Abstract
Reversible electroporation is used to increase the uptake of chemotherapeutic drugs in local tumor treatment (electrochemotherapy) by applying the pulsing protocol (8 rectangular pulses, 1000 V/cm, 100 µs) standardized in the framework of the European Standard Operating Procedure on Electrochemotherapy multicenter trial. Currently, new electrochemotherapy strategies are under development to extend its applicability to tumors with different histology. Electrical parameters and drug type are critical factors. A possible approach is to test pulse parameters different from European Standard Operating Procedure on Electrochemotherapy but with comparable electroporation yield (European Standard Operating Procedure on Electrochemotherapy-equivalent protocols). Moreover, the use of non-toxic drugs combined with electroporation represents the new frontier for electrochemotherapy applications; calcium electroporation has been recently proposed as a simple tool for anticancer therapy. In vitro investigations facilitate the optimization of electrical parameters and drugs for in vivo and clinical testing. In this optimization study, new pulsing protocols have been tested by increasing the pulse number and reducing the electric field with respect to the standard. European Standard Operating Procedure on Electrochemotherapy-equivalent protocols have been identified in HL-60 and A431 cancer cell models, and a higher sensitivity in terms of electroporation yield has been recorded in HL-60 cells. Moreover, cell killing efficacy of European Standard Operating Procedure on Electrochemotherapy-equivalent protocols has been demonstrated in the presence of increasing calcium concentrations on both cell lines. Equivalent European Standard Operating Procedure on Electrochemotherapy protocols can be used to optimize the therapeutic effects in the clinic, where different regions of the same cancer tissue, with different electrical properties, might result in a differential electroporation yield of the standard protocol over the same tissue, or, eventually, in an override of the operational limits of the instrument. Moreover, using calcium can help overcome the drawbacks of standard drugs (side effects, high costs, difficult handling, preparation, and storage procedures). These results support the possibility of new treatment options in both standard electrochemotherapy and calcium electroporation, with clear advantages in the clinic.
Keywords: calcium electroporation, ESOPE, equivalent protocols, in vitro, HL-60 cells, A431 cells
Introduction
The application of external pulsed electric fields (PEFs) of sufficient amplitude and short duration to mammalian cells results in an increase in the transmembrane potential of the cell above a critical value, leading to the permeabilization of the plasma membrane (electroporation or electropermeabilization [EP]).1
Depending on the PEF exposure parameters (electric field amplitude, pulse duration, repetition rate, and number of pulses), membrane permeabilization can be either transient (reversible EP) or permanent, leading to cell death (irreversible electroporation [IRE]).
Based on these characteristics, different EP-based strategies have been developed for applications in the clinical field. The use of IRE as a nonthermal modality for cancer and tissue ablation has substantially increased over the past decade, and in vitro, translational, and clinical studies are being carried out with the aim of proving its validity and safety in clinical practice.2
Reversible EP is used in DNA transfection for DNA vaccination and gene therapy, although standard procedures have not yet been defined.3 Reversible EP is also used to increase the uptake of cytotoxic hydrophilic drugs in local tumor treatment, termed electrochemotherapy (ECT).4
Electrochemotherapy, which is highly effective even with lower doses of the chemotherapeutic agent (and concomitant reductions in side effects), has become a standard practice for the treatment of cutaneous and subcutaneous metastatic tumor nodules or deep-seated tumors of various histologies at different body sites, and is currently adopted in over 140 clinical centers in Europe.5–7
Electrochemotherapy treatment was standardized in the framework of the European Standard Operating Procedure on Electrochemotherapy (ESOPE) multicenter trial, first released in 20064,8 and recently updated.9 The ESOPE protocol (8 rectangular pulses, 1000 V/cm, 100 µs) defines the standard pulsing conditions on human patients, in combination with a nonpermeant (bleomycin) or a low-permeant (cisplatin) anticancer drug with a very high intrinsic cytotoxicity, and pulses applied just after, or few minutes after, the injection of the drug.5,6
New ECT strategies are currently being evaluated to extend its applicability to tumors with different histology, including drug-resistant cancer types.10 The physical and biological heterogeneity of cancer cells leads to the expectation that different cancer cells within a tumor may require different electrical parameters for the optimum therapeutic effect. Indeed, different regions of the same cancer tissue might be, for example, characterized by differences in conductivity, resulting in variations in EP yield of the standard protocol over the same tissue. Moreover, in necrotic areas exhibiting a lower electrical impedance, an increase in the current above the operational limits of the instrument may occur, leading to an interruption of the treatment.11,12 Furthermore, following the ESOPE protocol application, different degrees of adverse reactions have been reported, such as electrically burnt skin and uncontrollable muscle contraction.13,14 The impracticability of testing new combinations on patients makes in vitro investigations a suitable approach for selecting electrical parameters for evaluation in in vivo and clinical studies. It has been demonstrated that ECT parameters optimized in vitro are applicable in vivo.15–17
Accordingly, an important challenge in this framework is the definition of new combinations of pulsing protocols and drugs which address the above mentioned issues without reducing the efficiency of the standard treatment.
Several papers tested in vitro pulsing protocols different from ESOPE. Saczko and coworkers tested a combination of 5-fluorouracil and cisplatin in a human ovarian carcinoma cell model, resistant to cisplatin.18 Ongaro and coworkers identified, in a human osteosarcoma cell line, different combinations of electric field amplitude and pulse number, able to attain EP efficiency comparable to the one achieved by the standard ESOPE protocol (ESOPE-equivalent protocols).12
Recently, a new EP-based therapy called calcium electroporation (calcium EP) has been demonstrated.19–23 In this method, lethal levels of intracellular calcium (Ca2+) are generated by electropermeabilizing tumor tissue in the presence of extracellular calcium administered locally. Calcium signaling, which regulates many vital cell functions and is necessary for cell survival, depends on the carefully regulated distribution of Ca2+ among intracellular compartments and the maintenance of a very low Ca2+ concentration overall in the cytoplasm. The loss of calcium homeostatic control, which occurs after the severe intracellular calcium overload associated with calcium EP, can result in necrotic, apoptotic, or autophagic cell death.24
Calcium EP kills tumor cells in vitro with an efficiency similar to that of the chemotherapeutic agent bleomycin,7,19 and calcium EP is more effective in inducing cell death in cancer than in healthy cells, possibly due to different membrane repair capacities, different membrane compositions, different energy reserves, and other factors that distinguish cancer cells from healthy cells.23,25–28 Calcium EP is also effective in vivo,20 with preliminary indications of an induced immune response in addition to direct killing of tumor cells.29,30 Calcium EP requires only a simple aqueous calcium solution. Because it does not use chemotherapeutic drugs, calcium EP avoids the complications of specific pharmacological preparation, handling, and storage procedures, and it can be easily incorporated into the services of nononcological centers, such as dermatology clinics, and even into field facilities lacking a biomedical technological infrastructure.7 The first clinical trial on calcium use in ECT was initiated in 2013, and the results have been recently published.28
The goal of the present study is to identify optimized ESOPE-equivalent pulsing protocols for 2 different cancer cell models and to test their efficacy with calcium as the therapeutic agent. Human acute promyelocytic leukemia (HL-60) cells and human epidermoid carcinoma (A431) cells were subjected to 100-microsecond electric pulses with a 5-kHz repetition rate, and the EP efficiency was evaluated for several protocols with increased pulse number and reduced electric field amplitude relative to the ESOPE standard, and with several concentrations of calcium.
