Abstract
Targeted immunotherapy using dendritic cell vaccine has been employed for the treatment of solid tumors. Topical 5-aminolevulinic acid-mediated photodynamic therapy, an established approach for topical cancers, can induce an effective antitumor immune response. We have previously shown that 5-aminolevulinic acid-mediated photodynamic therapy–induced tumor lysates could considerably enhance antigen-presenting capacity of ex vivo-generated dendritic cells. The current study further demonstrates that 5-aminolevulinic acid-mediated photodynamic therapy dendritic cell vaccine can induce immune responses against cancers. Dendritic cells pulsed by photodynamic therapy–treated skin squamous cell carcinoma cells inhibited squamous cell carcinoma to a greater extent than tumor lysates treated by photodynamic therapy alone or dendritic cells pulsed by freeze–thawed treated tumor cells. Immunohistochemistry showed that photodynamic therapy dendritic cell vaccine could increase the activity of CD4+ and CD8+ T cells in the tumor implantation sites. Flow cytometry assays showed that CD4+ and CD8+ T cells in the spleens of photodynamic therapy dendritic cell vaccine immunized mice increased significantly. Furthermore, we observed increased amounts of interleukin 12 and Interferon gamma (IFN-γ) and decreased amounts of interleukin 10 in the splenocytes and peripheral blood of photodynamic therapy dendritic cell vaccine immunized mice by enzyme linked immunosorbent assay (ELISA). Taken together, our findings suggest that photodynamic therapy dendritic cell vaccination is an effective prophylactic therapy for squamous cell carcinoma.
Keywords: dendritic cells, photodynamic therapy, 5-aminolevulinic acid, PDT-DC vaccine, immune effects
Introduction
Cancer immunotherapy encompasses various techniques that aim to activate the immune system to control tumor growth.1 Other cancer treatments, such as chemotherapy, radiation, and surgery, all cause great damage to the body. These traditional treatment methods have shown limited success for treating metastatic cancers such as skin squamous cell carcinoma (SCC). However, immunotherapy has shown progress in treating metastatic cancers by using the body’s own immune system to target and destroy cancer cells.2 Tumor cells have the ability to evade the innate and adaptive immune responses through changes in their surface antigens and synthesis of antigen-presenting cell (APC) suppressive factors, leading to inhibition of T-cell responses.3 One of the most promising approaches to cancer immunotherapy is the use of APCs, such as dendritic cells (DCs).4,5 Dendritic cells are the most potent APCs, capable of uptaking, transporting, processing, and presenting antigens to T cells.6,7 Thus, significant advances have been made in the development of DC-based vaccines.8 Studies have shown that DCs pulsed with tumor lysates could enhance therapeutic antitumor immune responses after vaccination.9–11
Dendritic cells are effective in inducing antigen-specific immune responses by presenting tumor antigens to T cells.12 Activated CD4+ T cells initiate and amplify CD8+ T-cell responses by providing cytokines or upregulating a number of molecules on the APCs that provide accessory signals for T-cell activation, resulting in tumor-specific immunity.13
Our previous study shows that 5-aminolevulinic acid (ALA)-mediated photodynamic therapy (PDT)-induced immunogenic apoptotic tumor cells can be more effective in enhancing DC-based cancer vaccines.14 Photodynamic therapy has been shown to be an effective antitumor therapy that uses a photosensitizer and light to induce damage to tumor tissues.15,16 One reason why PDT is an effective antitumor therapy is due to the high precision of energy delivery obtained with the assistance of photosensitizers.16 Photosensitizers used in PDT are generally nontoxic molecules that preferably aggregate in cancer cells and become reactive oxygen species when excited by a light source of appropriate wavelength and energy.17 Our previous study showed that PDT could induce immune response by activating the immune cells and feasible molecular mechanisms.18–20 It was also reported that PDT-treated tumor cells tend to induce effective antitumor immunity in vivo compared to tumor cell lysates produced by treatments like ionizing irradiation or freeze–thaw (FT) therapy.21 When combined with DC-based vaccines, the antitumor effects of PDT could be enhanced to decrease cancer metastasis and target cancer cells throughout the body.
Numerous trials of DC-based vaccines have been conducted in various types of cancers, including malignant melanoma, prostate cancer, renal cell carcinoma, non-Hodgkin lymphoma, multiple myeloma, colorectal cancer, and adenocarcinoma of the lung.22 Studies have shown that antigen-pulsed DC vaccination is a safe and promising tool in the treatment of cancer.23 However, the efficacy and immunological effects of PDT-DC-based vaccines for prevention of SCC has not been determined. In this study, we extended our previous experiments in order to determine the efficacy and immunological mechanism of PDT-DC-based vaccines for SCC.
