Abstract
Background and Aims:
Nasal surgery under desflurane anaesthesia is more prone to develop emergence agitation (EA). The present study aimed to evaluate the efficacy of dexmedetomidine for prevention of EA.
Methods:
A total of 72 patients were randomised to group C and group D. Group C patients received placebo while group D patients received dexmedetomidine 1.0 μg/kg bolus followed by 0.4 μg/kg/h after induction of anesthesia. End tidal desflurane was adjusted to keep the bispectral index (BIS) 45–55. Study drug was stopped at extubation. EA was evaluated from extubation till the patient was shifted to postanaesthesia care unit (PACU). Primary outcome was incidence of EA. Secondary outcome measures were requirement of desflurane, haemodynamic stability, and recovery after anaesthesia. The results were analyzed using SPSS version 21.
Results:
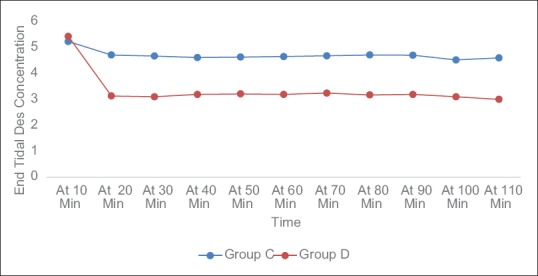
Infusion of dexmedetomidine significantly reduced the incidence of EA (Group C 52.8%; Group D 5.6%) by 89.5% (P = 0.00001). The endtidal desflurane concentration was significantly lower and there was an average 28.87% reduction in requirement of desflurane in group D compared to group C (P < 0.001). The mean heart rate was significantly higher in Group C (P < 0.001). In group C time to extubation, time to achieve BIS 90 and time to response on verbal command was significantly lesser compared to group D (P < 0.0001).
Conclusion:
Dexmedetomidine significantly reduced the incidence of EA and requirement of desflurane in patients undergoing nasal surgery. However, it was associated with delayed extubation, residual sedation, and prolonged PACU stay.
Keywords: Desflurane, dexmedetomidine, emergence agitation, nasal surgery
INTRODUCTION
Inhaled anaesthetic agents constitute the backbone of anaesthetic practice. The discovery of newer inhalational agents aimed at perfect agent, one that rapidly induces anesthesia, smells pleasant, safe, and is free of side effects. Over the past more than one and a half century anaesthesiology has come a considerable way to finding such an agent. Yet, even with the newest agents like sevoflurane and desflurane, there are side effects that keep it from being the ‘perfect’ anaesthetic agent. Desflurane has been shown to decrease time to awakening with faster eye opening, response to verbal command, orientation to person, place and time.[1] Therefore, its use can often cause emergence agitation (EA) during recovery from general anaesthesia.
EA may be associated with physical injury as well as negative postoperative behaviours. Although it occurs for short duration, it may require pharmacological intervention. Various agents including ketamine, propofol, clonidine, opioids, etc., have been used to prevent EA. However, these medications may increase sedation after anaesthesia, cause slow awakening, and in some cases are associated with undesirable side effects, such as nausea and vomiting.[2]
Dexmedetomidine acts on α2-adrenergic receptors and produces sedative, hypnotic, and anxiolytic effects without significant respiratory depression.[3] In children, it has been used extensively to reduce the incidence of EA.[4] Limited numbers of studies have evaluated the efficacy of dexmedetomidine for prevention of EA in adult patients undergoing nasal surgery.[5]
We hypothesize that dexmedetomidine infusion during the maintenance of anaesthesia leads to reduced incidence of EA in adult patients posted for nasal surgery under desflurane anaesthesia. This randomised, double blinded, placebo-controlled trial aimed to assess the effect of dexmedetomidine on incidence of EA, requirement of desflurane, intraoperative haemodynamics, and recovery after general anaesthesia.