Materials and Methods
Chemicals and Reagents
Roswell Park Memorial Institute (RPMI) medium, Dulbecco’s modified Eagle medium (DMEM), phosphate-buffered saline (PBS), foetal bovine serum (FBS), l-glutamine, penicillin–streptomycin, trypan blue, and trypsin were from Biowhittaker (Verviers, Belgium), and resazurin, propidium iodide (PI), calcein acetoxymethyl ester (CAM), hepes, sucrose, magnesium chloride (MgCl2), and calcium chloride (CaCl2) were from Sigma (St. Louis, Missouri).
Cell Culture and Maintenance
Both HL-60 and A431 cell lines were used as models of different cancer types. The HL-60 cells were acquired from the Biological Bank and Cell Factory of the IRCCS Azienda Ospedaliera Universitaria San Martino—IST National Institute for the Research on Cancer (Genova, Italy), while A431 cells were kindly provided by Prof. Paolo Abrescia from the University Federico II (Napoli, Italy). Cells were maintained under exponential growth conditions (37°C, 95% air, and 5% CO2) in standard complete growth medium consisting of RPMI (in the case of HL-60 cells) or DMEM (in the case of A431 cells) supplemented with 10% heat-inactivated FBS, 2 mmol/L l-glutamine, and 1% penicillin/streptomycin antibiotic. For consistency and reproducibility, HL-60 cells were subcultured 3 times per week, while A431 were routinely maintained as a monolayer by subculturing twice per week by trypsinization.
Experimental Procedures
Pulse Generator and Pulsing Conditions
A Cliniporator device (IGEA, SpA, Italy) was used to deliver 100-microsecond electric pulses, 5 kHz repetition rate, to 300 µL pulsing buffer containing 5 × 106 cells/mL in standard, 4-mm gap, EP cuvettes (Sigma Aldrich). Different pulsing media were used based on the specific analysis to be carried out and are described below. The following pulse number/electric field combinations were tested: 8 pulses, 1000 V/cm (ESOPE); 20 pulses, 750 V/cm; 40 pulses, 750 V/cm; 40 pulses, 500 V/cm; 60 pulses, 500 V/cm; and 80 pulses, 500 V/cm.
For each pulsing condition, each exposed sample was associated with a sham-exposed sample which served as control.
Analysis of EP Efficiency
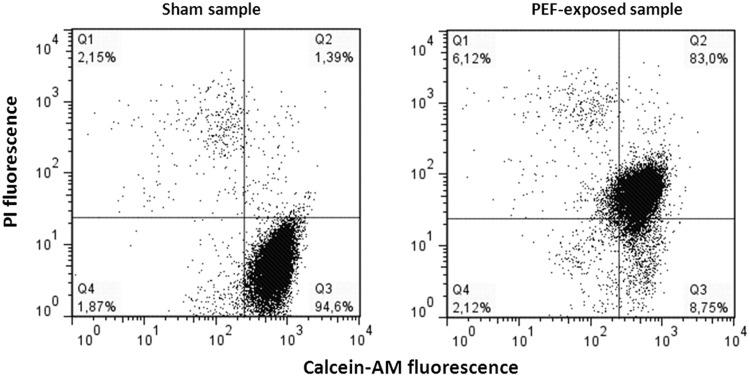
The EP efficiency was evaluated with a flow cytometric method based on double-staining samples with the fluorescent dyes CAM and PI. The CAM is an esterified anionic fluorochrome that enters viable cells freely and becomes trapped in the cytoplasm by an intact cell membrane following removal of the AM group. The PI is membrane impermeant and generally excluded from viable cells, but it passes through permeabilized membranes and fluoresces on binding to nucleic acids. The combined staining of cells with these 2 dyes enables quantification, in the same sample, of the percentage of viable and nonpermeabilized (CAM+/PI−; Q3), viable and permeabilized (CAM+/PI+; Q2), and dead (CAM−/PI+; Q1) cells. Unstained debris (CAM−/PI−; Q4) were not included in the analysis. A representative dot-plot graph is shown in Figure 1.
Figure 1.
Calcein-AM/PI flow cytometric assay. A representative dot-plot graph from analysis of PEF-exposed/sham-exposed samples, double-stained with calcein-AM and PI. Calcein-AM indicates acetoxymethyl ester; PI, propidium iodide.
For the experiments, cells were exposed in basic medium to electric pulses in the presence of PI (40 µmol/L) and then incubated for 5 minutes at room temperature (RT) to allow dye uptake by permeabilized cells. Subsequently, samples were washed in PBS and incubated for 10 minutes at RT in PBS containing CAM (0.2 µmol/L in the case of HL-60 cells and 0.02 µmol/L in the case of A431 cells). A BD FacsCalibur flow cytometer (Becton Dickinson, San Josè, CA, USA) (488 nm excitation wavelength) was used to process the samples. For each sample, 15 000 events were acquired using CELL QUEST software (Becton Dickinson, San Josè, CA, USA), and the raw fluorescence data were then quantitatively analyzed with the FlowJo analysis program (TreeStar, Oregon).
Cell permeabilization efficiency was calculated as electropermeabilized live cells / total live cells = Q2 / (Q2 + Q3). For each pulsing protocol, 4 experiments with triplicate samples were carried out.