Materials and Methods
Animal and Cell Line
SKH-1 mice (female, 8 weeks old, hair-less, immunocompetent), weighing approximately 30 g, were obtained from Shanghai Public Health Clinical (Shanghai Certificate number 2010-0024, Shanghai, China). The research was conducted in accordance with the Declaration of Helsinki and with the Guide for Care and Use of Laboratory Animals as adopted and promulgated by the United National Institutes of Health. All experimental protocols were approved by the Review Committee for the Use of Human or Animal Subjects of Shanghai Skin Disease Hospital. Forty mice were divided into 4 groups. The PECA cell line used in this study was SCC cell line obtained from the Cell Lines Service (Germany). PECA cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin (100 IU·mL−1), and streptomycin (100 µg·mL−1) at 37°C in an atmosphere of 5% CO2.
Chemicals and Reagents
RPMI 1640 cell culture medium, phosphate buffer saline (PBS), and penicillin/streptomycin were obtained from Hyclone (Thermo Scientific, Waltham, Massachusetts). Fetal bovine serum was obtained from Gibco (California, USA). 5-Aminolevulinic acid hydrochloride powder was obtained from Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co, Ltd (Shanghai, China). Cell Counting Kit-8 (CCK-8 kit) was obtained from Dojindo (Kumamoto, Japan). Mouse monoclonal anti-CD4 and mouse monoclonal anti-CD8 (Abcam, UK) were used for immunohistochemical studies. Rabbit anti-mouse CD3-PE, rabbit anti-mouse CD4-FITC, and rabbit anti-mouse CD8-PE/Cy5 were also used for flow cytometric analysis. In addition, we used mouse Interferon gamma (IFN-γ), interleukin 12 (IL-12), and IL-10 ELISA Kit (R&D Systems, Minnesota, USA), and 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di- phenytetrazoliumromide (MTT) assay kit (Sigma-Aldrich, St Louis, Missouri).
Preparation of PDT Tumor Lysates
For PDT, 1 × 107 PECA cells growing in 100-mm petri dishes were incubated in the dark with 0.5 mM ALA in serum-free medium for 5 hours, rinsed twice with PBS, and irradiated by a LED light (630 nm, Philips, the Netherlands) at a power density of 10 mW/cm2, with 0.5 J/cm2. The cells were then harvested 6 hours later and used as a source of antigen for DC generation.
Preparation of DCs
Dendritic cells were isolated and cultured according to the method of Inaba et al.24 Briefly, DCs were obtained from bone marrow precursors by flushing femur, tibia, and humerus bones of 8-week-old SKH-1 mice with RPMI-1640. Red blood cells were lysed with Tris–NH4Cl. Cells (1 × 107 cells/well) were then cultured in 6-well plates in fresh, complete medium, containing RPMI 1640 supplemented with 10% FBS, 20 ng/mL granulocyte macrophage colony-stimulating factor (PeproTech), and 10 ng/mL IL-4 (PeproTech, New Jersey, USA). After 48 hours, the culture medium was removed and fresh medium was added. On day 5, 50% of the medium was replaced with a fresh medium. Loosely adherent cells (immature dendritic cells [imDCs]) collected on day 7 were used for the experiments. For Bone Marrow-Derived Dendritic Cells maturation, day 6 DCs were incubated with PDT tumor lysates or FT tumor lysates at a ratio of 10 tumor cells to 1 DC (ie, 10:1) in RPMI 1640. The treated and untreated PECA cells were collected and incubated with imDCs for 24 hours, followed by the detection of major histocompatibility complex (MHC-II), CD80, and CD86 on the surface of DCs. After 24 hours of incubation, DCs were harvested, washed twice in PBS, and resuspended in normal saline or RPMI 1640 for further studies.
Maturation of DCs
PECA cells without treatment or treated by ALA-PDT or F/T were incubated with imDCs at a ratio of 20:1 (PECA:imDCs) for 24 hours. Immature DCs were used for a negative control and DCs incubated with 4 µg/mL Lipopolysaccharide for 24 hours were used for a positive control. After detachment and washing, the DCs were stained with the following antibodies: anti-mouse CD80-FITC, anti-mouse CD86-FITC, anti-mouse MHC-II-PE (eBioscience, California, USA), according to the manufacturer’s instructions. After the antibody staining, the cells were washed and analyzed with a FACScan flow cytometer (Becton Dickinson, Mountain View, California).
Immunization of Animals
Forty mice were divided into 4 groups. The mice were immunized with PDT-DC vaccine, PDT-PECA, or F/T-DC vaccine. Approximately 4 × 106 DCs in 0.2 mL PBS were injected subcutaneously into the same flank of mice. Immunization was done 3 times with a 7-day interval. Control mice were injected with 0.2 mL PBS.
Immunohistochemical Studies
Mice were immunized 3 times with a 7-day interval. Mice were killed 7 days after being challenged with viable PECA cells. Freshly isolated tissue was stored in formalin and 5 µm sections were dewaxed (30 minutes 56°C, 2 × 10 minutes xylene), followed by rehydration, antigen unmasking, and blocking. Then the samples were stained with anti-CD4 and anti-CD8 primary antibodies at 1 µg/mL in the blocking solution for 30 minutes at room temperature. Slides were rinsed in PBS and incubated with goat anti-rabbit Immunoglobulin G (IgG) secondary antibody (Boster, China) diluted in blocking solution for 30 minutes. Slides were inculcated with streptavidin–biotin complex (Boster, Wuhan) for 30 minutes, rinsed in PBS, stained using a diaminobezidin (DAB) chromogen and hematoxylin counterstain, and observed under a light microscope. PBS was used for negative control sections.