METHODS
After getting institutional ethical committee approval and informed written consent from patients, the present study was conducted on 72 patients belonging to American society of Anesthesiologist (ASA) physical status I or II, aged between 18 and 65 years and scheduled for elective nasal surgery of more than 1 h duration under desflurane anaesthesia. Patients having severe systemic illness (cardiac, hepatic, renal pulmonary, endocrinal, neurological, or psychiatric disease), with substance abuse disorder, having body mass index >35 kg/m2, on medication (beta-blockers, α2-agonists, opioids, clonidine, and tricyclic antidepressant), with known allergic reactions to study drugs and pregnant/breast feeding females were excluded from study.
All the patients were thoroughly evaluated a day prior to scheduled operation. In operating room, standard monitoring including noninvasive blood pressure (NIBP), electrocardiogram, peripheral oxygen saturation (SpO2), and BIS electrodes were applied and baseline parameters were recorded. Intravenous (IV) access was secured at two sites for all the patients, one for IV fluids and the other for infusion of study drug. Following preoxygenation, all the patients received midazolam 0.05 mg/kg IV and fentanyl 2μg/kg IV slowly. Anaesthesia was induced with propofol 2.0–2.5 mg/kg IV targeting BIS score of 45–50. Atracurium 0.5 mg/kg IV was used to facilitate tracheal intubation.
Patients were randomised into one of the two study groups using computer-generated random number sequence. Group C patients received desflurane in 50:50 air and oxygen with a placebo infusion of 0.9% normal saline bolus for 10 min and then maintenance infusion after intubation and Group D patients received desflurane in 50:50 air and oxygen with dexmedetomidine infusion 1 μg/kg for 10 min as a bolus than 0.4 μg/kg/h after intubation. To ensure allocation concealment, opaque envelope method was used which was opened prior to surgery and the study drug based on sequence revealed was prepared by one of the anaesthesiologist in 50 mL syringe labeled as test. The dilution of the drug was based on patient weight in such a way that all the patients would receive loading infusion at 120 mL/h for first 10 min followed by 8 mL/h till the end of surgery. The attending anaesthesiologists, recovery, and ward nurses, as well as the patients were blinded to the computer-generated randomization schedule.
In both groups, desflurane was started at 6% dial flow concentration with 50:50 air and oxygen mixture along with the infusion of study drug. During the surgery, dial flow concentration of desflurane was titrated to maintain BIS 45–55. Endtidal concentration of desflurane (EtDes) was noted every 10 min in both the groups. The lungs were ventilated with a tidal volume of 6–8 mL/kg and respiratory rate of 12 per minute to target endtidal CO2 (EtCO2) concentration of 35–40 mm Hg. Intermittent boluses of fentanyl 1 μg/kg IV and atracurium bromide 0.1mg/kg IV were used as analgesic and muscle relaxant, respectively. During surgery NIBP, heart rate (HR), SpO2, BIS, EtDes, and EtCO2 was recorded at every 10 min interval. Paracetamol 1 g IV over 30 min and ondensetron 0.1 mg/kg IV was administered half an hour before the completion of surgical procedure. Desflurane and study drug was stopped when surgical dressing was applied and the time (T0) was noted. Neuromuscular blockade was antagonized using neostigmine 0.05 mg/kg and glycopyrrolate 0.01 mg/kg IV. The trachea was extubated on recovery of adequate muscle power.