Analysis of Calcium EP-Induced Cell Death
Cell viability was assessed by the ability of cells to grow over a 24-hour period. The spectrofluorimetric method of the resazurin assay was used, in which the nonfluorescent compound resazurin is reduced to the highly fluorescent resorufin in the growth medium by cell activity, and a direct correlation exists between the reduction in resazurin and the metabolic activity of living cells.31
For the experiments, a previously standardized pulsing procedure was employed.21 Chilled (4°C) cuvettes were used to pulse cells in pulsing buffer (10 mmol/L hepes, 250 mmol/L sucrose, 1 mmol/L MgCl2 in sterile water), at RT, containing CaCl2 added 5 minutes before pulsing to yield a final concentration of 0, 1, 3, 5, or 10 mmol/L. To avoid calcium precipitation, this buffer does not contain phosphates.19 After 20 minutes postpulse incubation of cells in EP cuvettes at 37°C and 5% CO2, to allow the uptake of calcium, cells were diluted in 3 mL complete medium and incubated for 24 hours. At the end of the incubation period, HL-60 cells, collected by centrifugation (1200 rpm, 5 minutes), and A431 cell monolayers were incubated for 40 and 25 minutes, respectively, at 37°C with 10 μg/mL resazurin in PBS (assay medium). Resorufin production was analyzed in the assay medium with a fluorometer (Perkin-Elmer, LS50B; Perkin-Elmer Instruments, Norwalk, Connecticut) at an excitation and emission wavelength of 530 and 590 nm, respectively, and expressed as relative fluorescence unit (RFU). For each pulsing protocol, and for each calcium concentration, 3 experiments with triplicate samples were carried out.
Statistical Analysis
For each pulsing condition, EP occurrence was statistically analyzed by applying the Student t test between exposed and sham-exposed samples. The EP efficiency of each pulsing condition was compared to that of ESOPE to identify equivalent protocols by applying a 1-way analysis of variance (ANOVA) followed by a Bonferroni test.
The effect of calcium alone on cell viability was statistically analyzed by 1-way ANOVA, followed by Bonferroni. Statistical comparisons in cell viability after calcium EP among the groups of samples were conducted with 2-way ANOVA for multiple comparison at the 95% confidence level, followed by a Bonferroni test.
In all cases, data met the assumptions for ANOVA application (independence of samples, normality, and homogeneity of variances). In all cases, P <.05 was considered as an indication of statistical significance.
Results
Effect of Pulsing Parameters on EP Efficiency and Determination of ESOPE-Equivalent Protocols
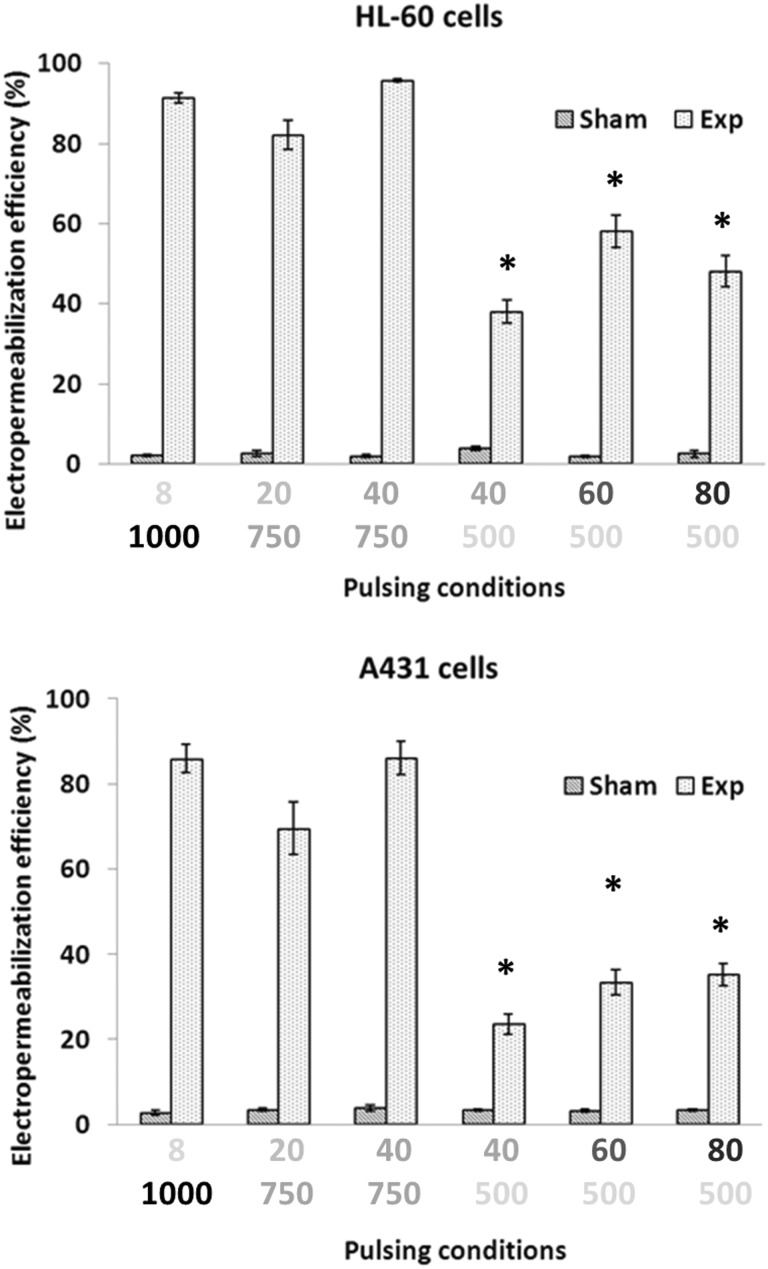
The EP efficiency under different pulsing conditions is presented in Figure 2 for HL-60 and A431 cells. In both cell lines, all the pulsing conditions were able to induce significant EP with respect to sham-exposed cells (P < .01, Student t test, not indicated in the figure to avoid excessive number of symbols), and the EP efficiency strictly depended on the pulse number–electric field combination. It can be pointed out that, at a fixed electric field, the EP efficiency increases upon increasing the pulse number, but a threshold effect is evident in terms of electric field level: 500 V/cm yields a lower EP efficiency (38% ± 3.0%, 58% ± 4.1%, and 48% ± 3.9% for HL-60 cells and 24% ± 2.3%, 33% ± 2.9%, and 35% ± 2.6% for A431 cells, when 40, 60, and 80 pulses were delivered, respectively), with respect to 750 V/cm (82% ± 3.6% and 96% ± 0.38% for HL-60 cells and 70% ± 6.2% and 86% ± 3.9% for A431 cells, when 20 and 40 pulses were delivered, respectively). Moreover, in both cell lines, 20 and 40 pulses delivered at an applied electric field of 750 V/cm were capable of inducing EP efficiency comparable to the one of ESOPE protocol and were identified as ESOPE-equivalent protocols. On the contrary, the 40, 60, and 80 pulses and 500 V/cm protocols resulted significantly different from ESOPE (P < .05 as indicated in Figure 2 with the * symbol).