Estimation of Splenocytes Cytotoxicity
Seven days after the mice challenged with viable PECA cells, mice were euthanized by cervical dislocation and spleens were aseptically removed and stripped of fat. Single-cell suspensions were obtained by grinding the spleens with a syringe plunger against a fine steel mesh. Erythrocytes were lysed with ammonium chloride hemolysis buffer (0.8% NH4Cl with 0.1 mM EDTA) and then washed twice in complete RPMI-1640 medium. Splenocytes were plated in triplicate in 96-well culture plates and cultured in RPMI-1640 medium supplemented with 10% FBS at 37°C in a humidified 5% CO2 incubator. Splenocytes were used as effector cells and PECA cells were used as target cells. Effector cells were cultured with target cells in different effector to target ratios (100:1, 80:1, 40:1, 20:1, 10:1) for 24 hours. PECA cells without treatment were used as a negative control. After washing, cell death rate was detected by CCK-8 assay according to the instructions.
Flow Cytometric Analysis of Splenocytes
Splenocytes were harvested as discussed above and stained with the following antibodies: anti-mouse CD3-PE, anti-mouse CD4-FITC, and anti-mouse CD8-PE/Cy5 according to the manufacturer’s instructions. Gating was performed on CD3+ T cells and the percentage of CD4+ or CD8+ T cells was calculated. After the antibody staining, the cells were washed and analyzed with a FACScan flow cytometer (Becton Dickinson, Mountain View, California).
Detection of Cytokines From Splenocytes and Peripheral Blood Serum
The peripheral blood serum of the mice was extracted and splenocytes were harvested 7 days after the mice were challenged with viable PECA cells. To evaluate systemic immune response, cytokines (IFN-γ, IL-12, and IL-10) were detected by enzyme linked immunosorbent assay (ELISA) according to the manufacturer’s instructions.
Evaluating the Efficacy of PDT-DC Vaccine In Vivo
To further evaluate DC vaccine-induced immune response in vivo, female SKH-1 mice, age 6 to 8 weeks, were randomly divided into 4 groups (10 per group). The mice were immunized (see section “Immunization of Animals”) and then challenged with 6 × 105 viable PECA cells in the right flank 7 days after the third immunization. Following the challenge, tumor volume was assessed every day throughout the study using calipers (width2 × length/2).
Statistical Analyses
Data are presented as mean (standard deviation; unless otherwise specified). Data were analyzed with GraphPad Prism 5 software. Statistical analyses were performed using t test and P <.05 was considered statistically significant.
Results
Maturation of DCs
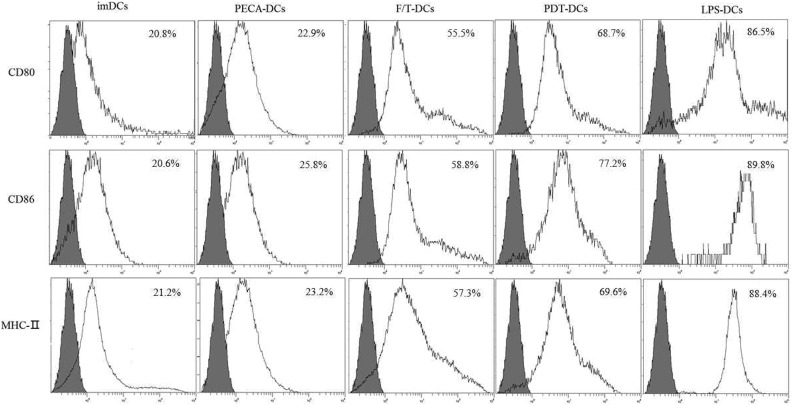
PECA cells treated by PDT have a much greater ability to upregulate expression of CD80, CD86, and MHC-II molecules on the surface of DCs than untreated PECA cells or F/T-treated PECA cells. The expression of CD80, CD86, and MHC-II molecules on DCs induced by PDT-treated PECA cells was significantly higher than that by untreated cells or cells treated by FT (Figure 1).
Figure 1.
Maturation of DCs. PECA cells treated by PDT have a much greater ability to upregulate the expression of CD80, CD86, and MHC-II molecules on the surface of DCs than untreated PECA cells or F/T-treated PECA cells. DC indicates dendritic cell; F/T, freeze–thawed; PDT, photodynamic therapy.