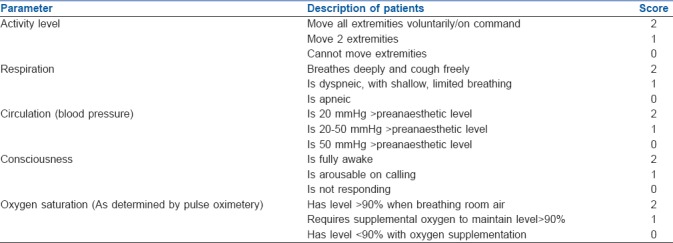
Emergence time was defined as the time interval from ‘T0’ till the patient was shifted to PACU once he/she tells his/her name. During this period, the level of agitation was evaluated using the Ricker sedation–agitation scale [Table 1],[6] and each patient's maximum agitation score was recorded. Simultaneously, the degree of pain was investigated through a numeric rating scale (NRS)[7] (0 = no pain, 10 = unimaginably severe pain). Patients with EA score of 5 and 6 required intervention in the form of verbal instruction and frequent verbal reminding of limit respectively, while patients with EA score of 7 required pharmacological interventions in the form of IV propofol 1 mg/kg. The measurements for EA and pain were repeated every 2 min to obtain the peak score. Time to extubation (TEXT) (time between T0 and extubation), time to achieve BIS of 90 (TBIS90) (time between T0 and recording of BIS value 90), time to response on verbal commands (TVERBAL) (time between T0 and patient respond by telling his/her name) and time to discharge from PACU (TDISCHARGE) [time between T0 and achievement of Aldrete score [Table 2][8] ≥9) were noted.
Table 1.
Ricker sedation-agitation scale
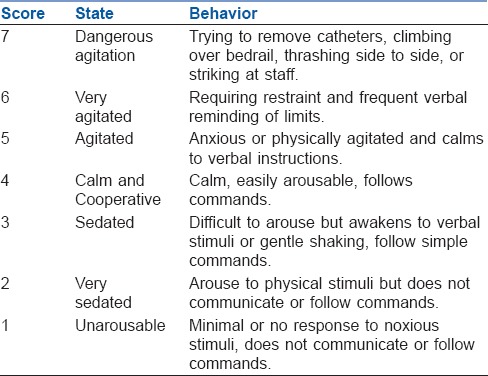
Table 2.
Aldrete score
Patients were also monitored by a blinded observer for residual sedation using Ramsay sedation scale,[9] nausea/vomiting, analgesic requirement, and other side effects like bradycardia, tachycardia, hypotension, hypertension, etc. The rescue analgesic (diclofenac sodium 75 mg intramuscular) was administered in PACU on patient request.
The primary outcome measure of our study was incidence of EA with dexmedetomidine, while the secondary outcome measures included requirement of desflurane, haemodynamic stability, recovery from general anaesthesia, residual sedation, analgesic requirement, and side effects.
Sample size calculation was based on our primary outcome measure i.e., incidence of EA. Kim et al. reported 52% incidence of EA in patients undergoing nasal surgery.[5] We assume that the 60% reduction in incidence of EA with dexmedetomedine would be of clinical relevance. The calculated sample size for the two sided test (α) of 5% and with power (1-β) of 80% would be 34 subjects in each group for proportional outcome.[10] We enrolled 36 subjects in each group. Summary data were tabulated and analysed using SPSS IBM software version 21 (IBM SPSS Advanced Statistics; Chicago, IL, USA). Categorical variables are expressed as percentages, whereas continuous data were checked for normal distribution by the Kolmogorov–Smirnov test and are reported as the means and standard deviations or the medians with 25th and 75th percentiles, when applicable. Categorical variables were analyzed by the Chi-square test. Comparisons of continuous data were performed by using the unpaired t-test for normally distributed variables and the Mann–Whitney U test for nonnormally distributed variables.
