Abstract
Background and Aims:
Several drug combinations have been tried in patients with acyanotic congenital heart disease (ACHD) undergoing transcatheter device closure in the cardiac catheterisation laboratory (CCL). Adequate sedation, analgesia, akinesia, cardiorespiratory stability, and prompt recovery are key requirements. Ketamine with propofol is used for this purpose. Dexmedetomidine carries a shorter recovery time. This study compared dexmedetomidine–propofol (DP) with ketamine–propofol (KP) in patients in the CCL.
Methods:
This was an open label randomised trial at a CCL over a 2-year period from August 2012 to August 2014. Fifty-six paediatric and 44 young adults with ACHD underwent device closure and were randomised to receive DP or KP. The primary outcome studied was time to regain full consciousness, airway and motor recovery.
Results:
Baseline characteristics were similar in the study groups. In the DP arm as compared to the KP arm, the time to recovery of consciousness (mean ± SD) was significantly faster in both paediatric patients [30 ± 15 vs. 58 ± 13 min (P < 0.001)] and in young adult patients [22 ± 10 vs. 35 ± 12 min (P < 0.001)]. There was significantly faster motor recovery also (mean ± SD) [paediatric: 25 ± 05 vs. 40 ± 14 (P < 0.001); young adult: 10 ± 05 vs. 22 ± 10 min (P < 0.001)].
Conclusion:
Procedural anaesthesia with DP in paediatric and young adult patients with ACHD undergoing device closure in the CCL resulted in faster recovery of consciousness and motor recovery compared to KP.
Keywords: Dexmedetomidine, ketamine, propofol
INTRODUCTION
Interventions performed in cardiac catheterisation laboratory (CCL) on paediatric and young adult patients are increasing due to better expertise, better availability of devices, non-operative advantage and shorter hospitalization with lesser morbidity. Though anaesthesia for device closure procedures comes with unique challenges and risks, there is no single best technique or a fixed drug combination regimen for it.[1] Anaesthetic technique should cater to the requirements of the patient, procedure, and the performer with a goal of ensuring sedation, analgesia, and akinesia, while maintaining spontaneous respiration along with haemodynamic stability. Multiple techniques and drug combinations such as ketamine–midazolam, ketamine–dexmedetomidine, ketamine–propofol (KP), and dexmedetomidine–propofol (DP) have been used successfully for anaesthesia in device closure procedures in CCL.[2,3,4,5] The emergence reactions of ketamine in children are less intense, making it appropriate for use in paediatric CCL procedures. However, the haemodynamic instability and prolonged recovery period make it less suitable. Propofol has long been recommended and used in CCL procedures due to rapid emergence. However, it is required to be combined with another drug as it does not have an analgesic effect. Dexmedetomidine is an α2-adrenoreceptor agonist that possesses sedative, analgesic, and anxiolytic properties with no or limited effects on respiratory depression.[6,7] It modulates the release of catecholamines from the sympathetic nervous system and decreases the central sympathetic outflow.[8,9] Dexmedetomidine has been approved by the US Food and Drug Administration for use in adults as well as in paediatrics for sedation in non-intubated patients prior to or during surgical procedures. It has been shown by various studies that addition of dexmedetomidine to propofol for sedation and analgesia is well tolerated with a shorter recovery time, decreased movement, and reduced need for airway interventions.[10,11] In our study, we compared DP with KP for anaesthesia in paediatric and young adult patients with acyanotic congenital heart lesions undergoing device closure procedures in CCL.