Immunological Effects of DC Vaccines for PECA SCC in a Mouse Model
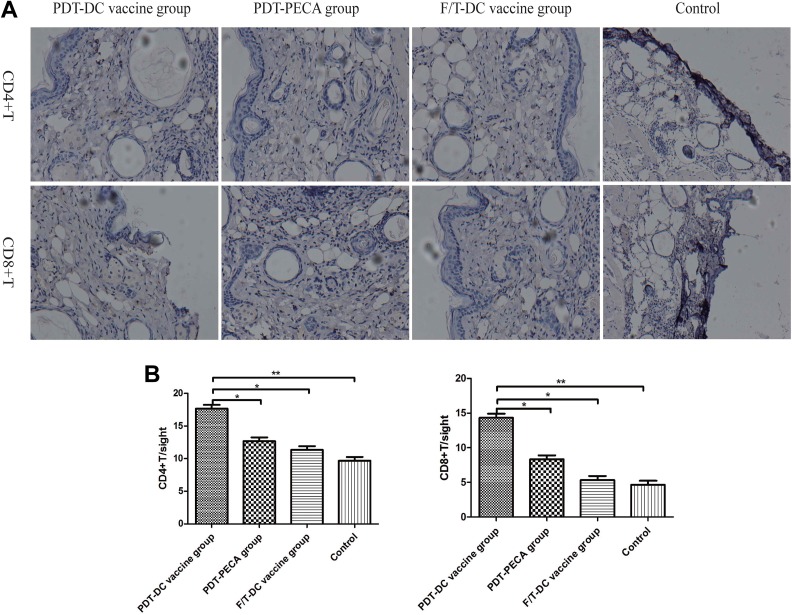
Naive mice were injected subcutaneously with different DC vaccines 3 times with a 7-day interval. Immediately following the third immunization, the mice were implanted with PECA cells. Seven days later, tissue samples from the tumor implantation sites were collected to observe expression of CD4+ and CD8+ T cells using immunohistochemistry. As shown in Figure 2, positive staining for CD4+ and CD8+ T were observed in PDT-DC vaccine group and PDT-PECA group.
Figure 2.
Immunological effects of DC vaccines for PECA SCC in a mouse model. Naive mice are injected with different DC vaccines 3 times with a 7-day interval. Immediately following the third immunization, the mice were implanted with PECA cells. Seven days later, tissue samples at the tumor implant sites were collected for histology. A, Histology of SCC tumors after different treatments stained for CD4+ and CD8+ T cells. B, The counts of CD4+ and CD8+ T cells after different treatments. **P < .005, *P < .05. DC indicates dendritic cell; SCC, squamous cell carcinoma.
Systemic Immunological Effects of PDT-DC Vaccine for PECA SCC
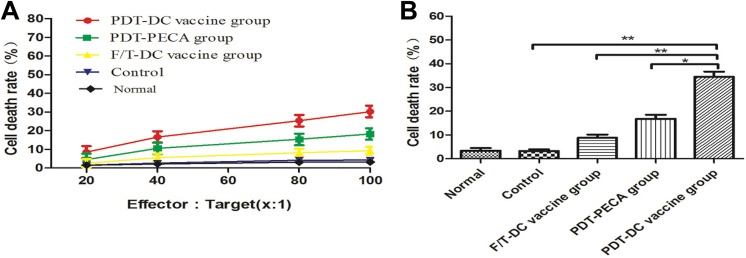
Seven days after the immunized mice were challenged with viable PECA cells, splenocytes were harvested and used for cytotoxic T cells (CTL) assays to investigate the system immunological effects. Different groups of splenocytes were incubated with PECA cells in different effector to target ratios (100:1, 80:1, 40:1, 20:1, 10:1). As shown in Figure 3A, splenocytes caused significant PECA cell death as a function of the effector to target ratio. Photodynamic therapy DC vaccination induced an effective CTL response (approximately 45% cell death) compared to PDT tumor lysates alone or F/T DC vaccination (Figure 3B).
Figure 3.
Splenocyte cytotoxicity after immunization of mice by different vaccines. Seven days after the immunized mice were challenged with viable PECA cells, splenocytes harvested from the mice were used as effector cells. Effector cells were cultured with target cells (PECA cells) in different ratios (100:1, 80:1, 40:1, 20:1, 10:1) for 24 hours. A, Cell death rates of PECA cells in different effector to target ratios. B, Cell death rates of PECA cells in different groups at a 100:1 effector to target ratio. **P < .005, *P < .05.
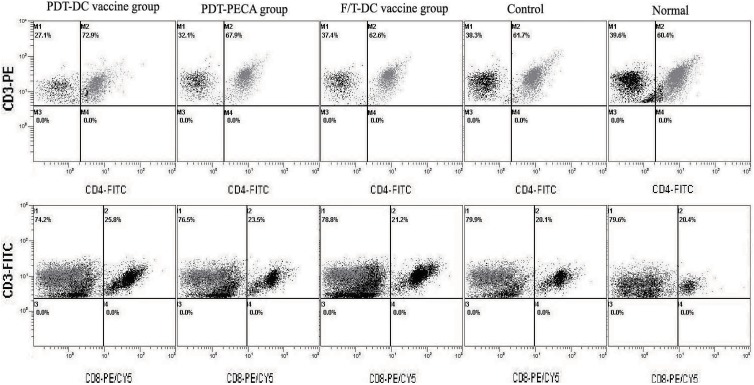
Moreover, the percentage of CD4+ T or CD8+ T cells in splenocytes was characterized by fluorescence activated cell sorter (FACS). In PDT-DC vaccine group, the percentage of CD4+ T or CD8+ T cells in splenocytes was 72.9% or 25.8%, respectively (Figure 4). In the PDT-PECA group, the percentage of CD4+ T or CD8+ T cells in splenocytes was 67.9% or 23.5%, respectively (Figure 4). In F/T-DC group, the percentage of CD4+ T or CD8+ T cells was 62.6% or 21.2%, respectively (Figure 3). In control group, the percentage of CD4+ T or CD8+ T cells was 61.7% or 20.1%, respectively (Figure 4). The percentage of CD4+ T or CD8+ T cells in normal splenocytes was 60.4% or 20.6%, respectively (Figure 4). Thus, vaccination with PDT-DC resulted in potent stimulation of CD4+ T and CD8+ T cells.