RESULTS
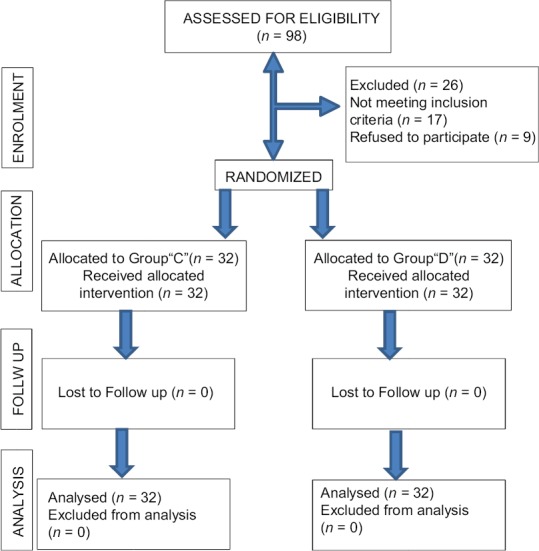
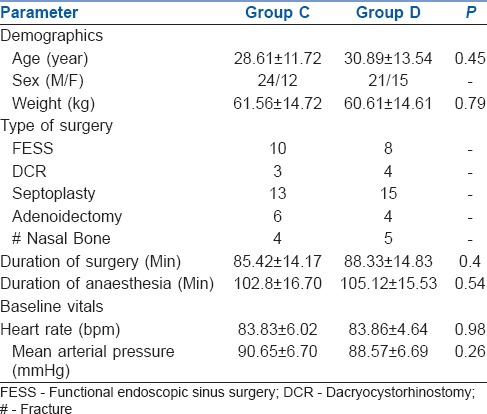
A total of 98 patients were screened, out of them 72 patients meeting inclusion criteria were enrolled in the study [Figure 1]. The demographic characteristics (age, weight, and gender), duration and type of surgery, duration of anesthesia, and baseline vitals were comparable between the groups (P > 0.05) [Table 3].
Figure 1.
Consort flow chart
Table 3.
Demographics, type and duration of surgery, duration of anesthesia, and baseline vitals of the groups
Nineteen (52.8%) patients in group C had EA out of which two (5.6%) patients had dangerous agitation while only two (5.6%) patients had EA and none of the patients had dangerous agitation in group D (P value = 0.00001) [Table 4]. Dexmedetomidine was effective in reducing the incidence of EA by 89.5%. Both the group displayed acceptable level of analgesia evaluated using NRS without any significant difference in the mean score [Table 4] (P = 0.179). Although there was no significant difference in MAP between the group, mean HR in group C was significantly higher compared to group D at all the time during observation period (P < 0.001). The EtDes concentration was significantly lower and there was an average 28.87% reduction in requirement of desflurane in group D compared to group C (P < 0.001) [Figure 2]. In group C TEXT, TBIS90, and TVERBAL (5.49 ± 0.41 min, 5.51 ± 0.39 min, and 5.64 ± 0.37 min, respectively) was significantly shorter compared to group D (8.54 ± 0.59 min, 8.87 ± 0.61 min, and 9.22 ± 0.60 min, respectively) (P < 0.0001) [Table 4].
Table 4.
Time to extubation, time to achieve BIS 90, time to telling name on verbal command, incidence of EA, residual sedation, analgesic, and antiemetic requirement in PACU and length of PACU stay in both groups
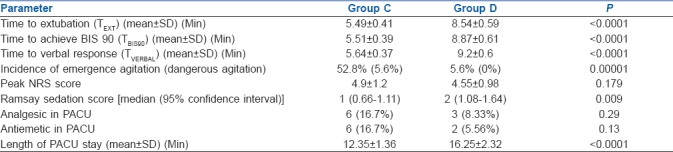
Figure 2.
EtDes recorded at different time interval in both groups
Patients in group C had significantly less residual sedation compared to group D (P value = 0.009) [Table 4]. The antiemetic requirement in groups C and D was 16.7% and 5.56% respectively (P = 0.13) [Table 4]. The analgesic requirement in groups C and D was 16.7% and 8.33% respectively (P = 0.29) [Table 4]. The length of PACU stay in group C was significantly less compared to group D (12.35 ± 1.36 min vs. 16.25 ± 2.32 min) (P < 0.01) [Table 4].
DISCUSSION
The results of our study suggest that intraoperative dexmedetomidine infusion (1 μg/kg over 10 min followed by 0.4 μg/kg/h) was effective in reducing the incidence of EA after nasal surgery. It also produced more stable haemodynamics during surgery as well as during extubation and significantly reduced the requirement of desflurane. However, it was associated with delayed extubation, residual sedation, and prolonged PACU stay.