Figure 2.
Electropermeabilization efficiency in HL-60 and A431 cells subjected to different pulsing protocols. Each data set is for p pulses at q V/cm, highlighted with different gray scales. Each data point is mean ± SE of 4 independent experiments with triplicates (*P < .05 with respect to ESOPE (8 pulses, 1000 V/cm); 1-way ANOVA for repeated measurements followed by Bonferroni test). ANOVA indicates analysis of variance; ESOPE, European Standard Operating Procedure on Electrochemotherapy; SE, standard error.
Taken together, these results also give some indications of a higher sensitivity (in terms of EP) of HL-60 with respect to A431 cells: EP efficiency in HL-60 cells always reached higher values for all the pulsing protocols investigated.
Effect of Extracellular Calcium Ions on Cell Viability
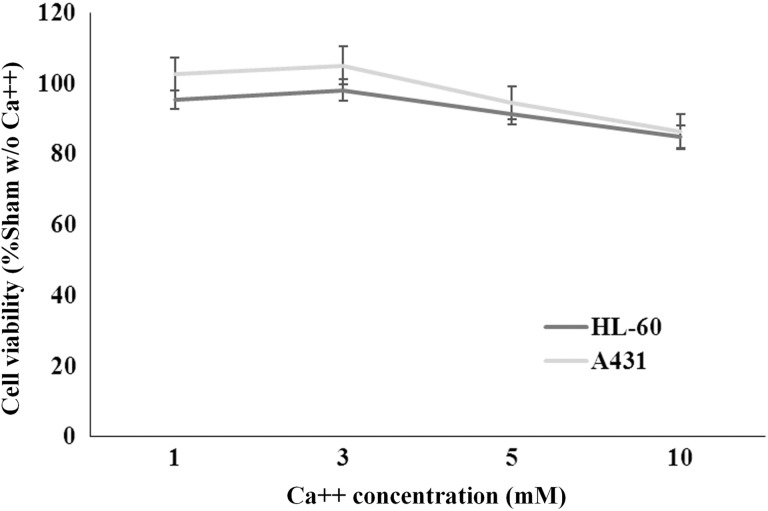
In both cell lines, calcium alone had no significant impact on cell viability at all the concentrations tested (1, 3, 5, and 10 mmol/L) as assessed by the resazurin assay at 24 hours after treatment (P > .05, 1-way ANOVA followed by Bonferroni test). Results are presented in Figure 3, as mean ± SE of 18 independent experiments with triplicates, obtained by pooling together calcium concentration data from all the experiments carried out.
Figure 3.
Cell viability of HL-60 and A431 cells under calcium treatment. Samples were treated with increasing calcium concentrations in the absence of pulsing. Each data is the mean ± SE of 18 independent experiments. SE indicates standard error.
Calcium EP-Induced Cell Death
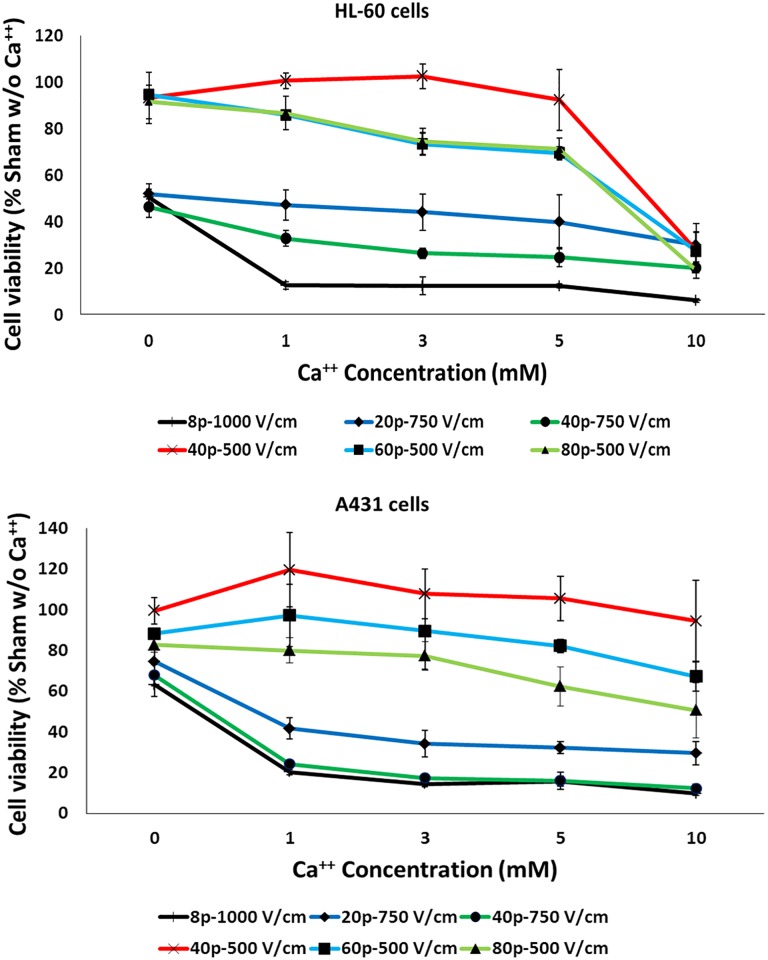
In Figure 4, cell viability (normalized to sham samples in the absence of calcium) of HL-60 and A431 cells, subjected to different pulsing protocols, in the presence of increasing calcium concentrations and assayed at 24 hours postpulse, is presented.
Figure 4.