Figure 4.
CD4+ T and CD8+ T cells in mouse splenocytes after immunization. Seven days after the immunized mice were challenged with viable PECA cells, splenocytes were harvested from the mice. Percentage of CD4+ T (upper panel) and CD8+ T (lower panel) in the splenocytes were evaluated using fluorescence activated cell sorter (FACS). Compared with other groups, vaccination with PDT-DC resulted in potent stimulation of CD4+ and CD8+ T cells. DC indicates dendritic cell; PDT, photodynamic therapy.
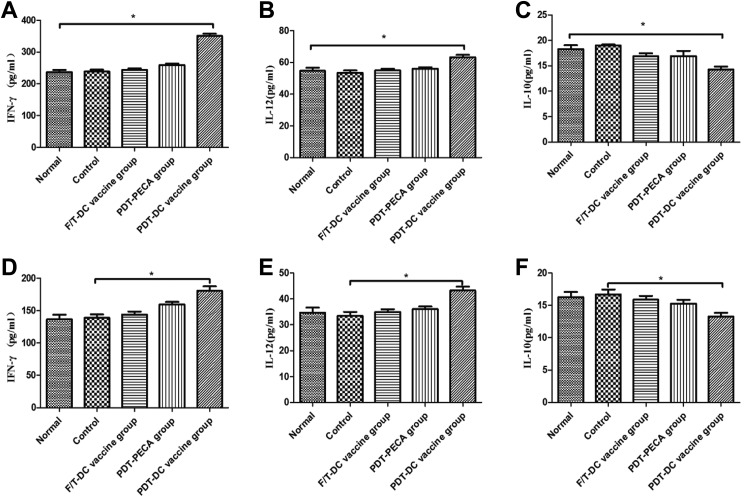
Expression of IFN-γ, IL-12, and IL-10 in Splenocytes and Peripheral Blood in Mice After Immunization
Seven days after the immunized mice were challenged with viable PECA cells, splenocytes were harvested and the peripheral blood of the mice was extracted. The expressions of IFN-γ, IL-12, and IL-10 from splenocytes and peripheral blood were evaluated by ELISA. As shown in Figure 4, PDT-DC vaccination induced significant high levels of IFN-γ and IL-12 secretion compared to PDT-PECA, F/T-DC vaccination, or PBS only. Decreased levels of IL-10 from splenocytes and peripheral blood were detected in the PDT-DC vaccine group and PDT-PECA group (Figure 5).
Figure 5.
Expression of IFN-γ, IL-12, and IL-10 in splenocytes and peripheral blood in mice after immunization. Seven days after the immunized mice were challenged with viable PECA cells, splenocytes were harvested and the peripheral blood of the mice was extracted. The expressions of IFN-γ, IL-12, and IL-10 were evaluated by enzyme linked immunosorbent assay (ELISA) from the splenocytes (A-C) and peripheral blood (D-F). The PDT-DC vaccination induced significantly high levels of IFN-γ and IL-12 as well as significantly low levels of IL-10, compared to that of PDT-PECA, F/T-DC, or phosphate buffer saline (PBS) only. *P < .05. DC, dendritic cell; IL, interleukin; PDT, photodynamic therapy.
