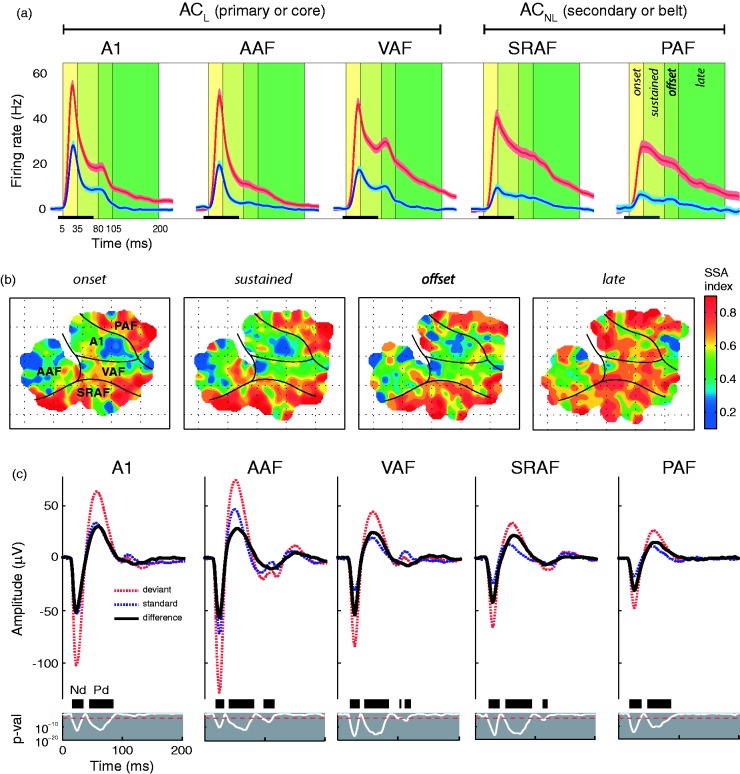
Deviance detection and prediction error are widespread in the AC. Yet, great differences can be observed between the neuronal response coming from lemniscal (primary or core) and nonlemniscal (secondary or belt) fields of the AC. On one hand, the primary AC is the first station in the lemniscal pathway to reliably exhibit deviance detection. SSA in lemniscal fields manifests more robustly in the late component of the neuronal response, as repetition suppression tends to almost abolish the tail of the standard-evoked responses while allowing the persistence of much of the onset component (Figure 7(a); Chen et al., 2015; Hershenhoren et al., 2014; Nieto-Diego & Malmierca, 2016; Taaseh et al., 2011; Ulanovsky et al., 2003, 2004). Prediction error, also evident at the late component (Chen et al., 2015), seems to account for about 25% of the overall deviance-detection activity recorded in the lemniscal AC (Figure 5; Parras et al., 2017). That is a bigger proportion than that found in the nonlemniscal divisions of subcortical nuclei, but only up to half of the component observed in its nonlemniscal counterpart (Figure 5; Parras et al., 2017), emphasizing the hierarchical disposition that exists between lemniscal (core) and nonlemniscal (belt) auditory fields.
Figure 7.
Variation of SSA index throughout time and cortical fields. (a) Grand-average multiunit responses (baseline-corrected firing rate, mean ± SEM) to standard (blue) and deviant (red) tones within each field and throughout four characteristic time windows. Many recordings showed significant late-component responses, beyond 100 ms after stimulus onset (check Figure 2(b) for a single-unit example). (b) Topographic distribution of SSA for the four different time windows. Note that only the late component of SSA index is high throughout the entire auditory cortex, suggesting intracortical hierarchical processing of deviance detection. (c) Grand-average LFP traces in response to deviant and standard tones, and the resulting difference wave (black), for each AC field. Two components of the difference wave were identified: the fast negative deflection (Nd) and the slower positive deflection (Pd). And additional small but significant deflection of the LFP was identified at longer latencies (>100 ms) in anteroventral fields (AAF, VAF, and SRAF). The thin white line below represents the p value of the difference wave, with a thick black bar marking the time intervals containing significant differences.
AAF = anterior auditory field; AC = auditory cortex; LFP = local field potential; PAF = posterior auditory field; SRAF = suprarhinal auditory field; SSA = stimulus-specific adaptation; VAF = ventral auditory field. Adapted from Nieto-Diego and Malmierca (2016).
On the other hand, the secondary AC is at the top of the auditory nonlemniscal pathway (Figure 4) and is where deviance detection and prediction error exhibit their uppermost expression. The belt fields of the AC show the greatest population indices of deviance detection (Figure 5), hosting a rich variety of context-driven responses, as it is characteristic of nonlemniscal neurons. SSA in the belt fields is not only the highest but also the swiftest in the AC, insofar as repetition suppression obliterates the standard-evoked response almost completely from its onset (Figure 7(a), (b); Nieto-Diego & Malmierca, 2016). Nonlemniscal AC is also the only part of the auditory system where prediction error equalizes or supersedes repetition suppression as the main component of deviance detection, with population ratios of 50% in anesthetized preparations (Figure 5(a), (b)) that swell up to 80% when stimulation intensity is near threshold (Figure 5(d)) or when animals are awake (Figure 5(c); Parras et al., 2017). This suggests that the nonlemniscal AC hosts the highest order populations of neurons within the auditory system. However, it is probable that the generative mechanism of hierarchical inference of auditory information is not exclusively constrained to the auditory system. Actually, the implication of areas beyond the auditory system is expected, continuing the neural processing hierarchy in charge of extracting increasingly abstract relationships between stimuli, as well as assembling multisensory perceptions by crossing information with other neural systems. As a matter of fact, local field potential (LFP) and ERP data from animal models have already pointed out the implication of prefrontal cortices in this auditory deviance-detection mechanism (Imada, Morris, & Wiest, 2013), as previously indicated by the prefrontal sources of the human MMN and the P3 (Dürschmid et al., 2016).
Participation of Cortical Microcircuitry: The Role of Interneurons
Cortical inhibitory interneurons are thought to play a crucial role in information processing and to shape how information is represented and transmitted within and between cortical neuronal populations (Yuste, 2015). The activity of excitatory neurons is shaped by feedback and recurrent networks that interweave complex excitatory-inhibitory interactions. Inhibitory neurons are remarkably diverse in their morphology and physiological properties, as well as in their complex connectivity patterns, targeting not only excitatory neurons but also other interneurons (Blackwell & Geffen, 2017; Isaacson & Scanziani, 2011). The two most common classes of GABAergic neurons are the parvalbumin- (PV) and somatostatin- (SOM) positive interneurons. PVs predominantly target the cell bodies of excitatory pyramidal neurons (Wang, Gupta, Toledo-Rodriguez, Wu, & Markram, 2002), whereas the majority of SOMs target the distal dendrites of excitatory pyramidal neurons (Figure 8(a); Ma, Hu, Berrebi, Mathers, & Agmon, 2006). Because PV and SOM in the AC have proven to contribute to tone frequency representation and behavioral selectivity in the AC (Blackwell & Geffen, 2017), and because cortical SSA is thought to emerge from a combination of thalamocortical depression and intracortical inhibitory–excitatory circuit effects (Nelken, 2014), the participation of interneurons in deviance detection has captured some attention as of late (Chen et al., 2015; Natan et al., 2015, 2017).
Figure 8.
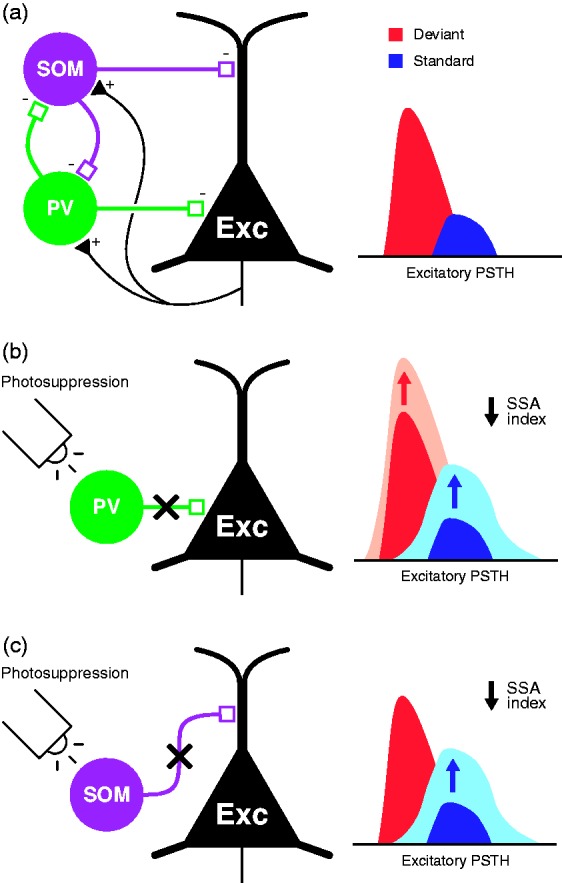
Interneurons exert inhibitory-mediated amplification effects over excitatory pyramidal neurons. (a) Schematic diagram showing only three common elements of the otherwise intricate cortical microcircuitry. PV-positive interneurons mostly inhibit perisomatic regions of pyramidal neurons, whereas SOM-positive interneurons mainly target the distal dendrites. In addition, both types of interneurons inhibit each other while targeted by excitatory recurrents of pyramidal neurons. The inhibition of both types of interneurons contributes to the amplification of the deviance detection signal, but in different manners. (b) Optogenetic photosuppression of PV-mediated inhibition leads to a nonspecific increase of the neuronal response, unveiling its gain-control action. (c) Optogenetic photosuppression of SOM-mediated inhibition increases the standard-evoked response, revealing a differential effect exerted on repetition suppression.
PSTH = peristimulus time histograms; PV = parvalbumin; SOM = somatostatin; SSA = stimulus-specific adaptation. Adapted from Natan et al. (2015).
In an optogenetic preparation using a multichannel probe perpendicularly crossing all cortical layers of mouse A1 for recording neuronal responses (Natan et al., 2015, 2017), the selective photosuppression of either PVs or SOMs found that both interneurons were amplifying deviance detection, but in distinctive manners. On one hand, when PV interneurons were deactivated, putative excitatory responses to both deviant and standard sounds increased equally. As PV interneurons inhibit perisomatic regions of pyramidal neurons, the general suppression of excitatory responses disregarding stimulus history amplifies SSA levels in a gain-control manner (Natan et al., 2015), which resembles the gain control of subcortical SSA exerted mainly by GABAergic receptors (Figure 8(b); Ayala & Malmierca, 2018; Duque et al., 2014; Pérez-González et al., 2012). Furthermore, even if also present in the supragranular and infragranular layers, the strongest effect of PVs on SSA was recorded in the granular layer (layer IV), where thalamocortical inputs are delivered (Figure 4; Natan et al., 2015). This is consistent with the relative distribution of PV interneurons in the AC and suggests a continuity of the gain-control mechanism of deviance detection over all levels of the auditory hierarchy.