Rapid recovery from anaesthesia has been associated with development of EA. It is commonly seen with the use of short acting volatile anesthetic agent like sevoflurane and desflurane. Also referred as emergence delirium or emergence excitement, it may lead to a dissociative state with altered cognitive perception, excitation, and agitation during recovery from anaesthesia. EA has been defined as a state of non-purposeful restlessness, non-cooperation, and inconsolability which is often accompanied by crying, screaming, thrashing, and disorientation.[11] Though agitation is observed more frequently in paediatric patients, the incidence in adults has been reported at 4.7% or 21.3%.[12,13] The wide variation in the reported incidence may be attributable to the use of different scoring scale for evaluating EA. An increase in noradrenaline release in the preoptic rat brains, especially in the locus coeruleus has been described.[14] However, association with other factors, such as pain, inhalational anaesthetics, preoperative benzodiazepine use, male gender, age, preoperative anxiety, and type of surgery has been suggested.[11]
EA is especially common after ENT surgery, where 55.4% of patients experienced agitation.[13] Various studies[13,15,16] reported that ENT surgical procedures particularly nasal surgeries in which intranasal packing is used, have a higher incidence of EA in both adults and children. The reported higher incidence may be attributed to a sense of suffocation during emergence from anaesthesia.[16] In present study, we enrolled the patients posted for nasal surgery under general anaesthesia with desflurane and were expected to have postoperative bilateral nasal packing.
Similar to the previously reported results, our study also demonstrated 52.8% incidence of EA in control group, whereas infusion of dexmedetomidine reduces the incidence by 89.5%.[5,13] Compared with control group, intraoperative dexmedetomidine infusion has been reported to significantly reduce the incidence of EA (risk ratio 0.34, 95% confidence interval 0.25–0.44, P < 0.00001) in children.[4] No systemic review and meta-analysis is available on dexmedetomidine infusion for prevention of EA in adults; however, few randomised controlled trials have shown reduction in the incidence of EA by 46% with dexmedetomidine compared to placebo.[5] Sedative property of dexmedetomidine is responsible for the reduction in incidence of EA; however, it also prolonged the time to extubation, time to achieve BIS 90, and time to response on verbal command. None of the patient developed respiratory depression in spite of its sedative property as dexmedetomidine does not depress respiratory drive so maintenance of dexmedetomidine infusion until extubation may be safe. Patients who developed EA required intervention in the form of verbal instruction only, while the patient who developed dangerous EA required intervention in the form of propofol. In present study, none of the patient receiving dexmedetomidine had dangerous agitation, while two patients receiving placebo had dangerous agitation.
Previous studies demonstrating effect of dexmedetomidine on EA in children used diverse protocol for administration of dexmedetomidine, e.g., loading of 0.5–2 mcg/kg followed by infusion of 0.4–0.7 mcg/kg/h, only loading of 0.5–4 mcg/kg, and only infusion of 0.2–1 mcg/kg/h,[4] and demonstrated significant advantage of dexmedetomidine in prevention of EA compared to placebo. For adults limited studies are available demonstrating effect of dexmedetomidine on EA, in a study dexmedetomidine infusion was administered at a dose of 0.4 mcg/kg/h without any bolus dose.[5] In our study, we administered dexmedtomidine 1 mcg/kg bolus followed by infusion of 0.4 mcg/kg/h.
Sympatholytic property of dexmedetomidine provides stable haemodynamic during surgery as well as after extubation. The mean HR in dexmedetomidine group was significantly lower than the control group at all time during observation. Our finding was in accordance with studies that found significant reduction in the intraoperative HR using dexmedetomidine.[17,18,19] Although hypotension is common after administration of the loading dose of dexmedetomidine,[20] we did not find any significant difference in the MAP between the group possibly because only ASA I and II patients were enrolled in our study. In a similar study, authors found significantly lower HR and MAP in patients receiving dexmedetomidine compared to control.[21] The observed difference in their MAP could be attributed to the higher age of the study population enrolled in the study (66.4 ± 8.2 years). Maintenance of dexmedetomidine until extubation provided more stable haemodynamic changes during emergence in our study.