Dendritic Cell Vaccines Against PECA SCC in a Mouse Model
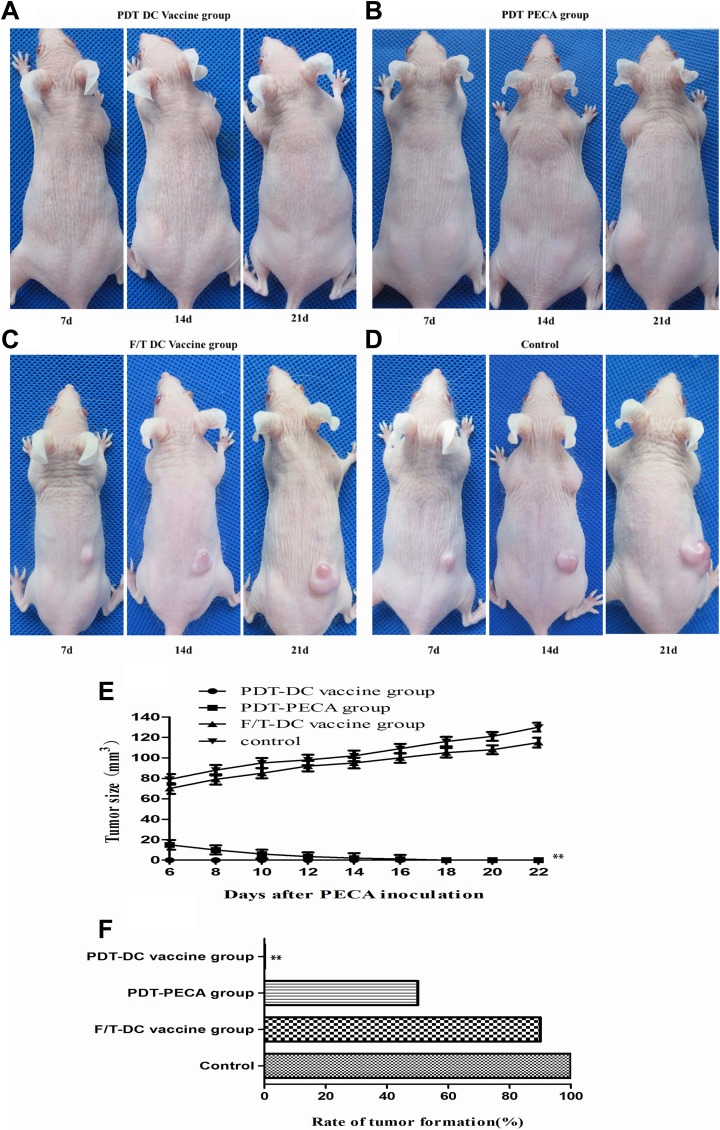
To further evaluate the prophylactic effects of PDT-DC vaccine for SCC, we immunized the SKH-1 hairless mice with a PDT-DC vaccine, PDT-PECA, or F/T-DC vaccine 3 times with a 7-day interval. Seven days after the third immunization, the mice were challenged with viable PECA cells in the other flank. As shown in Figure 6A-E, 21 days after injection with PECA cells, no tumors were observed in PDT-DC vaccine group, while all mice immunized with the F/T-DC vaccine or PBS experienced tumor growth. In PDT-PECA group, tumors were observed 7 days after tumor cells challenge, but faded away 7 days after tumor cells challenge. As shown in Figure 6F, the rate of tumor formation of the PDT-DC vaccine group was 0, whereas the PDT-PECA group, F/T-DC vaccine group, and control group represented high rate of tumor formation.
Figure 6.
Effects of DC vaccines for PECA SCC in a mouse model. Naive mice were injected subcutaneously with different DC vaccines 3 times with a 7-day interval. Immediately following the third immunization, the mice were implanted with PECA cells. A-D, Tumor growths in mice under immunization of different vaccines. No tumors were observed in PDT-DC vaccine group (A). In the PDT-PECA group, tumors were observed 7 days after tumor cells challenge, but faded away 14 days after tumor cells challenge (B). All mice in F/T-DC vaccine group (C) or control group (D) experienced tumor growth. E-F, Tumor volume and rate of tumor formation in different groups. n = 10 per group. **P < .05. DC indicates dendritic cell; PDT, photodynamic therapy; SCC, squamous cell carcinoma.
Discussion
Dendritic cell-based cancer vaccines are an attractive and promising form of cancer immunotherapy.25 Numerous trials have shown that DC-based vaccination is capable of inducing tumor-specific T-cell responses, yet overall, the therapeutic efficacy of this approach is limited due to the lack of ubiquitous tumor antigens.7
5-Aminolevulinic acid-mediated PDT is an established treatment for cutaneous cancers and precancerous lesions. It is demonstrated in our previous study that PDT can induce cell death and activate immune cells.18 It was reported that hypericin mediated PDT-induced immunogenic apoptosis characterized by phenotypic maturation and functional stimulation of DCs and enhanced antitumor immunity.26,27 Our previous study also showed that morphology maturation and functional activation of DCs could be potentiated by ALA-PDT-treated apoptotic PECA cells.14 Moreover, DCs pulsed with ALA-PDT-induced PECA lysates provided protection against skin SCC in mice.14
It was reported that DCs loaded with antigens evoke strong allogeneic stimulatory activity in mixed lymphocyte reactions and activate CD4+ and CD8+ T cells.28 Saji et al also reported that DCs injected into tumors following PDT treatment resulted in potent systemic antitumor immunity and regression of both directly injected and distant tumors, suggesting that the antitumor activity was mediated by CD8+ T cells.6 Immunohistochemistry showed positive staining for CD4+ T and CD8+ T cells in the tissues of PDT-DC vaccine group, in comparison with the PDT-PECA group (Figure 1). Our results were consistent with the previous reports14 and demonstrated that the PDT-DC vaccine induced immune effects by recruiting and activating CD4+ T and CD8+ T cells in the topical area of injecting viable PECA cells.