METHODS
The study was an open label randomised controlled trial conducted over 2 years from August 2012 to August 2014 in the CCL of a tertiary care hospital. Paediatric and young adult patients having congenital acyanotic heart diseases considered amenable for device closure by the Interventional Cardiologist were eligible. Cardiac lesions in the enrolled population included secundum atrial septal defects (ASD), ventricular septal defect (VSD), and patent ductus arteriosus (PDA). Paediatric cases below 7 years age, presence of haemodynamic instability, need for inotropic support, or assisted ventilation resulted in exclusion of the patient from the study. The study was approved by the Institutional Ethics Committee. Patients were stratified into paediatric (7–16 years) and young adult (17–25 years) categories. Patients were randomised into DP or KP group after stratification using an online service (www.randomization.com). Serially numbered opaque-sealed envelopes were used for allocation concealment. Masking of the intervention was not done. Pre-anaesthetic checkup was carried out diligently for all patients that included history and clinical examination, assessment of the airway, complete blood counts, renal function tests, prothrombin time, activated partial thromboplastin time, and international normalized ratio, electrocardiogram and, transthoracic echocardiography. Written informed consent for the procedure and enrollment for the study was taken from the parents. The cardiovascular evaluation involved history of easy fatigability, increasing shortness of breath, orthopnoea, paroxysmal nocturnal dyspnoea, and examination for signs of congestive heart failure such as hepatomegaly, pedal oedema, raised jugular venous pulsations, and basal crackles. Pre-procedure fasting was done as per protocol and an intravenous (IV) cannula was placed. On arrival in the CCL, ringers lactate infusion was started as determined by the patient's weight. All patients were pre-medicated with IV midazolam (0.05mg/kg) and glycopyrrolate (10 μg/kg) 10 min before the procedure. Strict compliance of pre-operative fasting guidelines was followed and when in doubt oro-gastric tube aspiration was done to empty the stomach contents. Ondansetron and ranitidine were given parenterally to all the patients before the procedure. Patients were pre-oxygenated before induction of anaesthesia. Following induction, ventilation was supported using face mask and Bain circuit for the period of transient apnoea. Once the patient was breathing spontaneously post induction, they were maintained on supplemental oxygen by face mask at 4-6 liters per minute targeting oxygen saturation (SpO2) of 98-100%. Supplemental oxygen was continued during the procedure. Respiratory depression and airway obstruction due to tongue fall or loss of airway tone was managed by head and neck positioning, Guedel oro-pharyngeal or nasopharyngeal airway, supplemental oxygen, and assisted ventilation. The recommended dose of all the drugs used in the study was similar (in mg/kg body weight) for both paediatric and young adult patients. Patients randomised to the DP group received a bolus of dexmedetomidine at 1 μg/kg and propofol at 2 mg/kg over 10 min followed by an infusion of dexmedetomidine 0.25–0.75 μg/kg/h and propofol at 4–6 mg/kg/h. Dexmedetomidine has linear and similar pharmacokinetics in paediatric age group and adults. Patients randomised to the KP group received a bolus of ketamine 1 mg/kg and propofol 2 mg/kg over 10 min followed by an infusion of ketamine at 0.5 mg/kg/h and propofol infusion at 4–6 mg/kg/h. Any additional requirement of sedation and/or analgesia was recorded. Following the bolus administration of the drugs as per the protocol and once the target modified Steward sedation scale (MSSS)[12] score of 1, i.e., an unconscious patient with spontaneous ventilation and akinesia, was obtained with the bolus, infusions of propofol and ketamine in group KP or dexmedetomidine and propofol in group DP was maintained at a constant rate. Depending on the therapeutic response and intra-procedural cardiorespiratory parameters, the dose of all the infusions was titrated individually near around the specified protocol doses so as to maintain a MSSS of 1 and maintain haemodynamic stability during the procedure. The heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), respiratory rate (RR), SpO2, and MSSS were recorded at baseline, after induction, and every 15 min. Invasive SBP, DBP, and MAP were continuously monitored during the procedure on the cardiac catheterisation console. Post-induction