Calcium electroporation-induced cell death. Cell viability of HL-60 and A431 cells exposed to different pulsing protocols in the presence of increasing calcium concentrations. Each data point is mean ± SE of 3 independent experiments with triplicates. SE indicates standard error.
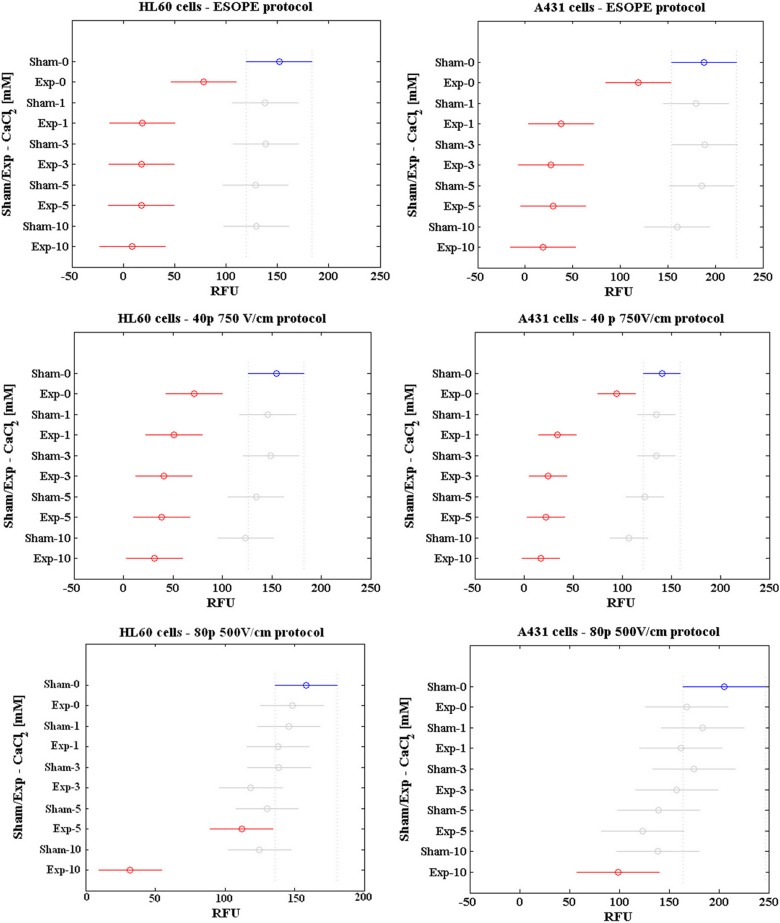
The results of the statistical analysis are reported in Figure 5 as mean of RFU values in sham/PEF-exposed samples in the presence of different calcium concentrations, with a 95% confidence interval for the mean. Overlapping intervals are representative of data points that are not statistically different between each other. The results of 3 representative pulsing conditions (ESOPE, 40 pulses at 750 V/cm and 80 pulses at 500 V/cm), for each cell line, are reported for the sake of brevity. The results relative to the remaining pulsing conditions are presented in Supplementary Figure S1.
Figure 5.
Results of the statistical analysis. Two-way ANOVA with Bonferroni test on cell viability data as obtained from resazurin assay. Data are reported as mean RFU. ANOVA indicates analysis of variance; RFU, relative fluorescence units.
In both cell lines, ESOPE and the ESOPE-equivalent protocols (20 and 40 pulses delivered at 750 V/cm with 0 mM CaCl2) resulted in a significant decrease in cell viability (P < .05 vs sham without calcium in the case of ESOPE and 40 pulses 750 V/cm protocol), with an average residual viability of 49% and 68% for HL-60 and A431 cells, respectively. When exposures were carried out in the presence of calcium, in both cell lines all the concentrations tested increased significantly the efficacy of ESOPE and ESOPE-equivalent protocols in inducing cell death (P < .05 vs sham without calcium), with no significant differences among the calcium concentrations (P > .05).
The remaining protocols (40, 60, and 80 pulses, 500 V/cm) did not induce significant reduction in HL-60 and A431 cell viability when delivered in the absence of calcium (P > .05 vs sham without calcium). When exposures were carried out in the presence of calcium, in the case of HL-60 cells, the 40 and 60 pulses, 500 V/cm protocols resulted in a statistically significant reduction in cell viability only in the presence of 10 mmol/L CaCl2 (P < .05), while the 80 pulses, 500 V/cm protocol was effective with both 5 and 10 mmol/L CaCl2 (P < .05). In the case of A431 cells, only the 80 pulses, 500 V/cm protocol with 10 mmol/L CaCl2 was effective in reducing cell viability (P < .05). These results confirm the higher sensitivity of HL-60 cells with respect to A431 cells.
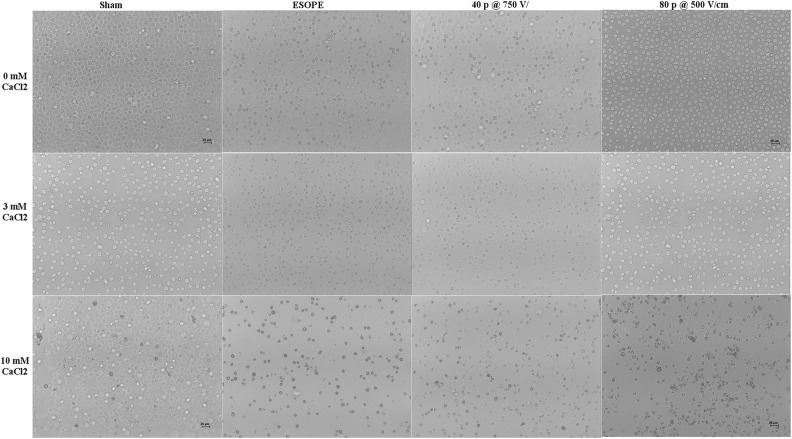
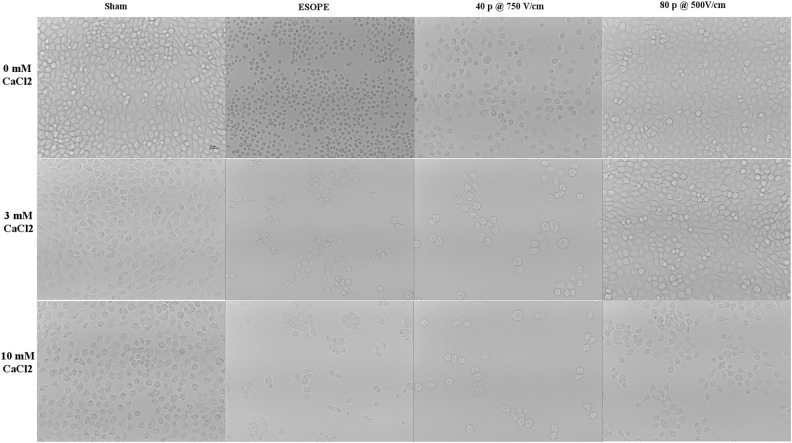
In Figures 6 and 7, the morphological features of HL-60 and A431 cells subjected to calcium EP under selected pulsing conditions are presented. In particular, in both cell lines, cells exposed to ESOPE or to 40 pulses, 750 V/cm protocols look shrunk, darker, and more sparse with respect to sham, with these features becoming more evident in the presence of 3 and 10 mmol/L calcium. Cells exposed to 80 pulses, 500 V/cm, look similar to respective sham, except when exposed in the presence of 10 mmol/L calcium.