On the other hand, when SOM interneurons were deactivated, differential effects were observed. Only the excitatory response to repetitive stimuli increased, while deviant-evoked responses remained unaffected, resulting in a decrease of SSA levels (Figure 8(c)). Although PV-mediated inhibition was constant throughout the tone train, SOM-mediated inhibition increased with the repeated presentations of the standard (Natan et al., 2015, 2017). Furthermore, the deactivation of SOM interneurons after several repetitions of the standard generated disinhibitory effects, which did not occur when PV interneurons were photosuppressed (Natan et al., 2017). Therefore, these data suggest that SOM interneurons modulate repetition suppression in excitatory neurons of A1 by exerting inhibition and short-term plasticity at their distal dendrites (Figure 8(c)). In fact, the strength of SOM-mediated inhibition on excitatory responses correlates with the magnitude of neuronal deviance detection (Natan et al., 2017). In spite of the great differences between auditory and visual systems, is worth mentioning an analogous preparation in the mouse primary visual cortex (Hamm & Yuste, 2016). In this study, pharmacogenetic silencing of SOM interneurons yielded similar reductions on deviance detection, implying its role is preserved across sensory modalities. However, the use of the many-standards control in this study revealed that this reduction was affecting mainly the prediction error component, leaving repetition suppression rather intact (Hamm & Yuste, 2016). This apparent discrepancy could be addressed by including many-standards or cascade controls in future SSA studies in the AC.
Moreover, both PV and SOM interneurons exhibited SSA themselves (Natan et al., 2015), something that could also be corroborated with precise whole-cell recordings in layer 2/3 of mouse A1 (Chen et al., 2015). SSA was especially rapid and pronounced in SOM interneurons, while in PV interneurons, the decrease of the response was more gradual (Chen et al., 2015; Natan et al., 2015). It is somewhat surprising to find that circuit elements, such as PV or SOM interneurons, are also repetition-sensitive, while still capable of modulating deviance detection in excitatory neurons. Nevertheless, considering excitatory and inhibitory neurons form tight recurrent networks in AC, it is possible to hypothesize that adapting interneurons can amplify deviance detection in excitatory neurons through differential postsynaptic integration by excitatory neurons (Natan et al., 2015). Consequently, GABAergic interneurons present in AC seem to play a prominent role regulating cortical deviance detection, thus adding yet another layer to the consecutive gain-control GABA-mediated mechanisms already present in subcortical deviance detection (Ayala & Malmierca, 2018; Duque et al., 2014; Pérez-González et al., 2012).
Linking Cortical SSA and MMN: In the Right Place at the Right Time
Deviance-detection activity and prediction error signals are generated all over the AC, as consistently confirmed by copious evidence from ERP studies analyzing middle-latency responses (MLRs) and long-latency responses (MMN) in human participants, as well as from cellular recordings (SSA) in animal models (Grimm et al., 2016). When SSA was first discovered in A1 (Ulanovsky et al., 2003), there were two initial concerns regarding its link with MMN: its temporal development and its anatomical location. The swift emergence of cortical SSA seemed relatively early when compared with the long latencies observed in MMN, neither A1 fitted well with the topography of MMN (Figure 2(b)), whose sources are usually pinned down within the region of the secondary AC in humans (Alho, 1995), cats (Pincze, Lakatos, Rajkai, Ulbert, & Karmos, 2001), and rats (Shiramatsu, Kanzaki, & Takahashi, 2013).
However, recent confirmation of more robust and enduring SSA (Nieto-Diego & Malmierca, 2016), as well as larger prediction error (Parras et al., 2017), emerging from cortical fields beyond A1 has better accounted for the generation of MMN. Although some deviance detection has been identified in the late component of the response of A1 neurons (Chen et al., 2015; Nieto-Diego & Malmierca, 2016), this late component is overall stronger and lasts longer (over 200 ms after stimulus onset) within nonlemniscal AC fields (Figure 7(a), (b)), also fitting better anatomically with the sources of MMN (Nieto-Diego & Malmierca, 2016). To put it simple, nonlemniscal cortical SSA and MMN are happening at the same time and place, further indicating both are two manifestations of the same physiological event.
Furthermore, LFPs were simultaneously recorded along the multiunit activity to provide an intermediate measure between cellular SSA and scalp-recorded MMN (Nieto-Diego & Malmierca, 2016; Parras et al., 2017). The difference wave extracted from the LFPs correlated in time and strength with the deviance detection (Nieto-Diego & Malmierca, 2016) and prediction error (Parras et al., 2017) observed in the multiunit recordings, confirming greater levels and longer persistence of both in nonlemniscal fields. These difference waves showed the same morphology in all cortical fields, with a fast negative deflection (Nd) followed by a positive one (Pd; Figure 7(c)). On one hand, the Nd occurred earlier and tended to be larger in lemniscal fields than in the nonlemniscal ones, suggesting a lemniscal origin. The time course of this early deflection suggests SSA in lemniscal AC could be related with the modulations of the scalp-recorded MLRs (Figure 2(a)) that correspond to the first response of the primary AC to a deviant event, which take place previous to the occurrence of the MMN (Grimm et al., 2016). On the other hand, the Pd peaked homogeneously along the AC, so its generation must hinge on intracortical processing and reciprocal interaction between lemniscal and nonlemniscal fields, further suggesting a hierarchical processing of deviance detection and a bottom-up propagation of prediction error. Most important, the Pd tended to peak between 60 and 80 ms (Figure 7(c)), well within the range of MMN-like potentials in the rat (50–100 ms; Harms et al., 2014; Harms, Michie, & Näätänen, 2016; Jung et al., 2013; Shiramatsu et al., 2013). This synchronicity finally allows to fully overcome the discrepancies in the time course and anatomical source of the SSA and the MMN, definitively ameliorating the acceptance of cortical SSA as the neuronal substrate of MMN (Nieto-Diego & Malmierca, 2016).
Further Linking SSA and MMN: The Key Role of N-Methyl-D-Aspartate Receptors
An additional connection between SSA and MMN could be established analyzing whether neurophamacological manipulations of the different neurotransmitter systems yields analogous effects in both, as it would be the case if they are manifestations of the same physiological mechanism of deviance detection. However, the clearest and most robust neurophamacological effect identified as of date in MMN is exerted through N-methyl-D-aspartate (NMDA) receptors (Kantrowitz, 2018). The NMDA-type glutamate receptors have a well-demonstrated critical role in controlling synaptic plasticity (Zorumski, Izumi, & Mennerick, 2016), so its participation in deviance detection is not surprising (Kantrowitz et al., 2018). The disruption of the normal function of NMDA receptors, using blocking substances such as ketamine in both humans (Todd et al., 2013) and animals (Javitt, Steinschneider, Schroeder, & Arezzo, 1996; Tikhonravov et al., 2008; Umbricht et al., 2000) or genetic alterations in animal models (Featherstone et al., 2015), induces significant reductions of MMN amplitude, along with other symptoms mimicking schizophrenia (Heekeren et al., 2008; Kreitschmann-Andermahr et al., 2001; Umbricht, Koller, Vollenweider, & Schmid, 2002; but see Oranje et al., 2000). Consequently, some studies have tested the effects of blocking NMDA receptors on SSA, in search of an analogous impairment.
A first attempt using systemic subcutaneous injections of the noncompetitive antagonist MK-801 of NMDA receptors failed to find that expected impairment in awake rats (Farley et al., 2010). Recording multiunit activity from A1, a strong suppressive effect was evoked proportionally in the whole response to both standard and deviant sounds, not affecting significantly the magnitude and dynamics of SSA. The suppression of neuronal excitability increased with the dose of MK-801, but SSA remained intact. Insensitivity of A1 to the systemic blockade was also confirmed by LFPs, leading the authors to argue that SSA in A1 was independent of NMDA receptors (Farley et al., 2010). Accordingly, SSA in A1 could maybe account for the generation of the early mismatch signals detected within the MLRs (Grimm et al., 2016), but not for the MMN (Farley et al., 2010), which had to emanate necessarily from the nonlemniscal AC (Maess, Jacobsen, Schröger, & Friederici, 2007; Nieto-Diego & Malmierca, 2016; Opitz, Schröger, & Von Cramon, 2005).
Recently, nonetheless, an elegant research using local applications of NMDA-receptor antagonists showed different effects. In a study conducted in layer 2/3 of the mouse A1 (Chen et al., 2015), intracellular injections of MK-801 did not induce changes in the SSA of PV interneurons, but a drastic reduction of the responses to deviant stimuli in excitatory pyramidal neurons was registered. Rare sounds still evoked a larger response than standard stimuli, but unlike what happened during systemic applications, overall SSA in pyramidal neurons was significantly reduced (Figure 6(e)), and especially affected the late component (Chen et al., 2015). Moreover, the SSA reduction entailed the disappearance of the prediction error component (Chen et al., 2015). Discrepancies between the two experimental approaches could be caused by the fact that systemic injections (Farley et al., 2010) block NMDA receptors in all brain regions, not just in a single neuron at a time (Chen et al., 2015). Intracortical processing might be essential to the emergence of the late component in the deviant-evoked response, as well as to the generation of prediction error (Chen et al., 2015; Nieto-Diego & Malmierca, 2016; Parras et al., 2017). Therefore, intracortical processing via NMDA-mediated dendritic integration is likely to trigger the long-lasting late component of deviance detection, as well as prediction error, in the pyramidal neurons in A1. Proven its dependence on NMDA receptors, SSA shares yet another feature with MMN, strengthening the evidence of SSA and MMN as two different scales for measuring the same physiological mechanism of deviance detection.
Closing Remarks: Some Insights Attained Through a Predictive Coding Perspective
SSA is the quantified index of change in the firing rate of a neuron in response to a rare tone (deviant) randomly presented embedded in a sequence with another common tone (standard), at varying presentation probabilities (usually, 10%–30%, deviant vs. 90%–70%, standard). This definition of SSA in terms of probabilities is rather similar to the classic conceptualization of MMN recorded in the human scalp (Näätänen et al., 1978). After four decades of research, however, MMN have expanded far beyond this narrow definition to comprise any discriminable change in the organization of an ongoing stimulation, somewhat blurring its theoretical relation with the much simpler SSA. Throughout the current review, we have tried to improve this connection by reinterpreting the available evidence on SSA from a predictive coding standpoint. That integrative endeavor has provided several interesting insights that may be useful to enrich the understanding of MMN generation as well.
There is consistent empirical evidence supporting that SSA takes place in the same cortical regions of the brain where MMN sources are located, as well as SSA time dynamics also encompass the pick of the MMN, implying they are happening synchronically. Moreover, SSA and MMN are rather sensitive to the manipulation of NMDA receptors, suggesting they are key to the generation of both. All of which reinforces the notion that SSA and MMN are the microscopic and macroscopic correlates of the same deviance-detection process and provides empirical justification to the use of a common framework and shared nomenclature to explain both (Table 1).
Table 1.