hypotension was managed with fluid boluses. All the drugs were administered after calculating the appropriate dose in mg/kg body weight. Towards the end of the procedure, in order to confirm the correct placement of the device transoesophageal echocardiography (TOE) was done. While inserting the probe the patient was given oxygen by nasal prongs. In case of gag reflex causing haemodynamic pressor response to the TEE probe or if the cardiologist was not satisfied with the sedation, a dose of rescue sedation was given using inj dexmedetomidine in a dose of 0.75 microgram/kg and inj ketamine in sub dissociative dose of 0.1-0.3 mg/kg in the respective groups while injection propofol was given in dose of 0.5 mg/kg in both the groups. Primary outcome of time to regain consciousness was defined as time in minutes required for the patient to be conscious and responding to verbal stimuli, airway recovery with return of gag reflex or cough, and motor recovery as purposeful movement of limbs. MSSS score of 6 indicated full consciousness. Secondary outcomes include respiratory depression, which was defined as decrease in respiratory rate or depth of respiration below the normal physiological limit with a drop in SpO2 of ≥10% from the baseline. Before labeling an event as respiratory depression, airway obstruction was ruled out. Respiratory support meant a need for assisted ventilation during the procedure. Need for rescue sedation or analgesia was defined as an increase in the target MSSS score from 1 manifesting as coughing, bucking, lacrimation, sudden movement, or variation in haemodynamic parameters (HR, BP) by >20% from baseline or a drop in SpO2 noted during TEE. Hypotension was defined as a blood pressure below 90/60 mmHg in young adults. In the pediatric population hypotension was defined as blood pressure below the fifth centile of the mean for age and gender.[13] Deviation of cardiorespiratory parameters from baseline and time to return to baseline was the change in the monitored parameters of HR, RR, SpO2, SBP, DBP, and MAP from the pre-procedure values and the time in minutes required for these parameters to return to baseline again. Fluid refractory hypotension was defined as persistence of hypotension despite two normal saline boluses of 20 ml/kg each, necessitating use of inotrope. Dopamine was started at 10 mcg/kg/min in patients with fluid refractory hypotension followed by dobutamine at the same dose if the blood pressure did not improve within 15 min. Maximum dose of both inotropes used was 20 mcg/kg/min each. Patient was managed for shock if there was failure to respond to a combination of these inotropes within 45 min. Post procedure patients were detained in the recovery room where HR, RR, SpO2, SBP, DBP, and MAP were continuously monitored using multi-function monitors. The parameters were recorded every 10 min. Patients were also monitored for recovery from anaesthesia by the MSSS. Monitoring of the limb where the arterial puncture was done for colour warmth and peripheral pulse was also done. Stratified random sampling for difference between two means was done for two-tailed hypothesis testing.
The primary outcome was time to regain full consciousness, airway and motor recovery. Secondary outcomes included episodes of respiratory depression, airway obstruction, need for respiratory support, need for additional sedation or analgesia, hypotension and deviation of cardiorespiratory parameters from baseline and time to return to baseline.
The average time required for recovery of majority of patients following interventional catheterisation procedure is 35–45 min. A significant difference in the time to recovery of consciousness and airway and motor recovery was taken as ≥10 min (difference in the expected mean time between the two study groups). The standard deviation of the primary outcome in the control arm was 20 min. To demonstrate a 20% reduction in the time to recovery with α-error of 5% and power of 80%, the requisite sample size was calculated to be 100 patients. Descriptive statistics were used to describe baseline variables. Categorical outcome variables were analyzed by Chi-square test or Fisher's exact test wherever one or more expected cell size was <5. Numerical variables were first tested for normality. Cardiorespiratory and haemodynamics data between the two groups were analyzed using Student's t-test. P value of <0.05 was taken as significant. Statistical analysis was done using statistical software package SPSS version 20.0 and Microsoft Excel. The analysis was an ‘intention to treat analyses.’