Dexmeditomidine also possess anaesthetic-sparing effects and a reduction in the requirement of anaesthetic agent has been reported in various studies.[22,23] In our study, EtDes was significantly lower (28.87% reduction) to achieve desired BIS in dexmedetomidine group compared to placebo.
The inherent sedative property of dexmedetomidine account for significantly prolonged time to extubation, time to response on verbal command, time to achieve BIS value of 90, and time to discharge from PACU. In a similar study, time to extubation and time to verbal response was longer in dexmedetomidine group compared to placebo.[5] However, the time to extubation was not significantly prolonged. This could be attributed to differences in protocol for dexmedetomidine infusion (no loading dose, only infusion at 0.4 mcg/kg/h) and infusion of study drug for shorter duration (77 ± 29 min) compared to longer duration (102.8 ± 16.70) in our study.
Patients in dexmedetomidine group had significantly higher sedation score in the PACU compared to placebo. Patients who required rescue analgesics or antiemetics in the form of diclofenac-Na and ondansetron respectively were lower in the dexmedetomidine group compared to placebo; however, difference was not significant (P > 0.05). This could be possibly because patients were studied for less than 16 min postoperatively. In a similar study, authors found decreased requirements of analgesic and antiemetic in the dexmedetomidine group; but difference was not significant.[5]
There are few limitations in our study. Possible effects of pain and preoperative anxiety on EA could not be ruled out as we did not assess preoperative anxiety and pain was our secondary outcome measure. However, severity of pain might be similar between the groups during emergence because pain scores after extubation and use of additional analgesics in the PACU were not significantly different between the groups. Second, we did not evaluate the long-term effect of intraoperative dexmedetomidine on EA. We focused solely on recovery characteristics and smooth emergence after anaesthesia with dexmedetomidine administration in the immediate postoperative period (12.35 ± 1.36 min vs. 16.25 ± 2.32 min in the two groups until Aldrete score of ≥9 was achieved). However, EA can develop up to few hours postoperatively; therefore, further evaluation employing long-term follow-up is needed. Third, sample size calculation was based on our primary outcome, i.e. incidence of EA, so the conclusion made for the secondary outcome measure could not be generalized.
CONCLUSION
Intraoperative dexmedetomidine infusion reduces the incidence of EA in immediate postoperative period in adult patients undergoing nasal surgery under desflurane anaesthesia. However, it could lead to delayed recovery in terms of prolonged time to extubation, time to achieve BIS value of 90, time to patient response on verbal command, and time to discharge from postanaesthesia care unit.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lim BG, Lee O, Ahn H, Lee DK, Won YJ, Kim HJ, et al. Comparison of the incidence of emergence agitation and emergence times between desflurane and sevoflurane anesthesia in children. Medicine. 2016;95:e4927. doi: 10.1097/MD.0000000000004927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahmani S, Stany I, Brasher C, Lejeune C, Bruneau B, Wood C, et al. Pharmacological prevention of sevoflurane and desflurane related emergence agitation in children: A meta-analysis of published studies. Br J Anaesth. 2010;104:216–23. doi: 10.1093/bja/aep376. [DOI] [PubMed] [Google Scholar]
- 3.Soliman R, Alshehri A. Effect of dexmedetomidine on emergence agitation in children undergoing adenotonsillectomy under sevoflurane anesthesia: A randomised controlled study. Egypt J Anaesth. 2015;31:283–9. [Google Scholar]