Definition of Main Terms in the Predictive Coding Framework.
| Predictive coding | Neurobiologically informed theory of general brain function based on a generative mechanism of Bayesian hierarchical inference. Based on a model of the current stimulation, predictive coding posits that higher order processing stations send predictions to lower hierarchical levels to aid the suppression of any ascending neuronal activity evoked by sensory events that can be anticipated. These lower stations also forward prediction errors to the higher hierarchical levels whenever their current predictions fail, to prioritize the processing of those inputs and update the perceptual model. Hence, perception emerges from a dynamic interaction between top-down expectations and bottom-up prediction errors. |
| Rule or regularity | Logical principle that organizes the relationships between successive stimuli featuring in an experimental sequence used to study the effects of predictive coding on perceptual processing. In the oddball paradigm, where two tones are presented successively with an uneven probability of appearance (usually 90% vs. 10%), that regularity would be generated by the repetition of the common tone. More complex (or even abstract) rules can be implemented in experimental sequences to analyze the effects of identifying a particular regularity (e.g., two-tone alternation paradigm). Furthermore, multiple rules could be simultaneously at play in a given sequence (e.g., in the local/global paradigm). |
| Regularity encoding | The ability of a processing network to extract regular interstimulus relationships and adjust them to a perceptual model accounting for the organization of the flow of incoming sensory inputs. Thereby, it generates an internal representation from which perception and predictions or expectations about upcoming events emerge. |
| Standard condition | A sensory event that fits in the experimental rule or regularity. In other words, a sensory input that can be predicted by the perceptual model encoded in a certain processing network. In the oddball paradigm, the standard condition would be the commonly repeating stimulus. |
| Deviant condition | A sensory event that represents a punctual violation of the experimental rule or regularity. In other words, a sensory input that cannot be predicted by the perceptual model encoded in a certain processing network. The absence of that sensory input, that is, an omission, can also constitute a deviant condition. In the oddball paradigm, the deviant condition would be the random rare stimulus. |
| Deviance detection | Response evoked by a stimulus that violates a regularity encoded in the processing system, when compared with the response evoked by that same stimulus when it fitted in the internal representation of that regularity. In other words, the response contrasts to a certain stimulus when it was predictable and when it could not be predicted by the perceptual model. In the oddball paradigm, its index corresponds to the difference wave (for LFPs and ERPs, like MMN) or the contrast in firing rate (for cellular recordings, like SSA) between the rare tone (deviant condition) and the common tone (standard condition). |
| Expectation suppression | Decrease in the response evoked by a stimulus that was predictable, based on the perceptual model held on a certain processing network. In other words, the attenuating effect that an encoded representation has on the response to a sensory input that is coherent with it. |
| Repetition suppression | Decrease in the evoked response of a stimulus due to its reoccurrence. It is the simplest form of expectation suppression and the result of assuming that the upcoming input will be similar to the previous one represented. In the oddball paradigm, its index corresponds to the component of deviance detection between the control and the standard conditions. |
| Prediction error | Increase in the response evoked by a stimulus (or its omission) that could not be predicted by the perceptual model established on a certain processing network. In other words, the enhancement of the response to a sensory input (or the absence of it) that violates an encoded regularity. It constitutes an effort to mobilize processing resources at higher level networks, to update the internal representation of the ongoing stimulation and better account for the incoming input. In the oddball paradigm, its index corresponds to the component of deviance detection between the deviant and the control conditions. |
| Mismatch negativity | Scalp-recorded ERP biomarker of deviance detection, usually peaking at 150 to 250 ms from deviance onset in humans. MMN-like or mismatch signals are also detectable in animal models. |
| Stimulus-specific adaptation | Index of change in the firing rate of a single neuron or a localized neuronal population in response to a rare (deviant) tone randomly presented as part of an oddball sequence with another commonly repeating (standard) tone. Index of deviance detection for the oddball paradigm most commonly used in animal models. |
Note. Most of the concept descriptions are illustrated taking as example the oddball paradigm, for its simplicity and popularity. Nevertheless, these terms are applicable to any experimental design in the study of deviance detection. ERP = event-related potential; LFPs = local field potentials; MMN = mismatch negativity; SSA = stimulus-specific adaptation.
Notwithstanding, SSA and MMN are only theoretically comparable in the context of an oddball paradigm, as the definition of SSA (Ulanovsky et al., 2003) describes the effects of representing a stimulus in the system and establishing the simple prediction that the following stimulus will be similar to the previous encoded, thereby suppressing the response to it. Thus, SSA by itself is inadequate to account for MMN signals generated in contexts where this repetition rule is not at play. SSA is an index for the violation of that particular representation, not a physiological mechanism that can be extrapolated to other stimulation contexts. The physiological mechanism is deviance detection as defined by predictive coding.
Prediction error and repetition suppression effects are confounded in the deviance-detection signal elicited by the oddball sequence. These components can be disentangled by the use of a control sequence that does not feature the standard-repetition rule, while generating a similar state of refractoriness in the system than the oddball. The proposed many-standards and cascade controls are applicable in SSA research, making possible to explain SSA in term of predictive coding empirically.
The application of control sequences has unveiled a predictive processing hierarchy in the auditory system, which roots go as profound as the cortices of the IC. The proportion of deviance detection accounted for by prediction error increases along two neuraxes in the auditory system: from lemniscal to nonlemniscal, and from the midbrain to the AC. Therefore, predictive activity is not exclusively cortical.
Within each processing station along the auditory pathway, the high heterogeneity in the functioning of single neurons exposed to deviant stimulation hinted the great importance of local microcircuitry in predictive processing. Along with the fact that deactivating the AC does not eliminate deviance detection, it follows that predictive activity must be emerging to a certain extent de novo at every level of the auditory pathway, instead of being just passed down from the cortex.
Neuromodulation exerts a tangible influence on the reciprocal interaction of processing levels that leads to deviance detection. The attenuating effect over repetition suppression that ACh modulates mainly via M1 muscarinic receptors is a good example of that, among others.
Inhibitory activity is key for an efficient deviance detection, unfolding gain-control mechanisms on predictive activity all over the auditory pathway, as well as facilitating repetition suppression.
Summary
After 15 years of thorough research since SSA was first discovered in the auditory cortex, there is a growing body of evidence indicating SSA is the neuronal correlate of MMN. It has been demonstrated that both cortical SSA and MMN occur over a similar time course and in similar cortical locations and that are both affected by the manipulation of NMDA receptors. Therefore, SSA and MMN must be, respectively, the microscopic and macroscopic manifestations of the same physiological mechanisms of deviance detection. Thus, the adoption of a common conceptual framework and shared nomenclature would be recommendable for explaining both dimensions of the same perceptual phenomenon. The predictive coding framework provides this needed integration by postulating a physiological generative mechanism of hierarchical inference founded on plastic changes in synaptic connectivity. That generative mechanism is not exclusively cortical as originally thought, inasmuch as new experimental approaches to SSA inspired in MMN research have unveiled the progressive emergence of prediction error signals from the IC and auditory thalamus. Many recent studies have highlighted the contribution of subcortical nuclei and the nonlemniscal pathway to the generation of SSA and prediction error. The emergence of prediction errors in a hierarchically organized network with roots in the IC expands the notion of predictive coding to subcortical brain structures and also challenges the prevailing corticocentric view of complex auditory processing and perceptual organization.
Acknowledgments
We thank Dr. Carles Escera for providing us a very explanatory figure about MLR and MMN recordings from the human scalp (Figure 2). We also thank three anonymous reviewers, whose comments on the original manuscript helped improving and expanding this work substantially.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been funded by Spanish MINECO (SAF2016-75803-P). GVC held a fellowship from the Spanish MICINN (BES-2017-080030).
References
- Abolafia J. M., Vergara R., Arnold M. M., Reig R., Sanchez-Vives M. V. (2011) Cortical auditory adaptation in the awake rat and the role of potassium currents. Cerebral Cortex 21(5): 977–990. doi:10.1093/cercor/bhq163. [DOI] [PubMed] [Google Scholar]
- Alain C., Woods D. L., Ogawa K. H. (1994) Brain indices of automatic pattern processing. NeuroReport 6(1): 140–144. doi:10.1097/00001756-199412300-00036. [DOI] [PubMed] [Google Scholar]
- Alho K. (1995) Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear and Hearing 16(1): 38–51. [DOI] [PubMed] [Google Scholar]
- Althen H., Grimm S., Escera C. (2011) Fast detection of unexpected sound intensity decrements as revealed by human evoked potentials. PLoS One 6(12): e28522 doi:10.1371/journal.pone.0028522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althen H., Grimm S., Escera C. (2013) Simple and complex acoustic regularities are encoded at different levels of the auditory hierarchy. European Journal of Neuroscience 38(10): 3448–3455. doi:10.1111/ejn.12346. [DOI] [PubMed] [Google Scholar]
- Altmann C. F., Hiraumi H., Terada S., Adachi T., Votinov M., Ono K., Fukuyama H. (2013) Preattentive processing of horizontal motion, radial motion, and intensity changes of sounds. NeuroReport 24(15): 861–865. doi:10.1097/WNR.0000000000000006. [DOI] [PubMed] [Google Scholar]
- Anderson L. A., Christianson G. B., Linden J. F. (2009) Stimulus-specific adaptation occurs in the auditory thalamus. Journal of Neuroscience 29(22): 7359–7363. doi:10.1523/JNEUROSCI.0793-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. A., Malmierca M. S. (2013) The effect of auditory cortex deactivation on stimulus-specific adaptation in the inferior colliculus of the rat. European Journal of Neuroscience 37(1): 52–62. doi:10.1111/ejn.12018. [DOI] [PubMed] [Google Scholar]