RESULTS
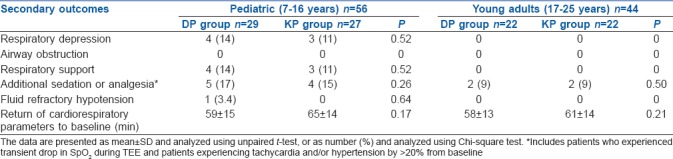
One hundred patients were enrolled in the study. There were 56 patients stratified as paediatric cases and 44 as young adult patients. Patients in both the stratified category were randomised to either DP or KP intervention arms. There were 29 paediatric patients in the DP arm and 27 in the KP arm. There were 22 young adult patients in the DP arm and a similar number in the KP arm [Figure 1]. The baseline characteristics were similar between the treatment arms of the stratified categories [Table 1]. There was no statistical difference in the nature of the cardiac lesions being addressed between the treatment arms. There was no difference in the variation of cardiorespiratory and haemodynamic parameters in patients between the two treatment arms in both the stratified categories. There were six patients in the DP arm [6/29 (21%)] and five in the KP arm [5/27 (18.5%)] of the pediatric cases who had disturbances in hemodynamic parameters, while two patients in the DP arm [2/22 (9%)] and five patients in the KP arm [5/22 (23%)] of the young adult group experienced hemodynamic disturbances. There was no difference in the incidence of hemodynamic disturbances in both the patient groups [Table 2]. The time to recovery of consciousness was significantly faster in patients randomised to the DP arm as compared to KP arm in paediatric patients (P < 0.001) as well as in young adult patients (P < 0.001). There was also a significantly faster motor recovery in patients in the DP arm as compared to KP arm in both the stratified populations (P < 0.001). There was no difference in the time to recovery of airway reflexes between the study groups [Table 3]. One paediatric case in the DP arm, one paediatric case and one young adult in the KP arm experienced a transient drop in SpO2 during TEE. All the secondary outcomes studied were comparable between the two arms in both the study groups. The need for rescue sedation or analgesia was seen in five children in the DP arm [5/29 (17%)] and four children in the KP arm [4/27 (14.8%)]. Among the young adult patients, two cases in both the DP and KP arm [2/22 (9%) each] required additional sedation or analgesia. There was no significant difference in the requirement of rescue sedation or analgesia in both the study groups. The time required for return of cardiorespiratory parameters to baseline was also not different between the patients in the two treatment arms in the stratified groups [Table 4].
Figure 1.
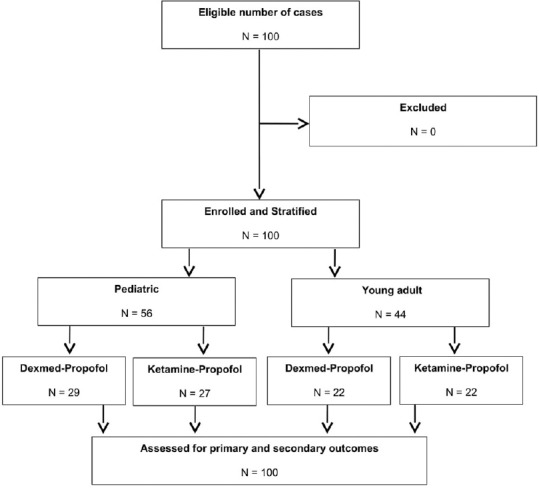
Flow of patients in the study
Table 1.
Baseline characteristics of the study population
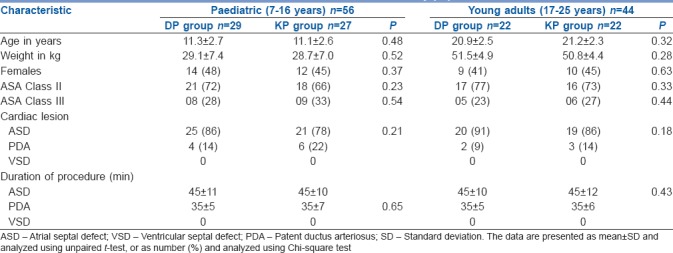
Table 2.
Incidence of disturbances in hemodynamic parameters
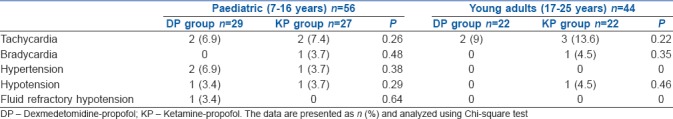
Table 3.