Figure 6.
Morphological features of HL-60 cell cultures (scale bar 10 µm). Images show samples exposed/sham exposed to ESOPE, 40 pulses 750 V/cm, 80 pulses 500 V/cm in the presence of CaCl2 0, 3, and 10 mM (×10 images acquired by a Leica ICC50W camera mounted on a Leica DMIL inverted microscope). ESOPE indicates European Standard Operating Procedure on Electrochemotherapy.
Figure 7.
Morphological features of A431 cell cultures (scale bar 10 µm). Images show samples exposed/sham exposed to ESOPE, 40 pulses 750 V/cm, 80 pulses 500 V/cm in the presence of CaCl2 0, 3, and 10 mmol/L (×10 images acquired by a Leica ICC50W camera mounted on a Leica DMIL inverted microscope). ESOPE indicates European Standard Operating Procedure on Electrochemotherapy.
Discussion
In this article, 2 different cancer cell lines have been used to identify ESOPE-equivalent pulsing protocols alone and in combination with calcium in such a way to expand and optimize the range of drugs and pulsing conditions that can be effective in EP-based treatments in the clinics.
We have demonstrated here that, with respect to the ESOPE standard, lower electric field amplitudes (750 V/cm) can be used, if a suitable number of pulses is applied, for inducing comparable cell membrane permeabilization. This confirms the results reported by Ongaro and coworkers.12 In that previous work, the extent of EP induced by different pulsing protocols was qualitatively evaluated by fluorescence microscopy in a single-cell model, based on the electropermeabilized area around the electrodes applied to cells in a culture dish. Here we have provided a quantitative evaluation of EP with 2 different cell models, by flow cytometric measurements, which yields more robust statistics. The possibility to compensate the decrease in pulse amplitude by increasing pulse numbers has been demonstrated in other publications.11,32
The demonstration here that changes in the value of 1 pulse parameter can be compensated by selected values for other parameters is relevant for the optimization of EP applications that require different pulse settings. In this respect, simple empirical mathematical relations between pulse parameters have been derived, such as power, logarithmic, and exponential functions.11,33
Moreover, we have demonstrated that ESOPE-equivalent protocols can be successfully applied to induce cell death when provided in the presence of calcium chloride, a compound already approved for clinical use.19 In accordance with previous studies, we have observed that calcium alone, at concentrations between 1 and 10 mmol/L, does not significantly affect cell viability.21 By applying equivalent EP protocols in combination with calcium, our results demonstrate the possibility of inducing substantial cell death with less intense pulsing protocols. In particular, in the presence of calcium, both the standard ESOPE protocol and the 2 ESOPE-equivalent protocols (20 pulses at 750 V/cm and 40 pulses at 750 V/cm) resulted in a strong reduction in cell viability in both cell lines. In these cases, varying the calcium concentration, in the range here considered, had no significant effect on viability. When pulses with the lower electric field value 500 V/cm were applied, cell viability decreased with increasing calcium concentration in both cell lines. However, in the case of HL-60 cells, the 40- and 60-pulse protocols significantly altered the cell viability only with 10 mmol/L calcium, while the 80-pulse protocol was effective already at 5 mmol/L calcium. In the case of A431 cells, only the 80-pulse protocol significantly reduced cell viability and only at 10 mmol/L calcium. It can be argued that with the more efficient EP protocols, the uptake of calcium is maximized already at lower external concentrations and that, as a consequence, the effect of different calcium concentrations on cell viability can be observed only in the case of milder pulsing protocols or also with the more efficient EP protocols but with lower calcium concentrations.
Interestingly, in our cell models, both ESOPE and ESOPE-equivalent protocols without calcium resulted in a decrease in cell viability at 24 hours postpulse, a time point at which any reversible EP effect can be ruled out, with an average 49% and 68% residual viability among the protocols for HL-60 and A431 cells, respectively. It is demonstrated that pH or temperature changes due to pulsing can affect cell viability. However, in our experimental conditions, pH changes were minimized by using a buffered, low conductivity solution and cuvettes with stainless steel electrodes,34,35 and a significant temperature increase can be excluded even in the highest energy level delivered to the sample.36 This finding is consistent with other studies, where a significant cell death was observed 24 hours postpulse, with a residual viability depending on the cell type.21,26,27 Thus, our observations confirm that determination of thresholds for reversible and irreversible EP is not straightforward, that it is dependent on cell type, and that the transition from one condition to the other is a continuum and not an on–off phenomenon.33
The 2 cell lines studied here exhibit different sensitivity to PEF exposure and to calcium EP-induced cell death, with HL-60 showing higher susceptibility than A431 cells. This is consistent with many reports showing that cells growing in suspension, like HL-60, are more sensitive to PEFs than cells growing in attachment to a substrate, like A430,37,38 although also other factors such as cell shape39 and size,40 membrane composition,41 population density,42 and many other physical and biological properties33 are known, from theory and practice, to affect EP efficiency.
In spite of the differences between in vitro and in vivo models, and in the clinical settings, it has been demonstrated that the ECT parameters identified in vitro are applicable in vivo.15–17 Indeed, in vitro testing enables the evaluation of a broad range of conditions, which would be impractical on patients, and the identification of optimized ECT settings ready to be applied in vivo and transferred to the clinics.