- Antunes F. M., Malmierca M. S. (2011) Effect of auditory cortex deactivation on stimulus-specific adaptation in the medial geniculate body. Journal of Neuroscience 31(47): 17306–17316. doi:10.1523/JNEUROSCI.1915-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes F. M., Malmierca M. S. (2014) An overview of stimulus-specific adaptation in the auditory thalamus. Brain Topography 27(4): 480–499. doi:10.1007/s10548-013-0342-6. [DOI] [PubMed] [Google Scholar]
- Antunes F. M., Nelken I., Covey E., Malmierca M. S. (2010) Stimulus-specific adaptation in the auditory thalamus of the anesthetized rat. PLoS One 5(11): e14071 doi:10.1371/journal.pone.0014071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki E., Semba R., Keino H., Kato K., Kashiwamata S. (1988) Glycine-like immunoreactivity in the rat auditory pathway. Brain Research 442(1): 63–71. doi:10.1016/0006-8993(88)91432-1. [DOI] [PubMed] [Google Scholar]
- Auksztulewicz R., Friston K. (2016) Repetition suppression and its contextual determinants in predictive coding. Cortex 80: 125–140. doi:10.1016/j.cortex.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala Y. A., Malmierca M. S. (2013) Stimulus-specific adaptation and deviance detection in the inferior colliculus. Frontiers in Neural Circuits 6: 89 doi:10.3389/fncir.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala Y. A., Malmierca M. S. (2015) Cholinergic modulation of stimulus-specific adaptation in the inferior colliculus. Journal of Neuroscience 35(35): 12261–12272. doi:10.1523/JNEUROSCI.0909-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala Y. A., Malmierca M. S. (2018) The effect of inhibition on stimulus-specific adaptation in the inferior colliculus. Brain Structure and Function 223(3): 1391–1407. doi:10.1007/s00429-017-1546-4. [DOI] [PubMed] [Google Scholar]
- Ayala Y. A., Pérez-González D., Malmierca M. S. (2016) Stimulus-specific adaptation in the inferior colliculus: The role of excitatory, inhibitory and modulatory inputs. Biological Psychology 116: 10–22. doi:10.1016/j.biopsycho.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Ayala Y. A., Udeh A., Dutta K., Bishop D., Malmierca M. S., Oliver D. L. (2015) Differences in the strength of cortical and brainstem inputs to SSA and non-SSA neurons in the inferior colliculus. Scientific Reports 5: 10383 doi:10.1038/srep10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo V. M., Leach N. D., Cordery P. M., Nodal F. R., King A. J. (2014) The cholinergic basal forebrain in the ferret and its inputs to the auditory cortex. European Journal of Neuroscience 40(6): 2922–2940. doi:10.1111/ejn.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldeweg T., Klugman A., Gruzelier J., Hirsch S. R. (2004) Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophrenia Research 69(2–3): 203–217. doi:10.1016/j.schres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Barascud N., Pearce M. T., Griffiths T. D., Friston K. J., Chait M. (2016) Brain responses in humans reveal ideal observer-like sensitivity to complex acoustic patterns. Proceedings of the National Academy of Sciences 113(5): E616–E625. doi:10.1073/pnas.1508523113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartha-Doering L., Deuster D., Giordano V., am Zehnhoff-Dinnesen A., Dobel C. (2015) A systematic review of the mismatch negativity as an index for auditory sensory memory: From basic research to clinical and developmental perspectives. Psychophysiology 52(9): 1115–1130. doi:10.1111/psyp.12459. [DOI] [PubMed] [Google Scholar]
- Bastos A. M., Usrey W. M., Adams R. A., Mangun G. R., Fries P., Friston K. J. (2012) Canonical microcircuits for predictive coding. Neuron 76(4): 695–711. doi:10.1016/j.neuron.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerle P., von der Behrens W., Kossl M., Gaese B. H. (2011) Stimulus-specific adaptation in the gerbil primary auditory thalamus is the result of a fast frequency-specific habituation and is regulated by the corticofugal system. Journal of Neuroscience 31(26): 9708–9722. doi:10.1523/JNEUROSCI.5814-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein T. A., Dehaene S., Rohaut B., Tadel F., Cohen L., Naccache L. (2009) Neural signature of the conscious processing of auditory regularities. Proceedings of the National Academy of Sciences 106(5): 1672–1677. doi:10.1073/pnas.0809667106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlot E., Formisano E., DeMartino F. (2018) Mapping frequency specific tone predictions in the human auditory cortex at high spatial resolution. Journal of Neuroscience 38(21): 4934–4942. doi:10.1523/JNEUROSCI.2205-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikov, N. G. (1977). [“Novelty” neurons in the frog auditory system]. Zh Vyssh Nerv Deiat Im I P Pavlova, 27(5), 1075–1082. [PubMed] [Google Scholar]
- Bidelman, G. M. (2018). Subcortical sources dominate the neuroelectric auditory frequency-following response to speech. NeuroImage , 175 , 56–69. 10.1016/j.neuroimage.2018.03.060. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M., Geffen M. N. (2017) Progress and challenges for understanding the function of cortical microcircuits in auditory processing. Nature Communications 8(1): 2165 doi:10.1038/s41467-017-01755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L. (1983) Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. British Journal of Pharmacology 78(1): 191–206. doi:10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brønnick K. S., Nordby H., Larsen J. P., Aarsland D. (2010) Disturbance of automatic auditory change detection in dementia associated with Parkinson’s disease: A mismatch negativity study. Neurobiology of Aging 31(1): 104–113. doi:10.1016/j.neurobiolaging.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Cacciaglia R., Escera C., Slabu L., Grimm S., Sanjuán A., Ventura-Campos N., Ávila C. (2015) Involvement of the human midbrain and thalamus in auditory deviance detection. Neuropsychologia 68: 51–58. doi:10.1016/j.neuropsychologia.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Cai R., Richardson B. D., Caspary D. M. (2016) Responses to predictable versus random temporally complex stimuli from single units in auditory thalamus: Impact of aging and anesthesia. Journal of Neuroscience 36(41): 10696–10706. doi:10.1523/JNEUROSCI.1454-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capano V., Herrmann H. J., De Arcangelis L. (2015) Optimal percentage of inhibitory synapses in multi-task learning. Scientific Reports 5(1): 9895 doi:10.1038/srep09895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo P. E., Younts T. J., Chávez A. E., Hashimotodani Y. (2012) Endocannabinoid signaling and synaptic function. Neuron 76(1): 70–81. doi:10.1016/J.NEURON.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I.-W., Helmchen F., Lutcke H. (2015) Specific early and late oddball-evoked responses in excitatory and inhibitory neurons of mouse auditory cortex. Journal of Neuroscience 35(36): 12560–12573. doi:10.1523/JNEUROSCI.2240-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennu S., Noreika V., Gueorguiev D., Shtyrov Y., Bekinschtein T. A., Henson R. (2016) Silent expectations: Dynamic causal modeling of cortical prediction and attention to sounds that weren’t. Journal of Neuroscience 36(32): 8305–8316. doi:10.1523/JNEUROSCI.1125-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheour M., Martynova O., Näätänen R., Erkkola R., Sillanpää M., Kero P., Hämäläinen H. (2002) Psychobiology: Speech sounds learned by sleeping newborns. Nature 415(6872): 599–600. doi:10.1038/415599b. [DOI] [PubMed] [Google Scholar]
- Choy B. D., Bishop D. C., Oliver D. L. (2015) Differential distribution of GABA and glycine terminals in the inferior colliculus of rat and mouse. Journal of Comparative Neurology 523(18): 2683–2697. doi:10.1002/cne.23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. (2013) Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behavioral and Brain Sciences 36(3): 181–204. doi:10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- Coffey E. B. J., Herholz S. C., Chepesiuk A. M. P., Baillet S., Zatorre R. J. (2016) Cortical contributions to the auditory frequency-following response revealed by MEG. Nature Communications 7: 11070 doi:10.1038/ncomms11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey, E. B. J., Musacchia, G., & Zatorre, R. J. (2017). Cortical correlates of the auditory frequency-following and onset responses: EEG and fMRI evidence. The Journal of Neuroscience , 37 (4), 830–838. 10.1523/JNEUROSCI.1265-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornella M., Leung S., Grimm S., Escera C. (2012) Detection of simple and pattern regularity violations occurs at different levels of the auditory hierarchy. PLoS One 7(8): e43604 doi:10.1371/journal.pone.0043604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaso K. A. M., Michie P. T., Todd J. (2015) Paying attention to MMN in schizophrenia. Brain Research 1626: 267–279. doi:10.1016/j.brainres.2015.06.031. [DOI] [PubMed] [Google Scholar]
- Davids N., Segers E., van den Brink D., Mitterer H., van Balkom H., Hagoort P., Verhoeven L. (2011) The nature of auditory discrimination problems in children with specific language impairment: An MMN study. Neuropsychologia 49(1): 19–28. doi:10.1016/j.neuropsychologia.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Meyniel F., Wacongne C., Wang L., Pallier C. (2015) The neural representation of sequences: From transition probabilities to algebraic patterns and linguistic trees. Neuron 88(1): 2–19. doi:10.1016/j.neuron.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Denham S. L., Winkler I. (2018) Predictive coding in auditory perception: Challenges and unresolved questions. European Journal of Neuroscience. . Advance online publication. doi:10.1111/ejn.13802. [DOI] [PubMed] [Google Scholar]
- Draganova R., Eswaran H., Murphy P., Huotilainen M., Lowery C., Preissl H. (2005) Sound frequency change detection in fetuses and newborns, a magnetoencephalographic study. NeuroImage 28(2): 354–361. doi:10.1016/j.neuroimage.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Draganova R., Eswaran H., Murphy P., Lowery C., Preissl H. (2007) Serial magnetoencephalographic study of fetal and newborn auditory discriminative evoked responses. Early Human Development 83(3): 199–207. doi:10.1016/j.earlhumdev.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Duque D., Ayala Y. A., Malmierca M. S. (2015) Deviance detection in auditory subcortical structures: What can we learn from neurochemistry and neural connectivity? Cell and Tissue Research 361(1): 215–232. doi:10.1007/s00441-015-2134-7. [DOI] [PubMed] [Google Scholar]
- Duque D., Malmierca M. S. (2015) Stimulus-specific adaptation in the inferior colliculus of the mouse: Anesthesia and spontaneous activity effects. Brain Structure and Function 220(6): 3385–3398. doi:10.1007/s00429-014-0862-1. [DOI] [PubMed] [Google Scholar]