Primary outcome in the study population
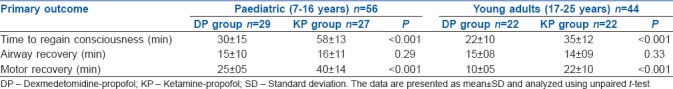
Table 4.
Secondary outcomes in the study population
DISCUSSION
This study was an open label randomised trial that compared anaesthesia using DP with KP in paediatric and young adult patients with acyanotic congenital heart disease lesions amenable to device closure in the CCL. The study included both paediatric and young adult patients together as both group of patients had stable cardiac functions, were on medical management, had similar indications for device closure, and were managed in the same setting. It was imperative that the anaesthetic technique allays pain, anxiety, and stress, which could lead to cardiorespiratory and haemodynamic compromise, and at the same time have a spontaneously breathing patient without an airway device for a successful procedural outcome. This was achieved by pre-procedure counseling, careful calculation of the drug dosage, appropriate pre-medication, prevention of hypothermia inside the cardiac catheterisation suite,[14] and other measures.
This study showed that there was a significantly faster recovery of consciousness and motor recovery in patients receiving anaesthesia with DP as compared to KP. There was also no significant difference in the incidence of respiratory depression, need for respiratory support, or loss of airway reflexes in patients between the two study arms. We found a very low incidence of respiratory depression in our study, possibly because of the younger age group with no associated co-morbidities. Patients in the DP arm of both the stratified groups showed an initial transient decrease in the SBP, DBP, and MAP, following initiation of anaesthesia, which soon returned to baseline without any additional intervention. The use of DP was well tolerated in the paediatric patients. These findings are in consonance with available evidence comparing dexmedetomidine with ketamine for various procedures.[4,15,16,17] Studies using ketamine anaesthesia for interventional cardiology procedure have reported lesser incidence of emergence reaction in paediatric patients,[18,19] but a longer recovery period and risk of haemodynamic instability putting it in disfavor.[20,21] Propofol has been shown to be effective for procedural sedation[22] and in combination with ketamine has been shown to be safe for CCL procedures.[23] This combination of ketamine with other anaesthetics has, however, been reported to have risk of haemodynamic instability in patients with left to right shunts undergoing cardiac catheterization.[24] A study comparing dexmedetomidine–ketamine with propofol–ketamine in a similar setting as ours showed no haemodynamic or respiratory adverse effects, but showed a longer time to recovery with dexmedetomidine–ketamine combination.[25] Another study comparing the effects of dexmedetomidine with propofol on cerebral oxygenation showed a statistically significant drop in the cerebral oxygenation between 5th and 10th minute into the procedure. However, the authors concluded that this drop was not clinically relevant, but in clinically unstable patients may be detrimental.[26] There is wide variation in the use and perception of procedural anaesthesia for CCL procedures largely influenced by culture, training, and geography.[27] In a study similar to ours, the authors reported more frequent agitation during recovery and a longer duration to regain baseline mental status in the ketamine group compared to the propofol group.[28] Our study did not show any significant difference in the cardiorespiratory or haemodynamic parameters patients in both treatment arms. There was overall greater tachycardia with KP as compared with DP and transient decrease in SBP, DBP, and MAP with reflex decrease in HR with DP, but neither of these findings were significantly different. There was also no difference in the time required for cardiorespiratory parameters to return to normal. Both the treatment strategies were found to be adequate with no significant difference in the need for additional sedation or analgesia. Transient episodes of drop in SpO2 noted during TEE were addressed by removing the probe, giving rescue sedation, and assisted ventilation with increased flow of oxygen. We preferred to use the MSSS for objectively scoring the primary outcome because of its simplicity and reproducibility, even though there is no consensus on the most appropriate scale for this purpose.[29]
The strength of this study was the adequate sample size, objective measure of outcomes of interest, wide range of patient population, and randomization of the intervention. The limitation of this study was the lack of masking of the intervention and that majority of the patients were clinically stable, thus limiting the application of the findings on clinically unstable patients with co-morbidities. Also, we are unable to report the cumulative dose of the drugs used between the study groups because of limitations in data collection.