- 4.Ni J, Wei J, Yao Y, Jiang X, Luo L, Luo D. Effect of dexmedetomidine on preventing postoperative agitation in children: A meta-Analysis. PLoS One. 2015;10:e0128450. doi: 10.1371/journal.pone.0128450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SY, Kim JM, Lee JH, Song BM, Koo BN. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth. 2013;111:222–8. doi: 10.1093/bja/aet056. [DOI] [PubMed] [Google Scholar]
- 6.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325–9. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Glossary. Spine. 2000;25:3200–2. [Google Scholar]
- 8.Aldrete JA. The postanesthesia recovery score revisited. J Clin Anesth. 1995;7:89–91. doi: 10.1016/0952-8180(94)00001-k. [DOI] [PubMed] [Google Scholar]
- 9.Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anaesthetic agent. Anesthesiology. 2004;101:787–90. doi: 10.1097/00000542-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 10.Chan YH. Randomised Controlled Trials (RCTs)- Sample Size: The Magic Number.? Singapore Med J. 2003;44:172–4. [PubMed] [Google Scholar]
- 11.Vlajkovic GP, Sindjelic RP. Emergence delirium in children: Many questions, few answers. Anesth Analg. 2007;104:84–91. doi: 10.1213/01.ane.0000250914.91881.a8. [DOI] [PubMed] [Google Scholar]
- 12.Lepouse C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96:747–53. doi: 10.1093/bja/ael094. [DOI] [PubMed] [Google Scholar]
- 13.Yu D, Chai W, Sun X, Yao L. Emergence agitation in adults: Risk factors in 2,000 patients. Can J Anaesth. 2010;57:843–8. doi: 10.1007/s12630-010-9338-9. [DOI] [PubMed] [Google Scholar]
- 14.Yasui Y, Masaki E, Kato F. Sevoflurane directly excites locus Coeruleus neurons in rate. Anesthesiology. 2007;107:992–1002. doi: 10.1097/01.anes.0000291453.78823.f4. [DOI] [PubMed] [Google Scholar]
- 15.Voepel-Lewis T, Malviya S, Tait AR. A prospective cohort study of emergence agitation in the paediatric postanesthesia care unit. Anesth Analg. 2003;96:1625–30. doi: 10.1213/01.ANE.0000062522.21048.61. [DOI] [PubMed] [Google Scholar]
- 16.Eckenhoff JE, Kneale DH, Dripps RD. The incidence and etiology of postanesthetic excitment. A clinical survey. Anesthesiology. 1961;22:667–73. doi: 10.1097/00000542-196109000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Bakhamees HS, El Halafawy YM, El Kerdawy HM, Gouda NM, Altemyatt S. Effects of dexmedetomidine in morbidly obese patients undergoing laparoscopic gastric bypass. Middle East J Anaesthesiol. 2007;19:537–51. [PubMed] [Google Scholar]
- 18.Patel A, Davidson M, Quraishi H. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg. 2010;111:1004–10. doi: 10.1213/ANE.0b013e3181ee82fa. [DOI] [PubMed] [Google Scholar]
- 19.Patel CR, Engineer SR, Shah BJ, Madhu S. Effect of intravenous infusion of dexmedetomidine on perioperative haemodynamic changes and postoperative recovery: A study with entropy analysis. Indian J Anaesth. 2012;56:542 6. doi: 10.4103/0019-5049.104571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: Dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17:576–84. doi: 10.1016/s1053-0770(03)00200-3. [DOI] [PubMed] [Google Scholar]
- 21.Kwon SY, Joo JD, Cheon GY, Oh HS, In JH. Effects of dexmedetomidine infusion on the recovery profiles of patients undergoing transurethral resection. Korean Med Sci. 2016;31:125–30. doi: 10.3346/jkms.2016.31.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J. Dexmedetomidine infusion during laparoscopic bariatric surgery: The effect on recovery outcome variables. Anesth Analg. 2008;106:1741–8. doi: 10.1213/ane.0b013e318172c47c. [DOI] [PubMed] [Google Scholar]
- 23.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian J Anaesth. 2011;55:352–7. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]