- Duque D., Malmierca M. S., Caspary D. M. (2014) Modulation of stimulus-specific adaptation by GABAA receptor activation or blockade in the medial geniculate body of the anaesthetized rat. Journal of Physiology 592(4): 729–743. doi:10.1113/jphysiol.2013.261941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque D., Perez-Gonzalez D., Ayala Y. A., Palmer A. R., Malmierca M. S. (2012) Topographic distribution, frequency, and intensity dependence of stimulus-specific adaptation in the inferior colliculus of the rat. Journal of Neuroscience 32(49): 17762–17774. doi:10.1523/JNEUROSCI.3190-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque D., Wang X., Nieto-Diego J., Krumbholz K., Malmierca M. S. (2016) Neurons in the inferior colliculus of the rat show stimulus-specific adaptation for frequency, but not for intensity. Scientific Reports 6(1): 24114 doi:10.1038/srep24114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürschmid S., Edwards E., Reichert C., Dewar C., Hinrichs H., Heinze H. J., Knight R. T. (2016) Hierarchy of prediction errors for auditory events in human temporal and frontal cortex. Proceedings of the National Academy of Sciences 113(24): 6755–6760. doi:10.1073/pnas.1525030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline J. M., Hars B., Maho C., Hennevin E. (1994) Transient and prolonged facilitation of tone-evoked responses induced by basal forebrain stimulations in the rat auditory cortex. Experimental Brain Research 97(3): 373–386. doi:10.1007/BF00241531. [DOI] [PubMed] [Google Scholar]
- Ells E. M. L., Rudolph E. D., Sculthorpe-Petley L., Abriel S. C., Campbell D. J., Tibbo P. G., Fisher D. J. (2018) Alterations of complex mismatch negativity (cMMN) elicited by a two-tone pattern paradigm in early-phase psychosis. Biological Psychology 135: 128–135. doi:10.1016/j.biopsycho.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Escera C., Corral M. J., Yago E. (2002) An electrophysiological and behavioral investigation of involuntary attention towards auditory frequency, duration and intensity changes. Cognitive Brain Research 14(3): 325–332. doi:10.1016/S0926-6410(02)00135-0. [DOI] [PubMed] [Google Scholar]
- Escera C., Malmierca M. S. (2014) The auditory novelty system: An attempt to integrate human and animal research. Psychophysiology 51(2): 111–123. doi:10.1111/psyp.12156. [DOI] [PubMed] [Google Scholar]
- Farley B. J., Quirk M. C., Doherty J. J., Christian E. P. (2010) Stimulus-specific adaptation in auditory cortex is an NMDA-independent process distinct from the sensory novelty encoded by the mismatch negativity. Journal of Neuroscience 30(49): 16475–16484. doi:10.1523/JNEUROSCI.2793-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone R. E., Shin R., Kogan J. H., Liang Y., Matsumoto M., Siegel S. J. (2015) Mice with subtle reduction of NMDA NR1 receptor subunit expression have a selective decrease in mismatch negativity: Implications for schizophrenia prodromal population. Neurobiology of Disease 73: 289–295. doi:10.1016/j.nbd.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Fisher D. J., Campbell D. J., Abriel S. C., Ells E. M. L., Rudolph E. D., Tibbo P. G. (2018) Auditory mismatch negativity and P300a elicited by the ‘optimal’ multi-feature paradigm in early schizophrenia. Clinical EEG and Neuroscience 49(4): 238–247. doi:10.1177/1550059418761459. [DOI] [PubMed] [Google Scholar]
- Fishman Y. I. (2014) The mechanisms and meaning of the mismatch negativity. Brain Topography 27(4): 500–526. doi:10.1007/s10548-013-0337-3. [DOI] [PubMed] [Google Scholar]
- Fishman Y. I., Steinschneider M. (2012) Searching for the mismatch negativity in primary auditory cortex of the awake monkey: Deviance detection or stimulus specific adaptation? Journal of Neuroscience 32(45): 15747–15758. doi:10.1523/JNEUROSCI.2835-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E., Hammerschmidt B., Kirsch J. (1997) Development of adult-type inhibitory glycine receptors in the central auditory system of rats. Journal of Comparative Neurology 385(1): 117–134. doi:10.1002/(SICI)1096-9861(19970818)385:1<117::AID-CNE7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Friston K. (2005) A theory of cortical responses. Philosophical Transactions of the Royal Society B: Biological Sciences 360(1456): 815–836. doi:10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J. B., Elhilali M., David S. V., Shamma S. A. (2007) Auditory attention – Focusing the searchlight on sound. Current Opinion in Neurobiology 17(4): 437–455. doi:10.1016/j.conb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Garrido M. I., Kilner J. M., Kiebel S. J., Stephan K. E., Baldeweg T., Friston K. J. (2009) Repetition suppression and plasticity in the human brain. NeuroImage 48(1): 269–279. doi:10.1016/j.neuroimage.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido M. I., Kilner J. M., Stephan K. E., Friston K. J. (2009) The mismatch negativity: A review of underlying mechanisms. Clinical Neurophysiology 120(3): 453–463. doi:10.1016/j.clinph.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris J., Braem S., Nijhof A., Rigoni D., Deschrijver E., Van de Cruys S., Brass M. (2018) Sensory prediction errors are less modulated by global context in autism spectrum disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. . Advance online publication. doi:10.1016/j.bpsc.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M. (1973) The thalamo-cortical projection of the so-called posterior nuclear group: A study with anterograde degeneration methods in the cat. Brain Research 49(2): 229–244. doi:10.1016/0006-8993(73)90420-4. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K., Henson R., Martin A. (2006) Repetition and the brain: Neural models of stimulus-specific effects. Trends in Cognitive Sciences 10(1): 14–23. doi:10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grimm S., Escera C., Nelken I. (2016) Early indices of deviance detection in humans and animal models. Biological Psychology 116: 23–27. doi:10.1016/j.biopsycho.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Grimm S., Recasens M., Althen H., Escera C. (2012) Ultrafast tracking of sound location changes as revealed by human auditory evoked potentials. Biological Psychology 89(1): 232–239. doi:10.1016/j.biopsycho.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Grotheer M., Kovács G. (2016) Can predictive coding explain repetition suppression? Cortex 80: 113–124. doi:10.1016/j.cortex.2015.11.027. [DOI] [PubMed] [Google Scholar]
- Haigh S. M., De Matteis M., Coffman B. A., Murphy T. K., Butera C. D., Ward K. L., Salisbury D. F. (2017) Mismatch negativity to pitch pattern deviants in schizophrenia. European Journal of Neuroscience 46(6): 2229–2239. doi:10.1111/ejn.13660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm J. P., Yuste R. (2016) Somatostatin interneurons control a key component of mismatch negativity in mouse visual cortex. Cell Reports 16(3): 597–604. doi:10.1016/j.celrep.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms L., Fulham W. R., Todd J., Budd T. W., Hunter M., Meehan C., Michie P. T. (2014) Mismatch negativity (MMN) in freely-moving rats with several experimental controls. PLoS One 9(10): e110892 doi:10.1371/journal.pone.0110892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms L., Michie P. T., Näätänen R. (2016) Criteria for determining whether mismatch responses exist in animal models: Focus on rodents. Biological Psychology 116: 28–35. doi:10.1016/j.biopsycho.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Hasselmo M. E., McGaughy J. (2004) High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Progress in Brain Research 145: 207–231. doi:10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Heekeren K., Daumann J., Neukirch A., Stock C., Kawohl W., Norra C., Gouzoulis-Mayfrank E. (2008) Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology 199(1): 77–88. doi:10.1007/s00213-008-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbron M., Chait M. (2017) Great expectations: Is there evidence for predictive coding in auditory cortex? Neuroscience. . Advance online publication. doi:10.1016/j.neuroscience.2017.07.061. [DOI] [PubMed] [Google Scholar]
- Heldmann M., Münte T. F., Paracka L., Beyer F., Brüggemann N., Saryyeva A., Tronnier V. M. (2017) Human subthalamic nucleus – Automatic auditory change detection as a basis for action selection. Neuroscience 355: 141–148. doi:10.1016/j.neuroscience.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Hershenhoren I., Taaseh N., Antunes F. M., Nelken I. (2014) Intracellular correlates of stimulus-specific adaptation. Journal of Neuroscience 34(9): 3303–3319. doi:10.1523/JNEUROSCI.2166-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohwy J. (2012) Attention and conscious perception in the hypothesis testing brain. Frontiers in Psychology 3: 96 doi:10.3389/fpsyg.2012.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth J., Czigler I., Winkler I., Teder-Sälejärvi W. A. (2007) The temporal window of integration in elderly and young adults. Neurobiology of Aging 28(6): 964–975. doi:10.1016/j.neurobiolaging.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Horváth J., Müller D., Weise A., Schröger E. (2010) Omission mismatch negativity builds up late. NeuroReport 21(7): 537–541. doi:10.1097/WNR.0b013e3283398094. [DOI] [PubMed] [Google Scholar]
- Hsieh C. Y., Cruikshank S. J., Metherate R. (2000) Differential modulation of auditory thalamocortical and intracortical synaptic transmission by cholinergic agonist. Brain Research 880(1–2): 51–64. doi:10.1016/S0006-8993(00)02766-9. [DOI] [PubMed] [Google Scholar]
- Hu B. (2003) Functional organization of lemniscal and nonlemniscal auditory thalamus. Experimental Brain Research 153(4): 543–549. doi:10.1007/s00221-003-1611-5. [DOI] [PubMed] [Google Scholar]
- Hudac C. M., DesChamps T. D., Arnett A. B., Cairney B. E., Ma R., Webb S. J., Bernier R. A. (2018) Early enhanced processing and delayed habituation to deviance sounds in autism spectrum disorder. Brain and Cognition 123: 110–119. doi:10.1016/j.bandc.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrizbegovic E., Hederstierna C., Rosenhall U. (2016) Mismatch negativity and ear laterality in Alzheimer’s disease and in mild cognitive impairment. Journal of Alzheimer’s Disease 53(4): 1405–1410. doi:10.3233/JAD-160323. [DOI] [PubMed] [Google Scholar]
- Imada A., Morris A., Wiest M. C. (2013) Deviance detection by a P3-like response in rat posterior parietal cortex. Frontiers in Integrative Neuroscience 6: 127 doi:10.3389/fnint.2012.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson J. S., Scanziani M. (2011) How inhibition shapes cortical activity. Neuron 72(2): 231–243. doi:10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jääskeläinen I. P., Ahveninen J., Belliveau J. W., Raij T., Sams M. (2007) Short-term plasticity in auditory cognition. Trends in Neurosciences 30(12): 653–661. doi:10.1016/j.tins.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Jacobsen T., Horenkamp T., Schröger E. (2003) Preattentive memory-based comparison of sound intensity. Audiology and Neuro-Otology 8(6): 338–346. doi:10.1159/000073518. [DOI] [PubMed] [Google Scholar]
- Javitt D. C., Steinschneider M., Schroeder C. E., Arezzo J. C. (1996) Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: Implications for schizophrenia. Proceedings of the National Academy of Sciences 93(21): 11962–11967. doi:10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt D. C., Sweet R. A. (2015) Auditory dysfunction in schizophrenia: Integrating clinical and basic features. Nature Reviews Neuroscience 16(9): 535–550. doi:10.1038/nrn4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Yan C., Qiao Z., Yao H., Jiang S., Qiu X., Zhang L. (2017) Mismatch negativity as a potential neurobiological marker of early-stage Alzheimer disease and vascular dementia. Neuroscience Letters 647: 26–31. doi:10.1016/j.neulet.2017.03.032. [DOI] [PubMed] [Google Scholar]
- Jones E. G. (2003) Chemically defined parallel pathways in the monkey auditory system. Annals of the New York Academy of Sciences 999: 218–233. doi:10.1196/annals.1284.033. [DOI] [PubMed] [Google Scholar]