CONCLUSION
In conclusion, our study shows that anaesthesia with DP as compared to KP in patients with acyanotic congenital heart lesions amenable to interventional cardiology device closure in the CCL have a significantly faster recovery of consciousness and motor activity. This combination was found to be safe and well tolerated in paediatric patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tosun Z, Akin A, Guler G, Esmaoglu A, Boyaci A. Dexmedetomidine-ketamine and propofol-ketamine combinations for anaesthesia in spontaneously breathing paediatric patients undergoing cardiac catheterization. J Cardiothorac Vasc Anesth. 2006;20:515–9. doi: 10.1053/j.jvca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Morray JP, Lynn AM, Stamm SJ, Herndon PS, Kawabori I, Stevenson JG. Haemodynamic effects of ketamine in children with congenital heart disease. Anesth Analg. 1984;63:895–9. [PubMed] [Google Scholar]
- 3.Williams GD, Jones TK, Hanson KA, Morray JP. The haemodynamic effects of propofol in children with congenital heart disease. Anesth Analg. 1999;89:1411–6. doi: 10.1097/00000539-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Ulgey A, Aksu R, Bicer C, Akin A, Altuntas R, Esmaoglu A, et al. Is the addition of dexmedetomidine to a ketamine-propofol combination in paediatric cardiac catheterization sedation useful? Pediatr Cardiol. 2012;33:770–4. doi: 10.1007/s00246-012-0211-1. [DOI] [PubMed] [Google Scholar]
- 5.Akin A, Esmaoglu A, Guler G, Demircioglu R, Narin N, Boyaci A. Propofol and propofol–ketamine in paediatric patients undergoing cardiac catheterization. Pediatr Cardiol. 2005;26:553–7. doi: 10.1007/s00246-004-0707-4. [DOI] [PubMed] [Google Scholar]
- 6.Belleville JP, Ward DS, Bloor BC. Effects of intravenous dexmedetomidine in humans: Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77:1125–33. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4:302–8. doi: 10.1186/cc712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandharipande P, Ely EW, Maze M. Alpha-2 agonists: Can they modify the outcomes in the postanaesthesia care unit? Curr Drug Targets. 2005;6:749–54. doi: 10.2174/138945005774574515. [DOI] [PubMed] [Google Scholar]
- 9.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bay) Univ Med Cent. 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young ET. Dexmedetomidine sedation in a paediatric cardiac patient scheduled for MRI. Can J Anaesth. 2005;52:730–2. doi: 10.1007/BF03016562. [DOI] [PubMed] [Google Scholar]
- 11.Tobias JD, Berkenbosch JW. Initial experience with dexmedetomidine in paediatric-aged patients. Paediatr Anaesth. 2002;12:171–5. doi: 10.1046/j.1460-9592.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- 12.Steward DJ. A simplified scoring system for the post-operative recovery room. Can Anaesth Soc J. 1975;22:111–3. doi: 10.1007/BF03004827. [DOI] [PubMed] [Google Scholar]
- 13.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents: The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(Suppl 4th Report):555–76. [PubMed] [Google Scholar]
- 14.Conway A, Kennedy W, Sutherland J. Inadvertent hypothermia after procedural sedation and analgesia in a cardiac catheterization laboratory: A prospective observational study. J Cardiothorac Vasc Anesth. 2015;29:1285–90. doi: 10.1053/j.jvca.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Koruk S, Mizrak A, Kaya Ugur B, Ilhan O, Baspinar O, Oner U. Propofol/dexmedetomidine and propofol/ketamine combinations for anaesthesia in paediatric patients undergoing transcatheter atrial septal defect closure: A prospective randomised study. Clin Ther. 2010;32:701–9. doi: 10.1016/j.clinthera.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Munro HM, Tirotta CF, Felix DE, Lagueruela RG, Madril DR, Zahn EM, et al. Initial experience with dexmedetomidine for diagnostic and interventional cardiac catheterization in children. Paediatr Anesth. 2007;17:109–12. doi: 10.1111/j.1460-9592.2006.02031.x. [DOI] [PubMed] [Google Scholar]