In order to evaluate the systemic immune effects, splenocytes were harvested from the mice treated by various vaccines. As shown in Figure 2, PDT-DC vaccination induced an effective CTL response (approximately 50% cell death) compared to PDT tumor lysates alone or F/T DC vaccination. FACS of splenocytes showed that the percentage of CD4+ T and CD8+ T cells increased significantly in PDT-DC vaccine group (Figure 3). Our data suggest that the antitumor activity of PDT-DC vaccine is mediated by CD4+ T and CD8+ T cells.
To get further insight into the systemic antitumor response of PDT-DC vaccine, we observed cytokines secreted from splenocytes and peripheral blood. As shown in Figure 4, the secretion of IFN-γ and IL-12 from both splenocytes and peripheral blood increased significantly in the PDT-DC vaccine group, whereas IL-10 decreased slightly. It was reported that the CD4+ T helper (Th) cells response not only generated naive CD8+ T cells into an effective CTL29 but also activated CD8+ memory T cells to a fully functional tumor killer cell.30 Among 2 predominant Th cell subtypes, Th1 cells are characterized by the secretion of IFN-γ and IL-12,28 while Th2 by secretion of IL-10.31 Interleukin 10 is known as an immunosuppressive cytokine.32 Therefore, SCC-specific secretion of IFN-γ and IL-12 in the PDT-DC vaccine immunized mice indicated that antigen-specific CD4+ Th cells were induced, resulting in generating effective CTL responses against the tumor challenge. However, the mice immunized with F/T-DC vaccine induced tumor growth after challenge. It may be due to the fact that F/T-DCs induce the increases of IL-10.
Since significant effects were observed in the findings above, we performed further experiments to evaluate the prophylactic effects of PDT-DC vaccine for SCC. The SKH-1 hairless mice were immunized 3 times with a 7-day interval. No tumors were observed after the mice were challenged with viable PECA cells, while all mice immunized with F/T-DC vaccine or PBS experienced tumor growth (Figure 5). Therefore, it was demonstrated that the PDT-DC vaccine prevented SCC growth, while the F/T-DC vaccine did not. Interestingly, tumors were observed 7 days after tumor cells challenge in PDT-PECA group but regressed and disappeared 14 days after tumor cells challenge (Figure 5). The result demonstrated that PDT-PECA induced poor immune effects that could not prevent tumors entirely.
Conclusions
This study confirms that ALA-PDT DC vaccine can induce systemic antitumor responses to provide protection against cutaneous SCC in mice. On the basis of these findings, we suggest that the PDT-DC vaccination may be developed as an immunotherapy for early-stage SCC.
Acknowledgments
The authors would like to thank all study participants for their cooperation.
Abbreviations
- ALA
5-aminolevulinic acid
- APC
antigen-presenting cell
- CCK-8 kit
Cell Counting Kit-8
- CTL
cytotoxic T cells
- DC
dendritic cell
- FBS
fetal bovine serum
- FT
freeze–thaw
- PDT
photodynamic therapy
- IL
interleukin
- imDCs
immature dendritic cells
- SCC
squamous cell carcinoma
- Th
T helper
Authors’ Note: Haiyan Zhang and Peiru Wang contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by National Natural Science Foundation of China (81472538, 81472796), the Key Project of Shanghai Municipal Commission of Health and Family Planning (20124034), the Advanced Suitable Technology Popularization Project of Shanghai Health System (2013SY007), Basic Research Project of Science and Technology of Shanghai (13JC1405101), the Project of Natural Science Foundation of Shanghai (15411950302), and the US National Institutes of Health (R21 EB0155091-01).