- Joshi Y. B., Breitenstein B., Tarasenko M., Thomas M. L., Chang W. L., Sprock J., Light G. A. (2018) Mismatch negativity impairment is associated with deficits in identifying real-world environmental sounds in schizophrenia. Schizophrenia Research 191: 5–9. doi:10.1016/j.schres.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung F., Stephan K. E., Backes H., Moran R., Gramer M., Kumagai T., Tittgemeyer M. (2013) Mismatch responses in the awake rat: Evidence from epidural recordings of auditory cortical fields. PLoS One 8(4): e63203 doi:10.1371/journal.pone.0063203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametani H., Kawamura H. (1990) Alterations in acetylcholine release in the rat hippocampus during sleep-wakefulness detected by intracerebral dialysis. Life Sciences 47(5): 421–426. doi:10.1016/0024-3205(90)90300-G. [DOI] [PubMed] [Google Scholar]
- Kantrowitz J. T. (2018) N-methyl-d-aspartate-type glutamate receptor modulators and related medications for the enhancement of auditory system plasticity in schizophrenia. Schizophrenia Research. . Advance online publication. doi:10.1016/j.schres.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Kantrowitz J. T., Swerdlow N. R., Dunn W., Vinogradov S. (2018) Auditory system target engagement during plasticity-based interventions in schizophrenia: A focus on modulation of N-methyl-d-aspartate-type glutamate receptor function. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. . Advance online publication. doi:10.1016/j.bpsc.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khouri L., Nelken I. (2015) Detecting the unexpected. Current Opinion in Neurobiology 35: 142–147. doi:10.1016/j.conb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- King A. J., Nelken I. (2009) Unraveling the principles of auditory cortical processing: Can we learn from the visual system? Nature Neuroscience 12(6): 698–701. doi:10.1038/nn.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C., von der Behrens W., Gaese B. H. (2014) Stimulus-specific adaptation in field potentials and neuronal responses to frequency-modulated tones in the primary auditory cortex. Brain Topography 27(4): 599–610. doi:10.1007/s10548-014-0376-4. [DOI] [PubMed] [Google Scholar]
- Knill D. C., Pouget A. (2004) The Bayesian brain: The role of uncertainty in neural coding and computation. Trends in Neurosciences 27(12): 712–719. doi:10.1016/j.tins.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Koelsch S., Heinke W., Sammler D., Olthoff D. (2006) Auditory processing during deep propofol sedation and recovery from unconsciousness. Clinical Neurophysiology 117(8): 1746–1759. doi:10.1016/j.clinph.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Koshiyama D., Kirihara K., Tada M., Nagai T., Fujioka M., Koike S., Kasai K. (2018) Association between mismatch negativity and global functioning is specific to duration deviance in early stages of psychosis. Schizophrenia Research 195: 378–384. doi:10.1016/J.SCHRES.2017.09.045. [DOI] [PubMed] [Google Scholar]
- Kraus N., Nicol T. (2009) Auditory evoked potentials. In: Binder M. D., Hirokawa N., Windhorst U. (eds) Encyclopedia of neuroscience, 1st ed Berlin, Germany: Springer-Verlag Berlin Heidelberg, pp. 214–218. [Google Scholar]
- Kreitschmann-Andermahr I., Rosburg T., Demme U., Gaser E., Nowak H., Sauer H. (2001) Effect of ketamine on the neuromagnetic mismatch field in healthy humans. Cognitive Brain Research 12(1): 109–116. doi:10.1016/S0926-6410(01)00043-X. [DOI] [PubMed] [Google Scholar]
- Kujala T., Leminen M. (2017) Low-level neural auditory discrimination dysfunctions in specific language impairment—A review on mismatch negativity findings. Developmental Cognitive Neuroscience 28: 65–75. doi:10.1016/j.dcn.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala T., Tervaniemi M., Schröger E. (2007) The mismatch negativity in cognitive and clinical neuroscience: Theoretical and methodological considerations. Biological Psychology 74(1): 1–19. doi:10.1016/j.biopsycho.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lee C. C., Winer J. A. (2008) Connections of cat auditory cortex: I. Thalamocortical system. Journal of Comparative Neurology 507(6): 1879–1900. doi:10.1002/cne.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. S., Mumford D. (2003) Hierarchical Bayesian inference in the visual cortex. Journal of the Optical Society of America A 20(7): 1434 doi:10.1364/JOSAA.20.001434. [DOI] [PubMed] [Google Scholar]
- Light G. A., Näätänen R. (2013) Mismatch negativity is a breakthrough biomarker for understanding and treating psychotic disorders. Proceedings of the National Academy of Sciences 110(38): 15175–15176. doi:10.1073/pnas.1313287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy D. H., Campbell K. B., De Lugt D. R., Elton M., Kok A. (2000) The mismatch negativity during natural sleep: Intensity deviants. Clinical Neurophysiology 111(5): 863–872. doi:10.1016/S1388-2457(00)00256-X. [DOI] [PubMed] [Google Scholar]
- Ma Y., Hu H., Berrebi A. S., Mathers P. H., Agmon A. (2006) Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. Journal of Neuroscience 26(19): 5069–5082. doi:10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald M., Campbell K. (2011) Effects of a violation of an expected increase or decrease in intensity on detection of change within an auditory pattern. Brain and Cognition 77(3): 438–445. doi:10.1016/j.bandc.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Maess B., Jacobsen T., Schröger E., Friederici A. D. (2007) Localizing pre-attentive auditory memory-based comparison: Magnetic mismatch negativity to pitch change. NeuroImage 37(2): 561–571. doi:10.1016/j.neuroimage.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Malmierca M. S. (2015) Chapter 29 – Auditory system. In: Paxinos G. (ed.) The rat nervous system, Cambridge, MA: Academic Press, pp. 865–946. [Google Scholar]
- Malmierca M. S., Anderson L. A., Antunes F. M. (2015) The cortical modulation of stimulus-specific adaptation in the auditory midbrain and thalamus: A potential neuronal correlate for predictive coding. Frontiers in Systems Neuroscience 9: 19 doi:10.3389/fnsys.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca M. S., Cristaudo S., Perez-Gonzalez D., Covey E. (2009) Stimulus-specific adaptation in the inferior colliculus of the anesthetized rat. Journal of Neuroscience 29(17): 5483–5493. doi:10.1523/JNEUROSCI.4153-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca, M. S., & Hackett, T. A. (2010). Structural organization of the ascending auditory pathway. In: D. R. Moore, A. Rees, & A. R. Palmer (Eds.), The Oxford handbook of auditory science: The Auditory Brain (pp. 9–42). Oxford University Press.
- Malmierca, M. S., & Ryugo, D. K. (2011). Descending connections of auditory cortex to the midbrain and brain stem. In J. A. Winer & C. E. Schreiner (Eds.), The auditory cortex (1st ed., pp. 189–208). Boston, MA: Springer US.
- Malmierca M. S., Sanchez-Vives M. V., Escera C., Bendixen A. (2014) Neuronal adaptation, novelty detection and regularity encoding in audition. Frontiers in Systems Neuroscience 8: 111 doi:10.3389/fnsys.2014.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone, B. J., & Semple, M. N. (2001). Effects of auditory stimulus context on the representation of frequency in the gerbil inferior colliculus. Journal of Neurophysiology, 86(3), 1113–1130. [DOI] [PubMed] [Google Scholar]
- Marrosu F., Portas C., Mascia M. S., Casu M. A., Fà M., Giagheddu M., Gessa G. L. (1995) Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Research 671(2): 329–332. doi:10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- May P. J. C., Tiitinen H. (2010) Mismatch negativity (MMN), the deviance-elicited auditory deflection, explained. Psychophysiology 47(1): 66–122. doi:10.1111/j.1469-8986.2009.00856.x. [DOI] [PubMed] [Google Scholar]
- Merchán M., Aguilar L. A., López-Poveda E. A., Malmierca M. S. (2005) The inferior colliculus of the rat: Quantitative immunocytochemical study of GABA and glycine. Neuroscience 136(3): 907–925. doi:10.1016/j.neuroscience.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Metherate R., Ashe J. H. (1993) Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse 14(2): 132–143. doi:10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Minks E., Jurák P., Chládek J., Chrastina J., Halámek J., Shaw D. J., Bareš M. (2014) Mismatch negativity-like potential (MMN-like) in the subthalamic nuclei in Parkinson’s disease patients. Journal of Neural Transmission 121(12): 1507–1522. doi:10.1007/s00702-014-1221-3. [DOI] [PubMed] [Google Scholar]
- Mittag M., Takegata R., Winkler I. (2016) Transitional probabilities are prioritized over stimulus/pattern probabilities in auditory deviance detection: Memory basis for predictive sound processing. Journal of Neuroscience 36(37): 9572–9579. doi:10.1523/JNEUROSCI.1041-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R. J., Campo P., Symmonds M., Stephan K. E., Dolan R. J., Friston K. J. (2013) Free energy, precision and learning: The role of cholinergic neuromodulation. Journal of Neuroscience 33(19): 8227–8236. doi:10.1523/JNEUROSCI.4255-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlet D., Fischer C. (2014) MMN and novelty P3 in coma and other altered states of consciousness: A review. Brain Topography 27(4): 467–479. doi:10.1007/s10548-013-0335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott D. (2015) The metabotropic GABAB receptors. In: Hammond C. (ed.) Cellular and molecular neurophysiology, 4th ed Cambridge, MA: Academic Press, pp. 245–269. [Google Scholar]
- Motts S. D., Schofield B. R. (2009) Sources of cholinergic input to the inferior colliculus. Neuroscience 160(1): 103–114. doi:10.1016/j.neuroscience.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R., Astikainen P., Ruusuvirta T., Huotilainen M. (2010) Automatic auditory intelligence: An expression of the sensory-cognitive core of cognitive processes. Brain Research Reviews 64(1): 123–136. doi:10.1016/j.brainresrev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Näätänen R., Gaillard A. W. K., Mäntysalo S. (1978) Early selective-attention effect on evoked potential reinterpreted. Acta Psychologica 42(4): 313–329. doi:10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Näätänen R., Kähkönen S. (2009) Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: A review. International Journal of Neuropsychopharmacology 12(1): 125–135. doi:10.1017/S1461145708009322. [DOI] [PubMed] [Google Scholar]
- Näätänen R., Kujala T., Escera C., Baldeweg T., Kreegipuu K., Carlson S., Ponton C. (2012) The mismatch negativity (MMN) – A unique window to disturbed central auditory processing in ageing and different clinical conditions. Clinical Neurophysiology 123(3): 424–458. doi:10.1016/j.clinph.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Näätänen R., Paavilainen P., Rinne T., Alho K. (2007) The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clinical Neurophysiology 118(12): 2544–2590. doi:10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Näätänen R., Petersen B., Torppa R., Lonka E., Vuust P. (2017) The MMN as a viable and objective marker of auditory development in CI users. Hearing Research 353: 57–75. doi:10.1016/j.heares.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Näätänen R., Sussman E. S., Salisbury D., Shafer V. L. (2014) Mismatch negativity (MMN) as an index of cognitive dysfunction. Brain Topography 27(4): 451–466. doi:10.1007/s10548-014-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R., Tervaniemi M., Sussman E., Paavilainen P., Winkler I. (2001) ‘Primitive intelligence’ in the auditory cortex. Trends in Neurosciences 24(5): 283–288. doi:10.1016/S0166-2236(00)01790-2. [DOI] [PubMed] [Google Scholar]
- Nashida T., Yabe H., Sato Y., Hiruma T., Sutoh T., Shinozaki N., Kaneko S. (2000) Automatic auditory information processing in sleep. Sleep 23(6): 821–828. [PubMed] [Google Scholar]