- 17.Hammer GB, Sam WJ, Chen MI. Determination of the pharmacodynamics interaction of propofol and dexmedetomidine during esophagogastroduodenoscopy in children. Paediatr Anaesth. 2009;19:138–44. doi: 10.1111/j.1460-9592.2008.02823.x. [DOI] [PubMed] [Google Scholar]
- 18.Laussen PC, Hansen DD, Perry SB, Fox ML, Javorski JJ, Burrows FA, et al. Transcatheter closure of ventricular septal defects: Haemodynamic instability and anesthetic management. Anesth Analg. 1995;80:1076–82. doi: 10.1097/00000539-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Kapoor MC, Sharma S, Sharma VK, Dugal JS, Singh C. Anaesthesia for percutaneous transcatheter closure of perimembranous ventricular septal defect. J Cardiothorac Vasc Anesth. 2006;20:202–8. doi: 10.1053/j.jvca.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Singh A, Girotra S, Mehta Y, Radhakrishnan S, Shrivastava S. Total intravenous anaesthesia with ketamine for paediatric interventional cardiac procedures. J Cardiothorac Vasc Anesth. 2000;14:36–9. doi: 10.1016/s1053-0770(00)90053-3. [DOI] [PubMed] [Google Scholar]
- 21.Reich DL, Silvay G. Ketamine: An update on the first 25 years of clinical experience. Can J Anaesth. 1989;36:186–97. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler DS, Vaux KK, Ponaman ML. The safe and effective use of propofol sedation in children undergoing diagnostic and therapeutic procedures: Experience in a paediatric ICU and a review of the literature. Pediatr Emerg Care. 2003;19:385–92. doi: 10.1097/01.pec.0000101578.65509.71. [DOI] [PubMed] [Google Scholar]
- 23.Kogan A, Efrat R, Katz J, Vidne BA. Propofol-ketamine mixture for anaesthesia in paediatric patients undergoing cardiac catheterization. J Cardiothorac Vasc Anesth. 2003;17:691–3. doi: 10.1053/j.jvca.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Malik M, Malik V, Chauhan S, Dhawan N, Kiran U. Ketamine-etomidate for children undergoing cardiac catheterization. Asian Cardiovasc Thorac Ann. 2011;19:143–8. doi: 10.1177/0218492311402132. [DOI] [PubMed] [Google Scholar]
- 25.Joshi VS, Kollu SS, Sharma RM. Comparison of dexmedetomidine and ketamine versus propofol and ketamine for procedural sedation in children undergoing minor cardiac procedures in cardiac catheterization laboratory. Ann Card Anaesth. 2017;20:422–6. doi: 10.4103/aca.ACA_16_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cetin M, Birbicer H, Hallioglu O, Orekeci G. Comparative study between the effects of dexmedetomidine and propofol on cerebral oxygenation during sedation at pediatric cardiac catheterization. Ann Card Anaesth. 2016;19:20–4. doi: 10.4103/0971-9784.173015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavi S, Jolly SS, Bainbridge D, Manji F, Randhawa V, Lavi R. Sedation, analgesia, and anaesthesia variability in laboratory-based cardiac procedures: An international survey. Can J Cardiol. 2014;30:627–33. doi: 10.1016/j.cjca.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 28.Ali NP, Kanchi M, Singh S, Prasad A, Kanase N. Dexmedetomedine–ketamine versus propofol–ketamine as anaesthetic agents in paediatric cardiac catheterization. J Armed Forces Med Coll Bangladesh. 2015;10:19–24. [Google Scholar]
- 29.Conway A, Page K, Rolley JX, Worrall-Carter L. A review of sedation scales for the cardiac catheterization laboratory. J Perianesth Nurs. 2014;29:191–212. doi: 10.1016/j.jopan.2013.05.017. [DOI] [PubMed] [Google Scholar]