ORCID iD: Xiuli Wang  http://orcid.org/0000-0002-2302-6022
http://orcid.org/0000-0002-2302-6022
References
- 1. Ferris RL. Progress in head and neck cancer immunotherapy: can tolerance and immune suppression be reversed? ORL J Otorhinolaryngol Relat Spec. 2004;66(6):332–340. [DOI] [PubMed] [Google Scholar]
- 2. Fowler DW, Copier J, Wilson N, Dalgleish AG, Bodman-Smith MD. Mycobacteria activate T-cell anti-tumour responses via cytokines from type 1 myeloid dendritic cells: a mechanism of action for cancer immunotherapy. Cancer Immunol Immunother. 2012;61(4):535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aptsiauri N, Cabrera T, Garcia-Lora A, Garrido F. Cancer immune escape: implications for immunotherapy, Granada, Spain, October 3-5, 2011. Cancer Immunol Immunother. 2012;61(5):739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106(3):263–266. [DOI] [PubMed] [Google Scholar]
- 5. Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106(3):271–274. [DOI] [PubMed] [Google Scholar]
- 6. Saji H, Song W, Furumoto K, Kato H, Engleman EG. Systemic antitumor effect of intratumoral injection of dendritic cells in combination with local photodynamic therapy. Clin Cancer Res. 2006;12(8):2568–2574. [DOI] [PubMed] [Google Scholar]
- 7. Jung NC, Kim HJ, Kang MS, et al. Photodynamic therapy-mediated DC immunotherapy is highly effective for the inhibition of established solid tumors. Cancer Lett. 2012;324(1):58–65. [DOI] [PubMed] [Google Scholar]
- 8. Zarnani AH, Torabi-Rahvar M, Bozorgmehr M, Zareie M, Mojtabavi N. Improved efficacy of a dendritic cell-based vaccine against a murine model of colon cancer: the helper protein effect. Cancer Res Treat. 2015;47(3):518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geiger JD, Hutchinson RJ, Hohenkirk LF, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61(23):8513–8519. [PubMed] [Google Scholar]
- 10. Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64(14):4973–4979. [DOI] [PubMed] [Google Scholar]
- 11. Lim DS, Kim JH, Lee DS, Yoon CH, Bae YS. DC immunotherapy is highly effective for the inhibition of tumor metastasis or recurrence, although it is not efficient for the eradication of established solid tumors. Cancer Immunol Immunother. 2007;56(11):1817–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264(5161):961–965. [DOI] [PubMed] [Google Scholar]
- 13. Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. [DOI] [PubMed] [Google Scholar]
- 14. Ji J, Fan Z, Zhou F, et al. Improvement of DC vaccine with ALA-PDT induced immunogenic apoptotic cells for skin squamous cell carcinoma. Oncotarget. 2015;6(19):17135–17146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang Z, Xu H, Meyers AD, et al. Photodynamic therapy for treatment of solid tumors-potential and technical challenges. Technol Cancer Res Treat. 2008;7(4):309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang XL, Wang HW, Wang HS, Xu SZ, Liao KH, Hillemanns P. Topical 5-aminolaevulinic acid-photodynamic therapy for the treatment of urethral condylomata acuminata. Br J Dermatol. 2004;151(4):880–885. [DOI] [PubMed] [Google Scholar]
- 17. Chen WR, Wei-Guo Z, Dynlacht JR, Lui H, Nordquist RE. Long-term tumor resistance induced by laser photo-immunotherapy. Int J Cancer. 1999;81(5):808–812. [DOI] [PubMed] [Google Scholar]
- 18. Song S, Zhou F, Chen WR, Xing D. PDT-induced HSP70 externalization up-regulates NO production via TLR2 signal pathway in macrophages. FEBS Lett. 2013;587(2):128–135. [DOI] [PubMed] [Google Scholar]
- 19. Li H, Liu L, Xing D, Chen WR. Inhibition of the JNK/Bim pathway by Hsp70 prevents Bax activation in UV-induced apoptosis. FEBS Lett. 2010;584(22):4672–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X, Xing D, Liu L, Chen WR. BimL directly neutralizes BclxL to promote Bax activation during UV-induced apoptosis. FEBS Lett. 2009;583(12):1873–1879. [DOI] [PubMed] [Google Scholar]
- 21. Garg AD, Nowis D, Golab J, Agostinis P. Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity. Apoptosis. 2010;15(9):1050–1071. [DOI] [PubMed] [Google Scholar]
- 22. Moon JH, Chung MK, Son YI. Immunotherapy with dendritic cells in an animal model of early pulmonary metastatic squamous cell carcinoma. Laryngoscope. 2012;122(11):2442–2446. [DOI] [PubMed] [Google Scholar]
- 23. Morisaki T, Matsumoto K, Onishi H, et al. Dendritic cell-based combined immunotherapy with autologous tumor-pulsed dendritic cell vaccine and activated T cells for cancer patients: rationale, current progress, and perspectives. Hum Cell. 2003;16(4):175–182. [DOI] [PubMed] [Google Scholar]
- 24. Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Z, Fan H, Wu Y, Chen B. Potent in vivo anti-tumor activity of isolated CD62 L (low) lymph node cells sensitized in vivo with tumor lysate-pulsed DC-based vaccines. Cytotherapy. 2005;7(4):353–362. [DOI] [PubMed] [Google Scholar]
- 26. Panzarini E, Inguscio V, Dini L. Immunogenic cell death: can it be exploited in PhotoDynamic Therapy for cancer? Biomed Res Int. 2013;2013:482160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garg AD, Krysko DV, Verfaillie T, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31(5):1062–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qiu L, Li J, Yu S, et al. A novel cancer immunotherapy based on the combination of a synthetic carbohydrate-pulsed dendritic cell vaccine and glycoengineered cancer cells. Oncotarget. 2015;6(7):5195–5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189(5):753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao FG, Khammanivong V, Liu WJ, Leggatt GR, Frazer IH, Fernando GJ. Antigen-specific CD4+ T-cell help is required to activate a memory CD8+ T cell to a fully functional tumor killer cell. Cancer Res. 2002;62(22):6438–6441. [PubMed] [Google Scholar]
- 31. Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54(8):721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang YJ, Choi JS, Choi JW. Antiangiogenic therapy impedes infiltration by CD4+ and CD8+ cells into an early colon tumor. J Cancer Prev. 2015;20(2):129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]