- Natan R. G., Briguglio J. J., Mwilambwe-Tshilobo L., Jones S. I., Aizenberg M., Goldberg E. M., Geffen M. N. (2015) Complementary control of sensory adaptation by two types of cortical interneurons. eLife 4: 1–27. doi:10.7554/eLife.09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natan R. G., Rao W., Geffen M. N. (2017) Cortical interneurons differentially shape frequency tuning following adaptation. Cell Reports 21(4): 878–890. doi:10.1016/j.celrep.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisser U. (1976) Cognition and reality: Principles and implications of cognitive psychology, New York, NY: W H Freeman/Times Books/ Henry Holt & Co.. [Google Scholar]
- Nelken I. (2014) Stimulus-specific adaptation and deviance detection in the auditory system: Experiments and models. Biological Cybernetics 108(5): 655–663. doi:10.1007/s00422-014-0585-7. [DOI] [PubMed] [Google Scholar]
- Nelken I., Ulanovsky N. (2007) Mismatch negativity and stimulus-specific adaptation in animal models. Journal of Psychophysiology 21(3–4): 214–223. doi:10.1027/0269-8803.21.34.214. [Google Scholar]
- Nieto-Diego J., Malmierca M. S. (2016) Topographic distribution of stimulus-specific adaptation across auditory cortical fields in the anesthetized rat. PLoS Biology 14(3): e1002397 doi:10.1371/journal.pbio.1002397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordby H., Roth W. T., Pfefferbaum A. (1988) Event-related potentials to breaks in sequences of alternating pitches or interstimulus intervals. Psychophysiology 25(3): 262–268. doi:10.1111/j.1469-8986.1988.tb01239.x. [DOI] [PubMed] [Google Scholar]
- Oceák A., Winkler I., Sussman E., Alho K. (2006) Loudness summation and the mismatch negativity event-related brain potential in humans. Psychophysiology 43(1): 13–20. doi:10.1111/j.1469-8986.2006.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz B., Schröger E., Von Cramon D. Y. (2005) Sensory and cognitive mechanisms for preattentive change detection in auditory cortex. European Journal of Neuroscience 21(2): 531–535. doi:10.1111/j.1460-9568.2005.03839.x. [DOI] [PubMed] [Google Scholar]
- Oranje B., Van Berckel B. N. M., Kemner C., van Ree J. M., Kahn R. S., Verbaten M. N. (2000) The effects of a sub-anaesthetic dose of ketamine on human selective attention. Neuropsychopharmacology 22(3): 293–302. doi:10.1016/S0893-133X(99)00118-9. [DOI] [PubMed] [Google Scholar]
- Paavilainen P. (2013) The mismatch-negativity (MMN) component of the auditory event-related potential to violations of abstract regularities: A review. International Journal of Psychophysiology 88(2): 109–123. doi:10.1016/j.ijpsycho.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Paavilainen P., Kaukinen C., Koskinen O., Kylmälä J., Rehn L. (2018) Mismatch negativity (MMN) elicited by abstract regularity violations in two concurrent auditory streams. Heliyon 4(4): e00608 doi:10.1016/j.heliyon.2018.e00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajani A., Kouider S., Roux P., de Gardelle V. (2017) Unsuppressible repetition suppression and exemplar-specific expectation suppression in the fusiform face area. Scientific Reports 7(1): 160 doi:0.1038/s41598-017-00243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadaniil C. D., Kosmidou V. E., Tsolaki A., Tsolaki M., Kompatsiaris I. Y., Hadjileontiadis L. J. (2016) Cognitive MMN and P300 in mild cognitive impairment and Alzheimer’s disease: A high density EEG-3D vector field tomography approach. Brain Research 1648: 425–433. doi:0.1016/j.brainres.2016.07.043. [DOI] [PubMed] [Google Scholar]
- Parras G. G., Nieto-Diego J., Carbajal G. V., Valdés-Baizabal C., Escera C., Malmierca M. S. (2017) Neurons along the auditory pathway exhibit a hierarchical organization of prediction error. Nature Communications 8(1): 2148 doi:0.1038/s41467-017-02038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel C. R., Redhead C., Cervi A. L., Zhang H. (2012) Neural sensitivity to novel sounds in the rat’s dorsal cortex of the inferior colliculus as revealed by evoked local field potentials. Hearing Research 286(1–2): 41–54. doi:0.1016/j.heares.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Pekkonen E. (2000) Mismatch negativity in aging and in Alzheimer’s and Parkinson’s diseases. Audiology & Neurotology 5(3–4): 216–224. doi:0.1159/000013883. [DOI] [PubMed] [Google Scholar]
- Pekkonen E., Jousmäki V., Reinikainen K., Partanen J. (1995) Automatic auditory discrimination is impaired in Parkinson’s disease. Electroencephalography and Clinical Neurophysiology 95(1): 47–52. doi:0.1016/0013-4694(94)00304-4. [DOI] [PubMed] [Google Scholar]
- Pekkonen E., Hirvonen J., Jääskeläinen I. P., Kaakkola S., Huttunen J. (2001) Auditory sensory memory and the cholinergic system: Implications for Alzheimer’s disease. NeuroImage 14(2): 376–382. doi:0.1006/nimg.2001.0805. [DOI] [PubMed] [Google Scholar]
- Pérez-González D., Hernández O., Covey E., Malmierca M. S. (2012) GABAA-mediated inhibition modulates stimulus-specific adaptation in the inferior colliculus. PLoS One 7(3): e34297 doi:0.1371/journal.pone.0034297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-González D., Malmierca M. S. (2012) Variability of the time course of stimulus-specific adaptation in the inferior colliculus. Frontiers in Neural Circuits 6: 107 doi:0.3389/fncir.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-González D., Malmierca M. S., Covey E. (2005) Novelty detector neurons in the mammalian auditory midbrain. European Journal of Neuroscience 22(11): 2879–2885. doi:0.1111/j.1460-9568.2005.04472.x. [DOI] [PubMed] [Google Scholar]
- Pincze Z., Lakatos P., Rajkai C., Ulbert I., Karmos G. (2001) Separation of mismatch negativity and the N1 wave in the auditory cortex of the cat: A topographic study. Clinical Neurophysiology 112(5): 778–784. doi:0.1016/S1388-2457(01)00509-0. [DOI] [PubMed] [Google Scholar]
- Quaedflieg C. W., Münte S., Kalso E., Sambeth A. (2014) Effects of remifentanil on processing of auditory stimuli: A combined MEG/EEG study. Journal of Psychopharmacology 28(1): 39–48. doi:0.1177/0269881113512036. [DOI] [PubMed] [Google Scholar]
- Raij T., McEvoy L., Mäkelä J. P., Hari R. (1997) Human auditory cortex is activated by omission of auditory stimuli. Brain Research 745(1–2): 134–143. doi:0.1016/S0006-8993(96)01140-7. [DOI] [PubMed] [Google Scholar]
- Ranganath C., Rainer G. (2003) Cognitive neuroscience: Neural mechanisms for detecting and remembering novel events. Nature Reviews Neuroscience 4(3): 193–202. doi:0.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Rao R. P. N., Ballard D. H. (1999) Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nature Neuroscience 2(1): 79–87. doi:0.1038/4580. [DOI] [PubMed] [Google Scholar]
- Recasens M., Grimm S., Wollbrink A., Pantev C., Escera C. (2014) Encoding of nested levels of acoustic regularity in hierarchically organized areas of the human auditory cortex. Human Brain Mapping 35(11): 5701–5716. doi:0.1002/hbm.22582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recasens M., Uhlhaas P. J. (2017) Test–retest reliability of the magnetic mismatch negativity response to sound duration and omission deviants. NeuroImage 157: 184–195. doi:0.1016/j.neuroimage.2017.05.064. [DOI] [PubMed] [Google Scholar]
- Rodríguez R. A., Bussière M., Froeschl M., Nathan H. J. (2014) Auditory-evoked potentials during coma: Do they improve our prediction of awakening in comatose patients? Journal of Critical Care 29(1): 93–100. doi:0.1016/j.jcrc.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Ruhnau P., Herrmann B., Schröger E. (2012) Finding the right control: The mismatch negativity under investigation. Clinical Neurophysiology 123(3): 507–512. doi:0.1016/j.clinph.2011.07.035. [DOI] [PubMed] [Google Scholar]
- Saarinen J., Paavilainen P., Schöger E., Tervaniemi M., Näätänen R. (1992) Representation of abstract attributes of auditory stimuli in the human brain. NeuroReport 3(12): 1149–1151. doi:0.1097/00001756-199212000-00030. [DOI] [PubMed] [Google Scholar]
- Saldaña E., Feliciano M., Mugnaini E. (1996) Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. Journal of Comparative Neurology 371(1): 15–40. doi:0.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sánchez-Vives M. V., Nowak L. G., McCormick D. A. (2000. a) Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. The Journal of Neuroscience 20(11): 4286–4299. doi:0.1523/JNEUROSCI.20-11-04286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Vives M. V., Nowak L. G., McCormick D. A. (2000. b) Membrane mechanisms underlying contrast adaptation in cat area 17 in vivo. Journal of Neuroscience 20(11): 4267–4285. doi:0.1523/JNEUROSCI.20-11-04267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M., Hasselmo M. E., Bruno J. P., Givens B. (2005) Unraveling the attentional functions of cortical cholinergic inputs: Interactions between signal-driven and cognitive modulation of signal detection. Brain Research Reviews 48(1): 98–111. doi:0.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Schall U. (2016) Is it time to move mismatch negativity into the clinic? Biological Psychology 116: 41–46. doi:0.1016/j.biopsycho.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Schofield B. R., Motts S. D., Mellott J. G. (2011) Cholinergic cells of the pontomesencephalic tegmentum: Connections with auditory structures from cochlear nucleus to cortex. Hearing Research 279(1–2): 85–95. doi:0.1016/j.heares.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröger E., Bendixen A., Denham S. L., Mill R. W., Bőhm T. M., Winkler I. (2014) Predictive regularity representations in violation detection and auditory stream segregation: From conceptual to computational models. Brain Topography 27(4): 565–577. doi:0.1007/s10548-013-0334-6. [DOI] [PubMed] [Google Scholar]
- Schröger E., Bendixen A., Trujillo-Barreto N. J., Roeber U. (2007) Processing of abstract rule violations in audition. PLoS One 2(11): e1131 doi:0.1371/journal.pone.0001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröger E., Marzecová A., SanMiguel I. (2015) Attention and prediction in human audition: A lesson from cognitive psychophysiology. European Journal of Neuroscience 41(5): 641–664. doi:0.1111/ejn.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröger E., Wolff C. (1996) Mismatch response of the human brain to changes in sound location. Neuroreport 7(18): 3005–3008. doi:0.1097/00001756-199611250-00041. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Shinn-Cunningham B., Tager-Flusberg H. (2018) Meta-analysis and systematic review of the literature characterizing auditory mismatch negativity in individuals with autism. Neuroscience and Biobehavioral Reviews 87: 106–117. doi:0.1016/j.neubiorev.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculthorpe L. D., Collin C. A., Campbell K. B. (2008) The influence of strongly focused visual attention on the detection of change in an auditory pattern. Brain Research 1234: 78–86. doi:0.1016/j.brainres.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Seer C., Lange F., Georgiev D., Jahanshahi M., Kopp B. (2016) Event-related potentials and cognition in Parkinson’s disease: An integrative review. Neuroscience & Biobehavioral Reviews 71: 691–714. doi:0.1016/j.neubiorev.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Shestopalova L. B., Petropavlovskaia E. A., Semenova V. V., Nikitin N. I. (2018) Mismatch negativity and psychophysical detection of rising and falling intensity sounds. Biological Psychology 133: 99–111. doi:0.1016/j.biopsycho.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Shiga T., Althen H., Cornella M., Zarnowiec K., Yabe H., Escera C. (2015) Deviance-related responses along the auditory hierarchy: Combined FFR, MLR and MMN evidence. PLoS One 10(9): 1–14. doi:0.1371/journal.pone.0136794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki N., Yabe H., Sato Y., Hiruma T., Sutoh T., Matsuoka T., Kaneko S. (2003) Spectrotemporal window of integration of auditory information in the human brain. Cognitive Brain Research 17(3): 563–571. doi:0.1016/S0926-6410(03)00170-8. [DOI] [PubMed] [Google Scholar]