In conclusion, the results presented here suggest that the application of ESOPE-equivalent protocols could provide new treatment options in both standard ECT and calcium EP that could be pursued to advantage in the clinic. In particular, applying a higher number of pulses with lower electric field amplitude with respect to the ESOPE standard protocol may (1) avoid the risk of exceeding the operational limits of pulse generators due to heterogeneity of impedance characteristics of large nodules; (2) reduce the intensity or the extent of muscle contractions, and may also provide a reduction in discomfort during pulse application,43 which can be also accomplished by the application of high-frequency bipolar pulses13; and (3) increase the electrode therapeutic range or develop electrodes for deep-seated tumors.44
Furthermore, the possibility of applying equivalent protocols in combination with calcium provides further support to the potential use of calcium EP in cancer therapy. Due to the pivotal role of calcium in defining the fate of cells, further investigations will be devoted to study the cellular mechanisms of such combined treatments and to give insight into the different response between healthy and cancer cells.
Supplemental Material
Supplemental Material, Fig_S1 for ESOPE-Equivalent Pulsing Protocols for Calcium Electroporation: An In Vitro Optimization Study on 2 Cancer Cell Models by Stefania Romeo, Anna Sannino, Maria Rosaria Scarfì, P. Thomas Vernier, Ruggero Cadossi, Julie Gehl, and Olga Zeni in Technology in Cancer Research & Treatment
Acknowledgment
The technical assistance of Dr Roberta Rispoli is gratefully acknowledged.
Abbreviations
- ANOVA
analysis of variance
- CAM
calcein acetoxymethyl ester
- DMEM
Dulbecco’s modified Eagle medium
- ECT
electrochemotherapy
- EP
electroporation or electropermeabilization
- ESOPE
European Standard Operating Procedure on Electrochemotherapy
- FBS
foetal bovine serum
- IRE
irreversible electroporation
- PEF
pulsed electric field
- PI
propidium iodide
- PBS
phosphate-buffered saline
- RFU
relative fluorescence unit
- RT
room temperature
- RPMI
Roswell Park Memorial Institute
- SE
standard error.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Regione Campania (DD N. 74 of 04/05/2015). Call title: POR-FESR 2007-2013—Bando per la realizzazione della rete delle biotecnologie in Campania. Project title: “Bersagli, sonde e segnali in terapia e diagnostica.”
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Weaver JC. Electroporation – a general phenomenon for manipulating cells and tissues. J Cell Biochem. 1993;51(4):426–435. [DOI] [PubMed] [Google Scholar]
- 2. Jiang CL, Davalos RV, Bischof JC. A review of basic to clinical studies of irreversible electroporation therapy. IEEE Trans Biomed Eng. 2015;62(1):4–20. [DOI] [PubMed] [Google Scholar]
- 3. Lambricht L, Lopes A, Kos S, Sersa G, Preat V, Vandermeulen G. Clinical potential of electroporation for gene therapy and DNA vaccine delivery (vol 13, pg 295, 2016). Expert Opin Drug Del. 2016;13(5):769–769. [DOI] [PubMed] [Google Scholar]
- 4. Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy — an easy, highly effective and safe treatment of cutaneous and subcutaneousmetastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Euro J Cancer. 2006;4(11):3–13. [Google Scholar]
- 5. Cadossi R, Ronchetti M, Cadossi M. Locally enhanced chemotherapy by electroporation: clinical experiences and perspective of use of electrochemotherapy. Future Oncol. 2014;10(5):877–890. [DOI] [PubMed] [Google Scholar]
- 6. Miklavcic D, Sersa G, Brecelj E, et al. Electrochemotherapy: technological advancements for efficient electroporation-based treatment of internal tumors. Med Biol Eng Comp. 2012;50(12):1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frandsen SK, Hansen HF, Gehl J. New drugs for electrochemotherapy with emphasis on calcium electroporation In: Miklavcic D, ed. Handbook of Electroporation. Switzerland: Springer International Publishing; 2016:13. [Google Scholar]
- 8. Mir LM, Gehl J, Sersa G, et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the Cliniporator (TM) by means of invasive or non-invasive electrodes. EJC Suppl. 2006;4(11):14–25. [Google Scholar]
- 9. Gehl J, Sersa G, Matthiessen LW, et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. [published ahead of print March 25, 2018]. doi:10.1080/0284186X.2018.1454602. [DOI] [PubMed] [Google Scholar]
- 10. Meschini S, Condello M, Lista P, et al. Electroporation adopting trains of biphasic pulses enhances in vitro and in vivo the cytotoxic effect of doxorubicin on multidrug resistant colon adenocarcinoma cells (LoVo). Euro J Cancer. 2012;48(14):2236–2243. [DOI] [PubMed] [Google Scholar]
- 11. Pucihar G, Krmelj J, Rebersek M, Napotnik TB, Miklavcic D. Equivalent pulse parameters for electroporation. IEEE Trans Biomed Eng. 2011;58(11):3279–3288. [DOI] [PubMed] [Google Scholar]
- 12. Ongaro A, Pellati A, Caruso A, et al. Identification of in vitro electropermeabilization equivalent pulse protocols. Technol Cancer Res Treat. 2011;10(5):465–473. [DOI] [PubMed] [Google Scholar]
- 13. Miklavcic D, Mali B, Kos B, Heller R, Sersa G. Electrochemotherapy: from the drawing board into medical practice. Biomed Eng Online. 2014;13(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Girelli R, Prejano S, Cataldo I, et al. Feasibility and safety of electrochemotherapy (ECT) in the pancreas: a pre-clinical investigation. Radiol Oncol. 2015;49(2):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larkin JO, Collins CG, Aarons S, et al. Electrochemotherapy – aspects of preclinical development and early clinical experience. Ann Surg. 2007;245(3):469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Breton M, Mir LM. Microsecond and nanosecond electric pulses in cancer treatments. Bioelectromagnetics. 2012;33(2):106–23. [DOI] [PubMed] [Google Scholar]
- 17. Sersa G, Miklavcic D, Cemazar M, Rudolf Z, Pucihar G, Snoj M. Electrochemotherapy in treatment of tumours. Eur J Surg Oncol. 2008;34(2):232–240. [DOI] [PubMed] [Google Scholar]