- Shiramatsu T. I., Kanzaki R., Takahashi H. (2013) Cortical mapping of mismatch negativity with deviance detection property in rat. PLoS One 8(12): e82663 doi:0.1371/journal.pone.0082663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S., Sterbing-D’Angelo S., Filipovic B., D'Angelo W. R., Oliver D. L., Kuwada S. (2004) GABAA synapses shape neuronal responses to sound intensity in the inferior colliculus. Journal of Neuroscience 24(21): 5031–5043. doi:0.1523/JNEUROSCI.0357-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoe E., Chandrasekaran B., Spitzer E. R., Wong P. C., Kraus N. (2014) Human brainstem plasticity: The interaction of stimulus probability and auditory learning. Neurobiology of Learning and Memory 109: 82–93. doi:0.1016/j.nlm.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Skoe E., Kraus N. (2010) Hearing it again and again: On-line subcortical plasticity in humans. PLoS One 5(10): e13645 doi:0.1371/journal.pone.0013645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoe E., Krizman J., Spitzer E., Kraus N. (2013) The auditory brainstem is a barometer of rapid auditory learning. Neuroscience 243: 104–114. doi:0.1016/j.neuroscience.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Smith P. H., Bartlett E. L., Kowalkowski A. (2007) Cortical and collicular inputs to cells in the rat paralaminar thalamic nuclei adjacent to the medial geniculate body. Journal of Neurophysiology 98(2): 681–695. doi:0.1152/jn.00235.2007. [DOI] [PubMed] [Google Scholar]
- Solís-Vivanco R., Ricardo-Garcell J., Rodríguez-Camacho M., Prado-Alcalá R. A., Rodríguez U., Rodríguez-Violante M., Rodríguez-Agudelo Y. (2011) Involuntary attention impairment in early Parkinson’s disease: An event-related potential study. Neuroscience Letters 495(2): 144–149. doi:0.1016/j.neulet.2011.03.058. [DOI] [PubMed] [Google Scholar]
- Sonnadara R. R., Alain C., Trainor L. J. (2006) Occasional changes in sound location enhance middle latency evoked responses. Brain Research 1076(1): 187–192. doi:0.1016/j.brainres.2005.12.093. [DOI] [PubMed] [Google Scholar]
- Southwell R., Baumann A., Gal C., Barascud N., Friston K., Chait M. (2017) Is predictability salient? A study of attentional capture by auditory patterns. Philosophical Transactions of the Royal Society B: Biological Sciences 372(1714): 20160105 doi:0.1098/rstb.2016.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M., Sitt J. D., King J.-R., Elbaz M., Azizi L., Buiatti M., Dehaene S. (2015) Disruption of hierarchical predictive coding during sleep. Proceedings of the National Academy of Sciences 112(11): E1353–E1362. doi:0.1073/pnas.1501026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield, C., Trittschuh, E. H., Monti, J. M., Mesulam, M.-M., & Egner, T. (2008). Neural repetition suppression reflects fulfilled perceptual expectations. Nature neuroscience, 11(9), 1004–1006. doi:10.1038/nn.2163. [DOI] [PMC free article] [PubMed]
- Sussman E., Ritter W., Vaughan H. G. (1998) Predictability of stimulus deviance and the mismatch negativity. Neuroreport 9(18): 4167–4170. doi:0.1097/00001756-199812210-00031. [DOI] [PubMed] [Google Scholar]
- Sussman E., Winkler I., Wang W. (2003) MMN and attention: Competition for deviance detection. Psychophysiology 40(3): 430–435. doi:0.1111/1469-8986.00045. [DOI] [PubMed] [Google Scholar]
- Sussman E. S., Chen S., Sussman-Fort J., Dinces E. (2014) The five myths of MMN: Redefining how to use MMN in basic and clinical research. Brain Topography 27(4): 553–564. doi:0.1007/s10548-013-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds R. M., Lee W. W., Kohn A., Schwartz O., Witkowski S., Sussman E. S. (2017) Distinguishing neural adaptation and predictive coding hypotheses in auditory change detection. Brain Topography 30(1): 136–148. doi:0.1007/s10548-016-0529-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski F. D., Garcia-Lazaro J. A., Schnupp J. W. H. (2009) Current source density profiles of stimulus-specific adaptation in rat auditory cortex. Journal of Neurophysiology 102(3): 1483–1490. doi:0.1152/jn.00240.2009. [DOI] [PubMed] [Google Scholar]
- Taaseh N., Yaron A., Nelken I. (2011) Stimulus-specific adaptation and deviance detection in the rat auditory cortex. PLoS One 6(8): e23369 doi:0.1371/journal.pone.0023369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervaniemi M., André J. S., Kruck S., Schröger E., Alter K., De Baene W., Friederici A. D. (2006) From air oscillations to music and speech: Functional magnetic resonance imaging evidence for fine-tuned neural networks in audition. Journal of Neuroscience 26(34): 8647–8652. doi:0.1523/JNEUROSCI.0995-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervaniemi M., Maury S., Näätänen R. (1994) Neural representations of abstract stimulus features in the human brain as reflected by the mismatch negativity. NeuroReport 5(7): 844–846. doi:0.1097/00001756-199403000-00027. [DOI] [PubMed] [Google Scholar]
- Tikhonravov D., Neuvonen T., Pertovaara A., Savioja K., Ruusuvirta T., Näätänen R., Carlson S. (2008) Effects of an NMDA-receptor antagonist MK-801 on an MMN-like response recorded in anesthetized rats. Brain Research 1203: 97–102. doi:0.1016/j.brainres.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Todd J., Harms L., Schall U., Michie P. T. (2013) Mismatch negativity: Translating the potential. Frontiers in Psychiatry 4: 171 doi:0.3389/fpsyt.2013.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic A., de Lange F. P. (2012) Repetition suppression and expectation suppression are dissociable in time in early auditory evoked fields. Journal of Neuroscience 32(39): 13389–13395. doi:0.1523/JNEUROSCI.2227-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic A., van Ede F., Maris E., de Lange F. P. (2011) Prior expectation mediates neural adaptation to repeated sounds in the auditory cortex: An MEG study. Journal of Neuroscience 31(25): 9118–9123. doi:0.1523/JNEUROSCI.1425-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolaki A. C., Kosmidou V., Kompatsiaris I., Papadaniil C., Hadjileontiadis L., Adam A., Tsolaki M. (2017) Brain source localization of MMN and P300 ERPs in mild cognitive impairment and Alzheimer’s disease: A high-density EEG approach. Neurobiology of Aging 55: 190–201. doi:0.1016/j.neurobiolaging.2017.03.025. [DOI] [PubMed] [Google Scholar]
- Ulanovsky N., Las L., Farkas D., Nelken I. (2004) Multiple time scales of adaptation in auditory cortex neurons. Journal of Neuroscience 24(46): 10440–10453. doi:0.1523/JNEUROSCI.1905-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N., Las L., Nelken I. (2003) Processing of low-probability sounds by cortical neurons. Nature Neuroscience 6(4): 391–398. doi:0.1038/nn1032. [DOI] [PubMed] [Google Scholar]
- Umbricht D., Koller R., Vollenweider F. X., Schmid L. (2002) Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biological Psychiatry 51(5): 400–406. doi:0.1016/S0006-3223(01)01242-2. [DOI] [PubMed] [Google Scholar]
- Umbricht D., Schmid L., Koller R., Vollenweider F. X., Hell D., Javitt D. C. (2000) Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers. Archives of General Psychiatry 57(12): 1139–1147. doi:0.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- Valdés-Baizabal C., Parras G. G., Ayala Y. A., Malmierca M. S. (2017) Endocannabinoid modulation of stimulus-specific adaptation in inferior colliculus neurons of the rat. Scientific Reports 7(1): 6997 doi:0.1038/s41598-017-07460-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaskamp C., Oranje B., Madsen G. F., Møllegaard Jepsen J. R., Durston S., Cantio C., Bilenberg N. (2017) Auditory processing in autism spectrum disorder: Mismatch negativity deficits. Autism Research 10(11): 1857–1865. doi:0.1002/aur.1821. [DOI] [PubMed] [Google Scholar]
- von der Behrens W., Bauerle P., Kossl M., Gaese B. H. (2009) Correlating stimulus-specific adaptation of cortical neurons and local field potentials in the awake rat. Journal of Neuroscience 29(44): 13837–13849. doi:0.1523/JNEUROSCI.3475-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacongne C., Changeux J.-P., Dehaene S. (2012) A neuronal model of predictive coding accounting for the mismatch negativity. Journal of Neuroscience 32(11): 3665–3678. doi:0.1523/JNEUROSCI.5003-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacongne C., Labyt E., van Wassenhove V., Bekinschtein T., Naccache L., Dehaene S. (2011) Evidence for a hierarchy of predictions and prediction errors in human cortex. Proceedings of the National Academy of Sciences 108(51): 20754–20759. doi:0.1073/pnas.1117807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Gupta A., Toledo-Rodriguez M., Wu C. Z., Markram H. (2002) Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cerebral Cortex 12(4): 395–410. doi:0.1093/cercor/12.4.395. [DOI] [PubMed] [Google Scholar]
- Winkler I. (2007) Interpreting the mismatch negativity. Journal of Psychophysiology 21(3–4): 147–163. doi:0.1027/0269-8803.21.34.147. [Google Scholar]
- Winkler I., Czigler I. (1998) Mismatch negativity: Deviance detection or the maintenance of the ‘standard’. NeuroReport 9(17): 3809–3813. doi:0.1097/00001756-199812010-00008. [DOI] [PubMed] [Google Scholar]
- Winkler I., Denham S. L., Nelken I. (2009) Modeling the auditory scene: Predictive regularity representations and perceptual objects. Trends in Cognitive Sciences 13(12): 532–540. doi:0.1016/j.tics.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Winkler I., Kushnerenko E., Horvath J., Ceponiene R., Fellman V., Huotilainen M., Sussman E. (2003) Newborn infants can organize the auditory world. Proceedings of the National Academy of Sciences 100(20): 11812–11815. doi:0.1073/pnas.2031891100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I., Schröger E. (2015) Auditory perceptual objects as generative models: Setting the stage for communication by sound. Brain and Language 148: 1–22. doi:0.1016/j.bandl.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Yabe H., Tervaniemi M., Reinikainen K., Näätänen R. (1997) Temporal window of integration revealed by MMN to sound omission. NeuroReport 8(8): 1971–1974. doi:0.1097/00001756-199705260-00035. [DOI] [PubMed] [Google Scholar]
- Yabe H., Winkler I., Czigler I., Koyama S., Kakigi R., Sutoh T., Kaneko S. (2001) Organizing sound sequences in the human brain: The interplay of auditory streaming and temporal integration. Brain Research 897(1–2): 222–227. doi:0.1016/S0006-8993(01)02224-7. [DOI] [PubMed] [Google Scholar]
- Yuste R. (2015) From the neuron doctrine to neural networks. Nature Reviews Neuroscience 16(8): 487–497. doi:0.1038/nrn3962. [DOI] [PubMed] [Google Scholar]
- Zaborszky L., van den Pol A., Gyengesi E. (2012) The basal forebrain cholinergic projection system in mice. In: Watson C., Paxinos G., Puelles L. (eds) The mouse nervous system, 1st ed Cambridge, MA: Academic Press, pp. 684–718. [Google Scholar]
- Zhao L., Liu Y., Shen L., Feng L., Hong B. (2011) Stimulus-specific adaptation and its dynamics in the inferior colliculus of rat. Neuroscience 181: 163–174. doi:0.1016/j.neuroscience.2011.01.060. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Rubio M. E., Tzounopoulos T. (2008) Distinct functional and anatomical architecture of the endocannabinoid system in the auditory brainstem. Journal of Neurophysiology 101(5): 2434–2446. doi:0.1152/jn.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski C. F., Izumi Y., Mennerick S. (2016) Ketamine: NMDA receptors and beyond. Journal of Neuroscience 36(44): 11158–11164. doi:0.1523/JNEUROSCI.1547-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]