- 18. Saczko J, Kaminska I, Kotulska M, et al. Combination of therapy with 5-fluorouracil and cisplatin with electroporation in human ovarian carcinoma model in vitro. Biomed Pharmacother. 2014;68(5):573–580. [DOI] [PubMed] [Google Scholar]
- 19. Frandsen SK, Gissel H, Hojman P, Eriksen J, Gehl J. Calcium electroporation in three cell lines: a comparison of bleomycin and calcium, calcium compounds, and pulsing conditions. BBA Gen Subj. 2014;1840(3):1204–1208. [DOI] [PubMed] [Google Scholar]
- 20. Frandsen SK, Gissel H, Hojman P, Tramm T, Eriksen J, Gehl J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012;72(6):1336–1341. [DOI] [PubMed] [Google Scholar]
- 21. Hansen EL, Sozer EB, Romeo S, Frandsen SK, Vernier PT, Gehl J. Dose-dependent ATP depletion and cancer cell death following calcium electroporation, relative effect of calcium concentration and electric field strength (vol 10, e0122973, 2015). PLoS One. 2015;10(5):e0122973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frandsen SK, Gehl J. Effect of calcium electroporation in combination with metformin in vivo and correlation between viability and intracellular ATP level after calcium electroporation in vitro. PLoS One. 2017;12(7):e0181839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frandsen SK, Krüger MB, Mangalanathan UM, et al. Normal and malignant cells exhibit differential responses to calcium electroporation. Cancer Res. 2017;77(16):4389–4401. in press. [DOI] [PubMed] [Google Scholar]
- 24. Zhivotovsky B, Orrenius S. Calcium and cell death mechanisms: a perspective from the cell death community. Cell Calcium. 2011;50(3):211–221. [DOI] [PubMed] [Google Scholar]
- 25. Frandsen SK, Gibot L, Madi M, Gehl J, Rols MP. Calcium electroporation: evidence for differential effects in normal and malignant cell lines, evaluated in a 3D spheroid model. PLoS One. 2015;10(12):e0144028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frandsen SK, McNeil AK, Novak I, McNeil PL, Gehl J. Difference in membrane repair capacity between cancer cell lines and a normal cell line. J Membr Biol. 2016;249(4):569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zielichowska A, Daczewska M, Saczko J, Michel O, Kulbacka J. Applications of calcium electroporation to effective apoptosis induction in fibrosarcoma cells and stimulation of normal muscle cells. Bioelectrochemistry. 2016;109:70–78. [DOI] [PubMed] [Google Scholar]
- 28. Falk H, Matthiessen LW, Wooler G, Gehl J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018;57(3):311–319. [DOI] [PubMed] [Google Scholar]
- 29. Falk H, Forde PF, Bay ML, et al. Calcium electroporation induces tumor eradication, long-lasting immunity and cytokine responses in the CT26 colon cancer mouse model. OncoImmunology. 2017;6(5):e1301332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Falk H, Lambaa S, Johannesen HH, Wooler G, Venzo A, Gehl J. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma – a case report. Acta Oncol. 2017;56(8):1126–1131. [DOI] [PubMed] [Google Scholar]
- 31. O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267(17):5421–5426. [DOI] [PubMed] [Google Scholar]
- 32. He HQ, Chang DC, Lee YK. Using a micro electroporation chip to determine the optimal physical parameters in the uptake of biomolecules in HeLa cells. Bioelectrochemistry. 2007;70(2):363–368. [DOI] [PubMed] [Google Scholar]
- 33. Kranjc M, Miklavčič D. Electric field distribution and electroporation threshold In: Miklavcic D, ed. Handbook of Electroporation. Switzerland: Springer International Publishing AG 2016; 2016:16. [Google Scholar]
- 34. Saulis G, Lape R, Praneviciute R, Mickevicius D. Changes of the solution pH due to exposure by high-voltage electric pulses. Bioelectrochemistry. 2005;67(1):101–108. [DOI] [PubMed] [Google Scholar]
- 35. Maglietti F, Michinski S, Olaiz N, Castro M, Suarez C, Marshall G. The role of pH fronts in tissue electroporation based treatments. PLoS One. 2013;8(11):e80167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weaver JC, Smith KC, Esser AT, Son RS, Gowrishankar TR. A brief overview of electroporation pulse strength-duration space: a region where additional intracellular effects are expected. Bioelectrochemistry. 2012;87:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pakhomova O, Gianulis E, Pakhomov A. Different cell sensitivity to pulsed electric field In: Miklavcic D, ed. Handbook of Electroporation. Switzerland: Springer International Publishing; 2016:1–17. [Google Scholar]
- 38. Vernier PT, Thu MMS, Marcu L, Craft CM, Gundersen MA. Nanosecond electroperturbation – mammalian cell sensitivity and bacterial spore resistance. IEEE Trans Plasma Sci. 2004;32(4):1620–1625. [Google Scholar]
- 39. Pucihar G, Kotnik T, Valic B, Miklavcic D. Numerical determination of transmembrane voltage induced on irregularly shaped cells. Ann Biomed Eng. 2006;34(4):642–652. [DOI] [PubMed] [Google Scholar]
- 40. Teissie J, Rols MP. An Experimental evaluation of the critical potential difference inducing cell-membrane electropermeabilization. Biophys J. 1993;65(1):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Levine ZA, Vernier PT. Calcium and phosphatidylserine inhibit lipid electropore formation and reduce pore lifetime. J Membr Biol. 2012;245(10):599–610. [DOI] [PubMed] [Google Scholar]
- 42. Pucihar G, Kotnik T, Teissie J, Miklavcic D. Electropermeabilization of dense cell suspensions. Eur Biophys J Biophy. 2007;36(3):173–185. [DOI] [PubMed] [Google Scholar]
- 43. Heller R, Cruz Y, Heller LC, Gilbert RA, Jaroszeski MJ. Electrically mediated delivery of plasmid DNA to the skin, using a multielectrode array. Hum Gene Ther. 2010;21(3):357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mahmood F, Gehl J. Optimizing clinical performance and geometrical robustness of a new electrode device for intracranial tumor electroporation. Bioelectrochemistry. 2011;81(1):10–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Fig_S1 for ESOPE-Equivalent Pulsing Protocols for Calcium Electroporation: An In Vitro Optimization Study on 2 Cancer Cell Models by Stefania Romeo, Anna Sannino, Maria Rosaria Scarfì, P. Thomas Vernier, Ruggero Cadossi, Julie Gehl, and Olga Zeni in Technology in Cancer Research & Treatment